FDA 铅含量测试方法
食品中铅的测定

23.2.1.4 含酒精性饮料或含二氧化碳饮料:
吸取10. 00 mL或20. 00 mL试样,置于250 mL~500 mL定 氮瓶中,加数粒玻璃珠,先用小火加热除去乙醇或二氧化 碳,再加5 mL-10 mL硝酸,混匀后,
以下,按23.2.1.1自“放置片刻··⋯”起依法操作,但定容 后的溶液每10 mL相当于2 mL试样。
称取05溶于50ml三氯甲烷中如不全溶可用滤纸过滤于250ml分液漏斗中用氨水199提取三次每次100ml将提取液用棉花过滤至500ml分液漏斗中用盐酸11调至酸性将沉淀出的二硫腙用三氯甲烷提取2次每次20ml合并三氯甲烷层用等量水洗涤两次弃去洗涤液在50水浴上蒸去三氯甲烷
食品中铅的测定
GB/T5009.12-2003 目次
21. 14 铅标准使用液: – 吸取1. 0 mL铅标准溶液,置于100 mL容量瓶中,加水 稀释至刻度。 – 此溶液,每毫升相当于10. 0 µg 铅, – 即:质量浓度ρ(Pb)= 10.0 µg/ml。
22 仪器
所用玻璃仪器
– 均用硝酸(10% -20%)浸泡24 h以上,用自来水反复冲 洗,最后用去离子水冲洗干净。
C02比色法概述.ppt
测定步骤
1. 静置分层后,三氯甲烷层(下层)经 脱脂棉滤入1 cm比色杯中,
2. 以纯三氯甲烷调节零点,于波长510 nm处测吸光度A,各点减去零管吸收 值后,绘制标准曲线或计算一元回归 方程,试样与曲线比较。
脱脂棉
721型分光光度计操作图.mht
表格形式
125ml的分液漏斗 0 1 2 3 4 5 样品液
21.9 硝酸(1+99) ;
– 量取1 mL硝酸,加人99 mL水中。
21. 10 二硫腙三氯甲烷溶液(0. 5 g/L)
铅测试方法

种植业产品
项目 方法
类型
前处理方 法
实验流程
仪器设备
称取试样0.2g~3g(精确至0.001g)于
锥形瓶中,加入10mL硝酸和0.5mL高
氯酸,在电热炉上消解。若消化液呈 □电子天平(SP2016—G621)
铅
GB 5009.12-2017 种植业产品 湿法消解 棕褐色,再加少量硝酸,消解至冒白 □石墨炉原子吸收
种植业产品
项目 方法
类型
前处理方 法
实验流程
仪器设备
称取试样0.2g~0.8g(精确至0.001g)
于微波消解罐中,加入5mL硝酸,
1mL过氧化氢,按照微波消解的操作
步骤(①130℃升温5min,恒温
10min②165℃升温5min恒温15min③
180℃升温5min,恒温15min)消解 □电子天平(SP2016—G621)
白烟,消化液呈无色透明或略带黄色,□电热板(SP2017—G1576)
冷却后用水定容至10mL,混匀备用。
同时做试剂空白试验。
计算公式
X=(C*f-C0)*V*1000/m/1000/1000 X:样品结果,mg/kg C:试样消化液中待测元素含量,µg/L C0:空白液中待测元素含量,µg/L V:样品消解液定容体积,mL f:稀释倍数 1000:换算系数
水产品
项目 方法
类型
铅
GB 5009.12-2017 水产品
前处理方 法
实验流程
仪器设备称取试样Leabharlann .2g~0.8g(精确至0.001g)
于微波消解罐中,加入5mL硝酸,
1mL过氧化氢,按照微波消解的操作
步骤(①130℃升温5min,恒温
铅标准储备液 比色法

铅标准储备液比色法是一种测定铅含量的方法,适用于食品、环境、药品等领域。
以下是该方法的步骤:
1. 配制铅标准储备液:准确称取适量的硝酸铅,溶解于适量的硝酸溶液中,转移到100毫升容量瓶中,用去离子水稀释至刻度。
该标准储备液的浓度为100微克/毫升。
2. 配制铅标准使用液:使用时,准确吸取一定量的铅标准储备液,加入适量的稀醋酸溶液,再加入适量的去离子水,制备成不同浓度的铅标准使用液。
3. 样品处理:将样品制备成溶液,进行前处理,以便与标准溶液进行比色测定。
4. 比色测定:在50毫升比色管中,分别加入不同浓度的铅标准使用液和样品溶液,加入适量的显色剂(如硫化钠),摇匀。
然后加入适量的醋酸溶液,再加入去离子水稀释至刻度。
5. 显色与测定:将上述溶液放置一定时间后,用紫外可见分光光度计进行比色测定,记录吸光度值。
6. 计算与分析:根据吸光度值与浓度之间的关系,绘制标准曲线。
通过比较样品溶液的吸光度值与标准曲线的差异,可以计算出样品中铅的含量。
同时,对结果进行误差分析,评估方法的可靠性。
需要注意的是,在实际操作中,要严格按照规定操作,
注意安全事项,避免误差的产生。
同时,为了提高测定的准确性和可靠性,可以进行多次测量和校准。
欧洲药典重金属检测

2.4.8 重金属下述方法需要使用硫代乙酰胺试剂。
作为另一种选择,硫化钠溶液(0.1ml)也常常适用。
由于各论中所述测试是使用硫代乙酰胺试剂研发出来的,如需用硫化钠溶液替代,需要包括方法A、方法B和方法H监测溶液,由测试规定的待测物的量进行配制,其已经加入了制备对照溶液规定量的铅标准溶液。
监测溶液至少要与对照溶液一样深,否则测试是无效的。
方法A供试溶液:12ml待测物水溶液。
对照溶液(标准):10ml规定的标准铅溶液(1ppm or 2ppm Pb)和2ml的待测液混合。
空白溶液:10ml的水和2ml的测试溶液混合。
向每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:相较于空白溶液,对照溶液呈浅棕色结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法B供试溶液:用含最少量水的溶剂(例如含15%水的二氧杂环乙烷或含15%水的丙酮)溶解成12ml待测液。
对照溶液(标准):10ml规定的铅标准溶液(1ppm or 2ppm Pb),加入2ml的待测液。
用待测物所用溶剂稀释100ppm Pb的铅标准溶液至1或2ppm Pb。
空白溶液:10ml待测物所用溶剂和2ml的待测溶液混合。
向每种溶液中,加入2ml pH为3.5的缓冲溶液。
混合后加1.2ml的硫代乙酰胺试液,立即混合。
2分钟后目测。
系统适用性:相较于空白溶液,对照溶液呈浅棕色结果:供试溶液的棕色不深于对照溶液。
若结果难以判断,进行膜过滤(孔径0.45μm)。
使用中等强度且恒定的压力缓慢且均匀地过滤。
比较不同溶液在过滤器上产生的斑点。
方法C供试溶液:规定量(不超过2g)的待测物质置于坩埚内,加4ml 250g/l硫酸镁的稀硫酸溶液。
玻璃棒搅拌混和,小心加热。
若混合物仍为液体,则在水浴中蒸发使其干燥。
铅的检测方法

铅的检测方法一、滴定法:原理:将一种已知准确浓度的试剂溶液,滴加到被测物质的溶液中,根据试剂溶液的浓度和用量,计算被测物质的含量。
例1:用盐酸-硝酸混合酸溶解试样,加入一定量的氯化钠防止铅析出,用氯化钠和盐酸稀释液稀释,在微酸性溶液中,用EDTA滴定法测定铅的含量。
例2:使Pb生成PbSO4沉淀与其它元素分离,在pH值5.5~6.0的醋酸-醋酸钠(铵)缓冲液中,使PbSO4转化为Pb(Ac)2,以二甲酚橙为指示剂,用EDTA标准溶液滴定。
(本法泛用于原矿、尾矿、精矿中含量在0.5以上的Pb)二、分光光度法:原理:分光光度法是通过测定被测物质在特定波长处或一定波长范围内光的吸光度或发光强度,对该物质进行定性和定量分析的方法。
基本定律是朗伯比尔定律。
例:以二溴羟基苯基卟啉为显色剂,配合物最大吸收波长为479nm,在最佳实验条件下绘制标准曲线,在0.06~1.00mg/ml范围内呈线性相关,线性回归方程为y=0.894x-0.022,相关系数为0.9994,摩尔吸光系数ε=2.8×105L·mol-1·cm-1,方法检出限为0.02ug/ml。
三、双波长分光光度法:双波长分光光度法是在传统分光光度法的基础上发展起来的,它的理论基础是差吸光度和等吸收波长。
它与传统分光光度法的不同之处,在于它采用了两个不同的波长即测量波长和参比波长同时测定一个样品溶液,以克服单波长测定的缺点,提高了测定结果的精密度和准确度。
四、双硫腙分光光度法:原理:双硫腙分光光度法是以双硫腙为螯合剂,使之与金属离子反应生成带色物质,而后用分光光度法测定该金属离子的方法。
这是环境监测中常用的一种间接、萃取分光光度法,是测定铅的常用方法,是基层单位经常采用的方法。
例:在水质标准中,采用双硫腙分光光度法对铅的测定:在pH为8.5-9.5的氨性柠檬酸盐-氰化物的还原性介质中,铅与双硫腙形成可被氯仿萃取的淡红色双硫腙铅螯合物,在510nm波长处可进行分光光度测定,从而求出铅的含量。
美国食品认证之 FDA测试项
Molecular weight Glass transition points
氯仿可溶提取物-去离子水
Net chloroform soluble extractives in distilled
Water
Net chloroform soluble
氯仿可溶提取物-50%乙醇 extractives in 50%
1680
FDA 21CFR 177.1580
—— US states law/regulation
1680
FDA 21CFR 177.1630
1120
660 *如果为内表面单面涂层必
须是可承装液体,否则需送
双面涂层或空白样品;
*如果外表面为涂层无法盛
装液体,需送送双面涂层或
660
空白样板,
*如果是胶水类样品,需要
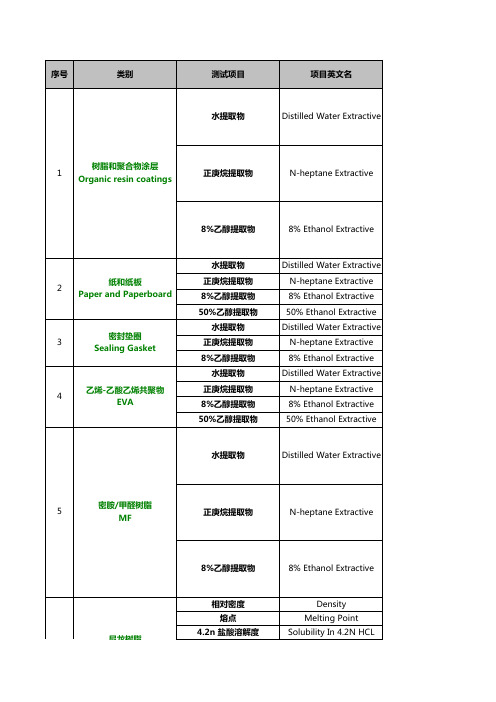
序号
类别
测试项目 水提取物
项目英文名 Distilled Water Extractive
1
树脂和聚合物涂层 Organic resin coatings
正庚烷提取物
N-heptane Extractive
8%乙醇提取物
8% Ethanol Extractive
2
纸和纸板 Paper and Paperboard
*如果外表面为涂层无法盛 装液体,需送送双面涂层或
660
空白样板,
*如果是胶水类样品,需要
客户自己做成涂层后送样检
测或提供处理方法。
660
1980
FDA 21CFR 175.300
560
560
560
560
需提供容器总面积,容器水
660
容量,样品为橡胶密封件
铅检测方法

铅检测方法本实验用的全部玻璃器具为硼硅酸玻璃制品或施行了甲基硅氨烷处理的器具(器具内壁用三甲基氯硅烷:吡啶:六甲基二硅氨烷(1:5:3)混合溶液浸湿,10分钟后水洗,干燥)。
另外,本试验中只能用除去铅的水和试剂。
1、试剂除下列试剂外,使用附录2所列试剂。
氨-氰化钾-亚硫酸钠溶液:在392mL氨水中,加入30mL氰化钾溶液和水至1000mL 后,加入1.5g亚硫酸钠溶解。
盐酸羟胺溶液:将20g盐酸羟胺溶于约65mL水中,移入分液漏斗内,加2~3滴麝香草酚蓝试液,加氨水至溶液呈黄色。
再加入10mL4%二乙基二硫代氨基甲酸钠,充分混匀后,静置,每次用10-15mL三氯甲烷提取,在5mL提取液中加入5滴硫酸铜溶液(1→100),振摇混合,不呈黄色时为终点。
然后,在抽提后的水溶液中加入稀盐酸至溶液变红色,再加入15mL三氯甲烷,振摇混匀后,静置,分取水层,加水至100mL。
灰化辅助液:将硝酸镁溶解在水中至饱和。
柠檬酸铵溶液:将250g柠檬酸铵溶于水中至500mL,滴加氨水,调节pH9。
随后,为了除铅,溶液中加入适量提取用双硫腙四氯化碳溶液,反复提取至双硫腙四氯化碳溶液的固有绿色不退为止。
溶液中残留的双硫腙先用氯仿,再用四氯化碳除去。
氰化钾溶液:将50g氰化钾溶于水中至100mL。
随后,为了除铅,溶液中加入适量的提取用双硫腙四氯化碳,反复提取至双硫腙四氯化碳溶液固有的绿色不褪为止。
溶液中残留的双硫腙先用氯仿,再用四氯化碳除去后,加水至500mL。
提取用双硫腙四氯化碳溶液:将40~50mg双硫腙溶于四氯化碳中至1000mL。
必要时,按下述方法配制。
将0.1g双硫腙溶于100mL氯仿中,移入分液漏斗内,加入100mL氨水溶液(1→100),振摇混合后,静置,收集氨提取液,用氨水重复提取2~3次,合并氨提取液,用少量四氯化碳洗涤数次后,加盐酸(1→2)使溶液稍呈酸性。
加入200mL四氯化碳,振摇混合后,静置,收集四氯化碳提取液,用四氯化碳重复提取2~3次,合并四氯化碳提取液,用50ml水洗涤后,加入适量的四氯化碳,调节至1000mL中含40~50mg双硫腙。
玩具产品材料中铅含量测试方法

玩具产品材料中铅含量测试方法一、前言美国总统布什于2008年8月14日签署了《消费品安全改进法案》(Consumer Product Safety Improvement Act of2008,简称CPSIA),该法案规定禁售任何对象为12岁或以下儿童、含铅量超过规定的产品。
法案对玩具产品材料中铅含量做出了明确的要求,因此如何测定玩具产品不同材料中的铅含量,给企业和测试机构提出了新的要求。
本文综述了目前国内外铅含量的主要测试方法,以供参考。
二、试剂除非另有说明,本文所使用的试剂均符合国家标准或行业标准,所使用的水均为去离子水或等同纯度的水(电阻率为18.2MΩ)。
所涉及的试剂及其纯度如下:2.1浓硝酸:优级纯;2.2过氧化氢(30%):分析纯;2.3浓盐酸:优级纯;2.4高氯酸:优级纯;2.5氢氟酸:分析纯;2.6硝酸(1+1,体积比):取1份浓硝酸(2.1)与1份水混合;2.7混合酸1(浓硝酸:高氯酸=4:1,体积比):取4份浓硝酸(2.1)与1份高氯酸(2.4)混合;2.8混合酸2(浓盐酸:浓硝酸:水=2:1:2,体积比):取2份浓盐酸(2.3)、1份浓硝酸(2.1)和2份水混合;2.9混合酸3(浓硝酸:氢氟酸=1:3,体积比):取1份浓硝酸(2.1)与3份氢氟酸(2.5)混合;2.10硝酸(2%):取2份浓硝酸(2.1)与65份水混合;2.11乙酸氨溶液(50%):取500g乙酸氨(NH4C2H3O2)溶于水中,定容至1L;2.12铅标准溶液:有证标准物质,浓度为100mg/L或1000mg/L。
三、设备试验中所用到的设备包括:3.1火焰原子吸收分光光度计(FLAA),配铅空心阴极灯;3.2电感耦合等离子体原子发射光谱仪(ICP-AES);3.3马弗炉;3.4微波消解炉;3.5可调式电热炉;3.6万分位电子分析天平。
四、样品处理目前,常见的样品处理有湿法消解、干法灰化和微波消解三种方法。
干法灰化法操作简便,但在高温下易损失;湿法消解法可以处理多种材料,但耗时长,同时需要使用高浓度的酸,造成的环境污染较严重;微波消解法操作简易,试验周期短,同时由于酸消耗量少,由酸引入的环境污染也大为降低,但同时处理样品的批量小,且仪器成本较高。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
J. Cosmet. Sci.,60, 405–414 (July/August 2009)Determination of total lead in lipstick: Development and validation of a microwave-assisted digestion, inductively coupled plasma–mass spectrometric methodNANCY M. HEPP, WILLIAM R. MINDAK, and JOHN CHENG,Offi ce of Cosmetics and Colors (N.M.H) and Offi ce of Regulatory Science(W.R.M., J.C.), Center for Food Safety and Applied Nutrition,U.S. Food and Drug Administration, College Park, MD 20740.Accepted for publication May 6, 2009.SynopsisRecent reports describing the presence of lead (Pb) in lipsticks have suggested that, under ordinary use, the potential amount of Pb exposure is harmful. To permit independent assessment of the Pb contamination, a method for determining total Pb in lipstick using microwave-assisted digestion and analysis employing in-ductively coupled plasma–mass spectrometry (ICP–MS) was developed and validated. Since lipsticks may contain fats, oils, pigments, dyes, and minerals, several reference materials (RM) were analyzed, including coal, wear metals in oil, organic Pb in oil, milk powder, and estuarine sediment. With the exception of the RM with mineral content (estuarine sediment), complete recovery of Pb from the RMs was obtained by simple nitric acid (HNO3) digestion. Complete recovery of Pb from estuarine sediment was achieved only when hydrofl uoric acid (HF) was added to the digestion mix, followed by treatment with excess boric acid (H3BO3) to neutralize the HF and to dissolve insoluble fl uorides. Commercial lipsticks were tested for total Pb by the validated method. The detection limit was estimated to be 0.04 µg Pb/g. The average value obtained for the lipsticks was 1.07 µg/g. Undigested material was present in some lipstick digests when only HNO3 was used, and generally lower Pb values were obtained. All of the Pb levels found by the U.S. Food and Drug Adminis-tration (FDA) were within the range the agency would expect to fi nd in lipsticks formulated with permitted color additives and other ingredients prepared under good manufacturing practice (GMP) conditions. This method will be useful for the FDA and industry in helping to ensure the safety of cosmetic products. INTRODUCTIONAlthough major sources of lead (Pb) contamination from leaded gasoline, Pb-based paints, Pb in public water systems, and Pb solder for sealing canned foods have been reduced through various regulatory actions, public concerns still exist over possible sources of Pb contamination. Pb from gasoline and paint can remain in soil and dust for many years, and imported foods and cosmetics may contain unsafe levels of Pb (1). The FDA issued warnings in 2003 for litargirio, a yellow- or peach-colored powder used in traditional remedies by people of Central America and the Caribbean region, particularly the Dominican Republic, because it contains up to 79% Pb. In 2003 and 2006 the FDA issued warnings for kohl, a traditional cosmetic eyeliner common in the Middle East, North Africa, Sub-Saharan Africa, and South Asia, because it frequently contains more than 50% Pb.405406JOURNAL OF COSMETIC SCIENCERecent media reports and e-mail hoaxes describing the presence of Pb in lipsticks have sug-gested that under conditions of ordinary use, the potential amount of Pb exposure is harm-ful (2–4). Pb contamination of lipsticks may originate from Pb solder or leaded paint in production equipment or from contaminated dust. Lipsticks also may be contaminated with Pb if they are manufactured with ingredients that naturally contain Pb or are produced under conditions that could introduce Pb into the ingredients. Dyes and pigments used as ingredients in lipsticks are regulated as color additives by the FDA and must undergo pre-market approval by the agency before they may be used in any cosmetics. The FDA controls potential Pb exposure from color additives by setting limiting specifications for Pb (5). Under current regulations, most color additives approved for cosmetic use are permitted to contain up to 20 µg Pb/g. In addition, certain color additives are required to be batch certi-fi ed by the FDA, and analysis for Pb is part of the certifi cation process.Other than color additives, the FDA does not have the statutory authority under the Federal Food, Drug, and Cosmetic Act (FD&C Act) to require pre-market approval of cosmetic products such as lipsticks or their ingredients. It is the responsibility of the manufacturer or distributor to ensure that cosmetic products and their ingredients are in compliance with requirements of the FD&C Act and other applicable laws and regula-tions (6). With the exception of color additives, a manufacturer may use any ingredient in the formulation of a cosmetic that does not cause the cosmetic to be adulterated or misbranded under the FD&C Act.Several methods have been reported for the analysis of Pb in lipstick and other cosmetics. Okamoto et al. (7) used a 1-gram portion of lipstick ignited at 500°C. The resulting ash was extracted with 20 ml and then 10 ml of 2N hydrochloric acid (HCl) and made up to 50 ml with 0.5% HCl. Pb was determined by atomic absorption spectrometry (AAS) using the standard addition method. A simple microwave-assisted acid extraction technique for determining Pb by inductively coupled plasma–optical emission spectrometry (ICP–OES) was reported by Besecker et al. (8). In their method, 0.15-g portions of several types of cosmetics were treated with 3.0 ml of HNO3 and heated in quartz vessels for a total time of 50 minutes at a maximum pressure of 74 bar. Accuracy was verifi ed by spike recoveries and by recoveries from an estuarine sediment reference material (RM) (National Institute of Standards and Technology, NIST, Estuarine Sediment SRM 1646). Besecker et al. men-tioned that their quartz vessels were cleaned with a mixture containing hydrofl uoric acid (HF). Others have noted an increased recovery of Pb when using vessels previously exposed to HF (9). Satisfactory recovery of Pb from the RM may not have occurred with vessels that had no prior HF exposure. The Lead Analysis Task Force of the Cosmetic, Toiletry, and Fragrance Association (CTFA, now the Personal Care Products Council) (10), developed a method for determining Pb in cosmetics using HNO3 and HF, microwave heating in sealed vessels, and for those cosmetics containing refractory materials, a subsequent treatment with boric acid (in smaller quantities than used in this study). This report presents a vali-dated method for determination of total Pb in lipstick, the Pb content of several lipstick products analyzed by the validated method, and a comparison with Pb content determined by other techniques.The FDA has developed and validated a method for determining Pb in lipstick in order to independently assess possible Pb contamination of lipstick products on the market. Analysis by x-ray fl uorescence (XRF) was initially investigated in order to avoid lengthy sample preparation. However, the technique is not sensitive enough with the availableDETERMINATION OF TOTAL LEAD IN LIPSTICK407 equipment and is subject to matrix absorption errors. AAS with electrothermal atomiza-tion analysis was also considered. However, since sample digestion would be necessary, inductively coupled plasma–mass spectrometry (ICP–MS) was chosen because of its po-tential for better sensitivity and speed. Thus, the effort focused on developing a method using microwave-assisted acid digestion for sample preparation and determination by ICP–MS.EXPERIMENTALCHEMICALS, REAGENTS, AND REFERENCE MATERIALSTwenty shades of lipstick sold in the United States under ten brand names were pur-chased from retail stores or provided by manufacturers. Multiple samples with the same lot number were obtained for several shades in order to compare analytical techniques. Six RMs were used for comparing different preparation techniques and for quality assurance: wear-metals in lubricating oil, SRM 1084a (NIST, Gaithersburg, MD); whole milk pow-der, SRM 8435 (NIST); estuarine sediment, SRM 1646a (NIST); lead in base oil 20 standard, ORG-PB8-2Y/Z (SPEX CertiPrep, Inc., Metuchen, NJ); base oil 20 standard (SPEX CertiPrep, Inc.); and trace elements in coal, SRM 1632c (NIST).American Society for Testing and Materials (ASTM) type 1 grade water was used to pre-pare reagents, standards, and analytical solutions. Pb standards (0, 0.1, 0.5, 1.0, and 10 ng Pb/ml), Pb stock (0.1 and 1.0 µg Pb/ml), and thallium internal standard (0.1 µg Tl/ml) solutions were prepared from commercial ICP–MS grade single-element analyte solu-tions (High-Purity Standards, Charleston, SC). Trace metals grade (TMG) HNO3 (Fisher Scientifi c, Pittsburgh, PA) was used for cleaning laboratory ware and digestion vessel lin-ers. Optima grade HNO3 and HF (Fisher Scientifi c) were used for sample, stock, and standard solutions. Boric acid (Puratronic grade, Alfa Aesar, Ward Hill, MA) was used to prepare 4% boric acid solution, which was conveniently dispensed with a bottle-top dis-penser. A 0.100-µg Pb/g in base oil 20 stock solution was prepared from 1000 µg Pb/g (SPEX ORG-PB8-2Y/Z organo-metallic standard solution) serially diluted to 10.00 and then to 0.100 µg Pb/g with SPEX base oil 20.Lipsticks were digested using XP-1500 Plus vessels in a MARS microwave digestion oven (CEM Corp., Matthews, NC). Pb determinations were performed on an Agilent 7500c ICP–MS (Agilent Technologies, Inc., Santa Clara, CA) equipped with a Peltier cooled Scott double-pass spray chamber and a MicroMist nebulizer (Glass Expansion, West Melbourne, Victoria, Australia). The built-in peristaltic pump was used to deliver the analytical and thallium internal standard solutions to the nebulizer at 0.17 ml/min and at 0.01 ml/min, respectively. The analytical and internal standard solutions were merged with a Tee fi tting. METHOD DEVELOPMENTLipstick is a challenging matrix of many ingredients including waxes, oils, dyes, and pigments (11). The pigments may include refractory minerals such as alumina, silica, titanium dioxide, and mica. Preliminary experiments with one lot of lipstick revealed an easily detectable amount of Pb, but quantitative results varied depending on preparation technique. In order to evaluate preparation techniques, a composite was prepared byJOURNAL OF COSMETIC SCIENCE408melting and mixing together eleven tubes of lipstick (same brand and shade, but several lot numbers). These lipsticks were placed in a beaker submerged in a water bath at 85°C and stirred with a propeller-type mixer.Initial attempts to completely dissolve a lipstick sample using typical microwave- assisted HNO3 digestion were unsuccessful. A cloudy, white suspension remained after the treatment. Also, replicate results for a single lot of lipstick showed variations in Pb recovery for different lipstick portion sizes and maximum digestion temperatures (see Table I).The portion size and temperature effects on Pb recovery suggested that microwave-as-sisted digestion with HNO3 was incomplete for Pb and that levels of Pb recovered would vary depending on digestion parameters. Therefore, other preparation procedures were investigated that might achieve total recovery of Pb. A dry ash procedure was tried in which a lipstick sample was heated gradually to 540°C and held at that temperature for an hour. The resulting residue was treated with HNO3 and HCl but would not com-pletely dissolve. The acid leachate was diluted with water and analyzed by ICP–MS. Results were variable and lower compared to values obtained with microwave-assisted acid diges-tion. Similarly, sodium carbonate fusion at 1075°C resulted in a residue that would not completely dissolve in HNO3 or HCl and produced variable values for Pb. The results indicated that some Pb must be associated with the undissolved refractory mineral matter in the lipsticks and suggested that HF would be necessary to break down the minerals. Based on work of other investigators (10), the initial HF dissolution procedure used 0.3-g portions of lipstick, 7 ml HNO3 + 2 ml HF, and sealed Tefl on microwave digestion ves-sels. A two-step procedure was used for digestion. The vessels were heated to 130°C over 15 minutes and held at that temperature for three minutes before ramping to 200°C over 15 minutes and holding for an additional 30 minutes. The vessels were allowed to cool to <50°C and then were vented. Initially 6 ml of 4% boric acid was then added to each vessel and the solutions were heated to 170°C over 15 minutes and held for ten minutes to complex the HF. Boron forms a strong complex with fl uoride according to the reaction shown in equation 1. After cooling and venting, the solutions were diluted to a fi nal vol-ume of 200 ml for ICP–MS analysis. However, the digests were still cloudy, with a ge-latinous precipitate appearing upon centrifugation. Also, results varied depending on the analytical portion. Equation 1 is as follows:+ B(OH)3→ HBF4+ 3H2O (1) 4HFTherefore, an excess of boric acid was used to dissolve any insoluble fl uorides. The revised HF digestion procedure used 30 ml of 4% boric acid and resulted in clear solutions for allTable IAnalytical Portion and Temperature Effects on Pb Determined After Digestion with Nitric Acid Portion size (g)Maximum digestion temperature (°C)µg Pb/g0.3240 1.40.1240 2.10.32000.5DETERMINATION OF TOTAL LEAD IN LIPSTICK 409lipstick samples and no variation in ICP–MS values for lipstick portion sizes ranging from 0.1 to 0.4 g (see Figure 1). The fi nal method parameters are outlined in Table II.For each lipstick sample, duplicate portions and portions fortifi ed at 0.5 and 1.0 µg Pb/g were digested. A Pb solution in 1% HNO 3 was used for fortifi cation. Each digestion batch of 12 vessels also included a blank, a blank + 0.02 µg Pb/g, an organic Pb RM (0.100 µg Pb/g in base oil 20), and an RM with mineral content (estuarine stediment, SRM 1646a).RESULTSMETHOD VALIDATIONAccuracy of the method was demonstrated by measuring recoveries of Pb from RMs and from fortifi ed lipstick samples. Since no lipstick-type RM was available, several RMs were analyzed representing varying matrix types. NIST 1635 trace elements in coal (complex matrix, organics); NIST 8435 whole milk powder (high fat matrix); NIST 1084a wear metals in oil and SPEX ORG-PB8-2Y/Z lead in base oil 20 (oily matrices Figure 1.Portion size effect before and after adding excess boric acid.● 0.3-g lipstick portion ● 2 ml HF + 7 ml HNO 3● CEM XP-1500+ vessels, MARS Microwave Digestion System ● Heat in two steps to 200°C, hold for 30 min:Stage Power (watts)Ramp (min)Pressure(psi)Temperature (°C)Hold (min)1120015:0008001303:002120015:00080020030:00● Cool to <50°C, vent, add 30 ml 4% boric acid ● Heat to 180°C, hold for 10 min ● Dilute to 200 mlJOURNAL OF COSMETIC SCIENCE410containing organically complexed Pb); and NIST 1646a estuarine sediment (refractory mineral matrix). Pb recoveries from RMs with and without HF are shown in Table III. Complete recovery of Pb from the RMs was obtained by simple HNO3digestion, with the exception of estuarine sediment, for which complete recovery was obtained only when HF was used in the digestion. Each lipstick sample was fortifi ed at two levels and analyzed following HNO3/HF digestion, with recoveries averaging 98.1%. Absence of matrix infl u-ence was shown by sequentially diluting several analytical solutions, with no signifi cant differences.Analytical solution stability was demonstrated by analysis of three representative analytical solutions over time. Two analytical solutions containing approximately 0.25 and 1.0 µg Pb/l were analyzed on days 1, 3, 7, and 14 using freshly prepared standard solutions on each day. There was <5% variation over the time period. A 10-µg Pb/l standard solution prepared on day 1 and analyzed with the analytical solutions on subsequent days behaved similarly.Method precision was demonstrated by between-day and within-day repeatability experiments. A 3% relative percent difference (RPD) was observed from analyzing 22 portions of lipstick composite over three days, and 2% RPD was obtained from analyzing 12 portions of one lipstick brand over three days. The precision of the instrument was tested by analyzing an analytical solution seven times on one day, yielding 2% RPD. The ruggedness of the method was demonstrated by varying the analytical parameters. There were no signfi cant differences in Pb results with portion size variations of 0.1 to 0.4 g. The volume of HF was varied from 0 to 4 ml (0, 0.5, 1.0, 2.0, 3.0, and 4.0 ml). Pb recov-ery became constant when ≥1 ml was used. The 4% boric acid solution amount was var-ied from 6 ml to 60 ml (6, 20, 30, 40, and 60 ml), with Pb recovery becoming constant when ≥20 ml was used. Solutions also became clear, eliminating the need for fi ltration or centrifugation before ICP–MS analysis. There was no signifi cant change in Pb recovery when the maximum disgestion temperature was lowered from 200°C to 180°C.As a measure of quality control, each digestion batch included two RMs: lead in base oil 20 (representing organic Pb in an oily matrix) and estuarine sediment (representing a mineral matrix). Recovery of Pb from the RMs is shown in Figure 2.Method blanks and method blanks spiked near the detection level were also included in each digestion batch. The average and standard deviation for blanks from fi fteen batchesTable IIILead Recoveries from Reference Materials and Lead Values from a Composited LipstickWith and Without HF*Certifi ed value(µg Pb/g)±95% C.I.HNO3 only(µg Pb/g)HF + HNO3(µg Pg/g)NIST 1635 (trace elements in coal) 1.90.2 1.8 (95%) 1.8 (95%) NIST 1084a (wear metals in oil)101.1 1.3103.7 (103%)SPEX ORG-PB8-2Y/Z (lead inbase oil 20)10001000 (100%)NIST 8435 (whole milk powder)0.110.050.10 (91%)0.11 (100%) NIST 1646a (estuarine sediment)11.7 1.28.2 (70%)10.8 (93%) Composited lipstick——0.29 2.91*Values are the average of 3 to 15 samples. Recoveries are indicated in parentheses.DETERMINATION OF TOTAL LEAD IN LIPSTICK 411are shown in Table IV , along with the aggregate RM results. Method blank results were used to estimate the detection limit of 0.04 μg Pb/g using equation 2 (12):Detection limit = (2 · t · σ · √(1 + 1/N)) (2)LIPSTICK SURVEY AND COMPARISON WITH VALUES BY OTHER METHODSTwenty-two lipstick samples (not including the composite), identifi ed by brand, shade, and lot number, were analyzed for Pb by the validated method. The results are summa-rized in Table V . All of the lipsticks contained detectable amounts of Pb, with values ranging from 0.09 to 3.06 µg/g and an average amount of 1.07 µg/g. Despite the limited size and color range of the survey samples (all were red shades), samples from a few manufacturers (A – C) appeared to contain the highest levels of lead.As stated above, recoveries from lipsticks fortifi ed with lead nitrate, Pb(NO 3)2, and ana-lyzed by the validated method (using HNO 3 and HF) averaged 98.1%. However, recov-eries from some lipsticks were equally good using digestion with HNO 3 alone (see Table III). Good recoveries were observed for all but one RM (the mineral-containing estuarine sediment) using either technique. This suggests that Pb-containing minerals were pres-ent in some but not all lipsticks.Figure 2.Lead recoveries from reference materials.NIST 1646a [estuarine sediment (µg/g)]SPEX ORG-PB8-2Y/Z [lead in base oil 20 (µg/g)]Method blank (µg/g)Blank + 0.02 µg Pb/g (µg/g)Composite lipstick Value(n = 13)Ref. value Value (n = 15)Ref. value Value (n = 15)Value (n = 22)Average10.8411.7 ± 1.20.1000.1000.0190.037 2.91S.D.0.36—0.007—0.0080.0090.09JOURNAL OF COSMETIC SCIENCE412The method developed here is for total Pb. Our studies showed that variable amounts of Pb can be extracted depending upon experimental conditions such as analytical portion, acids used, temperature, decomposition procedure, etc. Consistent results could usually be obtained only by including HF in the digestion procedure. To illustrate this, fi ve lipstick lots and the composite were analyzed by digestion with HNO 3 alone or with HNO 3 + HF. Substantially higher values were obtained for three of the lots and the composite using HNO 3 + HF compared to HNO 3 alone. Pb values for two different lots of the same lipstick were equivalent by digestion with HNO 3 alone or with HNO 3 + HF (see C-4, lots a and b). Results reported by the Campaign for Safe Cosmetics (CSC), in which several of the same brands and shades of lipsticks were analyzed (2), are also listed in Table V . The CSC method used a 0.5-g portion, extraction with sulfuric and nitric acids, and ICP–MS analysis. Lot numbers were not reported by the CSC.Differences among values obtained by the three digestion techniques can be explained by the presence of mineral content in some formulations, as well as by the fact that Pb is easily precipitated as a sulfate after sulfuric acid is used in the CSC extraction. Mica, a mineral permitted as a color additive, which frequently contains small amounts of Pb(13), would require HF for complete dissolution. The use of sulfuric acid in the CSC ex-Table V Lead Content in Lipsticks by Validated Method and by Two Other TechniquesProductShade Lot FDA: HF + HNO 3 (µg Pb/g)FDA: HNO 3 (µg Pb/g)CSC (µg Pb/g) (lot #s unknown)A 1a1.40b1.20, 1.220.12, 0.56c3.06 1.90d3.05B 1Composite2.910.29<0.02a2.38A 2a2.240.03, 0.03C 1a1.79<0.02, <0.02, 0.06A 3a1.760.28C 2a1.53b0.62, 0.680.50, 0.65c1.47 1.20B 2a1.370.91<0.02b0.83, 0.81C 3a1.210.19B 3a1.04<0.02, 0.03C 4a0.670.600.58b0.790.74, 0.80D 1a0.55<0.02D 3a0.480.03D 2a0.43<0.02E 1a0.330.09C 5a0.230.12F 1a0.170.12G 1a0.15<0.02, 0.04H 1a0.120.21I 1a0.10<0.02, 0.03J 1a 0.09<0.02DETERMINATION OF TOTAL LEAD IN LIPSTICK413 traction method may have reduced the soluble Pb available for ICP–MS analysis, thusresulting in the lower values reported by the CSC.The FDA has not set specifi cations for Pb in cosmetics, except that color additives per-mitted as ingredients are usually limited to 20 µg Pb/g (20 ppm) (5). The Pb levels found in these lipsticks, determined by the validated method, are within the range that might be expected from lipsticks formulated with permitted color additives and other ingredi-ents prepared under good manufacturing practice (GMP) conditions. CONCLUSIONSTypical microwave-assisted HNO3 digestion produced low-biased, inaccurate values for some lipstick samples and one of the RMs. All of the lipsticks analyzed in this work con-tained detectable amounts of Pb when digested with HNO3 and HF. Some of the Pb in certain lipstick samples appears to be incorporated in the refractory mineral pigments, which require HF for complete digestion. Pb levels found by the FDA are within the range that might be expected from lipsticks formulated with permitted color additives and other ingredients prepared under GMP conditions.ACKNOWLEDGMENTSThe authors thank Richard Jacobs of the FDA’s San Francisco District Lab for analyzing several lipsticks after a HNO3 extraction treatment. Nancy Hepp would like to thank Stephen Capar in the FD A’s Office of Regulatory Science and his group for allowing her to use his laboratories, equipment, and instrumentation to do the studies, and for providing helpful guidance. She would also like to thank Fred Hurley for advice and for sharing knowledge of cosmetics and lipsticks. Finally, we are very grateful to representa-tives from several cosmetic companies and the industry trade group, the Personal Care Product Council, for cooperation in providing samples and information about testing, and to Stanley Milstein in the FDA’s Offi ce of Cosmetics and Colors for coordinating all involved parties.REFERENCES(1) “Lead Poisoning,” March 15, 2007 accessed August 2008, </health/lead-poisoning/FL00068/ DSECTION=causes>.(2) “Campaign for Safe Cosmetics, A Poison Kiss: The Problem of Lead in Lipstick,” October 2007, ac-cessed October 2007, </your_health/poisonkiss.cfm>.(3) R. Paige, “Dangerous Levels of Lead in Lipstick, Lip Gloss?,” May 17, 2006, accessed July 2008, <http:///consumer/Lipstick.Lip.Gloss.2.516979.html>.(4) B. Thompson, “Is Lead Inside Lipstick,” July 24, 2006, accessed September 26, 2008, <http://www./print/9566833/detail.html>.(5) Code of Federal Regulations (2008) Title 21 (U.S. Government Printing Offi ce, Washington, DC), Sec-tions 73, 74, and 82.(6) Federal Food, Drug, and Cosmetic Act, Chapter XI, as amended January 2004.(7) M. Okamoto, M. Kanda, I. Matsumoto, and Y. Miya, Fast analysis of trace amounts of lead in cosmeticsby atomic absorption spectrophotometry, J. Soc. Cosmet. Chem.,22, 589–598 (1971).(8) K. D. Besecker, C. B. Rhoades, B. T. Jones, and K. W. Barnes, A simple closed-vessel nitric acid diges-tion method for cosmetic samples, Atom. Spectros.,19, 48–54 (1998).414JOURNAL OF COSMETIC SCIENCE(9) Y.-H. Xu, A. Iwashita, T. Nakajima, H. Yamashita, H. Takanashi, and A. Ohki, Effect of HF additionon the microwave-assisted acid-digestion for the determination of metals in coal by inductively coupled plasma–atomic emission spectrometry, Talanta,66, 58–64 (2005).(10) Cosmetics, Toiletries, and Fragrances Association, “Determination of Lead Content of Finished Cosmet-ics and Raw Materials by Closed-Vessel Microwave Digestion Graphite Furnace Atomic Absorption Spectrometry: Lead Method CTFA 1997.DOC JAW or SLW 2001” (internal document).(11) M. Schlossman, in Handbook of Cosmetic Science and Technology, 2nd ed., Paye, Barel, and Maibach, Eds.(CRC Press, Boca Raton, FL, 2006), pp. 579–580, 588–589.(12) L. A. Currie, Detection: International update, and some emerging dilemmas involving calibration, theblank, and multiple detection decisions, Chemomet. Intell. Lab. Syst.,37, 151–181 (1997).(13) Geochemical Reference Standards, USGS Certifi cate of Analysis Mica Schist, SDC-1, March 1995, accessedOctober 2008, </geo_chem_stand/mica.html>.。
