pGL3 basic哺乳动物表达载体说明
pGL3-Basic_Vector质粒图谱及其说明

Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·1.Description ..........................................................................................................22.Product Components and Storage Conditions ............................................23.pGL3 Vector Maps and Sequence Reference Points . (2)A.pGL3-Basic Vector................................................................................................3B.pGL3-Enhancer Vector........................................................................................4C.pGL3-Promoter Vector........................................................................................5D.pGL3-Control Vector...........................................................................................64.Cloning Methods .. (7)A.Cloning Strategies.................................................................................................7B.Preparation of pGL3 Vectors and Insert DNA for Cloning...........................8C.Transformation Protocols for pGL3 Vectors....................................................8D.Isolation of Plasmid DNA (8)5.Transfection of Mammalian Cells ..................................................................96.Assay of Luciferase Activity ............................................................................97.Sequencing of Luciferase Reporter Vectors .. (11)8.Appendix mon Structural Elements of the pGL3 LuciferaseReporter Vectors ................................................................................................12B.Advantages of the pGL3 Vectors.....................................................................13C.The pGL3 Vectors luc + Gene............................................................................14D.Mapping Genetic Elements Located Within DNA position of Buffers and Solutions............................................................16F.References............................................................................................................17G.pGL3-Basic Vector Restriction Sites.................................................................18H.pGL3-Enhancer Vector Restriction Sites.........................................................20I.pGL3-Promoter Vector Restriction Sites.........................................................23J.pGL3-Control Vector Restriction Sites............................................................26K.Related Products. (28)pGL3 Luciferase Reporter VectorsAll technical literature is available on the Internet at: /tbs/ Please visit the web site to verify that you are using the most current version of this Technical Bulletin. Please contact Promega Technical Services if you have questions on useofthissystem.E-mail:********************1.DescriptionThe pGL3 Luciferase Reporter Vectors(a–c)provide a basis for the quantitativeanalysis of factors that potentially regulate mammalian gene expression. Thesefactors may be cis-acting, such as promoters and enhancers, or trans-acting,such as various DNA-binding factors. The backbone of the pGL3 LuciferaseReporter Vectors is designed for increased expression, and contains a modifiedcoding region for firefly (Photinus pyralis) luciferase that has been optimized formonitoring transcriptional activity in transfected eukaryotic cells. The assay ofthis genetic reporter is rapid, sensitive and quantitative. In addition, theseLuciferase Reporter Vectors contain numerous features aiding in the structuralcharacterization of the putative regulatory sequences under investigation.2.Product Components and Storage ConditionsProduct Size Cat.# pGL3-Control Vector20μg E1741 pGL3-Basic Vector20μg E1751 pGL3-Promoter Vector20μg E1761 pGL3-Enhancer Vector20μg E1771 Information on related products, including the Luciferase Assay System, is provided inSections 4–7 and 8.K.Storage Conditions:Store the pGL3 Luciferase Reporter Vectors at –20°C.3.pGL3 Vector Maps and Sequence Reference PointsThe listings of restriction sites for the pGL3 Luciferase Reporter Vectors areprovided in Section VIII.G–J.Note: The specific transcriptional characteristics of the pGL3 Vectors willvary for different cell types. This may be particularly true for COS cells,which contain the SV40 large T antigen. The SV40 large T antigen promotesreplication from the SV40 origin, which is found in the promoter of thepGL3-Promoter and pGL3-Control Vectors. The combination of large T antigenand SV40 origin will result in a higher copy number of these vectors in COScells, which in turn may result in increased expression of the reporter genecompared to other cell and vector combinations.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·3.A. pGL3-Basic VectorThe pGL3-Basic Vector lacks eukaryotic promoter and enhancer sequences,allowing maximum flexibility in cloning putative regulatory sequences.Expression of luciferase activity in cells transfected with this plasmid depends on insertion and proper orientation of a functional promoter upstream from luc +. Potential enhancer elements can also be inserted upstream of the promoter or in the BamHI or SalI sites downstream of the luc + gene.Figure 1. pGL3-Basic Vector circle map. Additional description: luc +, cDNAencoding the modified firefly luciferase; Amp r , gene conferring ampicillin resistance in E. coli ; f1 ori, origin of replication derived from filamentous phage; ori, origin of replication in E. coli.Arrows within luc + and the Amp r gene indicate the direction of transcription; the arrow in the f1 ori indicates the direction of ssDNA strand synthesis.pGL3-Basic Vector Sequence Reference Points:Promoter (none)Enhancer(none)Multiple cloning region 1–58Luciferase gene (luc +)88–1740GLprimer2 binding site 89–111SV40 late poly(A) signal 1772–1993RVprimer4 binding site2080–2061ColE1-derived plasmid replication origin 2318β-lactamase gene (Amp r )3080–3940f1 origin4072–4527upstream poly(A) signal 4658–4811RVprimer3 binding site4760–4779Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·(for 0746V A 08_4AThe pGL3-Enhancer Vector contains an SV40 enhancer located downstream of luc + and the poly(A) signal. This aids in the verification of functional promoter elements because the presence of an enhancer will often result in transcription of luc + at higher levels.Figure 2. The pGL3-Enhancer Vector circle map. Additional description: luc +,cDNA encoding the modified firefly luciferase; Amp r , gene conferring ampicillin resistance in E. coli ; f1 ori, origin of replication derived from filamentous phage; ori,origin of plasmid replication in E. coli . Arrows within luc + and the Amp r gene indicate the direction of transcription; the arrow in f1 ori indicates the direction of ssDNA strand synthesis.pGL3-Enhancer Vector Sequence Reference Points:Promoter(none)Multiple cloning region 1–58Luciferase gene (luc +)88–1740GLprimer2 binding site 89–111SV40 late poly(A) signal 1772–1993Enhancer2013–2249RVprimer4 binding site2307–2326ColE1-derived plasmid replication origin 2564β-lactamase gene (Amp r )3329–4186f1 origin4318–4773upstream poly(A) signal 4904–5057RVprimer3 binding site5006–5025Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·0745V A 08_4AThe pGL3-Promoter Vector contains an SV40 promoter upstream of theluciferase gene. DNA fragments containing putative enhancer elements can be inserted either upstream or downstream of the promoter-luc + transcriptional unit.Figure 3. The pGL3-Promoter Vector circle map. Additional description: luc +,cDNA encoding the modified firefly luciferase; Amp r , gene conferring ampicillin resistance in E. coli ; f1 ori, origin of replication derived from filamentous phage; ori,origin of plasmid replication in E. coli . Arrows within luc + and the Amp r gene indicate the direction of transcription; the arrow in f1 ori indicates the direction of ssDNA strand synthesis.pGL3-Promoter Vector Sequence Reference Points:Enhancer(none)Multiple cloning region 1–41Promoter48–250GLprimer2 binding region 281–303Luciferase gene (luc +)280–1932SV40 late poly(A) signal 1964–2185RVprimer4 binding region2253–2272ColE1-derived plasmid replication origin 2510β-lactamase gene (Amp r )3272–4132f1 origin4264–4719Upstream poly(A) signal 4850–5003RVprimer3 binding region4952–4971Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·51115212832360748V A 08_4AThe pGL3-Control Vector contains SV40 promoter and enhancer sequences,resulting in strong expression of luc + in many types of mammalian cells. This plasmid is useful in monitoring transfection efficiency, in general, and is a convenient internal standard for promoter and enhancer activities expressed by pGL3 recombinants.Figure 4. pGL3-Control Vector circle map. Additional description: luc +, cDNA encoding the modified firefly luciferase; Amp r , gene conferring ampicillin resistance in E. coli ; f1 ori, origin of replication derived from filamentous phage; ori, origin of plasmid replication in E. coli . Arrows within luc + and the Amp r gene indicate the direction of transcription; the arrow in f1 ori indicates the direction of ssDNA strand synthesis.pGL3-Control Vector Sequence Reference Points:Multiple cloning region 1–41Promoter48–250Luciferase gene (luc +)280–1932GLprimer2 binding site 281–303SV40 late poly(A) signal 1964–2185Enhancer2205–2441RVprimer4 binding site2499–2518ColE1-derived plasmid replication origin 2756β-lactamase gene (Amp r )3518–4378f1 origin4510–4965upstream poly(A) signal 5096–5249RVprimer3 binding site5198–5217Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·(for 0747V A 08_4AFigure 5. pGL3 Vector multiple cloning regions. Shown are the upstream and downstream cloning sites and the locations of the sequencing primers (GLprimer2,RVprimer3 and RVprimer4). The large primer arrows indicate the direction ofsequencing. The positions of the promoter (in the pGL3-Promoter and pGL3-Control Vectors) and the enhancer (in the pGL3-Enhancer and pGL3-Control Vectors) are shown as insertions into the sequence of the pGL3-Basic Vector. (Note that the promoter replaces four bases [AAGT] of the pGL3-Basic Vector.) The sequence shown is of the DNA strand generated from the f1 ori.4.Cloning Methods4.A. Cloning StrategiesThe restriction sites for XhoI and SalI have compatible ends, as do BglII and BamHI. Therefore, cloning into the XhoI or BglII sites upstream of luc +, or the downstream SalI or BamHI sites, allows easy interchange of DNA insertsbetween upstream and downstream positions relative to the luciferase reporter gene. Thus, positional effects of a putative genetic element may be readily tested. Cloning fragments into a single site will generally yield both possible orientations relative to the reporter gene, making these effects also readily testable.The other upstream restriction sites may be used for cloning. However, note that some of the sites are required for generating nested deletions (see Section 8.D). Specifically, the KpnI or SacI site is needed to generate a 3´ overhang upstream of the insert.Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·CATTCCGGTACTGTTGGTAAAGCCACCATGGAAGACGCCAAAAACATAAAG . . . (1892bp) . . . GGATCCGTCGACRVprimer35′GGTACCGAGCTCTTACGCGTGCTAGCCCGGGCTCGAGATCTGCGATCTAAGTAAGCTTGG . . .KpnI Acc65ISacIMluINheIXmaI SmaIXhoIBglIIHindIIISV40Enhancerluc+ Coding Region ′BamHI SalI0756M A 08_4A4.B.Preparation of pGL3 Vectors and Insert DNA for CloningThe fragment and vector DNA should be digested with restriction enzymesthat will generate compatible ends for cloning. In some cases, the ends of theDNA fragment may require modification, either by using synthetic linkers, bya PCR amplification using primers containing sites for appropriate restrictionenzymes, or by filling in the restriction site overhangs. It may be advantageousto treat the vector DNA with calf intestinal alkaline phosphatase (CIAP; Cat.#M2825) or TSAP Thermosensitive Alkaline Phosphatase (Cat.# M9910) toremove 5´ phosphate groups, thus preventing reclosure of the vector on itselfwithout an insert. Sufficient DNA should be prepared to perform controlreactions for digestion, ligation and transformation steps.To ensure capture of the correct insert DNA, the desired restriction fragmentcan be purified by electrophoresis on an acrylamide or agarose gel and thenrecovered from the gel by one of several methods, such as using the Wizard®PCR Preps DNA Purification System Technical Bulletin#TB118. Alternatively,unfractionated restriction fragments can be cloned into the target plasmid, andthe desired recombinant then can be identified by gel electrophoresis ofplasmid DNA.Protocols for restriction digestion, alkaline phosphatase treatment, linkerligation and transformation of competent cells can be found in MolecularCloning, A Laboratory Manual(1).4.C. Transformation Protocols for pGL3 VectorsBecause the Luciferase Reporter Vectors are supplied as modified DNA, E. colihosts may be either restriction + or restriction –. The use of a rec A host such asJM109 is preferred because this prevents undesirable recombination betweenthe insert and the host chromosomal DNA. A strain that has an F´ episome isrequired for ssDNA production.Grow JM109 on minimal plates (M-9) supplemented with 1.0mM thiamine-HCl prior to preparation of competent cells and transformation. This selectsfor the presence of the F´ episome.4.D. Isolation of Plasmid DNAThe Wizard®Plus SV Minipreps DNA Purification System (Cat.# A1340,A1470) may be used for small-scale preparation of plasmid DNA for screeningclones. DNA suitable for transfection may be purified using the PureYield™Plasmid Midipreps System (Cat.# A2492, A2495).Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·5.Transfection of Mammalian CellsTransfection of DNA into eukaryotic cells may be mediated by cationic lipid compounds (2), calcium phosphate (3,4), DEAE-dextran (3,5), or electroporation (4). Transfection systems based on cationic lipids (TransFast™ Transfection Reagent, Transfectam®Reagent and Tfx™ Reagents) and calcium phosphate (Profection®Mammalian Transfection System) are available from Promega. Formore information on these transfection reagents, please request the TransFast™Transfection Reagent Technical Bulletin(#TB260), the Transfectam®Reagent Technical Bulletin(#TB116), the Tfx™-Reagents Technical Bulletin(#TB216) or the ProFection®Mammalian Transfection System Technical Manual(#TM012). All of thesedocuments are available on our web site at: /tbs/6.Assay of Luciferase ActivityExperimental strategies using firefly luciferase may involve the analysis of afew samples per day or as many as several thousand samples per hour, and equipment used to measure luminescence may vary from inexpensive, single-sample luminometers to high-end CCD luminometers. To support this widerange of applications, we have developed three luciferase assays with different,but complementary, characteristics: Luciferase Assay System (Cat.# E1500),Bright-Glo™ Luciferase Assay System (Cat.# E2610), Steady-Glo®LuciferaseAssay System (Cat.# E2510), and ONE-Glo™ Luciferase Assay System (Cat.#E6110). Reagent choice depends on the relative importance of experimentalformat, assay sensitivity, and luminescence duration.Table 1. Characteristics of Promega Luciferase Assay Reagents.LuciferaseBright-Glo™Steady-Glo®Assay ONE-Glo™Reagent Reagent Reagent ReagentFormat NH or H NH or H NH NH or HProcess continuous batch bench scale batch orcontinuous Number of Steps1141Sensitivity highest lower higher highSignal Half-Life~30 minutes~5 hours~12 minutes~50 minutes Precision High High High HighestCell Lysis Time~2 minutes~5 minutes NA~3 minutesmaximum maximumNH = nonhomogeneous (first create a lysate); H = homogeneous; NA = not applicablePromega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·6.Assay of Luciferase Activity (continued)The Luciferase Assay System has long been the standard reagent for routine laboratory analysis. Before using this reagent, cells from which the luciferase is to be measured must be washed and lysed. This reagent was optimized for high sensitivity in nonhomogeneous, single-sample measurements. The Luciferase Assay System requires a luminometer fitted with injectors to efficiently measure luminescence in 96-well plates.The Bright-Glo™, Steady-Glo®and ONE-Glo™ Reagents were developed to perform assay reactions within multiwell plates and in the presence of complete cell culture medium: no cell preparation steps such as washing or lysing are required before the luminescence reaction is initiated. All of these are single-step reagents, requiring only addition of the reagent before measuring luminescence. This makes them ideal reagents for efficient and precise quantitation in 96-, 384- and 1536-well plates.The Bright-Glo™ and Steady-Glo®Reagents are complementary in their characteristics based on the inverse relationship between luminescence duration and assay sensitivity (6). Generally, as the half-life of the luminescence increases, assay sensitivity decreases. The Steady-Glo®Reagent provides long luminescence duration (changing only about 10% per hour); however, toachieve this long luminescence duration, the assay sensitivity must be reduced. This reagent was designed for experiments in which many microplates are processed as a batch.In contrast, the Bright-Glo™ Reagent provides high assay sensitivity with shorter luminescence duration (<10% decrease per 5 minutes). This reagent is designed for general research applications and for experiments using robotics for continuous sample processing. Furthermore, as a result of increased sample capacity, the Bright-Glo™ Reagent provides greater assay sensitivity than the Luciferase Assay Reagent in most applications (6).The ONE-Glo™ Reagent provides the ultimate performance for luciferase assays. It features a high-sensitivity assay with extended duration. TheONE-Glo™ Reagent also demonstrates more robust performance and provides reagent handling enhancements.The Luciferase Assay System, Bright-Glo™ Reagent, Steady-Glo®Reagent and ONE-Glo™ Reagent provide the highest standards in assay quantitation, sensitivity and convenience. Since these reagents are based on the same underlying design principles, different reagents can be used as experimental needs change. For more information, request the Luciferase Assay System Technical Bulletin#TB281, the Steady-Glo®Luciferase Assay System Technical Manual#TM051, the Bright-Glo™ Luciferase Assay System Technical Manual#TM052, or the ONE-Glo™ Luciferase Assay System Technical Manual#TM292.When studying promoter functionalities, it is often desirable to include asecond reporter (e.g., Renilla luciferase) as an internal control for normalization. Plasmids derived from pGL3 or pGL4 vectors can be co-transfected with Renilla luciferase vectors, such as phRL-TK, and assayed using the Dual-Luciferase®Reporter Assay System (Cat.# E1910) or the Dual-Glo™ Luciferase AssaySystem (Cat.# E2920).Table 2. Characteristics of Promega Dual-Luciferase Assays.Dual-Luciferase®Dual-Glo™Assay AssayFormat NH HProcess bench scale batchNumber of Steps52Sensitivity higher lowerSignal Half-Life—firefly~9 minutes~2 hoursSignal Half-Life—Renilla~2 minutes~2 hoursPrecision High HighCell Lysis Time~10 minutes~15 minutesmaximum maximumNH = nonhomogeneous (first create a lysate); H = homogeneous7.Sequencing of Luciferase Reporter VectorsYou may desire to sequence the DNA inserted into the Luciferase Reporter Vectors. Two examples of such applications are to determine the exact positionof generated deletions and to confirm production of a site-specific mutation.Three primers are available for sequencing the pGL3 Vectors: RVprimer3 (Reporter Vector Primer 3) for sequencing clockwise across the upstreamcloning sites, RVprimer4 for sequencing counterclockwise across the BamHIand SalI cloning sites downstream of luc+, and GLprimer2 for sequencing counterclockwise upstream of luc+.RVprimer35´-CTAGCAAAATAGGCTGTCCC-3´RVprimer45´-GACGATAGTCATGCCCCGCG-3´GLprimer25´-CTTTATGTTTTTGGCGTCTTCCA-3´RVprimer3 is especially useful for identifying positions of nested deletions.Note:All three primers can be used for dsDNA sequencing, but onlyRVprimer4 and GLprimer2 also may be used for ssDNA sequencing.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·8.Appendix8.A. Common Structural Elements of the pGL3 Luciferase Reporter VectorsExcept for the inclusion of promoters and enhancers, the four pGL3 Luciferase Reporter Vectors are structurally identical. Each plasmid’s distinguishing features are summarized in Section 3. The pGL3 Vectors each contain a high-copy-number prokaryotic origin of replication for maintenance in E. coli , an ampicillin-resistance gene for selection, and a filamentous phage origin of replication (f1 ori) for single-stranded DNA (ssDNA) production. Restriction sites for insertion of DNA fragments are located upstream and downstream of the luciferase gene. Two of the upstream sites (XhoI and BglII) yield cohesive ends compatible with the downstream sites (SalI and BamHI, respectively),allowing the interchange of the DNA insert for rapid analysis of positional effects.Figure 6. Comparison of luciferase activities expressed in HeLa cells transfected with the pGL2-Control and pGL3-Control Reporter Vectors. The expression level of luc + is dramatically higher with the pGL3-Control Vectors. In repeatedexperiments with several cell lines, we observed 20- to 100-fold higher luciferase activity from cells transfected with pGL3-Control. Luciferase activity was measured with a Turner Designs luminometer. (Absolute light values and relative expressionprofiles may vary between different cell types.)1,4001,2001,0008006004002000Improved Expression Level with the pGL3-Control VectorConstruct TransfectedpGL3-ControlVectorpGL2-ControlVectorA v e r a g e R e l a t i v e L i g h t U n i t sFigure 7. A representative experiment comparing luciferase activities expressed in HeLa cells transfected with the pGL2 and pGL3 Vector series. The increase in luciferase expression observed with these new vectors provides greater sensitivity,while maintaining relatively low background luciferase expression.8.B. Advantages of the pGL3 VectorsThe pGL3 Reporter Vectors contain a modified firefly luciferase cDNAdesignated luc + and a redesigned vector backbone. These changes were made to increase luciferase expression, improve in vivo vector stability, and provide greater flexibility in performing genetic manipulations. The modified reporter vectors have resulted in luciferase expression levels dramatically higher than those obtained with pGL2 Reporter Vectors (Figure 6), while maintaining relatively low background luciferase expression (Figure 7).The substantial increase in the expression of luciferase observed with the pGL3Vectors provides greater sensitivity. It may now be possible to obtainmeasurable luciferase expression in cell types that are difficult to transfect or when studying weak promoter elements. Users of the pGL2 and pGL3 Vectors should be aware, however, that absolute light unit values and relative expression profiles vary between different cell types (7). Therefore, it is important to include the appropriate control vectors in all experiments.Further refinements have been made since the pGL3 Vectors became available.Our newest series of luciferase reporter vectors, the pGL4 Luciferase Vectors,provide additional features and benefits as compared to the pGL3 Vectors. For more information, see the pGL4 Luciferase Reporter Vectors Technical Manual #TM259 available at:/tbs/Promega Corporation ·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·80604020Construct TransfectedControl Basic Enhancer PromoterA v e r a g e R e l a t i v e L i g h t U n i t sA v e r a g e R e l a t i v e L i g h t U n i t sConstruct TransfectedControl Basic Enhancer Promoter 08.C. The pGL3 Vectors luc+ GeneModifications that distinguish the luc+ gene from the native luciferase genegenerally fall into four categories: i) the C-terminal tripeptide has beenremoved to eliminate peroxisome targeting of the expressed protein; ii) codon usage was improved for expression in plant and animal cells; iii) two potential sites of N-glycosylation were removed; and iv) several DNA sequence changes were made to disrupt extended palindromes, remove internal restriction sites, and eliminate consensus sequences recognized by genetic regulatory binding proteins, thus helping to ensure that the reporter gene itself is unaffected by spurious host transcriptional signals. (For a detailed description of themodifications to the luc+ gene, see reference 8.)Four major modifications were made to the vector backbone: i) the SV40 early poly(A) signal has been replaced with the SV40 late poly(A) signal to increase the efficiency of transcription termination and polyadenylation of theluciferase transcripts (9); ii) a synthetic poly(A) and transcriptional pause site (10,11) have been placed upstream of the multiple cloning site to terminatespurious transcription, which may initiate within the vector backbone; iii) the small T intron has been removed to prevent reduced reporter gene expression due to cryptic RNA splicing (12,13); and iv) a Kozak consensus sequence (14) has been inserted to increase the efficiency of translation initiation of theluciferase gene (7; Table 3).There is a newer luciferase gene available, luc2. The luc2gene not only shares the same features as luc+, but the sequence was codon-optimized forexpression in mammalian cells. For further information about the luc2genepresent in the pGL4 Luciferase Vectors, see Technical Manual #TM259available at: /tbs/Table 3. Changes Made to the pGL3 Vectors.Changes Made Purpose of Modification Reference Modifications made to Changes eliminate peroxisome(8)the luciferase gene targeting of expressed protein,(luc to luc+).eliminate consensus bindingsequences for various geneticregulatory proteins, improvecodon usage for mammalianand plant cells, and provideconvenient restriction sites.A unique NcoI site created Ability to create N-terminalat 5´ end of luc+ gene. NcoI gene fusions with luc+sites removed from SV40 using unique NcoI site.enhancer and promoterregions.Intron from SV40 small Intron from SV40 small T (12,13)T antigen removed. antigen can reduceexpression when placed 3´of certain genes due tocryptic splicing.Poly(A) site for back-Avoids possible recombination (9,10)ground reduction changed between two SV40 poly(A)from SV40 early site to a sequences in thesynthetic poly(A) and same plasmid.transcriptional pause site.Poly(A) signal for luc+ Late SV40 poly(A) signal is(7)changed from early to more efficient than early SV40late SV40 poly(A) signal.poly(A).Kozak consensus Provides optimal (14) sequence created translation efficiency.immediately 5´ of theluc+ gene.Unique XbaI site User convenience; facilitatescreated just downstream subcloning of the luc+ gene.of the luc+ gene.SmaI site moved to User convenience; blunt-ended insertsinternal position in can now be cleaved on either sidemultiple cloning region.by restriction endonucleases.Promega Corporation·2800 Woods Hollow Road ·Madison, WI 53711-5399 USA·Fax 608-277-2516 ·。
pgl3basic载体原理

pgl3basic载体原理
pGL3 Basic载体是一种荧光素酶报告载体,其原理是基于荧光素酶基因的
表达。
荧光素酶基因能够将无荧光的荧光素转化为有荧光的荧光素,这个过程伴随着光子的释放。
pGL3 Basic载体被广泛用于基因表达和基因克隆的
研究中,它具有多克隆位点和高拷贝的特点,可以方便地插入外源DNA片段,并实现高表达。
通过荧光素酶基因的表达,研究人员可以监测外源
DNA插入后的转录活性,从而了解基因的表达情况。
以上信息仅供参考,如需了解更多信息,建议查阅相关文献或咨询专业人士。
哺乳动物基因表达腺病毒载体

哺乳动物基因表达腺病毒载体
腺病毒载体能高效转导大多数的哺乳动物细胞,并以游离的DNA 形式存在于宿主细胞中。
该载体系统是把外源基因导入体内的优先使用方法,经常用于基因治疗和疫苗。
腺病毒载体来源于诱发普通感冒的腺病毒,野生型腺病毒基因组是线性双链DNA。
腺病毒重组载体构建完成后转染进入包装细胞。
在包装过程中,位于两个反向重复序列(ITRs)之间的DNA片段与由包装细胞表达的病毒蛋白进一步包装成病毒颗粒。
当病毒转导宿主细胞时,两个ITR之间的外源DNA及病毒基因组一起进入细胞,并以游离DNA的形式存在于细胞核中。
哺乳动物细胞表达系统

哺乳动物细胞表达系统按照宿主细胞的类型,可将基因表达系统大致分为原核、酵母、植物、昆虫和哺乳动物细胞表达系统。
与其它系统相比,哺乳动物细胞表达系统的优势在于能够指导蛋白质的正确折叠,提供复杂的N型糖基化和准确的O型糖基化等多种翻译后加工功能,因而表达产物在分子结构、理化特性和生物学功能方面最接近于天然的高等生物蛋白质分子。
从最开始以裸露DNA直接转染哺乳动物细胞至今的30余年间,哺乳动物细胞表达系统不仅已成为多种基因工程药物的生产平台,在新基因的发现、蛋白质的结构和功能研究中亦起了极为重要的作用。
本文主要从表达系统及其两个组成部分一一表达载体和宿主细胞等方面,简要介绍哺乳动物细胞表达系统和相关的研究进展。
研究现状①部分蛋白在哺乳动物细胞中的表达已从实验室研究迈向生产或中试生产阶段。
②已有许多重要的蛋白及糖蛋白利用哺乳动物细胞系统表达和大量制备、生产。
如人组织型血纤蛋白酶原激活因子、凝血因子皿、干扰素、乙肝表面抗原、红血球生成激素、人生长激素、人抗凝血素出,集落刺激因子等。
有些产品已投入临床应用或试用。
③虽然经过多年努力,哺乳动物细胞表达系统的表达水平有大幅度增高,但从整个水平上看仍偏低,一般处在杂交瘤细胞单克隆抗体蛋白产率的下限,即1-30^g/l08细胞/24小时。
有人认为其限速步骤可嚣是在工程细胞中(对于重组蛋白来讲,常是异源的),重组蛋白的分泌效率较低。
1表达载体1. 1表达栽体的类型哺乳动物细胞表达外源重组蛋白可利用质粒转染和病毒载体的感染。
利用质粒转染获得稳定的转染细胞需几周甚至几个月时间,而利用病毒表达系统则可快速感染细胞,在几天内使外源基因整合到病毒载体中,尤其适用于从大量表达产物中检测出目的蛋白。
根据进入宿主细胞的方式,可将表达载体分为病毒载体与质粒载体。
病毒载体是以病毒颗粒的方式,通过病毒包膜蛋白与宿主细胞膜的相互作用使外源基因进入到细胞内。
常用的病毒载体有腺病毒、腺相关病毒、逆转录病毒、semliki森林病毒(sFv)载体等。
pDsRed2-Bid哺乳动物表达载体说明
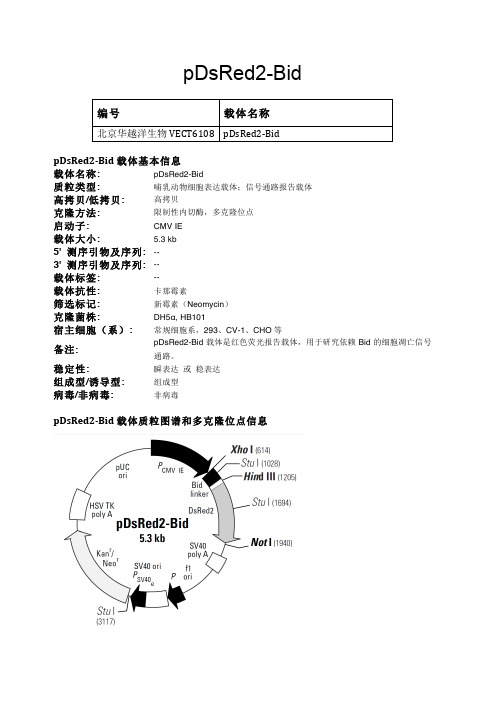
pDsRed2-Bid编号 载体名称北京华越洋生物VECT6108 pDsRed2-‐BidpDsRed2-‐Bid载体基本信息载体名称: pDsRed2-Bid质粒类型: 哺乳动物细胞表达载体;信号通路报告载体高拷贝/低拷贝: 高拷贝克隆方法: 限制性内切酶,多克隆位点启动子: CMV IE载体大小: 5.3 kb5' 测序引物及序列: --3' 测序引物及序列: --载体标签: --载体抗性: 卡那霉素筛选标记: 新霉素(Neomycin)克隆菌株: DH5α, HB101宿主细胞(系): 常规细胞系,293、CV-1、CHO等备注: pDsRed2-Bid载体是红色荧光报告载体,用于研究依赖Bid的细胞凋亡信号通路。
稳定性: 瞬表达或稳表达组成型/诱导型: 组成型病毒/非病毒: 非病毒pDsRed2-‐Bid载体质粒图谱和多克隆位点信息pDsRed2-Bid载体描述pDsRed2-Bid is a mammalian expression vector that encodes a fusion of Discosoma sp. red fluorescent protein (DsRed2; 1, 2) and Bid, a member of the Bcl-2 “pro-apoptosis” family (3). Developed for our ApoAlert line, pDsRed2-Bid is designed to help researchers study Bid-dependent apoptosis pathways. Because of its fluorescent label, the Bid-DsRed2 fusion is easily detected by microscopy, allowing researchers to track its movements in response to certain apoptosis inducing agents (e.g., TNF-α).Bid’s activity is closely tied to its location in the cell. In healthy, non-apoptotic cells, Bid normally resides in the cytosol. But soon after the induction of apoptosis, it translocates to mitochondria, where it stimulates the release of cytochrome c—a key amplification step in the apoptotic cascade (4–7). The translocation is triggered by caspase-8, which, when activated by a death signal, cleaves Bid to produce a 15-kDa C-terminal fragment. The fragment, often referred to as truncated Bid or tBid, transmits the death signal further by translocating to the mitochondria. You can monitor this event visually by expressing Bid as a DsRed2 fusion. Bid-DsRed2, thefusion expressed by this vector, for example, emits red fluorescence—even after truncation—so it can be followed as it moves from the cytosol to the mitochondria. To drive expression of Bid-DsRed2, this vector contains the immediate early promoter of cytomegalovirus (PCMV IE), positioned just upstream of the Bid sequence. A short linker joins the Bid coding sequence to the 5'-end of DsRed2. Farther downstream, the vector contains a pair of SV40 polyadenylation signals, which direct proper processingof the 3'-end of the Bid-DsRed2 mRNA. The vector also contains an SV40 origin for replication in mammalian cells expressing the SV40 T antigen, a pUC origin of replication for propagation in E. coli, and an f1 origin for single-stranded DNA production. A neomycin-resistance cassette (Neor), consisting of the SV40 early promoter, the neomycin/kanamycin resistance gene of Tn5, and polyadenylation signals from the Herpes simplex virus thymidine kinase (HSV TK) gene, allows stably transfected eukaryotic cells to be selected using G418 (8). A bacterial promoter upstream of the cassette confers kanamycin resistance (Kanr) to E. coli.Cells transfected with pDsRed2-Bid constitutively express Bid as an N-terminal fusionto DsRed2. pDsRed2-Bid can be introduced into a variety of mammalian cell lines using any standard transfection method. If required, stable transfectants can be selected using G418 (8).Propagation in E. coliSuitable host strains: DH5α, HB101 and other general purpose strains. Single-stranded DNA production requiresa host containing an F plasmid such as JM109 or XL1-Blue.Selectable marker: plasmid confers resistance to kanamycin (50 μg/ml) to E. coli hosts.E. coli replication origin: pUCCopy number: ~500Plasmid incompatibility group: pMB1/ColE1Red Fluorescent Protein (DsRed2)Excitation/Emission Maxima: 558 nm / 583 nm其他哺乳动物表达载体:pCHO1.0 pBApo-CMV-Pur pOPRSVIpcDNA3.1/His C pcDNA5/FRT/V5-His-TOPO pREP4pcDNA3.1/His B pcDNA5/FRT/TO-TOPO pDual-GCpcDNA3.1/His A pcDNA5/TO pBK-RSVpIRESpuro3 pcDNA5/FRT/TO pBK-CMVpIRES2-EGFP pcDNA5/FRT pBI-CMV4pTT5 pFLAG-CMV2 pcDNA4/TO/Myc-His/LacZ pNFkB-DD-tdTomato pcDNA3.1/CT-GFP-TOPO pOPI3CATpBI-CMV5 pcDNA3.1/NT-GFP-TOPO pGene/V5-His B pSEAP2-Basic pOptiVEC-TOPO pSwitchpSEAP2-Control pCMV-MEKK1 pCMVLacIpBI-CMV3 pCMV-MEK1 pVgRxRpBI-CMV2 pCMV-PKA pINDpBI-CMV1 pcDNA6.2/nTC-Tag-DEST pTRE3G-Luc pNFκB-MetLuc2-Reporter pcDNA6.2/cTC-Tag-DEST pTRE3GpCRE-MetLuc2-Reporter pcDNA3.2/V5/GW/D-TOPO pTRE2-hygro pAcGFP1-Actin pcDNA6.2/V5/GW/D-TOPO pTRE-Tight pAcGFP1-N In-Fusion Ready pcDNA6.2/nGeneBLAzer-GW/D-TOPO pTK-hyg pAcGFP1-C3 pcDNA6.2/C-YFP-DEST pTet-On pAcGFP1-C pcDNA6.2/cGeneBLAzer-DEST pTet-Off pAcGFP1-p53 pcDNA6/V5-His A pTet on advanced pAcGFP1-Mito pcDNA6/V5-His B pRevTRE pAcGFP1-Mem pcDNA6/V5-His C pRevTet-On pAcGFP1-Lam pcDNA6/myc-His C pRevTet-Off pAcGFP1-Golgi pcDNA6/myc-His A pCMV-Tet3G pAcGFP1-F pcDNA6/myc-His B pTRE2 pAcGFP1-Hyg-C1 pcDNA6.2/nGeneBLAzer-DEST pBD-NF-κB pAsRed2-N1 pcDNA4/HisMax-TOPO pCMV-AD ptdTomato-N1 pcDNA6.2/nLumio-DEST pCMV-BD pCMV-tdTomato pcDNA6.2/cLumio-DEST pBIND-Id Control pCRE-DD-tdTomato pcDNA4/myc-His C pBINDpCMV-DsRed-Express2 pcDNA4/HisMax C pG5 luciferase pEF1α-tdTomato pcDNA4/HisMax A pACT-MyoD pCRE-hrGFP c-Flag pcDNA3 pACT ptdTomato-C1 pcDNA4/HisMax B pCMV-SPORT6 pAsRed2-C1 pcDNA4/myc-His B pGL4.13pGL3-Promoter pcDNA4/myc-His A pGL4.19pGL3 basic pcDNA4/His C pGL4.26 pAcGFP1-C2 pcDNA4/His B pGL4.20 pAcGFP1-C1 pcDNA4/His A pGL4.29 pAcGFP1-N3 pcDNA6/TR pGL4.30 pAcGFP1-N2 pcDNA4/TO/Myc-His A pGL4.27 pAcGFP1-N1 pcDNA4/TO pGL4.75 pAcGFP1-C In-Fusion Ready pcDNA4/TO/Myc-His B pGL4.10pCRE-DD-AmCyan1 pcDNA4/TO/Myc-His C pGRN145 pNFkB-DD-AmCyan1 pcDNA3.3-TOPO pSecTag2 A pDsRed2-Bid pBudCE4.1 pEBVHis B pDsRED2-Mito pFLAG-CMV-4 pEBVHis ApDD-AmCyan1 Reporter pFLAG-CMV-3 pCMV-Tag 3C pAmCyan1-N1 pFLAG-CMV-2 pCMV-Tag 3A pAmCyan1-C1 pFLAG-CMV-5a pCMV-Tag 3B pEF1α-IRES-DsRed-Express2 p3XFLAG-CMV-9 pCMV-Tag 5C pEF1α-DsRed-Monomer-N1 p3xFLAG-CMV-10 pCMV-Tag 5A pDsRED-Monomer-N1 p3XFLAG-CMV-8 pCMV-Tag 4ApDsRed-Express-N1 p3XFLAG-CMV-7.1 pCMV-Tag 5Bp3XFLAG-CMV-7 pDsRed-Monomer-N In-Fusion Ready pCMV-Tag 4B pDsRed-Express-C1 p3XFLAG-CMV-13 pCMV-Tag 2C pIRES2-ZsGreen1 p3XFLAG-CMV-14 pCMV-Tag 2B pDsRed-Express2-C1 plRES2-ZsGreen1 pCMV-Tag 2A pDsRed-Express2-N1 pBApo-EF1α-pur pCMV-LacZpEF1α-DsRed-Express2 pBApo-EF1α-neo pCMV-MycpIRES2-DsRed-Express pBApo-CMV pEF1α-IRES-AcGFP1 pIRES2-DsRed-Express2 pBApo-CMV-neo pEF1α-IRES-ZsGreen1 pIRES-hrGFP-1a pIRES-EGFP pEF1α-AcGFP1-N1 pIRESneo2 pIRESneo3 pIRES2-DsRed2 pIRESneo pDsRed-Monomer pIRES2-AcGFP1 pIREShyg3 pIRES。
蛋白质表达在动物细胞中的应用利用哺乳动物细胞表达重组蛋白质的优势

蛋白质表达在动物细胞中的应用利用哺乳动物细胞表达重组蛋白质的优势蛋白质是生命机体中最重要的组成部分之一,在生物学、医学和工业领域都具有广泛的应用。
蛋白质表达则是将基因信息转化为蛋白质的过程,这一过程对于基础研究和工业化生产都具有重要作用。
在动物细胞中,蛋白质表达利用哺乳动物细胞表达重组蛋白质具有一系列优势。
一、哺乳动物细胞表达的优势哺乳动物细胞是表达重组蛋白质的理想载体,其优势主要有以下几点:1.真核生物中的哺乳动物细胞能够在所有重组蛋白质修饰过程中提供最高水平的质量。
由于哺乳动物细胞在体内合成蛋白质时,可以发生多种复杂的修饰过程,例如酰化、糖基化和磷酸化等。
这些修饰可以提高蛋白质的稳定性、可溶性和生物活性,使重组蛋白质更加适合用于医学和工业方面。
相比之下,原核生物(如大肠杆菌)表达的蛋白质缺乏这些修饰过程,因此其生物活性和稳定性较低。
此外,哺乳动物细胞中的重组蛋白质也很少产生抗原性,因此更适合用于医学应用。
2.哺乳动物细胞中的蛋白质折叠和修饰过程更加类似于人类和其他哺乳动物中蛋白质合成的过程。
由于哺乳动物细胞与人类和其他哺乳动物有很大相似性,因此表达的重组蛋白质更符合人类进化历史和生物学功能。
这也意味着可以更好地预测重组蛋白质在生理环境下的稳定性和效力,缩短临床试验的时间和成本。
3.哺乳动物细胞表达的重组蛋白质能够以天然状态的形式分泌到培养基中。
重组蛋白质如果能够以天然状态的形式分泌到培养基中,可以节省纯化步骤和工艺流程,降低生产成本,提高重组蛋白质的产量和纯度。
而哺乳动物细胞中的重组蛋白质通常能够实现这一点。
此外,哺乳动物细胞的培养和维护工艺已经比较成熟,使用更加方便。
二、哺乳动物细胞表达重组蛋白质的方法哺乳动物细胞表达重组蛋白质的方法有多种,主要有以下几种:1.哺乳动物细胞内表达哺乳动物细胞内表达是将重组质粒导入哺乳动物细胞内部,利用细胞自身的基因转录、转译和修饰等机制,表达出重组蛋白质。
哺乳动物细胞表达重组蛋白

哺乳动物细胞大规模生产重组蛋白的关键之处
表达载体 改进表达载体,提高表达水平和产量 分泌表达 下游纯化
宿主细胞 新型细胞株的建立
培养工艺
一、 真核表达载体
人工构建的哺乳动物细胞表达载体为穿梭载体,含: 原核DNA序列:大肠杆菌复制子及抗生素抗性基因,便于 载体在原核细胞中扩增和大量制备。 转录相关元件:哺乳动物细胞中有效转录的启动子和增强子 元件,以及终止子和加polyA信号序列。。 翻译相关元件:有效的mRNA翻译信号 其它:视实验需要加入标志基因、内含子、内部核糖体进入 位点等。
利用代谢工程,改进培养工艺,降低生产成本
采用无血清/无蛋白培养基(SFM/PFM)来降低细胞培养和产 品纯化的成本。双无培养基
但SFM/PFM缺乏生长刺激因子、黏附因子、扩展因子以及其 他细胞生长存活所必需的成分,在培养过程中细胞常表现出活力降 低、贴壁性差等现象,进而导致分泌目的蛋白的能力下降。
通过将生长刺激因子、黏附因子基因导入CHO细胞中,让 CHO细胞本身提供其自身生长所需要的成分,使其能在SFM/ PFM中生长良好。
抑制细胞凋亡,延长培养周期
细胞在大规模培养初期, 目的蛋白的表达和细胞的增殖速率呈正 相关。
但当反应器中细胞的密度达到饱和后,细胞继续增殖会导致养分 和氧的大量消耗以及乳酸、氨等有毒代谢产物的大量积累,细胞逐渐 凋亡,重组蛋白表达量逐渐降低。
哺乳动物细胞大规模培养生产重组蛋白中, 常用的细胞系有 中国仓鼠卵巢细胞(CHO) 人胚肾细胞HEK293
中国仓鼠卵巢细胞(CHO的优势 遗传背景清楚,生理代谢稳定 与人的亲缘关系接近,外源蛋白修饰准确 基因转移和载体表达系统完善 耐受剪切力,便于大规模培养 被美国FDA确认为安全的基因工程受体细胞
载体——精选推荐
NEB
原核表达 载体
NEB
配套大肠 杆菌
Amersham 原核表达载体
Amersham 原核表达载体
Amersham 原核表达载体
Invitrogen 原核表达载体
Lucigen
原核表达 菌株
Stratagene 原核表达菌株
Qiagen
原核表达 载体
Qiagen
原核表达 载体
Invitrogen 基因载体
700 元 现货 800 元 现货
900 元 现货
800 元 现货 800 元 现货 800 元 现货 800 元 现货 800 元 现货 700 元 现货 800 元 现货 800 元 现货 800 元 现货 800 元 现货 900 元 现货 1000 元 现货 1000 元 现货 1000 元 现货 900 元 现货 1000 元 现货 1500 元 现货 600 元 现货 300 元 现货 300 元 现货 600 元 现货 600 元 现货 800 元 现货 900 元 现货 900 元 现货 900 元 现货
BL21(DE3) BL21(DE3)plysS Rosetta(DE3) Rosetta(DE3)plysS Rosetta-gami(DE3) Rosetta-gami B(DE3)pLysS Orgami(DE3) OrgamiB(DE3) BLR(DE3) pTWIN1 pTYB11 pMXB10 pTXB1 ER2566 pGEX-6P-1 pGEX-4T-2 pGEX-4T-3 pRset-a C41(DE3) BL21 codonplus(DE3)RIPL TAGZyme pQE-2 pQE-Trisystem pOG44 pORF-lacZ pORF-HSV1tk pSP73 pUC18 pUC19 pBR322 pSP64 pBluescript II SK(+) SuperCos 1 pGAPZα A pPICZα A
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
pGL3 basic编号 载体名称北京华越洋生物VECT6057 pGL3 b asicpGL3 b asic载体基本信息载体名称: pGL3 basic, pGL3basic, pGL3-Basic质粒类型: 荧光素酶报告载体高拷贝/低拷贝: --启动子: --克隆方法: 多克隆位点,限制性内切酶载体大小: 4818bp5' 测序引物及序列: RVprimer3: CTAGCAAAATAGGCTGTCCC 3' 测序引物及序列: --载体标签: --载体抗性: Ampicillin筛选标记: --备注: --稳定性: --组成型: --病毒/非病毒: 非病毒pGL3 b asic载体质粒图谱和多克隆位点信息pGL3 b asic载体序列ORIGIN1 GGTACCGAGC TCTTACGCGT GCTAGCCCGG GCTCGAGATC TGCGATCTAA GTAAGCTTGG 61 CATTCCGGTA CTGTTGGTAA AGCCACCATG GAAGACGCCA AAAACATAAA GAAAGGCCCG 121 GCGCCATTCT ATCCGCTGGA AGATGGAACC GCTGGAGAGC AACTGCATAA GGCTATGAAG 181 AGATACGCCC TGGTTCCTGG AACAATTGCT TTTACAGATG CACATATCGA GGTGGACATC 241 ACTTACGCTG AGTACTTCGA AATGTCCGTT CGGTTGGCAG AAGCTATGAA ACGATATGGG 301 CTGAATACAA ATCACAGAAT CGTCGTATGC AGTGAAAACT CTCTTCAATT CTTTATGCCG 361 GTGTTGGGCG CGTTATTTAT CGGAGTTGCA GTTGCGCCCG CGAACGACAT TTATAATGAA 421 CGTGAATTGC TCAACAGTAT GGGCATTTCG CAGCCTACCG TGGTGTTCGT TTCCAAAAAG 481 GGGTTGCAAA AAATTTTGAA CGTGCAAAAA AAGCTCCCAA TCATCCAAAA AATTATTATC 541 ATGGATTCTA AAACGGATTA CCAGGGATTT CAGTCGATGT ACACGTTCGT CACATCTCAT 601 CTACCTCCCG GTTTTAATGA ATACGATTTT GTGCCAGAGT CCTTCGATAG GGACAAGACA 661 ATTGCACTGA TCATGAACTC CTCTGGATCT ACTGGTCTGC CTAAAGGTGT CGCTCTGCCT 721 CATAGAACTG CCTGCGTGAG ATTCTCGCAT GCCAGAGATC CTATTTTTGG CAATCAAATC 781 ATTCCGGATA CTGCGATTTT AAGTGTTGTT CCATTCCATC ACGGTTTTGG AATGTTTACT 841 ACACTCGGAT ATTTGATATG TGGATTTCGA GTCGTCTTAA TGTATAGATT TGAAGAAGAG 901 CTGTTTCTGA GGAGCCTTCA GGATTACAAG ATTCAAAGTG CGCTGCTGGT GCCAACCCTA 961 TTCTCCTTCT TCGCCAAAAG CACTCTGATT GACAAATACG ATTTATCTAA TTTACACGAA 1021 ATTGCTTCTG GTGGCGCTCC CCTCTCTAAG GAAGTCGGGG AAGCGGTTGC CAAGAGGTTC 1081 CATCTGCCAG GTATCAGGCA AGGATATGGG CTCACTGAGA CTACATCAGC TATTCTGATT 1141 ACACCCGAGG GGGATGATAA ACCGGGCGCG GTCGGTAAAG TTGTTCCATT TTTTGAAGCG 1201 AAGGTTGTGG ATCTGGATAC CGGGAAAACG CTGGGCGTTA ATCAAAGAGG CGAACTGTGT 1261 GTGAGAGGTC CTATGATTAT GTCCGGTTAT GTAAACAATC CGGAAGCGAC CAACGCCTTG 1321 ATTGACAAGG ATGGATGGCT ACATTCTGGA GACATAGCTT ACTGGGACGA AGACGAACAC 1381 TTCTTCATCG TTGACCGCCT GAAGTCTCTG ATTAAGTACA AAGGCTATCA GGTGGCTCCC 1441 GCTGAATTGG AATCCATCTT GCTCCAACAC CCCAACATCT TCGACGCAGG TGTCGCAGGT 1501 CTTCCCGACG ATGACGCCGG TGAACTTCCC GCCGCCGTTG TTGTTTTGGA GCACGGAAAG 1561 ACGATGACGG AAAAAGAGAT CGTGGATTAC GTCGCCAGTC AAGTAACAAC CGCGAAAAAG 1621 TTGCGCGGAG GAGTTGTGTT TGTGGACGAA GTACCGAAAG GTCTTACCGG AAAACTCGAC 1681 GCAAGAAAAA TCAGAGAGAT CCTCATAAAG GCCAAGAAGG GCGGAAAGAT CGCCGTGTAA 1741 TTCTAGAGTC GGGGCGGCCG GCCGCTTCGA GCAGACATGA TAAGATACAT TGATGAGTTT 1801 GGACAAACCA CAACTAGAAT GCAGTGAAAA AAATGCTTTA TTTGTGAAAT TTGTGATGCT 1861 ATTGCTTTAT TTGTAACCAT TATAAGCTGC AATAAACAAG TTAACAACAA CAATTGCATT 1921 CATTTTATGT TTCAGGTTCA GGGGGAGGTG TGGGAGGTTT TTTAAAGCAA GTAAAACCTC 1981 TACAAATGTG GTAAAATCGA TAAGGATCCG TCGACCGATG CCCTTGAGAG CCTTCAACCC 2041 AGTCAGCTCC TTCCGGTGGG CGCGGGGCAT GACTATCGTC GCCGCACTTA TGACTGTCTT 2101 CTTTATCATG CAACTCGTAG GACAGGTGCC GGCAGCGCTC TTCCGCTTCC TCGCTCACTG 2161 ACTCGCTGCG CTCGGTCGTT CGGCTGCGGC GAGCGGTATC AGCTCACTCA AAGGCGGTAA 2221 TACGGTTATC CACAGAATCA GGGGATAACG CAGGAAAGAA CATGTGAGCA AAAGGCCAGC 2281 AAAAGGCCAG GAACCGTAAA AAGGCCGCGT TGCTGGCGTT TTTCCATAGG CTCCGCCCCC 2341 CTGACGAGCA TCACAAAAAT CGACGCTCAA GTCAGAGGTG GCGAAACCCG ACAGGACTAT 2401 AAAGATACCA GGCGTTTCCC CCTGGAAGCT CCCTCGTGCG CTCTCCTGTT CCGACCCTGC 2461 CGCTTACCGG ATACCTGTCC GCCTTTCTCC CTTCGGGAAG CGTGGCGCTT TCTCAATGCT2521 CACGCTGTAG GTATCTCAGT TCGGTGTAGG TCGTTCGCTC CAAGCTGGGC TGTGTGCACG 2581 AACCCCCCGT TCAGCCCGAC CGCTGCGCCT TATCCGGTAA CTATCGTCTT GAGTCCAACC 2641 CGGTAAGACA CGACTTATCG CCACTGGCAG CAGCCACTGG TAACAGGATT AGCAGAGCGA 2701 GGTATGTAGG CGGTGCTACA GAGTTCTTGA AGTGGTGGCC TAACTACGGC TACACTAGAA 2761 GGACAGTATT TGGTATCTGC GCTCTGCTGA AGCCAGTTAC CTTCGGAAAA AGAGTTGGTA 2821 GCTCTTGATC CGGCAAACAA ACCACCGCTG GTAGCGGTGG TTTTTTTGTT TGCAAGCAGC 2881 AGATTACGCG CAGAAAAAAA GGATCTCAAG AAGATCCTTT GATCTTTTCT ACGGGGTCTG 2941 ACGCTCAGTG GAACGAAAAC TCACGTTAAG GGATTTTGGT CATGAGATTA TCAAAAAGGA 3001 TCTTCACCTA GATCCTTTTA AATTAAAAAT GAAGTTTTAA ATCAATCTAA AGTATATATG 3061 AGTAAACTTG GTCTGACAGT TACCAATGCT TAATCAGTGA GGCACCTATC TCAGCGATCT 3121 GTCTATTTCG TTCATCCATA GTTGCCTGAC TCCCCGTCGT GTAGATAACT ACGATACGGG 3181 AGGGCTTACC ATCTGGCCCC AGTGCTGCAA TGATACCGCG AGACCCACGC TCACCGGCTC 3241 CAGATTTATC AGCAATAAAC CAGCCAGCCG GAAGGGCCGA GCGCAGAAGT GGTCCTGCAA 3301 CTTTATCCGC CTCCATCCAG TCTATTAATT GTTGCCGGGA AGCTAGAGTA AGTAGTTCGC 3361 CAGTTAATAG TTTGCGCAAC GTTGTTGCCA TTGCTACAGG CATCGTGGTG TCACGCTCGT 3421 CGTTTGGTAT GGCTTCATTC AGCTCCGGTT CCCAACGATC AAGGCGAGTT ACATGATCCC 3481 CCATGTTGTG CAAAAAAGCG GTTAGCTCCT TCGGTCCTCC GATCGTTGTC AGAAGTAAGT 3541 TGGCCGCAGT GTTATCACTC ATGGTTATGG CAGCACTGCA TAATTCTCTT ACTGTCATGC 3601 CATCCGTAAG ATGCTTTTCT GTGACTGGTG AGTACTCAAC CAAGTCATTC TGAGAATAGT 3661 GTATGCGGCG ACCGAGTTGC TCTTGCCCGG CGTCAATACG GGATAATACC GCGCCACATA 3721 GCAGAACTTT AAAAGTGCTC ATCATTGGAA AACGTTCTTC GGGGCGAAAA CTCTCAAGGA 3781 TCTTACCGCT GTTGAGATCC AGTTCGATGT AACCCACTCG TGCACCCAAC TGATCTTCAG 3841 CATCTTTTAC TTTCACCAGC GTTTCTGGGT GAGCAAAAAC AGGAAGGCAA AATGCCGCAA 3901 AAAAGGGAAT AAGGGCGACA CGGAAATGTT GAATACTCAT ACTCTTCCTT TTTCAATATT 3961 ATTGAAGCAT TTATCAGGGT TATTGTCTCA TGAGCGGATA CATATTTGAA TGTATTTAGA 4021 AAAATAAACA AATAGGGGTT CCGCGCACAT TTCCCCGAAA AGTGCCACCT GACGCGCCCT 4081 GTAGCGGCGC ATTAAGCGCG GCGGGTGTGG TGGTTACGCG CAGCGTGACC GCTACACTTG 4141 CCAGCGCCCT AGCGCCCGCT CCTTTCGCTT TCTTCCCTTC CTTTCTCGCC ACGTTCGCCG 4201 GCTTTCCCCG TCAAGCTCTA AATCGGGGGC TCCCTTTAGG GTTCCGATTT AGTGCTTTAC 4261 GGCACCTCGA CCCCAAAAAA CTTGATTAGG GTGATGGTTC ACGTAGTGGG CCATCGCCCT 4321 GATAGACGGT TTTTCGCCCT TTGACGTTGG AGTCCACGTT CTTTAATAGT GGACTCTTGT 4381 TCCAAACTGG AACAACACTC AACCCTATCT CGGTCTATTC TTTTGATTTA TAAGGGATTT 4441 TGCCGATTTC GGCCTATTGG TTAAAAAATG AGCTGATTTA ACAAAAATTT AACGCGAATT 4501 TTAACAAAAT ATTAACGTTT ACAATTTCCC ATTCGCCATT CAGGCTGCGC AACTGTTGGG 4561 AAGGGCGATC GGTGCGGGCC TCTTCGCTAT TACGCCAGCC CAAGCTACCA TGATAAGTAA 4621 GTAATATTAA GGTACGGGAG GTACTTGGAG CGGCCGCAAT AAAATATCTT TATTTTCATT 4681 ACATCTGTGT GTTGGTTTTT TGTGTGAATC GATAGTACTA ACATACGCTC TCCATCAAAA 4741 CAAAACGAAA CAAAACAAAC TAGCAAAATA GGCTGTCCCC AGTGCAAGTG CAGGTGCCAG 4801 AACATTTCTC TATCGATA//其他哺乳动物表达载体:pCHO1.0 pBApo-CMV-Pur pOPRSVIpcDNA3.1/His C pcDNA5/FRT/V5-His-TOPO pREP4pcDNA3.1/His B pcDNA5/FRT/TO-TOPO pDual-GCpcDNA3.1/His A pcDNA5/TO pBK-RSVpIRESpuro3 pcDNA5/FRT/TO pBK-CMVpIRES2-EGFP pcDNA5/FRT pBI-CMV4pTT5 pFLAG-CMV2 pcDNA4/TO/Myc-His/LacZ pNFkB-DD-tdTomato pcDNA3.1/CT-GFP-TOPO pOPI3CATpBI-CMV5 pcDNA3.1/NT-GFP-TOPO pGene/V5-His B pSEAP2-Basic pOptiVEC-TOPO pSwitchpSEAP2-Control pCMV-MEKK1 pCMVLacIpBI-CMV3 pCMV-MEK1 pVgRxRpBI-CMV2 pCMV-PKA pINDpBI-CMV1 pcDNA6.2/nTC-Tag-DEST pTRE3G-LucpNFκB-MetLuc2-Reporter pcDNA6.2/cTC-Tag-DEST pTRE3GpCRE-MetLuc2-Reporter pcDNA3.2/V5/GW/D-TOPO pTRE2-hygropAcGFP1-Actin pcDNA6.2/V5/GW/D-TOPO pTRE-TightpAcGFP1-N In-Fusion Ready pcDNA6.2/nGeneBLAzer-GW/D-TOPO pTK-hygpAcGFP1-C3 pcDNA6.2/C-YFP-DEST pTet-OnpAcGFP1-C pcDNA6.2/cGeneBLAzer-DEST pTet-OffpAcGFP1-p53 pcDNA6/V5-His A pTet on advanced pAcGFP1-Mito pcDNA6/V5-His B pRevTREpAcGFP1-Mem pcDNA6/V5-His C pRevTet-OnpAcGFP1-Lam pcDNA6/myc-His C pRevTet-OffpAcGFP1-Golgi pcDNA6/myc-His A pCMV-Tet3GpAcGFP1-F pcDNA6/myc-His B pTRE2pAcGFP1-Hyg-C1 pcDNA6.2/nGeneBLAzer-DEST pBD-NF-κBpAsRed2-N1 pcDNA4/HisMax-TOPO pCMV-ADptdTomato-N1 pcDNA6.2/nLumio-DEST pCMV-BDpCMV-tdTomato pcDNA6.2/cLumio-DEST pBIND-Id ControlpCRE-DD-tdTomato pcDNA4/myc-His C pBINDpCMV-DsRed-Express2 pcDNA4/HisMax C pG5 luciferasepEF1α-tdTomato pcDNA4/HisMax A pACT-MyoDpCRE-hrGFP c-Flag pcDNA3 pACTptdTomato-C1 pcDNA4/HisMax B pCMV-SPORT6 pAsRed2-C1 pcDNA4/myc-His B pGL4.13pGL3-Promoter pcDNA4/myc-His A pGL4.19pGL3 basic pcDNA4/His C pGL4.26pAcGFP1-C2 pcDNA4/His B pGL4.20pAcGFP1-C1 pcDNA4/His A pGL4.29pAcGFP1-N3 pcDNA6/TR pGL4.30pAcGFP1-N2 pcDNA4/TO/Myc-His A pGL4.27pAcGFP1-N1 pcDNA4/TO pGL4.75pAcGFP1-C In-Fusion Ready pcDNA4/TO/Myc-His B pGL4.10pCRE-DD-AmCyan1 pcDNA4/TO/Myc-His C pGRN145pNFkB-DD-AmCyan1 pcDNA3.3-TOPO pSecTag2 ApDsRed2-Bid pBudCE4.1 pEBVHis B pDsRED2-Mito pFLAG-CMV-4 pEBVHis ApDD-AmCyan1 Reporter pFLAG-CMV-3 pCMV-Tag 3C pAmCyan1-N1 pFLAG-CMV-2 pCMV-Tag 3A pAmCyan1-C1 pFLAG-CMV-5a pCMV-Tag 3BpEF1α-IRES-DsRed-Express2 p3XFLAG-CMV-9 pCMV-Tag 5CpEF1α-DsRed-Monomer-N1 p3xFLAG-CMV-10 pCMV-Tag 5A pDsRED-Monomer-N1 p3XFLAG-CMV-8 pCMV-Tag 4A pDsRed-Express-N1 p3XFLAG-CMV-7.1 pCMV-Tag 5Bp3XFLAG-CMV-7 pDsRed-Monomer-N In-Fusion Ready pCMV-Tag 4B pDsRed-Express-C1 p3XFLAG-CMV-13 pCMV-Tag 2C pIRES2-ZsGreen1 p3XFLAG-CMV-14 pCMV-Tag 2B pDsRed-Express2-C1 plRES2-ZsGreen1 pCMV-Tag 2A pDsRed-Express2-N1 pBApo-EF1α-pur pCMV-LacZpEF1α-DsRed-Express2 pBApo-EF1α-neo pCMV-MycpIRES2-DsRed-Express pBApo-CMV pEF1α-IRES-AcGFP1 pIRES2-DsRed-Express2 pBApo-CMV-neo pEF1α-IRES-ZsGreen1 pIRES-hrGFP-1a pIRES-EGFP pEF1α-AcGFP1-N1 pIRESneo2 pIRESneo3 pIRES2-DsRed2 pIRESneo pDsRed-Monomer pIRES2-AcGFP1 pIREShyg3 pIRES。
