Controlled Fabrication of Iron Oxide Mesoporous Silica Core
DAPI染色液说明书

DAPI染⾊液说明书DAPI染⾊液产品简介:DAPI染⾊液(DAPI Staining Solution)是经过精⼼优化⼏乎适⽤于所有常见细胞和组织细胞核染⾊的染⾊液。
DAPI,即2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride,也称DAPI dihydrochloride,分⼦式为C16H15N5 ·2HCl ,分⼦量为350.25 ,CAS Number 28718-90-3。
DAPI是⼀种可以穿透细胞膜的蓝⾊荧光染料。
和双链DNA结合后可以产⽣⽐DAPI⾃⾝强20多倍的荧光。
和EB(ethidium bromide)相⽐,对双链DNA的染⾊灵敏度要⾼很多倍。
DAPI染⾊常⽤于细胞凋亡检测,染⾊后⽤荧光显微镜观察或流式细胞仪检测。
DAPI也常⽤于普通的细胞核染⾊以及某些特定情况下的双链DNA染⾊。
DAPI的最⼤激发波长为340nm,最⼤发射波长为488nm;DAPI和双链DNA结合后,最⼤激发波长为364nm,最⼤发射波长为454nm。
本DAPI染⾊液可以直接⽤于固定细胞或组织的细胞核染⾊。
保存条件:-20℃避光保存,⼀年有效。
注意事项:本DAPI 染⾊液的浓度经过碧云天的优化,确保可以满⾜各种常规染⾊的需要。
如需使⽤特定浓度的DAPI,请选购碧云天的DAPI(C1002)。
荧光染料都存在淬灭的问题,建议染⾊后尽量当天完成检测。
为减缓荧光淬灭可以使⽤抗荧光淬灭封⽚液。
抗荧光淬灭封⽚液(P0126)可以向碧云天订购。
DAPI对⼈体有⼀定刺激性,请注意适当防护。
为了您的安全和健康,请穿实验服并戴⼀次性⼿套操作。
使⽤说明:1.对于细胞或组织样品,固定后,适当洗涤去除固定剂。
随后如果需要进⾏免疫荧光染⾊,则先进⾏免疫荧光染⾊,染⾊完毕后再按后续步骤进⾏DAPI染⾊。
如果不需要进⾏其它染⾊,则直接进⾏后续的DAPI染⾊。
2.对于贴壁细胞或组织切⽚,加⼊少量DAPI染⾊液,覆盖住样品即可。
纳米零价铁材料的表面修饰及其检测典型环境污染物

Surface modification of nanoscale zerovalent iron particles and their applications in enrichment and determination of typicalenvironmental pollutantsABSTRACTSafarikova and Safarik first proposed magnetic solid phase extraction (MSPE) in 1999, and introduced it into environmental sample pre-treatment field. As a new mode of SPE. Magnetic solid phase extraction has merits such as simplicity, rapidness, high enrichment factor, short extraction time and low consumption of organic solvents. Nanoscale zero-valent iron (NZVI) has been widely used in environmental engineering field due to its high reactivity, large specific surface area, and abundant reactive sites, but there are very few reports on its applications in environmental analytical chemistry due to the fact that bare NZVI particles are easily oxided and agglomerated. Functional modification is a good way to enhance the various applicabilities for various pollutants and dispersities, and which make NZVI have great application prospect in the field of environmental analytical chemistry.In this paper, NZVI particles are modified with several functional materials. The new synthesized magnetic nanoparticles are investigated as the magnetic solid phase extraction adsorbents for preconcentration and determination of typical environmental pollutants prior to high performance liquid chromatography coupled with variable wavelength detection. The main contents are as follows:(1) Fe@MgAl-LDH magnetic nanoparticles were synthesized and characterized with TEM, XRD techniques, which was investigated to develop a novel method for proconcentration and determination of bisphenol A, 4-octylphenol and 4-nonylphenol from environmental water samples. Under the optimal conditions, linear ranges were obtained in the range of 0.5-200 μg·L-1, the detection limits were in the range of 0.24-0.34 μg·L-1, and the spiked recoveries were achieved in the range of 96.0-99.3%.(2) Fe@MOF-5 magnetic nanoparticles were prepared and characterized with TEM, EDS, XRD techniques. This new magnetic material was used as the adsorbent todevelop a new method for enrichmment and determination of carbazole, 9-methylcarbazole, dibenzothiophene, 4-methyldibenzothiophene and 4,6-dimethyldibenzothiophene from environmental water samples. Under the optimal conditions, there were good linearship between the concentration and the peak area in the range of 0.05-200 μg·L-1, the detection limits were in the range of 0.025-0.033 μg·L-1. Real water samples were used to validate the applicability of the proposed method, the spiked recoveries were in the range of 92.6-97.3%.(3) Fe@MIL-101(Cr) magnetic nanoparticles were prepared and characterized with TEM, EDS, XRD techniques, which was used to establish a magnetic solid phase method for proconcentration and determination of naphthalene, anthracene, fluoranthene and pyrene from environmental water samples. Under the optimal conditions, linear ranges were achieved in the range of 0.1-500 μg·L-1, the detection limits were in the range of 0.044-0.064 μg·L-1, and the spiked recoveries were in the range of 85.7-97.3%.(4) Fe@Ag@DMB magnetic nanoparticles were synthesized and characterized with TEM, EDS techniques. This new material was used to develop a novel method for simultaneous proconcentration and determination of Pb(II), Cd(II) and Hg(II) ions from environmental water samples. Under the optimal conditions, linear ranges were in the range of 0.05-200 μg·L-1, the detection limits were in the range of 0.011-0.031 μg·L-1, and the spiked recoveries were in the range of 97.5-103.2%.(5) Magnetic and thermos dual-responsive Fe@SiO2@p(NIPAM-co-DMMA) nanoparticles were prepared and characterized with TEM, XRD techniques, which was used as the adsorbent for developing a novel method for proconcentration and determination of four Sudan Red dyes from environmental water samples. Under the optimal conditions, linear ranges were obtained in the range of 0.05-500 μg·L-1, the detection limits were in the range of 0.005-0.013 μg·L-1, and the spiked recoveries were in the range of 97.4-102.6%.Keywords:nanoscale zero-valent iron core-shell materials;phenols;polycyclic aromatic hydrocarbons;heavy metal ions;Sudan Red dyes目录硕士学位论文独创性声明 (I)硕士学位论文版权使用授权书 (I)摘要 (II)ABSTRACT (IV)第1章绪论 (1)1.1 磁性固相萃取技术 (1)1.2 磁性纳米颗粒表面修饰及功能化 (2)1.2.1 碳材料 (2)1.2.2 聚合物 (3)1.2.3 金属有机骨架材料 (4)1.2.4 氧化物 (5)1.3 磁性材料在环境污染物检测中的应用 (6)1.3.1 多环芳烃 (6)1.3.2 重金属离子 (7)1.3.3 染料 (8)1.3.4 农药 (8)1.3.3 酚类化合物 (9)1.4 论文构思及研究内容 (10)1.4.1 研究内容 (10)1.4.2 技术路线 (10)1.4.3 研究总工作量 (11)第2章磁性固相萃取-高效液相色谱法测定环境水样中的双酚A、4-辛基酚和4-壬基酚 (12)2.1 实验部分 (12)2.1.1 试剂和仪器 (12)2.1.2 Fe@MgAl-LDH的制备 (13)2.1.3 磁性固相萃取(MSPE)过程 (13)2.1.4 高效液相色谱仪(HPLC)分析 (14)2.2 结果与讨论 (14)2.2.1 材料表征 (14)2.2.2 实验条件的优化 (15)2.2.3 材料可重复利用性研究 (22)2.2.4 方法评价和实际水样分析 (23)2.3 小结 (25)第3章金属有机骨架材料MOF-5修饰纳米零价铁磁性固相萃取富集检测痕量含氮及含硫多环芳烃 (26)3.1 实验部分 (26)3.1.1 试剂和仪器 (26)3.1.2 Fe@MOF-5的制备 (27)3.1.3 磁性固相萃取(MSPE)过程 (28)3.1.4 高效液相色谱仪(HPLC)分析 (29)3.2 结果与讨论 (29)3.2.1 材料表征 (29)3.2.2 实验条件的优化 (31)3.2.3 材料可重复利用性研究 (37)3.2.4 方法评价和实际水样分析 (37)3.3 小结 (40)第4章金属有机骨架材料修饰纳米零价铁磁性固相萃取富集检测环境水样中痕量多环芳烃 (41)4.1 实验部分 (41)4.1.1 试剂和仪器 (41)4.1.2 Fe@MIL-101(Cr)的制备 (42)4.1.3 磁性固相萃取(MSPE)过程 (43)4.1.4 高效液相色谱仪(HPLC)分析 (43)4.2 结果与讨论 (43)4.2.1 材料表征 (43)4.2.2 实验条件的优化 (45)4.2.3 材料可重复利用性研究 (52)4.2.4 方法评价和实际水样分析 (53)4.3 小结 (55)第5章Fe@Ag@DMB磁性固相萃取高效液相色谱联用同时检测水中Pb(II)、Cd(II)和Hg(II) (56)5.1 实验部分 (56)5.1.1 试剂和仪器 (56)5.1.2 Fe@Ag@DMB的制备 (57)5.1.3 磁性固相萃取(MSPE)过程 (57)5.1.4 高效液相色谱仪(HPLC)分析 (58)5.2 结果与讨论 (58)5.2.1 材料表征 (58)5.2.2 实验条件的优化 (59)5.2.3 材料可重复利用性研究 (64)5.2.4 方法评价和实际水样分析 (64)5.3 小结 (66)第6章Fe@SiO2@p(NIPAM-co-DMMA) 磁性温度双重响应材料富集检测环境水样中的苏丹红染料 (67)6.1 实验部分 (67)6.1.1 试剂和仪器 (67)6.1.2 Fe@SiO2@p(NIPAM-co-DMMA)的制备 (68)6.1.3 磁性固相萃取(MSPE)过程 (68)6.1.4 高效液相色谱仪(HPLC)分析 (69)6.2 结果与讨论 (69)6.2.1 材料表征 (69)6.2.2 实验条件的优化 (71)6.2.3 材料可重复利用性研究 (78)6.2.4 方法评价和实际水样分析 (78)6.3 小结 (80)第7章结论 (81)参考文献 (83)致谢 (95)攻读硕士期间发表论文情况 (96)第1章绪论目前环境污染是全世界共同关注的问题。
超顺磁性杂化铁氧体纳米微球的制备与表征

超顺磁性杂化铁氧体纳米微球的制备与表征王宇航【摘要】采用经济环保的一步水热法制备了杂化铁氧体MFe2O4(M=Mg、Zn、Mn、Ni)磁性纳米微球,通过调节反应物配比控制其粒径、内部孔道结构和组成,通过SEM、TEM、VSM、XRD对其形貌、内部孔道结构及比饱和磁化强度进行分析测试.结果表明,一步水热法制备的MFe2O4(M=Mg、Zn、Mn、Ni)磁性纳米微球具有高比饱和磁化强度和良好的水溶性,其粒径、组成可随反应物配比进行调控.%We prepared hybridization ferrite MFe2O4(M=Mg,Zn,Mn,Ni) magnetic nanoparticles by an economical and green one-step hydrothermal method.We controlled the particle size,internal pore structure,and composition of nanoparticles by regulating reactant ratio.Moreover,we analyzed and determined their morphology,internal pore structure,and specific saturation magnetization by SEM,TEM,VSM,and XRD.The results showed that MFe2O4(M=Mg,Zn,Mn,Ni) magnetic nanoparticles had high specific saturation magnetization and good water solubility,and their particle sizes and compositions could be controlled by regulating reactant ratio.【期刊名称】《化学与生物工程》【年(卷),期】2017(034)008【总页数】4页(P44-47)【关键词】杂化铁氧体纳米微球;超顺磁性;比饱和磁化强度【作者】王宇航【作者单位】陕西学前师范学院化学与化工系,陕西西安 710100【正文语种】中文【中图分类】O614.8随着科技的发展,无机功能化纳米微球的应用范围逐步扩大[1-4],构筑粒径可控的单分散性无机功能化纳米微球成为研究热点。
氧化钛纳米片材料的合成及其催化应用进展

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2017年第36卷第7期·2488·化 工 进展氧化钛纳米片材料的合成及其催化应用进展李路1,2,徐金铭2,齐世学1,黄延强2(1烟台大学化学与化工学院,山东 烟台 264005;2中国科学院大连化学物理研究所,航天催化与新材料研究室,辽宁 大连 116023)摘要:氧化钛纳米片材料为一种新兴的二维层状材料,在催化、环境、能源和电子领域引起人们广泛的关注。
本文从催化研究的角度出发,综述了氧化钛纳米片材料的结构、制备方法、金属及非金属元素的掺杂、纳米片基复合材料和其在光催化、光电催化和热催化等方面的应用进展。
分析表明氧化钛纳米片材料拥有特殊的形貌和特别的物理化学性质,通过控制材料的组成及结构变化,能够实现氧化钛纳米片材料的多种功能化。
指出氧化钛纳米片材料虽然有着优良的性能,但是在实际应用中远不能满足要求。
因此,优化合成和探索新形式的二氧化钛纳米片材料,对其表面进行改性及开发具有特殊功能纳米复合材料是解决其瓶颈的有效途径。
探索催化反应过程中的反应机理,开发氧化钛纳米片基工业应用催化剂将是今后重要的研究方向。
关键词:氧化钛纳米片;层状钛酸盐;催化;合成;纳米材料中图分类号:O611.4 文献标志码:A 文章编号:1000–6613(2017)07–2488–09 DOI :10.16085/j.issn.1000-6613.2016-2340Recent advances in titanium oxide nanosheets for catalytic applicationsLI Lu 1,2,XU Jinming 2,QI Shixue 1,HUANG Yanqiang 2(1College of Chemistry and Chemical Engineering ,Yantai University ,Yantai 264005,Shandong ,China ;2Laboratory of Catalysts and New Materials for Aerospace ,Dalian Institution of Chemical Physics ,Chinese Academy of Science ,Dalian 116023,Liaoning ,China )Abstract: As a new class 2D layered materials ,Titanium oxide nanosheets have attracted great interest inthe fields of catalysis ,environment ,energy and electronics. In this work ,we provide an overview of the recent advance of titanium oxide nanosheets on their layered structure ,synthetic methods ,doping with metals or nonmetal ,as well as their nanocomposites and applications in catalysis. Recent researches indicate that titanium oxide nanosheets with unique structure and special physical and chemical properties can achieve multiple functions by controlling their compositions and structures. Although titanium oxide nanosheets have a lot of advantages ,they are still far from practical applications. Therefore it is demanded to explore new synthesis ,doping and modification methods ,and develop new composite materials. In addition ,the reaction mechanism in the catalytic reaction process and the industrial application of titanium oxide nanosheets will be important research directions in the future. Key words :titanium oxide nanosheets ;layered titanate compounds ;catalysis ;synthesis ;nanomaterials助理研究员,从事有序介孔材料合成及表面修饰和生物质催化转化制化学品相关科研工作。
冶金工程专业英语 炼铁名词 英文排序

炼铁名词 2018.11.10.a batch of burden ,一批料Absolute pressure绝对压力absolute temperature ,绝对温度absorbing sheath吸音罩accelerate wear on the lining ,加速砖衬磨损acceleration / gravity ,加速度acid slag ,酸性渣active coke zone ,活动焦炭带adding machine ,加法器age hardening ,时效硬化air flow indicator ,空气流量指示器allotropy ,同素异形alloy / ferro,合金alumina ,Al2O3 aluminum ,AlAnchor point ,支点annular gap scrubber for gas c;eaning环缝式洗涤塔anthracite ,无烟煤antimony,锑apparent porosity,显气孔apparent specific gravity , ρ堆比重(容重)apron feeder带式给料机argon ,氩arsenic ,砷available ,有效弹axial blower ,轴流式风机back draft stack ,回压管ball mill ,球磨机banking ,封炉basicity 碱度batch weight ,批重batches charging ,分批装料bed charge ,底料bed coke ,底焦bell clearance ,炉喉间隙Bell’s reaction ,贝尔反应Bell-less top,无钟bellows expansion joint,膨胀器belly炉腰BF settler ,炉前big bell hopper ,大料斗bin料仓black coke ,空焦blast ,鼓风blast control 风量控制blast furnace ,高炉blast furnace dissection ,高炉解剖Blast furnace lines ,高炉内衬blast pressure ,风压blast system送风系统blast temperature,风温blast velocity 风速blast volume / blast rate ,风量blasting爆破bleeder ,放散管bleeder value / snuffle valve,放风伐 放散伐blending the tuyere ,堵风口bloomers / bloomery ,熟铁吹炼炉bloomery furnace / stuckofen ,块炼炉blow off ,停炉blow off valve,放风伐 废气伐blow on ,开炉blow pipe ,直吹管Blow through 吹透高炉blowing down ,降料面blowing in ,送风blowing in burden ,开炉配料blowing out休风停炉boron ,硼bosh 炉腹bosh gas 炉腹煤气boundary layer ,边界层break down temperature ,碎裂温度break through ,穿破breakage ,烧穿Brick lined ,砖衬brick work ,砌砖Bridging悬料briquetted pig iron热压铁块brittle zone ,脆化层bucked elevator(hoist),料罐捲揚机bull specific gravity ,容积比重burden炉料burden calculation /calculate a charge ,配料计算burden program ,装料程序burden sheet ,配料表burden structure炉料结构burdening ,配料Burner ,燃烧器burnt-out ,烧坏bustle pipe ,环管by-pass ,冷风支管by-product ,付产品calcia CaO calcination ,焙烧 煅烧calcium oxide ,生石灰calorie / calory ,卡campaign life ,寿命carbide ,碳化物carbon ,碳Carbon block ,碳砖carbon deposition碳沉积carbon dioxide ,二氧化碳carbon monoxide ,一氧化炭carbon solution loss碳融损carbonate ,炭酸盐carburizer ,渗碳剂carburizing ,渗碳法cast house ,出铁场cast iron / foundry pig ,铸铁casting machine ,铸铁机catalan furnace ,熟铁炉cement carbon ,渗碳central gas flow ,中心气流centrifugal blower ,离心式风机CFB Circulating fluidized bed ,环形流动床chamotte粘土砖Channeling ,管道characteristic number of batch weight , D 批重特徵数characteristic number of stock distribution E 布料特徵数charcoal ,木炭charcoal blast furnace ,木碳高炉charge / charging / filling ,装料 上料charge charactor ,炉料特性charging sequence(order) ,装料次序check checking坐料Checker brick ,格子砖chemical composition ,化学成分chert ,燧石chevron人字纹,人字型断口Chill ,凉 冷却chilled hearth ,炉冷chimney valve ,废气伐chlorine ,氯chrominum ,铬circumference direction ,圆周方向cking coal ,粘结煤clean the walls ,洗炉closel loop soft water cooling loop冉水密闭循环冷却系统closely spaced copper plate ,密集铜水箱coagulum凝结物coal injection ,喷煤coal iron gasfication process ,铁浴造气炼铁法coarse coke ,焦炭coarse ore ,粗粒矿cobalt ,钴coefficient of internal friction ,内摩擦系数cohesive zone软融带coke ,焦炭coke base ,焦炭批重coke base (road) ,焦炭负荷coke blank ,净焦coke breeze焦粉Coke Chimney焦炉烟囱;coke gas ,焦炉煤气coke layer ,矿层coke rate (ratio) ,焦比coke slits ,焦窗coking coal ,焦煤cold blast header ,冷风管cold blast valve ,冷风伐cold screen 冷筛collector ,收集器combustibility ,可燃性combustible,易燃的Combustion chamber ,燃烧室combustion zone ,燃烧带compact coke zone ,死料堆 死焦带compaign live寿命composition of pig iron , 生铁成分compressibility ,可压缩性compressibility factor,压缩系数compressor ,压缩机conditions furnace ,炉况conprehensive utilization综合利用continuous tapping ,连续出铁control room ,值班室convection ,对流Coolant ,冷却剂cooler plate (bosh) ,炉腹冷却板Cooling intensity ,冷却强度cooling plate (stack) / plate cooler ,冷却板Cooling rate ,冷却速度cooling system ,冷却系统coranit ,刚玉氮化物corrosion ,(化学)侵蚀Counting variable ,计数变量crew room ,休息室CRI coke reaction index焦炭反应性critical batch weight ,W0临界批重Cross section ,剖面图crucible steel ,坩埚钢crusher / rock crusher ,破碎机crystal ,结晶水CSR coke strength after reduction还原后焦炭强度Cupola ,化铁炉cyanides ,氰化物dam ,闸 坝deformation ,变形Dehumidifying ,脱湿Deign drawing / plan ,设计图density ,密度 浓度deoxidation ,脱氧deoxidizer ,脱氧剂dephosphotize ,脱P depressurizing valve ,减压伐desulphurization ,脱硫differentia ltemperature of cooling water冷却水温差diffusion ,扩散direct reduction 直接还原direction reduction process ,直接还原炼铁法discharge hole ,出料口discharge side ,出料口一侧distributing chute ,溜槽distribution of burden ,布料distributor / top distributor ,布料器dolomite ,白云石dosing 配料double peak melting zone ,双峰型软熔带down comer(take),下降管draft control ,回压伐Drawing ,草图DRI Direct reduction iron ,海绵铁, 直接还原铁drilling 钻孔driving rate ,作业率dropping zone ,滴落带dross ,铁屑dry cleaning干法除尘dry coke quenching ,干熄焦drying ,烘干ductility ,韧性dump hopper料斗durability ,耐久性dust ,炉尘dust catcher ,除尘器dust catcher ,灰尘捕集器dust collector ,灰尘收集器dynamic response ,动态响应early slag ,初渣effective working volume ,有效容积effective(useful) height ,有效高度elective current ,电流electric furnace ,电炉electric furnace ironmaking ,电炉炼铁electrostatic precipitator ,电除尘器elephant foot erosion ,象脚侵蚀elephant foot wear ,象脚磨损energy-saving and emission 节能减排environment protection环保equalizing valve均压阀equivalent diameter ,当量直径Erection drawing ,安装图erosion ,(机械)侵蚀evaporation ,蒸发exothermic reaction ,放热反应explosion door ,安全防爆门external cooled ,外部冷却extraction metallurgy ,提取(萃区)冶金fabric(bag)filter ,布袋除尘器Fabrication drawing ,制造图Fahrenheit ,华氏feeder spout ,导料管fell down ,脱落ferric oxide ,Fe2O3ferrite ,铁素体ferro manganese ,锰铁ferro silicon ,硅铁fettling 补炉fettling time ,铸锭时间fine(power) ore ,矿粉finery ,精炼fire clay ,耐火泥fire clay brick ,耐火砖first order reaction ,一级反应fixed shaft probe ,炉身固定探测器flactuation,波动 起伏flame temperature ,火焰温度floating ,浮动的 流动的flooding ,液泛flow control valve(gate) ,节流伐Flow sheet /flow diagram ,流程图(表)Flue ,烟道Flue gas ,废气fluidity / runnability / flowability ,流动性fluidization ,流态化fluidized bed ironmaking ,流态化炼铁fluorite / fluorspar / fluor ,萤石flushing furnace洗炉flux / agent of fusion ,熔剂foamed slag ,泡沫渣foldspar ,长石Forced circulation ,强制循环foundation ,炉基foundry ,铸造franklinite ,锌铁矿free running temperature ,自由流动温度free standing BF ,自立式高炉freeze line ,凝固线friction coefficient ,摩擦系数fuel consumption ,燃料消耗fuel injection ,喷吹燃料fuel rate(ratio) ,燃料比fundamental law ,基本规律fundamental principle ,基本原理Furnace "kicking" 静压波动fusibility ,易熔性 熔化性fusion curve ,熔化曲线fusion zone / cohesive zone / melting zone软熔带gangue ,脉石尾矿gas cleaner ,煤气清洗器gas distribution ,煤气分布Gas fired regenerative furnace ,煤气发生炉gas flow ,煤气流gas main ,煤气总管gas profile(curve) ,煤气曲线gas seal (sealing)valve ,气密伐gas uptake上升管gas utilization ,煤气利用gas utilization rate ,ηco煤气利用率gas washing tower ,洗涤塔gauge rod探料尺General layout ,总图glass slag ,玻璃渣go out of blast ,停风grain size ,粒度granite ,花岗岩granulating pit水渣池granulating slag水渣Graphite ,石墨graphitization ,石墨化作用Gray iron ,灰口铁growth ,膨胀half reverse charging (filling) ,半倒装hand charging / hand feed ,人工装料Hanging ,悬料hardness ,硬度harmful elements有害元素HBI Hot briquetted iron ,热压块hearth / well of BF ,炉缸hearth accumulation炉缸堆积hearth bottom ,炉底hearth deposition炉缸堆积hearth freeze-up炉缸冻结hearth layer gate铺底料闸门hearth layer material 铺底料hearth-layer feeding铺底料给料系统Heat balance ,热平衡heat conductivity ,导热率heat function ,热函 焓heat loss ,热损失heat of fusion ,熔化热heat treatment ,热处理heating up烘炉heavy burdener ,重料hematite ore / iron glance ,赤铁矿High alumina brick ,高铝砖Hi-QIP High quality iron peebble ,高质量铁块H-iron processes ,氢铁法hot blast main ,热风管Hot blast stove ,热风炉hot blast valve热风阀hot condition ,热状态Hot metal ,铁水Hot metal ladle ,铁水罐hot spot ,(炉皮)红点hot stove热风炉H-rion process 氢铁法humidified blast蒸汽鼓风humidity ,湿度hydraulic diameter ,水力学直径hydrogen ,氢HYL process HYL直接炼铁[法] igniter ,点火器ignition furnace 点火炉ignition temperature of fuel ,燃料着火点IISI= International Iron and Steel Institut国际钢铁协会ilmenite ,钛磁铁矿impact point ,碰点impact zone ,碰撞区impregnation ,穿透 入侵incomplete combustion ,不完全燃烧Index of BF height ,高炉高度指数Index of Fuel Smelting Intensity ,高炉强度指数Index of Smelting Intensity ,强化指数indicator ,指示器indicator for carbon dioxide ,二氧化碳指示器indirect(indirect) reduction间接还原inertia ,惯性inertial ,惯性的injector喷射器instrument ,仪器insulation ,绝缘 绝热interface effect ,界面效应inwall ,内墙inwall batter ,内墙斜度iron ladle铁[水]罐Iron notch / tap(tapping) hole ,铁口iron notch drill开铁口机iron oxide / ferrous oxide ,FeO 氧化铁iron penetration zone ,滲铁层iron runner铁沟iron silicatte ,硅酸铁iron-bath process铁浴法iron-carbon equilibrium diagram ,铁碳平衡图Ironmaking ,炼铁isotherm ,等温线iss-viscosity line ,等粘度线jacket ,炉皮jasper ,碧玉kaolin / clay ,粘土kiln calcination ,煅烧炉kiln ironmaking ,回转炉炼铁Kinematics viscosity ,动力粘度kinetic energy ,动能Krupp rotary kiln iron-making克虏伯回转窑炼铁[法] lamp ore / sized ore ,块矿Large bell ,大钟large sized blast furnace大高炉leak humting捡漏leaking tuyere ,风口漏水life-ending period ,末期lighting ,点火lignite / brown coal褐煤lime ,石灰limestone ,石灰石limonite / morass ore ,褐铁矿lining ,炉衬loop ,循环 闭环Low phosphorus iron ,低磷铁Low shaft furnace ,矮身高炉lower heat exchanger下部的热交换luppen ,粒铁magneium oxide / magnesia ,MgOmagnesite ,镁砂 菱镁矿magnesium ,镁magnet ,磁, 磁体 磁铁magnetic concentration ,磁选magnetite ,磁铁矿Major capital ,大修malleable cast iron / forge pig ,可锻铸铁malleable iron ,锻铁manganese ,锰manganese oxide ,MnOMantle ,环梁 支圈marble ,大理石Material balance ,物平衡Mckee Revolving ,马基式布料器measures on operation ,操作措施mechanical stress ,机械应力mechanism ,机械作用melting point / fusion point ,熔点melting viscosity ,熔化粘度mesh ,筛孔metallography ,金相学Metallurgy ,冶金meteoric iron /陨铁methane ,甲烷micropore(microprorous) carbon , 微孔碳砖midrex process米德法直接炼铁[法mild steel ,软钢milk of line ,石灰浆miscellaneous weigh hopper ,杂矿称量罐mix consolidation 混合料压实mixed charging ,混装mixed injection混合喷吹mixed layer ,混合层mixer ,混合器mixer main ,混风管mixer selector valve混风阀mixing calculation配料计算mixing drum混料器modification ,η 修正(变换)系数Module ,模数 模量modulus of elasticity ,弹性系数Moisture removal from blast ,脱湿molecule ,分子molybdenum ,钼Monkey / slag notch / cinder notch ,渣口mother oil ,原油movable armor/ adjustable armors ,活动炉喉 /变径炉喉Mud gun / clay gun ,泥砲mushroom wear ,蘑菇状磨损near net shape ,近净化成型nickel ,镍nitrogen ,氮nodulizing ore / briquitting ,团矿 压块non-coke iron making非焦炭炼铁nut coke ,焦丁off grade iron ,出格铁oil injection ,喷油on blast of stove, on blast送风期on gas of stove, on gas燃烧期opaline ,蛋白石open pit mines 露天矿开采operation rate of blast furnace高炉作业率ore ,矿石ore body矿体ore layer ,焦层ore layer ,矿石层ore size矿区的规模outside combustion stove外燃式燃烧炉outward batter ,外倾overland conveyor 皮带传输oxidation ,氧化oxide ,氧化物oxidizing zone ,氧化带oxygen氧Oxygen enrichment ,富氧率packed bad ,固定床 填充床packed coke in slag ,渣内堆积焦particles rebounding off wall ,弹离炉墙PCI: pulverized coal injection 喷煤比peak position ,堆尖位置pearlite ,珠光体Peephole ,窥孔pelletizing ,造球penetration ,渗透percolator ,渗透层periodic table ,周期表periphery / outside ,边缘permeability ,透气性permeability index ,透气性指数permeable layer ,透气层petrified wood ,木化石phologiston ,燃素phosphorous ,磷physical metallurgy ,物理冶金pig bed ,砂床Pig iron ,生铁pillaring ,料柱作用, 冷料柱pipe stove ,管式热风炉porosity ,孔隙度porous ,多孔的 疏松的positive measures ,切实措施potential heat ,潜热PR= production rate,还原率Prereduction ,预还原Pressure drop ,压降pressure drop in furnace炉内压差pressure equalizing valve ,均压伐pressure gauge ,压力表pressure entry hearth曲损Pressurized ,充压pressurizing valve ,充压伐productivity ,利用系数 生产率profile ,furnace lines炉型property ,特性Protecting brick ,保护砖puddled steel ,搅拌钢 熟铁pursuit of low Si operation ,追求低硅操作pyrite ,黄铁矿pyrometer ,高温计quench ,熄火quench ,淬火quick lime ,活性石灰raceway ,循环区 回旋区radiation ,辐射rator assembly ,转子raw material ,原料raw(original) coal ,原煤reactivity ,反应率receiving hopper ,受料斗Recuperater ,蓄热室 换热器red shortness ,热裂现象redistribution ,再分布reduced burden ,轻料reducibility ,还原速率reduction ,还原reduction velocity / reducibility ,还原速度reference frame 座标refinery ,提纯refractor ,耐火材料regular charging ,正装regular joint (mixed ) charging ,正同装regular unit(layer) charging(filling) / nor mal uni c ,正分装regulating valve ,调解伐regulator ,调解器remained brickworks ,残砖厚度Removable trough cover ,活动沟盖replacement ratio ,替换比repose angle ,自然堆角Reserve zone ,稳定区 热储备区residual brickworks ,残砖厚度residual thickness ,剩余厚度resistance coefficient (drag coefficient )阻力系数reverberatory furnace ,反射炉reverse charging(filling) ,倒装reverse joint (mixed ) charging 倒同装reverse unit(layer) charging(filling) ,倒分装revolving hopper ,旋转料斗revolving (rotating ) distributor ,旋转布料器Ring pattern 布料矩阵riser pipe / uptake / gas off take ,上升管Rist diagram ,R式操作图roll scale ,轧钢皮rotary hearth iron making 转底炉炼铁run number ,试验号 编号SAF Submerged arc furnace ,潜弧炉Salamander ,炉底积铁Scabs / skull /.accretion ,炉瘤Scaffolding ,结瘤scale car ,称量车scar炉瘤,炉结scarp ,废铁scouring effect ,摩擦作用screening分选筛self coating ,自结sementite ,渗碳体semi-coke ,半焦sensible heat ,显热sensor ,传感器serpentine ,蛇纹石Set up data ,设定数据Shaft / Stack ,炉身Shaft furnace ,竖炉Shop drawing ,施工图shrinkage ,收缩shut off valve / blast isolation ,切断伐silica ,菱铁矿silicate ,硅酸盐silicide ,硅化物silicomanganese ,硅酸锰single(dual- or multi-)ring charging ,单(双或多)环布料sinter / agglomerate ,烧结矿sinter strand ,带式烧结机sintering / agglomerate ,烧结sintering machine ,烧结机size segregation’ ,粒度偏析Skimmer / Iron dam ,撇渣器skimming the metal ,渣铁分离Skip ,料车skip hoist ,料车捲揚机slag ,炉渣Slag car / pot ,渣罐slag crust渣皮slag crust fall off渣皮脱落Slag dam ,渣坝slag irrigates the tuyere 涌渣slag notch cooler渣口水套Slag pit ,渣坑Slag runner ,渣沟slag specimen ,渣样Slag thimble ,渣口套slag to iron ratio,slag ratio渣比Slag volume ,渣量slaked lime ,消石灰slight change zone ,微变区Slip ,塌料slow change zone ,缓变区sludge ,尘泥sluggish tuyere挂渣风口slugs slugging洗炉Slurry injection ,喷煤浆smelting ,熔炼smelting cycle ,冶炼周期smelting intensity ,冶炼强度Smooth running / unformity of process ,顺行snort valve ,消音伐soda ash ,苏达soft coal ,烟煤soft-melting zone软融带solid solution ,固溶体solidification凝固solution loss ,溶碳损失sow主铁沟space speed(velocity) ,空区速度specific gravity(weight) ,比重specific heat ,比热specular hematite / specularite ,镜铁矿speed of burden ,料速speed of rotation ,旋转速度spiegel iron ,镜铁Spiegeliesen / spiegel ,镜铁spilt(layer) charging / separating charging分装splasher ,防溅板Sponge iron ,海绵铁spray cooling / external shower cooling喷水冷却spray water ,喷水spray zone喷水带stack angle炉身角Stack valve ,烟道伐stack(lumpy)zone ,块状带Static pressure ,静压力stave cooler ,冷却壁stock heap(pile) ,料堆stock house ,捲揚机室stock indicator ,料线指示器stock level monitoring system ,料线指示器stock line / stock level ,料线stock rod ,料尺Stock s raw ,斯托克定律stock yard ,料场stock(charge) column / pillaring ,料柱stockpile料堆storage bin ,料仓streamline ,流线 流线型streamline flow ,层流边缘气流strong wall flow (working)/ peripheral gas flo ,suction 抽风sulphide(sulfide) ,硫化物sulphur(sulfur) ,硫sump depth ,铁口到炉底深度suppress the wind憋风surface tension ,表面张力surplus(excess) air coefficient ,空气过剩系数swan neck /goose neck ,鹅颈管switching runner摆动溜槽tap hole铁口Taphole drill ,开口机tapping ,出铁teeming time ,补炉时间temperature conversion ,温度转换temperature gradient ,温度梯度tensile strength ,抗张强度Test room ,试验室the mining method开采的方法theorem ,定理theoretical combustion(flame) temperature理论燃烧温度theoretical flame(burning) temperature理论燃烧温度theoretical investigation ,理论研究theory ,理论thermal capacity ,热容量thermal compensation 热补偿thermal reserve zone热储区thermal Reserve zone ,热稳定区,热储备区thermal road ,热负荷thermal state ,热状态thermo-camera ,热成象thermo-chemical attack ,热化学侵蚀thermocuple ,热电偶therom-stress ,热应力throat 炉喉throttle ,节流伐throttling valve ,节流伐through put ,生产能力throwing effect ,抛掷作用Ti(C,N) crystols钛碳、氮化物晶体Ti-containing material钛化物Tie rod ,吊杆time study ,时间测定titanium ,钛title converter ,傾动式转炉top combustion stove顶燃式热风炉top gas ,blast furnace gas炉顶煤气 高炉煤气top gas analysis (recorder) ,煤气分析器Top gas pressure ,炉顶压力Top gas Recovery (expansion)Turbine ,TRT 高炉煤气回收 torpedo car鱼雷车total carbon ,全碳tramp element ,残余元素Trial plant内 ,试验厂trial rod ,料尺true specific gravity ,真比重try hole ,料尺孔tube mill ,棒磨机tumbler test , 转鼓试验 转鼓强度tungsten ,钨turbine ,涡轮机turbo blower ,涡轮风机tuyere风口Tuyere cap ,风口小盖tuyere cooler风口水套Tuyere loss ,风口坏tuyere region ,风口带tuyere sonde sampling ,风口取样器tuyere stock风口弯头two 2 bell valve seal,双钟双伐Two bell top ,two-bells system charging 双钟 双料钟式装料ultimate analysis , 元素分析ultimate capacity ,最高产量under blowing ,慢风underground mine 地下矿开采upper heat exchanger上部热交换upper sealing valve ,上密封伐utilization coefficient利用系数V type melting zone ,V型软熔带V(M)-shaped layer(profile),V 型料面valuation of iron ores ,铁矿石评价vanadium ,钒vaporization cooling汽化冷却Variable ,变量Vector ,矢量velocity / speed ,速度velocity constant ,速度常数venturi meter ,流量计Venturi throat ,文式管vibrating feeder ,震动给料器violent change zone ,激变区viscosimeter , 粘度计viscosity ,粘度viscosity index ,粘度指数voids ,孔隙volatile matter挥发物washer / scrubber洗涤塔water equivalent thermal flow ,水当量water trough ,水槽weathering of iron ores ,铁矿石风化Wedged key ,梢子weigh hopper ,称量罐weighed accurately ,称量精度well depth ,死铁层深度wet cleaning ,湿法除尘wet concentration ,湿选whirler / syclone ,旋转除尘器wool / slag wool ,渣棉Working drawing ,工作图working instruction ,操作规程熟铁working volume工作容积wrought iron ,熟铁zinc ,,锌zone of relatively constant temperature热稳定区风口曲损。
Zhang CV Nov 2014 张波教授 简历
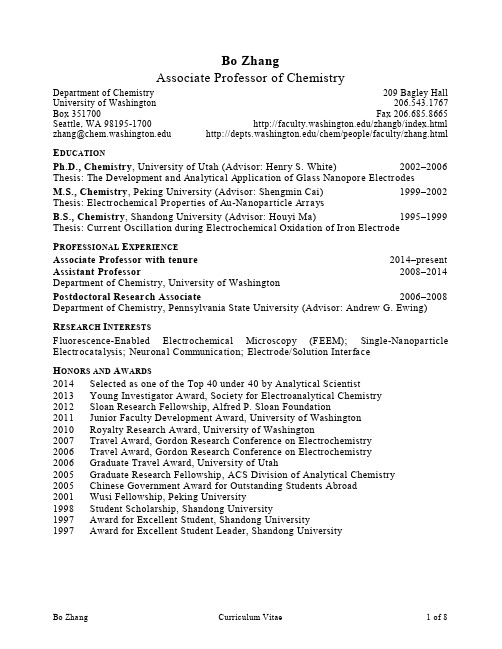
Bo Zhang Associate Professor of ChemistryDepartment of Chemistry University of Washington Box 351700Seattle, WA 98195-1700 zhang@209 Bagley Hall206.543.1767Fax 206.685.8665 /zhangb/index.html /chem/people/faculty/zhang.htmlE DUCATIONPh.D., Chemistry, University of Utah (Advisor: Henry S. White) 2002–2006 Thesis: The Development and Analytical Application of Glass Nanopore ElectrodesM.S., Chemistry, Peking University (Advisor: Shengmin Cai) 1999–2002 Thesis: Electrochemical Properties of Au-Nanoparticle ArraysB.S., Chemistry, Shandong University (Advisor: Houyi Ma) 1995–1999 Thesis: Current Oscillation during Electrochemical Oxidation of Iron ElectrodeP ROFESSIONAL E XPERIENCEAssociate Professor with tenure2014–present Assistant Professor2008–2014 Department of Chemistry, University of WashingtonPostdoctoral Research Associate2006–2008 Department of Chemistry, Pennsylvania State University (Advisor: Andrew G. Ewing)R ESEARCH I NTERESTSFluorescence-Enabled Electrochemical Microscopy (FEEM); Single-Nanoparticle Electrocatalysis; Neuronal Communication; Electrode/Solution InterfaceH ONORS AND A WARDS2014 Selected as one of the Top 40 under 40 by Analytical Scientist2013 Young Investigator Award, Society for Electroanalytical Chemistry2012 Sloan Research Fellowship, Alfred P. Sloan Foundation2011 Junior Faculty Development Award, University of Washington2010 Royalty Research Award, University of Washington2007 Travel Award, Gordon Research Conference on Electrochemistry2006 Travel Award, Gordon Research Conference on Electrochemistry2006 Graduate Travel Award, University of Utah2005 Graduate Research Fellowship, ACS Division of Analytical Chemistry2005 Chinese Government Award for Outstanding Students Abroad2001 Wusi Fellowship, Peking University1998 Student Scholarship, Shandong University1997 Award for Excellent Student, Shandong University1997 Award for Excellent Student Leader, Shandong UniversityP UBLICATIONS(*corresponding author; ‡undergraduate coworkers)40. Oja, S. M.; Zhang, B.* “Imaging Transient Formation of Diffusion Layers withFluorescence-Enabled Electrochemical Microscopy.” Anal. Chem.revision submitted.39. Oja, S. M.; Guerrette, J. P.; David, M. R.; ‡ Zhang, B.* “Electrochemical Oxidation ofDihydroresorufin as a New Indicator Reaction for Fluorescence-Enabled Electrochemical Microscopy.” Anal. Chem. 2014, 86, 6040–6048.38. Percival, S. J.; Zhang, B.* “Study of the Formation and Quick Growth of Thick OxideFilms Using Platinum Nanoelectrodes as a Model Electrocatalyst.” Langmuir 2014, 30, 11235–11242.37. Guo, Z. H.; Percival, S. J.; Zhang, B.* “Chemically-Resolved Transient CollisionEvents of Single Electrocatalytic Nanoparticles” J. Am. Chem. Soc. 2014,136, 8879–8882.36. Percival, S. J.; Vartanian, N.;‡ Zhang, B.* “Laser-pulled Ultralong Metal Nanowires.”RSC Advances2014, 4, 10491–10498.35. Cox, J. T.; Gunderson, C.; and Zhang, B.* “The effects of Outer Sphere RedoxSpecies to the Kinetics of Carbon-Fiber Microelectrodes.” Electroanalysis2013, 25, 2151–2158.34. Percival, S. J.; Zhang, B.* “Electrocatalytic Activity of Single Pt Nanowir eElectrode.” J. Phys. Chem.2013, 117, 13928–13935.33. Park, J. H.; Thorgaard, S. N.; Zhang, B.; Bard, A. J.* “Single Particle Detection byArea Amplification–Single Wall Carbon Nanotube Attachment to a Nanoelectrode.” J.Am. Chem. Soc.2013, 135,5258–5261.32. Park, J. H.; Zhou, H.; Percival, S. J.; Zhang, B.; Fan, F-R. F.; Bard, A. J.* “OpenCircuit (Mixed) Potential Changes Upon Contact Between Different Inert Electrodes–Size and Kinetic Effects.” Anal. Chem. 2013, 85, 964–970.31. Guerrette, J. G.; Percival, S. J.; Zhang, B.* “Fluorescence Coupling forElectrochemical Heterogeneity.” J. Am. Chem. Soc.2013, 135, 855–861.30. Oja, S. M.; Percival, S. J.; Zhang, B.* “Nanoscale Electrochemistry.” Anal. Chem.2013, 85, 473–486. (Invited Review)29. Percival, S. J.; Zhang, B.* “Electrocatalysts under the Microscope.” Nature Nanotech.2012, 7, 615–616. (News & Views)28. Cox, J. T.; Zhang, B.* “Nanoelectrodes, Recent Advances and New Directions.” Annu.Rev. Anal. Chem.2012, 5, 253–272.(Invited)27. Cox, J. T.; Guerrette, J. P.; Zhang, B.* “Steady-State Voltammetry of aMicroelectrode in a Closed Bipolar Cell.” Anal. Chem.2012, 84, 8797–8804.26. Guerrette, J. G.; Oja, S. M.;‡Zhang, B.* “Coupled Electrochemical Reactions atBipolar Microelectrodes and Nan oelectrodes.” Anal. Chem.2012, 84, 1609–1616.25. Guerrette, J. G.; Percival, S. J.; Zhang, B.* “Voltammetric Behavior of GoldNanotrench Electrodes.” Langmuir2011, 27, 12218–12225.24. Lan, W. J.; Holden, D. A.; Zhang, B.; White, H. S.* “Nanoparticle Tr ansport inConical-Shaped Nanopores.” Anal. Chem.2011, 83,3840–3847.23. Kwon, S. J.; Zhou, H. J.; Fan, F. F.-R.; Vorobyev, V.; Zhang, B.; and Bard, A. J.*“Stochastic electrochemistry with electrocatalytic nanoparticles at inert ultramicroelectrodes–the ory and experiments.” Phys. Chem. Chem. Phys.2011, 13, 5394–5402.22. Adams, K. L.; Jena, B. K.; Percival, S. J.; Zhang, B.* “Highly-Sensitive Detection ofExocytotic Dopamine Release using a Gold-Nanoparticle-Network Microelectrode.”Anal. Chem.2011, 83,920–927.21. Zhang, B.; Heien, M. L. A. V.; Santillo, M. F.; Mellander, L.; Ewing, A. G.*“Temporal Resolution in Electrochemical Imaging on Single PC12 Cells Using Amperometry and Voltammetry at Microelectrode Arrays.” Anal. Chem. 2011, 83, 571–577.20. Guerrette, J. P.; Zhang, B.* “Scan-Rate Dependent Current Rectification of Cone-Shape Silica Nanopores in Quartz Nanopipettes.” J. Am. Chem. Soc.2010, 132, 17088–17091.19. Jena, B. K.; Percival, S. J.; Zhang, B.* “Au Nanoelectrode by ElectrochemicalDep osition in a Nanopore.” Anal. Chem.2010, 82,6737–6743.18. Nelson, T.; Zhang, B.; and Prezhdo, O. V.* “Detection of Nucleic Acids withGraphene Nanopores: Ab Initio Characterization of a Novel Sequencing Device.”Nano Lett.2010, 10,3237–3242.17. Li, Y.; Cox, J. T.; and Zhang, B.* “Electrochemical Response and Electrocatalysis atsingle Au Nanoparticles.” J. Am. Chem. Soc. 2010, 132, 3047–3052.16. Adams, K. L.; Engelbrektsson, J.; Voinova, M.; Zhang, B.; Eves, D. J.; Karlsson, R.;Heien, M.; Cans, A. S.; Ewing, A. G.* “Steady-State Electrochemical Determination of Lipidic Nanotube Diameter Utilizing an Artificial Cell Model.” Anal. Chem.2010, 82, 1020–1026.15. Zhang, B.*; Wood, M.; Lee, H. “A Silica Nanochannel and Its Applications in Sensingand Mole cular Transport.” Anal. Chem.2009, 81, 5541–5548.14. Li, Y.; Bergman, D.°; and Zhang, B.* “The Preparation and Electrochemical Responseof 1-3 nm Pt Electrodes.” Anal. Chem.2009, 81, 5496–5502.Prior to the University of Washington13. Zhang, B.; Adams, K. L.; Luber, S.; Heien, M., Ewing, A. G. “Spatially andTemporally Resolved Single-Cell Exocytosis with Individually-Addressable Carbon Microelectrode-Arrays.” Anal. Chem.2008, 80, 1394–1400. (Accelerated Article) 12. Zhang, B.; Galusha, G.; Shiozawa, P. G.°; Wang, G.; Bergren, A. J.; Johns, R. M.;White, R. J.; Ervin E. N.; Cauley, C. C.; White, H. S. “A Bench-Top Method for Fabricating Glass-Sealed Nanodisk Electrodes, Glass Nanopore Electrodes, and Glass Nanopore Membranes of Controlled Size.” Anal. Chem.2007, 79, 4778–4787.(Accelerated Article)11. White, R. J.; Zhang, B.; Daniel, S.; Tang, J.; Ervin, E. N.; Cremer, P. S.; White, H. S.“Ionic Conductivity of the Aqueous Layer Separating a Lipid Bilayer Membrane and a Glass Support.” Langmuir2006, 22, 10777–10783.10. Wang, G. L.; Zhang, B.; Wayment, J. R.; Harris, J. M.; White, H. S. “Electrostatic-Gated Transport in Chemically Modified Glass Nanopore Electrodes.” J. Am. Chem.Soc.2006, 128, 7679–7686.9. Zhang, B.; Zhang, Y.; White, H. S. “Steady-State Voltammetric Response of theNanopore Electrode.” Anal. Chem.2006, 78, 477–483.8. Zhang, Y.; Zhang, B.; White, H. S. “Electrochemistry of Nanopore Electrodes in LowIonic Strength Solutions.” J. Phys. Chem. B. 2006,110, 1768–1774.7. Watkins, J. J.; Zhang, B.; White, H. S. “Electrochemistry at Nanometer-ScaledElectrodes.” J. Chem. Edu.2005, 82, 712–719.6. Zhang, B.; Zhang, Y.; White, H. S. “The Nanopore Electrode.”Anal. Chem. 2004, 76,6229–6238.5. Wang. W. L.; Zhai, J.; Jiang, L.; Bai, F. L.; Ren, Y. J.; Zhang, B.; Cai, S. M. “NovelPhotoactive Self-Assembled Rigid Monolayer of a Perylene Derivative: Fabrication and Characterization.” Colloids Surf., A 2005, 257-258, 489–495. (Special Issue)4. Wang. W. L.; Zhai, J.; Jiang, L.; Bai, F. L.; Ren, Y. J.; Zhang, B.; Cai, S. M.“Perylene Derivative Based Self-Assembled Monolayer on ITO Electrode Surfaces.”International Journal of Nonlinear Sciences and Numerical Simulation2002, 229–232. (Special Issue)3. Yan, J.; Li, J. J.; Zhang, B.; Cai, S. M. “Direct Electrochemistry of Cytochrome C-551from Pseudomonas Aeruginosa at ITO Electrodes.” Acta Physico-Chimica Sinica 2001, 17, 1126–1128.2. Zhang, B.; Zhang, Z. J.; Wang, B.; Yan, J.; Li, J. J.; Cai, S. M. “Preparation of GoldNanoelectrode Array on Silicon Substrate and Its Electrochemical Properties-Probe into Biosensors Based on Electroluminescence of Porous Silicon.” Acta Chimica Sinica2001, 59, 1932–1936.1. Zhao, S. Y.; Zhang, B.; Ma, H. Y.; Chen, S. H. “A Study of Designed CurrentOscillations of Fe in H2SO4Solution.” Acta Chimica Sinica2000,58, 1670–1673. Book Chapters1. Bo Zhang, Gangli Wang, and Henry S. White, “Glass Nanopore Electrodes,” in Handbook ofElectrochemistry, C.G. Zoski, Ed.;Elsevier, Amsterdam, 2007, Chapter 6, Section 6.3.11, pp 254–260.Patents4. “Fluorescence-Enabled Electrochemical Detection and Imaging.” Bo Zhang, Joshua P.Guerrette, Stephen J. Percival. UW Patent Discloser, submitted on March 21, 2013.3. “DNA Sequencing with a Graphene Nanopore.” Bo Zhang, Stephen P ercival, Marissa Wood.UW Patent Discloser, submitted on November 24, 2009.2. “A Silica Nanopore Sensor.” Bo Zhang. UW Patent Discloser, submitted on May 15, 2009. 1. “Nanopore Electrode and Nanopore Membrane, Methods of Preparation and SurfaceModificati on, and Use Thereof.” Henry S. White, Bo Zhang, Ryan J. White, Eric N. Ervin, and Gangli Wang. U.S. Patent: 7,849,581.P RESENTATIONSInvited Seminars at Universities and Other Research Institutions29. Department of Chemistry, Shanghai Jiaotong University, Shanghai, China July 201528. Department of Chemistry, Busan university, Busan, Korea Aug 201527. Department of Chemistry, Seoul National University, Seoul, Korea Aug 201526. Department of Chemistry and Biochemistry, University of Arizona, Tucson, AZ Feb., 2015 25. Department of Chemistry, Oregon State University, Nov., 201424. Department of Chemistry, Tsinghua University, Beijing, China, Oct. 25, 2013.23. Department of Chemistry, Peking University, Beijing, China, Oct. 25, 2013.22. Department of Chemistry, University of Illinois at Urbana–Champaign, Sep. 13, 2013.21. Department of Chemistry & Biochemistry, University of Maryland at Baltimore County,Baltimore, MD, Sep. 10, 2013.20. Department of Chemistry, Georgia State University, Atlanta, GA, Mar. 15, 2013.19. Department of Chemistry, University of Pittsburgh, Pittsburgh, PA, Jan. 24, 2013.18. Department of Chemistry, University of North Carolina, Chapel Hill, Nov. 12, 2012.17. Department of Chemistry & Biochemistry, University of Texas, Austin, Nov. 8, 2012.16. Department of Chemistry, Texas A&M University, College Station, TX, Nov. 6, 2012.15. Department of Chemistry, Vanderbilt University, Nashville, TN, Sep. 17, 2012.14. Department of Chemistry, Beijing Normal University, Beijing, China, Sep. 2, 2012.13. Dept. of Chemistry & Biochemistry, University of Notre Dame, South Bend, IN, Aug. 30, 2012.12. Department of Chemistry, Indiana University, Bloomington, IN, Aug. 28, 2012.11. Department of Chemistry & Biochemistry, University of Maryland, Baltimore County,Baltimore, MD, Mar. 27, 2012. (Cancelled and Rescheduled due to flight cancellation)10. Department of Chemistry, University of Utah, Salt Lake City, UT, Feb. 28, 2011.9. Center for Electrochemistry, University of Texas at Austin, Austin, TX, Feb. 19, 2011.8. Department of Physics, University of Washington, Seattle, Dec. 2, 2009.7. Department of Chemistry, University of Idaho, Moscow, ID, Dec. 1, 2009.6. Center for Nanotechnology, University of Washington, Seattle, Feb. 17, 2009.5. Department of Chemistry, University of Washington, Seattle, Jan. 7, 2008.4. Department of Chemistry, University of Massachusetts, Amherst, Dec. 2007.3. Department of Chemistry, Iowa State University, Ames, IA, Dec. 2007.2. Department of Chemistry & Biochemistry, Texas Tech University, Lubbock, TX, Dec.6, 2007.1. Department of Chemistry, University of Kansas, Lawrence, KS, Nov. 2007.Invited Presentations at Conferences and Other Scientific Meetings19. IUPAC Symposium on “Electroanalysis and Nanobio sensor” Busan, Korea Aug 201518. Pittcon, New Orleans, LA, March 10, 2015.17. Pittcon, Chicago, IL, March 15, 2014.16. Keynote address, Beijing Conference and Exhibition on Instrumental Analysis (BCEIA)Meeting, Beijing, China, October 24, 2013.15. Charles N. Reilley and Young Investigator Award Symposium, Pittcon, Philadelphia, PA,March 18, 2013.14. Pittcon, Orlando, FL, March 15, 2012.13. Pittcon, Orlando, FL, March 12, 2012.12. ACS Southwest Regional Meeting, Austin, TX, November 10, 2011.11. BCEIA Meeting, Beijing, China, October 12, 2011.10. Catalysis Science Program Meeting, Department of Energy, Washington, DC, Oct. 2, 2011.9. 7th Potter’s Lodge Meeting on Electrochemistry, Blue Mountain Lake, NY, September 7, 2011.8. Pittcon, Atlanta, GA, March 13, 2011.7. NIH NHGRI Meeting, Chapel Hill, NC, March 10, 2010.6. Pittcon, New Orleans, LA, March 2, 2010.5. Pittcon, New Orleans, LA, March 1, 2010.4. Pittcon, New Orleans, LA, February 28, 2010.3. 6th Potter’s Lodge Meeting on Electrochemist ry, Blue Mountain Lake, NY, September 9, 2009.2. Pittcon, Chicago, IL, March 10, 2009.1. 12th International Conference on in vivo methods, Vancouver, BC, August 12, 2008. Other Presentations and Posters at Conferences7.Joshua Guerrette, Department of Chemistry, University of Hawaii, Feb. 20, 2013. (invitedpresentation)6. Poster at Pittcon by graduate student Joshua Guerrette, Orlando, FL, March 12, 2012.5. Poster at Pittcon by graduate student Jonathan Cox, Pittcon, Atlanta, GA, March 13, 2011.4. Attendee, Pittcon, Chicago, IL, February 2007.3. Poster at Gordon Research Conference on Electrochemistry, Ventura, CA, Jan. 2007.2. Poster at Gordon Research Conference on Electrochemistry, Buellton, CA, Feb. 2006.1. Poster at the Joint Regional Meeting of the Northwest and Rocky Mountain Sectionsof the American Chemical Society, Logan, UT, June 2004.R ESEARCH S UPPORTCurrentAir Force Office of Scientific Research (MURI) (Co-PI) 1/2014–1/2019 $1,000,000 Electrochemical Imaging and Mechanistic Studies on the Nanometer ScaleNational Science Foundation (PI) 8/2012–8/2015 $230,000 Fluorescence-Enabled Electrochemical DetectionNational Institutes of Health (PI) 4/2012–3/2017 $1,384,856 New Electroanalytical Methods for Single-Cell ExocytosisDefense Threat Reduction Agency (Co-PI) 8/2011–8/2016 $500,000 Fundamental Aspects of Single Molecule and Zeptomole ElectroanalysisPendingNational Science Foundation (PI) 8/2015–7/2018 $480,000 Fluorescence-Enabled Electrochemical MicroscopyPastAlfred P. Sloan Foundation (PI) 9/2012–9/2014 $50,000 Single-Particle ElectrocatalysisUniversity of Washington Royalty Research Fund (PI) 6/2010-05/2011 $30,000 Single-Nanoparticle ElectrochemistryP ROFESSIONAL A CTIVITIES AND S ERVICEProfessional ServiceFeatures Panel, Analytical Chemistry 2015-2018Board of Directors, Society of Electroanalytical Chemistry, 2013–2017Conference OrganizationDiscussion Leader, Gordon Research Conference on Electrochemistry, Ventura, CA, January 2014.“Electrochemical Imaging in Neurochemistry with Microelectrodes and Nanoelectrodes” Symposium, Pittcon, Orlando, FL, March 2012.“Electrochemistry at Nanoscale and Single Nanoparticles” Symposium, Pittcon, Atlanta, GA, March 2011.Professional Society MembershipAmerican Chemical SocietySociety of Electroanalytical ChemistryReviewer for Journals:ACS Nano, ACS Applied Materials & Interfaces, Analyst, Analytical Chemistry, Analytical Letters, Analytical Methods, Chemical Science, Electroanalysis, Journal of Electroanalytical Chemistry, Journal of the American Chemical Society, Langmuir, Nano Letters, Nature Nanotechnology, Scientific Reports, TalantaReviewer for Funding Agencies:NSF review panel (March 2013), NIH (March 2014)Department and University ServiceChemistry Graduate Applications and Recruiting Committee, 2008–present Chemistry Graduate Exam Committees, 2008–presentM ENTORINGCurrent Graduate StudentsChu Han 2013-presentYunshan,Fan 2013-presentChristopher Gunderson 2012–presentStephen M. Oja 2012–presentStephen J. Percival 2009–presentCurrent Postdoctoral Researchers and VisitorsDr. Rui Hao 9/2013–presentDr. Ming Zhou 1/2013–presentDr. Jin Lu 7/2014-presentDr. Gang Xue 8/2014-presentDr. Cairong Gong 8/2014-presentCurrent Undergraduate ResearchersPh.D. Dissertations SupervisedDr. Marissa Wood 2008–2014Current position: University of WashingtonDr. Joshua Guerrette 2009–2013Current position: Postdoctoral Associate, University of North CarolinaDr. Jonathan T. Cox 2008–2012Current position: Postdoctoral Associate, PNNLFormer Postdoctoral Research Associates and VisitorsDr. Zhihui Guo 10/2012–01/2014Current position: Associate Professor, Shaanxi Normal University ChinaDr. Joshua Guerrette 2013Current position: Postdoctoral Associate, University of North CarolinaDr. Kelly Adams 2010–2011Current position: Senior Research Scientist, Albany Molecular Research, Inc.Dr. Bikash Kumar Jena 2010–2011Current position: Scientist, Institute of Minerals and Materials Technology, India Dr. Yongxin Li 2008–2010Current position: Professor, Anhui Normal University, ChinaM.S. Students SupervisedErnest Tomlinson 2011–2012 Graduate Student (M.S. 2012) Jin Chen 2008–2010 Graduate Student (M.S. 2011) Former Undergraduate Researchers (*denotes Amgen Scholars)Matt J. Bates* 6/2014-8/2014 (Undergraduate, Oregon State Univ.) Noah Vartanian 2012-2014 (Seattle, WA)Michelle David*6/2013–8/2013 (Undergraduate, Washington State Univ.)David Galvan* 6/2012–8/2012 (Ph.D. student, UW Chemical Engineering) Laura Belluzzi 3/2012–3/2013 (Undergraduate, Univ. Washington)Ben Shipley 3/2012–3/2013Kelsey Musgrove 3/2011–9/2011 (M.D. student, Florida International University) Stephen Oja* 2011–2012 (Ph.D. student, UW Chemistry)Kayla N. Eychner 2/2010–3/2011Ye Long 12/2009–1/2011Chris Chou* 9/2009–9/2010 (M.D. student, Washington U. School of Medicine) Sean Chang 2009–2010David Bergman 9/2008–5/2009 (High school teacher in Seattle, WA)Jung An Hong 9/2008–12/2008Hyunae Lee 9/2008–6/2010 (Ph.D. student, University of Chicago)C LASSROOM T EACHINGCHEM 152: General Chemistry (Undergraduate)CHEM 426:Instrumental Analysis (Undergraduate)CHEM 520: Special Topics in Analytical Chemistry (Graduate)CHEM 592: Seminar in Analytical Chemistry (Graduate) (coordinator)。
三氧化钨纳米线的制备及对染料的选择性吸附
三氧化钨纳米线的制备及对染料的选择性吸附郑华均;赵浙菲;李世雄;黄益操【摘要】以Na2WO4为前驱体,K2SO4为结构导向剂,采用水热法制备WO3纳米线.利用X射线衍射(XRD)、扫描电镜(SEM)和红外光谱(IR)等方法表征了材料的形貌、结构和化学组成;测定了WO3纳米线对染料的吸附性能,研究了影响WO3纳米线吸附亚甲基蓝的因素.结果表明:所得WO3纳米线具有较大的比表面积(864.20m2/g);WO3纳米线对印染废水中的有机物具有较好的吸附选择性,对亚甲基蓝的吸附能力较强;当溶液pH为4时,未经煅烧的WO3纳米线对亚甲基蓝的吸附量高达148.6 mg/g,是商业WO3的50倍.【期刊名称】《浙江工业大学学报》【年(卷),期】2015(043)002【总页数】5页(P119-123)【关键词】三氧化钨;纳米线;选择性吸附;亚甲基蓝【作者】郑华均;赵浙菲;李世雄;黄益操【作者单位】浙江工业大学绿色化学合成技术国家重点实验室培育基地,浙江杭州310014;浙江工业大学绿色化学合成技术国家重点实验室培育基地,浙江杭州310014;浙江工业大学绿色化学合成技术国家重点实验室培育基地,浙江杭州310014;浙江工业大学绿色化学合成技术国家重点实验室培育基地,浙江杭州310014【正文语种】中文【中图分类】O643随着印染行业的发展,印染废水已经严重影响生态环境及人类健康.印染废水大多是具有复杂的芳环结构的有机物,在环境中很难自降解[1-3].工业上处理废水的常用方法有吸附、氧化、生物降解和絮凝沉淀等方法[4-6].由于吸附法具有成本低、效率高、能耗少以及简单易行的优点,被广泛应用于有机废水的处理.近年来,有研究表:具有纳米结构的三氧化钨(WO3)材料对有机染料,特别是亚甲基蓝具有较好的去除作用[7].目前,应用于对亚甲基蓝吸附的WO3材料的形貌主要有一维棒状[8-9],二维片状[10-11],以及三维结构的花状[12]和海胆状[13].但这些结构材料普遍存在比表面积较小,对亚甲基蓝的吸附能力不强的不足.如Wang等[9]以偏钨酸铵为原料,通过低温搅拌、高温煅烧的方法,制备六方相的WO3纳米棒,其比表面积为27 m2/g,对亚甲基蓝的饱和吸附量仅为87.80 mg/g。
孙丰强教授简况-华南师范大学
孙丰强研究员简介孙丰强,博士,研究员,2007年度“新世纪优秀人才支持计划”入选者,广东省“千百十”人才工程省级培养对象,华南师范大学一类岗位特聘教授。
主持国家自然科学基金3项,省自然科学基金1项,广东省自然科学基金团队项目核心成员;申请国家发明专利6项。
研究领域:1、光化学,涉及无机、聚合物的光化学合成;材料的光催化性能;2、聚合物微球材料,涉及合成、印迹构筑与吸附性能研究;3、传感器,主要包括气体传感器、化学传感器的构筑与性能研究;4、电化学合成,控制合成各种半导体和聚合物材料,并研究其敏感性能与光化学性能;5、多孔材料,利用模板构筑大孔、介孔材料,并研究其性能。
工作经历:1.2004.8-2004.12:中国科学院固体物理研究所,助理研究员;2.2005.1-2006.1:香港中文大学,博士后;3.2006.3-今:华南师范大学,化学与环境学院,研究员教育背景:1.1994.9-1998.7:山东轻工业学院,材料系,硅酸盐工程专业(本科);2.1998.9-2001.7:吉林大学,地球科学学院,矿物学、岩石学、矿床学专业(硕士);3.2001.9-2004.7:中国科学院固体物理研究所,凝聚态物理专业(博士);主要论文:1.Fengqiang Sun, Jimmy C.Yu,Xinchen Wang, “Construction ofSize-Controllable Hierarchical Nanoporous TiO2 Ring Arrays and TheirModifications”, Chemistry of Materials, 2006, 18, 3774-3779;2.Fengqiang Sun, Weiping Cai, Yue Li, Lichao Jia, Fang Lu, “Direct growth ofmonoand multilayer nanostructured porous films on curved surfaces and theirapplication as gas sensors”Advanced Materials, 2005, 17, 2872-2877;3.Fengqiang Sun, Weiping Cai, Yue Li, Bingqiang Cao, Fang Lu, Guotao Duan,Lide Zhang, “Morphology Control and Transferability of Orderly ArrangedThrough-Pore Arrays Based on Electrodeposition of Colloidal Monolayer”,Advanced Materials, 2004,16(13),1116~1121;4.Fengqiang Sun, Weiping Cai, Yue Li, Bingqiang Cao, Yong Lei, Lide Zhang,“Morphology-Controlled Growth of Large Area 2D Ordered Pore Array”,Advanced Functional Materials, 2004, 14(3), 283~288;5.Fengqiang Sun, Weiping Cai, Yue Li, Guotao Duan, W. T. Nichols, ChanghaoLiang, N. Koshizaki, Qi Fang, “Laser Morphological manipulation of goldnanopartilces periodically arranged on solid supports”, Applied Physics B:Lasers and Optics, 2005, 81,765-768;6.Fengqiang Sun, Weiping Cai, Yue Li, Bingqiang Cao, Yong Lei, Lide Zhang,“Morphology controlled growth of large area ordered porous film”, MaterialScience and Technology, 2005, 21(4), 500~504;7.Yu Lin, Fengqiang Sun, and Lide Zhang, “Sol–gel ElectrophoreticDeposition andOptical Properties of Fe2O3 Nanowire Arrays”, AppliedPhysics A: Materials Science & Processing, 2004,78,1197;8.Bingqiang Cao, Fengqiang Sun, Weiping Cai, “Electrodeposition-inducedhighly oriented zinc oxide ordered pore arrays and their ultraviolet emissions”, Electrochemical and Solid State Letters, 2005, 8(9), G237~G240;9.Bingqiang Cao, Weiping Cai, Fengqiang Sun, Yue Li, Yong Lei and LideZhang, “Fabrication of large-area zinc oxide ordered pore arrays withcontrollable morphology”,Chemical Communications 2004, 14,1604;10.Cuncheng Li, Weiping Cai, Bingqiang Cao, Fengqiang Sun, Yue Li, CaixiaKan, Zhang Lide, “Mass Synthesis of Large, Singel-Crystal Au NanosheetsBased on a Polyol Process”Advanced Functional Materials, 2006, 16, 83-90 主要专利:1.孙丰强,蔡伟平,李越,曹丙强,张立德,“溶液浸渍-单层胶体晶体模板法制备形态可控有序多孔功能薄膜材料”,专利号:CN200410044978.4;2.孙丰强,蔡伟平,李越,曹丙强,张立德,“电化学沉积法合成形态可控、可转移的有序通孔薄膜”,专利号:CN200410044976.5;3.孙丰强,蔡伟平,贾丽超,曹丙强,李越,“纳米结构有序多孔薄膜型气敏元件及其制备方法”,申请号:200510095606.9。
半导体工艺流程英文缩写
半导体工艺流程英文缩写英文回答:Semiconductor Fabrication Process Acronyms.ALD: Atomic Layer Deposition.APCVD: Atmospheric Pressure Chemical Vapor Deposition. CMP: Chemical Mechanical Polishing.CVD: Chemical Vapor Deposition.DIB: Dry Ion Beam.DRAM: Dynamic Random Access Memory.EB: Electron Beam.EBL: Electron Beam Lithography.EPI: Epitaxial Growth.EUV: Extreme Ultraviolet.FIB: Focused Ion Beam.FPD: Flat Panel Display.IC: Integrated Circuit.LPCVD: Low Pressure Chemical Vapor Deposition. MEMS: Microelectromechanical Systems.MOCVD: Metalorganic Chemical Vapor Deposition. MOS: Metal-Oxide-Semiconductor.MPSG: Multilevel Photoresist Spin.OFE: Oxide Free Etch.PECVD: Plasma Enhanced Chemical Vapor Deposition. PVD: Physical Vapor Deposition.RIE: Reactive Ion Etching.RTP: Rapid Thermal Processing.SOG: Spin-On Glass.SOI: Silicon-On-Insulator.STI: Shallow Trench Isolation.TEOS: Tetraethyl Orthosilicate.UHV: Ultra High Vacuum.UV: Ultraviolet.中文回答:半导体工艺流程英文缩写。
两步法制备核壳结构Fe3O4@PDA@BSA纳米复合材料
两步法制备核壳结构Fe3O4@PDA@BSA纳米复合材料陈天弟;刘辉;马阜生;陶彩虹【摘要】采用溶剂热法制备了Fe3O4纳米粒子,再经两步法制备了核壳结构Fe3O4@PDA@BSA纳米复合材料,并利用X-射线衍射仪(XRD)、透射电镜(TEM)、振动样品磁强计(VSM)时样品形貌及磁性能进行了表征.结果表明,所制备的Fe3O4纳米粒子粒径为3~21 nm;核壳结构Fe3O4@PDA@BSA纳米复合材料的壳层厚度为10~20 nm,比饱和磁化强度为58.8 emu·g-1,具有良好的磁性能和生物安全性.该方法简单、反应条件温和、绿色环保,具有较好的适用性.【期刊名称】《化学与生物工程》【年(卷),期】2018(035)010【总页数】5页(P26-30)【关键词】四氧化三铁(Fe3O4);聚多巴胺(PDA);牛血清白蛋白(BSA);层层自组装;磁性能;生物安全性【作者】陈天弟;刘辉;马阜生;陶彩虹【作者单位】兰州交通大学化学与生物工程学院,甘肃兰州730070;兰州交通大学化学与生物工程学院,甘肃兰州730070;兰州交通大学化学与生物工程学院,甘肃兰州730070;兰州交通大学化学与生物工程学院,甘肃兰州730070【正文语种】中文【中图分类】O614磁性纳米粒子不但具有纳米材料所特有的性质,而且具有磁响应性,具有广泛的应用前景,尤其是在生物技术领域以及靶向药物传递领域[1]。
当Fe3O4纳米粒子粒径小至16 nm时就具有超顺磁性[2],超顺磁性Fe3O4纳米粒子无毒,具有良好的生物安全性、生物降解性和生物相容性,被广泛用于药物载体、催化剂载体、碳吸附剂[3]和靶向磁共振成像[4]等研究。
目前制备Fe3O4纳米粒子有共沉淀法、微乳液法、水热合成法、溶剂热法和溶胶-凝胶法等,其中溶剂热法是制备高性能Fe3O4纳米粒子的常用方法。
在磁性纳米粒子表面修饰靶向药物,通过外加磁场,可以定向运输,靶向传递,提高药物的利用率[5-7],但磁性纳米粒子表面需要进行特殊的改性和修饰。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
EXPERIMENTAL SECTION Materials. All of the chemicals were of analytical grade and used as received without any further purification. Ferric nitrate
Received: May 20, 2013 Revised: August 26, 2013 Published: September 5, 2013
INTRODUCTION The controlled fabrication of core−shell nanocomposites has attracted much attention because of the combined functionality of cores and shells and great application potential in various fields.1−3 Iron oxide@silica core−shell nanostructures possess controllable magnetism, low cytotoxicity, and a chemically modifiable surface. They have shown great potential in applications such as drug delivery, magnetic resonance imaging (MRI), and catalysis.4−7 Many efforts have been made to fabricate iron oxide@silica colloidal materials.8−13 However, few of these studies organically couple iron oxide synthesis routes with a silica sol−gel process because of the different sensitive processes for either the iron oxide synthesis or the silica sol−gel process.2 In these studies, the formation of the iron oxide core and the silica shell was achieved in different processes. Iron oxide was first prepared and separated from the mother liquid in order to remove impurities. Second, the asobtained iron oxide was redispersed in silica sol−gel after surface modification for the deposition of silica shell. Such complicated procedures usually led to poor dispersion and irregular agglomeration of the obtained nanostructures and made scaling-up more difficult, which would limit the application of the final colloidal materials. Therefore, the facile and scalable synthesis of core−shell nanocomposites with a dimensionally and morphologically well-defined iron oxide
Article /JPCC
Controlled Fabrication of Iron Oxide/Mesoporous Silica Core−Shell Nanostructures
Mingwei Zhang,†,‡ Kegong Fang,† Minggui Lin,† Bo Hou,† Liangshu Zhong,§ Yan Zhu,§ Wei Wei,*,§ and Yuhan Sun*,†,§
21529
/10.1021/jp4049583 | J. Phys. Chem. C 2013, 117, 21529−21538
■
The Journal of Physical Chemistry C (Fe(NO3)3·9H2O), acetic acid, ethanol, tetraethyl orthosilicate (TEOS) and cetyltrimethylammonium bromide (CTAB) were purchased from Tianjin Chemical Reagent Co., Ltd. (Tianjin, China). Polyvinylpyrrolidone (PVP, Mw = 1 300 000) was obtained from Sigma-Aldrich. Preparation of Monodisperse Iron Oxide Nanoparticles. The iron oxide nanocubes were synthesized by a novel solvothermal approach. In brief, 1.01 g of Fe(NO3)3· 9H2O, 1.01 g of PVP, and 2 mL of acetic acid were dissolved in 100 mL of ethanol under magnetic stirring at room temperature. Then the formed solution was transferred into a Teflon-lined stainless steel autoclave. The autoclave was sealed and maintained at 200 °C for 24 h. Afterward, the autoclave was allowed to cool to room temperature, and the iron oxide nanocubes were obtained. Preparation of Iron Oxide Nanoparticles Embedded in Mesoporous Silica Spheres (Fe2O3@SiO2 Core−Shell Composites). The as-prepared iron oxides in ethanol (100 mL) were dissolved in distilled water (100 mL), and an additional 100 mL of ethanol was added. Then 9 mL aqueous ammonia (25 wt %) was added to this solution to adjust the pH to 9. Afterward, cetyltrimethylammonium bromide (CTAB, 0.2 g) was dissolved in the above solution. Finally, tetraethoxysilane (TEOS, 0.625 mL) was added slowly. After the solution was stirred for 24 h at room temperature, the nanoparticles were separated by filtering, washed thoroughly with water and ethanol, and then dried in an oven at 100 °C. The calcination of as-prepared core−shell composite nanoparticles was carried out for 5 h at 550 °C in the presence of static air. Then iron oxides coated with mesoporous silica (20 nm shell thickness) composites with perfect core−shell structure were at last obtained. Additionally, two samples were prepared with an identical procedure but with increased amounts of TEOS (1.25 mL, 2.5 mL) and CTAB (0.4g, 0.8g) to change the thickness of the SiO2 shell (35 nm, 60 nm). To investigate the effects of various synthesis conditions in the sol−gel process, a series of experiments were conducted by changing the volume ratio of alcohol to water (VE/W), the feeding amount of aqueous ammonia, and CTAB. Preparation of Hollow Silica Particles. Hollow SiO2 spheres were prepared by etching Fe2O3@SiO2 nanoparticles with 18% HCl. In a typical etching process, one gram of core− shell powder was added to 20 mL of an 18% HCl solution, and the suspension was treated by ultrasonication for 2 h. During this process, the mother solution turned a yellowish color, indicating that ferric ions were leaching out. Finally, the prepared hollow-type silica particles were collected by centrifugation and washed with deionized water and ethanol to remove FeCl3. Preparation of Magnetic Core/Mesoporous Silica Shell (MCMS) Particles. Magnetic core/mesoporous silica shell (MCMS) particles were obtained by H2 reduction of Fe2O3@SiO2 nanoparticles. The reduction was carried out by the thermal treatment of the Fe2O3@SiO2 particles in mixed H2 and Ar (5:95) gases at different temperature (350, 450, or 650 °C) for 4 h to obtain MCMS particles with different compositions and magnetic properties. The synthesis process of Fe2O3@SiO2 and its derivative nanoparticles is illustrated in Scheme 1. Characterization. X-ray powder diffraction (XRD) measurements were performed on a D8 Advance Bruker AXS diffractometer, using a Cu Kα radiation (λ = 1.5406 Å) at 40 kV, 40 mA, employing a scanning rate of 0.02° s−1 in the 2θ
