Fabrication of block copolymer brushes on hollow sphere surface via reverse iodine
高分子专业英语词汇英汉对照关键词:英语高分子词汇英汉对照序号

高分子专业英语词汇英汉对照关键词:英语高分子词汇英汉对照序号中文英文1 高分子 macromolecule, polymer 又称"大分子"。
2 超高分子 supra polymer3 天然高分子 natural polymer4 无机高分子 inorganic polymer5 有机高分子 organic polymer6 无机-有机高分子 inorganic organic polymer7 金属有机聚合物 organometallic polymer8 元素高分子 element polymer9 高聚物 high polymer10 聚合物 polymer11 低聚物 oligomer 曾用名"齐聚物"。
12 二聚体 dimer13 三聚体 trimer14 调聚物 telomer15 预聚物 prepolymer16 均聚物 homopolymer17 无规聚合物 random polymer18 无规卷曲聚合物 random coiling polymer19 头-头聚合物 head-to-head polymer20 头-尾聚合物 head-to-tail polymer21 尾-尾聚合物 tail-to-tail polymer22 反式有规聚合物 transtactic polymer23 顺式有规聚合物 cistactic polymer24 规整聚合物 regular polymer25 非规整聚合物 irregular polymer26 无规立构聚合物 atactic polymer27 全同立构聚合物 isotactic polymer 又称"等规聚合物"。
28 间同立构聚合物 syndiotactic polymer 又称"间规聚合物"。
29 杂同立构聚合物 heterotactic polymer 又称"异规聚合物"。
刚柔嵌段共轭聚合物自组装体系

收稿:2008年12月,收修改稿:2009年3月*国家重点基础研究发展计划(973)项目(No.2009CB930602)、国家自然科学基金项目(No.90406021,20874048)、霍英东青年教师基金项目(No.111051)和江苏省自然科学基金(No.BK2008453)资助**Corresp onding author e mail:iamqlfan@njup ;wei huang @刚柔嵌段共轭聚合物自组装体系*李 迪 张 龙 范曲立**黄 维**(江苏省有机电子与信息显示重点实验室南京邮电大学信息材料与纳米技术研究院 南京210046)摘 要 刚柔嵌段共轭聚合物的自组装是超分子化学研究的热点之一。
本文综述了近年来刚柔嵌段共轭聚合物自组装体系的研究进展。
根据共轭刚性段的不同分类进行阐述,综述了聚芴、二(苯乙烯) 蒽、聚对苯撑、聚对苯乙烯撑、聚对苯撑乙炔、聚(2,5 苯甲酮)、聚噻吩、聚苯基喹啉等作为刚性链段的刚柔嵌段共轭聚合物自组装体系,介绍了刚柔嵌段共轭聚合物的合成和光物理性质;重点评述了刚柔嵌段共轭聚合物在不同溶剂、浓度、温度等条件下自组装形成一维、二维以及三维的周期性微结构,且具有方便的可控性。
概括了刚柔嵌段共轭聚合物自组装体系广阔的应用前景,尤其在光电器件领域有着潜在的应用价值。
最后展望了刚柔嵌段共轭聚合物自组装体系研究和发展的方向。
关键词 刚柔嵌段共聚物 共轭聚合物 自组装中图分类号:O631 1+3;TN383+1 文献标识码:A 文章编号:1005 281X(2009)12 2660 14Self Assembly of Conjugated Rod Coil Block CopolymersLi Di Zhang Long Fan Quli **Huang Wei**(Jiangsu Key Laboratory for Organic Elec tronics &Information Displays and Institute of Advanced Materials,Nanjing University of Posts and Telecommunications,Nanjing 210046,China)Abstract The self assembly of conjugated rod coil block copolymers has recently become one of the hot topics in the research of supramolecular che mistry.This article revie ws the program on the self assembly of conjugated rod coil block copolymers with different rod blocks,including polyfluorene,polydi(styryl) anthracene,poly(para phenylene),poly (para phenyleneethynylene ),poly (para phenylenevinylene ),poly (2,5 benzophenone ),polythiophene,poly (phenylquinoline ),etc.The syntheses and photophysical properties of conjugated rod c oil block c opolymers are introduced.The formation and regulation of well defined one ,two ,or three dimensional conjugated domains in nanoscale dimensions by the self assembly of these copolymers in different solvents,temperature and concentration are reviewed emphatically.The potential applications of the self assembly of conjugated rod c oil block copolymers in many fields,particularly in optoelectronic device,are summarized.Finally,the prospects for the self asse mbly of conjugated rod coil block copolymers are stated.Key words rod c oil block copolymers;conjugated polymers;self assemblyContents1 Introduction2 The syste m of conjugated rod c oil block copolymers2.1 Fluorene basedconjugatedrod coilblockcopolymers2.2 Di (styryl ) anthracene based c onjugated rod coilblock copolymers第21卷第12期2009年12月化 学 进 展PROGRESS I N C HE MISTRYVol.21No.12 Dec.,20092.3 Poly (para phenylene )or oligo (para phenylene)based conjugated rod coil block c opolymers 2.4 Poly (para phenyleneethynylene )or oligo (paraphenyleneethynylene ) based conjugated rod coilblock copolymers2.5 Poly (para phenylenevinylene )or oligo (paraphenylenevinylene) based conjugated rod coil block copolymers2.6 Poly(2,5 benzophenone) based c onjugated rod coil block copolymers 2.7 Polythiophene basedconjugatedrod coilblockcopolymers2.8 Poly(phenylquinoline) based conjugated rod coil block copolymers 2.9 Others 3 Conclusion1 引言在材料科学和有机电子学的研究中,设计大小和形状规则的纳米结构引起了科学家们的广泛兴趣。
DiblockCopolymer中层状结构之.

Diblock Copolymer中層狀結構之Shear Alignment現象中央大學物理系陳培亮e-mail: peilong@.tw前言Diblock Copolymer之層狀結構方向在剪流下之反應是一在學術與應用上均為極為重要之課題,本文簡介目前實驗觀察之現象以及理論上對此問題之分析。
自聚合(self-assembly)系統近年來是在物理,化學及材料科學上積極研究的領域。
此類材料由於分子之間之特殊交互作用,在適當條件下會自動形成毫米(micrometer)尺寸以下之結構,省去了人為製造奈米(nanometer)結構在成本,精確度,製造時間上等等之限制。
因此在現今科學與技術研究對於奈米結構系統重視之時代趨勢下,自聚合材料自然佔有一極為重要之地位。
現有比較普遍之自聚合材料主要有block copolymer, surfactant system, 與液晶材料,本文將集中討論block copolymer。
Block copolymer 是指在一高分子系統中,其中每一個長鏈高分子不再是由單一重複之monomer所組成,而是由不同monomer組成之之鏈串接而成。
如圖一顯示了一些不同block copolymer之可能。
每一個不同粗細之線段可代表了數百以至於數十萬以上monomer之串鏈。
由圖一最簡單之block copolymer為AB copolymer,僅僅是由兩種monomer所組成,一般稱之為diblock copolymer。
一些更複雜之組成也如圖所示,而且我們可以想像為無窮無盡。
Diblock copolymer中自聚合現象通常是說當系統在高溫時,兩種不同monomers(稱之為A與B)可互相混合而形成一均勻材料。
熱力學上之解釋是此時系統之entropy克服了A與B分子間之排斥作用。
當系統溫度降低時,entropy之作用減少,A與B之排斥使系統產生相分離,也就是說系統會產生由A與由B組成之兩個不同之相(phases)。
METHODS FOR MANUFACTURING BLOCK COPOLYMERS AND ART
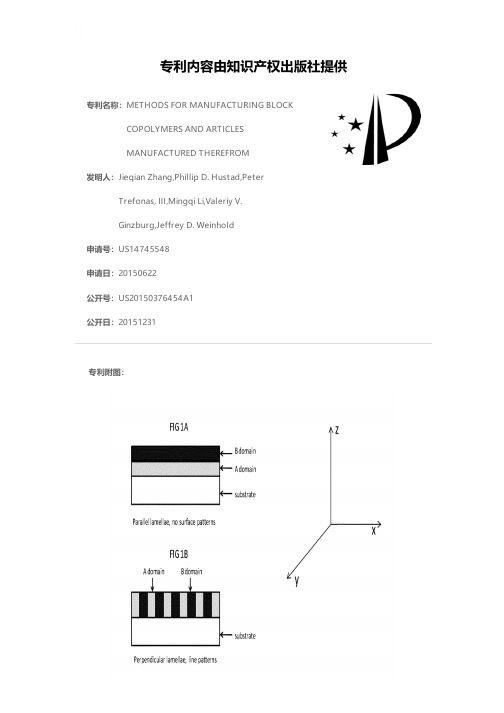
专利名称:METHODS FOR MANUFACTURING BLOCK COPOLYMERS AND ARTICLESMANUFACTURED THEREFROM发明人:Jieqian Zhang,Phillip D. Hustad,PeterTrefonas, III,Mingqi Li,Valeriy V.Ginzburg,Jeffrey D. Weinhold申请号:US14745548申请日:20150622公开号:US20150376454A1公开日:20151231专利内容由知识产权出版社提供专利附图:摘要:Disclosed herein is an article comprising a substrate; upon which is disposed a composition comprising: a first block copolymer that comprises a first block and a second block; where the first block has a higher surface energy than the second block; a second block copolymer that comprises a first block and a second block; where the first block of the first block copolymer is chemically the same as or similar to the first block of the second block copolymer and the second block of the first block copolymer is chemically the same as or similar to the second block of the second block copolymer; where the first and the second block copolymer have a chi parameter greater than 0.04 at a temperature of 200° C.申请人:DOW GLOBAL TECHNOLOGIES LLC,ROHM AND HAAS ELECTRONIC MATERIALS LLC地址:Midland MI US,MARLBOROUGH MA US国籍:US,US更多信息请下载全文后查看。
人大考研-化学系研究生导师简介-金朝霞教授

爱考机构-人大考研-化学系研究生导师简介-金朝霞教授金朝霞教授金朝霞,1970年出生。
北京大学化学系理学学士(1991年);北京大学化学系助理工程师(1991年)、工程师(1996年);新加坡国立大学化学系哲学博士(2002年);韩国国立汉城大学物理系博士后研究(2001年-2002年)。
中国人民大学化学系副教授(2004年6月),教授(2011年7月)。
主要研究方向:a.限域条件下聚合物纳米结构的制备、性质与功能的研究 b.碳纳米材料与聚合物的复合材料的生物医学应用主要科研项目与课题在研课题:国家自然科学基金面上项目21074149(2011.1-2013.12),51173201(2012.1-2015.12)中国人民大学明德学者计划(2009.12-2012.12)北京分子科学国家实验室开放课题(2009.10-2011.12)已完成课题:国家自然科学基金青年项目(2005年,项目号50503025)其他科研项目:中国人民大学科研启动基金教育部归国留学人员启动基金已发表论文:1.S.L.Mei,L.Wang,X.D.Feng,Z.X.Jin*,Swellingofblockcopolymernanoparticles---apro cesscombiningdeformationandphaseseparation,Langmuir2013,29,4640-4646.2.S.L.Mei, Z.X.Jin*,Mesoporousblock-copolymernanospherespreparedbyselectiveswelling,Small2 013,9,322-329.3.S.L.Mei,X.D.Feng,Z.X.Jin*,Polymernanofibersbycontrollableinfilt rationofvapourswollenpolymersintocylindricalnanopores,SoftMatter,2013,9,945-951 .4.X.D.Feng,S.L.Mei,Z.X.Jin*,Wettabilitytransitioninducedtransformationandentra pmentofpolymernanostructuresincylindricalnanopores,Langmuir2011,27,14240-14247.5.S.L.Mei,X.D.Feng,Z.X.Jin*,FabricationofPolymerNanospheresBasedonRayleighInsta bilityinCapillaryChannels,Macromolecules2011,44,1615-1620.6.L.Zhang,D.A.Zha,T.T .Du.S.L.Mei,Z.J.Shi,Z.X.Jin*,Formationofsuperhydrophobicmicrospheresofpoly(viny lidenefluoride-hexafluoropropylene)/graphenecompositeviagelation,Langmuir2011,2 7,8943-8949.7.D.A.Zha,S.L.Mei,Z.Y.Wang,H.J.Li,Z.J.ShiandZ.X.Jin*,Superhydrophob icpolyvinylidenefluoride/grapheneporousmaterials,Carbon2011,49,5166-5172.8.K.K. Zhao,Z.Y.Wang,Z.J.Shi,Z.N.Gu,Z.X.Jin*,Fillingdouble-walledcarbonnanotubeswithWO 3andWnanowiresviaconfinedchemicalreactions,J.Nanosci.Nanotechnol.2011,11,2278-2 282.9.H.L.Fan,L.L.Wang,K.K.Zhao,N.Li,Z.J.Shi,Z.G.Ge,andZ.X.Jin*,Fabrication,Mec hanicalProperties,andBiocompatibilityofGraphene-ReinforcedChitosanComposites,Bi omacromolecules2010,11,2345-2351.10.Q.C.Zhao,J.Yin,X.D.Feng,Z.J.Shi,Z.G.GeandZ. X.Jin*,Abiocompatiblechitosancompositecontainingphosphotungsticacidmodifiedsing le-walledcarbonnanotubes,J.Nanosci.Nanotechno.2010,10,7126-7129.11.X.D.Feng,Z.X .Jin*,SpontaneousFormationofNanoscalePolymerSpheres,Capsules,orRodsbyEvaporatio nofPolymerSolutionsinCylindricalAluminaNanopores,Macromolecules2009,42,569-572.12.Q.C.Zhao,X.D.Feng,S.L.MeiandZ.X.Jin*,Carbonnanotubeassistedhighloadingandcon trolledreleaseofpolyoxometalatesinbiodegradablemultilayerthinfilm,Nanotechnolog y2009,20,105101.13.Z.G.Ge,Z.X.JinandT.Cao,Manufactureofdegradablepolymericscaff oldsforboneregeneration,Biomed.Mater.2008,3,22001.14.Z.X.Jin*,Z.YWang,Z.J.Shi,H .J.Lee,Y.W.ParkandK.Akagi,Thehierarchicalmicrostructureofhelicalpolyacetylenena nofibers,Curr.App.Phys.2007,7,367.15.H.J.Lee,Z.X.Jin,A.N.Aleshin,J.Y.Lee,M.J.Go h,K.Akagi,Y.S.Kim,D.W.Kim,Y.W.Park,"Dispersionandcurrent-voltagecharacteristics ofhelicalpolyacetylenesinglefiber",J.Am.Chem.Soc.2004,126,16722.16.Z.X.Jin,S.H. Goh,G.Q.Xu,Y.W.Park,Dynamicmechanicalpropertiesofmulti-walledcarbonnanotube/poly(acrylicacid)-surfactantcomplex,Synth.Met.2003,135(Sp.Iss.),735-736.17.Z.X.Jin ,K.PPramoda,G.Q.Xu,S.HGoh,Poly(vinylidenefluoride)-assistedmelt-blendingofmulti -walledcarbonnanotube/poly(methylmethacrylate)composites,Mater.Res.Bull.,2002,3 7,271-278.18.Z.X.Jin,L.Huang,S.H.Goh,G.Q.Xu,W.Ji,Size-dependentopticallimitingb ehaviorofmulti-walledcarbonnanotubes,Chem.Phys.Lett.,2002,352,328-333.19.Z.X.Ji n,K.PPramoda,G.Q.Xu,S.HGoh,Dynamicmechanicalbehaviorofmelt-processedmulti-walle dcarbonnanotube/poly(methylmethacrylate)composites,Chem.Phys.Lett.,2001,337,43-47.20.Z.X.Jin,L.Huang,S.H.Goh,G.Q.Xu,W.Ji,Characterizationandnonlinearpropertie sofapoly(acrylicacid)-surfactant-multi-walledcarbonnanotubecomplex,Chem.Phys.Le tt.,2000,332,461-466.21.Z.X.Jin,X.Sun,G.Q.Xu,S.H.Goh,Nonlinearopticalproperties ofsomepolymer/multi-walledcarbonnanotubecomposites,Chem.Phys.Lett.,2000,318,505 -510.22.Z.X.Jin,G.Q.Xu,S.H.Goh,Apreferentiallyorderedaccumulationofbromineonmul ti-wallcarbonnanotube,Carbon2000,38,1135-1139.。
材料科学专业英语词汇(S1)_材料专业英语词汇

s-n curvess-n 曲线saccharin 糖精sacrificial anodes 牺牲阳电极sacrificial red 祭红saddle 鞍座(陶)safety glass 安全玻璃safflower oil 红花子油saggar 匣钵sagging 下垂sago starch 西米淀粉sags 表膜不匀sails 帆salicylaldehyde hydrazone 柳醛? salicylanilide 柳醛苯胺saligenin 水杨醇salt bridge 盐效应salt cake 盐饼salt effect 无盐聚电解salt glaze 盐岫salt rejection 盐挡阻salt solutions 盐溶液saltfree polyelectrolyte solutions 质溶夜saltlike complexes 盐状复体saltpeter 硝石sampling 取样sampling probe function 取样探测功能sand 砂sand cloth 砂布sand filter 砂砾过滤器sand grinder 砂磨sand paper 砂纸sand seal 砂封sand-faced 砂面的sand-lime brick 砂灰砖sand-stone 砂石sanding 铺砂磨sanding disc 金刚砂研磨盘sandpaper 砂纸sandwich cells 夹层电池sandwich complex 夹层复体sandwich construction 夹层建筑sandwich panels 夹层仪表板sang de boeuf 宝石红sanitary earthenware 卫生陶器sanitary landfills 卫生填土santicizers 消毒剂saponification numbers 皂化值saponified acetate process 皂化醋纤法saponified acetate rayon 皂化醋纤嫘萦saponified poly 皂化聚醋酸乙烯sapphire 蓝宝石sapwood 边材saran 赛冉sarcosine 靛蓝 = 磺酸sarfish-type initiators 星鱼型引发剂satin glaze 缎光釉satin white 缎光白sato etching 佐藤蚀刻saturated calomel electrode 饱和甘汞电极saturated hydrocarbon polymers 饱聚体saturation coefficient 饱和系数saturation curve 饱和曲线saturn space vehicle 土星太空舱saucer pit defect 碟状坑缺陷sausage model 烧瓶型saw mark 锯痕saw wire 线锯钢线sawdust 锯屑scab 疤scale 垢(玻)scale wax 鳞状腊,块蜡scaling 片落scan bus method 扫描汇流排法scan path method 扫描路径法scan path test 扫描路径试验scaning electron microscopy 扫描电子显微镜scanned beam current 扫描波束电流scanning acoustic tomograph 超音波断层扫描摄影装置scanning auger electron microscope 扫描型奥格电子显微镜scanning electron microscope 扫描型电子显微镜scanning function 扫描功能scanning projection aligner 扫描型投影对准曝光器scanning transmission electron microscope 扫描透射型电子显微镜scanning tunneling microscopy 扫描隧道型显微镜学scarfjoint 嵌接scattering 散射scattering factor 散射引数scattering loss 散射损失scavenger 清扫机schematic editor 简图编辑器schiff bases 希夫咸schlieren optical system 暗线照像光学系统schreiner calendering 施赖讷压光scintillation 闪烁scintillation counters 闪烁计数机scission 切开scission yields 切开产率scorch time 焦化时间scotch kiln 苏格兰窑scotching 捣打scouring 洗涤scrap 残余物scrap rubber 废橡皮scraping 刮scrapings 刮料scraps 废坯scratch 刮线scratch tests 画痕试验screen 筛screen analysis 筛析screen pack 网包screen printing 网板印染screen printing inks 网板印染油墨screenback hardboard 单面光硬板screening 筛选screening agents 掩蔽剂screening tests 筛分试验screw closures 螺旋盖头screw extrusion 螺杆挤压screw injection-molding machine 螺杆射出模制机screw plasticating injection molding 螺杆塑炼射出模制screw plasticators 螺杆塑炼机screw preplasticators 螺杆预塑机screw press 螺旋压机screw-plunger perplastication system 螺杆活塞预塑系统screwless extruders 无螺杆挤压机screws 螺杆scribing 划片,划割,划线scroop 挤丝机scrubbing 洗气sculpture 雕刻sculpture techniques 雕刻技巧scum 浮霜(陶);浮沫(玻)scumming 乏光(搪)scurf 碳积sea of gate 标准闸门电子组件sea plants 海生植物sea-water magnesia 海水苦土seal 封sealant 封闭剂sealant-grade polysulfide polymers 封闭级多硫化物聚体sealed glass tubes 封闭玻璃管sealers 涂封物sealing 密封sealing glass 熔封玻璃seals 封印seals, cryogenic 低温封印seam 缝seamless flooring 无缝地板铺设search level 搜抹速度search speed 焊接工具保持部下降量,搭接深度season cracking 季候缝裂seasoning 风乾处理seat 座seaweed gums 海藻胶sebacic acid 皮脂酸sebacic acid derivatives 皮脂酸衍sebacic acid esters 皮脂酸酯secco etching 射哥蚀刻second bond off 第二接合点剥离second bonding 第二接合,第二压接second moment 次级偶甩second virial coefficient 第二展向系数second-order fluids 二级流体second-order termination 二级终止second-order transition 二级转变second-surface decorating 亮件背面装饰secondary acetate 仲醋纤secondary air 辅空气;二次空气secondary amines 仲胺secondary antioxidants 副抗氧化剂secondary cellulose acetate 仲醋纤secondary charge effect 二次充电效应secondary crusher 二次辊碎机secondary crystallization 二级晶化secondary electron emission 次生电发射secondary electron image survey function 二次电子影像观测功能secondary emulsifiers 次级乳化剂secondary ion mass spectroscopy 二次离子质谱学secondary mechanical relaxations 二次机械松弛secondary plasticizers 辅助塑化剂secondary structure 际会构架sectioning 割截sedimentary claysecondary clay sedimentation 沈积sedimentation coefficient 沈积系数sedimentation coefficient distribution 沈积系数分布sedimentation equilibrium 沈积平衡sedimentation velocity 沈积速率sedimentation-velocity 沈积速度法see saw type wire saw 交互转换式线锯seed chuck 籽晶夹头seed crystal 籽晶seed cut 种子棒切割seed fibers 种籽纤维seed flax 亚麻种籽seed lift rate 籽晶升降速率seed lift travel 籽晶升降行程seed rotation rate 籽晶旋转速率seed shaft 籽晶轴seeding 播种seedlac 粗虫胶seedmeal glues 籽肉胶seedmeal proteins 籽肉蛋白质segment anisotropies 段间向异性segment fraction of polymer 聚体片段segment-interaction parameters 段间互应变根segmental friction factors 段间磨擦因素segmental jump concept 段间跳跃概念segmented polyurethanes 段间聚胺酯segregation 偏析segregation of noncrystallizable impurities 不结晶什质的分离selection of materials 物料选择selective deposition 选择淀积selective precipitation 选择沈淀selectivity 选择性selectivity coefficient 选择系数selenium polymers 晒化物聚体selevtive elution 选择洗提self bias 自给偏压self diagnostic function 自我诊断功能self-aligned contact etching 自我对准接解孔蚀刻self-extinguishing 自行熄灭self-extinguishing property 自熄特性self-ignition temperature 自燃温度self-nucleating technique 自核技巧self-organization 自引组合self-potting tubing 自熔制管semi full cutting 半全切割semi-conducting glaze 半导性釉semi-conductor 半导体semi-continuous kiln 半连续窑semi-porcelain 半瓷semi-silica refractory 半矽质耐火物semi-vitreous 半瓷化;半玻化semiautomatic controls 半自动控制semiautomatic molds 半自动模semibleached pulp 半漂白纸浆semicarbazide 氨基semichemical pulp 半化学纸浆semichermical pulping 半化学纸浆法semiconducting jacket 半导包套semiconduction 半导性semiconductive polymers 半导聚体semiconductor bonding wafer 半导体接合晶圆semiconductor devices 半导体设备semiconductors 半导体semiconduting properties 半导特性semicrystalline polymers 半晶聚体semicustom ic 客户半定制ic semidurable fire-retardant finish 半永久性防火尾工semigloss paints 半有光油漆semigloss wall paints 半有光壁漆semimechanical pulping 半机械纸浆法semipermanent storage structures 半永久储存构架semipermeable membranes 半透膜semipositive molds 半溢式模semirigid cellular materials 半硬多孔物料semirigid cellular plastics 半硬多孔塑胶semitransparent materials 半透明物料sender 发送机sensitization 敏化sensitizers 敏化剂sentinel pyrometer 示温锭separan 赛派栏絮凝剂separate feeding 隔开输送separate-pot mold 分罐模separation by implantation of oxygen soi wafersimox soi 晶圆separation factor 分离因素sephadex chromatography 赛发呆移差术sepiolite 海泡石septaphosphate 七磷酸盐sequence 次序sequence control 次序控制sequence copolymers 定序共聚体sequence distribution 序列分配sequence, nonrandom 非随意序列sequence-length distribution 序列长度分配sequential damper 时序风门,时序排气器sequential pattern generator 序列图案产生器sericin 丝胶sericite 绢云母series-zone model 层域模式serine proteinases 丝氨酸蛋白质serpentine 蛇纹石serrated saddle 齿状垫座serum albumin 血清蛋白serving of cable 辫里sesquimethylolurea 倍半甲基set setting 定型,凝结set values 定型值seter 托架seting 装窑;凝结setting length of tool 压接头至超音波叭头之设定长度setting-up agent 釉稠调节剂setup boxes 装置匣sewage treatment 污水处理sewer brick 污[水]沟砖sewer pipe 污水管sewing 缝合sgraffito 刮花shadow wall 隔火墙shaft 炉颈shaft kiln 竖窑shale 页岩shale planer 开石机shallow pit defect 浅坑缺陷shape birefringence 气式双折射shape of beam 光束形状shape, cross-sectional 截面形状shaped articles 特型制品sharp fold surface 锐摺面sharpening 削尖shear 剪切shear creep 剪切蠕变shear degradation 剪切退解shear loading 剪切负荷shear modulus 剪切损失模数shear rate 剪切速率shear relaxation 剪切松弛shear storage modulus 剪切储存模数shear strain 剪应变shear strength 剪切强度shear stress 剪应力shear test 剪切试验shear waves 剪切波shear-cone preplasticator 剪锥预塑机shear-thickening materials 剪力增稠物料shear-thinning fluids 剪力减稠液shearing action 剪切作用shearing mode/failure mode 剪切模式/故障模式sheath 皮鞘sheath-and-core bicom-ponent fibers 鞘蕊双重纤维sheathing-siding 鞘边sheep stock 羊群sheepskins 羊皮纸sheet calendering 全张压延sheet casting 全张浇铸sheet extrusion 全张挤压sheet forming 全张成型sheet glass 平板玻璃sheet molding compounds 全张模制化物sheet polymers 成片聚体sheet rubber 成片橡胶sheeting 压片sheets 板片shelf aging 搁置老化shelf life 搁置寿命shellshelling shell flour 粉shell molding 箱模制shell moulding 壳模制造shellac 虫胶shellac modified 变性虫胶shellolic acids 脑酸shield 掩体shielding glass 屏遮玻璃(从原子能)shielding solvents 掩蔽溶剂shift factor 转移因素shikimic acid 草酸shock isolation 震荡隔离shock resistance 耐冲击性shoe applications 鞋靴用途shoe heels 鞋跟shoe parts 鞋靴零件shoe products 鞋靴产品shoe sloes 鞋底shoe-upper material 鞋面物料shore durometer 鞋靴硬度计shore hardness tester 萧氏硬度[试验]计short-fiber substrates 短纤维衬底short-oil alkyd resins 短油醇酸树脂short-term fracture 短期破断shorten material 减黏材料shortstops 急速中止shot 注射shot capacity 注射能力shoulder 肩部shoulder angle 过肩角shredding 撕裂shrend 水淬shrink mark 收缩记号shrinkage 收缩shrinkage volumetric 容量收缩shrinking stress 收缩应力shrinkproofing 防缩shut-off nozzles 停闭喷咀shutter 快门光闸shuttle kiln 梭动窑side arch 侧拱砖side chains 侧链side etching 侧面蚀刻side lap 侧搭side-by-side bicomponent fibers 并排双重纤维side-seam cements 边缝水泥siderite 菱铁矿sidewall protection layer 侧壁保护层siege 台座sieve 筛sigma-blade mixer 弓刀混合机标志sign off 签字保证sign off simulator 签字保证模拟器signal glass 号志玻璃signal strength 信号强度signs 标志silane diols 矽烷双醇silanemonols 矽烷单醇silanes 矽烷silanolates 矽烷醇衍silanols 矽烷醇silastic 矽橡胶silazane polymers 矽氮烷聚体silex 燧石silica 氧化矽,矽石silica and silicates 矽石及矽酸盐silica fabrics 矽石织物silica fibers 矽石纤维silica fireclay 矽石火黏土silica foam 矽石泡沫silica gel 矽凝胶silica glass 矽石玻璃silica glass membranes 矽石玻璃膜silica mm 矽石silica modulus 矽石模数silica retractory 矽石耐火物silica sand 矽砂silica sol 矽石溶胶silica, amorphous 非晶形矽石silica, synthetic 合成矽石silica-water solutions 矽石水溶液silicate 矽酸盐silicate bond 矽酸黏合剂silicate cement 矽酸盐水泥silicate glasses 矽酸盐玻璃silicon 矽素silicon carbide 碳化矽silicon carbide whiskers 碳化矽晶丝silicon carbide-tungsten wire composite properties 碳矽钨线混合体silicon compiler 矽晶自动编辑器silicon compounds 矽化物silicon oxyhydride 矽氧氢化物silicon polymers 矽聚体silicon-bridged polymers 矽乔聚体silicon-carbon bond 碳矽互silicon-nitrogen polymers 矽氮聚体silicon-nitrogen polymers, linear prepn 线式矽氮聚体silicon-oxygen polymers types 氧矽聚体silicon-oxygen tetrahedron 氧矽四面体silicone 矽峒silicone elastomers 聚矽氧弹体silicone emulsions 聚矽氧乳液silicone fluids 聚矽氧液体silicone gel 聚矽氧凝胶silicone greases 聚矽氧润膏silicone monomers 矽氧单体silicone rubber 聚矽氧橡胶silicone surfactants 聚矽氧界面活化剂silicones 聚矽氧silicones, cellular 蜂窝聚矽氧silicones, reinforced 加强聚矽氧silicones, rtv 室温硫化聚矽氧silicosis 矽肺病silk 蚕丝silk fibroin 蚕丝纤silk gum 蚕丝胶silk protein 丝蛋白silk-screen printing 丝网印刷silk-screen process 丝网印刷法;绢印法sillmanite 矽线石siloxanes 氧矽烷siloxazane polymers 环氧矽氮烷聚体silphenylenes 对一双甲矽烷苯silsesquioxanes silver 倍半氧矽烷银silt density index(sdi)淤泥密度指标(sdi) silver halides 卤化银silver ions 银离子silver luster 银光料silver nitrate 硝酸银silvering 上银silvichemicals 森林化物sily1 hydrides 甲矽烷基氢化物silylamine polymers 甲矽烷基胺聚体silylamines 甲矽烷基胺silylation 甲矽烷化silylization system 甲矽烷基化处理系统simple 凹坑,表面微凹simple extension 简单延伸simple microscope 普通显微镜simple proteins 简单simple shear 简单剪切simple shearing 简单剪切simulated annealing 模拟退火simultaneous grafting 同时接技simultaneous irradiation reactions 同时照射反应sin echo method 旋转回音化sinapy1 alcohol 芥子醇singeing 燃芒single cassette rotor 单个套装匣转子single crystal 单晶体single crystals 单晶single plate 单板single point boding 单端子接合single point tab bonding tool 单点tab 接合工具single side lapping machine 单面磨光机single side polishing machine 单面抛光机single station cleaning equipment 单站洗涤装置single t state 单介状态single wafer processing 单晶圆处理方式single wafer processing cleaner 单晶圆加工洗涤机single-base propellants 单基推进剂single-bridged coordination polymers 单桥配位聚合single-bridged polymers 单桥聚体single-bucket excavator 单斗挖掘机single-cavity centor-gated mold 单穴心闸模single-cavity hot-runner mold 单穴热道模single-crystal fibers 单晶纤维single-crystal patterns 单晶图案single-orifice designs 单孔设计single-point methods 单点法single-screw extruders 单螺杆挤压机single-screw, single-stage extruders 单螺杆单段挤压机single-spindle rotational-molding machine 单心轴回转模型机single-tab gate 单顶闸single-toggle jaw crusher 单肘颚轧机singulation/separate 分离sink mark 沈标sinter 烧结sintered glass 烧结玻璃sintering 熔结sinusoidal experiments 正弦试验sio2 film fluorine doped silicon dioxide 掺杂氟素二氧化矽膜sirtl etching 沙特蚀刻sisal fibers 剑麻纤维sit array 区分地段阵列site 区分地段,划分地段site array 区分地段阵列site binding 定位结合site flatness 区分地段平面度site fpd 区分地段焦点平面偏差site size 区分地段大小site tir 区分地段总指示器读数size 大小;度分;胶料size analysis 粒度分折sizing 上胶sizing materials 上胶物料skein dyeing 纱束染色skein-dyeing machine 纱束染色机skeleton oven 骨架炉skew 相位差,时间偏差skew rays 歪斜光skewback 拱座skid tests 滑距试验skiing 滑溜skim coat 平板纸光skimmed milk 脱脂奶skimmer 撇渣器skimmer block 阻渣砖skin 皮skins 皮类skip measurement 跳越测试skiving 削片slab glass 光学玻璃板slabstock 板材slack waxes 松蜡slag 熔渣slag cement 熔渣水泥slag notch 放渣口slag pocket 积渣室slaking 水化slashing 割裂sleeper wall 地龙墙sleeve 套筒sleeves 套筒slef-extinguishing plastics 自熄塑胶slew rate 转动数率,变化率sliced wafer 已切割晶圆slicing machine 切割机slide-off transfer 胶模印花纸slider-pad extruder 滑垫挤压机slip 滑脱slip additives 助滑添料slip agents 助滑剂slip depressants 抑滑剂slip glaze 泥釉slip joint 滑接头slip plane 滑动面slipware 泥釉陶slotting wheel 起槽磨轮slow pumping/slow roughing 缓慢排气slow vent 缓慢通气sludge removal system 淤泥排除系统slug 泥饼(陶);结块(玻);圆柱媒(泥) slugged bottom 厚底slugging 缓涌slump 坍度(泥);流动度(搪)slump test 流动度试验slurry 泥状研磨剂slurry polymerization 浆状聚合slurry separator 研磨剂分离器slurrying 浆化slush casting 熔附铸造slush cating slushing 熔附模制slushing 减水small-angle electron diffraction 小角电子绕射smalt 花?青;大青smart model 精灵模型smear test 画素之电荷流量试验smeatic state 碟状液晶态smectic phase 碟状液晶相smoke-gray film 烟灰薄膜smokeless powder 无烟火药smokes 烟雾smoothness 平滑度snack foods 小吃食品snakeskin glaze 蛇皮釉snap cure 快速硬化snitaryware 卫生陶瓷snubbing pin 制止销snyder process 斯奈德程序soak time 热炼时间soaking 浸热soaps 肥皂soapstone 皂石socket board 插座基板socket type contract 插座型接触soda cellulose 钠纤维素soda process 钠法soda pulping 钠制浆sodium 钠sodium 2,3,4,6,-tetrachlorophenoxide 2,3,4,6,-四氯苯酚钠sodium acetylide 乙炔钠sodium acrylate 丙烯酸钠sodium alginate 藻酸钠sodium amide 氨基化钠sodium azodiformate 偶氮双甲酸钠sodium bicarbonate 碳酸氢钠sodium borohydride 硼氢化钠sodium carbonate 碳酸钠sodium carboxymethy1-cellulose 甲基纤维素钠sodium carboxymethy1-hydroxyethylcellulose 甲基乙基纤维素钠sodium carrier 钠载体sodium caseinate 酪酸钠sodium cellulose glycolate 乙酸钠纤维素sodium ch1oroacetate 氯醋酸钠sodium chlorite 亚氯酸钠sodium chlorite bleaching 亚氯酸钠漂白sodium dichromate 重铬酸钠sodium dithionite 双硫研酸钠sodium doclecyl sulfate 双硫研酸钠sodium ethylenesulfonate 乙烯研酸钠sodium ethylenesulfonate polymers 乙烯磺钠聚体sodium gallate 没食子酸钠sodium hydrosulfite 次硫酸氢钠sodium hydroxide 氢氧化钠sodium hypotchlorite 次氯酸钠sodium metaborate 偏硼酸钠sodium methacrylate 甲基丙烯酸钠sodium methoxide 甲醇钠sodium o-phenylphenoxide 邻-苯基苯酚钠sodium p-styrenesulfonate 对-苯乙烯磺酸钠sodium perborate 高硼酸钠sodium peroxide 过氧化钠sodium phosphate glasses 磷酸钠玻璃sodium polyacrylate 聚丙烯酸钠sodium polyphosphate 聚磷酸钠sodium polyphosphate solution 聚酸钠溶液sodium polysulfides 多硫化钠sodium protocatechuate 原儿茶酸钠sodium sulfate 硫酸钠sodium tetraborate 四硼酸钠sodium thiocyanate 硫氰酸钠sodium tungstate 钨酸钠sodium-naphthalene complex 钠复体soft fibers 软纤维soft fibrids 软原纤质soft landing 软性着陆soft macro cell 软性巨集功能电路胞soft resin shellac 软树脂虫胶soft roughmg 软性排气soft vent 软性通气soft x rays 软x 光线soft-bake 软性烘烤处理soft-paste porcelain 软质瓷softeners 软化剂softening point 软化点softening points 软化点softpmud process 软泥法software error 软体错误softwood 软材soi lwood lignin 软木质素soil redeposition 土壤调节剂soil release 免污soil releasers 免污剂soil repellents 驱污剂soil retardancy 阻污剂soil retardants 阻污soil stabilization 土壤安定sol-air temperature 溶胶空气温度sol-to-gel transitions 溶胶凝胶转移solar absorptivity 阳光吸收性solar furnace 太阳炉solar radiation 阳光照射solar ulatraviolet radiation 阳光紫外线照射solarization 老化作用solder bonding 焊剂接合solder dip test system 浸焊测试系统solder dipping machine 浸焊剂装置solder plating machine 镀焊装置solder sealing equipment 焊料密封装置solder sleeve 焊铁套筒soldier block 立砌砖sole 炉底solid casting 实铸法solid dolution 固溶体solid fatty polyamides 固体脂肪聚醯胺solid fiber boxes 固体纤维盒solid phase epitaxial growth system 固相磊晶生长系统solid polysulfide elastomers 固体聚硫化物弹体solid propellants 啦体推进剂solid vaporizer 固体蒸发源solid-propellant motors 固体推进剂马达solid-propellant rockets 固体推进剂火箭solid-state measurements 固态测剂solid-state polymerization 固态聚合solidification ratio 固化比率solidification shrinkage crack solidus sols 溶胶soltion viscosity 溶液粘度solubility 溶度solubility coefficient 溶度系数solubility evaluation 溶度评估solubility fractionation 溶度分级solubility of polymers 聚体溶度solubility parameters 溶度参数solubility spectra 溶度谱solubilization 溶化solubilizer removal method 助溶剂除去法solubilizer-deficient feed method 助溶剂不足加料法solubilizing ability 溶化能力soluble polyurethane elastomers 可溶聚氨基甲酸乙弹性体solute 溶质solute-solvent interaction 溶质-溶剂相互作用solution adhesives 溶液粘着剂solution blending 溶液掺合solution ceramics 陶瓷护层solution chlorination 溶液氯化solution coating 溶液涂膜solution condensation 溶液缩合solution copolymerization 溶液共聚合solution dyeing 溶液染色solution extrusion 溶液挤压solution grafting 溶液接技solution heat treatment solution measurements 溶液测定solution polycondensation 溶液聚缩合solution polymrization 溶液聚合solution properties 溶液特性solution spinning 溶液纺丝solution techniques 溶液技术solution-solvent viscosity ratio 溶液溶剂粘度比solution-spun fibers 溶液纺丝纤维solutions 溶液solvent adhesives 溶剂粘着剂solvent bonding 溶剂黏合solvent casting 溶剂浇铸solvent cement 溶剂粘合剂solvent coating 溶剂涂膜solvent cracking 溶剂裂开solvent crazing 溶剂隙裂solvent extraction 溶剂萃取solvent finishing 溶剂尾工solvent gradient 溶剂坡度solvent hydrogen bonding 溶剂氢结合solvent molding 溶剂模制solvent processing 溶剂加工solvent release 溶剂脱离solvent resistance 溶剂抗性solvent selection 溶剂选择solvent transfer coefficients 溶剂移动系数solvent treatment 溶剂处理solvent welding 溶剂焊接solvent-based coatings 溶剂基涂膜solvent-based polishes 溶剂基擦亮剂solvent-bleeding resistance 抗溶剂渗出solvent-polymer systems 溶剂聚体系统solvent-segment interactions 溶剂段节相互反应solventless coating 无溶剂涂膜solvents 溶剂solvents for polymerization 聚合用溶液sonic measurements 声音测定sorbic acid 山梨酸sorbitol 山梨糖醇sorel cement 苏鲁水泥soret effect 抹瑞效应sori 弯度sori control system 弯度控制系统sorption 吸着sorption equilibrium 吸着平衡。
呼吸图案法制备聚苯乙烯有序多孔膜

呼吸图案法制备聚苯乙烯有序多孔膜陈春华;宁文生;沈荷红;金杨福【摘要】有序多孔膜是一种可以应用于众多领域的功能性材料,建立简单、低廉和安全的制备方法能够强有力地推进多孔膜材料的广泛利用.本文用水作为模板剂,以溶解于甲苯的工业通用级聚苯乙烯(PS,PG-22)溶液为成膜材料,利用呼吸图案法成功地制备出多孔膜,并采用扫描电镜(SEM)对所制的多孔膜形貌进行了观察,分别研究了动态和静态气氛、制膜液用量、质量浓度、环境湿度等条件对孔径大小和分布的影响.结果表明,制备有序多孔膜的适宜条件为:在环境温度25℃和用甲苯为溶剂时,聚合物质量浓度为25 mg/mL,用量0.8 mL,环境湿度85%(相对湿度),并保持静态气氛.采用聚苯乙烯溶液多次涂覆方法可以增加多孔膜厚度,有利于提高其机械强度,也为有序多孔碳材料的制备奠定了基础.%Ordered porous film is one kind of functional materials which can be applied in many fields. The preparation method, easy, low-cost and safe, is able to promote porous films into wide application. Breath figure method was used to prepare porous films from polystyrene ( PS, PG-22 ) solved in toluene with water as templating agent. The morphology of the porous film was observed by scanning electron microscopy ( SEM) . The influences of polymer concentration, dosage, humidity, and dynamic or static atmosphere on the structures of porous films were investigated. The results show that the optimal parameters to prepare ordered porous polystyrene films are 25 mg/mL (polymer concentration), 0.8 mL (the dosage of PS solution) and 85% RH( environmental humidity ) under 25 ℃ static atmosphere and toluene as solvent. Multi-coating process with polystyrene can increase the thicknessand the strength of ordered porous films, which lays a foundation for preparation of porous bulk carbon materials.【期刊名称】《材料科学与工艺》【年(卷),期】2016(024)006【总页数】6页(P67-72)【关键词】聚苯乙烯;甲苯;呼吸图案法;有序多孔膜;多次涂覆【作者】陈春华;宁文生;沈荷红;金杨福【作者单位】浙江工业大学化学工程学院,杭州310032;浙江工业大学化学工程学院,杭州310032;浙江工业大学化学工程学院,杭州310032;浙江工业大学材料科学与工程学院,杭州310032【正文语种】中文【中图分类】O631有序多孔材料在微容器和微反应器[1]、图案化模板[2]、细胞培养支架[3]、超疏水表面[4]、催化剂载体[5]、光电材料[6]等研究领域有着重要的应用前景.在各种制备有序多孔材料的方法中,呼吸图案法擅长于制备聚合物多孔结构材料,其孔径介于几百纳米到几十微米之间,与传统的模板法相比,呼吸图案法具有实验操作简单、条件温和且可以通过实验条件对孔径大小实现调控等优点,同时,通过对这些成膜材料进行二次化学处理可以极大地扩展多孔膜所能应用的领域,这些独特的优势使其在多孔材料制备领域备受欢迎.1994年,Francois等[7]首次以PS苯撑嵌段聚合物为成膜材料,利用呼吸图案法成功制备出了蜂窝状多孔膜.在此之后的很长一段时间内,人们都认为只有以星型结构或者能实现胶束组装的嵌段聚合物为成膜材料才能通过呼吸图案法制备出有序多孔结构膜[8-9].随着对呼吸图案法研究的深入,包括线形聚合物[10]、星形聚合物[11]、梳形共聚物[12]、两亲性共聚物[13]、棒线嵌段共聚物[14]、聚合物-冠醚嵌段共聚物[15]、有机/无机杂化物[16]和聚离子复合物[17]在内的一系列聚合物有序多孔薄膜被制备出来.这些聚合物存在着共同的特点,聚合物分子中都含有亲水和疏水基团,在液滴的冷凝阶段,聚合物在亲水/疏水的平衡作用下沉淀析出的同时也有效地保持了水滴的有序排列.Cui等[18]以聚乙烯基吡咯烷酮(PVP)与PS共混制备出了蜂窝状有序膜.Tian等[19]以不含强极性基团的疏水性线性聚合物聚苯醚为成膜材料,获得了蜂窝状多孔结构膜.唐林等[20]以自制的聚(苯乙烯-b-丙烯腈)(PS-b-PAN)嵌段共聚物为成膜材料,利用呼吸图案法成功制备出了以六方阵列形式排列的有序多孔膜.Li课题组[21]在PS共聚物中添加表面活性剂聚苯乙烯-b-聚二甲基硅氧烷,在甲醇蒸汽气氛中对其孔结构进行了研究,最终得到了圆柱状的有序多孔结构.申延明等[22]以不含极性端基的工业线性 PS(Mw =235 000)为膜材料,实现了利用呼吸图案法在高湿度条件下制备蜂窝状多孔膜.本文以工业通用级PS(PG-22,Mw=150 000)为成膜材料,探讨利用呼吸图案法在高湿度环境中制备有序多孔膜的可能性,并在单层膜的基础上进行多次涂覆,研究多层膜的形成规律,为后续有序多孔碳材料的制备及应用奠定了基础.1.1 多孔膜的制备1.1.1 试剂及原料聚苯乙烯(PG-22,Mw=150 000,奇美实业有限公司),甲苯(分析纯,杭州双林化工试剂厂).1.1.2 多孔膜的制备称取一定量的PS于容量瓶当中,往容量瓶中加入一定体积的甲苯,密封后经过超声波震荡溶解成制膜溶液备用.利用注射器将一定量的溶液均匀涂抹在5 cm×5 cm 的干净玻璃上,将玻璃片在一定的湿度条件下放置于25℃气氛中,等待甲苯挥发完毕后,得到PS有序多孔膜.多次涂覆制膜的操作步骤为:在第一层多孔膜制备完成后,利用注射器再在其上均匀涂抹相同量的制膜溶液,并在相同的条件下进行成膜操作,以此类推,得到不同涂覆次数的样品.1.2 样品的表征膜的结构观察:日本Hitachi公司的S-4700扫描电镜(SEM),加速电压15 kV,观察前对样品表面进行镀铂处理.2.1 制膜溶液量对孔结构的影响呼吸图案法是以低沸点有机溶剂挥发导致的水滴凝结为动态模板来制备有序多孔结构,实验的关键在于制备过程中水滴是否稳定,而制膜溶液量、溶剂种类、环境温度、湿度、聚合物浓度、动静态气氛等因素对这个动态过程都有着一定的影响,决定着有序多孔结构能否形成以及膜的多孔结构与形态[23].为了研究制膜溶液用量对膜多孔形貌的影响,分别将0.8、1.0、1.2 mL的PS甲苯溶液均匀地涂抹在5 cm×5 cm的玻璃板基底上,其中溶质的质量浓度为25 mg/mL,再将玻璃板放置在25℃、环境湿度85%的静态气氛中成膜,结果如图1所示.3个膜中都形成了贯穿孔,制膜液用量为0.8 mL时,所形成的孔径相近;随着制膜液用量的增加,出现了大孔和小孔并存的现象,其中的大孔直径变得更大.聚合物成膜时,由于玻璃基板的面积保持不变,制膜溶液一次注入量的多少决定了溶液层厚度,从而影响甲苯溶剂完全挥发所需的时间,相应地决定了水滴的生长时间[24].水滴半径与生长时间的关系为[25]式中:R为水滴半径;t为水滴的生长时间.当制膜溶液用量较少时,溶液层厚度也小,甲苯挥发所需的时间较短,溶液表面冷凝的水滴大小适中,因此得到的膜具有直径相近的孔;随着制膜溶液用量的增加,溶剂完全挥发所需的时间被延长,水滴在溶液表面凝结和生长的时间也就越长,不同阶段形成的水滴直径不同,而且其中的部分相邻水滴发生相互接触而融合成更大的水滴,它是图1(b)和1(c)中出现大孔的原因,因此得到的多孔膜形貌变得越来越不规则.2.2 制膜溶液浓度对孔结构的影响在温度25℃、相对湿度85%的静态气氛中,固定制膜溶液量为0.8 mL,考察了溶液中PS质量浓度对多孔膜的影响,图2是PS质量浓度分别为15、25、35和45 mg/mL时所制得的多孔膜的SEM照片.由图2可知,随着聚合物质量浓度逐渐增大,膜的孔径逐渐减小,4种情况下所获得的孔径都比较均匀,孔直径分别为20、10、2.5、1.5 μm.根据拉乌尔定律式中:P0为纯溶剂的蒸汽压;P为溶剂蒸汽压;χB为PS的质量浓度.当制膜溶液中PS质量浓度增大时,溶剂甲苯蒸汽压P减小,导致甲苯的挥发速度变慢,使得溶液表面与环境间的温度差变小,由于单位时间内液滴半径的增加与温度差成正比[26],遵循公式式中:ΔT=Tr-Ts,Tr为环境温度,Ts为表面温度.因此,随着溶液质量浓度的增加,膜的孔径越来越小.2.3 环境湿度对多孔结构的影响呼吸图案法是以低沸点有机溶剂挥发导致的水滴凝结为动态模板来制备有序多孔结构,一定的环境湿度是形成水滴模板的前提条件,因此环境湿度的大小必然对所形成多孔膜的结构产生直接的影响.在环境温度25℃、制膜液用量0.8 mL时,采用25 mg/mL的PS甲苯溶液研究环境湿度(相对湿度分别为60%、85%、95%)对薄膜多孔结构与形貌的影响.由图3可得知:在环境相对湿度为60%条件下所制得的聚合物膜为无色透明的,没有形成通透的孔;85%环境湿度时,形成了孔径均匀的膜;但环境湿度增加到95%,由于冷凝到聚合物表面的液滴增多,相邻液滴之间融合、生长,孔径也就逐渐增大,分布较广.2.4 流动气氛对孔结构的影响成膜环境气氛的流动性对所形成膜的孔结构有着显著的影响.在保持和图2相同的湿度、温度和制膜溶液用量的前提下,研究了PS质量浓度分别为15、25、35mg/mL制膜溶液在流动气氛中所形成的膜形貌,结果见图4.与在静态气氛下制备的膜(图2)相比,流动气氛制的膜是大孔和小孔同时共存,PS质量浓度为15 mg/mL时,孔的形状不规则;PS质量浓度为25和35 mg/mL时,孔都呈现圆形.这可能是因为,随着PS质量浓度的提高,甲苯挥发时间缩短,有利于孔形成速度加快,而溶液粘度增加有利于冷凝下来的水滴保持圆形.比较图2和图4可知,在制膜溶液的浓度相同时,在静态气氛下得到的膜孔径均小于对应的动态气氛下形成的膜.呼吸图案法实验的关键在于对水滴的控制[27],当样品处于流动气氛中时,容易造成局部的湿度偏高,液滴的生长过程难以控制,气流也能引起水滴之间的聚结,因此得到直径大小不等的孔.2.5 涂覆次数对多孔结构的影响在确定了制备具有均匀孔径的单层膜方法基础上,进一步开展了通过多次涂覆制膜溶液制备多层复合膜的工作,以探讨涂覆次数对于多孔膜结构与形貌影响.实验是在25℃和相对湿度为85%的静态气氛下进行的,PS甲苯溶液质量浓度为25mg/mL,制膜溶液每次的用量为0.8 mL,所制的多孔膜形貌如图5所示.从图5可以直观地看到,随着涂覆次数的增加,多次涂覆后的孔形成位置是在第一层膜孔的上方形成的,而孔径呈现逐渐增大的趋势,但如其中的插图所示,多次涂覆后的孔径分布仍然较窄,孔径分别处于10~20 μm、20~30 μm、30~50μm和50~60 μm的范围.利用游标卡尺测量这4个样品的厚度,分别为30、50、80、100 μm,随着膜厚度的增加,膜的机械强度也能提高,将能延长膜的使用寿命.本文以工业通用PS(PG-22)为成膜材料,利用呼吸图案法成功制备出了有序多孔膜.通过对制膜液用量、溶液浓度、环境湿度、动静态气氛等成膜条件的研究,发现适宜的制备多孔膜条件为:在环境温度为25℃,甲苯为溶剂时,制膜溶液量为0.8mL,溶液质量浓度为25 mg/mL,环境湿度85%,并保持静态气氛.在单层膜的基础上,利用多次涂覆的方法成功制备出了厚度在30~100 μm内的孔结构相对有序的多孔聚合物膜,它可以提高膜的机械性能和使用寿命.【相关文献】[1]ZHANG W X,WAN L S,MENG X L,et al.Macro⁃porous,protein⁃containing films cast from water⁃in⁃oil emulsions featuring a block⁃copolymer[J].Soft Mat⁃ter,2011,7(9):4221-4227.[2]GALEOTTI F,MROZ W,BOLOGNESI A.CdTe nanocrystal assemblies guided by breath figure tem⁃plates[J].Soft Matter,2011,7(8):3832-3836.[3]LI L,CHEN C,LI J.Breath figures:fabrication of honeycomb porous films induced by marangoni instabilities[J].Mater Chem,2009,19(18):2789.[4]YABU H,SHIMOMURA M.Single⁃step fabrication of transparent superhydrophobic porous polymer films[J].Chemistry of Materials,2005,17(21):5231-5234.[5]WAN L S,LI Q L,CHEN P C.Patterned biocatalytic films via one⁃step self⁃assembly [J].Chem Commun,2012,48(37):4417-4419.[6]WANG J,SHEN H X,WANG C F.Multifunctional ionomer⁃derivedhoneycomb⁃patterned architectures and their performance in light enhancement oflight⁃emitting diodes[J].J Mater Chem,2012,22(9):4089-4092.[7]WIDAWSKI Q,RAWISO M,FRANCOIS B.Self⁃organized honeycomb morphology ofstar⁃polymer polystyrene films[J].Nature,1994,369:387-389.[8]STENZEL⁃ROSENBAUM M H,DAVIS T P,FANE A G,etal.Porouspolymerfilmsand honeycomb structures made by the self⁃organization of well⁃defined macromolecular structures created by living radical polymerization techniques we acknowledge a DAAD (German Academic Exchange Service)scholarship(HSPIII)forDr.M.H.stenzel⁃rosenbaum[J].Angew Chem Int Ed,2001,40:3428-3430.[9]JENEKHE S A,CHEN X L.Self⁃assembly of ordered microporous materials fromrod⁃coil block copolymers[J].Science,1999,283(5400):372-375.[10]PENG J,HAN Y,YANG Y.The influencing factors on the macroporous formation in polymer films by water droplet templating[J].Polymer,2004,45:447-449.[11]LORD H T,QUINN J F,ANGUS S D.Microgel stars via reversible addition fragmentation chain transfer(RAFT)polymerisation⁃a facile route to macroporous membranes, honeycomb patterned thin filmsand inverse opal substrates[J].J Mater Chem,2003,13(11):2819-2824.[12]HERNÁNDEZ⁃GUERRERO M,DAVIS T P,BARN⁃ER⁃KOWOLLIK C,et al.Polystyrene comb polymers built on cellulose or poly(styrene⁃co⁃2⁃hydroxyethyl⁃methacrylate)backbones as substrates for the prepara⁃tion of structured honeycomb films[J].Eur Polym J,2005,41(10):2264-2277.[13]WANG C,LIU Q,SHAO X,et al.One step fabrica⁃tion of nanoelectrode ensembles formed via amphiphilic block copolymers self⁃assembly and selectivevoltam⁃metric detection of uric acid in the presence of high as⁃corbic acid content [J].Talanta,2007,71(71):178-185.[14]WIDAWSKI G,RAWISO B,FRANCOIS B.Membra⁃nas porosas con estructura de panal formadas a partir de condensación de agua[J].Nature,1994,369(2):387-391.[15]PENG J,HAN Y C,FU J,et al.Formation of regular hole pattern in polymer films [J].Macromolecular Chem Phys,2003,204(1):125-130.[16]LI X,ZHANG L,WANG Y,et al.A bottom⁃up approach to fabricate patterned surfaces with asymmetrical TiO2microparticles trapped in the holes of honeycomblike polymer film[J].J Am Chem Soc,2011,133(11):3736-3739.[17]MARUYAMA N,KOITO T,SAWADAISHI T.Water⁃assisted formation of micrometer⁃size honeycomb patterns of polymers[J].Langmuir,2000,15(16):6072-6082.[18]CUI L,PENG J,DING Y,et al.Ordered porous polymerfilms via phase separation in humidity environment[J].Polymer,2005,46(14):5334-5338.[19]TIAN Y,JIAO Q,DING H,et al.The formation of honeycomb structure in polyphenylene oxide films[J].Polymer,2006,47(11):3866-3873.[20]唐林,马晓燕,宋颖,等.利用呼吸图案法制备聚(苯乙烯-b-丙烯腈)有序多孔薄膜[J].物理化学学报,2013,29(5):1107-1114.TANG Lin,MA Xiaoyan,SONG Ying,et al.Ordered porous films prepared by the breath figure method based on polystyrene⁃b⁃polyacrylonitrile[J].Acta Phys⁃Chim Sin,2013,29(5):1107-1114. [21]ZHANG A,WANG J,LI L,et al.Formation of breath figure arrays in methanol vapor assisted by surface active agents[J].ACS Appl,Mater:Interfaces,2014,6(11):8921-8927.[22]申延明,刘东斌,李士凤,等.聚苯乙烯蜂窝状多孔膜的制备及应用[J].功能材料,2012,12(43):1548-1552.SHEN Yanming,LIU Dongbin,LI Shifeng,et al.Synthesis and application of polystyrene porous film with honeycomb pattern[J].Journal of Functional Materials,2012,12(43):1548-1552.[23]栗志广,马晓燕,洪清,等.呼吸图案法制备蜂窝状有序多孔薄膜及其功能化应用[J].物理化学学报,2015,31(3):393-411.LI Zhiguang,MA Xiaoyan,HONG Qing,etal.Functional applications of ordered honeycomb⁃patterned porous films based on the breath figure technique[J].Acta Phys⁃Chim Sin,2015,31(3):393-411.[24]孙航,吴立新.水滴模板法构筑蜂窝状有序多孔膜[J].化学进展,2010,9(22):1784-1798.SUN Hang,WU Lixin.Ordered honeycomb⁃patterned films via breath figures [J].Progress in Chemistry,2010,9(22):1784-1798.[25]FRANCOIS B,PITOIS O.Crystallization of condensa⁃tion droplets on a liquid surface[J].Colloid Polym Sci,1999,277:574-578.[26]BEYSENS D,STEYER A,GUENOUN P,et al.How does dew form[J].Phase Transitions,1991,31(1):219-246.[27]SRINIVASARAO M,COLLINGS D,PHILIPS A,et al.Three⁃dimensionally ordered array of air bubbles in a polymer film[J].Science,2001,292(5514):79-83.。
材料化工专业英语生词本

材料化工专业英语生词本Synthesis 合成Properties 性质Anatase 锐钛矿rutile 金红石brookite板钛矿Crystalline 结晶的nanometer 纳米nanorods/wires纳米棒/线nanocrystals 纳米晶体nanocarriers 纳米载体nanoparticles (NPs)纳米颗粒nanocomposite纳米复合Hierarchical Nanostructures 分层纳米材料titanium dioxide TiO2 polymorphs of titania 多晶型 TiO2 amorphous 非晶的Three-dimensional 3Dfacile and controlled 容易控制hydrothermal 热液的annealing 退火investigate 调查,研究radially 放射状地petal 花瓣thin 薄的thick 厚的morphology 形态The surface area 表面积adsorption-desorption 吸附-解析(ads)orption isotherms 吸附等温线the Brunauer-Emmett-Teller BET 比表面积测试法specific surface areas 比表面积sensitivity 灵敏、灵敏性ethanol 乙醇、酒精ethylene glycol 乙二醇EG化学式C2H6O2分子式:HOC2H4OHsensor 传感器、感应器solar cells太阳能电池biosensors 生物传感器catalyst 催化剂Catalysis 催化photo-catalytic 光催化的inorganic 无机的objective 目标optimize 使完善、使优化optical 光学的magnetic 磁的application 应用bandgap 带隙transition metal oxides 过渡金属氧化物paint 油漆、颜料gas sensor 气敏元件、气敏传感器Li-ion battery 锂离子电池Electrochromic 电致变色的Photochromism 光致变色macro/mesoporous materials 宏/介孔材料CVD(Chemical Vapor Deposition, 化学气相沉积)Anodic 阳极的hydrothermal method 水热法Template 样板、模板oriented attachment 定向附着primary nanoparticle 初级纳米粒子anisotropic非等方性的、各向异性的capping agents 盖髓剂kirkendall effect柯肯达尔效应tetragonal structure 四方结构photovoltaic cells 光伏电池smart surface coatings 智能表面涂层single-phase 单相precursor 先驱、前导Herein 在此处、鉴于、如此 Nanoflakes 纳米片metal-enhanced fluorescence 金属增强荧光fluorophores 荧光团The Royal Society of Chemistry 英国皇家化学学会ESI (Electronic Supplementary Material) 电子补充材料 Innovative 创新的 Polymer 聚合物 Chemical 化学品 Silica 硅 FITC (fluorescein isothiocyanate )荧光异硫氰酸酯EiTC ( Eosin isothiocyanate ) 异硫氰酸曙红Fluorescence spectra 荧光光谱 control sample 对照样品 Dissolve 溶解Characterization 表征 analytical grade 分析纯 ethanol 乙醇ethylene glycol 乙二醇 ammonia aqueous solution (28 wt %)氨水溶液(100公斤里含28公斤) acetone 丙酮分子式:C3H6O 简式:CH3COCH3EtoH 乙醇 ( PS :Et 代表乙基CH3CH2- Me 代表甲基CH3-)TEOS (tetraethyl orthosilicate ) 原硅酸四乙酯the TEOS concentration TEOS 浓度 CTAB (hexadecyltrimethylammonium bromide ) 十六甲基溴化铵The CTAB surfactant CATB 表面活性剂Sinopharm Chemical Reagent Co. 国药集团化学试剂有限公司Polyvinylpyrrolidone (PVP, Mw = 55000) 聚乙烯吡咯烷酮(PVP ,MW = 55000=兆瓦,百万瓦特(megawatt))Rhodamine B (Rh B) 玫瑰精,若丹明B poly(allylamine hydrochloride) (PAH, Mw = 56000) 聚(烯丙胺盐酸盐) Deionized water 去离子水PAH ( polycyclic aromatic hydrocarbon )多环芳族烃 Via 经由、通过the three-neck flask 三颈烧瓶 oil bath 油浴precipitate 沉淀centrifugation 离心分离 rpm 每分钟转数 core-shell 核-壳a surfactant-templating sol-gel approach 表面活性剂模板溶胶 - 凝胶法homo-dispersed solution 均聚物分散夜agitate 搅拌ultrasonically and mechanically 超声波地、机械地solvent extraction method 溶剂萃取法reflux 回流an impregnation method 浸渍方法 vial 小瓶 dilute 稀释composite 合成物、复合物TEM (Transmission electron microscopy )透射电子显微镜copper grids 铜网carbon films 碳膜SEM(Scanning electron microscopy)扫描电子显微镜Spray 喷FESEM(Field-emission scanning el ectron microscopy)场发射扫描电子显微镜LCSM(Laser confocal scanning microscopy )激光共聚焦扫描显微镜X-ray diffraction (XRD) X 射线衍射X-ray diffractometer X射线衍射仪Nitrogen 氮Micromeritcs n. 微晶(粒)学,粉末工艺学;粉体学degas除去瓦斯vacuum 真空BET(The Brunauer-Emmett-Teller) pore volume 孔体积spectrofluorometer 荧光分光剂spectrophotometer分光光度计bandpass 带通PMT voltage (Photomultiplier Tube)光电倍增管电压Confocal luminescence images共聚焦荧光图像Silver 银silica spacer 硅垫片fabricate制造; 伪造; 组装; 杜撰the metal-enhancedMEF(the metal-enhanced fluorescence )金属增强荧光Fluorescence quenching 荧光猝灭FRET (Fo¨rs ter resonance energy transfer )福斯特共振能量转移Optimization 最佳化; 最优化excited-state 激发态plasmon 等离子基元quantum yields 量子产率quantum dots 量子点resonance n.共振,共鸣, 反响, 回声donor–acceptor pairs 给体- 受体对proximity 接近efficiency 效率the transfer distances 传输距离deposite 被沉淀,存放plastic planar substrate塑料平面基板photoluminescence (PL)光致发光luminescent 发光的single nanoparticle sensing单一纳米粒子传感dielectric电介质; 绝缘体adj.非传导性的RE complexes稀土复合Polyelectrolytes聚合高分子电解质Electrolyte电解质Multilayer 多层Concentric 同中心的functionalized organic molecules 官能有机分子conjugation 结合,配合tedious and fussy繁琐和挑剔obstacle n.障碍, 阻碍, 妨害物controlled release,控释detection and probe applications 检测和探头应用general一般的; 综合的; 普通的universal普遍的, 通用的, 全体的Inspired 启发Possess 拥有Pore 孔drugs and macro-molecules 药物和大分子herein在此处, 鉴于, 如此Ag@SiO2@mSiO2(Ag-core@silica-spacer@mesoporo us silica )The preparation procedure编制程序Water-soluble可溶于水的; 水溶性的,微溶于水A high-temperature solvothermal method一种高温溶剂热法Solvent 溶剂Esolution 分辨率twinned structures 联动结构,孪生结构concentration 浓度tune 调节is ascribed to 归因于dilute稀释spherical morphology 球形形态type-IV curves IV型曲线polyelectrolytesodium chloride食盐; 氯化钠plasmonic absorption电浆吸收an intuitive way 以直观的方式unambiguous 不含糊的, 明白的demonstrate 证明antibody 抗体NSF(National Sanitation Foundation)美国国家卫生基金会PRC(The People's Republic of China)中华人民共和国Shanghai Municipality上海市Shanghai Leading Academic Discipline Project上海重点学科建设项目Tri-functional hierarchical三官能分层DSSCs(dye-sensitized solar cells)染料敏化太阳能电池DOI(Digital Object Unique Identifier)是一种数字对象标识体系acid thermal method 酸热法titanium n-butoxid正丁醇钛acetic acid乙酸、醋酸kinetic 动能light-scattering 光散射photoelectrodes 光电极opto-electronic 光电的calcine煅烧short-circuit photocurrent density短路光电流密度open-circuit voltage开路电压compared to 相比,把什么比作什么electron 电子recombination rates 重组率oxide 氧化物inorganic 无机的sub-microspheres 亚微球beads珠子To date 迄今a ruthenium complex light-harvester钌络合物的光收割机volatile 挥发性的photoanode光阳极superior 好的,卓越的photons 光子photovoltaic performance光伏性能In addition to 除。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
This article appeared in a journal published by Elsevier.The attached copy is furnished to the author for internal non-commercial research and education use,including for instruction at the authors institutionand sharing with colleagues.Other uses,including reproduction and distribution,or selling or licensing copies,or posting to personal,institutional or third partywebsites are prohibited.In most cases authors are permitted to post their version of thearticle(e.g.in Word or Tex form)to their personal website orinstitutional repository.Authors requiring further informationregarding Elsevier’s archiving and manuscript policies areencouraged to visit:/copyrightFabrication of block copolymer brushes on hollow sphere surface via reverse iodine transfer polymerizationLi-Ping Wang a ,b ,Li-Hua Dong a ,c ,Jing-Cheng Hao a ,⇑,Xin-Hu Lv b ,Wen-Zhi Li b ,Yu-Chao Li b ,Jin-Ming Zhen b ,Yu-Cheng Hao b ,Fei Ma baKey Laboratory of Colloid and Interface Chemistry,Shandong University,Ministry of Education,Jinan 250100,China bCollege of Materials Science and Engineering,Liaocheng University,Liaocheng 252059,China cSchool of Chemistry and Chemical Engineering,Taishan Medical University,Taian,Shandong 271000,Chinaa r t i c l e i n f o Article history:Received 1March 2011Accepted 1May 2011Available online 9May 2011Keywords:Polymer brushes Hollow sphere RITP‘‘Living’’characteristica b s t r a c tThe block copolymer brushes grafted from hollow sphere surface via reverse iodine transfer polymeriza-tion (RITP)were investigated in this work.A sufficient amount of azo initiator was introduced onto hol-low sphere surface firstly.Then the monomer methyl methacrylate (MMA)was polymerized via surface-initiated reverse iodine transfer polymerization (RITP)using azo group modified hollow sphere as initi-ator.The microstructure of the samples was characterized by FT-IR,1H NMR,respectively.Results indi-cated that the poly(methyl methacrylate)(PMMA)with end functionality of alkyl iodine group had grafted from hollow sphere surface.TEM observations showed that the average diameter of hollow core was central at 1.3–1.4l m and the average wall thickness increased from 103nm to 138nm and 172nm after grafting polymerization of MMA and Tb complex,respectively.The closely linear plots of molecular weight (M n )versus conversion,linear kinetic plots for the free polymer formed in solution and the ability to extend the chains by sequential addition of monomer indicated that the RITP was a controlled process with a ‘‘living’’characteristic.Crown Copyright Ó2011Published by Elsevier Inc.All rights reserved.1.IntroductionControlled/‘‘living’’free radical polymerization is one of the most effective routes to prepare well-defined polymers (predeter-mined molecular weight,narrow distribution,and tailored archi-tectures).The most prominent living free radical polymerization techniques are iniferter (initiation-transfer-termination)method [1],nitroxide-mediated polymerization (NMP)[2],atom transfer radical polymerization (ATRP)[3,4],reversible addition-fragmen-tation chain transfer (RAFT)[5,6],iodine transfer polymerization (ITP)[7],and reverse iodine transfer polymerization (RITP)[8–10].Iodine transfer polymerization (ITP)was discovered by Tatemoto in late 1970s [11],and studied with much interest since the mid-1990s.By using an initiating radical,iodofluorocom-pounds could enter in a controlled process,based on a degenera-tive transfer (DT).The ITP process was first applied to the copolymerization of vinylidene fluoride with hexafluoropropene and was rapidly extended to other common nonhalogenated vinyl monomers.However,ITP exhibits two important limitations [12,13].Firstly,in the initiation system,the chain transfer agents (such as 2-iodo-perfluoropropane,1-iodo-1-chloroethane and 1-phenylethyl iodide)used in ITP are unstable which is due to the weak C–I bond and thus lead to inconvenience upon storage.Secondly,ITP of monomers involving tertiary propagating radicals (such as methacrylates with 1-phenylethyliodide as transfer agent)was not successful because it would require more activated iodoal-kyl compounds such as ethyl 2-iodo-2-methylpropionate,which are inherently even more unstable.To overcome these drawbacks,Tatemoto and Nakagawa [11]reported a new process called re-verse iodine transfer polymerization (RITP)which is based on a di-rect reaction of radicals with molecular iodine I 2.In this way,the reversible chain transfer agent is generated in situ in the reaction mixture,thus avoiding its preliminary synthesis and storage.At present,the study of RITP is relative few and mostly focuses on the investigations of the mechanism.In this work,we wish to report the first successful use of RITP technique to synthesize well-controlled polymer brushes from inorganic substrate.Hollow sphere is a kind of inorganic microspheres with abundant hydroxyl on its surface.It has large inner void,low density and high surface area which account for its actual and potential applications in imaging [14],wave absorber [15],acoustic [16],sensing devices [17],controlled drug-delivery carriers [18]and so on [19,20].Therefore,hollow sphere appears very attractive to be used as the inorganic substrate.The overall synthesis route is listed in Scheme 1.0021-9797/$-see front matter Crown Copyright Ó2011Published by Elsevier Inc.All rights reserved.doi:10.1016/j.jcis.2011.05.003⇑Corresponding author.E-mail address:wangliping5@ (J.-C.Hao).2.Experimental2.1.ReagentsUnless otherwise indicated,chemicals were obtained from commercial suppliers and used as received.The monomer methyl methacrylate(MMA)(AR,Shanghai Chemical Reagent Plant)was washed with10%NaOH and ion-free water,stirred over CaH2 and distilled under reduced pressure prior to use.4,40-azobis (4-cyanopentanoic acid)(ACPA)was purchased from Sigumas Co. The hollow sphere and the4,40-azobis(4-cyanopentanoic acid chloride)(ACPAC)were synthesized following a previously de-scribed procedure respectively[21,22].2.2.InstrumentationThe structure of samples was characterized by Nicolet-5700 Fourier transform infrared spectroscopy(FT-IR)from400cmÀ1to 4000cmÀ1by the KBr pellet methods.The molecular weights and polydispersities(PDI)of the polymers were determined via an alli-ance GPCV2000(Waters,USA)gel permeation chromatographer (GPC)using THF as the eluent at aflow rate of1.0mL minÀ1and operated at40°C.The compositions and the structures of the poly-mers obtained by RITP of MMA were determined by Varian Mer-cury Plus400MHz nuclear magnetic resonance instrument,using CDCl3as a solvent and tetramethylsilane(TMS)as internal refer-ence.Transmission electron microscopy(TEM)analysis was per-formed on a JEOL JEM-1400transmission election microscope. The elemental analyses(EA)for C,H,and N were performed on a GmbH VarioEL elemental analyse system.Fluorescence excitation and emission spectra were recorded on an Edinburgh Instruments FLS920spectrofluorimeter from a450W stable xenon lamp.The thermal decomposition behavior of the materials was examined by means of thermogravimetry(TG)with a heating rate of 10°C minÀ1in the nitrogen atmosphere on a model STA449C simultaneous DSC–TGA(Netzsch Instruments,Germany).L.-P.Wang et al./Journal of Colloid and Interface Science361(2011)400–4064012.3.Synthesis of azo groups immobilized hollow sphereThe target compound was synthesized according to our previ-ous work[23].Typically,hollow sphere(1g),(3-aminopropyl)tri-ethoxysilane(KH-550)(1mL)and toluene20mL were mixed and heated at80°C for11h under nitrogen atmosphere.The mixture wasfiltrated and thefiltrate was washed with toluene to remove excess compound KH-550to give amino-functionalized hollow sphere(Hollow sphere-NH2),which was dried at25°C in vacuum. Elem.Anal.Calcd.(%):C,18.6;H,2.9;N,2.8.Hollow sphere-NH2(1g)was dispersed in dichloromethane (10mL)and0.2g ACPAC was added.The reaction mixture was stir-red at room temperature for6h and stopped to remove the excess ACPAC byfiltration.The whitefiltrate was washed thoroughly with mixed solvent of ethanol and water(V/V=1:1),ethanol and ether to afford azo groups immobilized hollow sphere.Elem.Anal.Calcd. (%):C,19.6;H,3.1;N,4.5.The amount of surface immobilized azo group can be tenta-tively estimated(N@N/hollow sphere=I c)by the following relation:I c¼WN@NÀWN14Â2ð1Þwhere W N@N and W N is the nitrogen weight(per centum)of azo groups immobilized hollow sphere and amino-functionalized hol-low sphere resulted from the element analysis data,respectively. The estimated I c for hollow sphere is0.60mmol gÀ1.2.4.Synthesis of Tb complexSalicylic acid(1.10g,8mmol)and MMA(1.42ml,4mmol)were dissolved in anhydrous ethanol.Terbium nitrate(1.38g,4mmol) was added under stirring and refluxing for6h resulting in the Tb complex(Scheme2).2.5.PMMA brushes growing from hollow sphere surface by RITP methodA mixture of azo groups immobilized hollow sphere(0.11g,azo group content:$0.066mmol)and cyclohexanone(6mL)in the flask was ultrasonically agitated for30minutes and then iodine (0.008g,0.031mmol)and degassed MMA(6mL,0.056mol)were added.After three freeze–thaw-pump cycles,theflask was heated at70°C in an oil bath.The polymerization was conducted in the dark under nitrogen atmosphere with magnetic stirring.After reac-tion,the colloidal stability of the composite hollow spheres isfine because of the kind compatibility of PMMA grafted from hollow sphere surface and the PMMA formed in solution.Theflask was cooled in an ice bath and the mixture was diluted with tetrahydro-furan(THF).The hollow sphere substrate with grafted PMMA(hol-low sphere-g-PMMA)was separated by centrifugation.The crude product was extracted with THF and dried at40°C in vacuum to afford hollow sphere-g-PMMA.2.6.Diblock copolymer brushes on the hollow sphere surfaceAs shown in Scheme1,the synthesis of diblock copolymer brushes was carried out on the hollow sphere-g-PMMA substrate. The procedures were similar to those used for synthesis of the hol-low sphere-g-PMMA hybrid material,except using hollow sphere-g-PMMA hybrid particles as macro chain transfer agent and Tb complex as the second monomer via a conventional ITP process.A general procedure is as follows:The mixture of hollow sphere-g-PMMA hybrid particles(0.02g)and16mL ethanol inflask was ultrasonically agitated for30min and then the AIBN(0.0066g, 0.04mmol)and Tb complex(4mmol)were added.Theflask was subsequently evacuated andflushed with nitrogen.The polymeri-zation was carried out under nitrogen in a70°C oil bath for16h, stopped byfiltration and washing with excess THF to remove un-grafted Tb complex.Finally,the resulting brown powder was dried at40°C in vacuum.3.Results and discussion3.1.Surface-initiated RITP on the functionalized hollow sphere substrateThe hollow sphere-g-PMMA hybrid materials were prepared using RITP method.The chemical state and the topography of the402L.-P.Wang et al./Journal of Colloid and Interface Science361(2011)400–406hollow sphere-g-PMMA hybrid materials were probed by FT-IR,1H NMR and TEM,respectively.Fig.1shows the infrared spectrum of hollow sphere before(Fig.1a)and after(Fig.1b and c)grafting polymerization.In Fig.1a,the peak at3414cmÀ1was attributed to the O–H stretching vibrations of hydroxyl group in hollow sphere,the peak at1087cmÀ1was assigned to the Si–O–Si stretch-ing vibration and the peak at962cmÀ1was a result of Si–OH vibra-tion.The peaks at3000–2800cmÀ1resulted from the C–H stretching vibrations and the peak at1619cmÀ1came from C@C stretching vibrations of benzene ring,which was a result of the small quantity of undissolved polystyrene in hollow sphere.As for Fig.1b,the disappeared peaks at3000–2800cmÀ1and 1619cmÀ1was due to the polystyrene in hollow sphere dissolved in cyclohexanone during reaction.The absorption peak at 2957cmÀ1came from C–H stretching vibration and the peak at 1730cmÀ1represented the C@O stretching vibration of PMMA which indicated the successful grafting between hollow sphere and PMMA.After grafting copolymerization for16h on the hollow spheres-g-PMMA substrate(Fig.1c),several changes were ob-served,the new peak at1615cmÀ1belonged to C@C stretching vibrations of benzene ring in salicylic acid and the strong new peak at1399cmÀ1means COOÀof salicylic acid complexes with Tb3+. The existence of benzene ring and COOÀgives the direct proof for successful grafting of Tb complex on hollow spheres-g-PMMA substrate.The ability to extend the chains by sequential addition of monomer indicated that the process of grafting polymerization on hollow spheres surface is a controlled/‘‘living’’polymerization.In order to get more information about the microstructures of hollow spheres,TEM was performed.The hollow nature of the microspheres was observed in the contrast between the thin dark edge and pale center in TEM observations,as shown in Fig.2.From the TEM images,we can alsofind that the dark edge become thick-er with the process of grafting polymerization.The average diam-eter of the hollow spheres before grafting polymerization centered at1.3–1.4l m with an average wall thickness of103nm indicated by the size distribution histogram.After grafting polymerization of MMA and Tb complex(Fig.2b and c),the average wall thickness in-creased to138nm and172nm,respectively,according to the size distribution histogram,which corresponded to the successful grafting process.L.-P.Wang et al./Journal of Colloid and Interface Science361(2011)400–4064031H NMR spectrum of PMMA formed in solution was performed to verify the reverse iodine transfer polymerization mechanism re-ported previously(Fig.3)[12,13].The signal at2.9ppm is assignedto the methylene(–CH2–C(CH3)(CO2CH3)–I)in the b position of io-dine(c)at the chain end.The signals centered at0.84,1.81,and 3.60ppm are attributed to the methyl–CH3(b),the methylene –CH2–(a),and the methoxy–OCH3(d)groups of the monomer units in PMMA chain respectively.The1H NMR results are consis-tent with chemical shifts of[–CH2–C]nÀ1–(CH3)(CO2CH3)–CH2–C (CH3)(CO2CH3)–I given in the literature[13].3.2.Controlled graft polymerization of PMMA on hollow sphere surfaceA living polymerization is characterized by narrow polydisper-sity products,linear increase in molecular weight with conversion, and the ability to extend the chains by sequential addition of monomer.Table1summarizes the results of all polymerization under various experimental conditions.They offer the narrowTable1Polymerization of methyl methacrylate(MMA)by reverse iodine transfer polymerization(RITP)performed at70°C.Sample Time(h)M n a(Â10À4g molÀ1)M w a(Â10À4g molÀ1)PDI b Conv.(%)Polymer content(wt%)HS-g-PMMA-4h49.7214.78 1.5229.5713.4 HS-g-PMMA-6h612.3516.41 1.3248.0216.2 HS-g-PMMA-8h812.7516.29 1.2451.2119.3 HS-g-PMMA-12h1214.6317.88 1.2280.6920.1 HS-g-PMMA-b-Tb complex-16h c16 5.75 6.44 1.1248.0428.4a Free polymer formed in the solution,obtained by GPC with standard polystyrene as reference.b PDI:molecular weight distribution is calculated from Mw/M n.c HS-g-PMMA-b-complex-16h:using HS-g-PMMA-12h as macroinitiator.polydispersity,the molecular weight increased with the polymeri-zation time.To prove the living nature of reverse iodine transfer polymerization fatherly,the relationship plot between molecularweight and monomer conversion and the kinetic plots are drawed according to Table1.In Fig.4,the linear relationship between the molecular weight of the free polymer formed in the solution and the monomer con-version was observed.Although the exact molecular weight of polymer grafted on the inorganic substrate is not known,its molec-ular weight is expected to be proportional to that of the polymer formed in the solution[24].The closely linear increase in molecu-lar weight with monomer conversion indicated that the process of surface-initiated reverse iodine transfer polymerization of MMA is controlled.The kinetic plots of Ln[M]0/[M](where[M]is the concentration of monomer)versus polymerization time was shown in Fig.5.The linearfirst-order kinetics plot suggested that the radical concentra-tion was constant throughout the polymerization.However,there exist about a two hours induction period.3.3.The ability to form block copolymer brushes on hollow sphere substrateAccording to the mechanism of RITP,the polymer prepared via the RITP-mediated process has an end functionality of alkyl iodine group.Thus,the graft chains prepared in this way on hollow sphere substrate can be used as macroinitiator for subsequent block polymerization or further functionalization.The Tb complex was chosen for the block copolymerization as the Tb complex repeat unit contains MMA group,which can serve as a monomer in the following iodine transfer polymerization(ITP).After the graft copolymerization proceeded for16h on the hol-low sphere-g-PMMA substrate in an ethanol medium,the diblock polymer brushes grafted from hollow sphere surface(hollow sphere-g-PMMA-b-Tb)came into being.Thefluorescence proper-ties of the sample were determined by spectrofluorimeter.Fig.6 shows the excitation spectra of hollow sphere-g-PMMA-b-Tb com-plex.The excitation spectrum of the resulting hybrid materials was obtained by monitoring the emission of Tb3+ions at548nm.The excitation spectrum is dominated by a broad band from290to 330nm in narrow region and the maximum peak is at310nm, which can be attributed to the characteristic absorption of the lan-thanide complexes arising from the efficient transition based on the conjugated double bonds of the aromatic cycle of salicylic acid ligand.These excitation spectra bands are the effective absorption for the luminescence of Tb3+.As a result,the strong green lumines-cence was observed(see Fig.7),indicating that the effective energy transfer took place between the aromatic ligand and the chelated Tb3+ions.In the emission spectrum,there are four characteristic fluorescence emission bands associated with Tb3+.The band at 489nm is assigned to the5D4?7F6electron transition of Tb3+.L.-P.Wang et al./Journal of Colloid and Interface Science361(2011)400–406405The band at around544nm is associated with the5D4?7F5tran-sition of Tb3+,and the bands at about583nm and621nm corre-sponded to the5D4?7F4and5D4?7F3electron transition of Tb3+respectively.Thefluorescence properties analysis and the EDS results conformed that the Tb complex has grafted from the hollow sphere-g-PMMA substrate successfully,which indicated that most of the PMMA chains were living and took part in the de-sired graft copolymerization.3.4.The grafted polymer content of the hybrid materialsFig.8displays the TGA curves of hollow sphere,HS-g-PMMA and HS-g-PMMA-b-Tb complex.The data show three loss zones associated with the evaporation of excess H2O molecules under 100°C(zone I),organic groups on hollow sphere(zone II)and the inorganic salts is remained(zone III).The organic content can be determined from the difference in weight loss between100 and500°C.The polymer contents calculated for the hybrid mate-rial are also summarized in Table1.The value obtained are rela-tively high(13.5–20.1wt%)which accords with the relatively high grafting rate.The grafted polymer content(calculated from TGA data)vs polymerization time was plotted as illustrated in Fig.9.The grafted polymer content exhibited a nearly linear in-crease of polymerization pared with the weight loss curves of the HS-g-PMMA,the loss ratio of HS-g-PMMA-b-Tb com-plex at zone II is larger and the quantity of last remains is smaller than that of the HS-g-PMMA.The calculated organic content of HS-g-PMMA-b-Tb complex(using HS-g-PMMA-12h as macroinitiator) is28.4wt%which is larger than that of HS-g-PMMA-12h,20.1wt%. That is,the Tb complex grafted on hollow sphere is about8.3wt%. The Tb3+content in the hollow spheres can be estimated by the following relation:Tb3þwt%¼M TbTb complexÂ8:3wt%¼159Â8:3wt%¼2:5wt%ð2Þwhere M Tb and M Tb complex is the molecular weight of Tb and Tb complex respectively.The calculated Tb3+wt%in the hollow spheres is about2.5wt%.4.ConclusionThe optical-functional diblock copolymer brushes grafted from hollow sphere surface were synthesized by surface-initiated re-verse iodine transfer polymerization(RITP).After immobilization of the azo initiator,PMMA chains were successfully grafted from the surface of hollow sphere.Moreover,the PMMA grafted from hollow sphere surface had the ability to extend the chains by sequential addition of Tb complex,illustrating the living nature of the polymerization process.TEM images clearly revealed the hollow structure of the hollow spheres and the increase of the average wall thickness with the grafting polymerization.Fluores-cence spectra confirmed that the PMMA-b-Tb complex grafted hol-low sphere hybrid materials exhibited strongfluorescence properties.This work presented a new method to synthesize opti-cal-functional hybrid material with controlled molecular weights and‘‘well-defined’’structures,which may extend potential appli-cations of hollow sphere and RITP.AcknowledgmentThis research was supported by scientific research start up fund for doctor of Liao-Cheng University.References[1]T.Sato,S.Tsuji,H.Kawagauchi,Ind.Eng.Chem.Res.47(2008)6358–6361.[2]Y.K.Chong(bill),F.Ercole,G.Moad,E.Rizzardo,S.H.Thang,A.G.Anderson,Macromolecules32(1999)6895–6903.[3]K.Matyjaszewski,J.Xia,Chem.Rev.101(2001)2921–2990.[4]C.Y.Lin,M.L.Coote,A.Gennaro,K.Matyjaszewski,J.Am.Chem.Soc.130(2008)12762–12774.[5]S.Liu,K.D.Hermanson,E.W.Kaler,Macromolecules39(2006)4345–4350.[6]P.He,L.He,Biomacromolecules10(2009)1804–1809.[7]C.Boyer,D.Valade,L.Sauguet,B.Ameduri,B.Boutevin,Macromolecules38(2005)10353–10362.[8]J.Tonnar,croix-Desmazes,B.Boutevin,Macromolecules40(2007)186–190.[9]J.Tonnar,croix-Desmazes,B.Boutevin,Macromolecules40(2007)6076–6081.[10]B.N.Patra,D.Rayeroux,croix-Desmazes,React.Funct.Polym.70(2010)408–413.[11]M.Tatemoto,T.Nakagawa,Daikin Kogyo Co.,Ltd.,Japan.DE.2729671,1978.[12]croix-Desmazes,R.Severac,B.Boutevin,Macromolecules38(2005)6299–6309.[13]C.Boyer,croix-Desmazes,J.J.Robin, B.Boutevin,Macromolecules39(2006)4044–4053.[14]Q.Peng,Y.Dong,Y.Li,Angew.Chem.Int.Ed.42(2003)3027–3033.[15]M.Han,Y.Ou,L.Deng,J.Magn.Magn.Mater.321(2009)1125–1129.[16]M.Y.Shatalov,S.V.Joubert,C.E.Coetzee,I.A.Fedotov,J.Sound Vib.322(2009)1038–1047.[17]Z.Wu,M.Zhang,K.Yu,S.Zhang,Y.Xie,Chem.Eur.J.14(2008)5346–5352.[18]S.H.Im,U.Jeong,Y.N.Xia,Nat.Mater.4(2005)671–675.[19]X.He,W.Yang,L.Yuan,X.Pei,J.Gao,Mater.Lett.63(2009)1138–1140.[20]C.Oh,Y.G.Lee,C.U.Jon,S.G.Oh,Colloid Surface A337(2009)208–212.[21]M.Chen,L.Wu,S.Zhou,B.You,Adv.Mater.18(2006)801–806.[22]G.Zhai,W.H.Yu,E.T.Kang,K.G.Neoh,C.C.Huang,D.J.Liaw,Ind.Eng.Chem.Res.43(2004)1673–1680.[23]L.P.Wang,W.Z.Li,L.M.Zhao,C.J.Zhang,Y.D.Wang,L.L.Kong,L.L.Li,Mater.Res.Bull.45(2010)1314–1318.[24]W.H.Yu,E.T.Kang,K.G.Neoh,Ind.Eng.Chem.Res.43(2004)5194–5202.406L.-P.Wang et al./Journal of Colloid and Interface Science361(2011)400–406。
