头孢地尼胶囊说明书(美国,FDA,英文)
头孢地尼的服用方法

头孢地尼的服用方法
头孢地尼是一种口服抗生素药物,一般按照医生的指导进行服用。
以下是一般的头孢地尼服用方法:
1. 阅读药品说明书:在使用头孢地尼之前,仔细阅读药品说明书上的用法和剂量指导,遵循医生的建议和药品说明。
2. 在饭前或饭后服用:头孢地尼可以在饭前或饭后服用,根据医生的建议选择最佳服用时间。
某些情况下,医生可能会建议您在饭后服用以减少胃部不适。
3. 按照医嘱进行剂量:根据医生的指示和药品说明书上的剂量指导,按照准确的剂量服用头孢地尼。
通常情况下,成年人每次服用剂量为250毫克至500毫克,每天服用两到三次。
对于儿童,剂量将根据年龄和体重而变化。
4. 使用药杯:使用提供的药杯、药匙或标明剂量的器具测量头孢地尼的剂量。
避免使用家用餐具,以确保准确的剂量。
5. 完整完成疗程:即使症状在服用头孢地尼的几天内得到缓解,也请依照医生的建议完成整个疗程。
在停药之前,不要中断治疗或减少剂量,以防止细菌耐药。
6. 不与特定食物一起服用:某些食物可能会影响头孢地尼的吸收或药效,因此请在服用头孢地尼期间避免与酒精、牛奶、果汁或含胃酸药物一起服用。
若有需
要,咨询医生或药剂师。
请记住,以上仅为一般性建议,具体的头孢地尼服用方法应该根据医生的指导和药品说明进行。
如果有任何疑问或困惑,请直接向医生或药剂师咨询。
头孢地尼的抗菌活性和药动学研究

头孢地尼的抗菌活性和药动学研究[摘要]目的观察头孢地尼的抗菌活性和药动学研究。
方法36例儿童细菌性呼吸道感染患者口服头孢地尼制剂治疗,观察其抗菌性、疗效与药动学。
结果治疗结束后,细菌清除率分别为96.6%(28/29),治疗有效率为94.4%,患儿的半衰期(t1/2)、峰浓度(Cmax)、达峰时间(tmax)分别为(1.71±0.5)h、(1.05±0.12)h和(14.26±2.09)?g.h/ml。
结论头孢地尼颗粒是治疗细菌性呼吸道感染安全、有效的药物。
[关键词]头孢地尼;儿童;细菌性呼吸道感染;药动学细菌性呼吸道感染是儿童常见的疾病,主要表现为化脓性扁桃体炎和支气管肺炎等。
近年来,由于抗生素的滥用导致细菌耐药性增高,对之有效的抗生素已经越来越少。
头孢地尼是(Cefdinir,CDR)日本藤泽药品株式会社开发研制的第3代口服头孢菌素类抗菌药。
其抗菌谱较广,对革兰阳性菌和阴性菌均有较好的抗菌作用,对不同细菌引起的呼吸系统、泌尿系统及皮肤软组织感染均有较好的治疗效果。
本文收集了我地区一家三甲和一家二甲医院2008年5-12月采用头孢地尼治疗儿童细菌性呼吸道感染36例的抗菌活性与药动学结果,现报告如下。
1临床资料1.1一般资料选择临床症状体征、实验室检查、X胸片和细菌性检查确诊为细菌性呼吸道感染的我地区一家三甲和一家二甲医院,住院患儿病例36例,其中男26例,女10例,年龄2-7岁,平均年龄4.5岁。
所有患儿家属理解本研究的性质及目的,愿意按规定服药并接受采血,并签署知情同意书。
1.2给药方案所有患儿采用双周期交叉试验,于清晨空腹分别单剂量口服头孢地尼制剂2粒(含头孢地尼500mg),以200ml温开水送服,每日3次,7d为1疗程。
同时于服药前和服药后0.25h、0.5h、0.75h、1h、1.5h、2h、2.5h、3h、4.5h、7h抽取肘静脉血2.0ml。
肝素抗凝,转速4000r、min,离心半径8cm,离心5min,分取血浆置-20℃保存待测。
头孢地尼胶囊说明书

头孢地尼胶囊说明书【药品名称】头孢地尼胶囊【药品成分】每粒头孢地尼胶囊含有头孢地尼二水合物等有效成分。
【药理作用】头孢地尼属于第三代头孢菌素类抗生素,具有广谱抗菌活性。
它通过抑制细菌细胞壁的合成,阻断细菌生长和繁殖,从而达到抗菌作用。
头孢地尼可有效对抗多种革兰阳性、革兰阴性菌以及某些厌氧菌的感染。
【适应症】头孢地尼胶囊适用于以下疾病的治疗:- 呼吸道感染:包括上呼吸道感染(如鼻窦炎、扁桃体炎等)和下呼吸道感染(如肺炎、支气管炎等)。
- 尿路感染:包括膀胱炎、尿道炎等。
- 皮肤和组织感染:如蜂窝织炎、创伤感染等。
- 腹部感染:如胆囊炎、胆道感染等。
【用法和用量】成人和12岁以上儿童:一般建议每次口服头孢地尼胶囊250mg,每天2次。
病情严重或由耐药菌引起的感染,可考虑每次口服头孢地尼胶囊500mg,每天2次。
【不良反应】头孢地尼胶囊在正常用量下一般耐受性良好,不良反应较少。
少数患者可能出现以下不良反应:- 消化系统方面:包括恶心、呕吐、腹泻、胃肠道不适等。
- 过敏反应:少数患者可能出现皮疹、荨麻疹、瘙痒等过敏症状。
- 肝功能异常:极少数患者可能出现肝功能异常,表现为黄疸、肝酶升高等。
- 其他:个别患者可能出现头晕、头痛、口干等不适症状。
【注意事项】- 对头孢菌素类药物过敏的患者禁用本品。
- 对本品过敏或其他过敏体质的患者应谨慎使用。
- 在使用头孢地尼胶囊期间,如出现严重的不良反应,应立即停药并就医。
- 孕妇、哺乳期妇女和儿童应在医生指导下使用。
- 使用头孢地尼胶囊期间,患者应升服充分的水分,以保持良好的排尿量。
- 头孢地尼胶囊可能对肝功能产生影响,患者应定期监测肝功能指标。
【贮藏方法】头孢地尼胶囊应存放在阴凉干燥处,避免阳光直射。
【包装规格】每瓶装有XX粒头孢地尼胶囊。
【生产厂家】XX药业有限公司【有效期】请参照包装上的有效期。
【总结】头孢地尼胶囊是一种广谱抗生素,适用于呼吸道感染、尿路感染、皮肤和组织感染、腹部感染等疾病。
头孢克肟胶囊说明书

积大希夫头孢克肟胶囊说明书【药品名称】通用名称: 头孢克肟胶囊英文名称: Cefixime Capsules汉语拼音: Toubaokewo Jiaonang【成份】本品关键成份为头孢克肟。
化学名称: ( 6R, 7R)-7-[[( Z)-2-( 2-氨基-4-噻唑基)-2-[( 羧甲氧基)亚氨基]乙酰基]氨基]-8-氧代-3-乙烯-5-硫杂-1-氮杂二环[4.2.0]-辛-2-烯-2-羧酸三水合物。
分子式: C16H15N5O7S2·3H2O分子量: 507.50【性状】本品内容物为白色至淡黄色粉末。
【适应症】本品适适用于对头孢克肟敏感对链球菌属(肠球菌除外), 肺炎球菌、淋球菌、卡她布兰汉球菌、大肠杆菌、克雷伯杆菌属、沙雷菌属、变性杆菌属, 流感杆菌等引发下列细菌感染性疾病:1. 支气管炎、支气管扩张症(感染时), 慢性呼吸系统感染疾病继发感染、肺炎;2. 肾盂肾炎、膀胱炎、淋球菌性尿道炎;3. 胆囊炎、胆管炎);4. 猩红热;5. 中耳炎、副鼻窦炎。
【规格】(1)50 mg (2)100 mg【使用方法与用量】口服。
成人及体重30千克以上儿童用量:毎次100 mg, 毎日2次; 成人重症感染者可增加至毎次200 mg, 毎日2次。
儿童:按毎次每千克1.5~3.0 mg计算给药量, 毎日2次。
或遵医嘱。
【不良反应】临床研究资料表明, 本品关键不良反应包含腹泻等消化道反应(0.87%), 皮疹等皮肤症状(0.23%)。
临床检验值异常包含GPT升高(0.61%), GOT升高(0.45%), 嗜酸细胞增多(0.20%)等。
具体以下:(1)严重不良反应①休克: 因为引发休克(<0.1%)可能性, 应亲密观察, 如有出现不适感, 口内异常感、哮喘、眩晕、便意、耳鸣、出汗等现象, 应停止给药, 采取合适处理;②过敏样症状: 有出现过敏样症状(包含呼吸困难、全身潮红、血管性水肿、荨麻疹等)(<0.1%)可能性, 应亲密观察, 如有异常发生时停止给药, 采取合适处理;③皮肤病变: 有发生皮肤粘膜眼症候群, (Stevens-Johnson症候群<0.1%), 中毒性表皮坏死症(Lyell症候群, <0.1%)可能性, 应亲密观察, 如有发生发烧、头痛、关节痛、皮肤或粘膜红斑、水泡、皮肤担心感、灼热感、疼痛等症状, 应停止给药, 采取合适处理;④血液障碍: 有发生粒细胞缺乏症(<0.1%, 早期症状: 发烧、咽喉疼、头疼、倦怠感等), 溶血性贫血(<0.1%, 早期症状: 发烧、血红蛋白尿、贫血等症状), 血小板降低(<0.1%, 早期症状: 点状出血、紫斑等)可能性, 且有其她头孢类抗生素造成全血细胞降低汇报, 所以应亲密观察, 比如进行定时检验等, 有异常发生时应停止给药, 采取合适处理;⑤肾功效障碍: 因为引发急性肾功效不全等严重肾功效障碍(<0.1%)可能性, 所以应亲密观察, 比如定时进行检验等, 如有异常发生时, 应停止给药, 采取合适处理;⑥结肠炎: 可能引发伴有血便严重大肠炎比如伪膜性结肠炎等(<0.1%)。
专业英语药品说明书

药品的适用范围 Indications
说明药品对哪些病菌、疾病有效或无效,是说明书的重点。 适应症 Indication (适应症) Uses(用途) Major indication, principal indication(主要适应症)
用法 Adminstration, direction (用法) Dosage, posology:剂量 Usage and dosage /Administration and dosage:用法与剂量 常用表达 Daily, per day, a day, every day; Every 8 hours, 8 hours apart, eight-hourly, at intervals of 8 hours; Once daily, twice daily, three times daily, every other day/every second day 50 mg per kilo of body weight daily
安替司丁 治疗过敏性病症的抗组胺剂 性质 安替司丁可减弱或抑制组胺作用,组胺在激发过敏性病症中起主要作用。本品的适应症正是根据这种实验证明的抗组胺作用来确定的。 适应症 荨麻疹、食物过敏、枯草热、血管舒缩性鼻炎;由于皮肤病症引起的搔痒包括湿疹、搔痒病和血清病。
Administration and dosage Tableis adults; 1tablet 3-4 time daily. Small children; ½ tablet 2-3 time daily. The tablets should be taken during meals and swallowed whole with a little fluid. Ampoules 1 ampoule, given by intramuscular or slow intravenous injection, 2-3 times daily. For children the doses should be correspondingly reduced服法与剂量 片剂 成人:每次1片,一日3-4次。 小儿:每次1/2片,一日1次。 本片剂应在餐间服用,以少许水整个吞服。 针剂 每次1瓶,一日2-3次,肌肉或缓慢静注射。儿童剂量应相应减少。
头孢地尼胶囊在健康人体的生物等效性研究

头孢地尼胶囊在健康人体的生物等效性研究陈芬;朱超然;翟学佳;冯霞;邓桂萍;郭卿;蒋立芬;吕永宁【摘要】目的:评价健康人餐后服用两种头孢地尼胶囊的生物等效性。
方法24例男性健康志愿者餐后随机交叉单剂量口服头孢地尼胶囊受试制剂或参比制剂,头孢地尼体内血药浓度采用液相色谱-质谱串联( LC-MS/MS)法测定,药动学参数及等效性采用药动学软件DAS计算和评价。
结果受试制剂和参比制剂的主要药动学参数如下:AUCt分别为(4.35±1.09)和(4.12±1.22)μg��h��mL-1,AUC0-∞分别为(4.53±1.12)和(4.53±1.73)μg��h��mL-1,t1/2分别为(1.74±0.29)和(2.13±1.65) h,tmax分别为(4.44±0.86)和(4.54±1.16) h,Cmax分别为(900±250)和(876±269) ng��mL-1。
结论头孢地尼胶囊受试制剂与参比制剂具有生物等效性。
%Objective To evaluate postprandial pharmacokinetics and bioequivalence of two preparations of cefdinir capsules in Chinese healthy volunteers. Methods In a two-way cross-over study, 24 healthy male volunteers were divided into two groups randomly and a single dose of cefdinir capsules of test and reference preparation were administered orally, respectively.The concentration in plasma was determined by LC-MS/MS. Pharmacokinetic parameters and bioequivalence were calculated and evaluated by DAS. Results The main pharmacokinetic parameters of test and reference were as follows: AUCt (4.35±1.09) μg��h��mL-1 and (4.12±1.22) μg��h��mL-1, AUC0-∞(4.53±1.12) and (4.53±1.73)μg��h��mL-1, t1/2 (1.74±0.29) h and (2.13±1.65) h, tmax(4.44±0.86) h and (4.54 ±1.16) h, Cmax(900±250) ng��mL-1 and (876±269) ng��mL-1 . Conclusion The test and reference preparation of cefdinir capsules are bioequivalent.【期刊名称】《医药导报》【年(卷),期】2015(000)010【总页数】4页(P1288-1291)【关键词】头孢地尼胶囊;液相色谱-质谱串联联用;药动学;生物等效性【作者】陈芬;朱超然;翟学佳;冯霞;邓桂萍;郭卿;蒋立芬;吕永宁【作者单位】华中科技大学同济医学院附属协和医院药剂科,武汉 430022;华中科技大学同济医学院附属协和医院药剂科,武汉 430022;华中科技大学同济医学院附属协和医院药剂科,武汉 430022;华中科技大学同济医学院附属协和医院药剂科,武汉 430022;华中科技大学同济医学院附属协和医院药剂科,武汉 430022;石家庄市华新药业有限责任公司,石家庄050091;河北省人民医院体检中心,石家庄 050051;华中科技大学同济医学院附属协和医院药剂科,武汉 430022【正文语种】中文【中图分类】R978.11;R969.1头孢地尼是第3代头孢菌素类抗菌药物,1988年由日本藤泽药品工业公司合成,1991年在日本上市,1997年被美国食品药品管理局(FDA)批准临床使用[1]。
头孢地尼治疗感染的适应症及使用说明

头孢地尼治疗感染的适应症及使用说明头孢地尼是一种广谱抗生素,常用于治疗各种细菌感染。
本文将详细介绍头孢地尼的适应症和使用说明,以帮助患者正确使用此药物并获得最佳疗效。
一、头孢地尼的适应症头孢地尼适用于对该药敏感的细菌引起的以下感染:1. 呼吸道感染:包括社区获得性肺炎、慢性阻塞性肺疾病合并感染、支气管炎等。
2. 泌尿系统感染:如尿路感染、膀胱炎、肾盂肾炎等。
3. 皮肤和软组织感染:如蜂窝组织炎、褥疮、脓疱疮等。
4. 腹部感染:如阑尾炎、胆囊炎、腹膜炎等。
5. 骨和关节感染:如骨髓炎、关节炎、骨折引起的感染等。
二、使用说明1. 治疗前请咨询医生:在使用头孢地尼之前,请务必咨询医生并根据医生的建议进行使用。
医生会根据感染的程度、部位和患者的具体情况来决定剂量和疗程。
2. 用法用量:按照医生的处方指导进行使用。
头孢地尼通常以口服或静脉注射的形式给予。
剂量的大小和用药频率会根据感染的严重程度和患者的年龄、体重等因素而异。
3. 用药时间:头孢地尼通常需持续使用一段时间才能有效治疗感染。
患者应确保按时、按量服药,并不得自行停药。
即使感觉症状已消失,也不宜提前停药,以免导致病情复发或细菌耐药。
4. 饭前或饭后:口服头孢地尼时,可以根据个人喜好选择是否餐前或餐后服用。
如果胃不适,可在饭后使用以减轻胃部刺激。
5. 药物不良反应:头孢地尼可能会引起一些不良反应,如恶心、呕吐、腹泻、皮疹等。
如果出现严重的过敏反应(如呼吸困难、皮肤荨麻疹等),应立即停药并就医。
6. 注意禁忌:头孢地尼对青霉素过敏者禁用。
同时,患者应告知医生个人的过敏史和药物使用情况,包括正在使用的处方药、非处方药和补充剂等。
7. 孕妇和哺乳期妇女:头孢地尼在妊娠期和哺乳期需慎用。
如果怀孕或正在哺乳期的患者需要使用头孢地尼,应在医生的指导下使用。
8. 耐药性和复发:头孢地尼是一种广谱抗生素,长期或不恰当的使用可能导致细菌耐药。
患者在使用过程中应按照医生的建议正确使用,并遵循疗程至其结束,以避免感染复发。
头孢地尼的抗菌活性和药动学概述
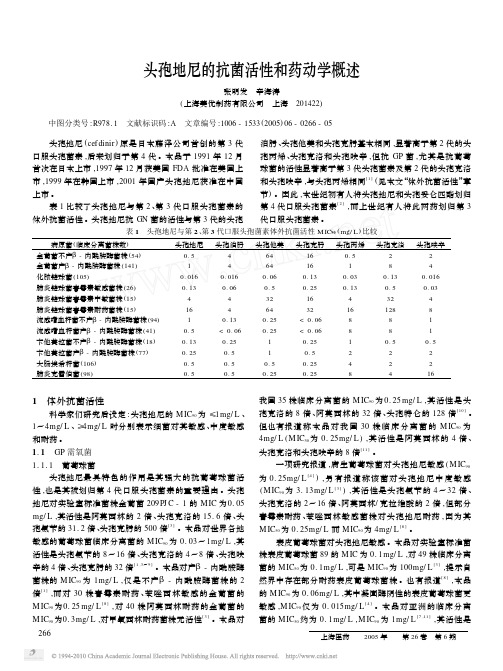
头孢氨苄的 15~267 倍 、头孢克洛的 8~133 倍 、头孢呋辛 的 32~67 倍 、头孢克肟的 62~533 倍 、阿莫西林的 2~8 倍。
头葡萄球菌 ( S. capitis) 也对头孢地尼敏感 ( M IC90 为 0. 5mg/ L [8] ) 。本品对人葡萄球菌 ( S. huminis) 的 M IC90 为 0. 125mg/ L [8] 或 0. 39mg/ L ,其活性是头孢氨苄的 64 倍 、头 孢克洛的 16 倍 、头孢克肟的 128 倍 、阿莫西林的 4 倍[3] 。 溶血葡萄球菌 ( S. hemolyticus) 虽对头孢地尼敏感 ,但也存 在耐药菌株 ,2 项研究报道称本品的 M IC50 和 M IC90 分别为 0. 25 mg/ L 和 32mg/ L [8] 及 0. 39 mg/ L 和 100mg/ L ,其活 性是头孢氨苄的 8 倍 、头孢克洛的 4 倍 、头孢克肟的 128 倍 、阿莫西林的 2 倍[3 ] 。 1. 1. 2 链球菌及其它 GP 菌
头孢克肟 16 16 0. 13 0. 25 16 32
< 0. 06 < 0. 06 0. 25
0. 5 0. 25 0. 25
头孢丙烯 0. 5 1 0. 03 0. 13 4 16 8 8 1 2 4 8
头孢克洛 2 8
0. 13 0. 5 32 128
8 8 0. 5 2 2 4
头孢呋辛 2 4
上市 。
节) 。因此 ,本世纪初有人将头孢地尼和头孢妥仑匹酯划归
表 1 比较了头孢地尼与第 2 、第 3 代口服头孢菌素的 第 4 代口服头孢菌素[2] ,而上世纪有人将此两药划归第 3
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
OMNICEF®(cefdinir) capsulesOMNICEF®(cefdinir) for oral suspensionTo reduce the development of drug-resistant bacteria and maintain the effectiveness ofOMNICEF and other antibacterial drugs, OMNICEF should be used only to treat orprevent infections that are proven or strongly suspected to be caused by bacteria.DESCRIPTIONProprietary name: OMNICEFEstablished name: cefdinirRoute of administration: ORAL (C38288)Active ingredients (moiety): cefdinir (cefdinir)# Strength Form Inactive ingredients1 300 MILLIGRAM CAPSULE (C25158) carboxymethylcellulose calcium, NF, polyoxyl 40 stearate, NF,magnesium stearate, NF, FD&C Blue #1, FD&C Red #40, D&C Red#28, titanium dioxide, NF, gelatin, NF, silicon dioxide, NF, sodium laurylsulfate, NF2 125 MILLIGRAM POWDER, FOR SUSPENSION (C42975) sucrose, NF, citric acid, USP, sodium citrate, USP, sodium benzoate,NF, xanthan gum, NF, guar gum, NF, artificial strawberry and creamflavors, silicon dioxide, NF, magnesium stearate, NF3250 MILLIGRAM POWDER, FOR SUSPENSION (C42975) sucrose, NF, citric acid, USP, sodium citrate, USP, sodium benzoate,NF, xanthan gum, NF, guar gum, NF, artificial strawberry and cream flavors, silicon dioxide, NF, magnesium stearate, NF OMNICEF (cefdinir) capsules and OMNICEF (cefdinir) for oral suspension contain theactive ingredient cefdinir, an extended-spectrum, semisynthetic cephalosporin, for oraladministration. Chemically, cefdinir is [6R-[6α, 7β (Z)]]-7-[[(2-amino-4thiazolyl)(hydroxyimino)acetyl]amino]-3-ethenyl-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene2-carboxylic acid. Cefdinir is a white to slightly brownish-yellow solid. It is slightly soluble in dilute hydrochloric acid and sparingly soluble in 0.1 M pH 7.0 phosphate buffer. Theempirical formula is C 14H 13N 5O 5S 2 and the molecular weight is 395.42. Cefdinir has thestructural formula shown below:OMNICEF Capsules contain 300 mg cefdinir and the following inactive ingredients:carboxymethylcellulose calcium, NF; polyoxyl 40 stearate, NF; and magnesium stearate,NF. The capsule shells contain FD&C Blue #1; FD&C Red #40; D&C Red #28; titaniumdioxide, NF; gelatin, NF; silicon dioxide, NF; and sodium lauryl sulfate, NF.OMNICEF for Oral Suspension, after reconstitution, contains 125 mg cefdinir per 5 mL or250 mg cefdinir per 5 mL and the following inactive ingredients: sucrose, NF; citric acid,USP; sodium citrate, USP; sodium benzoate, NF; xanthan gum, NF; guar gum, NF; artificial strawberry and cream flavors; silicon dioxide, NF; and magnesium stearate, NF. CLINICAL PHARMACOLOGYPharmacokinetics and Drug MetabolismAbsorptionOral BioavailabilityMaximal plasma cefdinir concentrations occur 2 to 4 hours postdose following capsule or suspension administration. Plasma cefdinir concentrations increase with dose, but the increases are less than dose-proportional from 300 mg (7 mg/kg) to 600 mg (14 mg/kg). Following administration of suspension to healthy adults, cefdinir bioavailability is 120% relative to capsules. Estimated bioavailability of cefdinir capsules is 21% following administration of a 300 mg capsule dose, and 16% following administration of a 600 mg capsule dose. Estimated absolute bioavailability of cefdinir suspension is 25%. Cefdinir oral suspension of 250 mg/5 mL strength was shown to be bioequivalent to the125 mg/5 mL strength in healthy adults under fasting conditions.Effect of FoodThe C max and AUC of cefdinir from the capsules are reduced by 16% and 10%, respectively, when given with a high-fat meal. In adults given the 250 mg/5 mL oral suspension with a high-fat meal, the C max and AUC of cefdinir are reduced by 44% and 33%, respectively. The magnitude of these reductions is not likely to be clinically significant because the safety and efficacy studies of oral suspension in pediatric patients were conducted without regard to food intake. Therefore, cefdinir may be taken without regard to food.Cefdinir CapsulesCefdinir plasma concentrations and pharmacokinetic parameter values following administration of single 300- and 600-mg oral doses of cefdinir to adult subjects are presented in the following table:Mean (± SD) Plasma Cefdinir Pharmacokinetic Parameter Values Following Administration ofCapsules to Adult SubjectsDose Cmax(µg/mL) t max(hr)AUC(µg•hr/mL)300 mg 1.60 2.9 7.05(0.55) (0.89) (2.17)600 mg 2.87 3.0 11.1(1.01) (0.66) (3.87)Cefdinir SuspensionCefdinir plasma concentrations and pharmacokinetic parameter values following administration of single 7- and 14-mg/kg oral doses of cefdinir to pediatric subjects (age 6 months-12 years) are presented in the following table:Mean (± SD) Plasma Cefdinir Pharmacokinetic Parameter Values Following Administration ofSuspension to Pediatric SubjectsDose Cmax(µg/mL) t max(hr)AUC(µg•hr/mL)7 mg/kg 2.30(0.65)2.2(0.6)8.31(2.50)14 mg/kg 3.86(0.62)1.8(0.4)13.4(2.64)Multiple DosingCefdinir does not accumulate in plasma following once- or twice-daily administration to subjects with normal renal function.DistributionThe mean volume of distribution (Vd area) of cefdinir in adult subjects is 0.35 L/kg (± 0.29); in pediatric subjects (age 6 months-12 years), cefdinir Vd area is 0.67 L/kg (± 0.38). Cefdinir is 60% to 70% bound to plasma proteins in both adult and pediatric subjects; binding is independent of concentration.Skin BlisterIn adult subjects, median (range) maximal blister fluid cefdinir concentrations of0.65 (0.33-1.1) and 1.1 (0.49-1.9) µg/mL were observed 4 to 5 hours following administration of 300- and 600-mg doses, respectively. Mean (± SD) blister C max and AUC (0-∞) values were 48% (± 13) and 91% (± 18) of corresponding plasma values.Tonsil TissueIn adult patients undergoing elective tonsillectomy, respective median tonsil tissue cefdinir concentrations 4 hours after administration of single 300- and 600-mg doses were 0.25 (0.22-0.46) and 0.36 (0.22-0.80) µg/g. Mean tonsil tissue concentrations were 24% (± 8) of corresponding plasma concentrations.Sinus TissueIn adult patients undergoing elective maxillary and ethmoid sinus surgery, respective median sinus tissue cefdinir concentrations 4 hours after administration of single 300- and 600-mg doses were < 0.12 (< 0.12-0.46) and 0.21 (< 0.12-2.0) µg/g. Mean sinus tissue concentrations were 16% (± 20) of corresponding plasma concentrations.Lung TissueIn adult patients undergoing diagnostic bronchoscopy, respective median bronchial mucosa cefdinir concentrations 4 hours after administration of single 300- and 600-mg doses were 0.78 (< 0.06-1.33) and 1.14 (< 0.06-1.92) µg/mL, and were 31% (± 18) of corresponding plasma concentrations. Respective median epithelial lining fluid concentrations were 0.29 (< 0.3-4.73) and 0.49 (< 0.3-0.59) µg/mL, and were 35% (± 83) of corresponding plasma concentrations.Middle Ear FluidIn 14 pediatric patients with acute bacterial otitis media, respective median middle ear fluid cefdinir concentrations 3 hours after administration of single 7- and 14-mg/kg doses were 0.21 (< 0.09-0.94) and 0.72 (0.14-1.42) µg/mL. Mean middle ear fluid concentrations were 15% (± 15) of corresponding plasma concentrations.CSFData on cefdinir penetration into human cerebrospinal fluid are not available. Metabolism and ExcretionCefdinir is not appreciably metabolized. Activity is primarily due to parent drug. Cefdinir is eliminated principally via renal excretion with a mean plasma elimination half-life (t½) of 1.7 (± 0.6) hours. In healthy subjects with normal renal function, renal clearance is 2.0 (± 1.0) mL/min/kg, and apparent oral clearance is 11.6 (± 6.0) and 15.5 (± 5.4) mL/min/kg following doses of 300- and 600-mg, respectively. Mean percent of dose recovered unchanged in the urine following 300- and 600-mg doses is 18.4% (± 6.4) and 11.6% (±4.6), respectively. Cefdinir clearance is reduced in patients with renal dysfunction (see Special Populations - Patients with Renal Insufficiency).Because renal excretion is the predominant pathway of elimination, dosage should be adjusted in patients with markedly compromised renal function or who are undergoing hemodialysis (see DOSAGE AND ADMINISTRATION).Special PopulationsPatients with Renal InsufficiencyCefdinir pharmacokinetics were investigated in 21 adult subjects with varying degrees of renal function. Decreases in cefdinir elimination rate, apparent oral clearance (CL/F), and renal clearance were approximately proportional to the reduction in creatinine clearance (CL cr). As a result, plasma cefdinir concentrations were higher and persisted longer in subjects with renal impairment than in those without renal impairment. In subjects withCL cr between 30 and 60 mL/min, C max and t½ increased by approximately 2-fold and AUC by approximately 3-fold. In subjects with CL cr < 30 mL/min, C max increased by approximately 2-fold, t½ by approximately 5-fold, and AUC by approximately 6-fold. Dosage adjustment is recommended in patients with markedly compromised renal function (creatinine clearance < 30 mL/min; see DOSAGE AND ADMINISTRATION). HemodialysisCefdinir pharmacokinetics were studied in 8 adult subjects undergoing hemodialysis. Dialysis (4 hours duration) removed 63% of cefdinir from the body and reduced apparent elimination t½ from 16 (± 3.5) to 3.2 (± 1.2) hours. Dosage adjustment is recommended in this patient population (see DOSAGE AND ADMINISTRATION).Hepatic DiseaseBecause cefdinir is predominantly renally eliminated and not appreciably metabolized, studies in patients with hepatic impairment were not conducted. It is not expected that dosage adjustment will be required in this population.Geriatric PatientsThe effect of age on cefdinir pharmacokinetics after a single 300-mg dose was evaluated in 32 subjects 19 to 91 years of age. Systemic exposure to cefdinir was substantially increased in older subjects (N = 16), C max by 44% and AUC by 86%. This increase was due to a reduction in cefdinir clearance. The apparent volume of distribution was also reduced, thus no appreciable alterations in apparent elimination t½ were observed (elderly: 2.2 ± 0.6 hours vs young: 1.8 ± 0.4 hours). Since cefdinir clearance has been shown to be primarily related to changes in renal function rather than age, elderly patients do not require dosage adjustment unless they have markedly compromised renal function (creatinine clearance < 30 mL/min, see Patients with Renal Insufficiency, above).Gender and RaceThe results of a meta-analysis of clinical pharmacokinetics (N = 217) indicated no significant impact of either gender or race on cefdinir pharmacokinetics.MicrobiologyAs with other cephalosporins, bactericidal activity of cefdinir results from inhibition of cell wall synthesis. Cefdinir is stable in the presence of some, but not all, β-lactamase enzymes. As a result, many organisms resistant to penicillins and some cephalosporins are susceptible to cefdinir.Cefdinir has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in INDICATIONS AND USAGE.Aerobic Gram-Positive MicroorganismsStaphylococcus aureus (including β-lactamase producing strains)NOTE: Cefdinir is inactive against methicillin-resistant staphylococci.Streptococcus pneumoniae (penicillin-susceptible strains only)Streptococcus pyogenesAerobic Gram-Negative MicroorganismsHaemophilus influenzae (including β-lactamase producing strains)Haemophilus parainfluenzae (including β-lactamase producing strains)Moraxella catarrhalis (including β-lactamase producing strains)The following in vitro data are available, but their clinical significance is unknown. Cefdinir exhibits in vitro minimum inhibitory concentrations (MICs) of 1 µg/mL or less against (≥ 90%) strains of the following microorganisms; however, the safety and effectiveness of cefdinir in treating clinical infections due to these microorganisms have not been established in adequate and well-controlled clinical trials.Aerobic Gram-Positive MicroorganismsStaphylococcus epidermidis (methicillin-susceptible strains only)Streptococcus agalactiaeViridans group streptococciNOTE: Cefdinir is inactive against Enterococcus and methicillin-resistant Staphylococcusspecies.Aerobic Gram-Negative MicroorganismsCitrobacter diversusEscherichia coliKlebsiella pneumoniaeProteus mirabilisNOTE: Cefdinir is inactive against Pseudomonas and Enterobacter species.Susceptibility TestsDilution TechniquesQuantitative methods are used to determine antimicrobial minimum inhibitoryconcentrations (MICs). These MICs provide estimates of the susceptibility of bacteria toantimicrobial compounds. The MICs should be determined using a standardizedprocedure. Standardized procedures are based on a dilution method(1) (broth or agar) orequivalent with standardized inoculum concentrations and standardized concentrations ofcefdinir powder. The MIC values should be interpreted according to the following criteria:For organisms other than Haemophilus spp. and Streptococcus spp:MIC (µg/mL) Interpretation≤ 1 Susceptible (S)(I)2 Intermediate≥ 4 Resistant (R)For Haemophilus spp:aMIC (µg/mL) Interpretation b≤ 1 Susceptible (S)a These interpretive standards are applicable only to broth microdilution susceptibility tests with Haemophilus spp.using Haemophilus Test Medium (HTM).(1)b The current absence of data on resistant strains precludes defining any results other than "Susceptible." Strainsyielding MIC results suggestive of a "nonsusceptible" category should be submitted to a reference laboratory for furthertesting.For Streptococcus spp:Streptococcus pneumoniae that are susceptible to penicillin (MIC ≤ 0.06 µg/mL), orstreptococci other than S. pneumoniae that are susceptible to penicillin (MIC≤ 0.12 µg/mL), can be considered susceptible to cefdinir. Testing of cefdinir againstpenicillin-intermediate or penicillin-resistant isolates is not recommended. Reliableinterpretive criteria for cefdinir are not available.A report of "Susceptible" indicates that the pathogen is likely to be inhibited if theantimicrobial compound in the blood reaches the concentration usually achievable.A report of "Intermediate" indicates that the result should be considered equivocal, and,if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the testshould be repeated. This category implies possible clinical applicability in body siteswhere the drug is physiologically concentrated or in situations where high dosage ofdrug can be used. This category also provides a buffer zone which prevents smalluncontrolled technical factors from causing major discrepancies in interpretation. A reportof "Resistant" indicates that the pathogen is not likely to be inhibited if the antimicrobialcompound in the blood reaches the concentrations usually achievable; other therapyshould be selected.Standardized susceptibility test procedures require the use of laboratory controlmicroorganisms to control the technical aspects of laboratory procedures. Standardcefdinir powder should provide the following MIC values:Microorganism MIC Range (µg/mL)Escherichia coli ATCC 25922 0.12-0.5Haemophilus influenzae ATCC 49766c 0.12-0.5Staphylococcus aureus ATCC 29213 0.12-0.5c This quality control range is applicable only to H. influenzae ATCC 49766 tested by a broth microdilution procedureusing HTM.Diffusion TechniquesQuantitative methods that require measurement of zone diameters also providereproducible estimates of the susceptibility of bacteria to antimicrobial compounds. Onesuch standardized procedure(2) requires the use of standardized inoculum concentrations.This procedure uses paper disks impregnated with 5-µg cefdinir to test the susceptibility ofmicroorganisms to cefdinir.Reports from the laboratory providing results of the standard single-disk susceptibility testwith a 5-µg cefdinir disk should be interpreted according to the following criteria:For organisms other than Haemophilus spp. and Streptococcus spp:dZone Diameter (mm) Interpretation≥ 20 Susceptible (S)(I)17-19 Intermediate≤ 16 Resistant (R)d Because certain strains of Citrobacter, Providencia , and Enterobacter spp. have been reported to give falsesusceptible results with the cefdinir disk, strains of these genera should not be tested and reported with this disk.For Haemophilus spp:eZone Diameter (mm) Interpretation f≥ 20 Susceptible (S)e These zone diameter standards are applicable only to tests with Haemophilus spp. using HTM.f The current absence of data on resistant strains precludes defining any results other than "Susceptible." Strainsyielding MIC results suggestive of a "nonsusceptible" category should be submitted to a reference laboratory for furthertesting.For Streptococcus spp:Isolates of Streptococcus pneumoniae should be tested against a 1-µg oxacillin disk.Isolates with oxacillin zone sizes ≥ 20 mm are susceptible to penicillin and can be considered susceptible to cefdinir. Streptococci other than S. pneumoniae should be tested with a 10-unit penicillin disk. Isolates with penicillin zone sizes ≥ 28 mm are susceptible to penicillin and can be considered susceptible to cefdinir.As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms to control the technical aspects of laboratory procedures. For the diffusion technique, the 5-µg cefdinir disk should provide the following zone diameters in these laboratory quality control strains:Organism Zone Diameter (mm) Escherichia coli ATCC 25922 24-28 Haemophilus influenzae ATCC 49766g 24-31 Staphylococcus aureus ATCC 25923 25-32g This quality control range is applicable only to testing of H. influenzae ATCC 49766 using HTM.INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of OMNICEF and other antibacterial drugs, OMNICEF should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.OMNICEF (cefdinir) capsules and OMNICEF (cefdinir) for oral suspension are indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below.Adults and AdolescentsCommunity-Acquired Pneumoniacaused by Haemophilus influenzae (including β-lactamase producing strains), Haemophilus parainfluenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including βlactamase producing strains) (see CLINICAL STUDIES).Acute Exacerbations of Chronic Bronchitiscaused by Haemophilus influenzae (including β-lactamase producing strains), Haemophilus parainfluenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including βlactamase producing strains).Acute Maxillary Sinusitiscaused by Haemophilus influenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including β-lactamase producing strains).NOTE: For information on use in pediatric patients, see Pediatric Use and DOSAGE AND ADMINISTRATION.Pharyngitis/Tonsillitiscaused by Streptococcus pyogenes (see CLINICAL STUDIES).NOTE: Cefdinir is effective in the eradication of S. pyogenes from the oropharynx. Cefdinir has not, however, been studied for the prevention of rheumatic fever following S. pyogenes pharyngitis/tonsillitis. Only intramuscular penicillin has been demonstrated to be effective for the prevention of rheumatic fever.Uncomplicated Skin and Skin Structure Infectionscaused by Staphylococcus aureus (including β-lactamase producing strains) and Streptococcus pyogenes.Pediatric PatientsAcute Bacterial Otitis Media caused by Haemophilus influenzae (including β-lactamase producing strains), Streptococcus pneumoniae (penicillin-susceptible strains only), and Moraxella catarrhalis (including β-lactamase producing strains).Pharyngitis/Tonsillitiscaused by Streptococcus pyogenes (see CLINICAL STUDIES).NOTE: Cefdinir is effective in the eradication of S. pyogenes from the oropharynx. Cefdinir has not, however, been studied for the prevention of rheumatic fever following S. pyogenes pharyngitis/tonsillitis. Only intramuscular penicillin has been demonstrated to be effective for the prevention of rheumatic fever.Uncomplicated Skin and Skin Structure Infectionscaused by Staphylococcus aureus (including β-lactamase producing strains) and Streptococcus pyogenes.CONTRAINDICATIONSOMNICEF (cefdinir) is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.WARNINGSBEFORE THERAPY WITH OMNICEF (CEFDINIR) IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFDINIR, OTHER CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF CEFDINIR IS TO BE GIVEN TO PENICILLINSENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSSHYPERSENSITIVITY AMONG β-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFDINIR OCCURS, THE DRUG SHOULD BE DISCONTINUED. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, INTRAVENOUS FLUIDS,INTRAVENOUS ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, AS CLINICALLY INDICATED.Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including OMNICEF, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.PRECAUTIONSGeneralPrescribing OMNICEF in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.As with other broad-spectrum antibiotics, prolonged treatment may result in the possible emergence and overgrowth of resistant organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate alternative therapy should be administered.Cefdinir, as with other broad-spectrum antimicrobials (antibiotics), should be prescribed with caution in individuals with a history of colitis.In patients with transient or persistent renal insufficiency (creatinine clearance< 30 mL/min), the total daily dose of OMNICEF should be reduced because high and prolonged plasma concentrations of cefdinir can result following recommended doses (see DOSAGE AND ADMINISTRATION).Information for PatientsPatients should be counseled that antibacterial drugs including OMNICEF should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When OMNICEF is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by OMNICEF or other antibacterial drugs in the future.Antacids containing magnesium or aluminum interfere with the absorption of cefdinir.If this type of antacid is required during OMNICEF therapy, OMNICEF should be taken at least 2 hours before or after the antacid.Iron supplements, including multivitamins that contain iron, interfere with the absorption of cefdinir. If iron supplements are required during OMNICEF therapy, OMNICEF should be taken at least 2 hours before or after the supplement.Iron-fortified infant formula does not significantly interfere with the absorption of cefdinir. Therefore, OMNICEF for Oral Suspension can be administered with iron-fortified infant formula.Diabetic patients and caregivers should be aware that the oral suspension contains2.86 g of sucrose per teaspoon.Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.Drug InteractionsAntacids (aluminum- or magnesium-containing)Concomitant administration of 300-mg cefdinir capsules with 30 mL Maalox® TC suspension reduces the rate (C max) and extent (AUC) of absorption by approximately 40%. Time to reach C max is also prolonged by 1 hour. There are no significant effects on cefdinir pharmacokinetics if the antacid is administered 2 hours before or 2 hours after cefdinir. If antacids are required during OMNICEF therapy, OMNICEF should be taken at least 2 hours before or after the antacid.ProbenecidAs with other β-lactam antibiotics, probenecid inhibits the renal excretion of cefdinir, resulting in an approximate doubling in AUC, a 54% increase in peak cefdinir plasma levels, and a 50% prolongation in the apparent elimination t½.Iron Supplements and Foods Fortified With IronConcomitant administration of cefdinir with a therapeutic iron supplement containing60 mg of elemental iron (as FeSO4) or vitamins supplemented with 10 mg of elemental iron reduced extent of absorption by 80% and 31%, respectively. If iron supplements are required during OMNICEF therapy, OMNICEF should be taken at least 2 hours before or after the supplement.The effect of foods highly fortified with elemental iron (primarily iron-fortified breakfast cereals) on cefdinir absorption has not been studied.Concomitantly administered iron-fortified infant formula (2.2 mg elemental iron/6 oz) has no significant effect on cefdinir pharmacokinetics. Therefore, OMNICEF for Oral Suspension can be administered with iron-fortified infant formula.There have been reports of reddish stools in patients receiving cefdinir. In many cases, patients were also receiving iron-containing products. The reddish color is due to the formation of a nonabsorbable complex between cefdinir or its breakdown products and iron in the gastrointestinal tract.Drug/Laboratory Test Interactions。
