SCHUR-MONOTONIC HSS APPROXIMATIONS OF SYMMETRIC POSITIVE DEFINITE MATRICES
Consensus[1]希罗莫斯机理
![Consensus[1]希罗莫斯机理](https://img.taocdn.com/s3/m/4538c8373968011ca300910a.png)
12
CNI转换为西罗莫司的指征-3
肝功能损害
除去肾毒性以外,在使用环孢素或者他克莫司的部分患 者中可以出现胆红素和转氨酶升高等肝功能损伤的表现。 在使用环孢素的患者中尤为明显 在这部分患者中,转换使用西罗莫司可以较好的改善患 者的肝脏功能和其他不良反应。改善患者的生活质量。
13
CNI转换为西罗莫司的指征-4
6
降低雷帕鸣®浓度
利福布汀,利福喷丁, 利福平
雷帕鸣®与其他免疫抑制剂比较
西罗莫司
作用机理 mTOR阻断 阻断
环孢素
钙调磷酸酶阻 断
普乐可复
钙调磷酸酶阻 断
骁悉
抗代谢
布累迪宁
抗代谢
定位 阻断信号
基础用药 第2,3信号 , 信号 G1-S期 期
基础用药 第1信号 信号 G0-G1期 期
基础用药 第1信号 信号 G0-G1期 期
CsA和FK506抑制钙调 和 抑制钙调 磷酸酶,阻断IL-2激 磷酸酶,阻断 激 活的细胞凋亡
1. Sehgal S.N., et al. Curr. Op. Nephrol. Hypertens. 1995; 4: 482-487. 2. Kelly P.A.,et al. Pharmacotherapy 1997; 17(6): 1148-1156.
随着肝移植,心脏移植,胰腺移植等患者存活时间的改善,近年 来发现越来越多长期服用CNI的肝移植患者的肾功能受到影响, 甚至在晚期出现肾功能衰竭 西罗莫司是FDA批准的第一个非CNI类的基础免疫抑制剂。在上 述患者中使用西罗莫司,不仅可以保持足够的免疫抑制强度,不 增加患者出现急排的危险,更可以有效改善患者的肾功能。
21
8
CNI转换为西罗莫司的指征
Development and Validation of a Liquid Chromatogra

J. Chem. Chem. Eng. 5 (2011) 1-6.Development and Validation of a LiquidChromatography–Tandem Mass Spectrometry Method for Determination of Artemisinin in Rat PlasmaElhassan Gamal1,2, Yuen Kah1, Wong Jiawoei1, Chitneni Mallikarjun1,3, Al-Dahli Samer1, Khan Jiyauddin1 and Javed Qureshi31. School of Pharmaceutical Sciences, Universiti Sains Malaysia, Minden 11800, Penang, Malaysia2. Local Pharmaceutical Manufacturing Department, General Pharmacy Directorate, MOH, 11111, Khartoum-Sudan3. School of Pharmacy and Health Sciences, International Medical University, 5700, Kula Lumpur, MalaysiaReceived: September 03, 2010 / Accepted: October 11, 2010 / Published: January 10, 2011.Abstract: Artemisinin is a potent anti-malarial drug isolated from traditional Chinese medicinal herb, Artemisia annua. The objective of this study was to develop and validate a sensitive and specific LC-MS/MS method for the determination of artemisinin in rat plasma using amlodipine as Internal Standard. The method consist of a simple liquid-liquid extraction with methyl tertiary butyl ether (MTBE) with subsequent evaporation of the supernatant to dryness followed by the analysis of the reconstituted sample by LC-MS/MS with a Z-spray atmospheric pressure ionization (API) interface in the positive ion-multiple reaction monitoring mode to monitor precursor→product ions of m/z 282.70→m/z 209.0 for artemisinin and m/z 408.9→m/z 237.0 for amlodipine respectively. The method was linear (0.999) over the concentration range of 7.8–2000 ng/mL in rat plasma. The intra and inter-day accuracy were measured to be within 94-104.2% and precision (CV) were all less than 5%. The extraction recovery means for internal standard and all the artemisinin concentrations used were between 82-85%.Key words: Artemisinin, LC-MS/MS, amlodipine, plasma, accuracy and precision.1. IntroductionArtemsinin is the name given to the active principle of qinghaosu, an extract of the Chinese medicinal plant qinghaosu or green Artemisia (Artemisinin annua L.) which has been used for many years centuries in Chinese traditional medicine for treatment of fever and malaria [1]. In 1972, Chinese researchers isolated artemisinin from Artemisia annua L. sweet wormwood) and its structure was elucidate in 1979 as show in Fig. 1.The determination of artemisinin and its derivatives in biological matrices have previously been characterized using several analytical techniques suchCorresponding author: Gamal Osman Elhassan Ph.D., research field: pharmaceutical technology. E-mail: ******************.as LC, HPLC, GC-MS etc [3-8]. However, some of these methods suffer from few drawbacks. In particulars, interference with endogenous constituents in the plasma at the absorption wave length of the derivatized compounds may render these techniques unsatisfactory and few of them lacked the required sensitivity to be used for measurement of drugFig. 1 The chemical structure of artemisinin [2].ll Rights Reserved.Development and Validation of a Liquid Chromatography–Tandem Mass Spectrometry Method forDetermination of Artemisinin in Rat Plasma2concentration in blood sample obtained from clinical investigation [9].To increase the specificity and sensitivity of HPLC-UV method, some workers combined it with a mass spectrometry (MS) and the total system is described as LC-MS technique [10, 11]. The development of LC-tandem mass spectrometry (LC-MS/MS) has made a more specific and sensitive analysis of artemisinin and its derivatives possible [12, 13]. The objective of this study was to develop a sensitive and specific LC-MS/MS method for the determination of artemisinin in rat plasma by simple liquid-liquid extraction procedure.2. Materials and Methods2.1 MaterialsArtemisinin was purchased from Kunming Pharmaceutical Corporation (Kunming, China). Amlodipine was obtained from Sigma Chemical (Louis, USA). Acetonitrile (ACN), formic acid and methyl tertiary butyl ether (MTBE) were purchased from J.T Baker (USA).3. Methods3.1 Instrumentation and ConditionsThe instrumentation comprised of Quattro-micro tandem mass spectrometer with Z-spray atomospheric pressure ionization (API) source (Micromass, Manchester, UK) using electrospray ionization (ESI) operated at positive mode. Chromatography was performed on an Alliance 2,695 separation module (Waters, M.A, USA). The delivery system consisted of an autosampler and a column heater. The chromatographic separation was obtained using an X Terra MS C8 encapped (5 μm) (150 × 2.1 mm) analytical column (Water, USA).3.2 Sample PreparationA 250 μL aliquot of plasma was pipetted into a screw-capped culture tube, followed by 100 μL of internal standard solution (50 ng/mL). To each tube, 5 mL (MTBE) extraction solvent was then added and the mixture was vortexed for 2.5 minutes followed by centrifuging for 15 minutes at 3,500 rpm. The upper layer was transferred to a reactive vial and dried under nitrogen flow at 40 °C. The residue was then reconstituted with 250 μL of mobile phase and 20 μL was injected into the LC-MS/MS system.3.3 Assay ValidationCalibration curve at a concentration range of 7.8–2,000 ng/mL were constructed by spiking blank human plasma with a known amount of artemisinin. Plasma sample spiked with artemisinin at these concentrations 7.8, 62.5, 250, 2,000 ng/mL were used to determine the within and between-day accuracy and precision. For within-day accuracy and precision, replicates analysis (n = 6) for each concentration were performed in a single day. For between-day evaluation, analysis was carried out with a single sample of each concentration daily over 6 days, with calibration curve constructed on each day of analysis. The extraction recovery of artemisinin was estimated by comparing the peak height obtained after extraction of the samples from plasma with that of aqueous artemisinin solution of the corresponding concentration.4. Results and DiscussionBoth electrospray (TIS) and atmospheric pressure chemical ionisation (APCI) methods have been reported previously for the quantification of artemisinin derivatives in biological fluids [11, 12, 14-16]. According to the previously reported methods TIS was found to be superior to APCI for the quantification of artesunate and dihydroartemisinin (DHA) mainly because of improved linearity [16]. Therefore in this method electrospray ionization was used. When artemisinin and amlodipine were injected directly into the mass spectrometer along with mobile phase in the positive mode, the protonated molecules of artemisinin and amlodipine were set as precursorll Rights Reserved.Development and Validation of a Liquid Chromatography–Tandem Mass Spectrometry Method forDetermination of Artemisinin in Rat Plasma3(a)(b)Fig. 2 (a) Positive-ionization electrospray mass spectra of precursor ion for artemisinin; (b) Positive-ionization electrospray mass spectra of product ion for artemisinin.ions with m/z of 282.7 and 408.7, respectively. The product ion that gave the highest intensity was m/z of 209.0 for artemisinin and 237.7 for amlodipine. Fig 2(a) shows the spectra precursor ion, 2(b) production for artemisinin.Artemisinin and amlodipine have retention time of approximately 6.9 and 1.65 minutes, respectively (Fig.3). The peak was well resolved and free from interference from endogenous compounds in rat plasma (Fig. 4).ll Rights Reserved.Development and Validation of a Liquid Chromatography–Tandem Mass Spectrometry Method forDetermination of Artemisinin in Rat Plasma4Fig. 3 Plasma spiked with 500 ng/ml artemisinin and amlodipine 50 ng/mL.Fig. 4 Chromatograms for analysis of artemisinin in plasma (Rat blank plasma).Calibration curve was linear over the entire range of calibration curves with a mean correlation coefficient greater than 0.9995 (Fig. 5).The limit of quantification (LOQ) of the assay method was 7.8 ng/mL being the lowest concentration used to construct the calibration curve whereas the limit of detection (LOD) was 3.9 ng/mL at a signal to noise ratio of 3. The validation data demonstrated a good precision, accuracy and recovery. The extraction recovery means for internal standard and all artemisinin concentrations used were 75-85% (Table 1). The within-day and between-day accuracy and precision values are given in Table 2.Neither artemisinin nor the internal standard producedll Rights Reserved.Development and Validation of a Liquid Chromatography–Tandem Mass Spectrometry Method forDetermination of Artemisinin in Rat Plasma5Fig. 5 Mean calibration curve of artemisinin (ng/mL).Table 1 Extraction recovery.Concentration (ng/mL) Mean recovery (%) CV (%)7.81 75.081.5062.50 82.161.94250.00 82.03 2.072000.00 85.23 1.48Table 2 Within-day and between-day precision andaccuracy.Added (ng/mL)Within-day Between-day Accuracy (%) C.V (%) Accuracy (%) C.V (%)7.81 96.00 4.60 104.11 2.30 62.50 98.10 1.60 94.10 2.20 250.00 98.10 1.50 98.10 1.60 2000.00 96.10 2.50 97.10 1.80any detectable carry-over after three injections of upper limit of quantification. Blank rat plasma showed no interference with artemisinin. Interfering signals from blank plasma contributed less than 20% of the artemisinin signal at LOQ. There was no interference of artemisinin on the internal standard or vice versa. A small enhancement for artemisinin and the internal standard could be detected when references in neat injection solvent were compared with references in extracted blank biological matrix. The normalized matrix effects (artemisinin/internal standard) were close to 1 with a low variation in accordance with international guidelines. Post-column infusion experiments confirmed the absence of regions with severe matrix effects (i.e., no sharp drops or increases in the response) for blank human plasma extracted with the developed method.Xing et al. used artmether as an internal standard for the analysis of artemisinin [17]while for the analysis of artemisinin derivatives; artemisinin was used as internal standard [14]. In the present study amlodipine was found to be suitable because it could be separated chromatographically, ionized and fragmented under the conditions that optimized the intensity of artemisinin peak (Fig. 3).The analysis of artemisinin and its derivatives with mass spectrometry are most often performed with a different mode of ionization. Xing et al. used ESI inletin the positive ion-multiple reaction monitoring mode which relatively producing a higher sensitivity than in the SIM mode. Therefore, the mass spectrometry was operated at positive ion-MRM mode.4. ConclusionThe LC-MS/MS method described in this work is suitable for the determination of artemisinin in plasma. The assay procedure is simple with a relatively shortll Rights Reserved.Development and Validation of a Liquid Chromatography–Tandem Mass Spectrometry Method forDetermination of Artemisinin in Rat Plasma6retention time allowing sufficient sample to beprocessed to be applied to pharmacokinetic and bioavailability studies of artemisinin. The accuracy and precision of the assay method, as well as the recovery of extraction procedure were found to be satisfactory.References[1] D.L. Klayman, Qinghasou (Artemisinin): An antimalaria drug from China, Science 228 (1985) 1049-1055.[2] X.D. Luo, C.C. Shen, The chemistry, pharmacology andclinical applications of Qinghaosu (artemisinin) and it’sderivatives, Med. Res. Rev. 7 (1987) 29-52.[3] K.T. Batty, M. Ashton, K.F. Llett, G . Edwards, T.M. Davis,Selective high-performance liquid chromatography ofartesunate and α-and β-dihydroartemisinin in patients withfalciparum malaria, J. Chromatog. B 677 (2-3) (1996)345-350.[4] J. Karbwang, K. Na-Bangchang, P. Molunto, V . Banmairuroi, Determination of artemisinin and its majormetabolite, dihydroartemisinin, in plasma usinghigh-performance liquid chromatography withelectrochemical detector, J. Chromatog. B 7 (1-2) (1997)259-265.[5] K.L. Chan, K.H. Yuen, H. Takayanki, S. Jinandasa, K.K. Peh, Polymorphism of artemisinin from Artemisia annua,Phytochemistry 46 (7) (1997) 1209-1214.[6] G .Q. Li, T.O. Peggins, L.L. Fleckenstein, K. Masonic,M.H. Heiffles, T.G . Brewer, The pharmacokinetics andbiovailability of dihydroartemisinin, arteether, artemether,artesunic acid and artelinic acid in rats, J. Pharm.Pharmacol 5 (1998) 173-182.[7] B.A. Avery, K.K. Venkatesh, M.A. Avery, Rapid determination of artemisinin and related analogues usinghigh-perfomance liquid chromatography and anevaporative light scattering detector, J. Chromat. B 730 (1)(1999) 71-80.[8] S.S. Mohamed, S.A. Khalid, S.A. Ward, T.S.M. Wan,H.P.O. Tang, M. Zheng, R.K. Haynes, G . Edwards,Simultaneous determination of artemether and its majormetabolite dihydroartemisinin in plasma by gaschromatography-mass spectrometry-selected ionmonitoring, J. Chromat. B 731(1999) 251-260.[9] K.T. Batty, M. Ashton, K.F. Llett, G . Edward, T.M. Davis,The pharmacokinetics of artemisinin (ART) and artesunate (ARTS) in healthy volunteers, Am J. Trop Med. Hyg. 58(2) (1998) 125-126.[10] C. Souppart, N. Gouducheau, N. Sandenan, F. Richard,Development and validation of a high-performance liquid chromatography-mass spectrometry assay for the determination of artemisinin and its metabolite dihydraartemisinin in human plasma, J. Chromat. B 774(2002) 195-203.[11] H. Naik, D.J. Murry, L.E. Kirsch, L. Fleckenstein,Development and validation of high-performance liquid chromatography-mass spectroscopy assay for determination of artesunate and dihydrroartemisinin in human plasma, J. Chromat. B 816 (1-2) (2005) 233-242. [12] J. Xing, H. Yan, S. Zhang, G . Ren, Y . Gao, A high-performance liquid chromatography/tandem mass spectrometry method for the determination of artemisinin in rat plasma, Rapid Commun in Mass Spectro. 20 (9) (2006) 1463-1468. [13] J. Xing, H.X. Yan, R.L. Wang, L.F. Zhang, S.Q. Zhang,Liquid chromatography-tandem mass spectrometry assay for the quantitation of β-dihydroartemisinin in rat plasma, J. Chromat. B 852 (1-2) (2007) 202-207. [14] M. Rajanikanth, K.P. Madhusudanan, R.C. Gupta, An HPLC-MS method for simultaneous estimation of alpha, beta-arteether and its metabolite dihydroartemisinin, in rat plasma for application to pharmacokinetic study, J Biomed. Chromat. 17 (7) (2003) 440-446. [15] Y . Gu, Q. Li, M.V . Elendez, P. Weina, Comparison of HPLC with electrochemical detection and LC–MS/for the separation and validation of artesunate and dihydroartemisinin in animal and human plasma, J. Chromatogr B 867 (2008) 213-218. [16] W. Hanpithakpong, B. Kamanikom, A.M. Dondorp, P.Singhasivanon, N.J. White, N.P. Day, N. Lindegardh, A liquid chromatographic-tandem mass spectrometric method for determination of artesunate and its metabolite dihydroartemisinin in human plasma, J. Chromatogr. B 876 (2008) 61-68. [17] Y . Xing, H. Yan, S. Zhang, G . Ren, Y . Gao, A high-performance liquid chromatography/tandem mass spectrometry method for the determination of artemisinin rat plasma, Rapid Communication in Mass Spectrometry 20 (9) (2006) 1463-1468.ll Rights Reserved.。
前列腺不典型小腺泡增生

ASAP与前列腺微小癌 (m inimal volume p rostatic adeno2 carcinoma,癌占活检组织总量的 5%以下 )之间的鉴别标准 中 ,腺泡数目和病灶大小是最主要的一条 , ASAP腺泡的数目 是癌腺泡数目的 2 /3 (11、17) , ASAP病灶比癌性病灶小一半 (014 mm、018 mm ) 。核增大 、明显的核仁 、核分裂象 、腔内蓝 色黏液及并存 P IN等形态特征在前列腺微小癌中更明显 ,但 核深染及中 ~重度萎缩在 ASAP 比癌中更为常见 (分别为 44%、9%和 59%、35% ) 。 100%前列腺微小癌呈浸润性生 长 ,但浸润性的生长方式也存在于 75%的 ASAP病例中 。嗜 酸性颗粒性分泌物与类晶体在两者无明显差异 [12 ] 。
前列腺癌占男性恶性肿瘤的第 2位 ,在发达国家 ,前列 腺癌占全部恶性肿瘤的 19% ,在发展中国家为 513% [1 ] 。前 列腺穿刺活检是发现和确诊前列腺癌的重要手段 ,但穿刺标 本中经常会遇到少量不典型腺泡 ,疑似癌却又不能确定为 癌 ,这便是前列腺不典型小腺泡增生 ( atyp ical small acinar p roliferation, ASAP) 。现将 ASAP形态特征 、诊断标准 、发病 率 、临床意义以及对发现前列腺癌的预测价值等作一综述 。
1 A SA P的病理特征及应用现状
ASAP也称不典型腺体 ( atyp ia / atyp ical glands) [2 ] ,是由 Bostw ick等 [3 ]于 1993年首次提出的一个描述不典型腺样前 列腺增生的诊断术语 。4 年后这一诊断的临床意义得到首 次阐述 [4 ] 。
ASAP为不典型腺泡病变 ,表现为排列紧密的灶性增生 的小腺泡集落 。这些小腺泡被覆一层几近透明的分泌细胞 上皮 ,而基底细胞呈断片状或消失 (可经 34βE12 免疫组化 证实 ) 。组织特点为 : ①有限数量的腺体 ; ② 极少腺体出现 细胞不典型性 ,包括核增大 、核仁增大 ; ③ 组织异型 :缺乏核 异型的小腺泡杂乱无章地排列 ; ④ 腔内可见蓝色黏液 、结晶 体或粉红色蛋白样分泌物 [5 ] 。这些腺泡的结构形态和 /或细 胞形态类似于分化较好的前列腺癌 ,但数量太少 ,只是怀疑 为癌但不能明确诊断 。不足以诊断为癌而做出 ASAP这一 诊断主要见于两种情况 [6 ] : ①质的方面 ,缺乏足够的前列腺 癌细胞和组织结构特点 。例如一个病灶可能包括 12 个腺 泡 ,腺泡缺乏基底细胞层 ,呈浸润性生长 ,但细胞形态和组织 结构上尚未达到癌的诊断标准 (如缺少明显的核仁和明显 的核增大 ) ; ②量的方面 ,包含的腺泡数量太少 ,腺泡的细胞 和组织结构方面已经达到癌的诊断标准 ,但病灶的大小是其 主要限制 (如 1~3个腺泡 ) 。
以色列二穗短柄草谷胱甘肽过氧化物酶活性分析

Y N J n’ X EW nto, E Y W N i — e , H N u. ag , H N a -i ( . o A u , U e— H a , A GXn m i Z O GG i in 。 C E GJ npn a e g x i g 1 C1 .
ro . Alo,sg i c n i ee c r b e v d a n 0 c tp s ot s i f a tdf rn eweeo s r e mo g1 5 e oy e .Ex e tr o ,e c ft eGS P cii n i c p o t a h o h H— x a tv—
由遗传 决定的 , 为模式植物的遗传 差异性对于禾谷 类植物 的遗传和基 因比较分析 等极具价值。 作 关键词 : 式植物 ; 色列; 模 以 二穗短 柄草 ; 胱甘肽过氧化物酶 谷
中 图分 类 号 :9 53 Q 4 .5 文献标识码 : A 文 章 编 号 : 0 —05 (0 1 0 0 9 0 1 8 4 7 2 1 ) 3— 18— 5 0
严 俊 , 薛文韬 何 , 跃 王兴梅 钟桂香 程剑 平 , , ,
395 10 ;
( .贵州大学 生命科学学 院, 1 贵州 贵阳 502 ;. 505 2 海法大学 进化研究所 , 以色列 海法 3 贵 州大 学 农 学 院 , 州 贵 阳 5 02 ) . 贵 5 05
摘
要: 二穗短柄草是一种理想的研 究温带禾谷类植物和牧 草的模 式植物。本文对 来源于以 色列 4个不 同地 区
e c s O h H— x a tvte fro ,se a d la at fB.di a h o r w n te n tr lmai o - n e n t e GS P cii so o t tm n e fp rso i s c y n g e i h au a ci t c n t l c
青少年特发性脊柱侧凸康复治疗现状与进展

青少年特发性脊柱侧凸康复治疗现状与进展王莉;黄晓琳;谢凌锋;徐群【期刊名称】《中国康复》【年(卷),期】2017(032)003【总页数】5页(P249-253)【关键词】脊柱侧凸;康复治疗【作者】王莉;黄晓琳;谢凌锋;徐群【作者单位】华中科技大学同济医学院附属同济医院康复医学科,武汉 430030;华中科技大学同济医学院附属同济医院康复医学科,武汉 430030;华中科技大学同济医学院附属同济医院康复医学科,武汉 430030;华中科技大学同济医学院附属同济医院康复医学科,武汉 430030【正文语种】中文【中图分类】R49;R687脊柱侧凸是一种三维的脊柱和躯干扭转异常,包括在冠状面上的侧方弯曲,水平面上椎体旋转和矢状面上脊柱正常生理曲度改变[1]。
青少年特发性脊柱侧凸(Adolescent Idiopathic Scoliosis,AIS)是脊柱侧凸中最常见的类型,发病率约为2%~3%[1],最新调查显示其在我国的发病率为5.2%[2]。
目前AIS病因尚未明确,研究显示可能与遗传基因、不良姿势、某些激素水平等相关[3-4]。
青少年处于生长发育的高峰期,脊柱增长迅速,如不及时诊治,脊柱侧凸的程度可能会随生长发育的进行逐渐加重,继而出现背部疼痛、心肺功能障碍等问题,严重者可导致瘫痪。
因此早发现、早诊断、早治疗非常重要。
目前脊柱侧凸常用的康复治疗方法有:支具、运动疗法、牵引、手法治疗、电刺激等。
关于AIS的治疗,2011国际脊柱侧凸矫形和康复治疗协会(The International Scientific Society on Scoliosis Orthopaedic and Rehabilitation Treatment,SOSORT)指南推荐:Cobb角<10°时只需观察随访;Cobb角10°~20°时,一般选择特定性运动疗法;Cobb角20°~45°时,推荐支具治疗,同时配合运动疗法;Cobb角>45°时可考虑手术治疗[1]。
药物分析英文词汇

药物分析英文词汇adsorbent 吸附剂adsorption 吸附affinity chromatography 亲和色谱法aliquot (一)份alkalinity 碱度alumina 氧化铝ambient temperature 室温ammonium thiocyanate 硫氰酸铵药物分析英语词汇analytical quality control(AQC)分析质量控制Abbe refractometer 阿贝折射仪anhydrous substance 干燥品 absorbance 吸收度anionic surfactant titration 阴离子表面活性剂滴定法absorbance ratio 吸收度比值absorption 吸收antibiotics-microbial test 抗生素微生物检定法absorption curve 吸收曲线absorption spectrum 吸收光谱 antioxidant 抗氧剂 absorptivity 吸收系数 appendix 附录 accuracy 准确度 application of sample 点样 acid-dye colorimetry 酸性染料比色法area normalization method 面积归一化法acidimetry 酸量法 argentimetry 银量法 acid-insoluble ash 酸不溶性灰分 arsenic 砷 acidity 酸度 arsenic stain 砷斑 activity 活度 ascending development 上行展开additive 添加剂ash-free filter paper 无灰滤纸(定量滤纸)additivity 加和性adjusted retention time 调整保留时间assay 含量测定assay tolerance 含量限度 bromate titration 溴酸盐滴定法atmospheric pressure ionization(API) 大气压离子化bromimetry 溴量法bromocresol green 溴甲酚绿 attenuation 衰减bromocresol purple 溴甲酚紫 back extraction 反萃取bromophenol blue 溴酚蓝 back titration 回滴法bromothymol blue 溴麝香草酚蓝 bacterial endotoxins test 细菌内毒素检查法bulk drug, pharmaceutical product 原料药band absorption 谱带吸收buret 滴定管 baseline correction 基线校正by-product 副产物 baseline drift 基线漂移calibration curve 校正曲线 batch, lot 批calomel electrode 甘汞电极 batch(lot) number 批号calorimetry 量热分析 Benttendorff method 白田道夫(检砷)法capacity factor 容量因子capillary zone electrophoresis (CZE) 毛细管区带电泳between day (day to day, inter-day) precision 日间精密度capillary gas chromatography 毛细管气相色谱法between run (inter-run) precision 批间精密度carrier gas 载气 biotransformation 生物转化cation-exchange resin 阳离子交换树脂bioavailability test 生物利用度试验ceri(o)metry 铈量法 bioequivalence test 生物等效试验characteristics, description 性状 biopharmaceutical analysis 体内药物分析,生物药物分析check valve 单向阀chemical shift 化学位移 blank test 空白试验chelate compound 鳌合物 boiling range 沸程chemically bonded phase 化学键合相British Pharmacopeia (BP) 英国药典chemical equivalent 化学当量 coefficient of distribution 分配系数Chinese Pharmacopeia (ChP) 中国药典coefficient of variation 变异系数color change interval (指示剂)变色范围Chinese material medicine 中成药Chinese materia medica 中药学 color reaction 显色反应 Chinese materia medica preparation 中药制剂colorimetric analysis 比色分析colorimetry 比色法 Chinese Pharmaceutical Association (CPA) 中国药学会column capacity 柱容量column dead volume 柱死体积 chiral 手性的column efficiency 柱效 chiral stationary phase (CSP) 手性固定相column interstitial volume 柱隙体积chiral separation 手性分离column outlet pressure 柱出口压 chirality 手性column temperature 柱温 chiral carbon atom 手性碳原子column pressure 柱压 chromatogram 色谱图column volume 柱体积 chromatography 色谱法column overload 柱超载 chromatographic column 色谱柱column switching 柱切换 chromatographic condition 色谱条件committee of drug evaluation 药品审评委员会chromatographic data processor 色谱数据处理机comparative test 比较试验 chromatographic work station 色谱工作站completeness of solution 溶液的澄清度clarity 澄清度compound medicines 复方药 clathrate, inclusion compound 包合物computer-aided pharmaceutical analysis 计算机辅助药物分析 clearance 清除率concentration-time curve 浓度,时间曲线clinical pharmacy 临床药学confidence interval 置信区间 deflection point 拐点confidence level 置信水平 degassing 脱气 confidence limit 置信限deionized water 去离子水 congealing point 凝点 deliquescence 潮解 congo red 刚果红(指示剂) depressor substances test 降压物质检查法content uniformity 装量差异derivative spectrophotometry 导数分光光度法controlled trial 对照试验correlation coefficient 相关系数 derivatization 衍生化 contrast test 对照试验 descending development 下行展开counter ion 反离子(平衡离子)desiccant 干燥剂 cresol red 甲酚红(指示剂)detection 检查 crucible 坩埚detector 检测器crude drug 生药developer, developing reagent 展开剂crystal violet 结晶紫(指示剂) cuvette, cell 比色池 developing chamber 展开室 cyanide 氰化物deviation 偏差 cyclodextrin 环糊精 dextrose 右旋糖,葡萄糖 cylinder, graduate cylinder, measuring cylinder 量筒diastereoisomer 非对映异构体diazotization 重氮化 cylinder-plate assay 管碟测定法2,6-dichlorindophenol titration 2,6-二氯靛酚滴定法daughter ion (质谱)子离子dead space 死体积 differential scanning calorimetry (DSC) 差示扫描热量法dead-stop titration 永停滴定法differential spectrophotometry 差示分光光度法dead time 死时间decolorization 脱色 differential thermal analysis (DTA) 差示热分析decomposition point 分解点differentiating solvent 区分性溶剂 deflection 偏差diffusion 扩散 electrophoresis 电泳digestion 消化 electrospray interface 电喷雾接口diphastic titration 双相滴定electromigration injection 电迁移进样disintegration test 崩解试验dispersion 分散度 elimination 消除 dissolubility 溶解度 eluate 洗脱液 dissolution test 溶出度检查 elution 洗脱 distilling range 馏程emission spectrochemical analysis 发射光谱分析distribution chromatography 分配色谱enantiomer 对映体 distribution coefficient 分配系数 end absorption 末端吸收 dose 剂量 end point correction 终点校正 drug control institutions 药检机构 endogenous substances 内源性物质drug quality control 药品质量控制enzyme immunoassay(EIA) 酶免疫分析drug release 药物释放度drug standard 药品标准 enzyme drug 酶类药物 drying to constant weight 干燥至恒重enzyme induction 酶诱导enzyme inhibition 酶抑制 dual wavelength spectrophotometry 双波长分光光度法eosin sodium 曙红钠(指示剂) duplicate test 重复试验 epimer 差向异构体 effective constituent 有效成分 equilibrium constant 平衡常数effective plate number 有效板数 equivalence point 等当点 efficiency of column 柱效 error in volumetric analysis 容量分析误差electron capture detector 电子捕获检测器excitation spectrum 激发光谱 electron impact ionization 电子轰击离子化exclusion chromatography 排阻色谱法expiration date 失效期 fluorescence polarization immunoassay(FPIA) external standard method 外标法荧光偏振免疫分析 extract 提取物fluorescent agent 荧光剂 extraction gravimetry 提取重量法fluorescence spectrophotometry 荧光分光光度法extraction titration 提取容量法extrapolated method 外插法,外推法fluorescence detection 荧光检测器factor 系数,因数,因子 fluorimetyr 荧光分析法 feature 特征 foreign odor 异臭Fehling’s reaction 费林反应 foreign pigment 有色杂质 field disorption ionization 场解吸离子化formulary 处方集fraction 馏分 field ionization 场致离子化freezing test 结冻试验filter 过滤,滤光片funnel 漏斗 filtration 过滤fused peaks, overlapped peaks 重叠峰fineness of the particles 颗粒细度fused silica 熔融石英 flame ionization detector(FID) 火焰离子化检测器gas chromatography(GC) 气相色谱法flame emission spectrum 火焰发射光谱gas-liquid chromatography(GLC) 气液色谱法flask 烧瓶gas purifier 气体净化器 flow cell 流通池gel filtration chromatography 凝胶过滤色谱法flow injection analysis 流动注射分析gel permeation chromatography 凝胶渗透色谱法flow rate 流速fluorescamine 荧胺 general identification test 一般鉴别试验fluorescence immunoassay(FIA) 荧光免疫分析general notices (药典)凡例general requirements (药典)通则 hydrophilicity 亲水性hydrophobicity 疏水性 good clinical practices(GCP) 药品临床管理规范hydroscopic 吸湿的hydroxyl value 羟值 good laboratory practices(GLP) 药品实验室管理规范hyperchromic effect 浓色效应 good manufacturing practices(GMP) 药品生产质量管理hypochromic effect 淡色效应规范identification 鉴别 good supply practices(GSP) 药品供应管理规范ignition to constant weight 灼烧至恒重gradient elution 梯度洗脱immobile phase 固定相 grating 光栅immunoassay 免疫测定 gravimetric method 重量法impurity 杂质 Gutzeit test 古蔡(检砷)法inactivation 失活 half peak width 半峰宽index 索引 [halide] disk method, wafer method, pellet method 压片法indicator 指示剂 head-space concentrating injector 顶空浓缩进样器indicator electrode 指示电极inhibitor 抑制剂 heavy metal 重金属injecting septum 进样隔膜胶垫 heat conductivity 热导率injection valve 进样阀 height equivalent to a theoretical plate 理论塔板高度instrumental analysis 仪器分析 height of an effective plate 有效塔板高度insulin assay 胰岛素生物检定法integrator 积分仪 high-performance liquid chromatography (HPLC) 高效液相色谱法 intercept 截距 high-performance thin-layer chromatography (HPTLC) interface 接口高效薄层色谱法interference filter 干涉滤光片 hydrate 水合物intermediate 中间体 hydrolysis 水解internal standard substance 内标物质Kjeldahl method for nitrogen 凯氏定氮法international unit(IU) 国际单位 Kober reagent 科伯试剂 in vitro 体外Kovats retention index 科瓦茨保留指数in vivo 体内labelled amount 标示量 iodide 碘化物leading peak 前延峰 iodoform reaction 碘仿反应least square method 最小二乘法 iodometry 碘量法leveling effect 均化效应 ion-exchange cellulose 离子交换纤维素licensed pharmacist 执业药师 ion pair chromatography 离子对色谱limit control 限量控制limit of detection(LOD) 检测限 ion suppression 离子抑制limit of quantitation(LOQ) 定量限ionic strength 离子强度limit test (杂质)限度(或限量)试验ion-pairing agent 离子对试剂ionization 电离,离子化 limutus amebocyte lysate(LAL) 鲎试验ionization region 离子化区linearity and range 线性及范围 irreversible indicator 不可逆指示剂linearity scanning 线性扫描 irreversible potential 不可逆电位liquid chromatograph/mass spectrometer (LC/MS) 液质联用仪isoabsorptive point 等吸收点litmus paper 石蕊试纸 isocratic elution 等溶剂组成洗脱loss on drying 干燥失重 isoelectric point 等电点low pressure gradient pump 低压梯度泵isoosmotic solution 等渗溶液isotherm 等温线 luminescence 发光 Karl Fischer titration 卡尔?费歇尔滴定lyophilization 冷冻干燥main constituent 主成分 kinematic viscosity 运动黏度make-up gas 尾吹气maltol reaction 麦牙酚试验 microsyringe 微量注射器Marquis test 马奎斯试验 migration time 迁移时间 mass analyzer detector 质量分析检测器millipore filtration 微孔过滤minimum fill 最低装量 mass spectrometric analysis 质谱分析mobile phase 流动相modifier 改性剂,调节剂 mass spectrum 质谱图molecular formula 分子式 mean deviation 平均偏差monitor 检测,监测 measuring flask, volumetric flask 量瓶monochromator 单色器 measuring pipet(te) 刻度吸量管monographs 正文 medicinal herb 草药mortar 研钵 melting point 熔点moving belt interface 传送带接口melting range 熔距multidimensional detection 多维检测metabolite 代谢物multiple linear regression 多元线性回归metastable ion 亚稳离子methyl orange 甲基橙multivariate calibration 多元校正 methyl red 甲基红natural product 天然产物 micellar chromatography 胶束色谱法Nessler glasses(tube) 奈斯勒比色管micellar electrokinetic capillary chromatography(MECC, Nessler’s reagent 碱性碘化汞钾试液MEKC) 胶束电动毛细管色谱法micelle 胶束neutralization 中和 microanalysis 微量分析nitrogen content 总氮量 microcrystal 微晶nonaqueous acid-base titration 非水酸碱滴定microdialysis 微透析micropacked column 微型填充柱 nonprescription drug, over the counter drugs (OTC drugs)非处方药 microsome 微粒体nonproprietary name, generic name 非专有名nonspecific impurity 一般杂质 orthogonal test 正交试验non-volatile matter 不挥发物 orthophenanthroline 邻二氮菲 normal phase 正相 outlier 可疑数据,逸出值 normalization 归一化法 overtones 倍频峰,泛频峰 notice 凡例 oxidation-reduction titration 氧化还原滴定nujol mull method 石蜡糊法oxygen flask combustion 氧瓶燃烧octadecylsilane chemically bonded silica 十八烷基硅烷键合硅胶packed column 填充柱 octylsilane 辛(烷)基硅烷packing material 色谱柱填料 odorless 无臭palladium ion colorimetry 钯离子比色法official name 法定名official specifications 法定标准 parallel analysis 平行分析 official test 法定试验 parent ion 母离子on-column detector 柱上检测器 particulate matter 不溶性微粒 on-column injection 柱头进样 partition coefficient 分配系数 on-line degasser 在线脱气设备 parts per million (ppm) 百万分之几on the dried basis 按干燥品计pattern recognition 模式识别 opalescence 乳浊peak symmetry 峰不对称性 open tubular column 开管色谱柱peak valley 峰谷 optical activity 光学活性peak width at half height 半峰宽 optical isomerism 旋光异构percent transmittance 透光百分率optical purity 光学纯度optimization function 优化函数 pH indicator absorbance ratio method pH指示剂吸光度比值法organic volatile impurities 有机挥发性杂质pharmaceutical analysis 药物分析orthogonal function spectrophotometry 正交函数分光光度法 pharmacopeia 药典pharmacy 药学 prescription drug 处方药phenolphthalein 酚酞 pretreatment 预处理 photodiode arraydetector(DAD) 光电二极管阵列检测器primary standard 基准物质principal component analysis 主成分分析photometer 光度计pipeclay triangle 泥三角programmed temperature gas chromatography 程序升温气相色谱法 pipet(te) 吸移管,精密量取prototype drug 原型药物 planar chromatography 平板色谱法provisions for new drug approval 新药审批办法plate storage rack 薄层板贮箱purification 纯化 polarimeter 旋光计purity 纯度 polarimetry 旋光测定法pyrogen 热原 polarity 极性pycnometric method 比重瓶法polyacrylamide gel 聚丙酰胺凝胶quality control(QC) 质量控制 polydextran gel 葡聚糖凝胶quality evaluation 质量评价 polystyrene gel 聚苯乙烯凝胶quality standard 质量标准 polystyrene film 聚苯乙烯薄膜quantitative determination 定量测定porous polymer beads 高分子多孔小球quantitative analysis 定量分析 post-column derivatization 柱后衍生化quasi-molecular ion 准分子离子 potentiometer 电位计 racemization 消旋化 potentiometric titration 电位滴定法radioimmunoassay 放射免疫分析法precipitation form 沉淀形式 random sampling 随机抽样 precision 精密度rational use of drug 合理用药 pre-column derivatization 柱前衍生化readily carbonizable substance 易炭化物preparation 制剂 reagent sprayer 试剂喷雾器recovery 回收率 safety 安全性reference electrode 参比电极 Sakaguchi test 坂口试验 refractive index 折光指数 salt bridge 盐桥 related substance 有关物质 salting out 盐析 relative density 相对密度 sample applicator 点样器 relative intensity 相对强度 sample application 点样 repeatability 重复性 sample on-line pretreatment 试样在线预处理replicate determination 平行测定sampling 取样 reproducibility 重现性saponification value 皂化值 residual basic hydrolysis method 剩余碱水解法saturated calomel electrode(SCE) 饱和甘汞电极residual liquid junction potential 残余液接电位selectivity 选择性 residual titration 剩余滴定 separatory funnel 分液漏斗 residue on ignition 炽灼残渣 shoulder peak 肩峰 resolution 分辨率,分离度signal to noise ratio 信噪比 response time 响应时间significant difference 显著性差异 retention 保留 significant figure 有效数字 reversed phase chromatography 反相色谱法significant level 显著性水平significant testing 显著性检验 reverse osmosis 反渗透silanophilic interaction 亲硅羟基作用rider peak 驼峰rinse 清洗,淋洗 silica gel 硅胶 robustness 可靠性,稳定性 silver chloride electrode 氯化银电极routine analysis 常规分析similarity 相似性 round 修约(数字)simultaneous equations method 解线性方程组法ruggedness 耐用性size exclusion chromatography(SEC) 空间排阻色谱法 standard deviation 标准差standardization 标定 sodium dodecylsulfate, SDS 十二烷基硫酸钠standard operating procedure(SOP) 标准操作规程sodium hexanesulfonate 己烷磺酸钠standard substance 标准品stationary phase coating 固定相涂布sodium taurocholate 牛璜胆酸钠sodium tetraphenylborate 四苯硼钠starch indicator 淀粉指示剂statistical error 统计误差 sodium thiosulphate 硫代硫酸钠sterility test 无菌试验 solid-phase extraction 固相萃取stirring bar 搅拌棒 solubility 溶解度stock solution 储备液 solvent front 溶剂前沿stoichiometric point 化学计量点 solvophobic interaction 疏溶剂作用storage 贮藏 specific absorbance 吸收系数stray light 杂散光 specification 规格substituent 取代基 specificity 专属性substrate 底物 specific rotation 比旋度sulfate 硫酸盐 specific weight 比重sulphated ash 硫酸盐灰分 spiked 加入标准的supercritical fluid chromatography(SFC) 超临界流体色谱法 split injection 分流进样support 载体(担体) splitless injection 无分流进样suspension 悬浊液 spray reagent (平板色谱中的)显色剂swelling degree 膨胀度 spreader 铺板机symmetry factor 对称因子 stability 稳定性syringe pump 注射泵 standard color solution 标准比色液systematic error 系统误差system model 系统模型 thymol 百里酚(麝香草酚)(指示剂)system suitability 系统适用性thymolphthalein 百里酚酞(麝香草酚酞)(指示剂)tablet 片剂tailing factor 拖尾因子 thymolsulfonphthalein ( thymol blue) 百里酚蓝(麝香草酚蓝)(指示剂) tailing peak 拖尾峰titer, titre 滴定度 tailing-suppressing reagent 扫尾剂time-resolved fluoroimmunoassay 时间分辨荧光免疫法test of hypothesis 假设检验titrant 滴定剂 test solution(TS) 试液titration error 滴定误差 tetrazolium colorimetry 四氮唑比色法titrimetric analysis 滴定分析法 therapeutic drug monitoring(TDM) 治疗药物监测tolerance 容许限toluene distillation method 甲苯蒸馏法thermal analysis 热分析法thermal conductivity detector 热导检测器toluidine blue 甲苯胺蓝(指示剂)thermocouple detector 热电偶检测器total ash 总灰分total quality control(TQC) 全面质量控制thermogravimetric analysis(TGA) 热重分析法traditional drugs 传统药 thermospray interface 热喷雾接口traditional Chinese medicine 中药The United States Pharmacopoeia(USP) 美国药典transfer pipet 移液管 The Pharmacopoeia of Japan(JP) 日本药局方turbidance 混浊turbidimetric assay 浊度测定法 thin layer chromatography(TLC) 薄层色谱法turbidimetry 比浊法turbidity 浊度 thiochrome reaction 硫色素反应ultracentrifugation 超速离心 three-dimensional chromatogram 三维色谱图ultrasonic mixer 超生混合器ultraviolet irradiation 紫外线照射 xylenol orange 二甲酚橙(指示剂) undue toxicity 异常毒性zigzag scanning 锯齿扫描 uniform design 均匀设计zone electrophoresis 区带电泳 uniformity of dosage units 含量均匀度zwitterions 两性离子 uniformity of volume 装量均匀性(装量差异)zymolysis 酶解作用uniformity of weight 重量均匀性(片重差异)validity 可靠性variance 方差versus …对…,…与…的关系曲线viscosity 粘度volatile oil determination apparatus 挥发油测定器volatilization 挥发法volumetric analysis 容量分析volumetric solution(VS) 滴定液vortex mixer 涡旋混合器watch glass 表面皿wave length 波长wave number 波数weighing bottle 称量瓶weighing form 称量形式weights 砝码well-closed container 密闭容器xylene cyanol blue FF 二甲苯蓝FF(指示剂)。
开启片剂完整性的窗户(中英文对照)
开启片剂完整性的窗户日本东芝公司,剑桥大学摘要:由日本东芝公司和剑桥大学合作成立的公司向《医药技术》解释了FDA支持的技术如何在不损坏片剂的情况下测定其完整性。
太赫脉冲成像的一个应用是检查肠溶制剂的完整性,以确保它们在到达肠溶之前不会溶解。
关键词:片剂完整性,太赫脉冲成像。
能够检测片剂的结构完整性和化学成分而无需将它们打碎的一种技术,已经通过了概念验证阶段,正在进行法规申请。
由英国私募Teraview公司研发并且以太赫光(介于无线电波和光波之间)为基础。
该成像技术为配方研发和质量控制中的湿溶出试验提供了一个更好的选择。
该技术还可以缩短新产品的研发时间,并且根据厂商的情况,随时间推移甚至可能发展成为一个用于制药生产线的实时片剂检测系统。
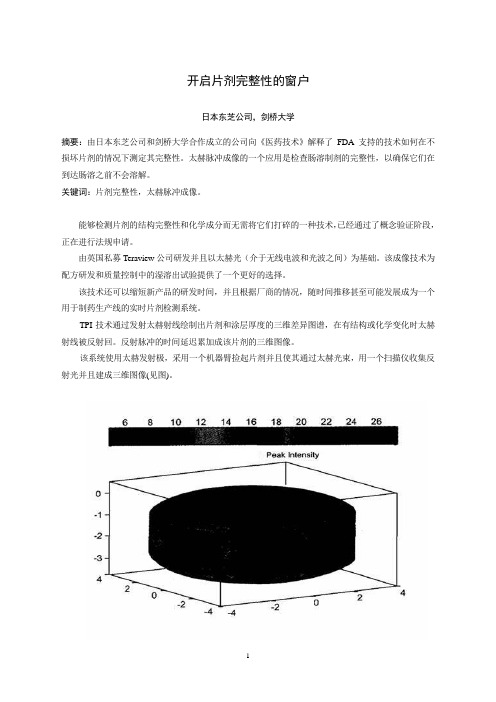
TPI技术通过发射太赫射线绘制出片剂和涂层厚度的三维差异图谱,在有结构或化学变化时太赫射线被反射回。
反射脉冲的时间延迟累加成该片剂的三维图像。
该系统使用太赫发射极,采用一个机器臂捡起片剂并且使其通过太赫光束,用一个扫描仪收集反射光并且建成三维图像(见图)。
技术研发太赫技术发源于二十世纪九十年代中期13本东芝公司位于英国的东芝欧洲研究中心,该中心与剑桥大学的物理学系有着密切的联系。
日本东芝公司当时正在研究新一代的半导体,研究的副产品是发现了这些半导体实际上是太赫光非常好的发射源和检测器。
二十世纪九十年代后期,日本东芝公司授权研究小组寻求该技术可能的应用,包括成像和化学传感光谱学,并与葛兰素史克和辉瑞以及其它公司建立了关系,以探讨其在制药业的应用。
虽然早期的结果表明该技术有前景,但日本东芝公司却不愿深入研究下去,原因是此应用与日本东芝公司在消费电子行业的任何业务兴趣都没有交叉。
这一决定的结果是研究中心的首席执行官DonArnone和剑桥桥大学物理学系的教授Michael Pepper先生于2001年成立了Teraview公司一作为研究中心的子公司。
TPI imaga 2000是第一个商品化太赫成像系统,该系统经优化用于成品片剂及其核心完整性和性能的无破坏检测。
大脑中动脉粥样硬化性狭窄支架置入术后高灌注脑出血的研究进展

基金项目:江苏省自然科学基金资助项目(BK20181240);江苏省医学重点人才培养(ZDRCA2016094)作者单位:211166南京医科大学金陵临床医学院(东部战区总医院)神经外科通信作者:张鑫,Email:zhangxsp@163.com ·综述·大脑中动脉粥样硬化性狭窄支架置入术后高灌注脑出血的研究进展袁斌张鑫吴琪文立利尤宗琦徐伟东陈姝娟邓金龙摘要:大脑中动脉(MCA)是颅内动脉粥样硬化性狭窄的常见部位,血管内治疗是MCA狭窄患者的治疗方式之一,但术后高灌注综合征(HPS)及高灌注脑出血(HICH)是严重的并发症,且与不良预后相关。
HICH的发生离不开HPS的病理生理学基础,脑血管自身调节机制受损、血-脑屏障结构受损以及一氧化氮和氧自由基的作用均可能参与了HICH的发生、发展。
结合HPS影像学征象有助于HICH的诊断。
虽然采用预测HPS的方法评估HICH的发生风险有局限性,但通过预测并防治HPS,有助于避免其进展为HICH。
未来尚需更多的研究以获得循证医学证据。
关键词:大脑中动脉;狭窄;血液灌注;颅内出血;综述doi:10.3969/j.issn.1672-5921.2019.03.010Advances on hyperperfusion-associated cerebral hemorrhage after stenting for middle cerebral artery stenosis Yuan Bin,Zhang Xin,Wu Qi,Wen Lili,You Zongqi,Xu Weidong,Chen Shujuan,Deng Jinlong.Department of Neurosurgery,Jinling Hospital,Jinling School of Clinical Medicine,Nanjing Medical University,Nanjing211166,ChinaCorresponding author:Zhang Xin,Email:zhangxsp@163.comAbstract:Intracranial atherosclerotic stenosis is commonly seen in middle cerebral artery(MCA).Endovascular treatment is one of the therapeutic strategies for MCA stenosis,with postoperative hyperperfusion syndrome(HPS)and hyperperfusion-associated intracranial hemorrhage(HICH)as its serious complications which are associated with poor prognosis.The occurrence of HICH can be understood from the pathophysiology of HPS.Impaired cerebral vascular autoregulation,damaged blood-brain barrier and the effects of nitric oxide and oxygen free radicals may be involved in the occurrence and development of bining HPS imaging features is helpful for the diagnosis of HICH.Despite using predictors of HPS to evaluate the risk of HICH has some limitations,it still can help to prevent HICH.In the future,further research is needed to obtain sufficient evidence.Key words:Middle cerebral artery;Stenosis;Hemoperfusion;Intracranial hemorrhages;Review颅内动脉粥样硬化性狭窄是缺血性卒中发生的重要原因,其中大脑中动脉(MCA)是常见的狭窄部位[1],MCA狭窄相关的缺血性卒中在中国人群中常见[2-5]。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
H1 =
U1
ˆ1 U
T1 ˆ1 T
≈ U1 T1 ≡ U1
T12
T14
T15
,
˜ij , T ˆij ). Note that a where Tij ’s are temporary matrices (also below, including any T tolerance is used in this approximate factorization. Then we can rewrite A as D U T T U1 T14 U1 T15 1 1 12 D1 (1) (1) (1) T T T12 A25 A24 A22 U1 A≈ (1)T (1) (1) , T T T14 I U1 A24 A44 A45 T T (1)T (1)T (1) T15 U1 A25 A45 A55 where A22 (1) T A24 (1)T A25 (b) Node 2.
Fig. 1.1. HSS tree.
an HSS matrix [9] also gives a superfast procedure to compute an approximate HSS factorization of the matrix. In fact, for a general SPD matrix A (not in HSS form) we can find an HSS approximation and in the meantime keep the positive definiteness of the matrix. Depending on the partition, HSS tree, and tolerance we may have different approximations. 2. Schur-monotonic HSS factorization. Given a matrix A and a partition sequence {mi } we compute a factorization A ≈ RT R where R is in postordering HSS
T D2 T T T T34 R2 U2 T T T T35 R2 U2
D1
I
U1 B1 V2T D2
U1 R1 T34 U2 R2 T34 (2) A44 (2)T A45
U1 R1 T35 U2 R2 T35 . (2) A45 (2) A55
(d) Node 4. Similarly compute
T A22 = D2 D2 , (1)
and write
T D1 T T T12 U1 A≈ T T T14 U1 T T T15 U1
T D2 T H2
I
D1
U1 T12 D2
U1 T14 U1 T15 H2 (1) (1) A45 A44 T − H2 H2 (1)T (1) A45 A55
SCHUR-MONOTONIC HSS APPROXIMATIONS OF SYMMETRIC POSITIVE DEFINITE MATRICES
S. CHANDRASEKARAN, M. GU, X. S. LI, J. XIA Abstract. Given a symmetric positive definite (SPD) matrix A, we compute an approximate factorization A ≈ RT R with any desired accuracy, where R is a hierarchically semi-separable (HSS) matrix. The factorization is both stable and efficient. The approximation RT R to A is an HSS matrix and is guaranteed to be positive definite.
A25
(1)
≈ U2
B2
T2
≡ U2
B2
T24 U1 T15 U2 T25 (2) . A45 (2) A55
T25
.
Now A becomes
T D1 T T T12 U1 A≈ T T T14 U1 T T U1 T15 (2) (2)
D1
T D2 T T T24 U2 T T U2 T25 (1)
,
(1) (1) A24 A25 . Now compress the second off-diagonal block row but ignoring any basis U, V (i.e. U1 here) T T12
−T where H2 = D2
H2
≡
T T12
A24
(1)
3
D1
U1 B1 V2T D2
U1 R1 T34 U2 R2 T34 D4
U1 R1 T35 U2 R2 T35 . H4 (2) T A55 − H4 H4
1
form. For simplicity we assume that R is associated with a complete tree such as in Fig. 1.1. We first demonstrate the procedure of constructing a 4 × 4 block HSS form (2.1) for R and {mi }: T T T T T D1 U1 B1 U2 U1 R1 B3 R4 U4 U1 R1 B3 R5 U5 T T T T D2 U2 R2 B3 R4 U4 U2 R2 B3 R5 U5 (2.1) R= T , D4 U4 B4 U5 D5 First we partition the matrix H into a A11 AT 12 A= AT 14 AT 15 4 × 4 block form A12 A22 AT 24 AT 25 A14 A24 A44 AT 45 A15 A25 , A45 A55
−T T A44 = D4 D4 , H4 = D4 A45 , T D1 T U2 B2 U1 A≈ T T T T34 R1 U1 T T T T35 R1 U1 (2) (2)
T D2 T T T T34 R2 U2 T T T T35 R2 U2 T D4 T H4
I
I
(1)
U1 T12 D2
U1 T14 U2 T24 (2) A44 (2)T A45
A45 A44 A45 A44 T T2 . − T2 = (1)T (1) (2)T (2) A45 A55 A45 A55 (c) Node 3. Node 3 is in upper level (level 1) with children node 1 and 2. A has two block rows/columns in terms of level 1. The off-diagonal block row corresponding to node 3 can be obtained by merging appropriate blocks of the off-diagonal block rows of node 1 and 2. We identify and compress it (ignoring any U basis) where T14 T24 Now A becomes T D1 T U2 B2 U1 A≈ T T T T34 R1 U1 T T T T35 R1 U1 T15 T25 = R1 R2 T34 T35 .
T A11 = D1 D1
and write A=
T D1 T H1
I
D1
Байду номын сангаас
A22 AT 24 AT 25
A24 A44 AT 45
H1 A25 , T A45 − H1 H1 A55
−T A12 A14 A15 . Now compress the first off-diagonal block row where H1 ≡ D1 in level 2 (bottom level) by a (rank revealing) QR factorization
2
(1)
A24 (1) A44 (1)T A45
(1)
(1) A25 A22 (1) A45 = AT 24 T (1) A 25 A55
A24 A44 AT 45
A25 T A45 − T1 T1 . A55
Now compute a Cholesky factorization
1. Introduction. For a given SPD matrix A [4] presents a fast and stable procedure to compute a sequentially semi-separable (SSS) approximation A ≈ RT R, where R is an upper triangular matrix in SSS form. See [1, 2, 3] for more details about SSS matrices. Here we show an algorithm that computes a factorization RT R where R is an HSS matrix. HSS structured are proposed by Chandrasekaran, Gu, et al. [6, 5] and they are generalizations of SSS structures and and H-matrices (see, e.g. [7, 8]). The generalizations of HSS matrices and HSS operations are included in [9]. Specifically [9] proposes a postordering HSS form T T T T V5 V4 U1 R1 B3 W5 U1 R1 B3 W4 D1 U1 B1 V2T T T T T V5 V4 U2 R2 B3 W5 D2 U2 R2 B3 W4 U2 B2 V1T (1.1) T T T T T . U4 R4 B6 W1 D4 U4 B4 V5 V1 U4 R4 B6 W2 V2 T T T T D5 U5 B5 V4T V2 V1 U5 R5 B6 W2 U5 R5 B6 W1 The postordering HSS form is a simplified version of the original HSS representation and more directly reflect the tree nature of the structure. Such a form is thus associated with an HSS tree. Fig. 1.1 shows a complete HSS tree for (1.1). Given
