ch5 Ion Implantation
光刻与刻蚀工艺

1、涂胶
第十六页,共118页。
1、涂胶
❖涂胶目的 ❖在硅片表面形成厚度均匀、附着性强、并
且没有(méi yǒu)缺陷的光刻胶薄膜。 ❖怎样才能让光刻胶粘的牢一些?
第十七页,共118页。
可以(kěyǐ)开始涂胶了……
❖ 怎么涂? ❖旋转涂胶法:把胶滴在硅片,然后使硅片
高速旋转,液态胶在旋转中因离心力作用 (zuòyòng)由轴心沿径向(移动)飞溅出去, 但粘附在硅表面的胶受粘附力的作用 (zuòyòng)而留下。在旋转过程中胶所含的 溶剂不断挥发,故可得到一层均匀的胶膜 ❖ 怎样才算涂的好?
❖一个英制等级100的洁净室相当于公制等级
第八页,共118页。
洁净室(4)
❖ 对一般的IC制造区 域,需要等级100的洁 净室,约比一般室内 空气低4个数量级。
❖ 在图形(túxíng)曝 光的工作区域,则需 要等级10或1的洁净室。
第九页,共118页。
lithography
❖Introduction ❖光刻 ❖洁净室 ❖工艺流程(ɡōnɡ yì liú chénɡ) ❖光刻机 ❖光刻胶 ❖掩膜版
第十四页,共118页。
resist substrate
maБайду номын сангаасk
negative tone
光刻工艺(gōngyì)过程
❖ 涂胶 coating ❖ 前烘 prebaking ❖ 曝光(bào guāng) exposure ❖ 显影 development ❖ 坚膜 postbake ❖ 刻蚀 etch ❖ 去胶 strip ❖ 检验 inspection
第二十八页,共118页。
❖显4、影之显后影的(x检iǎ查n yǐng)(Development)
妇科英语试题库及答案

妇科英语试题库及答案一、选择题1. Which of the following is a common symptom of endometriosis?A. Heavy menstrual bleedingB. Irregular periodsC. InfertilityD. All of the above答案:D2. What is the medical term for the removal of the uterus?A. HysterectomyB. OophorectomyC. SalpingectomyD. Cystectomy答案:A3. The hormone responsible for the development of female secondary sexual characteristics is:A. EstrogenB. ProgesteroneC. TestosteroneD. Insulin答案:A二、填空题4. The process of a fertilized egg implanting into the________ is known as implantation.答案:endometrium5. The medical condition characterized by the presence of endometrial-like tissue outside the uterus is called ________. 答案:endometriosis6. A ________ is a surgical procedure to remove the ovaries.答案:oophorectomy三、判断题7. Polycystic ovary syndrome (PCOS) is a condition thataffects only the ovaries.答案:错误8. Menopause is the permanent cessation of menstruation,which occurs naturally in women around the age of 50.答案:正确9. The use of oral contraceptives can help prevent the development of ovarian cysts.答案:错误四、简答题10. Describe the function of the cervix in the female reproductive system.答案:The cervix serves as a passageway for sperm to enterthe uterus and for the baby to be delivered during childbirth.It also produces mucus that helps to facilitate the movement of sperm towards the egg.11. What are the typical symptoms of premenstrual syndrome (PMS)?答案:Typical symptoms of PMS include mood swings,irritability, bloating, breast tenderness, fatigue, and headaches, among others.五、案例分析题12. A 35-year-old woman presents with a history of severe pelvic pain, painful intercourse, and infertility. She has been trying to conceive for the past two years without success. What condition might she be suffering from, and what diagnostic tests could be performed to confirm the diagnosis?答案:The woman may be suffering from endometriosis. Diagnostic tests that could be performed include a pelvic examination, transvaginal ultrasound, and possibly a laparoscopy to visualize the endometrial-like tissue outside the uterus.六、翻译题13. 翻译以下医学术语:- 子宫肌瘤 (Uterine fibroids)- 子宫颈涂片 (Pap smear)- 卵巢囊肿 (Ovarian cyst)答案:- 子宫肌瘤 (Uterine fibroids)- 子宫颈涂片 (Pap smear)- 卵巢囊肿 (Ovarian cyst)七、论述题14. Discuss the importance of regular gynecological check-ups and the common procedures involved.答案:Regular gynecological check-ups are crucial for early detection and prevention of various gynecological disorders such as cervical cancer, ovarian cysts, and endometriosis. Common procedures involved include a pelvic examination, Pap smear for cervical cancer screening, breast examination, and discussions regarding sexual health and contraception.请注意,以上内容仅为示例,实际的试题库和答案应根据具体教学大纲和课程内容进行编制。
08工艺-刻蚀

Si
氮化硅和垫层氧化剥离
(c)
(e)
RCA 清洗
• 1960年, 年 Kern and Puotinen 在RCA开发 • IC fabs中最常用的清洗工艺 • SC-1溶液:NH4OH:H2O2:H2O按1:1:5到1:2:7 的比例配制并且温度在7 70 - 80 °C,用于去除 颗粒沾污. • SC-2 SC 2 溶液: 溶液 HCl:H2O2:H2O 按 1:1:6 到 1:2:8 比 例配制并且温度在 70-80 ° C ,用于去除无机 沾污。 沾污 • 去离子水冲洗 • HF 浸洗或 HF 蒸汽腐蚀去除自然氧化层
– – – – 硅片的清洗 无图形的薄膜去除 如氮化硅和钛的去除 无图形的薄膜去除,如氮化硅和钛的去除。 测试硅片的薄膜去除和清洗。 应用于 CVD膜质量的控制 (缓冲氧化层刻蚀 剂或BOE)
旋转甩干
SiO2的湿法刻蚀
• 氢氟酸溶液 (HF),极高的选择比。 • 通常用缓冲剂或去离子水稀释减少刻 蚀速率
垫层氧化 氮化硅 P-型衬底 垫层氧化,氮化硅淀积和图案形成
Si Si 生长SiO2/SiN 刻蚀 SiN/SiO2/Si
氮化硅 p+ P-型衬底
SiO2 p+
+ p 隔离掺杂
(a)
氧化 + CVD USG
(b)
去掉 SiN/SiO2 USG
LOCOS 局部氧化 SiO2 p+ P-型衬底 p+ 鸟嘴
硅化物退火
湿法去除钛
湿法刻蚀优缺点
• 高选择比 • 相对便宜的设备 • 批处理,高产出 批处理 高产出 • • • • 各向同性的形貌 不能形成3微米以下的图形 化学剂用量大 化学剂的危害性
病理学英文——精选推荐

第一章病理学单词绪论pathology etiologypathogenesispathological change 第二章细胞和组织的适应与损伤adaptationatrophyhypertrophy compensatory hypertrophy endocrine hypertrophy hyperplasia compensatory hyperplasia hormonal hyperplasia metaplasiainjuryischemiahypoxiagenetic variation reversible injury degenerationcellular swelling hydropic degeneration fatty change/steatosis fatty infiltration hyalinizationhyaline degeneration arteriolosclerosis amyloid changemucoid degerenation pathological pigmentation hemosiderinlipofuscinmelaninbilirubinpathologic calcificationdystrophic calcificationmetastatic calcificationirreversiblenecrosispyknosiskaryorrhexiskaryolysiscoagulative necrosiscaseous necrosisliquefactive necrosisfibrinoid necrosisgangreneerosionulcersinusfistulacavityorganizationencapsulationapoptosiscellular agingtelomere第三章损伤的修复repair regeneration cell cycle labile cells stable cells permanent cells extracellular matrix,ECM collagenelastinmatricellular proteins hyaluronanchaloneembryonic stem cell adult stem celltrans-differentiation mesenchymal stem cell granulation tissue myofibroblastscarwound hesalinghealing by first intentionhealing by second intentionbone fracturewoven bone第四章局部血液循环障碍hyperemia congestionarterial hyperemia venous hyperemia cyanosiscongestive edema congestive hemorrhage chronic congestion congestive sclerosis heart failure cells brown duration nutmeg liverIto cellscongestive liver cirrhosis hemorrhage hematomapetechiaepurpuraecchymosesbilirubinthrombosisthrombusadhesionrelease reaction aggregationblood hypercoagulabilitypale thrombusmixed thrombusmural thrombusred thrombushyaline thrombusrecanalizationcalcificationphlebolitharteriolithembolismembolusthromboembolismpulmonary embolismsaddle embolismfat embolismgas embolismair embolismdecompression sicknessamniotic fluid embolisminfarctionanemic infarctwhite infarcthemorrhagic infarctred infarctedema第五章炎症inflammation alteration exudation proliferation exudate transudateacute inflammation chronic inflammation stasis transcytosisleukocytic margination selectinemigrationchemotaxisrecognition and attachment opsoninengulfment phagolysosomekilling and degradation defensins inflammatory mediator炎serous inflammationfibrinous inflammationsuppurative or purulent inflammationpusempyemaphlegmonous inflammationabscesshemorrhagic inflammationbacteremiatoxemiasepticemiapyemiachronic granulomations inflammation 第五章肿瘤tumor, neoplasmmass aggressivenessbenign tumor malignant tumor cancerneoplasianeoplastic proliferation polyclonalclonalneoplastic transformation autonomy tumorigenic agent promotercarcinogenpapillaryvillouspolypoidnodularlobularinfiltratingulcerativecysticparenchymastromadifferentiationdegree of differentiation undifferentiatedatypia architectural atypia cellular atypiaanaplasiaanaplastic tumor adenomacarcinomasarcoma carcinosarcomainvasionmetastasisexpansile growth exophytic growth invasive growthwell-circumscribed capsuleencapsulated compression angiogenesis progression heterogeneitymalignant transformation direct spreading metastatic tumor, metastasis secondary tumorprimary tumorlymphatic metastasis hematogenous metastasis implantation metastasis precancerous diseaseinherited cancer syndrome atypical hyperplasia dysplasiaoncogenepoint mutationgene amplification chromosomal translocation tumor suppressor gene tumor immunology tumor-specific antigen tumor-associated antigen oncofetal antigen immunosurveillance文- 汉语汉字编辑词条文,wen,从玄从爻。
教师授课计划(半导体制造工艺与设备)
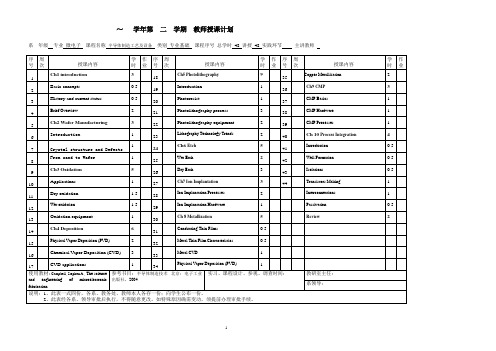
0.5
19
1
36
Ch9 CMP
3
3
History and curre
37
CMP Basics
1
4
Brief Overview
2
21
3
38
CMP Hardware
1
5
3
22
2
39
CMP Processes
1
6
Introduction
1
23
Lithography Technology Trends
10
1
27
Ch7 Ion Implantation
3
44
Transistors Making
1
11
1.5
28
Ion Implantation Processes
2
Interconnections
1
12
Wet oxidation
1.5
29
Ion Implantation Hardware
1
Passivation
1
使用教材:Campbell, Stephen A.,The science and engineering of microelectronic fabrication.
参考书目:半导体制造技术.北京:电子工业出版社,2004.
实习、课程设计、参观、调查时间:
教研室主任:
系领导:
说明:1、此表一式四份,各系、教务处、教师本人各存一份;向学生公布一份。
0.5
13
1
无机化合物的英文命名

* 阳 离 子 的 电 荷 数 用 斯 托 克 数 字 ( Stock number)来表示(只形成一种阳离子的元素 不必用). 例:CuCl: copper(I)chloride;
arseniteБайду номын сангаасAsO33-) phosphite(PO33-) sulfite(SO32-) nitrite(NO2-)
-ic
-ous
nitric含氮的/硝酸的 nitrous 含氮的(化合价低的) sulfuric含(六价)硫的 sulfurous含硫的
Note -ic: 指相似化合物中较高价态的,-ous:较低价的化合物
NaOH: sodium hydroxide KOH: potassium hydroxide
ammonium hydroxide
如果某元素能形成一种以上的阳离子,则使用斯 托克数字(Stock number)来表示其价态.
Fe(OH)3: iron(Ⅲ) hydroxide
6. Names of Salts(盐的命名):
PO3-(偏磷酸根):metaphosphate ion (S2O6)2- (焦硫酸根):pyrosulfate ion
3 ) Anions containing hydrogen ( 含 氢阴离子):hydrogen + 酸根离子名称 例:HCO3-:hydrogen carbonate ion
3. Names of oxides: 非金属氧化物的命名(nonmetals)
I-: iodide ion
I: iodine
H-: hydride ion
H:hydrogen
Design of Multiple Metal Doped Ni Based Catalyst for Hydrogen Generation from Biooil Reforming

CHINESE JOURNAL OF CHEMICAL PHYSICS VOLUME26,NUMBER1FEBRUARY27,2013ARTICLEDesign of Multiple Metal Doped Ni Based Catalyst for Hydrogen Generation from Bio-oil Reforming at Mild-temperatureLi-xia Yuan a,b,Fang Ding a,Jian-ming Yao a,Xiang-song Chen a,Wei-wei Liu a,Jin-yong Wu a,Fei-yan Gong b,Quan-xin Li b∗a.Institute of Plasma Physics,Chinese Academy Sciences,Hefei230026,Chinab.Anhui Key Laboratory of Biomass Clean Energy,University of Science and Technology of China,Hefei230026,China(Dated:Received on November16,2012;Accepted on December10,2012)A new kind of multiple metal(Cu,Mg,Ce)doped Ni based mixed oxide catalyst,synthesizedby the co-precipitation method,was used for efficient production of hydrogen from bio-oilreforming at250−500◦C.Two reforming processes,the conventional steam reforming(CSR)and the electrochemical catalytic reforming(ECR),were performed for the bio-oil reforming.The catalyst with an atomic mole ratio of Ni:Cu:Mg:Ce:Al=5.6:1.1:1.9:1.0:9.9exhibited veryhigh reforming activity both in CSR and ECR processes,reaching82.8%hydrogen yieldat500◦C in the CSR,yield of91.1%at400◦C and3.1A in the ECR,respectively.Theinfluences of reforming temperature and the current through the catalyst in the ECR wereinvestigated.It was observed that the reforming and decomposition of the bio-oil weresignificantly enhanced by the current.The promoting effects of current on the decompositionand reforming processes of bio-oil were further studied by using the model compounds of bio-oil(acetic acid and ethanol)under101kPa or low pressure(0.1Pa)through the time offlightanalysis.The catalyst also shows high water gas shift activity in the range of300−600◦C.The catalyst features and alterations in the bio-oil reforming were characterized by the ICP,XRD,XPS and BET measurements.The mechanism of bio-oil reforming was discussedbased on the study of the elemental reactions and catalyst characterizations.The researchcatalyst,potentially,may be a practical catalyst for high efficient production of hydrogenfrom reforming of bio-oil at mild-temperature.Key words:Hydrogen generation,Bio-oil,Ni based catalyst,Mild-temperatureI.INTRODUCTIONCurrently,hydrogen production from steam reform-ing of bio-oil has attracted considerable attention[1]. The conventional carbonbased fossil fuels,such as coal, oil,and natural gas are becoming depleted day by day, on the other hand,hydrogen production processes from catalytic steam reforming of nonrenewable materials such as natural gas and oilderived naphtha are accom-panied by high CO2emissions,which significantly con-tributes to the greenhouse effect[2].Consequently, biomass,the intriguing renewable resources could in principle be a candidate for hydrogen production[3]. Hydrogen can be produced from biomass mainly via two thermochemical processes,the gasification and the flash pyrolysis followed by steam reforming of the py-rolysis oil[4,5].The pyrolysis oil,known as bio-oil, generally,contains numerous and complex oxygenated organic compounds including acids,alcohols,aldehydes,∗Author to whom correspondence should be addressed.E-mail: liqx@ ketones,substituted phenolics and other oxygenates de-rived from biomass carbohydrates and lignin[6].From an economic and environmental view,steam reforming of pyrolysis oil(bio-oil)is one of the promising routes of hydrogen production from renewable sources.The bio-oil reforming reactions would take place in the re-forming reaction(Eq.(1))or the water-gas shift reaction (WGS)(Eq.(2))[7,8].C n H m O k+(n−k)H2O=n CO+n+m2−kH2(1) CO+H2O→CO2+H2(2) During the last decade,steam reforming of bio-oil and its model compounds has been studied continuously, various catalysts such as the Ni-based catalysts(sup-ported on such as Al2O3,MgO,CeO2and TiO2etc.) and noble metal-loaded catalysts(e.g.,Pt,Ru,Rh etc.) have been selected and used for production of hydro-gen from steam reforming of bio-oil and hydrocarbons [9−12].Conventional catalysts for the steam reforming of hydrocarbons are NiO supported on a mineral(e.g., alumina,magnesia)usually operating at high temper-ature(600−850◦C)[8,13].Noble metals are gener-DOI:10.1063/1674-0068/26/01/109-120109c 2013Chinese Physical Society110Chin.J.Chem.Phys.,Vol.26,No.1Li-xia Yuan et al.ally more effective than the Ni-based catalysts and less carbon depositing but not common in real applications because of their high cost.Lots of works on the steam reforming of bio-oil and model compounds(e.g.,methanol,ethanol,acetic acid etc.)have been extensively performed in the past[1,14, 15],but the exorbitant reforming temperature and the catalyst deactivation still remain serious challenging in the production of hydrogen from bio-oil.The process of bio-oil reforming is much more complex than that of single organic compound.Generally,the reforming temperature to achieve a high hydrogen yield for bio-oil over a given catalyst is much higher than that of a single oxygenated organic compound,the catalyst inac-tivity and the reaction channels in the bio-oil reforming are much more serious and intricate than those in the re-forming process of single oxygenated organic compound [7,16].Accordingly,developing non-noble metal cata-lysts suitable for the low-temperature reforming bio-oil, optimizing reforming conditions,and studying the re-action mechanism and the catalyst inactivation process are required and imperative.In our previous work,much attention has been paid to the fast pyrolysis of biomass[9],the production of hydrogen from the volatile fraction of the bio-oil and model compounds[17,18],and a new electrochemical catalytic reforming(ECR)bio-oil method was devel-oped[19].In this work,to solve the problems in hy-drogen production from bio-oil,including reducing re-forming temperature and deactivation of catalyst,the multiple metal doped Ni based mixed oxide catalyst was researched in electrochemical catalytic steam reforming of bio-oil.This non-noble catalyst showed good activity for reforming of bio-oil at lower operating temperature (250−500◦C)with a longer lifetime,as compared with the performance using the conventional NiO-Al2O3cat-alysts.The activity of water gas shift and decomposi-tion for the oxygenated organic compounds in the bio-oil over the research catalyst,and the influences of the current on the bio-oil reforming,decomposition and cat-alyst microcosmic properties were studied.The mech-anism of the bio-oil reforming was also discussed based on present investigations.II.EXPERIMENTSA.Catalyst preparation and characterizationThe mixed oxide catalysts were prepared by the side-by-side co-precipitation method at a constant pH us-ing respective metal nitrates as precursors and a mix-ture of NaOH and Na2CO3as precipitators.The main preparation procedures included:(i)preparation of re-spective metal nitrates solution,(ii)preparation of a mixture of NaOH(1mol/L)and Na2CO3(1mol/L), (iii)preparation of precipitates by the side-by-side co-precipitation of metal nitrates solution and the pre-cipitators at a constant pH(9.0±0.5)and80◦C,(iv)FIG.1Schematic setup of thefixed-bedflow reaction sys-tem for the bio-oil.ECR mode:as the Ni-Cr wire was passed through an ac electronic current for heating the catalyst and synchronously providing the electrons onto the cata-lyst.CSR mode:the current was shut offand the reactor was homogeneously heated by an outside furnace.the precipitates were aged for10h at25◦C,pumped and washed until pH=7,dried overnight in an oven at 110◦C,(v)then the dried precipitates were heated at 1◦C/min to450◦C and calcined at450◦C for6h in air to obtain the corresponding mixed oxide catalysts, (vi)finally,the mixed oxide catalysts were crushed into 0.1−0.2mm and ready for the reforming.The metallic contents in the prepared catalysts were measured by inductively coupled plasma and atomic emission spectroscopy(ICP/AES,Atom scan Advan-tage of Thermo Jarrell Ash Corporation,USA).X-ray diffraction(XRD)measurements were em-ployed to investigate the diffraction structure changes of the catalysts.XRD patterns of the catalysts were recorded on an X’pert Pro Philips diffractrometer,us-ing a Cu Kαradiation.The surface elements and their states were analyzed by X-ray photoelectron spectroscopy(XPS).The XPS measurements were performed on an ESCALAB-250 (Thermo-VG Scientific,USA)spectrometer with Al Kα(1486.6eV)irradiation source.The C1s peak at 284.6eV was generally used as a calibration standard for determining the peaks’position and the elemental concentration.The Brunauer-Emmett-Teller(BET)surface area and pore volume was determined by the N2physisorp-tion at−196◦C using a COULTER SA3100analyzer.B.Reaction systemAs shown in Fig.1,the bio-oil steam reforming ex-periments were carried out in the continuousflow sys-tems,using a quartzfixed-bed reactor under atmo-spheric pressure.The bio-oil was fed into the reactors using the multi-syringe pump(Model:TS2-60,Baod-ing Longer Precision Pump),the steam from a steam-generator was simultaneously fed into the reactors forDOI:10.1063/1674-0068/26/01/109-120c 2013Chinese Physical SocietyChin.J.Chem.Phys.,Vol.26,No.1Ni Based Catalyst for Hydrogen Generation from Bio-oil111 adjusting the S/C ratio(mole ratio of steam to carbonin bio-oil fed).The steam amount fed was controlledby the massflow controller,and the effluent gases fromthe reactors were measured byflow display.Tempera-ture and its distribution were measured by the ther-mocouples inserted into the catalyst beds.We per-formed the reforming experiments with following twoprocesses,i.e.,the conventional steam reforming(CSR)and the ECR.For the ECR,an annular Ni-Cr wire,which passed through a given ac electronic current,en-twined around a quartz column for heating the catalystand synchronously providing the electrons onto the cat-alyst,and installed in the center of the reactor.The cat-alyst was uniformly embedded around the Ni-Cr wire.To make a certain reforming temperature,the catalystbed was heated by a supplementary outside furnace.For the CSR,ac was shut offand the catalyst bed washomogeneously heated by an outside furnace.The prepared bio-oil described by a chemical formulaof CH2.03O0.67·0.89H2O[19].The products of the re-forming reactions were analyzed by two on-line gas chro-matographs(GC1and GC2)with thermal conductivitydetector(TCD).The intermediates desorbed from thecatalyst surface were mass analyzed by a time-of-flight(TOF)mass spectrometer.The experimental setup ofthe TOF system has been described in detail elsewhere[19,20].The hydrogen yield was calculated as a percentage ofthe stoichiometric potential,in case of complete conver-sion of carbon element in the bio-oil to CO2accordingto the reactionC n H m O k+(2n−k)H2O=2n+m2−kH2+n CO2(3)The potential yield of hydrogen is(2n+m/2−k)mole per mole of carbon in the feed.The carbon conversion was calculated by the total mol carbon in the gaseous products divided by the mol carbon in the fed bio-oil. Generally,all experiments were repeated three times. The difference for each repeating,in general,ranged from zero to about10%.Temperature distributions in the catalyst bed under different conditions werefirst measured by the thermocouples inserted into different positions in the catalyst bed before running reforming test. Obviously,the temperature of the surface of Ni-Cr wire is higher than that of the near.So we have carefully detected the temperature distribution in the catalyst bed both for the ECR and the CSR in elsewhere [19].The temperature in the center of the catalyst bed,generally,is almost close to the average value in our investigated range(250−500◦C).Generally, the averaged temperature in the catalyst bed was approximately used as the reaction temperature in the ECR and CSR experiments.TABLE I Weight percent of metal elements in the different reforming catalysts prepared.Catalyst Metal element/%Ni Cu Mg Ce Al Cat I:Cu-Mg-Ce-Al08.20 6.197.4337.20 Cat II:Ni-Mg-Ce-Al19.410 6.327.4129.50 Cat III:Ni-Mg-Cu-Al19.498.29 6.32028.80 Cat IV:Ni-Cu-Ce-Al18.237.630 6.9627.00 Cat V:Ni-Cu-Mg-Ce-Al25.95 5.50 3.7211.1521.29III.RESULTS AND DISCUSSIONA.Screening of reforming catalystsThe performance of the bio-oil reforming over var-ious selected mixed oxide catalysts was tested un-der typical reforming conditions(T=400◦C,I=0A, GHSV=10500h−1,S/C=6.9and P=111.1kPa),shown in Table I.Figure2shows the carbon conversion,the yield of hydrogen,the distribution of hydrogen and var-ious carbon-containing products over the selected cat-alysts.In the absence of the Ni element,the catalyst exhibited the lowest yield of hydrogen yield with the highest content of CH4.The Ni based catalyst pro-moted by Cu,Ce,and Mg shows the highest reforming activity—the carbon conversion(about60.5%)and the highest hydrogen yield(circa58.2%)from the bio-oil re-forming.In the above systems,the Ni active ingredient may play an important role in promoting C−C or C−H bonds rupture of oxygenated organic compounds,and the compositions of Cu/Ce/Mg may enhance the activ-ity of the WGS reaction.On the other hand,adding Ce and Mg to the reforming catalysts favors to reduce the carbon-formation during the bio-oil reforming.Ac-cording to present activity tests,the multiple metal doped Ni based catalyst with the atomic mole ratio of 5.6:1.1:1.9:1.0:9.9shows the highest activity for the bio-oil reforming.Thus,much attention in this work was paid to studying the production of hydrogen from bio-oil over the above catalyst,denoted as Cat V.B.Effect of current on reforming of bio-oilIn this work,the performance of the bio-oil reforming was significantly sensitive to the current I through the researched catalyst.Figure3(a)shows dependence of the carbon conversion on I at variousfixed reforming temperature.In the case of I=0(i.e.,CSR),the car-bon conversion was very low(about6.0%)at250◦C, and increased to79.8%at450◦C.This indicates that the mixed oxide catalyst shows good low temperature reforming activity for the bio-oil.When the current passed through the catalyst(i.e.,ECR),however,the carbon conversion was remarkably enhanced by the cur-rent,particularly at lower temperature(250−350◦C). The carbon conversion increased from28.3%to84.5%DOI:10.1063/1674-0068/26/01/109-120c 2013Chinese Physical Society112Chin.J.Chem.Phys.,Vol.26,No.1Li-xia Yuan etal. FIG.2The effect of the catalyst compositions on the bio-oil reforming.(a)The hydrogen yield,(b)the carbon conversion,(c) the concentration of H2,and(d)the concentration of carbon-containing gas over different catalysts respectively.Reforming conditions:T=400◦C,I=0A,GHSV=10500h−1,S/C=6.9and P=111.1kPa.Cat I:Cu-Mg-Ce-Al,Cat II:Ni-Mg-Ce-Al, Cat III:Ni-Mg-Cu-Al,Cat IV:Ni-Cu-Ce-Al,Cat V:Ni-Cu-Mg-Ce-Al.FIG.3Effect of the current on(a)the carbon conversion and(b)the hydrogen yield,measured as a function of current over the Cat V at differentfixed temperatures,other reforming conditions:GHSV=10500h−1,S/C=6.9,and P=111.1kPa.with the current increasing from0A to3.1A at the same temperature of350◦C,and reached nearly com-plete conversion(96.8%)at3.1A and450◦C.Figure 3(b)shows dependence of the hydrogen yield on cur-rent at given temperature.It was observed that the hydrogen yield was also promoted by the current. Figure4depicts the influence of the current on the composition of the gaseous products over the NiCuMgCeAl catalyst.Hydrogen(about62%−76%)and carbon dioxide(21%−29%)are the major products together with smaller amount by-products of carbon monoxide(2%−4%)and a trace amount of methane (<4%).It was found that the concentrations of H2 and CO slightly increased with the current increasing, accompanied by a decrease of concentrations of CO2 and CH4.The above results indicated that the pro-duction of hydrogen from the bio-oil reforming can be realized at low-temperature using the multiple metalDOI:10.1063/1674-0068/26/01/109-120c 2013Chinese Physical SocietyChin.J.Chem.Phys.,Vol.26,No.1Ni Based Catalyst for Hydrogen Generation from Bio-oil113 FIG.4The effect of the current on the distribution of the gas products for the bio-oil reforming over the Cat V.(a)The volume percentage of H2,(b)the volume percentage of CO2,(c)the volume percentage of CO,and(d)the volume percentage of CH4,respectively.Reforming conditions:T=250−450◦C,GHSV=10500h−1,S/C=6.9,and P=111.1kPa.doped Ni based catalyst and the ECR approach.This suggests that the researched catalyst may be one of most suitable candidates for the bio-oil reforming be-cause this non-noble metal catalyst can efficiently re-form the bio-oil to rich H2and CO2at low oper-ating temperature(350−450◦C),being much lower than that using the conventional NiO-based catalysts (600−850◦C).The carbon conversion of the bio-oil us-ing the18%NiO/Al2O3catalyst was very low(about 14.7%)at400◦C and only about60%even at600◦C. Even using ECR method,achieving90%hydrogen yield should perform at550−600◦C using Ni-Al2O3catalyst [19].However,it is possible to produce hydrogen from the bio-oil with high carbon conversion(>90%)and hy-drogen yield(>90%)at low reforming temperature by using the ECR method over the researched catalyst. Lower operating temperature using the researched cat-alyst in the bio-oil reforming would be attributed to higher activity for the bio-oil reforming reactions.The decomposition of the oxygenated organic compounds and the water-gas shift reaction over this catalyst would be described in the next sections.Moreover,the stability of the catalyst in the bio-oil reforming was tested by measuring the carbon conver-sion,the yield of hydrogen and the changes of the prod-ucts compositions as a function of the time on stream. As shown in Fig.5,no obvious changes in the carbon conversion,the yield of hydrogen and the products dis-tribution were observed for about the initial15h un-der typical reforming conditions(I=3.1A,T=400◦C, GHSV=10500h−1,S/C=6.9,and P=111.1kPa).A slight decrease of the catalytic activity was observed for a longer term test.For example,the hydrogen yield gradually decreased by about10%(from about91%to 81%)for30h reforming,and the carbon conversion slightly decreased from about95%to88%(Fig.5(a)). As can be seen from Fig.5(b),the composition alter-ation of the products seems to be trivial during the in-vestigated duration.The comparison of the activity and stability of selected catalysts in hydrogen production from steam reforming of bio-oil is shown in Table II.It is noticed that the catalyst inactivity of the Ni-based cat-alyst and other catalyst in the bio-oil reforming is much more serious as compared with present catalyst,which would be mainly attributed to lower carbon-deposition over the researched catalyst.C.Decomposition of acetic acid-model compound ofbio-oil over the researched catalystThe reaction pathways in the bio-oil steam reforming process are very complex,a lot of potential intermedi-ates and products may be formed.The reaction network may consist of a complex set of numerous reactions with multiple pathways depending on the bio-oil used and the catalyst selected and the operating conditions.AtDOI:10.1063/1674-0068/26/01/109-120c 2013Chinese Physical Society114Chin.J.Chem.Phys.,Vol.26,No.1Li-xia Yuan etal. FIG.5Stability of the Cat V during the bio-oil reforming(T=400◦C,I=3.1A,GHSV=10500h−1,S/C=6.9,P=111.1kPa).(a)The hydrogen yield and carbon conversion and(b)the gaseous products composition.TABLE II Comparison of activity and stability using the selected catalysts for hydrogen production from reforming of bio-oil.Catalyst Main operating condition Maximum value Stability∗T/C I/A S/C GHSV/h H2yield/%Carbon conv./%NiO-Al2O3[23]8250 4.912600090−About15min NiO-Al2O3[19]6000 5.8604852.765.4−Ni-Co/MgO-La2O3-Al2O3[23]8250 4.912600090−About30min 12%Ni/γ-Al2O3[29]8500 6.02600097.071.5−C12A7[18]7500 4.010*******−C12A7-O−/18%Mg[18]7500 4.010*******About3hPt/CeZrO2[30]795010.8309065−−1%Pt/γ-Al2O3[29]7000 6.02600098.375.0−Ni-Cu-Mg-Ce-Al5000 6.91050082.885.9−450 3.1 6.91050095.896.8−400 3.1 6.9105009194About30h∗The stability refers to the duration once the H2yield decreases to the initial values of50%.least three different types reactions should be consid-ered:(i)the steam reforming of the oxygenated organic compounds(C n H m O k)in the bio-oil(Eq.(1)),(ii)the decomposition of the oxygenated organic compounds (C n H m O k→C x H y O z+other fragments or gaseous small molecules),and(iii)the WGS reaction.To further reveal the reaction of bio-oil reforming over the researched catalyst and to avoid the influence of water on the decomposition of the bio-oil,because the crude bio-oil and the pretreated one contained about 20%−40%H2O[19],we selected the acetic acid as the model compound and studied on the thermal decompo-sition over the researched catalyst.As shown in Fig.6, at temperature lower than300◦C in the CSR,the con-version of the acetic acid decomposition was very low (<18%).With increasing temperature from300◦C to 600◦C,the conversion of the acetic acid remarkably increased from about18%to68%without the current increase(Fig.6(a)).The conversion of the acetic acid in-creased from21.3%to56.8%with the current increasing from0A to2.0A at400◦C,and the hydrogen yield si-multaneously increased with increasing of temperature and current(Fig.6(a)and(c)).Main dry gas prod-ucts from the acetic acid decomposition observed are H2,CO,CO2,CH4,and other hydrocarbons(C2and C2+hydrocarbons etc.)(Fig.6(b)and(d)).The com-position of H2slightly increased with increasing tem-perature and current.When the temperature increased from300◦C to400◦C at the current of2A,the content of CO decreased from10.8%to9.7%,while the content of methane increased from7.4%to22.2%,subsequently with temperature increasing,the content of CO began to increase,while the content of methane kept slightly increase until to500◦C then began to decrease,when the temperature increased to600◦C,the content of CO reached22.9%,while the content of methane de-creased to11.5%,however during the whole range of temperature increasing,the CO2content kept decreas-ing.The other hydrocarbons(C2and C2+hydrocar-bons etc.)kept decreasing with increasing temperatureDOI:10.1063/1674-0068/26/01/109-120c 2013Chinese Physical SocietyChin.J.Chem.Phys.,Vol.26,No.1Ni Based Catalyst for Hydrogen Generation from Bio-oil115 FIG.6The decomposition of the model compounds of bio-oil(acetic acid)over the researched catalyst.The effect of temperature on:(a)carbon conversion and hydrogen yield,I=0A,(b)the composition of the gaseous products,I=0A, (c)carbon conversion and hydrogen yield,I=2.0A,(d)the composition of the gaseous products from the decomposition of acetic acid,I=2.0A,respectively.Other conditions:T decomposition=300−600◦C,flow f(Ar)=108mL/min,flow f(acetic acid)=0.2mL/min,GHSV=5000h−1,and P=111.1kPa.and current.From a mechanistic viewpoint,the overall reactions system involved in acetic acid decomposition is very complex.The main reactions can take place, according to the following reactions including decom-position(Eq.(4)−(Eq.6))and Ketonization(Eq.(7))CH3COOH→2CO+2H2(4)CH3COOH→CO2+CH4(5)CH3COOH→C2H4+C2H6+coke(6)2CH3COOH→CH3COCH3+CO2+H2O(7)In addition,we deduced that methane can also be formed by the hydrogenation of CO x that is the metha-nation reaction[21]CO+3H2→CH4+H2O(8)∆H298=−205.9kJ/molCO2+4H2→CH4+2H2O(9)∆H298=−112.9kJ/molwhich was in the following water-gas shift(WGS)ex-periments,we did observe hydrogenation of CO over the researched catalyst).The above results indicated that the catalyst efficiently dissociates the oxygenated organic compounds in the bio-oil over300◦C.D.Water-gas shift activity of researched catalystThe WGS reaction(Eq.(2))is a very important side reaction that affects the equilibrium of both CO and CO2in the reforming of bio-oil.The WGS reaction is an encouraged elementary step for the reforming of bio-oil,for that it can enhance the hydrogen yield.To investigate the WGS activity over the researched cata-lyst,the conversion of CO to CO2was tested using a model mixture of CO/Ar/H2O(1:2:5.2in mole ratio) corresponding to the reforming process within the tem-perature range of interest.As shown in Fig.7,the con-version of CO increases with increasing temperature at low temperature(<400◦C),reaching a near complete conversion(about94.2%)at400◦C.Further increas-ing temperature over450◦C will lead to a decrease of the CO conversion.CO was mainly converted into CO2 with a selectivity of99.2%−100%through the WGS reaction(Eq.(2),∆H298=−41.1kJ/mol),accompanied by the formation of a trace amount of methane around 450◦C through the methanation reaction(Eq.(8),H2 was produced by WGS).The above experimental results suggests that the researched catalyst shows very high activity in WGS reaction in the range of300−600◦C, which agreed with the results that the CO content in thefinal products gases is much lower than that of CO2DOI:10.1063/1674-0068/26/01/109-120c 2013Chinese Physical Society116Chin.J.Chem.Phys.,Vol.26,No.1Li-xia Yuan etal. FIG.7WGS activity over the Cat-V.(a)CO conversion,(b)the selectivity to CO2and CH4,Other reaction conditions: I=0A,CO/Ar=1:2(volume ratio),f(CO+Ar)=120mL/min,f(H2O)=0.15mL/min,T reforming=300−600◦C,S/C=6.0, GHSV=5000h−1,and P=111.1kPa.FIG.8The effect of the current on the decomposition of bio-oil via the homogeneous experiments through the quartz bed in the absence of any catalyst.(a)The carbon conversion,(b)the hydrogen yield.Other conditions:f bio-oil=0.74mL/min, f Ar=210mL/min,GHSV=6000h−1,and P=111.1kPa.from the bio-oil reforming(Fig.4).E.Effect of current on the thermal decomposition ofbio-oilThe influence of current on the decomposition of bio-oil under111kPa was tested via the homogeneous ex-periments with and without the current through the quartz bed in the absence of any catalyst.As shown in Fig.8(a),the bio-oil conversion for the decomposition of bio-oil depends on both the temperature and the cur-rent.Without the current supplied(i.e.,I=0A),the carbon conversion was very low(about8%)at600◦C. However,the carbon conversion increased to34.6%at the same temperature with current increasing to3.2A. It was also observed that the hydrogen yield was en-hanced by the current(Fig.8(b)).To further study the dissociation and reforming pro-cesses,the species desorbed from the catalyst surface were detected by an anionic TOF mass spectrometry under the low-pressure(0.1Pa)using ethanol as a model compound of bio-oil for simplifying the reaction system.Without the current supplied to the catalyst, no ionic signal was observed.When the current passed through the catalyst in Ar,only one peak near the mass number of zero was observed(Fig.9(a)),corre-sponding to the thermal electrons desorbed from the electrified Ni-Cr wire through the catalyst bed.The number density of thermal electronsflowing through the catalyst bed was about8.0×108cm−3at480◦C and I Ni-Cr=3.2A,which was measured by a columned Au-coated Faraday-plate and a amperometer(Keithley model6485).When a mixture gas of ethanol and argon was fed onto the electrified catalyst,a series of peaks appeared in the TOF spectrum(Fig.9(b)), corresponding to e−,H−,CH x−(x=0−4),OH−,and C2H x−(x=0−5),respectively.The ionic fragments of the hydrocarbons(CH x−)and C2H x−(x=0−5) may form through the dissociation of ethanol by the thermal electrons on the catalyst surface(i.e., e−(s)+C2H5OH(s)→CH x−(s)+C2H x−(s)+OH−(s)+H−(s)...;In addition,when a mixture of ethanol/water/argon was fed onto the electrified catalyst,the CO2−peak was obviously observed (Fig.9(c)),which may arise from the ethanol reformingDOI:10.1063/1674-0068/26/01/109-120c 2013Chinese Physical Society。
半导体物理基础(6)

图1
p-n结基本结构
合金温度
降温再结晶
扩 散
缓变结与突变结
5.2 Equilibrium p-n Junction
(平衡状态下的结)
1 空间电荷区(Space charge region)的形成 当p型半导体和n型半导体接触在一起时,在两 者的交界面处存在着一个过渡区,通常称为p-n结.
(3)热击穿 禁带宽度较窄的半导体易发生这种击穿.
6. p-n结中的隧道效应 当p-n结的两边都是重掺杂时: (1) 费米能级分别 进入导带和价带. (2)势垒十分薄.
1.掺杂浓度:掺杂浓度大,击穿电压小.
2.势垒宽度:势垒宽度足够宽,击穿电压小 3.禁带宽度:禁带宽度越宽,击穿电压越大. 4.温度:温度升高,击穿电压增大.
(2)齐纳击穿(Zener berakdown)或隧道击穿
是掺杂浓度较高的非简并p-n结中的击穿机制.
XAB
XD
根据量子力学的观点,当势垒宽度XAB足够窄时,将有 电子穿透禁带.当外加反向电压很大时,能带倾斜严重,势 垒宽度XAB变得更窄.造成很大的反向电流.使p-n结击穿.
* 势垒高度~ ND、NA
4.空间电荷区宽度(Space charge region width)
突变结
N A x p N D xn
5 载流子分布( Carrier distributions)
nx nn0e
qVD qV ( x ) k0T
n p0
n( x ) nn 0
px pn0e
2 q V kT
CT与CD都与p-n结的面积A成正比,且随外加 电压而变化。
点接触式二极管面积很小, CT 、CD :0.5—1pF 面结型二极管中的整流管面积大, CT 、CD :几十—几百pF
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Eo = incident kinetic energy
More crystalline damage at end of range Sn > Se
2009-11-30 集成电路工艺基础
Less crystalline damage Se > S n
26
东华理工大学
Stopping Mechanisms
a.标准的高斯分布, b.峰值向深处移动(轻 的离子注入靶),尾部 向表面扩展; c.分布呈扁平
2009-11-30
集成电路工艺基础
33
东华理工大学
⎡ 1⎛x−R P N(x ) = N maX exp⎢ − ⎜ 2 ⎜ ΔR P ⎢ ⎝ ⎣
⎞ ⎟ ⎟ ⎠
2
⎤ ⎥ ⎥ ⎦
信电学院
N max =
0.4Q ≈ 2π ΔR P ΔR P
2009-11-30
集成电路工艺基础
31
东华理工大学
信电学院
标准偏差与入射能量的关系
2009-11-30
集成电路工艺基础
32
东华理工大学
信电学院
注入离子在靶中的分布 LSS理论(多晶靶)的结果是对称的高斯分布,不同 的杂质,不同程度地偏离对称的高斯分布.
N(x ) = ⎛ (x − R P )2 exp⎜ − 2 ⎜ 2ΔR P 2π ΔR P ⎝ Q ⎞ ⎟ ⎟ ⎠
2009-11-30
集成电路工艺基础
13
东华理工大学
信电学院
离子注入设备
离子注入机包含离子源、分离单元、 加速器、偏向系统、注入室等。
2009-11-30
集成电路工艺基础
14
东华理工大学
信电学院
离子注入机工作原理
首先把待搀杂物质如B,P,As等离子化 利用质量分离器(Mass Seperator)取出需要的杂质离 子。分离器中有磁体和屏蔽层。由于质量,电量的不 同,不需要的离子会被磁场分离,并且被屏蔽层吸收。 通过加速管,离子被加速到一个特定的能级,如 10∼500keV。 通过四重透镜,聚成离子束,在扫描系统的控制下, 离子束轰击在注入室中的晶圆上。 在晶圆上没有被遮盖的区域里,离子直接射入衬底材 料的晶体中,注入的深度取决于离子的能量。 最后一次偏转(deflect)的作用是把中性分离出去。 faraday cup的作用是用来吸收杂散的电子和离子。
东华理工大学
信电学院
离子注入 (Ion Implantation Process)
• • • • 离子注入的优缺点 离子注入设备 离子注入机理 离子注入的应用和 今后的发展趋势
2009-11-30
集成电路工艺基础
1
东华理工大学
信电学院
Q&A
• • • • • Why Semiconductor need to be doped? What is p-type dopant? What is n-type dopant? List at least two ways to dope? Diffusion and Ion Implantation
2009-11-30
集成电路工艺基础
17
东华理工大学
信电学院
离子注入机理
• • • • 离子注入参数:剂量(dose)、射程(range) 阻滞理论(Stopping Mechanism) 沟道效应(Channeling) 退火(Annealing)
2009-11-30
集成电路工艺基础
18
东华理工大学
2009-11-30
集成电路工艺基础
8
东华理工大学
பைடு நூலகம்
信电学院
Ion Implantation
• Independently control dopant profile (ion energy) and dopant concentration (ion current times and implantation time) • Anisotropic(各向异性的) dopant profile • Easy to achieved high concentration dope of heavy dopant atom such as phosphorus and arsenic.
2009-11-30 集成电路工艺基础 36
东华理工大学
信电学院
晶格损伤与退火 晶格损伤
2009-11-30
集成电路工艺基础
37
东华理工大学
信电学院
Implantation
2009-11-30
集成电路工艺基础
38
东华理工大学
信电学院
Annealing
2009-11-30
集成电路工艺基础
39
东华理工大学
Accelerator Voltage : 1 − 200kV Dose ~ 1011 − 1016 / cm 2 Accuracy of dose :< 0.5% Uniformity < 1% for 8" wafer
2009-11-30
集成电路工艺基础
16
东华理工大学
信电学院
Photograph of the Eaton HE3 High Energy Implanter, showing the ion beam hitting the 300mm wafer end-station
* (Charge collected by integrating cup current ) / (cup area) = dose
2009-11-30 集成电路工艺基础 20
东华理工大学
信电学院
Range and Projected Range
离子在半导体中行进的总距 离是射程R(range), 该射程在垂直轴上的投影就 是投影射程Rp。
信电学院
Strip and Clean
2009-11-30
集成电路工艺基础
7
东华理工大学
信电学院
Ion Implantation
• Used for atomic and nuclear research • Early idea introduced in 1950’s • Introduced to semiconductor manufacturing in mid-1970s (1973)
信电学院
Implantation Dose
For singly charges ions (e. g. As )
⎛ Ion Beam current in amps ⎞ ⎛ Implant ⎞ ⎟ ⎜ ⎟×⎜ ⎜ ⎟ ⎜ time ⎟ q ⎠ ⎝ ⎠ φ=⎝ [Implant area ] =# 2 cm
Electronic stopping
2009-11-30
集成电路工艺基础
23
东华理工大学
信电学院
a.电子阻滞
K e ∝ ZiZt Mi Mt Mi + Mt / Zi
3
3
(
2
3
+ Zt
2
3
)
Z为原子序数(质子数),M为杂质和靶原子的分子量。
b.核阻滞 Sn= 2.8×10-15eV·cm2 ( ZiZt /Z1/3)(Mi/ Mi+Mt) 其中 Z=(Zi2/3+Zt2/3 )3/2 一旦已知Sn (E)和S e(E),就可以用下式求RP 。
2009-11-30
集成电路工艺基础
2
东华理工大学
信电学院
Diffusion
• • • • First used to dope semiconductor Performed in high temperature furnace Using silicon dioxide mask Still used for dopant drive-in
2009-11-30
集成电路工艺基础
12
东华理工大学
信电学院
离子注入的优缺点
优点: • 掺杂的均匀性好 • 温度低工艺 • 可以精确控制杂质含量 • 可以注入各种各样的元素 • 横向扩散比纵向扩散要小 得多 • 注入的离子能穿过薄膜 • 无固溶度极限 缺点: • 入射离子对半导体晶格有 损伤 • 很浅和很深的注入分布难 以实现 • 对高剂量注入,产率受限 • 离子注入设备昂贵
2009-11-30 集成电路工艺基础 40
2009-11-30 集成电路工艺基础 15
东华理工大学
信电学院
Ion Implanter
e . g . AsH 3 As + , AsH + , H + , AsH 2
+
Magnetic Mass seperation
$3 − 4 M / implanter ~ 60 wafers / hour
信电学院
退火(annealing)
1)目的: a)消除晶格损伤; b)激活注入杂质. 2)退火条件: 离子注入时,又碰撞引 起能量传递,当传递的能 量大于晶格结合能时,晶 格就会受损伤。存在一个 阈值剂量φth.E> φth时,晶 格完全损伤,衬底表面呈 无定型状态。 φth值的大小与注入能量, 注入物质,靶材料及注入 过程中的衬底温度有关。
2009-11-30
集成电路工艺基础
35
东华理工大学
信电学院
沟道效应 当入射离子平行晶轴时,入射离子很少与晶格相碰,使Rp很大, 不符合LSS理论.实际应用时须有相应的措施,以避免沟道效应.
1.偏离入射方向一定的角度,一般为7°左右; 2.表面沉积多晶层(二氧化硅,多晶硅,氮化硅等); 3.靶处于高温等。
