DIN EN 12476-2001 ENG金属磷化转化涂层方法
EN12476

EN124764月2000ICS25.220.99英文版本磷酸盐转化膜的金属方法指明要求这份欧洲标准是有CEN提供的在2000年3月10日CEN成员都有义务遵守与CEN /CENELEC内部规章规定的条件,给这个欧洲标准的地位,国家标准,没有任何改动不断更新的名单和参考书目等有关国家标准可能获得的应用,向管理中心或任何CEN会员这份欧洲标准存在三种官方版本(英语,法语,德语)一份版本在任何其他语言所做出的翻译下的责任,CEN会员纳入其自己的语言,并通知管理中心具有相同的地位作为官方版本。
CEN成员是国家标准机构的有奥地利,比利时,捷克共和国,丹麦,芬兰,法国,德国,希腊,冰岛,意大利,卢森堡,荷兰,挪威,葡萄牙,西班牙,瑞典,瑞士和英国。
CEN欧洲标准化委员会秘书中心:rue de Stassart 36, B-1050 Brussels第二页EN 12476:2000内容前言 (1)1范围 (1)2规范参考 (1)3信息要由买方提供 (1)4涂层的类型和指定 (1)4.1涂层类型 (1)4.2转换涂层指定 (2)5采样 (3)6涂层的要求 (3)6.1外观 (3)6.2单位面积涂层的质量 (3)6.3腐蚀抵抗 (3)附件A(详实)指导 (4)附件B(详实)建议 (6)附件C(详实)后处理 (8)附件D(详实)测定的耐腐蚀性 (9)参考书目 (10)前言这份欧洲标准是由CEN/TC 262技术委员会提供的,“金属和无机涂料”,这是由BSI秘书处认证本欧洲标准应考虑的地位,一个国家标准组织对下列国家必将实施这项欧洲标准,本欧洲标准应考虑的地位,一个国家标准无论是出版相同的文字或背书最迟由2000年10月,和冲突的国家标准应撤回在最迟于2000年10月。
根据CEN /CENELEC的国际规则,下列国家的标准化组织必将为实施这项欧洲标准:奥地利,比利时,捷克共和国,丹麦,芬兰,法国,德国,希腊,冰岛,意大利,卢森堡,荷兰,挪威,葡萄牙,西班牙,瑞典,瑞士和英国。
金属表面的磷化处理方法

金属表面的磷化处理方法根据本发明的方法,将经过上述除油清洗的工件浸入酸性磷化液中,磷化的水溶液含有锌化合物,其含量相当于0.5克/升-1.5克/升的锌离子,相当于5-30克/升磷酸根离子的磷酸盐,相当于0.01至0.2克/升亚硝酸根离子的亚硝酸盐和(或)0.05至2克/升的芳族硝基化合物,溶液温度为40°-70℃,浸渍15至120秒。
然后以同样温度的同样磷化液喷2秒以上,通常在喷后依次用自来水及去离子水清洗。
磷化液中的主要成份是锌离子,含量可以是0.5-1.5克/升,最好是0.7-1.2克/升。
低于0.5克/升时,就不能产生均匀的磷化膜,而只形成不均匀的蓝膜。
含量高于1.5克/升时,会产生均匀磷化膜,但膜层易于含有叶片状结晶,就象普通喷淋工艺所形成的那样,因而就不宜于作阳离子电泳漆的底层。
锌离子的加入,可用氧化锌、碳酸锌、硝酸锌等。
磷酸盐离子含量可为5至30克/升,最好是10至20克/升。
低于5克/升时易于形成不均匀的膜层,超过30克/升时不会再有很大的作用。
磷酸根离子的来源可为磷酸、磷酸钠、磷酸锌、磷酸镍等。
作为磷化促进剂,可用亚硝酸根离子。
其含量为0.01-0.2克/升,最好是0.04-0.15 克/升。
或者是使用芳族硝基化合物,含量为0.05-2克/升,最好是0.1-1.5克/升。
亦可二者共用。
倘若这些促进剂含量低于下限,就不可能获得足够的磷化,而生成黄锈或类似的膜层。
如含量超过上限,就会形成不均匀的蓝色膜层。
亚硝酸根离子的来源可为亚硝酸钠、亚硝酸铵等。
至于芳族硝基化合物,则可用间-硝基苯磺酸盐类(如间硝基苯磺酸钠)、硝基苯甲酸、硝基间苯二酚等。
磷化液除含有上述锌离子、磷酸盐离子、亚硝酸盐离子及一种芳族硝基化合物外,还可以含有硝酸盐离子、氯酸盐离子、镍离子和钴离子。
这些任选的附加离子含量可为:硝酸盐1-10克/升,最好是2-8克/升;氯酸盐0.05-2克/升,最好是0.2-1.5克/升;镍离子0.05-2克/升,最好是0.2-1.5克/升;钴离子0.05-2克/升,最好是0.1至1克/升。
涂装工艺与设备(磷化)
第 3 章 涂装前处理
3.4 涂装前磷化处理
磷化膜的特点
属于多孔结晶性沉淀膜 与基体结合力较高
在潮湿条件下的耐蚀性较差
膜层强度低,润滑性好 绝缘性好
3
第 3 章 涂装前处理
3.4 涂装前磷化处理
磷化膜的作用
提高耐蚀性
保护膜 无覆膜 磷化膜 镀镍 镀铬 磷化膜加石蜡 两层烤漆 磷化膜加一层烤漆 长效防腐涂料 磷化膜加长效防腐涂料 在3%NaCl溶液中首先出现腐蚀的时间/h 0.1 1 10-13 23-24 60 70 500h实验无腐蚀 1000h实验无腐蚀 >2000h实验无腐蚀
1-40
颗粒-树枝-针状混合晶型, 空隙较少
密集颗粒状,空隙少
中型
(4.3~7.5)g/m2 轻型 (1.1~4.3)g/m2 最轻型 (0.3~1.1)g/m2
锰系 铁系 非晶相铁系
1-40 5-20 0.1-1.5
Fe5H2(PO4)4· 2O 4H Fe3(PO4)2· 2O 8H Fe2O3 FePO4
淤渣
磷化膜成分
8
第 3 章 涂装前处理
3.4 涂装前磷化处理
磷化膜的结构
9
第 3 章 涂装前处理
3.4 涂装前磷化处理
磷化处理的分类
规模商品化应用的主要类型
轻铁系 锌系 锰系 锌钙系
10
第 3 章 涂装前处理
3.4 涂装前磷化处理
磷化处理的分类
规模商品化应用的主要类型 磷化处理温度
不明确 现阶段对适合电泳工艺的磷化膜特性只能由实验确定
7
第 3 章 涂装前处理
3.4 涂装前磷化处理
磷化膜形成的反应过程
金属制品磷化防锈技术
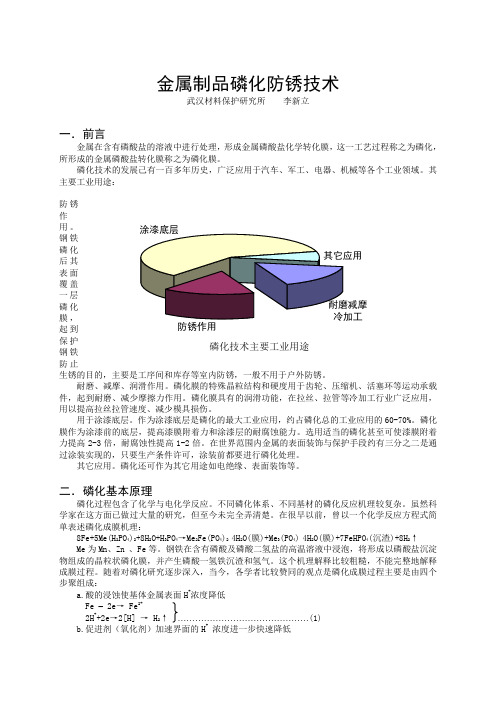
防锈作用 磷化技术主要工业用途金属制品磷化防锈技术武汉材料保护研究所 李新立一.前言金属在含有磷酸盐的溶液中进行处理,形成金属磷酸盐化学转化膜,这一工艺过程称之为磷化,所形成的金属磷酸盐转化膜称之为磷化膜。
磷化技术的发展己有一百多年历史,广泛应用于汽车、军工、电器、机械等各个工业领域。
其主要工业用途: 防锈作用。
钢铁磷化后其表面覆盖一层磷化膜,起到保护钢铁防止生锈的目的,主要是工序间和库存等室内防锈,一般不用于户外防锈。
耐磨、减摩、润滑作用。
磷化膜的特殊晶粒结构和硬度用于齿轮、压缩机、活塞环等运动承载件,起到耐磨、减少摩擦力作用。
磷化膜具有的润滑功能,在拉丝、拉管等冷加工行业广泛应用,用以提高拉丝拉管速度、减少模具损伤。
用于涂漆底层。
作为涂漆底层是磷化的最大工业应用,约占磷化总的工业应用的60-70%。
磷化膜作为涂漆前的底层,提高漆膜附着力和涂漆层的耐腐蚀能力。
选用适当的磷化甚至可使漆膜附着力提高2-3倍,耐腐蚀性提高1-2倍。
在世界范围内金属的表面装饰与保护手段约有三分之二是通过涂装实现的,只要生产条件许可,涂装前都要进行磷化处理。
其它应用。
磷化还可作为其它用途如电绝缘、表面装饰等。
二.磷化基本原理磷化过程包含了化学与电化学反应。
不同磷化体系、不同基材的磷化反应机理较复杂。
虽然科学家在这方面已做过大量的研究,但至今未完全弄清楚。
在很早以前,曾以一个化学反应方程式简单表述磷化成膜机理:8Fe+5Me(H 2PO 4)2+8H 2O+H 3PO 4→Me 2Fe(PO 4)2·4H 2O(膜)+Me 3(PO 4)·4H 2O(膜)+7FeHPO 4(沉渣)+8H 2↑ Me 为Mn 、Zn 、Fe 等。
钢铁在含有磷酸及磷酸二氢盐的高温溶液中浸泡,将形成以磷酸盐沉淀物组成的晶粒状磷化膜,并产生磷酸一氢铁沉渣和氢气。
这个机理解释比较粗糙,不能完整地解释成膜过程。
随着对磷化研究逐步深入,当今,各学者比较赞同的观点是磷化成膜过程主要是由四个步聚组成:a.酸的浸蚀使基体金属表面H +浓度降低Fe – 2e → Fe 2+2H ++2e →2[H] → H 2↑ (1)b.促进剂(氧化剂)加速界面的H +浓度进一步快速降低[氧化剂]+[H] → [还原产物]+H2OFe2++[氧化剂] → Fe3++[还原产物] (2)由于促进剂氧化掉第一步反应所产生的氢原子,加快了反应(1)的速度,进一步导致金属表面H+浓度急剧下降。
表面处理标准大众13750(中文)

保密,保留所有权力。
未经大众汽车集团标准部门的许可,不得复制或转换本文件的任何一部分。
合同当事方仅能通过B2B供应商平台“”获取本标准。
第2页删除了“带微小裂缝的光亮镀铬处理”表面保护类型(代码f320),(代替以Ofl-f350)。
制定了“镀锡处理”表面保护类型的涂层厚度(代码g...)。
增补了电解沉积铝和铝/猛合金涂层的表面保护类型(代码q600, q605, q610, q615)。
含六价铬Cr(VI)的“镀锌/铁合金处理”表面保护类型(代码r600, r605, r610, r620 和r630),限于可行的应用(不再允许用于新设计,有关替代类型请参见表A.1)。
增补了不含六价铬Cr(VI)的“镀锌/铁合金处理”表面保护类型(代码r301和r302)。
“镀锌/镍合金处理”表面保护类型(代码r640, r650, r660, r665, r670, r675),限于可行的应用(不再允许用于新设计,有关替代类型请参见表A.1)。
增补了不含六价铬Cr(VI)的“镀锌/镍合金处理”表面保护类型(代码r642, r643, r649, r672, r673和r677)。
增补了不含六价铬Cr(VI)的“复合镀层处理”表面保护类型(代码s611, s617, s621和s627)。
含六价铬Cr(VI)的“复合镀层处理”表面保护类型(代码s610, s615, s620和s625),限于可行的应用(不再允许用于新设计,有关替代类型请参见表A.1)。
“锌片涂层”表面保护类型(代码t300, t310, t320, t345, t600, t620和t645),限于可行的应用(不再允许用于新设计,有关替代类型请参见表A.1)。
增补了不含六价铬Cr(VI)的“锌片涂层”表面保护类型(代码t602, t611, t615和t647)。
重新增补了“表面镀黑抛光处理”表面保护类型(代码u110)。
含六价铬Cr(VI)的“渗铬处理”表面保护类型(代码v110),限于可行的应用(不再允许用于新设计,代替以Ofl-v111)。
《金属镀覆和化学处理表示方法》新旧标准对照
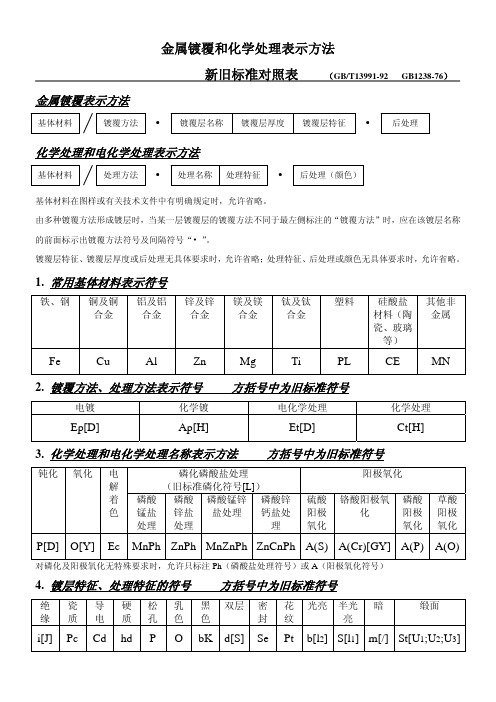
新旧标准对照表
(GB/T13991-92 GB1238-76)
金属镀覆表示方法
基Байду номын сангаас材料
镀覆方法 • 镀覆层名称 镀覆层厚度 镀覆层特征 • 后处理
化学处理和电化学处理表示方法
基体材料
处理方法 • 处理名称 处理特征 • 后处理(颜色)
基体材料在图样或有关技术文件中有明确规定时,允许省略。
由多种镀覆方法形成镀层时,当某一层镀覆层的镀覆方法不同于最左侧标注的“镀覆方法”时,应在该镀层名称
的前面标示出镀覆方法符号及间隔符号“• ”。
镀覆层特征、镀覆层厚度或后处理无具体要求时,允许省略;处理特征、后处理或颜色无具体要求时,允许省略。
1. 常用基体材料表示符号
铁、钢 铜及铜 铝及铝
合金
合金
锌及锌 合金
6. 颜色表示符号 方括号中为旧标准符号
黑 棕红橙黄绿
白
蓝 紫 灰 粉 金黄 青 银
(浅 (紫 (蓝 红
绿白
蓝) 红) 灰)
BK[H] BN RD OG YE GN WH[B] BU VT GY PK GD TQ SR
7. 独立加工工序名称符号 方括号中为旧标准符号
有机溶 化学除油 电解除 化学酸洗 电解酸 化学碱洗
4. 镀层特征、处理特征的符号 方括号中为旧标准符号
绝 瓷 导 硬 松 乳 黑 双层 密 花 光亮 半光 暗
缎面
缘 质 电 质孔色色
封纹
亮
i[J] Pc Cd hd P O bK d[S] Se Pt b[l2] S[l1] m[/] St[U1;U2;U3]
5. 后处理符号 方括号中为旧标准符号
色顺序为黑、红、金黄。
EN12476金属表面磷化处理
EN124764月2000ICS25.220.99英文版本磷酸盐转化膜的金属方法指明要求这份欧洲标准是有CEN提供的在2000年3月10日CEN成员都有义务遵守与CEN /CENELEC内部规章规定的条件,给这个欧洲标准的地位,国家标准,没有任何改动不断更新的名单和参考书目等有关国家标准可能获得的应用,向管理中心或任何CEN会员这份欧洲标准存在三种官方版本(英语,法语,德语)一份版本在任何其他语言所做出的翻译下的责任,CEN会员纳入其自己的语言,并通知管理中心具有相同的地位作为官方版本。
CEN成员是国家标准机构的有奥地利,比利时,捷克共和国,丹麦,芬兰,法国,德国,希腊,冰岛,意大利,卢森堡,荷兰,挪威,葡萄牙,西班牙,瑞典,瑞士和英国。
CEN欧洲标准化委员会秘书中心:rue de Stassart 36, B-1050 Brussels第二页EN 12476:2000内容前言 (1)1范围 (1)2规范参考 (1)3信息要由买方提供 (1)4涂层的类型和指定 (1)4.1涂层类型 (1)4.2转换涂层指定 (2)5采样 (3)6涂层的要求 (3)6.1外观 (3)6.2单位面积涂层的质量 (3)6.3腐蚀抵抗 (3)附件A(详实)指导 (4)附件B(详实)建议 (6)附件C(详实)后处理 (8)附件D(详实)测定的耐腐蚀性 (9)参考书目 (10)前言这份欧洲标准是由CEN/TC 262技术委员会提供的,“金属和无机涂料”,这是由BSI秘书处认证本欧洲标准应考虑的地位,一个国家标准组织对下列国家必将实施这项欧洲标准,本欧洲标准应考虑的地位,一个国家标准无论是出版相同的文字或背书最迟由2000年10月,和冲突的国家标准应撤回在最迟于2000年10月。
根据CEN /CENELEC的国际规则,下列国家的标准化组织必将为实施这项欧洲标准:奥地利,比利时,捷克共和国,丹麦,芬兰,法国,德国,希腊,冰岛,意大利,卢森堡,荷兰,挪威,葡萄牙,西班牙,瑞典,瑞士和英国。
磷化处理EN 12476标准(英文版)
ICS 25.220.99Phosphatierüberzüge auf Metallen – Verfahren für die Festlegung vonAnforderungen European Standard EN 12476:2000 has the status of a DIN Standard.A comma is used as the decimal marker.National forewordThis standard has been prepared by CEN/TC 262 ‘Metallic and other organic coatings’ (Secretariat: United Kingdom).The responsible German body involved in its preparation was the Normenausschuss Materialprüfung (Ma-terials Testing Standards Committee), Technical Committee Chemische und elektrochemische Überzüge .The committee decided to retain the specifications of subclause 7.2 (cf. clauses NA.1 to NA.5) and of tables 7 and 8 (cf. tables NA.1 and NA.2) of DIN 50942 by introducing them in the national annex below.DIN EN ISO 3892 is the standard corresponding to European Standard EN ISO 3892 referred to in clause 2of the EN.AmendmentsThis standard differs from the DIN EN 12476, July 2000 edition, and DIN 50942, September 1996 edition,in that a national annex has been introduced.Previous editionsDIN 50942: 1955-01, 1973-11, 1987-05, 1996-09; DIN EN 12476: 2000-07.National Annex NAStandard referred to(and not included in Normative references and Bibliography )DIN EN ISO 3892Conversion coatings on metallic materials – Determination of coating mass per unit areawith gravimetric methods (ISO 3892:1980)Ref.No.DIN EN 12476:2001-10English price group 09Sales No.110902.02DEUTSCHE NORM October 2001EN 12476{Continued on pages 2 and 3.EN comprises 12 pages.©No part of this standard may be reproduced without the prior permission ofDIN Deutsches Institut für Normung e.V., Berlin. Beuth Verlag GmbH , 10772 Berlin, Germany,has the exclusive right of sale for German Standards (DIN-Normen).Phosphate conversion coatings of metalsMethod of specifying requirementsEnglish version of DIN EN 12476Supersedes DIN EN 12476,July 2000 edition, and DIN 50942, September 1996 edition.Page 2DIN EN 12476:2001-10Notes on the use of this standardNA.1Chemical composition of coatingIn addition to the methods specified in this annex, other analytical methods with at least the same precision may be used.The detection of phosphate in a coating shall be taken as proof that it is a phosphate coating (cf.subclause NA.2).To establish that a coating is a manganese phosphate coating, it is sufficient to establish the presence of manganese (for ferrous substrates only) (cf. subclause NA.3).To establish that a coating is a zinc phosphate or a zinc calcium phosphate coating, it is sufficient to establish the presence of zinc or zinc and calcium, respectively (for zinc-free substrates) (cf. subclause NA.5).If neither manganese nor zinc has been detected in a phosphate coating on a substrate of ferrous material, then the coating is certain to be an iron phosphate coating.NA.1.1ReagentsThe following reagents, of analytical grade, and distilled or deionized water should be used.a)5% sodium hydroxide solution, NaOH, prepared by dissolving 50g of sodium hydroxide in one litre of water.b) 40% nitric acid solution, HNO 3, prepared by mixing four parts by volume of nitric acid (of density 1,4g/cm³) and 2,5 parts by volume of water.c)Ammonium molybdate solution, prepared by dissolving 88,3g of ammonium heptamolybdate tetrahydrate, (NH 4)6Mo 7O 24·4 H 2O, 30m l of 25% ammonia solution (of density 0,91g/cm³), and 240g of ammonium nitrate, NH 4NO 3, in water and making up to 1litre.d)25% hydrochloric acid solution, HC l , prepared by mixing 2,5 parts by volume of hydrochloric acid (of density 1,19g/cm³), and 1,2 parts by volume of water;e)5% potassium hexacyanoferrate (II ) solution, K 4[Fe(CN)6], prepared by dissolving 50g potassium hexacyanoferrate in one litre of water;f)17% nitric acid solution, HNO 3, prepared by mixing 1,7 parts by volume of nitric acid (of density 1,4g/cm 3) and 4,8 parts by volume of water.g)3% hydrogen peroxide solution, H 2O 2 (of density 1,3g/cm 3), prepared by mixing one part by volume of hydrogen peroxide and nine parts by volume of water;h)Bismuth sodium trioxide, NaBiO 3.i)Ammonium nitrate, NH 4NO 3.j)10% aqueous ammonia solution, NH 3, prepared by mixing two parts by volume of concentrated ammo-nia solution (of density 0,91g/cm³) and five parts by volume of water.k)Ammonium oxalate, (NH 4)2C 2O 4 · H 2O.l)Ammonium chloride, NH 4C l .m)10% hydrochloric acid solution, HC l , prepared by mixing one part by volume of hydrochloric acid (of density 1,19g/cm 3) and 2,7 parts by volume of water.n)45% sulfuric acid solution, H 2SO 4, prepared by mixing 4,5 parts by volume of sulfuric acid (of density 1,84g/cm 3) and 5,3 parts by volume of water.o)5% potassium permanganate solution, KMnO 4, prepared by dissolving 50g of potassium permanganate in one litre of water.NA.2Testing for the presence of phosphateTo test for the presence of phosphate in the coating, treat a coated test piece having a surface area of about 100 cm 2 with 100m l of a 5% sodium hydroxide solution at 80°C to 90°C until either the coating has been stripped or it has at least been noticeably attacked. If necessary, scrub the coating off with a rubber wiper. Filter the resulting solution and acidify 25m l of the filtrate with 40% nitric acid. Add 10m l of the ammonium molybdate solution and 5g of ammonium nitrate to the acidified filtrate, and leave the mixture to stand for at least 15 minutes. A yellow precipitate indicates the presence of phosphate.NA.3Testing for the presence of manganeseTo test for the presence of manganese, dissolve the filter residue obtained as described in subclause NA.2 with warm 17% nitric acid, to which a few drops of 3% hydrogen peroxide have been added. To decompose the excess hydrogen peroxide, boil the resulting solution for a few minutes and then cool it to ambient ing half of the solution, test for manganese by adding 0,5g of bismuth sodium trioxide, thus oxidizing any manganese present to permanganate; a reddish violet colour indicates the presence of manganese.Use the other half of the solution to test for the presence of calcium as described in subclause NA.5, if necessary.NA.4Testing for the presence of zincTo test for the presence of zinc (only for coatings on zinc-free substrates), use 25% hydrochloric acid to slightly acidify 25m l of the filtrate obtained as described in subclause NA.2 and then add 5m l of 5% potassium hexacyanoferrate (II ) solution. A white precipitate, which may have a slight green tinge due to the presence of a small amount of iron, indicates the presence of zinc.NA.5Testing for the presence of calciumTo test for the presence of calcium, take 5m l of the alkaline filtrate obtained as described in subclause NA.2and combine it with the remaining half of the solution obtained as described in subclause NA.3. Prepare a clear solution by adding a few drops of 17% nitric acid and then buffering it to a pH between 2 and 3 by adding a few drops of 10% ammonia solution (check the pH with special indicator paper, e.g. ion-specific indicator paper). After heating the solution to about 80°C, dissolve into it 1g of solid ammonium oxalate, thus changing the pH of the solution to between 5 and 6 (check again with indicator paper). If necessary, adjust the pH to the desired level by adding solid ammonium chloride.If calcium is present, a fine white crystalline precipitate of calcium oxalate forms when the solution is heated to 80°C. Filter off this precipitate after about 10 minutes of heating, rinse it with hot distilled water to which some ammonium oxalate (2g/l ) has been added, and then purify it by reprecipitation as follows: Redissolve the precipitate on the filter in hot 10% hydrochloric acid, heat the resulting solution to 80°C, adjusting it to a pH of between 5 and 6 by adding 10% ammonia solution; the calcium then reprecipitates as fine crystalline calcium oxalate. Filter this off after 10minutes of heating and rinse thoroughly with hot distilled water.To identify the purified white precipitate as calcium oxalate, remove it from the filter by spraying with hot distilled water, and then add about 50m l of distilled water. Add 5m l of 45% sulfuric acid to dissolve the precipitate and treat it at about 80°C by adding some drops of 5% potassium permanganate solution. De-coloration of the permanganate solution identifies the precipitate as calcium oxalate, thus indicating the pres-ence of calcium in the coating.Table NA.1:Minimum exposure times for phosphate coatings on ferrous materials withoutsupplemental coating, using the NSS type atmosphere as in ISO 9227Type of phosphate coatingZinc phosphate Manganese phosphateSymbolZnph Mnph Mass per unit area, in g/m 2Over 10Over 16Minimum exposure time, in hours 21,5Table 8:Minimum exposure times for phosphate coatings on test panels coated with standardreference oil 1), using using the NSS type atmosphere as in ISO 9227Type of phosphate coatingZinc phosphate Zinc phosphate Zinc calcium Manganese phosphatephosphate Symbol Znph ZnphZnCaph Mnph Mass per unit area, in g/m 2 5 to 10Over 105 to 15Over 16Minimum exposure time,24482436in hours 1)Information on sources of supply is obtainable from the Normenausschuss Materialprüfung (Materials Test-ing Standards Committee), Burggrafenstr. 6, 10787 Berlin, Germany.Page 3DIN EN 12476:2001-10English versionICS 25.220.99Management Centre: rue de Stassart 36, B-1050 BrusselsEuropean Committee for StandardizationComité Européen de NormalisationEuropäisches Komitee für NormungPhosphate conversion coatings of metalsMethod of specifying requirements©2000.CEN –All rights of exploitation in any form and by any means reserved worldwide for CEN national members.Ref. No. EN 12476:2000 ECouches de conversion phosphatéesdes métaux – Méthode de spécifica-tions des exigences Phosphatierüberzüge auf Metallen –Verfahren für die Festlegung von AnforderungenThis European Standard was approved by CEN on 2000-03-01.CEN members are bound to comply with the CEN/CENELEC Internal Regulations which stipulate the conditions for giving this European Standard the status of a national standard without any alteration.Up-to-date lists and bibliographical references concerning such national stand-ards may be obtained on application to the Management Centre or to any CEN member.The European Standards exist in three official versions (English, French, German).A version in any other language made by translation under the responsibility of a CEN member into its own language and notified to the Management Centre has the same status as the official versions.CEN members are the national standards bodies of Austria, Belgium, the Czech Republic, Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Italy,Luxembourg, the Netherlands, Norway, Portugal, Spain, Sweden, Switzerland,and the United Kingdom.ÈÉËEN 12476April 2000Page2EN12476:200023333345555568101112Page3 EN12476:2000Page4EN12476:2000Page5 EN12476:2000Page6EN12476:2000。
国外汽车镀锌板预磷化和磷化技术
国外汽车镀锌板预磷化和磷化技术世界金属导报/2008年/4月/22日/第008版绿色钢铁国外汽车镀锌板预磷化和磷化技术朱久发预磷化膜能较好地解决较易锈蚀的热镀锌板在仓储、运输期间的防锈问题,又能在汽车板的冲压成型过程中起到固体润滑膜的作用,明显地降低了热镀锌板材的表面摩擦系数,并有效减缓了热镀锌板与模具摩擦后易产生的锌层脱落、粉化等缺陷。
热镀锌预磷化板在欧、美、日等有些钢厂得到普遍推广,己成为解决难冲压件开裂的一种有效技术。
为了不断提高汽车车身用热镀锌板的防腐蚀性能,汽车车身的油漆底层仍将采用锌盐磷化处理技术,这就需要采用更加环保、更短处理时间和更低运行成本的锌盐磷化技术。
1、预磷化镀锌板生产工艺预磷化镀锌板是在钢厂热镀锌机组或电镀锌机组上添置的磷化装置所生产的。
在镀锌工艺段后,直接对锌层表面进行磷酸盐溶液处理,形成一层有利于冲压的固体磷酸盐润滑膜,并提高热镀锌板在仓储、运输及整车涂装工序前的耐蚀性。
磷化膜多为锌锰镍三元系的伪转化膜。
当前有3种典型的预磷化处理方式,即浸渍式、喷淋式和辊涂式预磷化工艺。
其中浸渍式和喷淋式适合于生产连续式预磷化板,辊涂式特别适合于生产非连续式预磷化板。
所谓“连续式”和“非连续式”,系指目前国际上流行的镀锌预磷化膜分为两类,即连续式和非连续式磷化膜的镀锌预磷化板。
1.1浸渍式预磷化工艺镀锌后的带钢先通过喷淋或浸渍处理上一层作为磷化晶体晶核的钛酸盐表面调整剂(以下简称为表调剂),以细化和均化磷化膜晶体的物理尺寸,再浸入装满磷化液的槽体内,在溶液中完成预磷化膜的生长过程,离开槽体后由去离子水充分漂洗,随后进行热风干燥。
浸渍式是传统磷化方式,磷化液多为锌锰镍改性,膜重一般控制在1.5~3.0g /m2范围内。
该工艺的优点是受设备状态的影响较小,带钢上下表面磷化膜的磷化指标,如膜重、结晶尺寸等没有差异。
但为了保证锌层与磷化液有足够的接触时间,必须要有体积足够大的磷化槽,还要解决含磷漂洗水所带来的环保问题。
防磷化工艺分类和应用 防锈磷化工艺流程
防磷化工艺分类和应用防锈磷化工艺流程磷化的分类1锌系磷化:又称灰磷,形成灰一暗灰色磷化膜,磷化膜重1.5-3.5g∕m2,主要用于涂装底层,提高涂层的结合力和防腐蚀能力。
2、铁系磷化:又称彩磷,形成蓝一金黄一彩虹色磷化膜,磷化膜重0.3-0.6g∕m2,主要用于涂装底层,提高涂层的结合力和防腐蚀能力。
3、镒系磷化:又称黑色磷化,形成深灰一黑色磷化膜,磷化膜重5.0-20g∕m2,浸防锈油或皂化后,用于零部件的长期防锈。
4、多功能磷化:又称三合一磷化,一次性完成除油、除锈、磷化功能,形成蓝一蓝紫一灰色磷化膜,用于大型设备、小批生产涂装前处理。
防锈磷化工艺磷化工艺的早期应用是防锈,钢铁件经磷化处理形成一层磷化膜,起到防锈作用。
经过磷化防锈处理的工件防锈期可达几个月甚至几年(对涂油工件而言),广泛用于工序间、运输、包装贮存及使用过程中的防锈,防锈磷化主要有铁系磷化、锌系磷化、镒系磷化三大品种。
铁系磷化的主体槽液成分是磷酸亚铁溶液,不含氧化类促进剂,并且有高游离酸度。
这种铁系磷化处理温度高于95℃z处理时间长达30min以上,磷化膜重大于10g∕m2,并且有除锈和磷化双重功能。
这种高温铁系磷化由于磷化速度太慢,现在应用很少。
镒系磷化用作防锈磷化具有最佳性能,磷化膜微观结构呈颗粒密堆集状,是应用最为广泛的防锈磷化。
加与不加促进剂均可,如果加入硝酸盐或硝基服促进剂可加快磷化成膜速度。
通常处理温度80-IOO o C,处理时间10~20min,膜重在7.5克/m2以上。
锌系磷化也是广泛应用的一种防锈磷化,通常采用硝酸盐作为促进剂,处理温度80~9(ΓC,处理时间10~15min,磷化膜重大于7.5g∕m2,磷化膜微观结构一般是针片紧密堆集型。
防锈磷化一般工艺流程:除油除锈一一水清洗一一表面调整活化一一磷化一一水清洗一一铭酸盐处理一一烘干一一涂油脂或染色处理通过强碱强酸处理过的工件会导致磷化膜粗化现象,采用表面调整活化可细化晶粒。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
NA.1 Chemical composition of coating
In addition to the methods specified in this annex, other analytical methods with at least the same precision may be used. The detection of phosphate in a coating shall be taken as proof that it Байду номын сангаасs a phosphate coating (cf. subclause NA.2). To establish that a coating is a manganese phosphate coating, it is sufficient to establish the presence of manganese (for ferrous substrates only) (cf. subclause NA.3). To establish that a coating is a zinc phosphate or a zinc calcium phosphate coating, it is sufficient to establish the presence of zinc or zinc and calcium, respectively (for zinc-free substrates) (cf. subclause NA.5). If neither manganese nor zinc has been detected in a phosphate coating on a substrate of ferrous material, then the coating is certain to be an iron phosphate coating.
NA.1.1 Reagents
The following reagents, of analytical grade, and distilled or deionized water should be used. a) 5 % sodium hydroxide solution, NaOH, prepared by dissolving 50 g of sodium hydroxide in one litre of water. b) 40 % nitric acid solution, HNO3, prepared by mixing four parts by volume of nitric acid (of density 1,4 g/cm³) and 2,5 parts by volume of water. c) Ammonium molybdate solution, prepared by dissolving 88,3 g of ammonium heptamolybdate tetrahydrate, (NH4)6Mo7O24 · 4 H2O, 30 ml of 25 % ammonia solution (of density 0,91 g/cm³), and 240 g of ammonium nitrate, NH4NO3, in water and making up to 1 litre. d) 25 % hydrochloric acid solution, HCl, prepared by mixing 2,5 parts by volume of hydrochloric acid (of density 1,19 g/cm³), and 1,2 parts by volume of water; e) 5 % potassium hexacyanoferrate (II) solution, K4[Fe(CN)6], prepared by dissolving 50 g potassium hexacyanoferrate in one litre of water; f) 17 % nitric acid solution, HNO3, prepared by mixing 1,7 parts by volume of nitric acid (of density 1,4 g/cm3) and 4,8 parts by volume of water. g) 3 % hydrogen peroxide solution, H2O2 (of density 1,3 g/cm3), prepared by mixing one part by volume of hydrogen peroxide and nine parts by volume of water; h) Bismuth sodium trioxide, NaBiO3. i) Ammonium nitrate, NH4NO3. j) 10 % aqueous ammonia solution, NH3, prepared by mixing two parts by volume of concentrated ammonia solution (of density 0,91g/cm³) and five parts by volume of water. k) Ammonium oxalate, (NH4)2C2O4 · H2O. l) Ammonium chloride, NH4Cl. m) 10 % hydrochloric acid solution, HCl, prepared by mixing one part by volume of hydrochloric acid (of density 1,19 g/cm3) and 2,7 parts by volume of water. n) 45 % sulfuric acid solution, H2SO4, prepared by mixing 4,5 parts by volume of sulfuric acid (of density 1,84 g/cm3) and 5,3 parts by volume of water. o) 5 % potassium permanganate solution, KMnO4, prepared by dissolving 50 g of potassium permanganate in one litre of water.
NA.2 Testing for the presence of phosphate
To test for the presence of phosphate in the coating, treat a coated test piece having a surface area of about 100 cm2 with 100 ml of a 5 % sodium hydroxide solution at 80 °C to 90 °C until either the coating has been stripped or it has at least been noticeably attacked. If necessary, scrub the coating off with a rubber wiper. Filter the resulting solution and acidify 25 ml of the filtrate with 40 % nitric acid. Add 10 ml of the ammonium molybdate solution and 5 g of ammonium nitrate to the acidified filtrate, and leave the mixture to stand for at least 15 minutes. A yellow precipitate indicates the presence of phosphate.
Previous editions DIN 50942: 1955-01, 1973-11, 1987-05, 1996-09; DIN EN 12476: 2000-07.
National Annex NA Standard referred to
(and not included in Normative references and Bibliography) DIN EN ISO 3892 Conversion coatings on metallic materials – Determination of coating mass per unit area
with gravimetric methods (ISO 3892 : 1980)
Continued on pages 2 and 3. EN comprises 12 pages.
© No part of this standard may be reproduced without the prior permission of DIN Deutsches Institut für Normung e. V., Berlin. Beuth Verlag GmbH, 10772 Berlin, Germany, has the exclusive right of sale for German Standards (DIN-Normen).
European Standard EN 12476 : 2000 has the status of a DIN Standard.
A comma is used as the decimal marker.
National foreword
This standard has been prepared by CEN/TC 262 ‘Metallic and other organic coatings’ (Secretariat: United Kingdom). The responsible German body involved in its preparation was the Normenausschuss Materialprüfung (Materials Testing Standards Committee), Technical Committee Chemische und elektrochemische Überzüge. The committee decided to retain the specifications of subclause 7.2 (cf. clauses NA.1 to NA.5) and of tables 7 and 8 (cf. tables NA.1 and NA.2) of DIN 50942 by introducing them in the national annex below. DIN EN ISO 3892 is the standard corresponding to European Standard EN ISO 3892 referred to in clause 2 of the EN.
