4-二甲胺合成
新型抗氧剂2,4-二(正辛基硫亚甲基)-6-甲基苯酚的合成

新型抗氧剂2,4-二(正辛基硫亚甲基)-6-甲基苯酚的合成摘要:研究了以邻甲酚、正辛硫醇、多聚甲醛为原料,二甲胺乙醇溶液为催化剂,DMF为溶剂,合成2,4-二(正辛基硫亚甲基).6.甲基苯酚的方法。
考察了投料比、催化剂用量、反应时间对反应产率的影响。
结果表明,当邻甲酚、正辛硫醇、多聚甲醛的摩尔比为1:2.20:4.20,催化剂用量占反应物总质量的4.79%,回流反应8 h时,2,4-二(正辛基硫亚甲基).6.甲基苯酚的收率达到90%。
用红外光谱和核磁共振氢谱对产品结构进行了表征。
关键词:2,4-二(正辛基硫亚甲基)-6-甲基苯酚;抗氧剂;合成1 实验部分1.1试剂及仪器二甲胺溶液(35%)、硅胶、乙酸乙酯:化学纯;无水乙醇、无水氯化钙、浓盐酸、碳酸钠、N,N-二甲基甲酰胺、多聚甲醛、溴化钾、无水乙醇、正己烷、正丁醇:分析纯;邻甲酚、正辛硫醇、活性炭:工业品。
FT IPJBaman红外光谱:美国Thermo Nicolet公司;DBX500型核磁共振谱仪:德国Bruker公司。
1.2催化剂的制备在装有温度计、球形冷凝管的两口烧瓶中加入质量浓度为35%的二甲胺溶液,以无水乙醇为吸收剂,加热,控制温度不超过90℃,使吸收瓶中气泡缓慢逸出。
利用酸碱滴定,测得二甲胺乙醇溶液的质量浓度为38%。
1.3实验方法1.3.1 2,4-二(正辛基硫亚甲基).6.甲基苯酚的合成在装有搅拌器的四口烧瓶中加入16 g(0.15mol)邻甲酚、18 g(0.60 m01)多聚甲醛、3.5 g(相当于0.03 mol二甲胺)二甲胺乙醇溶液(质量浓度38%)和22 mL Ⅳ,J7、r.二甲基甲酰胺,从恒压滴液漏斗滴加44 g(O.30 mol)正辛硫醇,通人N2保护,加热搅拌,回流8 h。
1.3.2粗产品的初步提纯将粗产品倒入分液漏斗,加入反应液双倍体积的正己烷,充分混合。
用沸水反复洗涤,直至水层透明。
常压蒸馏,除去溶剂(DMF、正己烷)。
二甲胺

EIN 204-697-4 ECS 号: 相 Pharmaceutical Intermediates;Compressed and Liquefied 关 GasesChemical Synthesis;Chemical Synthesis;Compressed and 类 Liquefied Gases;C2 to C6Chemical Synthesis;Amines;Nitrogen 别: Compounds;Organic Bases;Synthetic Reagents;二硫代氨基甲酸盐类杀
菌剂;杀菌剂中间体;农药中间体
Mol 124-40-3.mol 文 件:
二甲胺 性质
熔点 沸点 密度
−93 °C(lit.) 7 °C(lit.) 0.89 g/mL at 25 °C
蒸气密度 蒸气压 折射率
1.55 (vs air) 16.97 psi ( 55 °C) n20/D 1.37
闪点 储存条件 敏感性
二甲胺 用途与合成方法
化学 无色易燃气体或液体,高浓度或压缩液化时,具有强烈的令人不愉快的氨
性 臭,浓度极低时有鱼油的恶臭。 易溶于水,溶于乙醇和乙醚。
质
用 用作生产药物、染料、农药、皮革去毛剂、橡胶硫化促进剂、火箭推进剂
途 等的原料
用 二甲胺是农药的重要中间体,可以制备杀菌剂福美双、福美甲胂、福美锌、
中 二甲胺 文 名 称: 中 N-甲基甲胺;二甲胺;二甲胺水溶液,33%;二甲胺(无水);N-甲基甲胺溶液 ; 文 二甲胺水溶液(33%);二甲胺,溶液;氨基二甲烷(33%水溶液) 同 义 词: 英 Dimethylamine 文 名 称: 英 (CH3)2NH;ai3-15638-x;Dimethylamin;dimethylamine(solutions);dimeth 文 ylamine,anhydre;dimethylamine,anhydrous;dimethylamine,aqueoussol 同 ution;Dimethylaminesolution 义 词:
连续催化胺化制备四甲基乙二胺催化剂

CHEMICAL INDUSTRY AND ENGINEERING PROGRESS 2017年第36卷第4期·1294·化 工 进展连续催化胺化制备四甲基乙二胺催化剂吴军,钱俊峰,孙富安,吴中,崔爱军,何明阳,陈群(常州大学石油化工学院,江苏省精细石油化工重点实验室,江苏 常州 213164)摘要:以Cu/Ni 为主活性组分,研制出适合于N ,N -二甲基乙醇胺(DMEA )固定床连续催化胺化制备N ,N ,N’,N’-四甲基乙二胺(TMEDA )复合催化剂。
考察了催化剂载体类型、铜镍摩尔比、负载量、助剂、焙烧温度和时间等因素对催化剂性能的影响。
通过实验得出催化剂较佳的制备条件为:以γ-Al 2O 3球为载体,摩尔比Cu ∶Ni ∶Mn=4∶1∶0.2,负载量20%,500℃下焙烧6h 。
在反应温度240℃、常压、胺醇摩尔比1∶1、空速0.12h –1、氢气流量30mL/min 反应条件下,DMEA 转化率和TMEDA 选择性分别达到92.8%和82.9%;通过固定床连续500h 寿命测试,转化率和选择性分别稳定在90%和80%以上,显示催化剂具有较好的催化活性和稳定性。
关键词:N ,N -二甲基乙醇胺;N ,N ,N’,N’-四甲基乙二胺;催化剂;固定床;加氢中图分类号:TQ032.4 文献标志码:A 文章编号:1000–6613(2017)04–1294–07 DOI :10.16085/j.issn.1000-6613.2017.04.019Study on catalyst for continuous catalytic amination of TMEDAWU Jun ,QIAN Junfeng ,SUN Fu’an ,WU Zhong ,CUI Aijun ,HE Mingyang ,CHEN Qun(Key Laboratory of Fine Petrochemical Engineering ,School of Petrochemical Engineering ,Changzhou University ,Changzhou 213164,Jiangsu ,China )Abstract :A compound catalyst with Cu/Ni as the main activate component is applied for continuous catalytic amination DMEA to TMEDA. The influences of catalyst carrier type ,Cu/Ni molar ratio ,loading amount ,additives ,roasting time and temperature on the performance of the catalyst were investigated. The best preparation conditions of the catalyst were as follows :with the γ-Al 2O 3 ball as the carrier ,Cu ∶Ni ∶Mn=4∶1∶0.2(mole ratio ),the load amount 20%,and roasting 6h at 500℃. Under the conditions of the reaction temperature of 240℃,atmospheric pressure ,amine alcohol molarratio 1∶1,space velocity of 0.12h –1,hydrogen speed of 30 mL/min ,the conversion rate of DMEA and the selectivity of TMEDA could achieve 92.8% and 82.9%,respectively. Through 500h life test ,the conversion rate and selectivity were stable and maintained at above 90% and 80%,respectively. The experimental results showed that the catalyst had excellent catalytic activity and stability. Key words :DMEA ;TMEDA ;catalyst ;fixed-bed ;hydrogenationN ,N ,N ′,N ′-TMEDA 为无色透明液体,略有氨味,能与水、乙醇等有机溶剂混溶。
四(二甲氨基)乙烯的制备__解释说明

四(二甲氨基)乙烯的制备解释说明1. 引言1.1 概述在有机合成领域,四(二甲氨基)乙烯(TDAE)作为一种重要的有机化合物具有广泛的应用价值。
它是指含有两个二甲胺基团和一个乙烯基团的化合物,具有多种特性和功能。
该化合物可以通过多种方法制备,每种制备方法都有其独特优势和适用范围。
本文将详细介绍TDAE的制备方法、其特性及应用领域,并着重讨论实验步骤以及可能遇到的问题与解决方案。
1.2 文章结构本文共分为5个部分来阐述关于TDAE的制备方法和应用领域。
引言部分主要概述了文章内容,并对TDAE进行了简要介绍。
接下来的部分将依次介绍TDAE 的定义与特性、常见制备方法、以及其应用领域。
实验步骤部分详细描述了制备TDAE的具体实验操作步骤和注意事项。
实验结果与讨论部分将对反应产物进行分析与表征,并探究可能存在的副反应以及相应解决方案。
最后的结论部分总结了实验过程与结果所得出的主要结论,并提出改进实验条件和方法以提高产品质量或产率的建议。
1.3 目的本文旨在详细介绍制备TDAE的方法、特性以及应用领域。
通过对相关实验步骤和操作注意事项的说明,希望读者能够获得针对TDAE制备的具体指导,并了解可能遇到的问题及其解决方案。
同时,通过对反应产物进行分析和讨论,探究可能存在的副反应和反应机理,从而为今后进一步优化合成方法提供参考。
最终目标是全面展示关于TDAE制备方法与机理研究的最新进展,为相关领域科研工作者提供实质性的帮助和启发。
2. 四(二甲氨基)乙烯的制备:2.1 定义和特性:四(二甲氨基)乙烯,也被称为N,N,N',N'-tetramethylethylene-1,2-diamine (TMEDA),是一种无色液体,在有机合成反应中具有广泛的应用。
它具有较低的沸点和挥发性,以及很强的配位能力和碱性。
TMEDA在金属催化反应、配位化学和有机合成等领域中作为配体或碱催化剂的重要试剂。
2.2 常见制备方法:目前有多种合成四(二甲氨基)乙烯的方法,其中最常见的包括以下几种:①氮气族元素与乙烯基化合物反应法:将乙烯与甘胺或其衍生物在惰性气氛下经过酸碱中和反应得到四(二甲氨基)乙烯。
甲胺工艺流程

一、配料系统由于甲胺两装置配料系统工艺上有所差别,故将生产工艺分别说明: 1、甲胺一套:甲胺一套原料槽分别有:甲醇罐(25M3)一台,液氨罐(25M3)一台,不合格三甲胺槽(25 M3)一台,不合格一二甲胺槽(25 M3)一台,共沸物贮槽(25M3)两台。
通过计量泵按照相应的原料配比(N/C=1.5-2.5)打入配料缓冲罐,当缓冲罐的物料充满后,会从缓冲罐顶部进入配料槽,从配料槽底部出料,按照调节合成升压泵的冲程,调整系统负荷,由升压泵将合成物料打入合成系统。
2、甲胺二套:甲胺二套原料槽分别有:甲醇罐(25.3 M3)一台,液氨罐(25.3 M3)一台,混胺槽(50.1M3)一台,共沸物贮槽(50.1M3)一台。
通过调节计量泵的转数来调节各原料的量,按照相应的原料配比(N/C=1.5-2.5)打入合成缓冲罐,然后通过压差将物料压入至合成系统。
二、合成系统岗位任务:把配料岗位计量泵输送来的合格原料液经升压予热,送合成塔中,在适宜的温度、压力、空速、和催化剂存在的条件下,进行连续气相胺化脱水反应,制取混合粗胺,经换热,减压后送至精馏工序分离。
由于甲胺的生产反应是放热反应,所以在反应后必将放出大量的热,为了保护设备和生产的安全,在原料的配料过程中加入的液氨是过量的,就是为了物料平衡,将合成的反应温度、压力控制在有效的反应温度指标内。
原料进入合成系统后,首先进入合成缓冲罐,低温换热器,在低温换热器中,原料会和合成液进行换热,换热后原料液的温度将会从30℃加热至80℃;原料进入开工气化器,经11Kg蒸汽加热后,原料出口温度达到120℃;进入高温换热器a,原料液和合成液进行换热,换热后,原料液温度升至150℃;进入高温换热器b,原料液被合成液加热至200℃;进入高温换热器c,原料液被再次加热至300℃;进入电炉,进行高压短路盘管加热至320℃,从合成塔顶部进入合成反应器,进行合成反应。
由于采用甲胺触媒的不同(新系统用的是T-1221型,老系统用的是A6-2型)所以合成反应的操作温度指标也不相同,新合成塔触媒层温度控制指标为375±5℃,老系统指标为420±5℃。
二甲胺制备-概述说明以及解释

二甲胺制备-概述说明以及解释1.引言1.1 概述二甲胺是一种重要的有机化合物,具有广泛的应用领域。
它是一种无色气体,具有刺激性气味。
二甲胺的制备方法有多种,主要包括催化氰化物法、脂肪酸酯氨化法、甲酰胺还原法等。
这些方法各具特点,适用于不同的生产需求。
二甲胺在聚氨酯、染料、药物、植物保护剂等领域具有重要的应用价值。
在聚氨酯的合成中,二甲胺作为主要的中间体,参与了聚合反应,产生高分子量的聚合物。
染料合成中,二甲胺能够与染料中的氨基反应,形成带有特定功能的染料分子。
在药物领域,二甲胺可以用于合成多种药物,具有重要的生物活性。
在植物保护剂中,二甲胺可以作为有效的杀虫剂、杀菌剂使用,帮助农作物达到更好的生产效果。
总之,二甲胺的制备和应用领域都具有重要意义。
本文将重点介绍二甲胺的制备方法和其在不同领域的应用,以期让读者更好地了解二甲胺的重要性和潜在的发展前景。
1.2文章结构文章结构部分的内容如下:1.2 文章结构本文共分为三个部分:引言、正文和结论。
引言部分主要概述了本文的主题,即关于二甲胺制备的研究。
首先介绍了二甲胺的概念和特性,然后说明了文章的结构和目的。
通过引言部分,读者可以了解到本文的主要内容和研究意义。
正文部分包括了二甲胺的制备方法和应用领域两个子部分。
在制备方法部分,将详细介绍二甲胺的常用制备方法,包括化学合成方法和生物制备方法等。
每种方法都会列举具体的操作步骤和实验条件,以及相关的反应机理和优缺点。
在应用领域部分,将介绍二甲胺在工业化学、医药领域、农业领域等方面的广泛应用。
这部分将详细阐述二甲胺在各个领域的具体用途和重要性。
结论部分将对二甲胺制备的重要性进行总结,并展望二甲胺在未来的发展前景。
通过对已有研究成果的归纳总结,强调了二甲胺在不同领域的重要作用。
同时,根据当前的科学技术发展趋势和市场需求,对二甲胺在未来的发展方向和应用前景进行展望和预测。
通过以上三个部分的编排,本文将全面介绍二甲胺的制备方法和应用领域,旨在为读者提供全面、准确的信息,进一步推动二甲胺研究的深入发展。
3,4-二甲基苯胺的合成【毕业答辩】
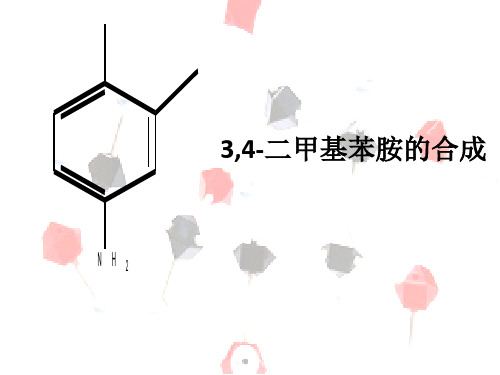
Organic Synthesis,2001,894-89. • [5]靖丹,曹亚峰,李沅,孟晨,大连工业大学学报,2012年9月,31(5),
原料N-(2-甲基-5-硝基苄基)乙酰胺氯 代得2-氯甲基对硝基甲苯
2-氯甲基对硝基甲苯催化加氢 得目标产物3,4-二甲基苯胺
实验步骤 第一步氯代反应
• 将18.72g(0.09mol)N-(2-甲基-5-硝基苄基)乙酰 胺加入分别装置搅拌器、温度计、回流冷凝管、干 燥管和尾气吸收装置的250ml四颈瓶中,再加入 6.50g催化剂A,150ml二甲苯,搅拌加热反应液至缓 慢回流后,用恒压滴液漏斗向反应液中滴加 29.01g(0.18mol)三氯氧磷,滴加完毕后,继续缓慢 回流1.5~2h,TLC跟踪至反应完成。
9.添加剂的影响
NHOH
3,4-二甲基苯基羟胺结构式
参考文献
• [1]陈颖,靳惠娟,3,4-二甲基苯胺的合成,河北化工,2006年第2期,30-33. • [2]Donald R.Maulding,KennethD.Lotts,SamuelA.Robinson,New procedure for
making 2-chloromethyl-4-nitrotoluene,.Chem.1983,48:2938-2939. • [3]吴崖迪,王桂林,3,4-二甲基苯胺合成进展,浙江化工,2003年第5期,22-
• 最后我再次感谢所有曾经帮助过我的同学和朋友们。 你们的鼓励和支持是我前进的动力,真心的谢谢你 们。
339-341. • [6]徐善利,陈宏博,李树德.催化加氢还原芳香硝基化合物制备芳胺的技术
二甲胺

Dimethylamine
(CH3)2NH;ai3-15638-x;Dimethylamin;dimethylamine(solutions);dimeth ylamine,anhydre;dimethylamine,anhydrous;dimethylamine,aqueoussol ution;Dimethylaminesolution
用 途 用 途 生产 方 法 生产 方 法
类别 有害气体 毒性 高毒 分级 急性 口服- 大鼠 LD50: 698 毫克/ 公斤; 口服- 小鼠 LD50: 316 毫克/ 公 毒性 斤 刺激 眼睛- 兔子 50 毫克/ 5 分 数据 爆炸 与空气混合明火、受热可爆 物危 险特 性 可燃 遇明火、高温、氧化剂易燃; 燃烧产生有毒氮氧化物烟雾 性危 险特 性 储运 库房通风低温干燥; 与氧化剂、酸类分开存放 特性 灭火 雾状水、泡沫、二氧化碳、四氯化碳、干粉 剂 职业 TWA 9 毫克/ 立方米; STEL 18 毫克/ 立方米 标准 安全信息 危险品标志 危险类别码 安全说明 危险品运输编号 WGK Germany RTECS 号 F HazardClass PackingGroup 毒害物质数据 F+,Xn,C,F,T 12-20-37/38-41-34-20/22-11-39/23/24/25-23/24/25 3-16-26-29-36/37/39-45-39 UN 2924 3/PG 2 2 IP8750000 3 3 II 124-40-3(Hazardous Substances Data)
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Journal of Inclusion Phenomena and Macrocyclic Chemistry46:105–110,2003.©2003Kluwer Academic Publishers.Printed in the Netherlands.105 The Intercalation of4-Aminopyridine into Layered FePS3XINGGUO CHEN1,LI ZOU1,XUAN ZHANG1,JINGUI QIN1,∗,MAKOTO INOKUCHI2and MINORU KINOSHITA21Department of Chemistry,Wuhan University,Wuhan430072,China;2Department of Materials Science and Engineering, Science University of Tokyo in Yamaguchi,Onoda,Yamaguchi756-0884,Japan(Received:28Octoer2002;infinal form:23May2003)Key words:intercalation,layered FePS3,4-aminopyridine,XRD,IR,paramagnetismAbstractA new intercalation compound,Fe0.81PS3(4-aminopyridineH)0.38,is synthesized by the direct reaction of4-aminopyridine with layered FePS3in the presence of acetic acid.From the XRD results it was found that there are two phases(Phase I and Phase II)in this intercalation compound and that4-aminopyridines as the guests adopt two different orientations between the interlayer region of the host(FePS3).In one of them with the lattice expansion( d)of6.0Åthe ring plane of the guest is perpendicular to the layer and in the other with d of3.4Åthe ring plane of the guest is parallel to the layer of the host. The IR spectra imply that the inserted guests take the protonated form to maintain the charge balance of the intercalation compound.Magnetic measurements indicate that Fe0.81PS3(4-aminopyridineH)0.38exhibits paramagnetism in the range of measurement temperature(1.8∼300K),where the magnetic behavior is well in agreement with the Curie-Weiss Law above 55K.IntroductionIn recent years much interest has focused on the inorganic-organic hybrid compounds because this offers perspectives for the realization of molecular-based materials,especially those that can combine different properties such as metal-like conductivity and bulk ferromagnetic property in a single material[1].Intercalation of organic species into layered inorganic solids represents one of the useful approaches to create the ordered molecular-based materials with some novel properties[2].The MPS3compounds,in which M is a divalent trans-ition metal,are a class of layered inorganic materials. They crystallize to space group C2/m,a monoclinic unite cell related to CdCl2-type structure with metal ions and phosphorus-phosphorus pairs occupying the cadmium pos-itions and sulfur ions occupying the chloride positions,so M ions construct a honeycomb lattice in the ab plane and they are separated by Van der Waals gap between hexagonal layers of sulfur ions[3].Some MPS3phases containing the paramagnetic M2+ions(Mn2+,S=5/2;Fe2+,S =2;Ni2+,S=1)show the quasi-two-dimensional anti-ferromagnetism with a Néel temperature of78K,120K and153K,respectively[4].When guest species are in-serted into the interlayer space of MPS3(M=Mn,Fe), they can lead to the occurrence of some intralamellar M2+ ion vacancies that makes the magnetic properties of the MPS3host lattice be changed and even dramatically altered [5–8].In this paper,the synthesis,structural characteriza-∗Author for correspondence.E-mail:jgqin@ tion and magnetic property of the intercalation compound (Fe0.81PS3(4-aminopyridineH)0.38)are presented and a pos-sible intercalation process is proposed for4-aminopyridine into layered FePS3.ExperimentalX-ray powder diffraction(XRD)patterns were obtained with Dmaxr A X-ray diffractometer using Cu Kαradiation(λ= 1.5418Å).Infrared spectrum was performed on a Nicolet SX Fourier transform spectrometer.Elemental analysis was performed with a Carlorba-1106microanalyzer,and the magnetic property was studied by a SQUID-magnetometer (MPMS,Quantum Design).Pure FePS3was synthesized as described by Taylor[9].It was identified by means of XRD and indexed ina monoclinic unit cell(space group C2/m,d=6.439Å, a=5.934Å,b=10.280Å,c=6.722Å,β=107.16◦) [10].4-aminopyridine was purchased from Aldrich and 4-aminopyridine·HCl was synthesized by the reaction of 4-aminopyridine with chloride acid(HCl36.5%,AR).The intercalation compound Fe0.81PS3(4-amino-pyridineH)0.38was prepared by stirring the mixture of FePS3 (0.20g,1.1mmol),4-aminopyridine(0.60g,6.4mmol)and acetic acid(HOAc)(1.0mL)in a Pyrex ampoule containing 12mL acetonitrile sealed under vacuum for two weeks at70◦C.The black powder wasfiltered off,and washed with acetonitrile,ethanol and distilled water,and then dried in air.Elemental analysis led to the formula Fe0.81PS3(4-106aminopyridineH)0.38(Found:C,11.00;H,1.05;N,4.80 (%).Calculated:C,10.94;H,1.29;N,5.10(%)).Results and discussionX-ray powder diffractionThe completion of intercalation was ascertained by X-ray powder diffraction(XRD).XRD reflection patterns show that the original00l reflection patterns of pure FePS3are totally absent and two new series of00l reflection patterns are observed in Fe0.81PS3(4-aminopyridineH)0.38(Figure 1(2)).This indicates that there are two phases(Phase I and Phase II)in this intercalation compound and the guest(4-aminopyridine)adopts two different orientations between the interlayer region of the host(FePS3).Phase I has the lattice spacing(d)of12.44Åcorresponding to the lat-tice expansion( d)of6.0Åindicating that the ring plane of the guest is perpendicular to the layer,which is in the similar case to that of mono-protonated2,2 -pyridine(2,2 -pyridineH+)inserted into FePS3[11].Phase II with lattice spacing(d)of9.8Å(expanded by3.4Å)corresponds to the pyridine ring plane parallel to the layer of the host,in which the guest is arranged in the similar orientation for pro-tonated pyridine(pyridineH+)to be intercalated into FePS3 [12](Scheme1Equation(3)).By comparison of the XRD reflection patterns of Fe0.81PS3(4-aminopyridineH)0.38with those of inter-calation compounds2,2 -bipyrindineH/FePS3[11]and pyridineH/FePS3[12]in detail,it was found that the re-flection patterns of two phases(Phase I and Phase II)in Fe0.81PS3(4-aminopyridineH)0.38can be readily indexed in the C2/m space group closely related to that of pristine FePS3,respectively.The calculated unit cell parameters are given in Table1.Among them,the calculated a,b andβvalues are almost identical with that of pristine FePS3except the expansion of the unit parameter c and lattice spacing d in both phases.This means that the layered structure of the host is maintained after intercalation.Infrared spectraIn general,the intercalation compounds based on MPS3(M =Mn,Fe,Cd etc)have two or three sharp absorptions in the range550∼610cm−1,which are assigned to theν(PS3) asymmetric stretching band coming from the splitting of 570cm−1in pure MPS3owing to departure of a fraction of metal ions into the solution during the intercalation.The occurrence of the intralamellar M2+vacancies makes the P–S bonds in the group of PS3unequivalent because some of PS3groups are surrounding the M2+vacancies and others are also coordinated to the M2+ions[13].Therefore,the intense bands at604and556cm−1can be attributed to the ν(PS3)asymmetric stretching band,which reflects the oc-currence of intralamellar Fe2+ion vacancies in Fe0.81PS3(4-aminopyridineH)0.38.There are numerous absorptions in the 700∼3500cm−1range that can be assigned to the guest.We also measured the spectra of4-aminopyridine and4-aminopyridine·HCl.By comparison of the IR spectra of Fe0.81PS3(4-aminopyridineH)0.38,4-aminopyridine and4-aminopyridine·HCl[14](Table2and Figure2),the spectrumof Fe0.81PS3(4-aminopyridineH)0.38exhibits some charac-teristic absorption bands at3319,3196,2964,2934,1651,1589,1526,1198,997,816and501cm−1etc.,which is similar to those of4-aminopyridine·HCl but different from4-aminopyridine.This suggests that the inserted guests arethe protonated4-aminopyridine cations formed by proton exchange between acetic acid and4-aminopyridine in thesolution.Magnetic propertiesThe magnetic properties of Fe0.81PS3(4-aminopyridineH)0.38were studied with SQUID.The antiferromagnetic transition at120K in pure FePS3no longer exists.Figure3displaysthe molar susceptibility(χ)and the inverse of the molarsusceptibility(1/χ)versus temperature(T)of Fe0.81PS3(4-aminopyridineH)0.38and Figure4shows the plot ofχTversus T.From Figure3it was found that Fe0.81PS3(4-aminopyridineH)0.38exhibits the paramagnetism in the range of the measured temperature(2∼300K).And thecurve ofχT vs.T in Figure4shows that theχT value mono-tonically decreases as the temperature decreases in the wholetemperature range indicating that the magnetic interactionbetween the localized Fe2+ions is antiferromagnetic one in the paramagnetic range.This behavior is confirmed by theinverse of the paramagnetic susceptibility as a function oftemperature of Fe0.81PS3(4-aminopyridineH)0.38displayed in Figure3.The inverse of the paramagnetic susceptibilityis well in agreement with Curie-Weiss Law above55K. The Curie constant is3.20cm3·K·mol−1and the effective magnetic moment per Fe2+ion is evaluated as5.0BM,which is closed to that of the spin-only value of high spin Fe2+ion(4.94BM)[15].This suggests that the Fe2+ionis still in the+2oxidation state after intercalation.Weissconstant(θ)is about−173K that also reflects the strong localized antiferromagnetic coupling interaction between the Fe2+ions.However,its antiferromagnetic coupling interaction between the Fe2+ions is much different from that of pure FePS3that shows the ferromagnetic coupling interaction in paramagnetic range(θ=+104K)[4].Due to the intercalation the existence of the intralayered the Fe2+ ion vacancies may change the magnetic interaction of the localized Fe2+ions of the host layer.From the above magnetic behavior,it is found that themagnetic property of Fe0.81PS3(4-aminopyridineH)0.38isdifferent from either its similar intercalation compounds such as pyridinium-FePS3,N-methylpyridinium-FePS3 that exhibit spontaneous magnetization at low temperat-ure[12]or Fe0.90PS3(phen)0.41showing antiferromagnetic transition with T N at about75K[11].It is also differ-ent from the pristine FePS3with the antiferromagnetic transition at T N of about120K[4].Why does Fe0.81PS3(4-aminopyridineH)0.38exhibits the paramagnetism?The main reason is that the occurrence of the intralayered Fe2+ion vacancies in the intercalation compound dilutes the magnetic107 Figure1.The XRD reflection patterns of different intercalation products and pure FePS3for comparison1–partial intercalation of4-aminopyridine withFePS3(∗Represents the reflection patterns of pristine FePS3);2full intercalation of4-aminopyridine with FePS3.ttice spacing(d)and calculated unit cell parametersCompound d(Å)a(Å)b(Å)c(Å)β(deg)FePS3 6.439 5.93410.280 6.722107.16Fe0.81PS3(4-aminopyridineH)0.38Phase I12.44 5.93510.28013.510113.00Phase II9.82 5.94010.30016.660113.10coupling interaction between the Fe2+ions.It was reported that Fe0.83PS3(2,2-bipyH)0.34[11]also exhibited paramag-netism in the range of its measured temperature(2∼300 K).In these two intercalation compounds Fe1−x PS3(G)2x (G stands for the guest with+1positive charge),the int-ralayered Fe2+ion vacancies are equal or more than0.17.It is clear that the dilution of the Fe2+ion vacancies is much stronger than that of other related intercalation compounds such as Fe0.88P0.99S3(pyridinium)0.24,Fe0.89P0.99S3(4-picolinium)0.20and Fe0.90PS3(1,10-phenanthroline)0.41, in which the intralayered Fe2+ion vacancies is less than 0.12.It is anticipated that the more the Fe2+ion vacancies, the stronger the dilution in the host of FePS3,which may drastically alter the magnetic properties of the intercalation compound and make an antiferromagnet of pure FePS3 become a paramagnet after intercalation.Synthesis and the possible intercalation processIn the study of the intercalation of4-aminopyridine with FePS3it was found that in the absence of acetic acid this intercalation did not occur,which was confirmed by XRD results where the product shows the same x-ray reflection patterns to those of pristine FePS3.This is because there does not exist4-aminopyridineH+in this intercalation.In or-der to obtain the intercalation compound containing only one phase(phase I or phase II),the synthesis of4-aminopyridine with layered FePS3has been tried three times through chan-ging the amount of HOAc used.It was also found if the amount of HOAc is less than0.5mL in the reaction only the partial intercalation compound is obtained,and the XRD measurement showed that patterns of Phase I and Phase II do exist and some FePS3still remained in this case of0.5 mL HOAc(Figure1in1).Obviously,the amount of HOAc plays a key role in the intercalation of4-aminopyridine with FePS3.From the experimental results,a possible intercalation process is proposed for4-aminopyridine intercalated into layered FePS3as Scheme1.As discussed,the infrared spectrum indicates that the in-serted guest is the protonated4-aminopyridine and there are some intralamellar Fe2+ion vacancies in the host.How does108Table2.The IR data of4-aminopyridine,4-aminopyridine·HCl and Fe0.81PS3(4-amino pyridineH)0.38 Assignments4-NH2Py4-NH2Py·HCl Fe0.81PS3(4-NH2PyH)0.38ν(NH)3437(3433)3303(3302)3314(3313)33193200(3195)31963140(3143)3144ν(CH)3081(3087)3089(3091)30883036(3038)3039(3042)2992(2995)ν(NH+)2966(2968)29642934(2936)2934δ(NH2or NH+2)1649(1645)1652(1650)16511638(1636)ν(C=N+or C=C)1601(1596)1595(1592)15891557(1555)1523(1523)1525(1527)15261507(1508)1474(1479)δ(C–H)1435(1435)14401406(1402)14141353(1353)1367(1364)1333(1334)1270(1270)1266(1262)1218(1219)11021191(1189)11981053(1052)1049(1050)10511022991(990)995(991)997885920842(842)847(846)822(824)805(801)816737715681(680)587536526500501ν(PS3)604556Note:The data in parentheses are cited from reference14.Scheme1.The possible intercalation process of FePS3with4-aminopyridin.109Figure 2.The infrared spectra of 4-aminopyridine,4-aminopyridine ·HCl and Fe 0.81PS 3(4-aminopyridineH)0.38.Figure 3.Temperature dependence of magnetic susceptibil-ity (χ)and the inverse of magnetic susceptibility (1/χ)of Fe 0.81PS 3(4-aminopyridineH)0.38(the plot of 1/χ-T above 55K is in good agreement with the Curie–Weiss Law).the protonated 4-aminopyridine come from?Because acetic acid is present in this intercalation,some 4-aminopyridine can obtain the protons from acetic acid to form the proton-ated 4-aminopyridine (4-aminopyridineH +)and acetic anion (Scheme 1Equation 1).And how does the intralamellar Fe 2+ion vacancies occur?It is known that pyridine and its derivatives such as β-or γ-picoline etc.,have the strong ability to coordinate with Fe 2+salts to form a series of complexes FePy 4L 2(Py =pyridine and its derivatives,L =HCOO,OAc,Cl,Br,I,SCN etc.)[16–20].Therefore,in this intercalation it may be inferred that 4-aminopyridine (4-NH 2Py)and acetic anion (OAc-)can remove some in-tralamellar Fe 2+ions to form the similar complex[Fe(4-Figure 4.The plot of χT −T of Fe 0.81PS 3(4-aminopyridineH)0.38.NH 2Py)4(OAc)2]into the solution.Thus,there are some intralamellar Fe 2+ion vacancies that makes the host have negative charges (as in Scheme 1Equation 2)and at the same time the protonated 4-aminopyridine will insert into the interlayer space of the host to maintain the charge bal-ance.So the driving force for this intercalation may be the static-charge attraction between the layered cluster anion of the host and the cation of the protonated 4-aminopyridine (Scheme 1Equation 3).In this process the initial intercala-tion is the parallel insertion of protonated 4-aminopyridine into the interlayer region of FePS 3(Phase I).And then the guest may be easily inserted into the interlayer space of the host because the van der Waals force holding the layers of the host together is destroyed,where protonated 4-aminopyridine will be arranged perpendicular to the layer (Phase II)due to the limited interlayer space of the host.Therefore,there coexist two phases during the intercalation.ConclusionA new intercalation compound,Fe 0.81PS 3(4-aminopyridineH)0.38,is synthesized by the direct reaction of 4-aminopyridine with layered FePS 3.The XRD results indicate that the guest (4-aminopyridineH)is arranged in two orientations between the interlayer region of the host (FePS 3),in which one arrangement with the lattice expansion ( d)of 6.0Åindicates that the molecular ring plane of the guest is perpendicular to the layer of the host and the other with 3.4Åmeans that the ring plane of the guest is perpendicular to the layer.The IR spectra imply that the inserted guest of 4-aminopyridine exists in the protonated form to maintain the charge balance of the in-tercalation compound.Magnetic measurement indicate that Fe 0.81PS 3(4-aminopyridineH)0.38exhibits paramagnetism in the range of measured temperature (2∼300K),where the magnetic behavior is well in agreement with the Curie-Weiss Law above 55K.A possible intercalation process was proposed for 4-aminopyridine inserted into layered FePS 3.110AcknowledgmentWe thank the National Natural Science Foundation of China forfinancial support.References1. E.Coronado,J.R.Galan-Mascaros, C.J.Gomez-Garcia and V.Laukhin:Nature408,470(2000).2.R.Chollhorn:Chem.Mater.8,1747(1996).3.V.Manriguez,A.Galdamez,A.Villanueva,P.Aranda,J.C.Galvanand E.Ruiz-Hitzky:Mater.Res.Bull.34(5),673(1999).4.R.Brec:Solid State Ionics22,3(1986).5.R.Clement,gadic,A.Leaustic,J.P.Audiere and L.Lomas:inP.Bernier et al.(eds.),Chemical Physics of Intercalation II,Plenum Press,New York(1993),p.315.6.P.A.Joy and S.Vasudevan:J.Am.Chem.Soc.114,7792(1992).7. C.Yang,X.Chen,J.Qin,K.Yakushi,Y.Nakazawa and K.Ichimura:J.Solid State.Chem.150,281(2000).8. C.Yang,X.Chen,J.Qin,K.Yakushi,Y.Nakazawa and K.Ichimura:Synth.Metals121,1802(2001).9. B.E.Taylor,J.Steger and A.Wold:J.Solid State Chem.7,461(1973).10.W.Klingen,G.Eulenberger and H.Hahn:Z.Anorg.Allg.Chem.401,97(1973).11.X.Chen,C.Yang,J.Qin,K.Yakushi,Y.Nakazawa and K.Ichimura:Chin.J.Chem.18(4),510(2000).12. A.Leaustic,J.P.Audiere,D.Cointereau,R.Clement,L.Lomas,F.Varret and H.Constant-Machado:Chem.Mater.8,1954(1996). 13.T.Coradin,R.Clement,croix and K.Nakatani:Chem.Mater.8,2153(1996).14. E.Spinner:J.Chem.Soc.3119(1962).15.P.A.Joy and S.Vasudevan:Phys.Rev.B46,5425(1992).16.H.D.Hardt and W.Moeller:Z.Anorg.U.Allgem.Chem.313,57(1961).17.L.M.Epstein:J.Chem.Phys.36,2731(1962).18.T.Tominaga,T.Morimoto,M.Takeda and N.Saito:Inorg.Nucl.Chem.Letters2(7),193(1966).19.N.S.Gill,R.H.Nuttall,D.E.Scaife and D.W.Sharp:J.Inorg.Nucl.Chem.18,79(1961).20.J.F.Duncan,K.F.Mok:Austrian J.Chem.19(4),701(1966).。
