TS 51.010-1 Pretest RSE report Deco mini 3
API 510-模拟试题 原稿

ASME SEC.9OPEN BOOK1 For corrosion-resistant weld overlay cladding, no open discontinuity exceeding ,measured in any direction, shall be permitted in the cladding, and no opendiscontinuity exceeding 1/8 in. (3 mm) shall be permitted along the approximate weld interface(A) 1/16 in.(B) 1/8 in.(C) 1/32 in.(D) 3/16 in.☞QW-163 耐蝕覆焊層彎曲試驗開口接受標準為1/16”,一般對焊為1/8”2 If a WPS is qualified using a base material that is 9" thick, the correct base metalthickness range shown in the WPS should be:(A) 3/16"-12" thick(B) 1/16"-14" thick(C) 3/16"-18" thick(D) 3/16"-9.9" thick(E) 3/16"-maximum to be welded☞QW-451.13 Two types of impact testing are permitted in ASME IX. One of these is:(A) Charpy V-notch(B) Etch-notch(C) Drop weight(D) Izon notch(E) Either A or C, above☞QW-170 NOTCH-TOUGHNESS TESTS4 A welding procedure is qualified on P5A to P5A steel. This WPS is then qualified toweld on:(A) P5A - P4 steels(B) P5A - P5B steels(C) P4 - P4 steels(D) All of the above☞QW-424 Base Metals Used for Procedure Qualification5 Which of the following is an essential variable for the GMAW process?(A) A. Wire diameter(B) Travel speed(C) Interpass temperature(D) Electrical characteristics (short arc to spray or vice-versa)(E) Groove design☞QW-256 WELDING VARIABLES PROCEDURE SPECIFICATIONS (WPS) Gas Tungsten-Arc Welding (GTAW)CLOSE BOOK1 A welder cannot be qualified by radiography when using:(A) P4X metals(B) P5A metals(C) P3X metals with GMAW-Spray Arc(D) P-1 metals with GMAW short-circuiting arc☞QW-304 Welders2 Supplementary Essential Variables (if applicable) must be addressed on the:(A) A. WPS only(B) WPQ only(C) PQR only(D) WPS, PQR and WPQ(E) WPS and PQR only☞QW-200 GENERAL3 If the WPS shows a single "Vee" groove is to be used and the PQR was qualified witha double "Vee" groove:(A) The WPS can be used without re-qualification(B) The WPS must be requalified(C) The PQR must be retested(D) The WPS must be modified to show the correct joint☞開槽方式通常不列入WPS必要變數4 A welder qualifies on a 3 NPS pipe in a 6G position. The piping is titanium, and theweld is made with SMAW. How many bend tests are required for this test weld?(A) 2(B) 4(C) 1(D) 3☞QW-452.1(a) NOTES:(1)5 Which welding test position with pipe axis horizontal and with the welding groove in avertical plane, welding shall be done without rotating the pipe.(A) 1G(B) 3G(C) 6G(D) 5GQW-122.3 Multiple Position 5G.6 Do the mechanical tests support qualification of this PQR?(A) Yes(B) No, one tensile test failed.(C) Face Bends and root Bends should have been performed instead of side bends.(D) The 3/32” defect in the heat effected zone on the side bend tests is over theacceptable limit.☞母材為A-516 Grade70 抗拉強度為70ksi;焊條等級為E-7018,抗拉強度為70ksi 兩支抗拉強度試片均斷在母材,其中1支不到母材95% (66403/70000)=0.9486☞QW-153.1 (d)7 Is joint dseign fully addressed on the WPS?(A) No, the sketch of the joint must also show weld layers & specify uphill ordownhill.(B) Yes(C) No, root spacing is not addressed.(D) No, spacing between backing strip & base metal must also be addressed.☞root spacing非屬必要變數,但須於WPS中明確標示QW-2538 The full range qualified for the base metal thickness that may be welded with thisWPS is:(A) 1/16” to 1 1/2”(B) 3/16” to 1 1/8”(C) As shown on the WPS(D) None of the above☞QW-451.1 查表9 The actual maximum throat dimension allowed for the weld metal thickness “t”forfillet welds:(A) Has been restricted by the WPS to 1” maximum throat.(B) Should be 0” to 8”(C) Is 1/16” to 3/4”(D) Is 3/16” to 1 1/2”☞(A)為4個答案中最適當之選項,但不是唯一☞QW-202.2(C)、QW-451.4,Butt Weld PQR取代Fillet Weld PQR之規定10 If a joint was made using this WPS and the welder put in a single pass with adeposited weld metal thickness, “t”, of 9/16:(A) It would not make any difference.(B) The welder would need to use a different electrode.(C) The WPS would need to be requalified with a new PQR.(D) Charpy production toughness tests would need to run.☞若單一堆焊層厚度大於1/2”,則母材合格厚度範圍受限於1.1T,在此1.1T為0.825”,無法support到1.5”☞QW-253,QW-403.911 The minimum preheat temperature that this WPS could specify without requalificationis:(A) 200°F(B) 300°F(C) 50°F(D) 100°F☞QW-253,QW-403.9☞PQR所列預熱溫度為200°F,預熱溫度容許變動範圍為100°F,因此下修之預熱溫度可低至100°F12 To minimum the full range qualified for“T”on the WPS to 3/16”to 2”:(A) The original coupon used for the PQR would have to have been 1” thick.(B) The WPS only needs editorial revision to allow the welding the thicker material.(C) The preheat temperature need to be increased to 300°F.(D) The method of back gouging must be restricted to grinding only.☞QW-451.1 查表☞QW-451.1幾個重要節點建議背起來13 The full range of A Number qualification which may be shown on the WPS is:(A) A-1 through A-11, P-34 and P-4X(B) As shown on the WPS(C) A-1, Groups 1,2 & 3 only(D) Not covered by ASME Section IX☞QW-253,QW-404.5ASME SEC.5OPEN BOOK1 The IQI image must be visible on sides when using a shim, per ASME V(A) 1(B) 2(C) 3(D) 4☞T-277.32 A weld will be radiographed using a source-side wire IQI. The weld is 3/8" thick with1/16" reinforcement on both sides. What ASTM IQI set will be required?(A) Set A(B) Set B(C) Set C(D) Set D(E) (A) and (D)☞焊道加計兩側焊冠厚度為1/2”。
OECD439 重建皮肤模型体外刺激性检测

OECD/OCDE439 Adopted: 22 July 2010© OECD, (2010) You are free to use this material for personal, non-commercial purposes without seeking prior consent from the OECD, provided the source is duly mentioned. Any commercial use of this material is subject to written permission from the OECD.OECD GUIDELINE FOR THE TESTING OF CHEMICALSIn Vitro Skin Irritation: Reconstructed Human Epidermis Test MethodINTRODUCTION1. Skin irritation refers to the production of reversible damage to the skin following the application of a test chemical for up to 4 hours [as defined by the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS)](1). This Test Guideline (TG) provides an in vitro procedure that may be used for the hazard identification of irritant chemicals (substances and mixtures) in accordance with UN GHS and EU CLP Category 2 (1) (2) (3). For member countries or regions that do not adopt the optional UN GHS Category 3 (mild irritants), this Test Guideline can also be used to identify non-classified chemicals, i.e. UN GHS and EU CLP “No Category”(1)(3). Depending on the regulatory framework and the classification system in use, this test method may be used to determine the skin irritancy of chemicals as a stand-alone replacement test for in vivo skin irritation testing, or as a partial replacement test, within a tiered testing strategy (4).2. The assessment of skin irritation has typically involved the use of laboratory animals [OECD TG 404; adopted in 1981 and revised in 1992 and 2002](4). In relation to animal welfare concerns, TG 404 was revised in 2002, allowing for the determination of skin corrosion/irritation by applying a tiered testing strategy, using validated in vitro or ex vivo test methods, thus avoiding pain and suffering of animals. Three validated in vitro test methods have been adopted as OECD TGs 430, 431 and 435 (5) (6) (7), to be used for the corrosivity part of the tiered testing strategy of TG 404 (4).3. This Test Guideline addresses the human health endpoint skin irritation. It is based on reconstructed human epidermis (RhE), which in its overall design (the use of human derived non-transformed epidermis keratinocytes as cell source and use of representative tissue and cytoarchitecture) closely mimics the biochemical and physiological properties of the upper parts of the human skin, i.e. the epidermis . This Test Guideline also includes a set of Performance Standards (PS)(Annex 2) for the assessment of similar and modified RhE-based test methods developed by EC-ECVAM (8), in accordance with the principles of Guidance Document No. 34 (9).4. There are three validated test methods that adhere to this Test Guideline. Prevalidation, optimisation and validation studies have been completed for an in vitro test method (10) (11) (12) (13) (14)(15) (16) (17) (18) (19) (20), using a RhE model, commercially available as EpiSkin™ (designated the Validated Reference Method – VRM). Two other commercially available in vitro skin irritation RhE test methods have shown similar results to the VRM according to PS-based validation (21), and these are the EpiDerm™ SIT (EPI-200) and the SkinEthic™ RHE test methods (22).5. Before a proposed similar or modified in vitro RhE test method other than the VRM, EpiDerm™ SIT (EPI-200) or SkinEthic™ RHE test methods can be used for regulatory purposes, its reliability, relevance (accuracy), and limitations for its proposed use should be determined in order to ensure that it can be regarded as similar to that of the VRM, in accordance with the requirements of the PS set out in this Test Guideline (Annex 2).439OECD/OCDE6. Definitions used are provided in Annex 1.INITIAL CONSIDERATIONS AND LIMITATIONS7. A limitation of the Test Guideline, as demonstrated by the validation study (16), is that it does not allow the classification of chemicals to the optional UN GHS Category 3 (mild irritants) (1). Thus, the regulatory framework in member countries will decide how this Test Guideline will be used. When used as a partial replacement test, follow-up in vivo testing may be required to fully characterize skin irritation potential (4). It is recognized that the use of human skin is subject to national and international ethical considerations and conditions.8. This Test Guideline addresses the in vitro skin irritation component of the tiered testing strategy of TG 404 on dermal corrosion/irritation (4). While this Test Guideline does not provide adequate information on skin corrosion, it should be noted that OECD TG 431 on skin corrosion is based on the same RhE test system, though using another protocol (6). This Test Guideline is based on RhE-models using human keratinocytes, which therefore represent in vitro the target organ of the species of interest. It moreover directly covers the initial step of the inflammatory cascade/mechanism of action (cell damage and tissue damage resulting in localised trauma) that occurs during irritation in vivo. A wide range of chemicals has been tested in the validation underlying this Test Guideline and the empirical database of the validation study amounted to 58 chemicals in total (16)(18)(23). The Test Guideline is applicable to solids, liquids, semi-solids and waxes. The liquids may be aqueous or non-aqueous; solids may be soluble or insoluble in water. Whenever possible, solids should be ground to a fine powder before application; no other pre-treatment of the sample is required. Gases and aerosols have not been assessed yet in a validation study (24). While it is conceivable that these can be tested using RhE technology, the current Test Guideline does not allow testing of gases and aerosols. It should also be noted that highly coloured chemicals may interfere with the cell viability measurements and need the use of adapted controls for corrections (see paragraphs 24-26).9. A single testing run composed of three replicate tissues should be sufficient for a test chemical when the classification is unequivocal. However, in cases of borderline results, such as non-concordant replicate measurements and/or mean percent viability equal to 50 ± 5%, a second run should be considered, as well as a third one in case of discordant results between the first two runs.PRINCIPLE OF THE TEST10. The test chemical is applied topically to a three-dimensional RhE model, comprised of non-transformed human-derived epidermal keratinocytes, which have been cultured to form a multilayered, highly differentiated model of the human epidermis. It consists of organized basal, spinous and granular layers, and a multilayered stratum corneum containing intercellular lamellar lipid layers representing main lipid classes analogous to those found in vivo.11. Chemical-induced skin irritation, manifested by erythema and oedema, is the result of a cascade of events beginning with penetration of the stratum corneum and damage to the underlying layers of keratinocytes. The dying keratinocytes release mediators that begin the inflammatory cascade which acts on the cells in the dermis, particularly the stromal and endothelial cells. It is the dilation and increased permeability of the endothelial cells that produce the observed erythema and oedema (24). The RhE-based test methods measure the initiating events in the cascade.12. Cell viability in RhE models is measured by enzymatic conversion of the vital dye MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Thiazolyl blue; CAS number 298-93-1], into a © OCDE, (2010) 2OECD/OCDE439 blue formazan salt that is quantitatively measured after extraction from tissues (25). Irritant chemicals areidentified by their ability to decrease cell viability below defined threshold levels (i.e.≤ 50%, for UN GHSCategory 2). Depending on the regulatory framework and applicability of the Test Guideline, chemicalsthat produce cell viabilities above the defined threshold level, may be considered non-irritants (i.e. > 50%,No Category).DEMONSTRATION OF PROFICIENCY13. Prior to routine use of any of the three validated test methods that adhere to this Test Guideline, laboratories should demonstrate technical proficiency, using the ten Proficiency Chemicals listed in Table1. For similar test methods developed under this Test Guideline or for modifications of any of the threevalidated test methods, the PS requirements described in Annex 2 of this Test Guideline should be metprior to using the test method for regulatory testing.14. As part of the proficiency exercise, it is recommended that the user verifies the barrier propertiesof the tissues after receipt as specified by the RhE model producer. This is particularly important if tissuesare shipped over long distance/time periods. Once a test method has been successfully established and proficiency in its use has been demonstrated, such verification will not be necessary on a routine basis. However, when using a test method routinely, it is recommended to continue to assess the barrier properties in regular intervals.The Proficiency Chemicals are a subset of the chemicals used in the validation study.2In vivo score in accordance with the OECD Test Guideline 404 (4).3Under this Test Guideline, the UN GHS optional Category 3 (mild irritants) (1) is considered as No Category.PROCEDURE3© OECD, (2010)439OECD/OCDE15. The following is a description of the components and procedures of a RhE test method for skin irritation assessment. A RhE model should be reconstructed, and can be in-house-prepared or obtained commercially. Standard Operating Procedures (SOPs) for the EpiSkin™, EpiDerm™ SIT (EPI-200) and SkinEthic™ RHE are available (26)(27)(28). Testing should be performed according to the following:R H E TEST METHOD COMPONENTSGeneral conditions16. Non-transformed human keratinocytes should be used to reconstruct the epithelium. Multiple layers of viable epithelial cells (basal layer, stratum spinosum, stratum granulosum) should be present under a functional stratum corneum. Stratum corneum should be multilayered containing the essential lipid profile to produce a functional barrier with robustness to resist rapid penetration of cytotoxic marker chemicals, e.g.sodium dodecyl sulphate (SDS) or Triton X-100. The barrier function should be demonstrated and may be assessed either by determination of the concentration at which a marker chemical reduces the viability of the tissues by 50% (IC50) after a fixed exposure time, or by determination of the exposure time required to reduce cell viability by 50% (ET50) upon application of the marker chemical at a specified, fixed concentration. The containment properties of the RhE model should prevent the passage of material around the stratum corneum to the viable tissue, which would lead to poor modelling of skin exposure. The RhE model should be free of contamination by bacteria, viruses, mycoplasma, or fungi. Functional conditionsViability17. The assay used for determining the magnitude of viability is the MTT-assay (25). The RhE model users should ensure that each batch of the RhE model used meets defined criteria for the negative control (NC). The optical density (OD) of the extraction solvent alone should be sufficiently small, i.e. OD<0.1. An acceptability range (upper and lower limit) for the negative control OD values (In the Skin Irritation Test Method conditions) are established by the RhE model developer/supplier, and the acceptability ranges for the 3 validated test methods are given in Table 2. It should be documented that the tissues treated with NC are stable in culture (provide similar viability measurements) for the duration of the test exposure period.Table 2:Acceptability ranges for negative control OD valuesBarrier function18. The stratum corneum and its lipid composition should be sufficient to resist the rapid penetration of cytotoxic marker chemicals, e.g. SDS or Triton X-100, as estimated by IC50 or ET50 (Table 3).© OCDE, (2010) 4OECD/OCDE439Morphology19. Histological examination of the RhE model should be performed demonstrating human epidermis-like structure (including multilayered stratum corneum).Reproducibility20. The results of the positive and negative controls of the test method should demonstrate reproducibility over time.Quality control (QC)21. The RhE model developer/supplier should ensure and demonstrate that each batch of the RhEmodel used meets defined production release criteria, among which those for viability (paragraph 17),barrier function (paragraph 18) and morphology (paragraph 19) are the most relevant. These data shouldbe provided to the test method users, so that they are able to include this information in the test report. An acceptability range (upper and lower limit) for the IC50 or the ET50 should be established by the RhE modeldeveloper/supplier (or investigator when using an in-house model). Only results produced with qualifiedtissues can be accepted for reliable prediction of irritation classification. As an example, the acceptabilityranges for the three validated test methods are given in Table 3.Table 3:Examples of QC batch release criteriaApplication of the Test and Control Chemicals22. At least three replicates should be used for each test chemical and for the controls in each run.For liquid as well as solid chemicals, sufficient amount of test chemical should be applied to uniformlycover the epidermis surface while avoiding an infinite dose, i.e.a minimum of 25 μL/cm2 or 25 mg/cm2should be used. For solid chemicals, the epidermis surface should be moistened with deionised or distilledwater before application, to improve contact between the test chemical and the epidermis surface.Whenever possible, solids should be tested as a fine powder. At the end of the exposure period, the testchemical should be carefully washed from the epidermis surface with aqueous buffer, or 0.9% NaCl. Depending on which of the three validated RhE test methods is used, the exposure period varies between15 and 60 minutes, and the incubation temperature between 20 and 37°C. These exposure periods and temperatures are optimized for each RhE test method and represent the different intrinsic properties of thetest methods, for details, see the Standard Operating Protocols (SOPs) for the test methods (26)(27)(28).5© OECD, (2010)439OECD/OCDE23. Concurrent NC and positive controls (PC) should be used in each run to demonstrate that viability (with the NC), barrier function and resulting tissue sensitivity (with the PC) of the tissues are within a defined historical acceptance range. The suggested PC chemical is 5% aqueous SDS. The suggested NC chemicals are water or phosphate buffered saline (PBS).Cell Viability Measurements24. The most important element of the test procedure is that viability measurements are not performed immediately after the exposure to the test chemicals, but after a sufficiently long post-treatment incubation period of the rinsed tissues in fresh medium. This period allows both for recovery from weak cytotoxic effects and for appearance of clear cytotoxic effects. The test optimisation phase (11) (12) (13) (14) (15) demonstrated that a 42 hours post-treatment incubation period was optimal.25. The MTT assay is a validated quantitative method which should be used to measure cell viability under this Test Guideline. It is compatible with use in a three-dimensional tissue construct. The tissue sample is placed in MTT solution of appropriate concentration (e.g.0.3 - 1 mg/mL) for 3 hours. The precipitated blue formazan product is then extracted from the tissue using a solvent (e.g.isopropanol, acidic isopropanol), and the concentration of formazan is measured by determining the OD at 570 nm using a filter band pass of maximum ± 30 nm.26.Optical properties of the test chemical or its chemical action on the MTT may interfere with the assay leading to a false estimate of viability (because the test chemical may prevent or reverse the colour generation as well as cause it). This may occur when a specific test chemical is not completely removed from the tissue by rinsing or when it penetrates the epidermis. If a test chemical acts directly on the MTT (MTT-reducer), is naturally coloured, or becomes coloured during tissue treatment, additional controls should be used to detect and correct for test chemical interference with the viability measurement technique. Detailed description of how to correct direct MTT reduction and interferences by colouring agents is available in the SOPs for the three validated test methods (26)(27)(28).Acceptability Criteria27. For each test method using valid RhE model batches (see paragraph 21), tissues treated with the NC should exhibit OD reflecting the quality of the tissues that followed shipment, receipt steps and all protocol processes. Control OD values should not be below historically established boundaries. Similarly, tissues treated with the PC, i.e.5% aqueous SDS, should reflect their ability to respond to an irritant chemical under the conditions of the test method (26) (27) (28). Associated and appropriate measures of variability between tissue replicates should be defined (e.g.if standard deviations (SD) are used they should be within the 1-sided 95% tolerance interval calculated from historical data; for the VRM SD < 18%).Interpretation of Results and Prediction Model28. The OD values obtained with each test chemical can be used to calculate the percentage of viability normalised to NC, which is set to 100%. The cut-off value of percentage cell viability distinguishing irritant from non-classified test chemicals and the statistical procedure(s) used to evaluate the results and identify irritant chemicals should be clearly defined, documented, and proven to be appropriate. The cut-off values for the prediction of irritation are given below:The test chemical is considered to be irritant to skin in accordance with UN GHS Category 2 ifthe tissue viability after exposure and post-treatment incubation is less than or equal (≤) to 50%.© OCDE, (2010) 6OECD/OCDE4397© OECD, (2010)Depending on the regulatory framework in member countries, the test chemical may be considered as non-irritant DATA AND REPORTINGto skin in accordance with UN GHS No Category if the tissue viabilityafter exposure and post-treatment incubation is more than (>) 50%.Data29. For each run, data from individual replicate tissues (e.g. OD values and calculated percentage cell viability data for each test chemical, including classification) should be reported in tabular form, including data from repeat experiments as appropriate. In addition means ± SD for each run should be reported. Observed interactions with MTT reagent and coloured test chemicals should be reported for each tested chemical.Test Report30.The test report should include the following information: Test and Control Chemicals:- C hemical name(s) such as CAS name and number, if known;- P urity and composition of the chemical (in percentage(s) by weight);-Physical-chemical properties relevant to the conduct of the study (e.g. physical state, stability,volatility, pH and water solubility if known);-Treatment of the test/control chemicals prior to testing, if applicable (e.g. warming,grinding);- S torage conditions;Justification of the RhE model and protocol used Test Conditions:- C ell system used;- C omplete supporting information for the specific RhE model used including its performance.This should include, but is not limited to;i) viabilityii) barrier functioniii) morphologyiv) reproducibility and predictivityv) Quality controls (QC) of the model- D etails of the test procedure used;- T est doses used, duration of exposure and post treatment incubation period;- D escription of any modifications of the test procedure;- R eference to historical data of the model. This should include, but is not limited to:i) acceptability of the QC data with reference to historical batch dataii) acceptability of the positive and negative control values with reference to positive andnegative control means and ranges439OECD/OCDE-D escription of evaluation criteria used including the justification for the selection of the cut-off point(s) for the prediction model;-Reference to historical control data;Results:-T abulation of data from individual test chemicals for each run and each replicatemeasurement;-I ndication of controls used for direct MTT-reducers and/or colouring test chemicals;-D escription of other effects observed;Discussion of the resultsConclusion© OCDE, (2010)8OECD/OCDE439LITERATURE1.UN (2009), United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS), Third revised edition, UN New York and Geneva. Available at:[/trans/danger/publi/ghs/ghs_rev03/03files_e.html]2.EC-ECVAM (2009), Statement on the “Performance under UN GHS of three in vitro assays forskin irritation testing and the adaptation of the Reference Chemicals and Defined Accuracy Values of theECVAM skin irritation Performance Standards”, issued by the ECVAM Scientific Advisory Committee(ESAC30), 9 April 2009. Available at: [http://ecvam.jrc.ec.europa.eu]3.EC (2008), REGULATION (EC) No 1272/2008 of the European Parliament and of the Councilof 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending andrepealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OfficialJournal of the European Union L353, 1-1355.4.OECD (2004), Acute Dermal Irritation/Corrosion, OECD Guideline for the Testing of Chemicals No. 404, OECD, Paris. Available at: [/env/testguidelines]5.OECD (2004), In Vitro Skin Corrosion: Transcutaneous Electrical Resistance (TER),OECDGuideline for the Testing of Chemicals No. 430, OECD, Paris. Available at:[/env/testguidelines]6.OECD (2004), In Vitro Skin Corrosion: Human Skin Model Test, OECD Guideline for theTesting of Chemicals No. 431, OECD, Paris. Available at: [/env/testguidelines]7.OECD (2006), In Vitro Membrane Barrier Test Method for Skin Corrosion, OECD Guideline forthe Testing of Chemicals No. 435, OECD, Paris. Available at: [/env/testguidelines]8.EC-ECVAM (2009), Performance Standards for in vitro skin irritation test methods based on Reconstructed human Epidermis (RhE)? Available at: [http://ecvam.jrc.ec.europa.eu]9.OECD (2005), Guidance Document on the Validation and International Acceptance of New orUpdated Test Methods for Hazard Assessment, OECD Series on Testing and Assessment No. 34, OECD,Paris. Available at: [/env/testguidelines]10.Fentem, J.H., Briggs, D., Chesné, C., Elliot, G.R., Harbell, J.W., Heylings, J.R., Portes, P.,Roguet, R., van de Sandt, J.J. M. and Botham, P. (2001), A prevalidation study on in vitro tests for acuteskin irritation, Results and evaluation by the Management Team, Toxicol. in Vitro 15, 57-93.11.Portes, P., Grandidier, M.-H., Cohen, C. and Roguet, R. (2002), Refinement of the EPISKINprotocol for the assessment of acute skin irritation of chemicals: follow-up to the ECVAM prevalidationstudy, Toxicol. in Vitro 16, 765–770.12.Kandárová, H., Liebsch, M., Genschow, E., Gerner, I., Traue, D., Slawik, B. and Spielmann, H. (2004), Optimisation of the EpiDerm test protocol for the upcoming ECVAM validation study on in vitroskin irritation tests, ALTEX 21, 107–114.13.Kandárová, H., Liebsch, M., Gerner, I., Schmidt, E., Genschow, E., Traue, D. and Spielmann, H. (2005), The EpiDerm test protocol for the upcoming ECVAM validation study on in vitro skin irritationtests – An assessment of the performance of the optimised test, ATLA 33, 351-367.9© OECD, (2010)439OECD/OCDE14.Cotovio, J., Grandidier, M.-H., Portes, P., Roguet, R. and Rubinsteen, G. (2005), The in vitro acute skin irritation of chemicals: optimisation of the EPISKIN prediction model within the framework of the ECVAM validation process, ATLA 33, 329-349.15.Zuang, V., Balls, M., Botham, P.A., Coquette, A., Corsini, E., Curren, R.D., Elliot, G.R., Fentem, J.H., Heylings, J.R., Liebsch, M., Medina, J., Roguet, R., van De Sandt, J.J.M., Wiemann, C. and Worth, A. (2002), Follow-up to the ECVAM prevalidation study on in vitro tests for acute skin irritation, The European Centre for the Validation of Alternative Methods Skin Irritation Task Force report 2, ATLA 30, 109-129.16.Spielmann, H., Hoffmann, S., Liebsch, M., Botham, P., Fentem, J., Eskes, C., Roguet, R., Cotovio, J., Cole, T., Worth, A., Heylings, J., Jones, P., Robles, C., Kandárová, H., Gamer, A., Remmele, M., Curren, R., Raabe, H., Cockshott, A., Gerner, I. and Zuang, V. (2007), The ECVAM international validation study on in vitro tests for acute skin irritation: Report on the validity of the EPISKIN and EpiDerm assays and on the skin integrity function test, ATLA 35, 559-601.17.Hoffmann, S. (2006), ECVAM skin irritation validation study phase II: Analysis of the primary endpoint MTT and the secondary endpoint IL1-α. Available at: [http://ecvam.jrc.ec.europa.eu]18.Eskes, C., Cole, T., Hoffmann, S., Worth, A., Cockshott, A., Gerner, I. and Zuang, V. (2007), The ECVAM international validation study on in vitro tests for acute skin irritation: selection of test chemicals, ATLA 35, 603-619.19.Cotovio, J., Grandidier, M.-H., Lelièvre, D., Roguet, R., Tinois-Tessonneaud, E. and Leclaire, J. (2007), In vitro acute skin irritancy of chemicals using the validated EPISKIN model in a tiered strategy - Results and performances with 184 cosmetic ingredients, AATEX, 14, 351-358.20.EC-ECVAM (2007), Statement on the validity of in vitro tests for skin irritation, issued by the ECVAM Scientific Advisory Committee (ESAC26), 27 April 2007. Available at: [http://ecvam.jrc.ec.europa.eu]21.EC-ECVAM (2007), Performance Standards for applying human skin models to in vitro skin irritation testing. Available at: [http://ecvam.jrc.ec.europa.eu]22.EC-ECVAM (2008), Statement on the scientific validity of in vitro tests for skin irritation testing, issued by the ECVAM Scientific Advisory Committee (ESAC29), 5 November 2008. Available at: [http://ecvam.jrc.ec.europa.eu]23.OECD (20xx), Explanatory background document to the OECD draft Test Guideline on in vitro skin irritation testing. To be published in OECD Series on Testing and Assessment, No. 1XX, OECD, Paris. Available at:24.Welss, T., Basketter, D.A. and Schröder, K.R. (2004), In vitro skin irritation: fact and future. State of the art review of mechanisms and models, Toxicol. in Vitro 18, 231-243.25.Mosmann, T. (1983), Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods 65, 55-63.26.EpiSkin™ SOP, Version 1.8 (February 2009), ECVAM Skin Irritation Validation Study: Validation of the EpiSkin™ test method 15 min - 42 hours for the prediction of acute skin irritation of chemicals. Available at: [http://ecvam.jrc.ec.europa.eu]© OCDE, (2010)1027.EpiDerm™ SOP, Version 7.0 (Revised March 2009), Protocol for: In vitro EpiDerm™ skin irritation test (EPI-200-SIT), For use with MatTek Corporation's reconstructed human epidermal model EpiDerm (EPI-200). Available at: [http://ecvam.jrc.ec.europa.eu]28.SkinEthic™ RHE SOP, Version 2.0 (February 2009), SkinEthic skin irritation test-42bis test method for the prediction of acute skin irritation of chemicals: 42 minutes application + 42 hours post-incubation. Available at: [http://ecvam.jrc.ec.europa.eu]29.Harvell, J.D., Lamminstausta, K., and Maibach, H.I. (1995), Irritant contact dermatitis, In: Practical Contact Dermatitis, pp 7-18, (Ed. Guin J. D.). Mc Graw-Hill, New York.30.EC (2001), Commission Directive 2001/59/EC of 6 August 2001 adapting to technical progress for the 28th time Council Directive 67/548/EEC on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances, Official Journal of the European Union L225, 1-333.31.Basketter, D.A., York, M., McFadden, J.P. and Robinson, M.K. (2004), Determination of skin irritation potential in the human 4-h patch test. Contact Dermatitis 51, 1-4.32.Jirova, D., Liebsch, M., Basketter, D., Spiller, E., Kejlova, K., Bendova, H., Marriott, M. and Kandarova, H. (2007), Comparison of human skin irritation and photo-irritation patch test data with cellular in vitro assays and animal in vivo data, AATEX, 14, 359-365.33.Jírová, D., Basketter, D., Liebsch, M., Bendová, H., Kejlová, K., Marriott, M. and Kandárová, H. (2010), Comparison of human skin irritation patch test data with in vitro skin irritation assays and animal data, Contact Dermatitis, 62, 109-116.11。
微生物屏障试验 DIN 58953-6_2010 Test report

Interlaboratory T est …Microbial barrier testing of packa ging materials for medical devices which are tobe ster ili ze d“according to DIN 58953-6:2010Test re portJanuary 2013Author: Daniel ZahnISEGA Forschungs- und Untersuchungsgesellschaft mbHTest report Page 2 / 15Table of contentsSeite1.General information on the Interlaboratory Test (3)1.1 Organization (3)1.2 Occasion and Objective (3)1.3 Time Schedule (3)1.4 Participants (4)2.Sample material (4)2.1 Sample Description and Execution of the Test (4)2.1.1 Materials for the Analysis of the Germ Proofness under Humidityaccording to DIN 58953-6, section 3 (5)2.1.2 Materials for the Analysis of the Germ Proofness with Air Permeanceaccording to DIN 58953-6, section 4 (5)2.2 Sample Preparation and Despatch (5)2.3 Additional Sample and Re-examination (6)3.Results (6)3.1 Preliminary Remark (6)3.2 Note on the Record of Test Results (6)3.3 Comment on the Statistical Evaluation (6)3.4 Outlier tests (7)3.5 Record of Test Results (7)3.5.1 Record of Test Results Sample F1 (8)3.5.2 Record of Test Results Sample F2 (9)3.5.3 Record of Test Results Sample F3 (10)3.5.4 Record of Test Results Sample L1 (11)3.5.5 Record of Test Results Sample L2 (12)3.5.6 Record of Test Results Sample L3 (13)3.5.7 Record of Test Results Sample L4 (14)4.Overview and Summary (15)Test report Page 3 / 15 1. General Information on the Interlaboratory Test1.1 OrganizationOrganizer of the Interlaboratory Test:Sterile Barrier Association (SBA)Mr. David Harding (director.general@)Pennygate House, St WeonardsHerfordshire HR2 8PT / Great BritainRealization of the Interlaboratory Test:Verein zur Förderung der Forschung und Ausbildung fürFaserstoff- und Verpackungschemie e. V. (VFV)vfv@isega.dePostfach 10 11 0963707 Aschaffenburg / GermanyTechnical support:ISEGA Forschungs- u. Untersuchungsgesellschaft mbHDr. Julia Riedlinger / Mr. Daniel Zahn (info@isega.de)Zeppelinstraße 3 – 563741 Aschaffenburg / Germany1.2 Occasion and ObjectiveIn order to demonstrate compliance with the requirements of the ISO 11607-1:2006 …Packaging for terminally sterilized medical devices -- Part 1: Requirements for materials, sterile barrier systems and packaging systems“ validated test methods are to be preferably utilized.For the confirmation of the microbial barrier properties of porous materials demanded in the ISO 11607-1, the DIN 58953-6:2010 …Sterilization – Sterile supply – Part 6: Microbial barrier testing of packaging materials for medical devices which are to be sterilized“ represents a conclusive method which can be performed without the need for extensive equipment.However, since momentarily no validation data on DIN 58953-6 is at hand concerns emerged that the method may lose importance against validated methods in a revision of the ISO 11607-1 or may even not be considered at all.Within the framework of this interlaboratory test, data on the reproducibility of the results obtained by means of the analysis according to DIN 58953-6 shall be gathered.1.3 Time ScheduleSeptember 2010:The Sterile Barrier Association queried ISEGA Forschungs- und Unter-suchungsgesellschaft about the technical support for the interlaboratory test.For the realization, the Verein zur Förderung der Forschung und Ausbildungfür Faserstoff- und Verpackungschemie e. V. (VFV) was won over.November 2010: Preliminary announcement of the interlaboratory test / Seach for interested laboratoriesTest report Page 4 / 15 January toDecember 2011: Search for suitable sample material / Carrying out of numerous pre-trials on various materialsJanuary 2012:Renewed contact or search for additional interested laboratories, respectively February 2012: Sending out of registration forms / preparation of sample materialMarch 2012: Registration deadline / sample despatchMay / June 2012: Results come in / statistical evaluationJuly 2012: Despatch of samples for the re-examinationSeptember 2012: Results of the re-examination come in / statistical evaluationNovember 2012: Results are sent to the participantsDecember 2012/January 2013: Compilation of the test report1.4 ParticipantsFive different German laboratories participated in the interlaboratory test. In one laboratory, the analyses were performed by two testers working independently so that six valid results overall were received which can be taken into consideration in the evaluation.To ensure an anonymous evaluation of the results, each participant was assigned a laboratory number (laboratory 1 to laboratory 6) in random order, which was disclosed only to the laboratory in question. The complete laboratory number breakdown was known solely by the ISEGA staff supporting the proficiency test.2. Sample Material2.1 Sample Description and Execution of the TestUtmost care in the selection of suitable sample material was taken to include different materials used in the manufacture of packaging for terminally sterilized medical devices.With the help of numerous pre-trials the materials were chosen covering a wide range of results from mostly germ-proof samples to germ permeable materials.Test report Page 5 / 15 2.1.1 Materials for the Analysis of Germ Proofness under Humidity according to DIN 58953-6, section 3:The participants were advised to perform the analysis on the samples according to DIN 58953-6, section 3, and to protocol their findings on the provided result sheets.The only deviation from the norm was that in case of the growth of 1 -5 colony-forming units (in the following abbreviated as CFU) per sample, no re-examination 20 test pieces was performed.2.1.2 Materials for the Analysis of Germ Proofness with Air Permeance according to DIN 58953-6, section 4:The participants were advised to perform the analysis on the samples according to DIN 58953-6, section 4, and to protocol their findings on the provided result sheets.2.2 Sample Preparation and DespatchFor the analysis of the germ proofness under humidity, 10 test pieces in the size of 50 x 50 mm were cut out of each sample and heat-sealed into a sterilization pouch with the side to be tested up.Out of the 10 test pieces, 5 were intended for the testing and one each for the two controls according to DIN 58953-6, sections 3.6.2 and 3.6.3. The rest should remain as replacements (e.g. in case of the dropping of a test piece on the floor etc.).For the analysis of the germ proofness with air permeance, 15 circular test pieces with a diameter of 40 mm were punched out of each sample and heat-sealed into a sterilization pouch with the side to be tested up.Test report Page 6 / 15 Out of the 15 test pieces, 10 were intended for the testing and one each for the two controls according to DIN 58953-6, section 4.9. The rest should remain as replacements (e.g. in case of the dropping of a test piece on the floor etc.).The sterilization pouches with the test pieces were steam-sterilized in an autoclave for 15 minutes at 121 °C and stored in an climatic room at 23 °C and 50 % relative humidity until despatch.2.3 Additional Sample and Re-examinationFor the analysis of the germ proofness under humidity another test round was performed in July / August 2012. For this, an additional sample (sample L4) was sent to the laboratories and analysed (see 2.1.2). The results were considered in the evaluation.For validation or confirmation of non-plausible results, occasional samples for re-examination were sent out to the laboratories. The results of these re-examinations (July / August 2012) were not taken into consideration in the evaluation.3. Results3.1 Preliminary RemarkSince the analysis of germ proofness is designed to be a pass / fail – test, the statistical values and precision data were meant only to serve informative purposes.The evaluation of the materials according to DIN58953-6,sections 3.7and 4.7.6by the laboratories should be the most decisive criterion for the evaluation of reproducibility of the interlaboratory test results. Based on this, the classification of a sample as “sufficiently germ-proof” or “not sufficiently germ-proof” is carried out.3.2 Note on the Record of Test Results:The exact counting of individual CFUs is not possible with the required precision if the values turn out to be very high. Thus, an upper limit of 100 CFU per agar plate or per test pieces, respectively, was defined. Individual values above this limit and values which were stated with “> 100” by the laboratories, are listed as 100 CFU per agar plate or per test piece, respectively, in the evaluation.Test report Page 7 / 153.3 Comment on the Statistical EvaluationThe statistical evaluation was done based on the series of standards DIN ISO 5725-1ff.The arithmetic laboratory mean X i and the laboratory standard deviation s i were calculated from the individual measurement values obtained by the laboratories.The overall mean X of the laboratory means as well as the precision data of the method (reproducibility and repeatability) were determined for each sample3.4 Outlier testsThe Mandel's h-statistics test was utilised as outlier test for differences between the laboratory means of the participants.A laboratory was identified as a “statistical outlier” as soon as an exceedance of Mandel's h test statistic at the 1 % significance level was detected.The respective results of the laboratories identified as outliers were not considered in the statistical evaluation.3.5 Record of Test ResultsOn the following pages, the records of the test results for each interlaboratory test sample with the statistical evaluation and the evaluation according to DIN 58953-6 are compiled.Test report Page 8 / 153.5.1 Record of Test Results Sample F1Individual Measurement values:Statistical Evaluation:Comment:Laboratory 4, as an outlier, has not been taken into consideration in the statistical Evaluation.Outlier criterion: Mandel's h-statistics (1 % level of significance)Overall mean X:91.0CFU / agar plateRepeatability standard deviation s r:17.9CFU / agar plateReproducibility standard deviation s R:19.8CFU / agar plateRepeatability r:50.0CFU / agar plateRepeatability coefficient of variation:19.6%Reproducibility R:55.5CFU / agar plateReproducibility coefficient of variation:21.8%Evaluation according to DIN 58953-6, Section 3.7:Lab. 1 - 6:Number of CFU > 5, i.e. the material is classified as not sufficiently germ-proof.Conclusion:All of the participants, even the Laboratory 4 which was identified as an outlier, came to the same results and would classify the sample material as “not sufficiently germ-proof”Test report Page 9 / 153.5.2 Record of Test Results Sample F2Individual Measurement values:Statistical Evaluation:Comment:Laboratory 4, as an outlier, has not been taken into consideration in the statistical Evaluation.Outlier criterion: Mandel's h-statistics (1 % level of significance)Overall mean X:0CFU / agar plateRepeatability standard deviation s r:0CFU / agar plateReproducibility standard deviation s R:0CFU / agar plateRepeatability r:0CFU / agar plateRepeatability coefficient of variation:0%Reproducibility R:0CFU / agar plateReproducibility coefficient of variation:0%Evaluation according to DIN 58953-6, Section 3.7:Lab. 1 – 3:Number of CFU = 0, i.e. the material is classified as sufficiently germ-proofLab. 4:Number of CFU ≤ 5, i.e. a re-examination on 20 test pieces would have to be done Lab. 5 – 6:Number of CFU = 0, i.e. the material is classified as sufficiently germ-proofConclusion:All of the participants, except for the Laboratory 4 which was identified as an outlier, came to the same results and would classify the sample material as “sufficiently germ-proof”.Test report Page 10 / 153.5.3 Record of Test Results Sample F3Individual Measurement values:Statistical Evaluation:Overall mean X:30.1CFU / agar plateRepeatability standard deviation s r:17.2CFU / agar plateReproducibility standard deviation s R:30.9CFU / agar plateRepeatability r:48.2CFU / agar plateRepeatability coefficient of variation:57.1%Reproducibility R:86.5CFU / agar plateReproducibility coefficient of variation:103%Evaluation according to DIN 58953-6, Section 3.7:Lab. 1 - 4:Number of CFU > 5, i.e. the material is classified as not sufficiently germ-proof. Lab. 5:Number of CFU = 0, i.e. the material is classified as sufficiently germ-proof. Lab. 6:Number of CFU > 5, i.e. the material is classified as not sufficiently germ-proof.Conclusion:Five of the six participants came to the same result and would classify the sample as “not sufficiently germ-proof”. Only laboratory 5 would classify the sample material as “sufficiently germ-proof”.Test report Page 11 / 153.5.4 Record of Test Results Sample L1Individual Measurement values:Statistical Evaluation:Overall mean X:0.09CFU / test pieceRepeatability standard deviation s r:0.32CFU / test pieceReproducibility standard deviation s R:0.33CFU / test pieceRepeatability r:0.91CFU / test pieceRepeatability coefficient of variation:357%Reproducibility R:0.93CFU / test pieceReproducibility coefficient of variation:366%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1 - 6:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof.Conclusion:All participants came to the same result and would classify the sample as “sufficiently germ-proof”.Test report Page 12 / 153.5.5 Record of Test Results Sample L2Individual Measurement values:Statistical Evaluation:Overall mean X:0.73CFU / test pieceRepeatability standard deviation s r: 1.10CFU / test pieceReproducibility standard deviation s R: 1.18CFU / test pieceRepeatability r: 3.07CFU / test pieceRepeatability coefficient of variation:151%Reproducibility R: 3.32CFU / test pieceReproducibility coefficient of variation:163%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1:Number of CFU > 15, i.e. the material is classified as not sufficiently germ-proof. Lab. 2 - 6:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof.Conclusion:Five of the six participants came to the same result and would classify the sample as “sufficiently germ-proof”. Only laboratory 1 exceeds the limit value slightly by 1 CFU, so that the sample would be classified as “not sufficiently germ-proof”.Test report Page 13 / 153.5.6 Record of Test Results Sample L3Individual Measurement values:Statistical Evaluation:Overall mean X:0.36CFU / test pieceRepeatability standard deviation s r: 1.00CFU / test pieceReproducibility standard deviation s R: 1.06CFU / test pieceRepeatability r: 2.79CFU / test pieceRepeatability coefficient of variation:274%Reproducibility R: 2.98CFU / test pieceReproducibility coefficient of variation:293%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1 - 6:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof.Conclusion:All participants came to the same result and would classify the sample as “sufficiently germ-proof”.Test report Page 14 / 153.5.7 Record of Test Results Sample L4Individual Measurement values:Statistical Evaluation:Overall mean X:35.1CFU / test pieceRepeatability standard deviation s r:18.8CFU / test pieceReproducibility standard deviation s R:42.6CFU / test pieceRepeatability r:52.7CFU / test pieceRepeatability coefficient of variation:53.7%Reproducibility R:119CFU / test pieceReproducibility coefficient of variation:122%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1 - 3:Number of CFU > 15, i.e. the material is classified as not sufficiently germ-proof. Lab. 4:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof. Lab. 5 - 6:Number of CFU > 15, i.e. the material is classified as not sufficiently germ-proof.Conclusion:Five of the six participants came to the same result and would classify the sample as“not sufficiently germ-proof”.Test report Page 15 / 15 4. Overview and SummarySummary:In case of four of the overall seven tested materials, a 100 % consensus was reached regarding the evaluation as“sufficiently germ-proof”and“not sufficiently germ-proof”according to DIN 58 953-6.As for the other three tested materials, there were always 5 concurrent participants out of 6 (83 %). In each case, only one laboratory would have evaluated the sample differently.It is noteworthy that the materials about the evaluation of which a 100 % consensus was reached were the smooth sterilization papers. The differences with one deviating laboratory each occurred with the slightly less homogeneous materials, such as with the creped paper and the nonwoven materials.。
STATEC 2011设备说明

0~999.9mV(Step 0.1 mV, Accuracy :ADC 16Bits) 2 Station Multiplexing AC Power:210~250VAC,50/60HZ,1Phase DC Power Supply:,+5V/+12V, ±15V,±24V DM2000A:±24V 2000B:±36V, Voltage control(40V~220V) 440mm(W) * 620 mm(D) * 600mm(H) 50Kg
Wide range VDS/VCB (1 to 200V) High Accuracy Sample & Holed (ADC 16Bits) Easy Operation & Maintenance Quick Set-up Installation Windows XP based Operation IE/ID – DM2000A:20A
Copyright © 2010 STATEC Co.,Ltd.
www.statec.co.kr
Ⅱ. Hardware
1. Discrete test system- DM2000A/B
The DM2000 test system series is used to measure the thermal resistance characteristics of Diodes, transistors, MOSFETs ,IGBTs The thermal resistance characteristics of the MOSFETs, Transistors, etc are measured as the temperature change (㎷) of the PN junction. Consideration of a contact check function and an oscillation detection function to prevent the wrong measurement. DM2000 is designed to have 2-channel multiplexing capability.
深圳市兴安科检测技术有限公司
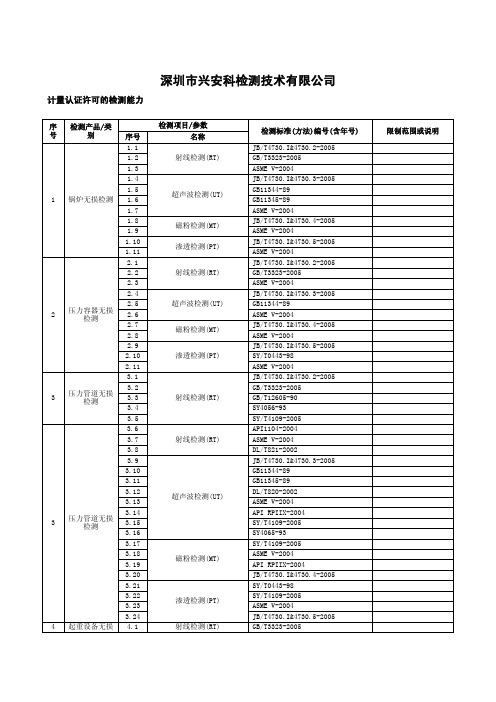
深圳市兴安科检测技术有限公司计量认证许可的检测能力序号 检测产品/类别检测项目/参数检测标准(方法)编号(含年号) 限制范围或说明 序号 名称1 锅炉无损检测1.1射线检测(RT)JB/T4730.I&4730.2-2005 1.2 GB/T3323-20051.3 ASME V-20041.4超声波检测(UT)JB/T4730.I&4730.3-2005 1.5 GB11344-891.6 GB11345-891.7 ASME V-20041.8磁粉检测(MT)JB/T4730.I&4730.4-2005 1.9 ASME V-20041.10渗透检测(PT)JB/T4730.I&4730.5-2005 1.11 ASME V-20042 压力容器无损检测2.1射线检测(RT)JB/T4730.I&4730.2-20052.2 GB/T3323-20052.3 ASME V-20042.4超声波检测(UT)JB/T4730.I&4730.3-20052.5 GB11344-892.6 ASME V-20042.7磁粉检测(MT)JB/T4730.I&4730.4-20052.8 ASME V-20042.9渗透检测(PT)JB/T4730.I&4730.5-20052.10 SY/T0443-982.11 ASME V-20043 压力管道无损检测3.1射线检测(RT)JB/T4730.I&4730.2-20053.2 GB/T3323-20053.3 GB/T12605-903.4 SY4056-933.5 SY/T4109-20053 压力管道无损检测3.6射线检测(RT)API1104-20043.7 ASME V-20043.8 DL/T821-20023.9超声波检测(UT)JB/T4730.I&4730.3-20053.10 GB11344-893.11 GB11345-893.12 DL/T820-20023.13 ASME V-20043.14 API RPIIX-20043.15 SY/T4109-20053.16 SY4065-933.17磁粉检测(MT)SY/T4109-20053.18 ASME V-20043.19 API RPIIX-20043.20 JB/T4730.I&4730.4-20053.21渗透检测(PT)SY/T0443-983.22 SY/T4109-20053.23 ASME V-20043.24 JB/T4730.I&4730.5-20054 起重设备无损 4.1 射线检测(RT) GB/T3323-20054.2 AWS DI.I/DI.IM:2004 4.3 GB/T12605-904.4超声波检测(UT) GB11344-894.5 AWS DI.I/DI.IM:2004 4.6 GB11345-894.7磁粉检测(MT) AWS DI.I/DI.IM:20044.8 JB/T6061-924.9渗透检测(PT) JB?T6062-924.10 AWS DI.I/DI.IM:20045 船舶无损检测 5.1射线检测(RT)GB/T3177-93 5.2 GB/T3558-93 5.3超声波检测(UT)GB/T3177-93 5.4 GB11344-89 5.5 GB/T3559-93 5.6 磁粉检测(MT) GB/T3958-2004 5.7 渗透检测(PT) GB/T3958-20046 钢结构无损检测6.1射线检测(RT)BS EN1435:19976.2 AWS DI.I/DI.IM:20046.3 BS2600-19836.4 GB/T3323-20056.5 GB/T2605-906.6超声波检测(UT)BS EN1714:19986.7 AWS DI.I/DI.IM:20046.8 BS3923-19866.9 GB11344-896.10 GB11345-896.11磁粉检测(MT)JB/T6061-926.12 AWS DI.I/DI.IM:20046.13 PSCO 10.08.046.14 PSCO 10.286.15 BS6072-19816.16 BS EN1290:19986.17渗透检测(PT)JB/T6062-926.18 AWS DI.I/DI.IM:20046.19 PSCO 10.276.20 BS EN571-1:19976.21外观检查(VT)BS EN970:19976.22 BS5135-19847 金属原材料、零部件无损检测7.1射线检测(RT)BS EN1435:19977.2 AWS DI.I-20047.3 GB/T3323-20057.4 GB/T12605-907.5超声波检测(UT)JB/T10554-20067.6 JB/T10555-20067.7 AWS DI.I-20047.8 GB11344-897.9 Pr EN12680-20007.10 BS EN1714:19987.11 DL/T694-19997.12 GB11345-897.13 JG/T-3034.1-19967.14 JG/T3034.2-19967.15磁粉检测(MT)JB/T6061-927.16 AWS DI.I-20047.17 BS EN1290:1998 7.18 PSCO 10.08.04 7.19 PSCO 10.287.20渗透检测(PT) JB/T6062-927.21 AWS DI.I-20047.22 BS EN571-1:19977.23 PSCO 10.277.24 外观检查(VT) BS EN970:19978 金属光谱分析 8.1 光谱分析 DL/T991-20069 金属硬度试验 9.1 硬度试验 GB/T17394-1998。
微生物屏障试验DIN58953-62010Testreport

Interlaboratory T est …Microbial barrier testing of packa ging materials for medical devices which are tobe ster ili ze d“according to DIN 58953-6:2010Test re portJanuary 2013Author: Daniel ZahnISEGA Forschungs- und Untersuchungsgesellschaft mbHTest report Page 2 / 15Table of contentsSeite1.General information on the Interlaboratory Test (3)1.1 Organization (3)1.2 Occasion and Objective (3)1.3 Time Schedule (3)1.4 Participants (4)2.Sample material (4)2.1 Sample Description and Execution of the Test (4)2.1.1 Materials for the Analysis of the Germ Proofness under Humidityaccording to DIN 58953-6, section 3 (5)2.1.2 Materials for the Analysis of the Germ Proofness with Air Permeanceaccording to DIN 58953-6, section 4 (5)2.2 Sample Preparation and Despatch (5)2.3 Additional Sample and Re-examination (6)3.Results (6)3.1 Preliminary Remark (6)3.2 Note on the Record of Test Results (6)3.3 Comment on the Statistical Evaluation (6)3.4 Outlier tests (7)3.5 Record of Test Results (7)3.5.1 Record of Test Results Sample F1 (8)3.5.2 Record of Test Results Sample F2 (9)3.5.3 Record of Test Results Sample F3 (10)3.5.4 Record of Test Results Sample L1 (11)3.5.5 Record of Test Results Sample L2 (12)3.5.6 Record of Test Results Sample L3 (13)3.5.7 Record of Test Results Sample L4 (14)4.Overview and Summary (15)Test report Page 3 / 15 1. General Information on the Interlaboratory Test1.1 OrganizationOrganizer of the Interlaboratory Test:Sterile Barrier Association (SBA)Mr. David Harding (director.general@)Pennygate House, St WeonardsHerfordshire HR2 8PT / Great BritainRealization of the Interlaboratory Test:Verein zur Förderung der Forschung und Ausbildung fürFaserstoff- und Verpackungschemie e. V. (VFV)vfv@isega.dePostfach 10 11 0963707 Aschaffenburg / GermanyTechnical support:ISEGA Forschungs- u. Untersuchungsgesellschaft mbHDr. Julia Riedlinger / Mr. Daniel Zahn (info@isega.de)Zeppelinstraße 3 – 563741 Aschaffenburg / Germany1.2 Occasion and ObjectiveIn order to demonstrate compliance with the requirements of the ISO 11607-1:2006 …Packaging for terminally sterilized medical devices -- Part 1: Requirements for materials, sterile barrier systems and packaging systems“ validated test methods are to be preferably utilized.For the confirmation of the microbial barrier properties of porous materials demanded in the ISO 11607-1, the DIN 58953-6:2010 …Sterilization – Sterile supply – Part 6: Microbial barrier testing of packaging materials for medical devices which are to be sterilized“ represents a conclusive method which can be performed without the need for extensive equipment.However, since momentarily no validation data on DIN 58953-6 is at hand concerns emerged that the method may lose importance against validated methods in a revision of the ISO 11607-1 or may even not be considered at all.Within the framework of this interlaboratory test, data on the reproducibility of the results obtained by means of the analysis according to DIN 58953-6 shall be gathered.1.3 Time ScheduleSeptember 2010:The Sterile Barrier Association queried ISEGA Forschungs- und Unter-suchungsgesellschaft about the technical support for the interlaboratory test.For the realization, the Verein zur Förderung der Forschung und Ausbildungfür Faserstoff- und Verpackungschemie e. V. (VFV) was won over.November 2010: Preliminary announcement of the interlaboratory test / Seach for interested laboratoriesTest report Page 4 / 15 January toDecember 2011: Search for suitable sample material / Carrying out of numerous pre-trials on various materialsJanuary 2012:Renewed contact or search for additional interested laboratories, respectively February 2012: Sending out of registration forms / preparation of sample materialMarch 2012: Registration deadline / sample despatchMay / June 2012: Results come in / statistical evaluationJuly 2012: Despatch of samples for the re-examinationSeptember 2012: Results of the re-examination come in / statistical evaluationNovember 2012: Results are sent to the participantsDecember 2012/January 2013: Compilation of the test report1.4 ParticipantsFive different German laboratories participated in the interlaboratory test. In one laboratory, the analyses were performed by two testers working independently so that six valid results overall were received which can be taken into consideration in the evaluation.To ensure an anonymous evaluation of the results, each participant was assigned a laboratory number (laboratory 1 to laboratory 6) in random order, which was disclosed only to the laboratory in question. The complete laboratory number breakdown was known solely by the ISEGA staff supporting the proficiency test.2. Sample Material2.1 Sample Description and Execution of the TestUtmost care in the selection of suitable sample material was taken to include different materials used in the manufacture of packaging for terminally sterilized medical devices.With the help of numerous pre-trials the materials were chosen covering a wide range of results from mostly germ-proof samples to germ permeable materials.Test report Page 5 / 15 2.1.1 Materials for the Analysis of Germ Proofness under Humidity according to DIN 58953-6, section 3:The participants were advised to perform the analysis on the samples according to DIN 58953-6, section 3, and to protocol their findings on the provided result sheets.The only deviation from the norm was that in case of the growth of 1 -5 colony-forming units (in the following abbreviated as CFU) per sample, no re-examination 20 test pieces was performed.2.1.2 Materials for the Analysis of Germ Proofness with Air Permeance according to DIN 58953-6, section 4:The participants were advised to perform the analysis on the samples according to DIN 58953-6, section 4, and to protocol their findings on the provided result sheets.2.2 Sample Preparation and DespatchFor the analysis of the germ proofness under humidity, 10 test pieces in the size of 50 x 50 mm were cut out of each sample and heat-sealed into a sterilization pouch with the side to be tested up.Out of the 10 test pieces, 5 were intended for the testing and one each for the two controls according to DIN 58953-6, sections 3.6.2 and 3.6.3. The rest should remain as replacements (e.g. in case of the dropping of a test piece on the floor etc.).For the analysis of the germ proofness with air permeance, 15 circular test pieces with a diameter of 40 mm were punched out of each sample and heat-sealed into a sterilization pouch with the side to be tested up.Test report Page 6 / 15 Out of the 15 test pieces, 10 were intended for the testing and one each for the two controls according to DIN 58953-6, section 4.9. The rest should remain as replacements (e.g. in case of the dropping of a test piece on the floor etc.).The sterilization pouches with the test pieces were steam-sterilized in an autoclave for 15 minutes at 121 °C and stored in an climatic room at 23 °C and 50 % relative humidity until despatch.2.3 Additional Sample and Re-examinationFor the analysis of the germ proofness under humidity another test round was performed in July / August 2012. For this, an additional sample (sample L4) was sent to the laboratories and analysed (see 2.1.2). The results were considered in the evaluation.For validation or confirmation of non-plausible results, occasional samples for re-examination were sent out to the laboratories. The results of these re-examinations (July / August 2012) were not taken into consideration in the evaluation.3. Results3.1 Preliminary RemarkSince the analysis of germ proofness is designed to be a pass / fail – test, the statistical values and precision data were meant only to serve informative purposes.The evaluation of the materials according to DIN58953-6,sections 3.7and 4.7.6by the laboratories should be the most decisive criterion for the evaluation of reproducibility of the interlaboratory test results. Based on this, the classification of a sample as “sufficiently germ-proof” or “not sufficiently germ-proof” is carried out.3.2 Note on the Record of Test Results:The exact counting of individual CFUs is not possible with the required precision if the values turn out to be very high. Thus, an upper limit of 100 CFU per agar plate or per test pieces, respectively, was defined. Individual values above this limit and values which were stated with “> 100” by the laboratories, are listed as 100 CFU per agar plate or per test piece, respectively, in the evaluation.Test report Page 7 / 153.3 Comment on the Statistical EvaluationThe statistical evaluation was done based on the series of standards DIN ISO 5725-1ff.The arithmetic laboratory mean X i and the laboratory standard deviation s i were calculated from the individual measurement values obtained by the laboratories.The overall mean X of the laboratory means as well as the precision data of the method (reproducibility and repeatability) were determined for each sample3.4 Outlier testsThe Mandel's h-statistics test was utilised as outlier test for differences between the laboratory means of the participants.A laboratory was identified as a “statistical outlier” as soon as an exceedance of Mandel's h test statistic at the 1 % significance level was detected.The respective results of the laboratories identified as outliers were not considered in the statistical evaluation.3.5 Record of Test ResultsOn the following pages, the records of the test results for each interlaboratory test sample with the statistical evaluation and the evaluation according to DIN 58953-6 are compiled.Test report Page 8 / 153.5.1 Record of Test Results Sample F1Individual Measurement values:Statistical Evaluation:Comment:Laboratory 4, as an outlier, has not been taken into consideration in the statistical Evaluation.Outlier criterion: Mandel's h-statistics (1 % level of significance)Overall mean X:91.0CFU / agar plateRepeatability standard deviation s r:17.9CFU / agar plateReproducibility standard deviation s R:19.8CFU / agar plateRepeatability r:50.0CFU / agar plateRepeatability coefficient of variation:19.6%Reproducibility R:55.5CFU / agar plateReproducibility coefficient of variation:21.8%Evaluation according to DIN 58953-6, Section 3.7:Lab. 1 - 6:Number of CFU > 5, i.e. the material is classified as not sufficiently germ-proof.Conclusion:All of the participants, even the Laboratory 4 which was identified as an outlier, came to the same results and would classify the sample material as “not sufficiently germ-proof”Test report Page 9 / 153.5.2 Record of Test Results Sample F2Individual Measurement values:Statistical Evaluation:Comment:Laboratory 4, as an outlier, has not been taken into consideration in the statistical Evaluation.Outlier criterion: Mandel's h-statistics (1 % level of significance)Overall mean X:0CFU / agar plateRepeatability standard deviation s r:0CFU / agar plateReproducibility standard deviation s R:0CFU / agar plateRepeatability r:0CFU / agar plateRepeatability coefficient of variation:0%Reproducibility R:0CFU / agar plateReproducibility coefficient of variation:0%Evaluation according to DIN 58953-6, Section 3.7:Lab. 1 – 3:Number of CFU = 0, i.e. the material is classified as sufficiently germ-proofLab. 4:Number of CFU ≤ 5, i.e. a re-examination on 20 test pieces would have to be done Lab. 5 – 6:Number of CFU = 0, i.e. the material is classified as sufficiently germ-proofConclusion:All of the participants, except for the Laboratory 4 which was identified as an outlier, came to the same results and would classify the sample material as “sufficiently germ-proof”.Test report Page 10 / 153.5.3 Record of Test Results Sample F3Individual Measurement values:Statistical Evaluation:Overall mean X:30.1CFU / agar plateRepeatability standard deviation s r:17.2CFU / agar plateReproducibility standard deviation s R:30.9CFU / agar plateRepeatability r:48.2CFU / agar plateRepeatability coefficient of variation:57.1%Reproducibility R:86.5CFU / agar plateReproducibility coefficient of variation:103%Evaluation according to DIN 58953-6, Section 3.7:Lab. 1 - 4:Number of CFU > 5, i.e. the material is classified as not sufficiently germ-proof. Lab. 5:Number of CFU = 0, i.e. the material is classified as sufficiently germ-proof. Lab. 6:Number of CFU > 5, i.e. the material is classified as not sufficiently germ-proof.Conclusion:Five of the six participants came to the same result and would classify the sample as “not sufficiently germ-proof”. Only laboratory 5 would classify the sample material as “sufficiently germ-proof”.Test report Page 11 / 153.5.4 Record of Test Results Sample L1Individual Measurement values:Statistical Evaluation:Overall mean X:0.09CFU / test pieceRepeatability standard deviation s r:0.32CFU / test pieceReproducibility standard deviation s R:0.33CFU / test pieceRepeatability r:0.91CFU / test pieceRepeatability coefficient of variation:357%Reproducibility R:0.93CFU / test pieceReproducibility coefficient of variation:366%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1 - 6:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof.Conclusion:All participants came to the same result and would classify the sample as “sufficiently germ-proof”.Test report Page 12 / 153.5.5 Record of Test Results Sample L2Individual Measurement values:Statistical Evaluation:Overall mean X:0.73CFU / test pieceRepeatability standard deviation s r: 1.10CFU / test pieceReproducibility standard deviation s R: 1.18CFU / test pieceRepeatability r: 3.07CFU / test pieceRepeatability coefficient of variation:151%Reproducibility R: 3.32CFU / test pieceReproducibility coefficient of variation:163%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1:Number of CFU > 15, i.e. the material is classified as not sufficiently germ-proof. Lab. 2 - 6:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof.Conclusion:Five of the six participants came to the same result and would classify the sample as “sufficiently germ-proof”. Only laboratory 1 exceeds the limit value slightly by 1 CFU, so that the sample would be classified as “not sufficiently germ-proof”.Test report Page 13 / 153.5.6 Record of Test Results Sample L3Individual Measurement values:Statistical Evaluation:Overall mean X:0.36CFU / test pieceRepeatability standard deviation s r: 1.00CFU / test pieceReproducibility standard deviation s R: 1.06CFU / test pieceRepeatability r: 2.79CFU / test pieceRepeatability coefficient of variation:274%Reproducibility R: 2.98CFU / test pieceReproducibility coefficient of variation:293%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1 - 6:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof.Conclusion:All participants came to the same result and would classify the sample as “sufficiently germ-proof”.Test report Page 14 / 153.5.7 Record of Test Results Sample L4Individual Measurement values:Statistical Evaluation:Overall mean X:35.1CFU / test pieceRepeatability standard deviation s r:18.8CFU / test pieceReproducibility standard deviation s R:42.6CFU / test pieceRepeatability r:52.7CFU / test pieceRepeatability coefficient of variation:53.7%Reproducibility R:119CFU / test pieceReproducibility coefficient of variation:122%Evaluation according to DIN 58953-6, Section 4.7:Lab. 1 - 3:Number of CFU > 15, i.e. the material is classified as not sufficiently germ-proof. Lab. 4:Number of CFU < 15, i.e. the material is classified as sufficiently germ-proof. Lab. 5 - 6:Number of CFU > 15, i.e. the material is classified as not sufficiently germ-proof.Conclusion:Five of the six participants came to the same result and would classify the sample as“not sufficiently germ-proof”.Test report Page 15 / 15 4. Overview and SummarySummary:In case of four of the overall seven tested materials, a 100 % consensus was reached regarding the evaluation as“sufficiently germ-proof”and“not sufficiently germ-proof”according to DIN 58 953-6.As for the other three tested materials, there were always 5 concurrent participants out of 6 (83 %). In each case, only one laboratory would have evaluated the sample differently.It is noteworthy that the materials about the evaluation of which a 100 % consensus was reached were the smooth sterilization papers. The differences with one deviating laboratory each occurred with the slightly less homogeneous materials, such as with the creped paper and the nonwoven materials.。
公路工程试验室仪器期间核查记录
公路工程试验室仪器期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH水泥胶砂试模期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH雷氏膨胀测定仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH雷氏夹期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH水泥抗压夹具期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH恒温恒湿养护箱期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH电热干燥箱期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH压碎指标值测定仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH混凝土坍落度筒期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH混凝土标准养护室期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH试验室用混凝土搅拌机期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH冷冻箱期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH混凝土及砂浆试模期间核查记录仪器编号:试模品种规格检测环境:温度℃,湿度%RH使用中混凝土及砂浆试模期间核查记录仪器编号:试模品种规格检测环境:温度℃,湿度%RH砂浆分层度仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH跳桌期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH砂浆稠度仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH道碴筛期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH道碴集料压碎率试模期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH规格型号:仪器编号:检测环境:温度℃,湿度%RH洛杉矶磨耗机期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH沥青软化点仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH沥青延度仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH沥青针入度仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH动力触探(标贯)仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH击实仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH光电式液、塑限测定仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH相对密度试验仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH灌砂仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RHK30平板载荷试验仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH比重瓶期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH玻璃仪器期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH金属线材反复弯曲试验机期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH钢筋冷弯弯芯期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH环刀期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH砼气压式含气量测定仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RHHG-80砼贯入阻力仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH砼维勃稠度仪期间核查记录规格型号:仪器编号:检测环境:温度℃,湿度%RH。
汽车电子EMC实验标准-按试验分类
各个车厂的EMC标准美国戴姆勒克莱斯勒Daimler ChryslerDC-10615:2004;DC-10614:2005福特FORDES-XW7T-1A278-AC通用GMWGMW3097-2006 GMW3100:2001 GMW 3172:2007德科T-752 DELCO日本日本汽车标准组织JASO D001-1994尼桑NISSAN 28400 NDS21(3) 28400NDS38[2],[3] 28401NDS02 马自达MAZDAMES PW 67600:2001欧洲标志,雪铁龙PSAB21 7110-2005 B21 7090大众汽车VOLKSWAGENTL 965 TL 82066 TL 82166 TL82366 TL82466 VW 801 01:2006菲亚特FIAT9.90110:2003罗孚MG ROVERMGR ES:62.61.627:2002TUV 7-Z0445:1995韩国大宇EDS-T-5006静电放电抗扰度试验ISO 10605:2001机动车抗静电放电骚扰试验方法GMW3100:2001通用标准电气/电子零部件和子系统电磁兼容验证部分ES-XW7T-1A278-AC:2003元件和子系统电磁兼容性全球要求和测试过程GMW3097:2006通用标准电气/电子零部件和子系统电磁兼容要求部分DC-10614:2002零部件电磁兼容性要求DC-10614:2005零部件电磁兼容性要求JASO D001-1994(第5.8条款)汽车零部件环境试验方法通用准则28400 NDS09:1996电子零部件的耐静电放电试验28400 NDS10:2000电子零部件的耐静电放电(操作部外加法)B21 7110:2001(第7条款)电子和电气设备有关环境的电气性能的通用技术标准MES PW 67600:2001电子器件7-Z0445:1995静电放电抗扰度试验9.90110:2003 (第2.7条款)汽车电子和电气设备MGR ES:62.61.627:2002汽车电磁兼容TL 824 66-2005静电放电抗扰度VW 801 01:2006机动车电子电气设施通用试验条件标准射频电磁场抗扰度试验ISO 11452-5:2002 机动车零部件由窄带辐射电磁能引起的骚扰的试验方法第五部分:带状线GMW3097:2006 通用标准电气/电子零部件和子系统电磁兼容要求部分GMW3100:2001 通用汽车标准电子/电气零部件和子系统电磁兼容通用标准验证部分DC-10614:2005 零部件电磁兼容性要求B21 7090:1993(第4条款)电气和电子装置环境的一般规定28400NDS05:2002 电子零部件的耐电波障碍性试验B21 7110:2001(第7条款) 电子和电气设备有关环境的电气性能的通用技术标准GB/T 17619-1998 机动车电子电器组件的电磁辐射抗扰性限值和测量方法MES PW 67600:2001 电子器件MGR ES:62.61.627:2002 汽车电磁兼容7-Z0448:2001 电子系统带状线电磁兼容试验VW 801 01:2006 机动车电子电气设施通用试验条件标准TL 821 66-2004 汽车电子零部件电磁兼容辐射干扰E/ECE/324 R10:2000+A1:1999 +A2:2004 机动车电磁兼容认证规定射频场骚扰感应的传导抗扰度试验ISO 11452-4:2005 机动车零部件由窄带辐射电磁能引起的骚扰的试验方法第四部分:大电流注入(BCI)GMW3097:2006 通用标准电气/电子零部件和子系统电磁兼容要求部分GMW3100:2001 通用标准电气/电子零部件和子系统电磁兼容验证部分ES-XW7T-1A278-AC:2003 元件和子系统电磁兼容性全球要求和测试过程DC-10614:2005 零部件电磁兼容性要求B21 7090:1993 (第4条款)电气和电子装置环境的一般规定28400NDS05:2002 电子零部件的耐电波障碍性试验B21 7110:2001 (第7条款)电子和电气设备有关环境的电气性能的通用技术标准EDS-T-5006:1993 电磁兼容零部件传导脉冲群敏感度试验程序MES PW 67600:2001 (第7.7条款) 电子器件7-Z0443:1997 电子系统耐电源线正弦波噪声试验(100kHz to 20MHz)7-Z0446:1995 电子系统电磁兼容大电流注入试验9.90110:2003 (第2.7条款) 汽车电子和电气设备MGR ES:62.61.627:2002 汽车电磁兼容传导骚扰CISPR 25: 2008 用于保护车载接收机的无线电骚扰特性的限值和测量方法GMW3097:2006 通用标准电气/电子零部件和子系统电磁兼容要求部分GMW3100:2001 通用标准电气/电子零部件和子系统电磁兼容验证部分ES-XW7T-1A278-AC:2003 元件和子系统电磁兼容性全球要求和测试过程DC-10614:2005 零部件电磁兼容性要求T-752 DELCO 产品试验标准电磁波频率干扰测量(CRFI)7-Z0470:1996 电子和电气系统电源线发射的静态噪声的测量B21 7090:1993 (第4条款)电气和电子装置环境的一般规定B21 7110:2001 (第7条款)电子和电气设备有关环境的电气性能的通用技术标准MES PW 67600:2001 电子器件9.90110:2003 (第2.7条款) 汽车电子和电气设备DIN 57 879:1981 德国标准汽车,汽车电器,内燃机的抗无线电干扰自抗干扰:汽车电器的测量TL 965:2004 近距离去扰要求VW 801 01:2006 机动车电子电气设施通用试验条件标准GB 18655-2002 用于保护车载接收机的无线电骚扰特性的限值和测量方法辐射骚扰CISPR 25: 2008 用于保护车载接收机的无线电骚扰特性的限值和测量方法GMW3097:2006 通用标准电气/电子零部件和子系统电磁兼容要求部分GMW3100:2001 通用标准电气/电子零部件和子系统电磁兼容验证部分ES-XW7T-1A278-AC:2003 元件和子系统电磁兼容性全球要求和测试过程DC-10614:2005 零部件电磁兼容性要求MIL-STD-461E:1999 子系统和设备的电磁干扰特性的控制要求B21 7090:1993(部分) (第4条款)电气和电子装置环境的一般规定7-Z0472:1996 电子和电气系统电波暗室辐射噪声的测量28400 NDS21:2002 电子零部件电磁发射B21 7110:2001 (第7条款)电子和电气设备有关环境的电气性能的通用技术标准MES PW 67600:2001 (第7.7条款) 电子器件9.90110:2003 (第2.7条款) 汽车电子和电气设备E/ECE/324 R10:2000 +A1:1999 +A2:2004 机动车电磁兼容认证规定GB 14023-2006 车辆、船和由内燃机驱动的装置无线电骚扰特性限值和测量方法GB 18655-2002 用于保护车载接收机的无线电骚扰特性的限值和测量方法电子负载ISO 16750-2:2003 机动车电子和电气设备环境条件和试验第二部分:电子负载GMW 3172:2007 汽车电子零部件通用要求分析/开发/验证程序环境、可靠性和性能要求DC 10615:2003 电气系统的电气或电子部件的性能要求ES-XW7T-1A278-AC:2003 元件和子系统电磁兼容性全球要求和测试过程9.90110:2003 汽车电子和电气设备(第2.7条款)7-Z0444:1999 电子/电气系统耐供电电压变化试验JASO D 001-1994 (第5.2~5.6条款) 汽车零部件环境试验方法通用准则B21 7090:1993 (第4条款)电气和电子装置环境的一般规定28400 NDS02:1999 耐电源波动试验标准28400 NDS81:1999 高速通信接口(500kbps)标准28400 NDS01:1992 耐异常电源波动B21 7110:2001 电子和电气设备有关环境的电气性能的通用技术标准(第7条款)MGR ES:62.61.627:2002 汽车电磁兼容MES PW 67600:2001 电子器件磁场敏感度MIL-STD-461E:1999 子系统和设备的电磁干扰特性的控制要求GMW3097:2006 通用标准电气/电子零部件和子系统电磁兼容要求部分ES-XW7T-1A278-AC:2003 元件和子系统电磁兼容性全球要求和测试过程DC-10614:2005 零部件电磁兼容性要求JASO D 001-1994(第5.10条款)汽车零部件环境试验方法通用准则9.90110:2003 (2.7条款) 汽车电子和电气设备7-Z0450:1996 电子系统磁场抗扰度试验28400 NDS22:1997 电子零部件的耐交流磁场试验B21 7110:2001(第7条款)电子和电气设备有关环境的电气性能的通用技术标准EDS-T-5514:1999 电子零部件和系统电磁抗扰度-磁场MES PW 67600:2001 (第7.7条款)电子器件点火噪声28400NDS08:1991 电子部件的耐点火噪声性B21 7110:2001 电子和电气设备有关环境的电气性能的通用技术标准(第7条款)MES PW 67600:2001电子器件导线耦合的传导瞬态脉冲抗扰度ISO 7637-1:1990 机动车传导耦合骚扰第一部分:12VDC供电的客车和小型商用车-沿电源线耦合的传导瞬态骚扰ISO 7637-2:2004 机动车传导耦合骚扰第二部分:沿电源线耦合的传导瞬态骚扰ISO 7637-2:1990 机动车传导耦合骚扰第二部分:24VDC供电的客车和小型商用车-沿电源线耦合的传导瞬态骚扰ISO 7637-3:2007 机动车传导耦合骚扰第三部分:供电电压为12V和24V沿除电源线外的导线通过容性和感性耦合的瞬态骚扰GMW3100:2001 通用标准电气/电子零部件和子系统电磁兼容验证部分GMW3097:2006 通用标准电气/电子零部件和子系统电磁兼容要求部分ES-XW7T-1A278-AC:2003 元件和子系统电磁兼容性全球要求和测试过程DC-10614:2005 零部件电磁兼容性要求JASO D 001-1994(第5.7条款) 汽车零部件环境试验方法通用准则9.90110:2003 汽车电子和电气设备(2.7条款)7-Z0440:1997 电子系统信号线瞬态噪声抗扰度试验7-Z0441:1997 电子系统电源线瞬态噪声抗扰度试验B21 7090:1993(第4条款)电气和电子装置环境的一般规定28400 NDS04:1997 耐高频脉动试验标准28400 NDS03耐低频浪涌试验MGR ES:62.61.627:2002 汽车电磁兼容MES PW 67600:2001 电子器件MES PW 67600:1995(第7.7条款)电子器件VW 801 01:2006 机动车电子电气设施通用试验条件标准TL 820 66-2004 汽车(kfz)电子部件的EMV(电磁兼容性)与导线相结合的干扰TL 823 66-2002 汽车电子部件敏感线路上的电磁兼容耦合干扰传导电压瞬态发射ISO 7637-1:1990 机动车传导耦合骚扰第一部分:12VDC供电的客车和小型商用车-沿电源线耦合的传导瞬态骚扰ISO 7637-2:2004 机动车传导耦合骚扰第二部分:沿电源线耦合的传导瞬态骚扰ISO 7637-2:1990 机动车传导耦合骚扰第二部分:24VDC供电的客车和小型商用车沿电源线耦合的传导瞬态骚扰GMW3100:2001 通用标准电气/电子零部件和子系统电磁兼容验证部分GMW3097:2006 通用标准电气/电子零部件和子系统电磁兼容要求部分ES-XW7T-1A278-AC:2003 元件和子系统电磁兼容性全球要求和测试过程DC-10614:2002 零部件电磁兼容性要求9.90110:2003 (第2.7条款) 汽车电子和电气设备7-Z0471:1996电子和电气系统供给线上瞬态噪声的测量B21 7090:1993(第4条款)电气和电子装置环境的一般规定28400NDS28:2003有感性负载的电子零部件浪涌源规则B21 7110:2001(第7条款)电子和电气设备有关环境的电气性能的通用技术标准MGR ES:62.61.627:2002 汽车电磁兼容MES PW 67600:2001电子器件低频传导抗扰度试验28400 NDS02:1999 电子零部件耐电源变化试验B21 7110:2001(第7条款)电子和电气设备有关环境的电气性能的通用技术标准MGR ES:62.61.627:2002 汽车电磁兼容MES PW 67600:2001电子器件汽车电磁兼容国际标准ISO 11451 道路车辆——窄带辐射电磁能量所产生的电气干扰——整车测试法(Road vehicles—Electrical disturbances by narrowband radiated electromagnetic energy—Vehicle test methods)ISO 11452 道路车辆——窄带辐射电磁能量所产生的电气干扰——零部件测试法(Road ISO vehicles—Electrical disturbances by narrowband radiated electromagnetic energy —Component test methods)ISO 7637 道路车辆——由传导和耦合产生的电气干扰(road vehicles—electrical disturbances by conduction and coupling)ISO 10605 道路车辆——静电放电产生的电气干扰(road vehicles—electrical disturbances from electrostatic discharge)CISPR 12 车辆、机动船和内燃发动机驱动装置的无线电骚扰特性的限值和测量方法(Vehicles,boats,and internal combustion engine driven devices radio disturbance characteristics limits and methods of measurement)CISPR 25 用于保护用在车辆、机动船和装置上车载接受机的无线电骚扰特性的限值和测量方法(Limits and methods of measurement of radio disturbance characteristics for the protection of receivers used on board vehicles,boats and on devices)欧洲汽车电磁兼容标准95/54/EC 对于车内点火发动机产生的无线电干扰的抑制(The suppression of radio interference produced by Dark-ignition engines fitted to motor vehicles95/56/EC 车辆保安系统(Vehicle security systems)97/24/EC 2/3轮式车辆(wheeled vehicles)2000/2/EC 森林和农用拖拉机(Forestry and agricultural Tractors)美国汽车工程学会(SAE)电磁兼容标准SAE J551-1 为车辆的装置的电磁兼容的限值和测试方法总则(60Hz~18GHz)SAE J551-2 为车辆,机动船和点火发动机驱动装置的无线电骚扰特性的限值及方法(30MHz~1GHz)SAE J551-3 窄带测量SAE J551-4 车辆和装置的宽窄带测量方法和限值(150kHz~IO00MHz)SAE J551-5 电动车宽带磁场和电场强度的限值和测量方法(9kHz~30MHz)SAE J551-11 来自车外干扰源的整车电磁抗扰度(100kHz~18GHz)SAE J551-12 来自车载发射机干扰源的整车抗扰度测量(1.8MHz一1.3GHz)SAE J551-13 大电流注入(1~400MHz)SAE J551-14 混响室SAE J551-15 为静电放电SAE J551-16 抗瞬态电磁干扰SAE J551-17 抗电源线磁场干扰(60Hz~30kHzSAE J1113-1 汽车零部件的电磁敏感性的测量过程及限值总则(60Hz~18GHz)SAE J1113-2 传导抗扰度测量~导线法(30Hz~250kHz)SAE J1113-3 传导抗扰度测量~射频(RF)功率直接注入法(250kHz~500kHz)SAE J1113-4 辐射电磁场抗扰度测量——BCI法SAE J1113-11 针对电源线的瞬态传导抗扰度SAE J1113-12 通过传导和耦合产生的电气干扰~耦合钳法SAE J1113-13 静电放电SAE J1113-21 用于电磁抗扰度测量的暗室(10kHz~18GHz)SAE J1113-22 由电源线产生辐射磁场的抗扰度测量(60Hz~30kHz)SAE J1113-23 辐射电磁场抗扰度测量——带状线法SAE J1113-24 为辐射电磁场抗扰度测量——TEM小室法(10kHz~200MHz)SAE J1113-25 辐射电磁场抗扰度测量——三层板法(10kHz~500MHz)SAE J1113-26 交流功率电场抗扰度测量(60Hz~30kHz)SAE J1113-27 辐射电磁场抗扰度测量——混响室法SAE J1113-41 用于保护车载接受机的车内零部件与组件的无线电干扰特性测量方法及限值SAE J1113-42 对于瞬态传导辐射的电磁敏感度国际电工委员会标准IEC 1000-4-3 辐射(射频)电磁场抗扰度试验。
压力管道报验资料全
压力管道报验资料焊接工艺评定报告焊接工艺评定报告编号: PQR05-01 焊接工艺评定指导书编号: WPS05-01 焊接方法:手工氩弧焊机械化程度(手工、半自动、自动)手工编制:日期审核:日期:批准:日期:中冶天工十三冶江阴兴澄特钢项目经理部焊接工艺评定报告共3页第1页接头简图:(坡口形式、尺寸、衬垫、每种焊接方法或焊接金属厚度)坡口形式 V垫板(材料及规格) /其它 /母材:材料标准GB700-88钢号Q235-B与材料标准GB700-88钢号Q235-B相焊类、组别号Ⅰ、Ⅰ-1与类、组别号Ⅰ、Ⅰ-1相焊厚度 6mm 直径 /其它 /填充金属:焊条标准: / 焊条牌号 / 焊丝标准:GB、T8110-1995 焊丝牌号 JQ.TG50焊材规格:焊丝:Ф2.0 焊缝金属厚度 6mm其它 /焊接位置:焊接位置:平焊方向(向上、向下) / 角焊位置: / 方向(向上、向下) /电特性:电流种类:直流极性:手工钨极氩弧焊:正极:焊接电流(A):手工钨极氩弧焊:100~120电弧电压(V):手工钨极氩弧焊:10~16钨极尺寸: Wce20 Ф3预热温度:预热温度(℃)室温层间温度(℃) 250℃其它: /附加说明:1、施焊记录见附表一;焊接工艺评定报告附表一焊评试验施焊记录表焊评编号(PQR №)PQR05-01焊工钢印检验员记录者母材名称钢号尺寸炉批号材质证明其它钢板Q235-B 6mm Z5-06774 5-5178 05A6-1 / / / / / /焊材牌号规格(mm)炉批号(或厂编号)烘干温度(℃)材质证明其它TG50 Ф2.0 04-9-135 / 43962 H04-06 / / / / / // / / / / // / / / / // / / / / /焊接位置平位施焊技术GTAW 预热温度室温层间温度250焊后热处理/ 后热处理/ 清根方法/ 保护气体Ar 脉冲频率/ 脉宽比/层焊接方法焊材牌号焊材规格电流种类及极性电流(A)电压(V)焊接速度cm/min热输入KJ/cm钨极直径喷嘴直径焊接工艺指导书焊接工艺评定报告编号:PQR05-1 焊接工艺评定指导书编号:WPS05-01 焊接方法:手工氩弧焊机械化程度(手工、半自动、自动):手工编制:日期:审核:日期:批准:日期:中冶天工十三冶兴澄特钢项目经理部焊接工艺指导书共2页第1 页焊接接头:对接简图:(接头形式、坡口形式与尺寸、焊层、焊道布置及顺序)坡口形式“V”型及其它垫板(材料及规格) / 其它 / 母材:类别号:Ⅰ组别号Ⅰ-1 与类别号Ⅰ组别号Ⅰ-1 相焊标准号:GB700-8 钢号 20#钢与标准号 GB700-8 钢号 20#钢相焊厚度围:母材:对接焊缝 1.5~12mm 角焊缝不限。
HCPL-0600(安华高)
CAUTION: It is advised that normal static precautions be taken in handling and assembly of this component to prevent damage and/or degradation which may be induced by ESD.Featuresx 15 kV/μs minimum Common Mode Rejection (CMR)at V CM = 1KV for HCNW2611, HCPL-2611, HCPL-4661, HCPL-0611, HCPL-0661x High speed: 10 MBd typical x LSTTL/TTL compatiblex Low input current capability: 5 mAx Guaranteed ac and dc performance over temper a ture: -40°C to +85°Cx Available in 8-Pin DIP , SOIC-8, widebody packages x Strobable output (single channel products only)x Safety approvalUL recognized - 3750 V rms for 1 minute and 5000 Vrms* for 1 minute per UL1577 CSA approved IEC/EN/DIN EN 60747-5-2 approved with V IORM = 560 V peak for 06xx Option 060 V IORM = 630 V peak for 6N137/26xx Option 060 V IORM = 1414 V peak for HCNW137/26X1x MIL-PRF-38534 hermetic version available (HCPL-56XX/66XX)Applicationsx Isolated line receiverx Computer-peripheral interfaces x Microprocessor system interfacesx Digital isolation for A/D, D/A conversion x Switching power supplyx Instrument input/output isolation x Ground loop eliminationx Pulse transformer replacementx Power transistor isolation in motor drives x Isolation of high speed logic systemsFunctional Diagram*5000V rms/1Minute rating is for HCNW137/26X1and Option 020(6N137,HCPL-2601/11/30/31,HCPL-4661)products only.A 0.1 P F bypass capacitor must be connected between pins 5 and 8.CATHODEANODE GNDV V CC O ANODE 2CATHODE 2CATHODE 1ANODE 1GNDV V CC O2V E V O16N137, HCPL-2601/2611HCPL-0600/0601/0611HCPL-2630/2631/4661HCPL-0630/0631/0661NC NCLED ON OFF ON OFF ON OFFENABLEH H L L NC NCOUTPUTL H H H L HTRUTH TABLE (POSITIVE LOGIC)LED ON OFFOUTPUTL HTRUTH TABLE (POSITIVE LOGIC)6N137, HCNW137, HCNW2601, HCNW2611, HCPL-0600,HCPL-0601, HCPL-0611, HCPL-0630, HCPL-0631, HCPL-0661,HCPL-2601, HCPL-2611, HCPL-2630, HCPL-2631, HCPL-4661High CMR, High Speed TTL Compatible OptocouplersData SheetDescriptionThe 6N137, HCPL-26XX/06XX/4661, HCNW137/26X1 are optically coupled gates that combine a GaAsP light emit-ting diode and an integrated high gain photo detector. An enable input allows the detector to be strobed. The output of the detector IC is an open collector Schottky-clamped transistor. The internal shield provides a guar-anteed common mode transient immunity specification up to 15,000 V/μs at Vcm=1000V.This u nique d esign p rovides m aximum a c a nd d c c ircuit i so-lation while achieving T TL compatibility. T he optocoupler ac and dc operational param e t ers are guaranteed from -40°C to +85°C allowing troublefree system performance.Lead (Pb) Free RoHS 6 fully compliantRo H S 6 f u lly comp -xxxE de n otes aThe 6N137, H CPL-26XX, H CPL-06XX, H CPL-4661, H CNW137,and HCNW26X1 are suitable for high speed logic interfac-ing, input/output buffering, as line receivers in environ-ments that conventional line receivers cannot tolerateand are recom m ended for use in extremely high groundor induced noise environments.Selection GuideWidebodyOn- Single Dual Single Dual SingleandDualdV/dt VCM CurrentOutput Channel Channel Channel Channel Channel Channel(V/μs)(V)(mA)Enable Package Package Package Package Package Packages 1000 10 5 Y E S 6N137[1][1]1,000 50 12.5 [3]HCPL-193X[1]HCPL-56XX[1]HCPL-66XX[1]Notes:1. Technical data are on separate Avago publications.2. 15 kV/μs with VCM = 1 kV can be achieved using Avago application circuit.3. Enable is available for single channel products only, except for HCPL-193X devices.Ordering InformationHCPL-xxxx is UL Recognized with 3750 Vrms for 1 minute per UL1577. HCNWxxxx is UL Rcognized with 5000 Vrms for 1 minute per UL1577.Part NumberOptionPackageSurfaceMountGullWingTape &ReelUL 5000 Vrms/1 MinuteRatingIEC/EN/DINEN 60747-5-2Quantity RoHSCompliantNon RoHSCompliant6N137-000E No option300milDIP-850 per tube -300E#300X X50 per tube -500E#500X X X1000 per reel -020E#020X50 per tube -320E#320X X X50 per tube -520E#520X X X X1000 per reel -060E#060X50 per tube -560E-560X X X X1000 per reelHCPL-2601-000E No option300milDIP-850 per tube -300E#300 X X50 per tube -500E#500X X X1000 per reel -020E#020X50 per tube -320E#320X X X50 per tube -520E#520X X X X1000 per reel -060E#060X50 per tube -360E-X X X50 per tubeHCPL-2611-000E No option300milDIP-850 per tube -300E#300X X50 per tube -500E#500X X X1000 per reel -020E#020X50 per tube -320E#320X X X50 per tube -520E#520X X X X1000 per reel -060E#060X50 per tube -360E#360X X X50 per tube -560E#560X X X X1000 per reelHCPL-2630-000E No option300milDIP-850 per tube -300E#300X X50 per tube -500E#500X X X1000 per reel -020E#020X50 per tube -320E#320X X X50 per tube -520E-520X X X X1000 per reelHCPL-2631 HCPL-4661-000E No option300milDIP-850 per tube -300E#300X X50 per tube -500E#500X X X1000 per reel -020E#020X50 per tube -320E#320X X X50 per tube -520E#520X X X X1000 per reelSchematicV FUSE OF A 0.1 μF BYPASS CAPACITOR CONNECTEDBETWEEN PINS 5 AND 8 IS RECOMMENDED (SEE NOTE 5).V CC V OGNDE6N137, HCPL-2601/2611HCPL-0600/0601/0611V V CC V O1V V O2GNDHCPL-2630/2631/4661HCPL-0630/0631/0661Part NumberOptionPackageSurface MountGull Wing Tape &ReelUL 5000 Vrms/1 Minute RatingIEC/EN/DIN EN 60747-5-2QuantityRoHS CompliantNon RoHS CompliantHCPL-0600HCPL-0601HCPL-0611-000E No option SO-8X 100 per tube -500E #500X X1500 per reel-060E #060X X100 per tube -560E #560X X X1500 per reel HCPL-0630HCPL-0631HCPL-0661-000E No option SO-8X100 per tube -500E #500X X1500 per reel HCNW137HCNW2601HCNW2611-000E No option 400 mil DIP-8XX 42 per tube -300E #300X X X X 42 per tube -500E#500XXXXX 750 per reelTo order, choose a part number from the part number column and combine with the desired option from the option column to form an order entry. Combination of Option 020 and Option 060 is not available.Example 1: HCPL-2611-560E to order product of 300mil DIP Gull Wing Surface Mount package in Tape and Reel packaging with IEC/EN/DIN EN 60747-5-2 Safety Approval in RoHS compliant.Example 2:HCPL-2630 to order product of 300mil DIP package in tube packaging and non RoHS compliant.Option datasheets are available. Contact your Avago sales representative or authorized distributor for information.Notes:The notation ‘#XXX’ is used for existing products, while (new) products launched since 15th July 2001 and RoHS compliant option will use ‘-XXXE‘.Package Outline Drawings8-pin DIP Package** (6N137, HCPL-2601/11/30/31, HCPL-4661)8-pin DIP Package with Gull Wing Surface Mount Option 300 (6N137, HCPL-2601/11/30/31, HCPL-4661)**JEDEC Registered Data (for 6N137 only).0.254+ 0.076- 0.051(0.010+ 0.003)- 0.002)DIMENSIONS IN MILLIMETERS AND (INCHES).*MARKING CODE LETTER FOR OPTION NUMBERS "L" = OPTION 020"V" = OPTION 060OPTION NUMBERS 300 AND 500 NOT MARKED.NOTE: FLOATING LEAD PROTRUSION IS 0.25 mm (10 mils) MAX.(0.025 ± 0.005)1.080 ± 0.320(0.100)BSCDIMENSIONS IN MILLIMETERS (INCHES).LEAD COPLANARITY = 0.10 mm (0.004 INCHES).+ 0.076- 0.051+ 0.003)- 0.002)NOTE: FLOATING LEAD PROTRUSION IS 0.25 mm (10 mils) MAX.Small-Outline SO-8 Package (HCPL-0600/01/11/30/31/61)8-Pin Widebody DIP Package (HCNW137, HCNW2601/11)0.228 ± 0.025(0.012)MIN.5.207 ± 0.254 (0.205 ± 0.010)DIMENSIONS IN MILLIMETERS (INCHES).LEAD COPLANARITY = 0.10 mm (0.004 INCHES) MAX.NOTE: FLOATING LEAD PROTRUSION IS 0.15 mm (6 mils) MAX.*1.80 ± 0.15NOTE: FLOATING LEAD PROTRUSION IS 0.25 mm (10 mils) MAX.0.254+ 0.076- 0.0051+ 0.003)- 0.002)8-Pin Widebody DIP Package with Gull Wing Surface Mount Option 300 (HCNW137, HCNW2601/11)Solder Reflow Temperature Profile0.(0..15.006)1.80 ± 0.15(0.071 ± 0.MAX .BSCDIMENSIONS IN MILLIMETERS (INCHES).LEAD COPLANARITY = 0.10 mm (0.004 INCHES).NOTE : FLOATING LEAD PROTRUSION IS 0.25 mm (10 m i l s ) MAX .TI ME (SECONDS)T E M P E R A T U R E (°C )ROOM T EMPERA T URENO T E: NON -H AL I DE F LU X S H OULD B E USED .Regulatory InformationThe 6N137, HCPL-26XX/06XX/46XX, and HCNW137/26XX have been approved by the following organizations:Recommended Pb-free IR ProfileInsulation and Safety Related Specifications 8-pin D I P Widebody(300 Mil) SO-8 (400 Mil) ParameterSymbol Value Value Value Units ConditionsMinimum External L(101) 7.1 4.9 9.6 mm Measured from input terminalsAir Gap (External to output terminals, shortest Clearance) distance through air. Minimum External L(102) 7.4 4.8 10.0 mm Measured from input terminals Tracking (External to output terminals, shortest Creepage)distance path along body.Minimum Internal 0.08 0.08 1.0 mm Through insulation distance, Plastic Gapconductor to conductor, usually (Internal Clearance) the direct distance between the photoemitter and photodetectorinside the optocoupler cavity. Minimum Internal NA NA 4.0 mm Measured from input terminals Tracking (Internal to output terminals, along Creepage) internal cavity. Tracking Resistance CTI200200200VoltsDIN IEC 112/VDE 0303 Part 1(Comparative Tracking Index) Isolation Group IIIa IIIa IIIa Material Group(DIN VDE 0110, 1/89, Table 1)Option 300 - surface mount classification is Class A in accordance with CECC 00802.ULRecognized under UL 1577, Component Recognition Program, File E55361.CSAApproved under CSA Component Acceptance Notice #5, File CA 88324.IEC/EN/DIN EN 60747-5-2Approved underIEC 60747-5-2:1997 + A1:2002 EN 60747-5-2:2001 + A1:2002DIN EN 60747-5-2 (VDE 0884 Teil 2):2003-01(Option 060 and HCNW only)T UAL T LT sma x T smin25T p TI MET E M P E R A T U R ENO T ES:TH E TI ME F ROM 25 °C t o PEA K T EMPERA T URE = 8 M I NU T ES MA X.T sma x = 200 °C, T smin = 150 °CNO T E: NON -H AL I DE F LU X S H OULD B E USED .* RECOMMENDED PEA K T EMPERA T URE F OR W I DE B OD Y 400mi l s PAC K AGE I S 245 °C(HCPL-06xx Option 060 Only) DescriptionSymbolCharacteristicUnitsInstallation classification per DIN VDE 0110/1.89, Table 1 for rated mains voltage ≤ 150 V rms I-IV for rated mains voltage ≤ 300 V rms I-III for rated mains voltage ≤ 600 V rms I-III Climatic Classification55/85/21Pollution Degree (DIN VDE 0110/1.89)2Maximum Working Insulation VoltageV IORM 567 V peak Input to Output Test Voltage, Method b*V IORM x 1.875 = V PR , 100% Production Test with t m = 1 sec, V PR 1063 V peakPartial Discharge < 5 pCInput to Output Test Voltage, Method a* V IORM x 1.5 = V PR , Type and Sample Test, V PR 851 V peakt m = 60 sec, Partial Discharge < 5 pC Highest Allowable Overvoltage (Transient Overvoltage, t ini = 10 sec)V IOTM 6000 V peakSafety Limiting Values(Maximum values allowed in the event of a failure) Case Temperature T S 150°C Input Current** I S,INPUT 150mA Output Power** P S,OUTPUT 600 mW Insulation Resistance at T S , V IO = 500 VR S ≥109:*Refer to the front of the optocoupler section of the current catalog, under Product Safety Regulations section, IEC/EN/DIN EN 60747-5-2, for a detailed description.Note: Isolation characteristics are guaranteed only within the safety maximum ratings which must be ensured by protective circuits in applica-tion.(HCPL-26xx ; 46xx ; 6N13x Option 060 Only) DescriptionSymbol Characteristic UnitsInstallation classification per DIN VDE 0110/1.89, Table 1 for rated mains voltage ≤ 300 V rms I-IV Climatic Classification55/85/21 Pollution Degree (DIN VDE 0110/1.89) 2 Maximum Working Insulation Voltage V IORM 630 V peak Input to Output Test Voltage, Method b*V IORM x 1.875 = V PR , 100% Production Test with t m = 1 sec, V PR 1181 V peakPartial Discharge < 5 pCInput to Output Test Voltage, Method a* V IORM x 1.5 = V PR , Type and sample test, V PR 945 V peakt m = 60 sec, Partial Discharge < 5 pC Highest Allowable Overvoltage* (Transient Overvoltage, t ini = 10 sec) V IOTM 6000 V peakSafety Limiting Values(Maximum values allowed in the event of a failure, also see Figure 16, Thermal Derating curve.) Case Temperature T S 175°C Input Current I S,INPUT 230mA Output PowerP S,OUTPUT 600 mW Insulation Resistance at T S , V IO = 500 VR S ≥109 :*Refer to the front of the optocoupler section of the current catalog, under Product Safety Regulations section, IEC/EN/DIN EN 60747-5-2, for adetailed description.Note: Isolation characteristics are guaranteed only within the safety maximum ratings which must be ensured by protective circuits in applica-tion.IEC/EN/DIN EN 60747-5-2 Insulation Related Characteristics (HCNW137/2601/2611 Only) DescriptionSymbolCharacteristic UnitsInstallation classification per DIN VDE 0110/1.89, Table 1 Climatic Classification (DIN IEC 68 part 1) 55/100/21Pollution Degree (DIN VDE 0110/1.89)2Maximum Working Insulation Voltage V IORM 1414 V peak Input to Output Test Voltage, Method b*V IORM x 1.875 = V PR , 100% Production Test with t m = 1 sec, V PR 2651 V peakPartial Discharge < 5 pCInput to Output Test Voltage, Method a* V IORM x 1.5 = V PR , Type and sample test, V PR 2121 V peakt m = 60 sec, Partial Discharge < 5 pC Highest Allowable Overvoltage* (Transient Overvoltage, t ini = 10 sec) V IOTM 8000 V peakSafety Limiting Values(Maximum values allowed in the event of a failure, also see Figure 16, Thermal Derating curve.) Case Temperature T S 150 °C Input Current I S,INPUT 400mA Output PowerP S,OUTPUT 700 mW Insulation Resistance at T S , V IO = 500 VR S ≥109 :*Refer to the front of the optocoupler section of the current catalog, under Product Safety Regulations section, IEC/EN/DIN EN 60747-5-2, for adetailed description.Note: Isolation characteristics are guaranteed only within the safety maximum ratings which must be ensured by protective circuits in applica-tion.Absolute Ma x imum Ratings* (No Derating Required up to 85°C) Parameter SymbolPackage** Min . Ma x. Units NoteStorage Temperature T S -55 125 °C Operating Temperature† T A -40 85 °C Average Forward Input Current I FSingle 8-Pin DIP20mA2Single SO-8Dual SO-8 Reverse Input Voltage V 8-Pin DIP , SO-8 5 V1Input Power Dissipation P I Widebody40 mW Supply Voltage V CC 7 V (1 Minute Maximum) Enable Input Voltage (Not to V ESingle 8-Pin DIPV CC + 0.5VE xceed V CC by more than Single SO-8E Output Collector Current I O50 mA 1 Output Collector Voltage V O 7 V 1 Output Collector Power P O Single 8-Pin DIP85mWDissipation Single SO-8Dual SO-8Lead Solder Temperature T LS 8-Pin DIP 260°C for 10 sec.,up to seating plane Solder Reflow Temperature SO-8 andSee Package Outline Profile (Surface Mount Parts Only)Option 300Drawings section*JEDEC Registered Data (for 6N137 only).**Ratings apply to all devices except otherwise noted in the Package column.†0°C to 70°C on JEDEC Registration.Recommended Operating Conditions ParameterSymbol Min . Ma x. UnitsInput Current, Low Level I FL * 0 250 μA Input Current, High Level [1] I FH ** 5 15 mA Power Supply Voltage V CC 4.5 5.5 V Low Level Enable Voltage† V EL 0 0.8 V High Level Enable Voltage† V EH 2.0 V CCVOperating Temperature T A -4085 °C Fan Out (at R L = 1 kΩ)[1]N 5 TTL Loads Output Pull-up ResistorR L 3304 k :*The off condition can also be guaranteed by ensuring that V FL ≤0.8 volts.**The initial switching threshold is 5 mA or less. It is recommended that 6.3 mA to 10 mA be used for best performance and to permit at least a 20% LED degradation guardband.†For single channel products only.Electrical SpecificationsOver recommended temperature (T A = -40°C to +85°C) unless otherwise specified. All Typicals at V CC = 5 V, T A = 25°C.All enable test conditions apply to single channel products only. See note 5.Parameter Sym . Package Min . Typ . Ma x. Units Test Conditions Fig . NoteHigh Level Output I OH * All 5.5 100 μA V CC = 5.5 V , V E = 2.0 V ,11, 6, CurrentV O = 5.5 V, I F = 250 mA19 Input Threshold I TH Single Channel 2.0 5.0 mA V CC = 5.5 V , V E = 2.0 V ,2, 319CurrentWidebody V O = 0.6 V,Dual Channel 2.5 I OL (Sinking) = 13 mA Low Level OutputV OL * 8-PinDIP 0.35 0.6 V V CC = 5.5 V, V E = 2.0 V, 2, 3, 1, 19Voltage SO-8 I F = 5 mA, 4, 5 Widebody 0.4 I OL (Sinking) = 13 mA High Level Supply I CCH Single Channel 7.0 10.0* mA V E = 0.5 V V CC = 5.5 V7Current 6.5 V E = V CC I F = 0 mA Dual Channel 10 15 Both Channels Low Level Supply I CCL Single Channel 9.0 13.0* mA V E = 0.5 V V CC = 5.5 V 8Current 8.5 V E = V CC I F = 10 mA Dual Channel 13 21 Both Channels High Level Enable I Single Channel-0.7 -1.6 mA V = 5.5 V , V = 2.0 VVoltage Input Forward V F 8-Pin DIP 1.4 1.5 1.75* V T A = 25°C I F = 10 mA 6, 7 1Voltage SO-8 1.3 1.80T A = 25°C1.22.05 Input Reverse BV R * 8-Pin DIP 5 V I R = 10 μA 1Breakdown SO-8 Voltage Widebody 3 I R = 100 μA, T A = 25°C Input Diode DV F /8-Pin DIP -1.6 mV/°C I F= 10 mA 7 1Temperature ∆T A SO-8 Coefficient Widebody -1.9 Input Capacitance C IN 8-Pin DIP 60pFf = 1 MHz, V F = 0 V1SO-8Widebody70*JEDEC registered data for the 6N137. The JEDEC Registration specifies 0°C to +70°C. HP specifies -40°C to +85°C.Switching Specifications (AC)Over Recommended Temperature (T A = -40°C to +85°C), V CC = 5 V, I F = 7.5 mA unless otherwise specified.All Typicals at T A = 25°C, V CC = 5 V .Parameter Sym . Package** Min . Typ . Ma x. Units Test ConditionsFig . NotePropagation Delay t PLH 20 48 75* ns T A = 25°C R L = 350 :8, 9, 1, 10,Time to High 100 C L = 15 pF 10 19 Propagation Delay t PHL 25 50 75* ns T A = 25°C 1, 11, Time to Low 10019f Time (90-10%)Propagation Delay t ELH SingleChannel 30 ns R L = 350 :, 13, 14 Time of Enable C L = 15 pF, 14 from V EH to V E L V EL = 0 V, V EH = 3 V Propagation Delay t EHLSingle Channel 20 ns 15Time of Enable from V EL to V EH*JEDEC registered data for the 6N137.**Ratings apply to all devices except otherwise noted in the Package column.Parameter Sym . DeviceMin . Typ . Units Test ConditionsFig . NoteLogic High |CM H | 6N137 1,000 10,000 V/μs |V CM | = 10 VV CC = 5 V, I F = 0 mA,15 1, 16, Common HCPL-2630 5,000 10,000 |V CM | = 1 kV V O(MIN) = 2 V,18, 19Mode HCPL-0600/0630 R L = 350 :, T A = 25°CTransient HCNW137 Immunity HCPL-2601/2631 10,000 15,000|V CM | = 1 kVHCPL-0601/0631 HCNW2601 HCPL-2611/4661 15,000 25,000|V CM | = 1 kVHCPL-0611/0661 HCNW2611 Logic Low |CM L | 6N137 1,000 10,000 V/μs |V CM | = 10 V V CC = 5 V, I F = 7.5 mA,15 1, 17, Common HCPL-2630 5,000 10,000 |V CM | = 1 kV V O(MAX) = 0.8 V,18, 19Mode HCPL-0600/0630 R L = 350 :, T A = 25°CTransient HCNW137 Immunity HCPL-2601/2631 10,000 15,000|V CM | = 1 kVHCPL-0601/0631 HCNW2601 HCPL-2611/4661 15,000 25,000|V CM | = 1 kVHCPL-0611/0661 HCNW2611Package Characteristics All Typicals at T A = 25°C.Parameter Sym . Package Min . Typ . Ma x. Units Test Conditions Fig . NoteInput-Output I I-O * Single 8-Pin DIP 1 P A 45% RH, t = 5 s,20, 21 InsulationSingle SO-8 V I-O = 3 kV dc, T A = 25°CInput-Output V ISO 8-Pin DIP , SO-83750 V rms RH ≤ 50%, t = 1 min,20, 21 Momentary With- Widebody 5000T A = 25°C 20, 22 stand Voltage** OPT 020† 5000Input-Output R I-O 8-Pin DIP , SO-8 1012 :V I-O = 500 V dc 1, 20, Resistance Widebody1012 1013T A = 25°C231011T A = 100°CInput-Output C I-O 8-Pin DIP , SO-8 0.6 pF f = 1 MHz, T A = 25°C1, 20, Capacitance Widebody 0.5 0.6 23 Input-Input I I-I Dual Channel 0.005 P A RH ≤ 45%, t = 5 s,24Insulation V I-I = 500 VI-I (Input-Input) Capacitance C I-I Dual 8-Pin DIP0.03pFf = 1 MHz24(Input-Input)Dual SO-80.25*JEDEC registered data for the 6N137. The JEDEC Registration specifies 0°C to 70°C. Avago specifies -40°C to 85°C.**The Input-Output Momentary Withstand Voltage is a dielectric voltage rating that should not be interpreted as an input-output continuous volt-age rating. For the continuous voltage rating refer to the IEC/EN/DIN EN 60747-5-2 Insulation Characteristics Table (if applicable), your equipment level safety specification or Avago Application Note 1074 entitled “Optocoupler Input-Output Endurance Voltage.”†For 6N137, HCPL-2601/2611/2630/2631/4661 only.Notes:1. Each channel.2. Peaking circuits may produce transient input currents up to 50 mA, 50 ns maximum pulse width, provided average current does not exceed 20 mA.3. Peaking circuits may produce transient input currents up to 50 mA, 50 ns maximum pulse width, provided average current does not exceed 15 mA.4. Derate linearly above 80°C free-air temperature at a rate of 2.7 mW/°C for the SOIC-8 package.5. Bypassing of the power supply line is required, with a 0.1 μF ceramic disc capacitor adjacent to each optocoupler as illustrated in Figure 17. Total lead length between both ends of the capacitor and the isolator pins should not exceed 20 mm.6. The JEDEC registration for the 6N137 specifies a maximum I OH of 250 μA. Avago guarantees a maximum I OH of 100 P A.7. The JEDEC registration for the 6N137 specifies a maximum I CCH of 15 mA. Avago guarantees a maximum I CCH of 10 mA.8. The JEDEC registration for the 6N137 specifies a maximum I CCL of 18 mA. Avago guarantees a maximum I CCL of 13 mA.9. The JEDEC registration for the 6N137 specifies a maximum I EL of –2.0 mA. Avago guarantees a maximum I EL of -1.6 mA.10. T he t PLH propagation delay is measured from the 3.75 mA point on the falling edge of the input pulse to the 1.5 V point on the rising edge of the output pulse.11. T he t PHL propagation delay is measured from the 3.75 mA point on the rising edge of the input pulse to the 1.5 V point on the falling edge of the output pulse.12. t PSK is equal to the worst case difference in t PHL and/or t PLH that will be seen between units at any given temperature and specified test conditions.13. S ee application section titled “Propagation Delay, Pulse-Width Distortion and Propagation Delay Skew” for more information.14. T he t ELH enable propagation delay is measured from the 1.5 V point on the falling edge of the enable input pulse to the 1.5 V point on the rising edge of the output pulse.15. T he t EHL enable propagation delay is measured from the 1.5 V point on the rising edge of the enable input pulse to the 1.5 V point on the falling edge of the output pulse.16. C M H is the maximum tolerable rate of rise of the common mode voltage to assure that the output will remain in a high logic state (i.e., V O > 2.0 V).17. C M L is the maximum tolerable rate of fall of the common mode voltage to assure that the output will remain in a low logic state (i.e., V O < 0.8 V).18. F or sinusoidal voltages, (|dV CM | / dt)max = S f CM V CM (p-p).19. N o external pull up is required for a high logic state on the enable input. If the V E pin is not used, tying V E to V CC will result in improved CMRperformance. For single channel products only.20. D evice considered a two-terminal device: pins 1, 2, 3, and 4 shorted together, and pins 5, 6, 7, and 8 shorted together.21. I n accordance with UL1577, each optocoupler is proof tested by applying an insulation test voltage ≥ 4500 V rms for one second (leakage detection current limit, I I-O ≤ 5 P A). This test is performed before the 100% production test for partial discharge (Method b) shown in the IEC/EN/DIN EN 60747-5-2 Insulation Characteristics Table, if applicable.22. I n accordance with UL 1577, each optocoupler is proof tested by applying an insulation test voltage ≥ 6000 V rms for one second (leakage detection current limit, I I-O ≤ 5 P A). This test is performed before the 100% production test for partial discharge (Method b) shown in the IEC/EN/DIN EN 60747-5-2 Insulation Characteristics Table, if applicable.23. M easured between the LED anode and cathode shorted together and pins 5 through 8 shorted together. For dual channel products only.24. M easured between pins 1 and 2 shorted together, and pins 3 and 4 shorted together. For dual channel products onlyI O H – H I G H L E V E L O U T P U T C U R R E N T – μAT A – TEMPERATURE – °C10155Figure 2. Typical output voltage vs . forward input current .Figure 3. Typical input threshold current vs . temperature .Figure 1. Typical high level output current vs . temperature .1623I F – FORWARD INPUT CURRENT – mA 0V O – O U T P U T V O L T A G E – V8-PIN DIP, SO-81623I F – FORWARD INPUT CURRENT – mAV O – O U T P U T V O L T A G E – VWIDEBODY6360T A – TEMPERATURE – °C20I T H – I N P U T T H R E S H O L D C U R R E N T – m A14563T A – TEMPERATURE – °C 20I T H – I N P U T T H R E S H O L D C U R R E N T – m A1458-PIN DIP, SO-8706060T A – TEMPERATURE – °C5020I O L – L O W L E V E L O U T P U T C U R R E N T – m A400.0.460T A – TEMPERATURE – °C 0.20V O L – L O W L E V E L O U T P U T V O L T A G E – V0.10.50.7WIDEBODY0.30.6Figure 7. Typical temperature coefficient of forward voltage vs . input current .Figure 4. Typical low level output voltage vs . temperature .Figure 5. Typical low level output current vs . temperature .Figure 6. Typical input diode forward characteristic .0.80.460T A – TEMPERATURE – °C 0.20V O L – L O W L E V E L O U T P U T V O L T A G E – V0.10.50.70.30.6I F – F O R W A R D C U R R E N T– m A0.001V F – FORWARD VOLTAGE – V 1.010000.010.110100I F - F O R W A R D C U R R E N T - m A0.001V F - FORWARD VOLTAGE - V1.010000.010.110100d V F /d T – F O R W A R D V O L T A G E T E M P E R A T U R E C O E F F I C I E N T – m V /°C0.1110100I F – PULSE INPUT CURRENT – mA -1.4-2.2-2.0-1.8-1.6-1.2-2.48-PIN DIP, SO-8d V F /d T – F O R W A R D V O L T A G E T E M P E R A T U R E C O E F F I C I E N T – m V /°C0.1110100I F – PULSE INPUT CURRENT – mA-1.9-2.2-2.1-2.0-1.8-2.3WIDEBODYFigure 8. Test circuit for t PHL and t PLH .Figure 9. Typical propagation delay vs . tem-perature .Figure 10. Typical propagation delay vs . pulse input current .Figure 11. Typical pulse width distortion vs . temperature .Figure 12. Typical rise and fall time vs . tempera-ture .10590I F – PULSE INPUT CURRENT – mA7530t P – P R O P A G A T I O N D E L A Y – n s6045OUTPUT V MONITORING NODEOOUTPUT V MONITORING NODEO.5 VI V .50 mA F.75 mA F*C L IS APPROXIMATELY 15 pF WHICH INCLUDES PROBE AND STRAY WIRING CAPACITANCE .10080T A – TEMPERATURE – °C600t P – P R O P A G A T I O N D E L A Y – n s4020t r , t f – R I S E , F A L L T I M E – n sT A – TEMPERATURE – °C4030T A - TEMPERATURE -o C20P W D - P U L S E W I D T H D I S T O R T I O N - n s100-10。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Report No.: P2012082901R01 1 of 26 Report Format Version 5.0.0 3GPP TS 51.010-1 V 10.1.0 12.2 Radiated Spurious Emission Test Report
MANUFACTURER : CT Asia BRAND NAME : BLU MODEL NAME : Deco mini 3 IMEI NUMBER : N/A STANDARD : 3GPP TS 51.010-1 V10.1.0 12.2 TEST DATE(S) : Aug. 13, 2012 TEST RESULT : PASS
The measurements shown in this test report were made in accordance with the procedures given in 3GPP TS 51.010-1 V10.1.0 and found to be in compliance with ETSI Standard EN 301 511 V9.0.2.
The test results in this report apply exclusively to the tested model / sample. Without written approval of Bureau Veritas Shenzhen Co., Ltd. Dongguan Branch ., the test report shall not be reproduced
except in full.
ISSUED BY: Bureau Veritas Shenzhen Co., Ltd. Dongguan Branch
LAB ADDRESS: No. 34, Chenwulu Section, Guantai Rd., Houjie Town, Dongguan City,Guangdong 523942, China Report No.: P2012082901R01 2 of 26 Report Format Version 5.0.0
Table of Contents 1 SUMMARY OF TEST RESULTS………………………………………………………...3 2 EQUIPMENT UNDER TEST (EUT) INFORMATION.………………………………….3 3 SUPPORT EQUIPMENT…………………………………………………….……………3 4 TEST PROCEDURE……………………………………………………………………....3 5 TEST RESULTS……………………………………………………………………………4 6 TEST DATA…………………………………………………………………………………8 7 TEST SETUP PHOTOGRAPHS…………………………………………………….….24 8 TEST EQUIPMENT CONFIGURATION INFORMATION……………………………25 9 MEASUREMENT UNCERTAINTY………………………………………………….….26 Report No.: P2012082901R01 3 of 26 Report Format Version 5.0.0
1 SUMMARY OF TEST RESULTS Description IMEI No. Result Remark
Radiated Spurious Emissions N/A PASS
1.The worse case is under limit 5.75 dB at 1417.2 MHz in GSM850 link mode on position Y Axis and vertical polarization.
2.The worse case is under limit 4.87 dB at 1489.83 MHz in GSM900 link mode on position Y Axis and Horizontal polarization.
3.The worse case is under limit 3.26 dB at 1844.6 MHz in DCS1800 link mode on position Y Axis and vertical polarization.
4.The worse case is under limit 4.44 dB at 1954.5 MHz in PCS1900 link mode on position Y Axis and Horizontal polarization.
2 EQUIPMENT UNDER TEST (EUT) INFORMATION The following information details the equipment under test (EUT) used for testing. Sample No. IMEI S/N Hardware Version Software Version Antenna Version Mech. Version
N/A N/A N/A N/A N/A N/A N/A
3 SUPPORT EQUIPMENT The following support equipment was used to exercise the EUT during testing. Description Type Model S/N Hardware Version
UNIVERSAL RADIO COMMUNICATION TESTER ROHDE & SCHWARZ CMU200 123259
N/A
4 TEST PROCEDURE a. The equipment under test (EUT) was placed on a rotated holder (Phi axis) on a turntable (Theta axis) inside a fully anechoic chamber. b. Established EUT link (dedicated mode) with system simulator (CMU200) and allocated a middle channel with maximum output power. c. Compensated the air loss in CMU 200, the set BS level to about – 75 dBm. d. The pre-calibration method has been performed ahead of testing so as to correct the readings from the spectrum analyzer using a peak detector and a various of RBW/VBW settings for each frequencies (Referred to 3GPP TS 51.010-1 table 12.8 for link mode and 12.10 for idle mode). Report No.: P2012082901R01 4 of 26 Report Format Version 5.0.0
e. The turntable (Theta axis) was rotated through 360 degrees for EUT X Y Z 3 Plane of rotation and the receive antenna was repeatedly tested for both horizontal and vertical polarizations. f. All spurious emissions radiated by the EUT were detected by the receive antenna and receiver is between the range of 30 MHz to 4 GHz. g. The test results were the maximum of those positions obtained over the whole sphere and only showed the worst data in this report ( final test).
5 TEST RESULTS Test Mode : GSM 850 Link Mode Temperature : 25℃ Sample No. : N/A Relative Humidity : 55%
Test Distance : 3m Test Engineer : Yuqiang
GSM850 Link CH189 mode : Y plane H Polarization V Polarization Frequency Limit Margin Frequency Limit Margin (MHz) EIRP (dBm) (dBm) (dB) (MHz) EIRP (dBm) (dBm) (dB)
30.630 -72.020 -36 -36.02 31.300 -75.540 -36 -39.54 114.500 -71.860 -36 -35.86 114.500 -69.450 -36 -33.45 624.460 -50.800 -36 -14.80 552.920 -48.670 -36 -12.67 798.600 -52.770 -36 -16.77 796.970 -54.450 -36 -18.45 811.230 -57.860 -36 -21.86 806.150 -58.400 -36 -22.40 819.930 -63.390 -36 -27.39 815.450 -63.560 -36 -27.56 825.590 -64.200 -36 -28.20 825.290 64.410 -36 100.41 834.530 -63.740 -36 -27.74 834.500 -65.250 -36 -29.25 839.980 -66.030 -36 -30.03 839.970 -67.470 -36 -31.47 844.910 -62.760 -36 -26.76 844.830 -63.120 -36 -27.12 852.500 -62.380 -36 -26.38 853.220 -63.330 -36 -27.33 861.200 -57.300 -36 -21.30 863.050 -58.080 -36 -22.08 873.220 -52.910 -36 -16.91 877.400 -51.430 -36 -15.43 1342.680 -42.630 -30 -12.63 1417.200 -41.750 -30 -11.75 1675.000 -39.280 -30 -9.28 1675.000 -43.080 -30 -13.08
