DEVELOPMENT REPORT - AIDS Drug Coalition
药品损害事件监测报告制度与流程

药品损害事件监测报告制度与流程英文回答:Drug Damage Incident Monitoring and Reporting System and Procedures.1. Monitoring System.The drug damage monitoring system is a systematic and continuous process of collecting, analyzing, and reporting information about adverse drug reactions (ADRs) and other drug-related problems. The primary objectives of the system are to:Detect and identify new and emerging ADRs.Assess the frequency and severity of ADRs.Identify risk factors for ADRs.Prevent and mitigate ADRs.Improve patient safety.2. Reporting Process.The drug damage reporting process typically involves the following steps:Healthcare professionals (HCPs) are required to report all suspected ADRs to the relevant regulatory authority.The regulatory authority reviews the reports and conducts an investigation to determine whether the ADR was caused by the drug.If the ADR is confirmed, the regulatory authority takes appropriate action to prevent or mitigate further ADRs.3. Data Analysis and Reporting.The data collected from the drug damage reportingsystem is analyzed to:Identify trends and patterns in ADRs.Develop risk management plans.Inform healthcare professionals and the public about ADRs.4. Regulatory Actions.Based on the data analysis, the regulatory authority may take the following actions:Issue safety warnings.Restrict the use of the drug.Withdraw the drug from the market.5. Public Education.The regulatory authority also plays an important role in educating the public about ADRs. The authority provides information about ADRs, including how to recognize them and report them.中文回答:药品损害事件监测报告制度与流程。
癌症临床试验(英文版

• Phase 4: From hundreds to thousands of people
– Usually takes place after drug is approved – Used to further evaluate long-term safety and
12
Clinical Trial Design
• Eligibility criteria: Can range from general (age, sex, type of cancer) to specific (prior treatment, tumor characteristics, blood cell counts, organ function); eligibility criteria also vary with trial phase
radioactive substances
7
Diagnostic Trials
• Develop better tools for classifying types and phases of cancer and managing patient care
• Possible benefits:
• Institutional review boards (IRBs) • Data and safety monitoring boards
(DSMBs)
– Minimize risks – Ensure integrity of data – Can stop study if necessary
– New technology may be better and less invasive – Earlier detection of recurrences
药学英语单词短语总结

药学英语单词短语总结药学英语单词短语总结1.具有成药性潜力的新化合物:New compounds with potential of drug resistance2.液相色谱-核磁共振联用法:liquid chromatography and nuclear magnetic resonance spectroscopy3.法定手册:Legal manual4.心血管、呼吸、内分泌和神经系统:Cardiovascular、Respiratory、Endocrine and nervous system5.新药的研究与开发:Research and development of new drugs6.针对癌症的高通量筛选:High throughput screening for cancer7.非处方药:Over-the-counter drugs(OTC)8.处方药:Prescription9.药品的用法和用量:Usage and dosage of drugs10.目标分析物的衍生化:Derivation of target analytes11.标准操作规程:standard operating procedures:12.药效学和药物代谢动力学:Pharmacodynamics and pharmacokinetics13.适应症和禁忌症:Indications and contraindications14.免疫原性和免疫毒性:Immunogenicity and immunetoxicity15.优质药品生产质量管理规范:Good manufacturing practice (GMP)16.紫外---可见分光光度法:UV Vis spectrophotometric method17.微生物发酵液:Microbial fermentation broth18.多药耐药:Multi drug resistance(MDR)19.肿瘤干细胞:Tumor stem cells20.脱氧核糖核酸和寡聚脱氧核苷酸:DNA and oligodeoxynucleotide21.糖尿病:Diabetes mellitus22.新药申请:New drug application23.二次代谢产物:second metabolites24.先导化合物:Lead compound25.胰岛素分泌:Insulin secretion26.统计学显著意义:Statistically significant27.缺血性中风:Ischemic stroke28.生理学和病理学:Physiology and pathology29.失效期:beyond-use date30.假劣药品:Counterfeit drugs31.出院后的药学护理:Pharmaceutical care after hospital discharge32.警告和注意事项:Warnings and precautions33.教学医院:T eaching hospital34.陆地植物:T errestrial plant35.多学科领域:Multidisciplinary field36.基于实验室的细胞和分子技术:Cell and molecular techniques based on laboratory37.帕金森病的流行病学:Epidemiology of Parkinson's disease38.相对标准偏差:relative standard deviation39.质量管理概念和药品生产质量管理规范:Quality management concept andGMP40.康复:Recure41.高效液相色谱法:High performance liquid chromatography (HPLC)42.免疫血液学:immunehematology43.心功能不全:Cardiac functional insufficiency44.免疫测定:Immunoassay。
美国卫生系统药师协会药品不良反应监测及报告指南

ቤተ መጻሕፍቲ ባይዱ
179 (收稿日期:2008-07-16责任编辑赵燕) Reporting) and Monitoring Reaction Drug Adverse on Guidelines (译自美国卫生系统药师协会ASH_P 患者)。 患者进行药物剂量调整(例如肾功能或肝功能损害的 数值并保证其在正常的治疗浓度范围内;④为特定的 危患者并在其医疗记录中记录;③监测药物血药浓度 患者提供ADR信息咨询;②鉴别易发生ADR的高 (2)药师直接向患者提供的服务内容包括:①向 重要的ADR。 (或)生产厂家报告严重ADR;⑨向医学界发表或介绍 播和使用从ADR计划中得到的信息;⑧向FDA和 内建立、维护和评估ADR档案;⑦在医疗机构内传 ADR计划进行相关内容的宣传教育;⑥在医疗机构 医务人员在其中的责任及相互之间的关系;⑤利用 明ADR计划中药师、医生、护士、风险管理者和其他 进药品不良反应监测及报告指南的政策及流程;④说 报告;②鉴别易发生ADR的高危药物及患者;③改 (1)药师应当开展的工作包括:①分析每—份ADR 部门和风险管理者。 来源于医疗人员、护理人员、质量监管人员、医疗记录 可和通过。在可能的情况下,指南设计的内容都应该 务人员执行委员会”)和机构管理部门得到正式的认 通过恰当的委员会(例如“药物和治疗委员会”或“医 展、巩固和持续评价中起主导作用,他们的地位应当 药师应当在药品不良反应监测及报告指南的发 告指南中药师的职责 4美国卫生系统药师协会药品不良反应监测及报 济上的益处。 少医疗机构的纠纷,显示出对ADR的防范所带来经 (7)通过降低住院率、更好和更经济地用药和减 的发现。 (6)为使用“药物应用评价计划”提供有质量保证 提高他们对ADR的认知水平。 (5)对医务人员和患者加强药物作用的教育,并 (4)衡量ADR的发生率。 药物的评估。 (3)评估药物治疗的安全性,尤其是对近期上市 少了医疗机构不必要的纠纷。 (2)作为医疗机构风险管理活动及工作的补充,减 进行预先监测,间接衡量了医疗机构临床药学的质量。 (1)通过发现可防范的ADR和对高危药物或患者 益,这些效益包括了(但不仅限于)以下方面: 为医疗机构、药师、其他医务人员和患者带来实际效 不断改进的药品不良反应监测及报告指南能够 告指南的效益 3美国卫生系统药师协会药品不良反应监测及报 促进患者获得好的预后。 药品不良反应监测及报告指南的根本目的应是 患者的影响。 预后和药品不良反应监测及报告指南对整体或个体 发生进行宣传教育;④评估处方模式、患者监测、患者 势、群发ADR及明显的个体ADR;③为防范ADR的 的医护人员进行信息反馈;②持续监测ADR的趋 动中去。这个过程应当包括以下方面:①向所有合适 应当被整合到医疗机构不断持续推进的质量改进活 续监测。从药品不良反应监测及报告指南发现的结果 (14)必须对患者的后续结果和ADR构成进行持 防范和改正的干预措施。 报告机制;④回顾总结ADR的构成和趋势;⑤建立 高大家对ADR重要性的认识;③建立ADR鉴别与 负有以下责任:①为本机构确定ADR的定义;②提 理小组领导、管理者和药师组成。此小组或委员会应 调的ADR小组或委员会,建议由医生、护士、质量管 (13)如有可能,在机构中应该设立一个由药房协 者隐私。 用的多学科综合评价。在整个过程中必须注意保护患 March.2009 V01.6,No.3 Pharrnacovigilance of Journal Chinese 中国药物警戒 2009年3月 第6卷第3期
FDA医药英文词汇
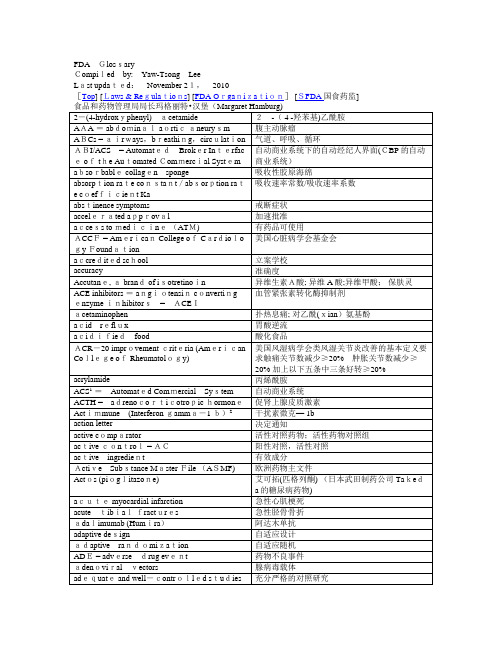
戒断症状
accelerated approval
加速批准
access to medicine (ATM)
有药品可使用
ACCF = American College of Cardiolo 美国心脏病学会基金会
gy Foundation
accredited school
立案学校
accuracy
准确度
Accutane, a brand of isotretinoin
FDA Glossary
Compiled by: Yaw-Tsong Lee
Last updated: November 21, 2010
[Top] [Laws & Regulations] [FDA Organizatபைடு நூலகம்on] [SFDA 国食药监]
食品和药物管理局局长玛格丽特•汉堡(Margaret Hamburg)
异维生素A酸; 异维 A 酸;异维甲酸; 保肤灵
ACE inhibitors = angiotensin converting 血管紧张素转化酶抑制剂
enzyme inhibitors = ACEI
acetaminophen
扑热息痛; 对乙酰(xian)氨基酚
acid reflux
胃酸逆流
acidified food
抗肿瘤坏死因子抑制剂 抗 TNF-α 治疗 美国围手术注册护士协会
主动脉疾病 主动脉瓣狭窄 亚太经济合作组织 动植物卫生检验局
美国感染控制和流行病专业协会
原料药
再生障碍性贫血 适当的保护水平
批准 已批准药物
approximate lethal dose = ALD aprotinin AQSIQ = China's General Administrati on for Quality Supervision, Inspectio n and Quarantine archival copy Area Under the Curve = AUC; area under the plasma concentration-time cur ve
医药研发常用英语词汇大全

医药研发常用英语词汇大全以下是一些医药研发领域常用的英语词汇,这些词汇涵盖了药物研发、临床试验、生物技术等多个方面。
请注意,这只是一个基本的参考,具体领域可能有更专业的术语。
1.Drug Discovery and Development(药物发现与开发):•Drug target: 药物靶点•Lead compound: 引导化合物•High-throughput screening: 高通量筛选•Hit compound: 命中化合物•Medicinal chemistry: 药物化学•Pharmacokinetics: 药代动力学•Pharmacodynamics: 药效动力学•Preclinical studies: 临床前研究2.Clinical Trials(临床试验):•Informed consent:知情同意•Placebo: 安慰剂•Randomized controlled trial (RCT): 随机对照试验•Double-blind study: 双盲研究•Phase I/II/III trials: Ⅰ/Ⅱ/Ⅲ期临床试验•Adverse events: 不良事件•Efficacy: 疗效•Safety: 安全性3.Biotechnology(生物技术):•Recombinant DNA technology: 重组DNA技术•Genetically modified organism (GMO): 转基因生物•Cloning: 克隆•Gene therapy: 基因治疗•Stem cells: 干细胞•Bioprocessing: 生物加工•Bioinformatics: 生物信息学4.Regulatory Affairs(法规事务):•Regulatory submission: 法规提交•Investigational New Drug (IND): 新药申请•New Drug Application (NDA): 新药上市申请•Good Manufacturing Practice (GMP): 良好生产规范•Good Clinical Practice (GCP): 良好临床实践5.Pharmacology(药理学):•Receptor: 受体•Agonist: 激动剂•Antagonist: 拮抗剂•Pharmacogenetics: 药理遗传学•Toxicology: 毒理学6.Quality Control(质量控制):•Batch release: 批释放•Quality assurance: 质量保证•Certificate of Analysis (CoA): 分析证书•Stability testing: 稳定性测试这些词汇只是医药研发领域中的一小部分,具体的词汇会根据不同的子领域而有所不同。
Drug discovery and development-药物发现与发展
Top Companies by R&D
Expense: Sr. No.
Company
R & D spend($bn),2010
1 Novartis
7.9
2 Merck & Co
8.1
3 Roche
7.8
4 GlaxoSmithKline
5.7
5 Sanofi
5.8
6 Pfizer
9.1
7 Johnson & Johnson
精选课件ppt
12
精选课件ppt
13
Target Selection
• Target selection in drug discovery is
defined as the decision to focus on
finding an agent with a particular
biological action that is anticipated to
Seeks to exploit the findings from the
sequencing of the human and other
genomes to fin精d选课n件peptw drug targets.
18
Genomics:
Drew’s estimates that the number of genes implicated in disease, both those due to defects in single genes and those arising from combinations of genes, is about 1,000
Pfizer
DEVELOPMENT REPORT - WHO Warns Against Misuse of Malaria Drug
DEVELOPMENT REPORT - WHO Warns Against Misuse of Malaria DrugBy Jill MossBroadcast: Monday, January 30, 2006This is Shep O'Neal with the VOA Special English Development Report.The World Health Organization is warning people not to use only one drug to treat malaria. That drug is artemisinin. W.H.O. officials say people should take it only incombination with other malaria drugs. The fear is that artemisinin could lose its effectiveness if it is misused.Arata Kochi is the new director of the malaria department at the W.H.O., the United Nations health agency. He says: "If we lose artemisinin, we will no longer have an effective cure for malaria." And if that happens, he says, it might take at least ten years before a new one could be discovered.Drug combinations are also used to treat diseases like AIDS andtuberculosis. Experts say combination treatments are not only moresuccessful than single-drug, or monotherapy. They also slow thedevelopment of resistance to medicines. The organisms that causemalaria have already developed resistance to many other drugs.The W.H.O. has called on eighteen drug manufacturers toimmediately halt the sale of artemisinin by itself. The companies arein China, India, Vietnam and other countries. The health agency cannot force them to obey. But there are steps it could take to pressure companies that continue to sell artemisinin as a monotherapy. For example, the W.H.O. could urge the World Bank, the Global Fund and other agencies not to buy drugs from those companies.Artemisinin comes from a plant called the sweet wormwood. Chinese researchersdiscovered it more than thirty years ago. The W.H.O. says artemisinin is more than ninety-five percent effective in curing malaria when used correctly with other anti-malarial drugs. Doctor Kochi says there have been no documented cases yet where treatment failedbecause of resistance to artemisinin. But he says there is concern about decreased reaction to the drug in Southeast Asia. That area is traditionally where resistance to anti-malariadrugs has first appeared.Doctor Arata KochiMalaria produces high body temperatures and a dangerous loss of fluids. The W.H.O. estimates there are more than three hundred million cases of malaria in the world each year. At least one million people die. Nine out of ten deaths happen in African countries south of the Sahara Desert. Most of the victims are young children.This VOA Special English Development Report was written by Jill Moss. Read and listen to our reports at . This is Shep O'Neal.。
不良反应科普宣传工作发言稿
不良反应科普宣传工作发言稿英文回答:Introduction.Adverse drug reactions (ADRs) are a major public health concern, affecting millions of people worldwide. They can range from mild and temporary to severe and life-threatening. The World Health Organization (WHO) estimates that ADRs account for up to 10% of hospital admissions and 5% of deaths.Importance of ADR Reporting.Reporting ADRs is essential for several reasons:Patient Safety: It helps identify and prevent future ADRs by providing data on the safety of medications.Drug Development: It informs drug manufacturers andregulatory agencies about the potential risks associated with their products, leading to safer drug design and labeling.Public Health Policy: It allows policymakers to make informed decisions about drug use and regulations.Common Types of ADRs.ADRs can affect different organ systems and manifest in various ways, including:Allergic Reactions: Hives, swelling, difficulty breathing.Gastrointestinal Problems: Nausea, vomiting, diarrhea.Cardiovascular Effects: Heart rhythm disturbances, blood pressure changes.Neurological Effects: Headache, dizziness, confusion.Liver and Kidney Damage: Jaundice, elevated liver enzymes.Factors Contributing to ADRs.Several factors can contribute to ADRs, including:Individual Susceptibility: Genetic factors, age, and underlying health conditions can increase the risk of ADRs.Drug Interactions: Combining certain medications can increase the likelihood of adverse effects.Dosage Errors: Taking too much or too little medication can lead to ADRs.Counterfeit and Substandard Drugs: Medications from unreliable sources may contain harmful impurities or incorrect dosages.Reporting ADRs.If you experience any unusual symptoms after taking medication, it is important to report it to your healthcare professional and the relevant regulatory authority. Reporting can be done through online portals, phone hotlines, or via healthcare professionals.Conclusion.ADR reporting is crucial for ensuring patient safety, improving drug development, and informing public health policy. By understanding the importance of ADR reporting and the factors that contribute to them, we can work together to minimize their impact and promote the safe use of medications.中文回答:引言。
制药工程专业英语7单元
Developing drugs from traditional medicinal plantsOver three quarters of the world's population relies mainly on plants andplant extracts for health care .Approximately one third of the prescription drugs in the US contain plant components, and more than 120important prescription drugs are derived from plants. Most of these drugswere developed because of their use in traditional medicine. Economically,this represents $8000-10,000M of annual consumer spending. Recent World Health Organization (WHO) studies indicate that over 30 per cent of the world's plant species have at one time or another been used for medicinalpurposes. Of the 250,000 higher plant species on Earth, more than 80,000species are medicinal. Although traditional medicine is widespread throughout the world , it is an integral part of each individual culture.Its practice is based mainly on traditional beliefs handed down from generation to generation for hundreds or even thousands of years. Unfortunately, much of this ancient knowledge and many valuable plantsare being lost at an alarming rate. The scientific study of traditional medicines and the systematic preservation of medicinal plants are thusof great importance.For quite a long time, the only way to use plant medicines was either directapplication or the use of crude plant extracts. With the development of organic chemistry at the beginning of this century, extraction and fractionation techniques improved significantly. It became possible to isolate and identify many of the active chemicals from plants. In the 1940s,advances in chemical synthesis enabled the synthesis of many plant components and their derivatives. In western countries, it was thoughtthat chemical synthesis of drugs would be more effective and economical than isolation from natural sources. Indeed, this is true in many cases.However, in many other cases, synthetic analogues are not as effectiveas their natural counterparts. In addition, some synthetic drugs cost manytimes more than natural ones. Inspired by these realisations, coupled withthe fact that many drugs with complex structures may be totally impossibleto synthesise, there is now a resurgent trend of returning to natural resources for drug developmentImportant prescription drugs from plantsEphedrine is the oldest and most classic example of a prescription drug developed from a traditional medicinal plant. It is derived from Ma Huang ,a leafless shrub. Used to relieve asthma and hay fever in China for over 5000 years, it was introduced into western medicine in 1924 by Chen and Schmidt. Ephedrine is an alkaloid closely related to adrenaline, the major product of the adrenal gland. Pharmacologically, Ephedrine is usedextensively to stimulate increased activity of the sympathetic nervous system. It is used as a pressor agent to counteract hypotension associatedwith anaesthesia, and as a nasal decongestant. The drug action of this medicine is based both on its direct effect on [alpha] and [beta] adrenergic receptors and on the release of endogenous noradrenaline.Digitalis is one of the most frequently used medications in the treatmentof heart failure and arrhythmia. It increases the contractility of the heart muscle and modifies vascular resistance. It also slows conduction through the atrioventricular node in the heart, making it useful in the treatment of atrial fibrillation and other rapid heart rhythms.Digitalis is found in the leaves and seeds of Digitalis purpurea and Digitalis lanata, commonly known as the foxglove plant. Foxglove has beenused in traditional medicine in many parts of the world - by African natives as arrow poisons, by the ancient Egyptians as heart medicine, andby the Romans as a diuretic, heart tonic, emetic and rat poison. The Chinese, who found this ingredient not only in plants but also in the dryskin and venom of the common toad, used it for centuries as a cardiac drug.In the western world, the foxglove was first mentioned in 1250 in the writing of a physician, Walsh, and it was described botanically in the 1500s.Digitalis is a glycoside containing an aglycone, or genin, linked to between one and four sugar molecules. The pharmacological activity resides in the aglycone, whereas the sugar residues affect the solubilityand potency of the drug. The aglycone is structurally related to bile acids,sterols, sex hormones and adrenocortical hormones.and its derivatives are the most frequently used drugsd-Tubocurarinein operating rooms to provide muscle relaxation and prevent muscle spasm.These agents interrupt the transmission of the nerve impulse at the skeletal neuromuscular junction. Curare, the common name for South American arrow poisons, has a long and interesting history. It has been used for centuries by Indians along the Amazon and Orinoco rivers for hunting. It causes paralysis of the skeletal muscles of animals and finally results in death. The methods of curare preparation were a secretentrusted only to tribal doctors. Soon after their discovery of the American continent, European explorers became interested in curare. Inthe late 16th century, samples of native preparations were brought to Europe for investigation. Curare, an alkaloid (see Figure), was found invarious species of Strychnos and certain species of Chondrodendron. The first use of curare for muscle relaxation was reported in 1942 by Griffithand Johnson. This drug offers optimal muscular relaxation without the useof high doses of anaesthetics. It thus emerged as the chief drug for use in tracheal intubation and during surgery.Vinblastine and vincristine (see Figure) are two of the most potent antitumour drugs. They are obtained from Catharanthus roseus, commonly known as the rosy periwinkle. This plant, indigenous to Madagascar, is also cultivated in India, Israel and the US. It was originally examined for clinical use because of its traditional use in treating diabetis. Theleaves and roots of this plant contain more than 100 alkaloids. Fractionation of these extracts yields four active alkaloids: vinblastine, vincristine, vinleurosine and vinresidine. These alkaloids areasymmetric dimeric compounds referred to as vinca alkaloids, but of these,only vinblastine and vincristine are clinically important antitumour agents.[9] These two alkaloids are cell-cycle specific agents that blockmitosis (cell division). Vincristine sulphate is used to treat acute leukaemia in children and lymphocytic leukaemia. It is also effective against Wilm's tumour, neuroblastoma. rhabdomyosarcoma (tumour ofvoluntary or striped muscle cells), reticulum cells sarcoma and Hodgkin'sdisease. Vinblastine sulphate is used in the treatment of Hodgkin's disease, lymphosarcoma, choriocarcinoma, neuroblastoma, carcinoma ofbreast, lung and other organs, and in acute and chronic leukaemia Emerging plant medicinesArtemisinin is the most recent anti-malaria drug developed fromplant-based traditional medicine. It is isolated from the leaves and flowers of Artemisia annua L. (Compositae), commonly known as the sweet wormwood, a cousin of tarragon. Indigenous to China, the extract of thisplant is traditionally known as the qinghao. It has been used to treat malaria in China for over 2000 years. Its active component, artemisinin,was first isolated in the 1970s by Chinese scientists. Unlike quinine and chloroquine, this compound is non-toxic, rapid in effect, and safe for pregnant women. Furthermore, it is effective againstchloroquine-resistant Plasmodium falciparum malaria and in patients withcerebral malaria. It kills the parasites directly so parasitaemia is quickly controlled. This work was confirmed by the WHO in Africa and otherparts of Southeast Asia.Artemisinin is an endoperoxide of the sesquiterpene lactone. Thestructure of this compound is too complex to be synthesised effectively.Artemisia is also found in many parts of the US, abudantly along the Potomac River in Washington DC, but the drug content of these varietiesis only about half that of the Chinese variety. Currently, the WHO and the US are jointly engaged in the cultivation of Chinese Artemisia forworldwide use. The recent development offers renewed hope for using traditional medicine to provide new drugs for future medicines从传统发展药品药用的植物在世界上有四分之三的人口主要依靠植物和植物提取物的保健作用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
DEVELOPMENT REPORT – January 20, 2003: AIDS Drug Coalition
By Jill Moss
This is the VOA Special English Development Report.
A new international coalition has been launched to help fight AIDS and the H-I-V virus that causes it. The new International H-I-V Treatment Access Coalition will help provide anti-retroviral drugs to people in poor countries. This medicine helps prevent H-I-V from developing in the body. These drugs have been used in rich countries since nineteen-ninety-six. They have resulted in a sharp drop in H-I-V and AIDS sickness and death in those countries. Coalition officials say poor countries in the developing world must now have the same drugs.
Coalition officials say no single organization can successfully spread anti-AIDS drugs around the world. Instead, a united group effort is required. The coalition plans to work together to share information about successful treatment programs in developing countries. It will also establish programs to buy the medicines and train health care workers about the drugs.
The coalition says the price of anti-retroviral drugs is now decreasing. A one-year treatment used to cost ten-thousand dollars for one person. Today, it is less than three-hundred dollars. This is still a high price for people in developing countries. However, coalition officials say more aid money is now being used to pay for the drugs. In addition, many governments have reduced import taxes on medicines. Coalition officials say this political and humanitarian support must now be expanded to make treatment a reality for all people with H-I-V and AIDS.
The World Health Organization estimates more than forty-million people have the disease. More than ninety-five percent live in poor and developing countries. Last year, nearly all of the more than three-million AIDS deaths were victims from these same poor countries.
The W-H-O says only about five percent of the people living with H-I-V in developing countries use anti-retroviral drugs. Coalition officials say their goal is to increase the number of patients on AIDS drugs during the next three years.
The International H-I-V Treatment Access Coalition has fifty-six members. They include governments, public health organizations, businesses, health researchers, humanitarian groups, victims, and their supporters. The W-H-O will supervise coalition efforts from its headquarters in Geneva, Switzerland.
This VOA Special English Development Report was written by Jill Moss.
Email this article to a friend
Printer Friendly Version。
