电阻率和掺杂浓度的比率关系表
单晶硅掺杂浓度和电阻率的关系

单晶硅掺杂浓度和电阻率的关系1. 引言嘿,朋友们!今天咱们要聊聊单晶硅和电阻率的那些事儿。
这可能听上去有点枯燥,但别急,咱们一边聊一边把这东西拆解开,肯定让你听得津津有味。
单晶硅,这可不是普通的硅,它就像一位超级明星,掺杂浓度是它的粉丝群体,电阻率则是它的实力等级。
掺杂得多了,电阻率就低了,这就好比你朋友圈子里的人越多,社交能力越强,明白吗?1.1 什么是单晶硅?首先,先给大家普及一下什么是单晶硅。
单晶硅就像是个乖乖牌,结构整齐划一,粒子之间没有太多杂质。
这就让它在电子设备里显得特别有用,比如手机、电脑这些高科技玩意儿。
想象一下,它就像是一个讲究的宴会厅,里面的每一个细节都要做到极致,这样才能让所有的“客人”——也就是电子,顺畅地进出。
1.2 掺杂浓度是个什么概念?再来说说掺杂浓度。
想象你在一个派对上,邀请了很多朋友来,结果大家都在一块嗨,气氛那叫一个好!掺杂浓度就是看你请了多少“朋友”——也就是杂质原子。
一般来说,咱们会掺一些三价元素,比如铝、镓;或者五价元素,比如磷、砷。
不同的元素会给单晶硅带来不同的“性格”,这就是掺杂浓度的魔力。
2. 电阻率的秘密好,咱们接下来谈谈电阻率。
电阻率就像是单晶硅的“脾气”,掺杂浓度越高,电阻率就越低。
这就像你在学校里,班上同学多了,合作也就多了,大家分工明确,效率高嘛!所以,掺杂浓度的增加,就像是给单晶硅加了个“助推器”,让它的“脾气”变得更加温和,电流通过的时候不再那么抗拒。
2.1 掺杂浓度与电阻率的关系来个简单的公式,你肯定听过吧?电阻率跟掺杂浓度成反比。
换句话说,掺杂浓度越高,电阻率越低,这就是“无事不登三宝殿”的道理。
想想看,越多的杂质原子就意味着越多的载流子,这些载流子就像是一群积极向上的年轻人,干劲十足,拉着电流一路狂奔,毫不犹豫!2.2 如何控制掺杂浓度?至于怎么控制掺杂浓度,这可是个技术活。
一般来说,咱们会用气相沉积、离子注入等方法。
这就像在厨房里调味料,掌握好每种元素的比例,才能做出一盘美味的菜肴。
硅电阻率随掺杂影响

二、实现只填充最低子带的条件
为提供量子霍尔实验的条件,首先应保证电子只限于最低子带。 假使电子过多,进入较高的子带,将使实验结果是几个子带的 叠加,造成分析困难。 故在制备样品时要控制载流子数目。
利用态密度公式 m*/ π h ,可知在最低子带 ε 0 与上一个子带 ε 1 之间的状态数为 m* ( ε 1 - ε0 ) / π h2 ,那么单位界面上 电子数目为
量子霍尔效应的两种形式
( a )霍尔电阻 ρ xy 与磁场 B 的关系 假设 n 不变,改变磁场 B ,测量霍尔电阻在某些 B 值 出现平台,如图 11-29 所示。平台 值是量子化的,精确地等于 h/e 2 的正整数分之一倍, 其精度可达 10 -6 以上。
( b )霍尔电阻与外电压 V G 的关系 这类实验是保持磁场 B 不变,通过加外电压改变载流子的数目, 一般这类实验是在半导体 MOS 器件的反型层上进行的,其典型 现象类似上面实验。在某些 V G 处,霍尔电阻出现平台,如图 所 示,平台是量子化的,精确地等于 h/e 2 的正整数分之一倍。 从理论上看,上述实验结果是显而易见的,即平台本身相当于填 满的朗道能级,这与公式完全吻合。但实际上仍存在许多费解的 问题,这些问题已经和正在被逐一解决。
2个 决定性因素
电阻率
杂质浓度
载流子浓度 载流子迁移率
2个 有关因素
温度
①杂质浓度较低时 1016~10 18 cm-3
电阻率与杂质浓度成反比关系, 即杂质浓度越高,电阻率越小。
1.电阻率和杂质 浓度的关系
②杂质浓度较高时
杂质在室温下不能全部电离, 在重掺杂的简并半导体中情况 更加严重; 迁移率随着杂质浓度的增加明 显下降
①对纯半导体材料
半导体电阻率及其与杂质浓度和温度
0
T
硅与T关系
精选2021版课件
11
电阻率与材料性质有关,禁带宽度越大,同一温 度下的本征载流子浓度就越低,进入本征导电的温度 也越高
锗器最高工作温度为100℃,硅为250℃,而砷化
镓可达450℃。
C
q m*
1
AT
3/2
BNi T 3/2
A B
0 硅与T关系
精选2021版课件
T
12
杂质变化的曲线(非补偿或轻补偿)。
精选2021版课件
3
A:轻掺(杂质浓度1016~1018cm-3),
迁移率随杂质浓度的变化较小
1
Ni
精选2021版课件
4
杂质浓度增高时,非线性曲线。原因: 一是杂质在室温下不能全部电离,重掺杂的简并 半导体中情况更加严重;
二是迁移率随杂质浓度的增加将显著下降。
精选2021版课件
Hale Waihona Puke 5由电阻率可确定所含杂质的浓度。材料越纯,电 阻率越高(不适于高度补偿的材料)。
精选2021版课件
6
4.4.2 电阻率随温度的变化
1)本征半导体
T ni (u不变)
1
nqn pq p
精选2021版课件
7
2)掺杂半导体:杂质电离、本征激发同时
存在,电离杂质散射和晶格散射机构的存在,电 阻率随温度的变化关系复杂。(AB BC C三段)
混合型
1
(4-64)
nqn pq p
本征 i
1
ni (qn
qp )
(4-65)
室温下,本征硅的约为2.3×105Ω·cm,本征锗
(禁宽小)约为47Ω·cm。
电阻率决定于载流子浓度和迁移率,与杂质浓度
半导体电阻率及其与杂质浓度和温度
杂质变化的曲线(非补偿或轻补偿)。
2021/6/3
3
A:轻掺(杂质浓度1016~1018cm-3),
迁移率随杂质浓度的变化较小
1
Ni
2021/6/3
4
杂质浓度增高时,非线性曲线。原因: 一是杂质在室温下不能全部电离,重掺杂的简并 半导体中情况更加严重;
二是迁移率随杂质浓度的增加将显著下降。
2021/6/3
5
由电阻率可确定所含杂质的浓度。材料越纯,电 阻率越高(不适于高度补偿的材料)。
2021/6/3
6
4.4.2 电阻率随温度的变化
1)本征半导体
T ni (u不变)
1
nqn pq p
2021/6/3
7
2)掺杂半导体:杂质电离、本征激发同时
存在,电离杂质散射和晶格散射机构的存在,电 阻率随温度的变化关系复杂。(AB BC C三段)
T
硅与T关系
2021/6/3
11
电阻率与材料性质有关,禁带宽度越大,同一温 度下的本征载流子浓度就越低,进入本征导电的温度 也越高
锗器最高工作温度为100℃,硅为250℃,而砷化
镓可达450℃。
C
q m*
1
AT
3/2
BNi T 3/2
A B
2021/6/3
0
T
硅与T关系
12
用于科普,若有不
当之处,请指正,感 谢您的下载。
混合型
1
(4-64)
nqn pq p
本征 i
1
ni (qn
qp )
(4-65)
室温下,本征硅的约为2.3×105Ω·cm,本征锗
(禁宽小)约为47Ω·cm。
掺杂浓度与电阻率关系

SEMI MF723-99Practice for Conversion Between Resistivity and Dopant Density for Boron-Doped, Phosphorus-Doped, and Arsenic-Doped SiliconThis standard was originally published by ASTM International as ASTM F 723-81. It was formally approved by ASTMballoting procedures and adhered to ASTM patent requirements. Though ownership of this standard has been transferredto SEMI, it has not been formally approved by SEMI balloting procedures and does not adhere either to SEMIRegulations dealing with patents or to SEMI Editorial Guidelines. Available at October 2003, to bepublished November 2003. Last published by ASTM International as ASTM F 723-99.INTRODUCTIONThe ability to convert between resistivity and dopant density in a semiconductor is important for a variety of applicationsranging from material inspection and acceptance to process and device modeling. Despite some experimentallimitations, the conversion is more readily established from an empirical data base than from theoretical calculations.Resistivity may be unambiguously determined throughout the desired resistivity range regardless of the dopantimpurity. However, it was necessary to use a variety of techniques to establish the complete dopant density scale ofinterest; these techniques do not all respond to the same parameter of the semiconductor.In the experimental work (1), (2), (3)1 supporting these conversions, capacitance-voltage measurements were used todetermine the dopant density of both boron- and phosphorous-doped specimens with dopant densities less than about1018 cm−3. The specimens were assumed to be negligibly compensated; hence, the data given by the capacitance-voltagemeasurements were taken to be a direct measure of the dopant density in the specimen. Hall effect measurements wereused to obtain dopant density values greater than 1018 cm−3. In addition, in this range neutron activation analysis andspectrophotometric analysis were used to determine phosphorus density, and the nuclear track technique was used todetermine boron density. Where there were discrepancies in the data from the analytical techniques, more weight wasgiven to the Hall effect results. Up to the highest densities measured, boron is expected to be fully electrically active.Therefore, the boron densities of these specimens were assumed equal to the carrier densities obtained from the Halleffect measurements with the use of an estimate for the Hall proportionality factor based on the best availableexperimental and theoretical information. In the case of specimens heavily doped with phosphorus, the Hallproportionality factor is unity, but there is considerable evidence that at densities above about 5 × 1019 cm−3 not all ofthe phosphorus is electrically active because of the formation of complexes. In the absence of data regarding the fractionof phosphorus atoms withdrawn from electrically active states due to complexing, the values of carrier density takenfrom the Hall effect measurements were assumed to be equal to the phosphorus density values. Consequently, theconversions based on these data may understate the total phosphorus density for stated values above about5 × 1019 cm−3.1. Scope1.1 This practice 2 describes a conversion between dopant density and resistivity for arsenic-, boron- and phosphorus-doped single crystal silicon at 23°C. The conversions are based primarily on the data of Thurber et al (1), (2), (3)2 taken on bulk single crystal silicon having dopant density values in the range from 3 × 1013 cm−3 to 1 × 1020 cm−3 for phosphorus-doped silicon and in the range from 1014 cm−3 to 1 × 1020 cm−3 for boron-doped silicon. The phosphorus data base was supplemented in the following manner: two bulk specimen data points of Esaki and Miyahara (4) and one diffused specimen data point of Fair and Tsai (5) were used to extend the data base above 1020cm−3, and an imaginary point was added at 1012 cm−3 to improve the quality of the conversion for low dopant density values. A conversion for arsenic, distint from that of phosphorus, is presented for the range 1019 to 6 by 1020cm−3.1.2 The self consistency of the conversion (resistivity to dopant density and dopant density to resistivity) (see Appendix X1) is within 3 % for boron from 0.0001 to 10 000 Ω·cm, (10 12 to 1021 cm−3) and within 4.5 % for phosphorus from 0.0002 to 4000 Ω·cm (1012 to 5 × 10 20cm−3). This error increases rapidly if the phosphorus conversions are used for densities above 5 × 1020 cm−3 .1.3 These conversions are based upon boron and phosphorus data. They may be extended to other dopants in silicon that have similar activation energies; although the accuracy of conversions for other dopants has not been established, it is expected that the phosphorus data would be satisfactory for use with arsenic and antimony, except when approaching solid solubility (see 6.3).1 Boldface numbers in parentheses refer to the list of references at the end of this practice.2 DIN 50434 is an equivalent method. It is the responsibility of DIN Committee NMP 221, with which Comittee F-1 maintains close technical liaison. DIN 50444, Testing of Materials for Semiconductor Technology: Conversion Between Resistivity and Dopant Density of Silicon, is available from Beuth Verlag GmbH Burggrafenstrasse 4-10, D-1000 Berlin 30, Federal Republic of Germany.1.4 These conversions are between resistivity and dopant density and should not be confused with conversions between resistivity and carrier density or with mobility relations.N OTE 1—The commonly used conversion between resistivity and dopant density compiled by Irvin (6) is compared with this conversion in Appendix X2. In this compilation, Irvin used the term “impurity concentration” instead of the term “dopant density.”1.5 This standard does not purport to address all of the safety concerns, if any, associated with its use. It is the responsibility of the user of this standard to establish appropriate safety and health practices and determine the applicability of regulatory limitations prior to use.2. Referenced Documents2.1 ASTM Standards:F 84 Test Method for Measuring Resistivity of Silicon Wafers with an In-Line Four-Point Probe 32.2 Adjuncts:Large Wall Chart 43. Terminology3.1 Definitions:3.1.1 carrier density, n (electrons);p (holes)—the number of majority carriers per unit volume in an extrinsic semiconductor, usually given in number/cm 3 although the SI unit is number/m3.3.1.2 compensation—reduction in number of free carriers resulting from the presence of impurities other than the majority dopant density impurity. Compensation occurs when both donor and acceptor dopant impurities are present in a semiconductor; in this case the net dopant density (which is equal to the carrier density provided that all the dopant impurities are ionized) is given by the absolute magnitude of the difference between the acceptor dopant density and the donor dopant density. Compensation may also occur if deep-level impurities or defects are present in quantities comparable with the dopant impurities; in this case the relationship between the carrier density and the dopant density (under the assumption of full ionization of the dopant impurity) depends on a variety of parameters (7). A semiconductor that exhibits compensation is said to be “compensated.”3.1.3 concentration—relative amount of a minority constituent of a mixture to the majority constituent (for example, parts per million, parts per billion, or percent) by either volume or weight. In the semiconductor literature, often used interchangeably with number density (for example, number per unit volume).3.1.4 deep-level impurity—a chemical element that when introduced into a semiconductor has an energy level (or levels) that lies in the mid-range of the forbidden energy gap, between those of the dopant impurity species.3.1.4.1 Discussion-Certain crystal defects and complexes may also introduce electrically active deep levels in the semiconductor.3.1.5 dopant density—in an uncompensated extrinsic semiconductor, the number of dopant impurity atoms per unit volume, usually given in atoms/cm3 although the SI unit is atoms/m3. Symbols: N D for donor impurities and N A for acceptor impurities.4. Summary of Practices4.1 The conversions between resistivity and dopant density are made using equations, tables, or graphs.5. Significance and Use5.1 Dopant density and resistivity of silicon are two important acceptance parameters used in the interchange of material by consumers and producers in the semiconductor industry. Therefore, a particular method of converting from dopant density to resistivity and vice versa must be available since some test methods measure resistivity while others measure dopant density.5.2 These conversions are useful in mathematical modeling of semiconductor processing and devices.3 Annual Book of ASTM Standards, Vol 10.05, ASTM International, 100 Barr Harbor Drive, West Conshohocken, PA 19428. Telephone: 610-832-9500, Fax: 610-832-9555, Website: 4 A large wall chart, “Conversion Between Resistivity and Dopant Density” is available from ASTM, 100 Barr Harbor Drive, West Conshohocken, PA 19428. Order Adjunct ADJF0723.6. Interferences6.1 Carrier Density— Attempts to derive carrier density values from resistivity values by using these conversions are subject to error. While dopant density and carrier density values are expected to be the same at low densities (up to about 1017 cm−3), the two quantities generally do not have the same value in a given specimen at moderate densities. At such moderate densities, (about 1017 cm−3 to 1019 cm−3), dopant densities are larger than carrier densities due to incomplete ionization. At densities above 10 19 cm−3, the population statistics become degenerate, and carrier densities would normally be equal to dopant densities. However, in this upper range of densities, the possibility of formation of compounds or complexes involving dopant atoms is more pronounced and would prevent some of the dopant atoms from being electrically active. Such formation of compounds or complexes is particularly likely in phosphorus- or arsenic-doped silicon. Precipitation occurs at dopant densities greater than solid solubility.6.2 Heavily Phosphorus-Doped Silicon—These conversions are given as functions of resistivity and of dopant density. For heavily phosphorus-doped specimens, primary emphasis was placed on Hall effect measurements for establishing the density values. However, since the Hall effect measures carrier density, it was assumed for these heavily doped specimens that all atoms were electrically active; that is, the dopant density was equal to the carrier density as measured by the Hall effect. The possible formation of phosphorus-vacancy pairs which are known to reduce the electrically active phosphorus atoms at high densities (5) was not tested or accounted for in the data base or the resulting conversions. The existence of such phosphorus-vacancy pairs would cause the Hall measurements to understate the total dopant density for the heavily phosphorus-doped specimens.6.3 Other Dopant Species—The applicability of these conversions to silicon doped with other than arsenic, boron or phosphorus has not yet been established. However, in the lightly doped range (<1017 cm−3) the conversions are expected to be reasonably accurate for other dopants. Between 10 17 and 1019 cm −3, the difference in activation energy of different dopant species will cause different resistivities to be measured for the same dopant density. In this range the differences in resistivities will be larger among the p-type dopants than among the n-type dopants due to larger differences in the activation energies among the p-type dopants. At high dopant densities (>1019 cm−3), the formation of complexes involving dopant atoms, lattice defects, and other impurities will lead to a modification in the number of charge carriers. The extent of this effect will depend upon the particular dopant species and is not well detailed in the literature for the various common dopants. Its onset is expected to be related to the density of the dopant compared to the solid solubility of that dopant in silicon. Therefore, in this upper dopant density range, the applicability of these conversions to dopants other than boron and phosphorus is unclear.6.4 Compensation— The specimens used to obtain the data base for these conversions were assumed to be uncompensated. The measured net dopant density was taken to be the total density of the intentional dopant in the specimen. For specimens in which significant compensation occurs, these conversions may not apply.6.5 Temperature— The conversions in this practice hold for a temperature of 23°C. Resistivity varies with temperature, but dopant density does not.N OTE 2—It is possible to obtain dopant density values from resistivity values that were not measured at 23°C by using Test Method F 84 to correct the resistivity values to 23°C. Also, the conversion from dopant density to resistivity may be made directly and the temperature correction for resistivity then made following Test Method F 84 to obtain the resistivity at other than 23°C.N OTE 3—References 1, 2, and 3 give values for the coefficients in the conversion equations at both 23°C and 300 K.6.6 Other Electrically Active Centers—Numerous other mechanisms exist that may modify the number of free carriers or noticeably alter carrier mobility, either of which will change the resistivity from the value given here for a given dopant density. Among these mechanisms are (1) lattice damage due to radiation (neutron transmutation doping or ion implantation), (2) formation of deep level centers due to chemical impurities (typically heavy metals, either unwanted or sometimes intentionally added for minority carrier lifetime control), and (3) unintentional doping due, for example, to electrically activated oxygen. When any of these effects is known or expected to be present, the conversions given here may not apply.6.7 Range of Arsenic-Doped Silicon Data—The conversion given for arsenic-doped silicon is from Fair and Tsai (8), covering the doping range of 1019 to 6 by 1020. This conversion was generated using Hall effect measurements. The principal reference for neutron activation data is that of Newman et al.(9). Neutron activation data give higher resistivity values for a given dopant density than do Hall data because of the assumption that the Hall coefficient R H = 1/ne. A more complicated relationship between R H and n is given in Ref (9). Care must be taken in using any conversion not to extrapolate beyond the range of the data fitted, as the formulas will diverge beyond that range. Other studies support the use of the conversion given here Refs (10, 11, 12, and 13), which means that the conversion to resistivity for phosphorus can be used for arsenic in this range. In the range 2 by 1019 to 1020, the Fair and Tsai fit matches the Irvin formula. The range beyond 6 by 1020 is discussed only in Ref (13).7. Procedure7.1 Convert Resistivity Values to Dopant Density Values— Follow 7.1.1 (graphical method), 7.1.2 (tabular method), or 7.1.3 (computation method). 57.1.1 Graphical Method:7.1.1.1 For boron-doped silicon, use the curve labeled “boron” in Fig. 1.7.1.1.2 For phosphorus-doped silicon, use the curve labeled “phosphorus” in Fig. 1.7.1.2 Tabular Method:7.1.2.1 For boron-doped silicon use Table 1.TABLE 1 Dopant Density as a Function of Resistivity for Boron-Doped SiliconN OTE 1—Entries in two significant digits are for regions of extrapolated data.TABLE 1 Continued7.1.2.2 For phosphorus-doped silicon use Table 2.7.1.3 Computational Method :7.1.3.1 For boron-doped silicon, calculate the dopant density value from the resistivity value as follows:N = 1.330 × 10 16ρ + 1.082 × 10 17ρ []1 + ()54.56ρ 1.105 (1)where:ρ= resistivity and N = dopant density.7.1.3.2 For phosphorus-doped silicon, calculate the dopant density from the resistivity as follows:N = 6.242 × 10 18ρ × 10 Z (2)where:Z = A 0 + A 1x + A 2x 2 + A 3x 31 + B 1x + B 2x2 + B 3x3 (3)where:x = log 10ρ,A 0 = −3.1083,A1 =−3.2626,A2 =−1.2196,A3 =−0.13923,B 1= 1.0265,B2= 0.38755, andB3= 0.041833.7.2 Convert Dopant Density Values to Resistivity Values—Follow 7.2.1 (graphical method), 7.2.2 (tabular method), or 7.2.3 (computational method).TABLE 2 Dopant Density as a Function of Resistivity for Phosphorus-Doped SiliconN OTE 1—Entries in two significant digits are for regions of extrapolated data.TABLE 2 Continued7.2.1 Graphical Method:7.2.1.1 For boron-doped silicon, use the curve labeled “boron” in Fig. 1.7.2.1.2 For phosphorus-doped silicon, use the curve labeled “phosphorus” in Fig. 1.7.2.2 Tabular Method:7.2.2.1 For boron-doped silicon, use Table 3.TABLE 3 Resistivity as a Function of Dopant Density for Boron-Doped Silicon N OTE 1—Entries in two significant digits are for regions of extrapolated data.TABLE 3 Continued7.2.2.2 For phosphorus-doped silicon, use Table 4.7.2.3 Computational Method :7.2.3.1 For boron-doped silicon, calculate the resistivity from the dopant density as follows:ρ = 1.305 × 10 16N + 1.133 × 10 17N []1 + ()2.58 × 10 −19 N −0.737(4) 7.2.3.2 For phosphorus-doped silicon, calculate the resistivity from the dopant density as follows: ρ = 6.242 × 10 18N × 10 Z (5)where:Z = A 0 + A 1y + A 2y 2 + A 3y 31 + B 1y + B 2y2 + B 3y3 (6)where:y = (log 10N ) − 16,A 0 = −3.0769,A 1 = 2.2108,A 2 = −0.62272,A 3 = 0.057501,B 1 = −0.68157,B 2 = 0.19833, andB3 =−0.018376.7.2.3.3 For arsenic-doped silicon, calculate the resistivity from the dopant density as follows:log10ρ = −6633.667 + AX + BX2 + CX3 + DX 4 + EX5 + FX6 + GX 7 + HX8 + IX9 + JX 10(7) where:log10N,X =768.2531,A =B =−25.77373,C= 0.9658177,D =−0.05643443,E =−8.008543 × 10 −4,F =9.055838 × 10 −5,G =−1.776701 × 10 −6,H =1.953279 × 10 −7,I =−5.754599 × 10 −9, andJ =−1.31657 × 10 −11.SEMI MF723-99 © SEMI 200311TABLE 4 Resistivity as a Function of Dopant Density for Phosphorus-Doped SiliconN OTE1—Entries in two significant digits are for regions of extrapolated data.SEMI MF723-99 © SEMI 2003 12TABLE 4 Continued8. Keywords8.1 arsenic; boron; dopant density; phosphorus; resistivity; siliconSEMI MF723-99 © SEMI 200313N OTE 1—The solid line shows the resistivity to dopant density conversion for the range of actual data. The chain dot line showsthe dopant density to resistivity conversion for the range of actual data. Dashed lines show regions of extrapolation from data.N OTE 2—On the scale of the figure as reproduced in this book, the solid and chaindot lines cannot be distinguished visually. Theyare distinguishable, however, on the wall chart available as Adjunct PCN 12-607230-46, wherever the self-consistency error (seeAppendix X1) becomes appreciable.FIG. 1 Preferred Conversion Between Resistivity and Total Dopant Density Values for Boron- and Phosphorus-Doped SiliconSEMI MF723-99 © SEMI 2003 14APPENDIXES(Nonmandatory Information)X1. DIFFERENCES IN CONVERSION SCALES ENCOUNTERED WHEN USING THE TABULAR ORCOMPUTATIONAL FORMS OF THIS PRACTICEX1.1 The conversion equations and the resulting tables are derived by fitting the experimental data using either resistivity or dopant density as the independent variables. This leads to complementary equations, for example, 7.1.3.1 and 7.2.3.1. These complementary equations are not exactly equivalent mathematically and small discrepancies can be found when using the equations or the tables derived from them. A given value of resistivity (for example, 1.00Ω· cm p -type) would convert to a dopant density value (1.46 × 1016cm −3), but the use of that dopant density in the complementary equation (or table) gives a resistivity (1.02 Ω·cm) which is different from the starting value. In this case, there is a 2 % (1.00 Ω·cm versus 1.02 Ω·cm) self-consistency error.X1.2 The self-consistency errors are plotted in Fig. X1.1.SEMI MF723-99 © SEMI 200315FIG. X1.1 Differences in Conversion Scales Encountered When Using this PracticeSEMI MF723-99 © SEMI 200316FIG. X1.2 Comparison of Conversions Between Resistivity and Dopant Density with Those of IrvinX2. COMPARISON OF CONVERSIONS WITH THOSE DUE TO IRVINX2.1 Irvin (6) reported conversion relations between resistivity and dopant density for n - and p -type silicon at 300 K. Irvin chose to use the term impurity concentration instead of the term dopant density. Irvin's analysis was based on a compilation of data by other authors based on several donor and acceptor dopants of similar energy levels in the silicon band gap and was supplemented by data taken by Irvin on heavily arsenic- and boron-doped specimens. All specimens were assumed to be uncompensated, but the possibility of compensation because of thermal activation of oxygen in the Czochralski crystals was recognized.X2.2 A comparison of the conversions given by this method with those due to Irvin is shown in Fig. X1.2.SEMI MF723-99 © SEMI 2003 17REFERENCES(1) Thurber, W. R., Mattis, R. L., Liu, Y. M., and Filliben, J. J., “Resistivity-Dopant Density Relationship for Phosphorus-Doped Silicon,” Journal of the Electrochemical Society , Vol 127, 1980, pp. 1807–1812.(2) Thurber, W. R., Mattis, R. L., Liu, Y. M., and Filliben, J. J., “Resistivity-Dopant Density Relationship for Boron-DopedSilicon,” Journal of the Electrochemical Society , Vol 127, 1980, pp. 2291–2294.(3) Thurber, W. R., Mattis, R. L., Liu, Y. M., and Filliben, J. J., Semiconductor Measurement Technology ,“ RelationshipBetween Resistivity and Dopant Density for Phosphorus- and Boron-Doped Silicon,” NBS Special Publication 400-64 (April 1981).(4) Esaki, L., and Miyahara, Y., “A New Device Using the Tunneling Process in Narrow p-n Junction,” Solid-StateElectronics , Vol 1, 1960, pp. 13–21.(5) Fair, R. B., and Tsai, J. C. C., “A Quantitative Model for the Diffusing of Phosphorus in Silicon and the Emitter DipEffect,” Journal of the Electrochemical Society, Vol 124, 1977, pp. 1107–1118.(6) Irvin, J. C.,“ Resistivity of Bulk Silicon and of Diffused Layers in Silicon,” Bell System Technical Journal, Vol 41, 1962,pp. 387–410.(7) Blakemore, J. S., Semiconductor Statistics, Pergamon Press, Oxford, 1962, pp. 153–161.(8) Fair, R. B., and Tsai, J. C. C., “The Diffusion of Ion-Implanted Arsenic in Silicon,” Journal of the ElectrochemicalSociety, Vol 122, No. 12, l975, p. 1689.(9) Newman, P. F., Hirsch, M. J., and Holcomb, D. F., “A Calibration Curve for Room-Temperature Resistivity versusDonor Atom Concentration in Si:As,” Journal of Applied Physics, Vol 58, No. 10, l985, p. 3779.(10) Furukawa, Y., “Impurity Effect Upon Mobility in Heavily Doped Silicon,” Journal of Physics Society, Japan, Vol 16,l961, p. 577.(11) Murota, J., Arai, E., Kobayashi, K., and Kudo, K., “Relationship Between Total Arsenic and Electrically Active ArsenicConcentrations in Silicon Produced by the Diffusion Process.” Journal of Applied Physics, Vol 50, No. 2, l979, p. 804.(12) Matsumoto, S., Niimi, T., Murota, J., and Arai, E., “Carrier Concentration and Hall Mobility in Heavily Arsenic-DiffusedSilicon,” Journal of Electrochemical Society, Vol 127, No. 7, l980, p. 1650.(13) Masetti, G., Severi, M., and Solmi, S., “Modeling of Carrier Mobility Against Carrier Concentration in Arsenic-,Phosphorus-, and Boron-Doped Silicon,” IEEE Trans. on Elec. Dev., ED-30, No. 7, l983, p. 764.NOTICE: SEMI makes no warranties or representations as to the suitability of the standards set forth herein for any particular application. The determination of the suitability of the standard is solely the responsibility of the user. Users are cautioned to refer to manufacturer's instructions, product labels, product data sheets, and other relevant literature, respecting any materials or equipment mentioned herein. These standards are subject to change without notice.By publication of this standard, Semiconductor Equipment and Materials International (SEMI) takes no position respecting the validity of any patent rights or copyrights asserted in connection with any items mentioned in this standard. Users of this standard are expressly advised that determination of any such patent rights or copyrights, and the risk of infringement of such rights are entirely their own responsibility.Copyright by SEMI® (Semiconductor Equipment and MaterialsInternational), 3081 Zanker Road, San Jose, CA 95134. Reproduction ofthe contents in whole or in part is forbidden without express writtenconsent of SEMI.。
半导体电阻率及其与杂质浓度和温度

nqn pq p
本征 i
1
ni (qn
qp )
(4-65)
室温下,本征硅的约为2.3×105Ω·cm,本征锗
(禁宽小)约为47Ω·cm。
电阻率决定于载流子浓度和迁移率,与杂质浓度
和温度有关。
4.4.1 电阻率和杂质浓度的关系
图4-15是锗、硅和砷化镓(温度定)300K时随
杂质变化的曲线(非补偿或轻补偿)。
4.4 电阻率及其与杂质浓度和温度的关系
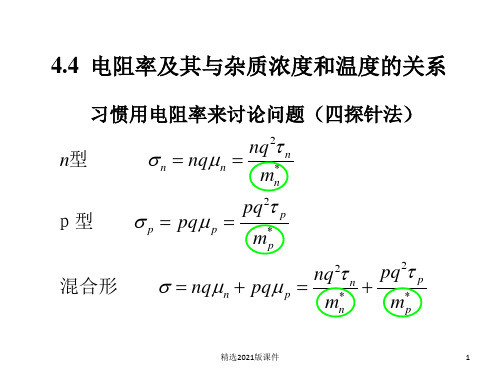
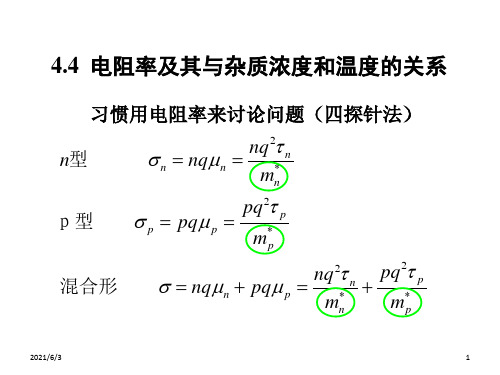
习惯用电阻率来讨论问题(四探针法)
n型 n
nqn
nq2 n
mn*
p型
p
pq p
pq2 p
m*p
混合形
ቤተ መጻሕፍቲ ባይዱ
nqn
pq p
nq2 n
mn*
pq2 p
m*p
n型 n
1
nqn
(4-62)
p型
p
1
pq p
(4-63)
混合型
1
(4-64)
主要由电离杂质决定,迁移率也随温度升高而增
大,所以,电阻率随温度升高而下降。
C
q
1
A B
m*
AT
3/2
BNi T 3/2
0 硅与T关系
T
T
BC段 温度继续升高,杂质全部电离,本征激发 还不十分显著,载流子基本上不随温度变化,晶 格振动散射上升为主要矛盾,迁移率随温度升高 而降低,所以,电阻率随温度升高而增大。
A:轻掺(杂质浓度1016~1018cm-3),
迁移率随杂质浓度的变化较小
1
Ni
杂质浓度增高时,非线性曲线。原因: 一是杂质在室温下不能全部电离,重掺杂的简并 半导体中情况更加严重;
电阻率与杂质浓度的关系
电阻率与杂质浓度的关系电阻率是材料的一个重要物理特性,它描述了材料电阻的大小。
而杂质则是指材料中的不纯物质,杂质浓度越高,材料的纯度越低。
那么这两者有什么关系呢?实验表明,杂质浓度与电阻率之间存在着一定的关系。
一般来说,杂质浓度越高,电阻率也越高。
这是因为杂质的存在会影响材料中的电子运动。
在一个纯净的材料中,电子很容易通过材料中的原子晶格运动,形成电流。
但是当杂质存在时,它们会影响材料中的原子晶格,导致电子的运动受到阻碍,因此电阻率会增加。
具体来说,杂质的影响是通过两种机制来实现的:散射和夹杂。
散射是指电子在材料中的运动被杂质原子的存在所影响。
当电子穿过材料时,它们会与杂质原子相互作用,导致电子的能量和动量发生变化。
这些变化会导致电子的运动方向发生改变,从而使电子的平均自由程减小。
因此,在杂质浓度增加时,散射的作用会变得更加显著,电子的平均自由程会减小,电阻率会增加。
夹杂是指杂质原子被嵌入到材料的晶格中,导致晶格的结构发生变化。
这种变化会导致电子在材料中的运动受到阻碍。
夹杂会导致材料的晶格变形,从而使材料的导电性能发生变化。
因此,在杂质浓度增加时,夹杂的作用会变得更加显著,电阻率会增加。
总的来说,杂质浓度对材料的电阻率影响非常大。
在一些应用中,需要尽可能地保持材料的纯度,以使电阻率尽可能地小。
例如,电子学中的半导体器件需要高度纯净的材料,以保证器件的性能和可靠性。
此外,在材料制备过程中,也需要采取一系列的措施,以减少杂质的存在,从而提高材料的质量。
电阻率与杂质浓度之间存在着一定的关系。
杂质的存在会影响材料中的电子运动,从而导致电阻率的增加。
因此,在材料的制备和应用中,需要尽可能地减少杂质的存在,以保证材料的性能和可靠性。
硅电阻率随掺杂影响
二维电子气:如果三维固体中电子的运动在某一个方向 (如z方向)上受到阻挡(限制),那么,电子就只能在另外两 个方向(x、y方向)上自由运动,这种具有两个自由度的自 由电子就称为二维电子气(2-DEG)。 2-DEG是许多场效应器件(例如MOSFET、HEMT)工 作的基础。
调制掺杂异质结一般用分子束外延法生长,结构如下图。 n阶的Ga0.68Al0.32As/GaAs能隙比GaAs能隙宽, 主要在导带形成300meV的台阶; Ga0.68Al0.32As/GaAs中导带的电子势必将流入GaAs, 从而在界面形成空间电荷区及三角形势垒。
2个 决定性因素
电阻率
杂质浓度
载流子浓度 载流子迁移率
2个 有关因素
温度
①杂质浓度较低时 1016~10 18 cm-3
电阻率与杂质浓度成反比关系, 即杂质浓度越高,电阻率越小。
1.电阻率和杂质 浓度的关系
②杂质浓度较高时
杂质在室温下不能全部电离, 在重掺杂的简并半导体中情况 更加严重; 迁移率随着杂质浓度的增加明 显下降
二、实现只填充最低子带的条件
为提供量子霍尔实验的条件,首先应保证电子只限于最低子带。 假使电子过多,进入较高的子带,将使实验结果是几个子带的 叠加,造成分析困难。 故在制备样品时要控制载流子数目。
利用态密度公式 m*/ π h ,可知在最低子带 ε 0 与上一个子带 ε 1 之间的状态数为 m* ( ε 1 - ε0 ) / π h2 ,那么单位界面上 电子数目为
掺杂对硅的电阻率的影响
一、电阻率和杂质浓度的关系
二、电阻率随温度的变化
电阻率:物质的电阻与横截面积的乘积与长度的比值 国际制单位是欧姆米,常用单位是“欧姆· 厘米”
电阻率是导体材料本身的电学性质,与温度有关。 1.载流子浓度 即电流载体的浓度, 半导体中有两种载流子: 电子和空穴。 2.迁移率 指单位电场强度下所产生的 载流子平均飘逸速度; 代表载流子导电能力的大小。
SiGe薄膜材料电阻率与掺杂浓度的关系研究
1145×1017 cm - 3, N D 为施主掺杂浓度。 212 P 型应变 S i1- xGex 材料空穴迁移率模型
Λp =
(
Λp 1+
,
m ax (N A
Λp
N
,
m0 )inΒ+
Λ ) p , m in
(1+ a1x + a2x 2+ a3x 3) x ≤0. 6
(4)
(4) 式 中, Λ = p ,m ax 625 cm 2 (V · s) , Λ = p,m in 40
其值为 116×10- 19库仑。
对于 N 型半导体, n > > p , 空穴对电流的贡献 可以忽略, 电导率近似为
Ρ= nq Λn
(6)
图 1 建立应变 Si1- x Gex 薄膜电阻率和掺杂浓度的 关系曲线
本文 采 用 M a tlab 语 言 编 程, 分 别 求 解 ( 10)、
x= 0
x (1- x ) exp (- 7. 68x ) 160 0< x ≤0. 2 (3)
exp (- 3. 86x ) 2151
0. 2< x ≤0. 6
( 2) 式中, Λ = n,m ax 1320 cm 2 (V · s) , Λ = n,m in 68
cm 2 (V ·s) , Α1 = 4131, Α2 = - 2128, Β= 0188, N 0 =
© 1994-2007 China Academic Journal Electronic Publishing House. All rights reserved.
252
戴显英等: SiGe 薄膜材料电阻率与掺杂浓度的关系研究
4.4 电阻率与杂质浓度和温度的关系(雨课堂课件)
离,可以认为导带电子全部由施主杂质电离而提供。此时,导
带电子浓度为
n0
N
。
D
根据热平衡载流子浓度的乘积公式
热平衡状态下价带空穴浓度
n0 p0 ni2
最后,掺杂锗材料的电阻p率0可由n下i2 面n0公式计算。
1 ....n p
n0 q n
解:本征半导体的电阻率
1
niq(n
....n
p)
p
ni
分析:本征半导体的电阻率
1
niq(n
....n
p)
p
ni
n 由此可得本征载流子浓度 i
V族元素锑(Sb)掺入锗中起施主杂质作用。已知每106个锗原
子中有一个杂质原子,而每立方厘米锗有 4.4 1022 个原子。
因此,施主杂质浓度
N D 4.4 10 22 10 6 cm3 。
当在本征锗中掺杂施主杂质锑后,假设室温下杂质全部电
图4-15 300K时Ge,Si, GaAs电阻率与杂质浓度的关系
D. B. Cutriss et al. Bell Sys. Tech.J. 41,387(1962)
高杂质浓度时,电阻 率与杂质浓度偏离线 性。(对数坐标) 理论分析与实验结果 相符合。
图4-15 300K时Ge,Si, GaAs电阻率与杂质浓度的关系
下面四块半导体硅单晶,除掺杂浓度不同外,其余条件均 相同,由下面给出的数据可知电阻率最小者是( )。
A NA = 3×1016 cm−3 B NA = 8×1015 cm−3, ND = 3×1016 cm−3 C NA = 2.2×1015 cm−3 D ND = 2.2×1015 cm−3
提交
6、室温下,本征锗的电阻率为47 • cm ,试求本征载流子
