对苯二硼酸4612-26-4
苯硼酸
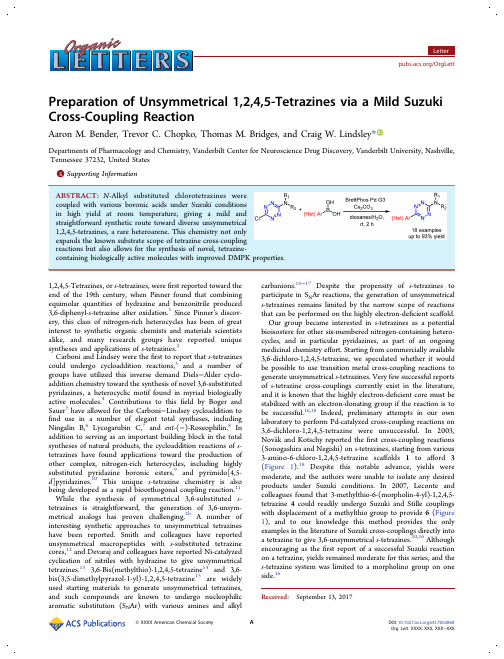
Preparation of Unsymmetrical1,2,4,5-Tetrazines via a Mild Suzuki Cross-Coupling ReactionAaron M.Bender,Trevor C.Chopko,Thomas M.Bridges,and Craig W.Lindsley*Departments of Pharmacology and Chemistry,Vanderbilt Center for Neuroscience Drug Discovery,Vanderbilt University,Nashville, Tennessee37232,United States*Supporting Informationproperties.1,2,4,5-Tetrazines,or s-tetrazines,werefirst reported toward the end of the19th century,when Pinner found that combining equimolar quantities of hydrazine and benzonitrile produced 3,6-diphenyl-s-tetrazine after oxidation.1Since Pinner’s discov-ery,this class of nitrogen-rich heterocycles has been of great interest to synthetic organic chemists and materials scientists alike,and many research groups have reported unique syntheses and applications of s-tetrazines.2Carboni and Lindsey were thefirst to report that s-tetrazines could undergo cycloaddition reactions,3and a number of groups have utilized this inverse demand Diels−Alder cyclo-addition chemistry toward the synthesis of novel3,6-substituted pyridazines,a heterocyclic motif found in myriad biologically active molecules.4Contributions to thisfield by Boger and Sauer5have allowed for the Carboni−Lindsey cycloaddition to find use in a number of elegant total syntheses,including Ningalin B,6Lycogarubin C,7and ent-(−)-Roseophilin.8In addition to serving as an important building block in the total syntheses of natural products,the cycloaddition reactions of s-tetrazines have found applications toward the production of other complex,nitrogen-rich heterocycles,including highly substituted pyridazine boronic esters,9and pyrimido[4,5-d]pyridazines.10This unique s-tetrazine chemistry is also being developed as a rapid bioorthogonal coupling reaction.11 While the synthesis of symmetrical3,6-substituted s-tetrazines is straightforward,the generation of3,6-unsym-metrical analogs has proven challenging.2b A number of interesting synthetic approaches to unsymmetrical tetrazines have been reported.Smith and colleagues have reported unsymmetrical macropeptides with s-substituted tetrazine cores,12and Devaraj and colleagues have reported Ni-catalyzed cyclization of nitriles with hydrazine to give unsymmetrical tetrazines.133,6-Bis(methylthio)-1,2,4,5-tetrazine14and3,6-bis(3,5-dimethylpyrazol-1-yl)-1,2,4,5-tetrazine15are widely used starting materials to generate unsymmetrical tetrazines, and such compounds are known to undergo nucleophilic aromatic substitution(S N Ar)with various amines and alkyl carbanions.14−17Despite the propensity of s-tetrazines to participate in S N Ar reactions,the generation of unsymmetrical s-tetrazines remains limited by the narrow scope of reactions that can be performed on the highly electron-deficient scaffold. Our group became interested in s-tetrazines as a potential bioisostere for other six-membered nitrogen-containing hetero-cycles,and in particular pyridazines,as part of an ongoing medicinal chemistry effort.Starting from commercially available 3,6-dichloro-1,2,4,5-tetrazine,we speculated whether it would be possible to use transition metal cross-coupling reactions to generate unsymmetrical s-tetrazines.Very few successful reports of s-tetrazine cross-couplings currently exist in the literature, and it is known that the highly electron-deficient core must be stabilized with an electron-donating group if the reaction is to be successful.16,18Indeed,preliminary attempts in our own laboratory to perform Pd-catalyzed cross-coupling reactions on 3,6-dichloro-1,2,4,5-tetrazine were unsuccessful.In2003, Nova k and Kotschy reported thefirst cross-coupling reactions (Sonogashira and Negishi)on s-tetrazines,starting from various 3-amino-6-chloro-1,2,4,5-tetrazine scaffolds1to afford3 (Figure1).18Despite this notable advance,yields were moderate,and the authors were unable to isolate any desired products under Suzuki conditions.In2007,Leconte and colleagues found that3-methylthio-6-(morpholin-4-yl)-1,2,4,5-tetrazine4could readily undergo Suzuki and Stille couplings with displacement of a methylthio group to provide6(Figure 1),and to our knowledge this method provides the only examples in the literature of Suzuki cross-couplings directly into a tetrazine to give3,6-unsymmetrical s-tetrazines.10,16Although encouraging as thefirst report of a successful Suzuki reaction on a tetrazine,yields remained moderate for this series,and the s-tetrazine system was limited to a morpholino group on one side.16Received:September13,2017We therefore wondered if the scope of this reaction could be expanded to include other amino-substituted s -tetrazines,speci fically amines not limited to a closed,tertiary ring system (i.e.,morpholino).Additionally,we were interested in the optimization of milder reaction conditions,as yields in the previous report for Suzuki couplings were increased only through microwave synthesis at 200°C.16For our optimization study,we therefore selected N -butyl-6-chloro-1,2,4,5-tetrazin-3-amine (7),synthesized under previously reported conditions from 3,6-dichloro-1,2,4,5-tetrazine (Table 1).19A screen of palladium catalysts (10%loading with phenylboronic acid and K 2CO 3as base)encouragingly indicated several promising systems,including Pd(dppf)Cl 2(entry 5)and the third generation BrettPhos palladacycle (entry 6),20both of which gave moderate yields of desired product 8after 1h at 100°C.Increasing the reaction time to 2h with both catalysts improved yield due to the complete consumption of starting material (entries 9and 10),particularly in the case of the BrettPhos palladacycle.Changing the base to Cs 2CO 3further improved yield using this catalyst (entry 11,92%yield),and we were encouraged to find that this reaction could be run with comparable yields both at 70°C and even at room temperature (entries 16and 18)and at a reduced catalyst loading of 5%at room temperature (entry 19,90%yield).A 1%catalyst loading at room temperature (entry 20)resulted in signi ficantly decreased yield compared to entry 19,although a 1%catalyst loading at 70°C proved successful (entry 21,81%yield).These mild reaction conditions and high yields are a testament to the robustness of this and other Buchwald palladacycles,partic-ularly for otherwise problematic or unreactive coupling partners,and the mild conditions reported for entry 19were selected for further substrate scope studies.Utilizing these optimized reaction conditions,8was isolated in 93%yield after column chromatography.With conditions optimized for 7and phenylboronic acid,we next sought to expand the scope of the boronic acid coupling partner (Scheme 1).Under the optimized conditions,2-,3-,and 4-methoxyphenylboronic acid all proved high yielding as a coupling partner (9a −c ),as did 4-methylphenylboronic acid (9d ).In cases of electron-de ficient aromatic rings such as 4-tri fluoromethyl (9e )and 4-nitrile (9f ),yields proved lower at room temperature (48%for 9e and 18%for 9f ),although the total recovery of 9f could be dramatically increased by running the reaction at 70°C under otherwise identical conditions (86%).Heteroaryl bicyclic ring systems (9g and 9h )were also tolerated,albeit in diminished yield for 6-quinoline 9g .The coupling of 7with potassium vinyltri fluoroborate (product 9i )proved sluggish at room temperature,although this product could also be isolated in high yield by increasing the temperature to 70°C (73%).This result was particularly encouraging,as vinyl-substituted s -tetrazines are of interest as a class of precursors to highly nitrogen-rich polymers,and to our knowledge there are no known previous examples of a successful cross-coupling between an s -tetrazine and a vinyl group.21This methodology should therefore prove straightfor-ward for the generation of new vinyltetrazines.Furthermore,protic substrates such as 4-hydroxyphenylboronic acid (9j ),smaller aromatic systems (methylpyrazole 9k )as well as 4-Boc-aminophenylboronic acid (9l )also proved compatible under our conditions with substrate 7,and all were isolatedinFigure 1.Previously reported Pd-catalyzed cross-coupling reactions of s -tetrazines.16,18Table 1.Optimization of the Suzuki Coupling Conditionsaentry catalyst catalyst loading base temp (°C)time (h)yield (%)b1RuPhos Pd G310K 2CO 31001502Pd(OAc)210K 2CO 31001123Pd(PPh 3)410K 2CO 31001534Pd 2(dba)310K 2CO 3100165Pd(dppf)Cl 2·DCM 10K 2CO 31001636BrettPhos Pd G310K 2CO 31001607tBuXPhos Pd G310K 2CO 3100138Pd(amphos)Cl 210K 2CO 31001599Pd(dppf)Cl 2·DCM 10K 2CO 310027110BrettPhos Pd G310K 2CO 310028511BrettPhos Pd G310Cs 2CO 310029212BrettPhos Pd G310K 3PO 410028913BrettPhos Pd G310DIPEA 10026514BrettPhos Pd G310CH 3COONa 10023115BrettPhos Pd G310Cs 2CO 313016316BrettPhos Pd G310Cs 2CO 37029717BrettPhos Pd G310Cs 2CO 35029418BrettPhosPd G310Cs 2CO 3rt 29019BrettPhos Pd G35Cs 2CO 3rt 29020BrettPhosPd G31Cs 2CO 3rt 2721BrettPhosPd G31Cs 2CO 370281a Reaction conditions:7(30mg,0.16mmol),PhB(OH)2(1.2equiv),base(3.0equiv),5:11,4-dioxanes/H 2O (1.0mL).b Yields determinedby LCMS using anisole as internal standard.moderate to high yield.Thus,the optimized conditions a fforded broad scope and general applicability to aryl,heteroaryl,and vinyl boronic acid coupling partners to deliver analogs 9.We next turned our attention to the replacement of the N -butyl substitution (10)(Scheme 2)and synthesized N -benzyl-6-chloro-1,2,4,5-tetrazin-3-amine (10a ),3-chloro-6-(1-piperid-yl)-1,2,4,5-tetrazine (10b ),6-chloro-N -phenyl-1,2,4,5-tetrazin-3-amine (10c ),4-(6-chloro-1,2,4,5-tetrazin-3-yl)morpholine (10d ),and 3-butoxy-6-chloro-1,2,4,5-tetrazine (10e )in con-ditions similar to those for the synthesis of 7(see Supporting Information ).These substrates were selected in order to probe the electronic requirements of the group donating electron density to the chloro -s -tetrazine core.Our conditions proved successful for benzylamine 11a ,piperidine 11b ,aniline 11c ,and morpholine 11d ,although no evidence of desired product could be detected by LCMS in the case of butoxy-s -tetrazine 11e (Scheme 2).These results indicate that our Suzuki conditions can tolerate a variety of di fferent amino-s -tetrazines while maintaining good yields,and optimization e fforts toward conditions for ether-containing tetrazine substrates are ongoing in our laboratory.There are few reported examples of tetrazine motifs in biologically active molecules,22and structure −activity relation-ship (SAR)studies that include this unusual heterocycle have historically been limited largely due to an inability to rapidly and consistently generate unsymmetrical substituted s -tetra-zines.We therefore became interested in utilizing our optimized Suzuki conditions to generate a direct s -tetrazine analog of an existing molecule (12)and were aware of a report describing 3,6-substituted pyridazines as acetylcholinesterase (AChE)inhibitors,structurally related to minaprine (Scheme 3).23Using a simple,two-step S N Ar/Suzuki-coupling sequence starting from amine 13(Scheme 3),we were able to generate a direct s -tetrazine analog (15)of the existing molecule (12)in high yield under mild conditions and to compare the human hepatic microsomal intrinsic clearance (CL int )and plasma protein binding (PPB)as the fraction unbound (fu )of each (Table 2),conducted as previously described.24The tetrazineanalog 15displayed greater than 3-fold improvement (i.e.,Scheme 1.Scope of the Suzuki Coupling with 7a a Standard reaction conditions:7(30mg,0.16mmol),RB(OH)2(1.2equiv),base (3.0equiv),5:11,4-dioxanes/H 2O (1.0mL).Isolated yields after column chromatography.b Reaction run at 70°C.Scheme 2.Scope of the Suzuki Coupling with 10a aStandard reaction conditions:10(30mg,0.16mmol),RB(OH)2(1.2equiv),base (3.0equiv),5:11,4-dioxanes/H 2O (1.0mL).Isolatedyields after column chromatography.Scheme 3.Synthesis of AChE Inhibitor Analog15Table parison of Human CLint ,Predicted CL hep ,PPB,andcLogP for Analogs 12and 15compd CL INT (hum)mL/min/kg CL HEP (hum)mL/min/kg f u (plasma)(%)cLogP a 124614 2.5 5.515148.3 2.6 4.4a Calculated using ChemDraw Professional version 16.0.reduction)in human hepatic intrinsic clearance compared to the pyridazine analog12,while both showed comparable f u values in the PPB pound15was also found to maintain activity as an AChE inhibitor(Scheme3).25 Incorporation of the s-tetrazine moiety can therefore maintain certain desirable drug metabolism and pharmacokinetic (DMPK)properties in existing molecules,and in this case even reduced the rate of metabolic clearance(in vitro). Additionally,replacement of the pyridazine with the tetrazine moves the molecule into an improved drug-like space in terms of cLogP(5.5vs4.4).In conclusion,the facile cross-coupling chemistry described herein should allow for the generation of new,drug-like, tetrazine-containing molecules and enable further SAR and DMPK studies around such scaffolds,as well as open avenues for the further incorporation and study of tetrazine cores as replacements for pyridazines,pyrimidines,and pyrazines inpharmaceuticals,agrochemicals,and for the material sciences.■ASSOCIATED CONTENT*Supporting InformationThe Supporting Information is available free of charge on the ACS Publications website at DOI:10.1021/acs.or-glett.7b02868.Experimental procedures,characterization data,and1H and13C NMR spectra for new compounds(PDF)■AUTHOR INFORMATIONCorresponding Author*E-mail:craig.lindsley@.ORCIDCraig W.Lindsley:0000-0003-0168-1445NotesThe authors declare no competingfinancial interest.■ACKNOWLEDGMENTSC.W.L.thanks the William K.Warren Family and Foundation for funding the William K.Warren,Jr.Chair in Medicine andsupport of our programs.■REFERENCES(1)Pinner,A.Ber.Dtsch.Chem.Ges.1893,26,2126.(2)For selected reviews:(a)Churakov,A.M.;Tartakovsky,V.A. Chem.Rev.2004,104,2601.(b)Saracoglu,N.Tetrahedron2007,63, 4199.(c)Clavier,G.;Audebert,P.Chem.Rev.2010,110,3299.(3)Carboni,R.A.;Lindsey,R.V.J.Am.Chem.Soc.1959,81,4342.(4)(a)Wermuth,C.G.MedChemComm2011,2,935.(b)Bender,A. M.;Weiner,R.L.;Luscombe,V.B.;Ajmera,S.;Cho,H.P.;Chang,S.; Zhan,X.;Rodriguez,A.L.;Niswender,C.M.;Engers,D.W.;Bridges, T.M.;Conn,P.J.;Lindsley,C.W.Bioorg.Med.Chem.Lett.2017,27, 3576.(5)(a)Boger,D.L.Chem.Rev.1986,86,781.(b)Sauer,J.;Pabst,G. R.;Holland,U.;Kim,H.-S.;Loebbecke,.Chem.2001, 2001,697.(6)Boger,D.L.;Soenen,D.R.;Boyce,C.W.;Hedrick,M.P.;Jin,Q. .Chem.2000,65,2479.(7)Oakdale,J.S.;Boger,.Lett.2010,12,1132−1134.(8)Boger,D.L.;Hong,J.J.Am.Chem.Soc.2001,123,8515.(9)Helm,M.D.;Moore,J.M.;Plant,A.;Harrity,J.P.A.Angew. Chem.,Int.Ed.2005,44,3889.(10)Galeta,J.;Sala,M.;Dracinsky,M.;Vrabel,M.;Havlas,Z.; Nencka,.Lett.2016,18,3594.(11)(a)Devaraj,N.K.;Weissleder,R.Acc.Chem.Res.2011,44,816.(b)Ramil,C.P.;Dong,M.;An,P.;Lewandowski,T.M.;Yu,Z.; Miller,L.J.;Lin,Q.J.Am.Chem.Soc.2017,139,13376.(12)(a)Brown,S.P.;Smith,A.B.J.Am.Chem.Soc.2015,137,4034.(b)Abdo,M.;Brown,S.P.;Courter,J.R.;Tucker,M.J.;Hochstrasser, R.M.;Smith,.Lett.2012,14,3518.(13)Yang,J.;Karver,M.R.;Li,W.;Sahu,S.;Devaraj,N.K.Angew. Chem.,Int.Ed.2012,51,5222.(14)Boger,D.L.;Sakya,.Chem.1988,53,1415.(15)Rusinov,G.L.;Latosh,N.I.;Ganebnykh,I.I.;Ishmetova,R.I.; Ignatenko,N.K.;Chupakhin,.Chem.2006,42,757.(16)Leconte,N.;Keromnes-Wuillaume,A.;Suzenet,F.;Guillaumet,G.Synlett2007,2007,0204.(17)Zhou,Q.;Audebert,P.;Clavier,G.;Miomandre,F.;Tang,J. RSC Adv.2014,4,7193.(18)Novak,Z.;Kotschy,.Lett.2003,5,3495.(19)(a)Bender,J.A.;Gentles,R.G.;Pendri,A.;Wang,A.X.; Meanwell,N.A.;Beno,B.R.;Fridell,R.A.;Makonen,B.;Nguyen,V. N.;Yang,Z.;Wang,G.;Kumaravel,S.;Thangathirupathy,S.;Bora,R. O.;Holehatti,S.M.;Mettu,M.R.;Panda,M.International Publication Number2016/WO172424A1,2016.(b)Novak,Z.;Bostai,B.; Csekei,M.;Lorincz,K.;Kotschy,A.Heterocycles2003,60,2653. (20)(a)Bruno,N.C.;Tudge,M.T.;Buchwald,S.L.Chem.Sci. 2013,4,916.(b)Bruno,N.C.;Niljianskul,N.;Buchwald,. Chem.2014,79,4161.(21)Pican,S.;Lapinte,V.;Pilard,J.;Pasquinet, E.;Beller,L.; Fontaine,L.;Poullain,D.Synlett2009,2009,731.(22)(a)Werbel,L.M.;McNamara,D.J.;Colbry,N.L.;Johnson,J. L.;Degnan,M.J.;Whitney,B.J.Heterocycl.Chem.1979,16,881.(b)Xu,F.;Yang,Z.;Ke,Z.;Xi,L.;Yan,Q.;Yang,W.;Zhu,L.;Lv,W.; Wu,H.;Wang,J.;Li,H.Bioorg.Med.Chem.Lett.2016,26,4580. (23)Contreras,J.;Rival,Y.M.;Chayer,S.;Bourguignon,J.; Wermuth,C.G.J.Med.Chem.1999,42,730.(24)(a)Wenthur,C.J.;Morrison,R.;Felts,A.S.;Smith,K.A.; Engers,J.L.;Byers,F.W.;Daniels,J.S.;Emmitte,K.A.;Conn,P.J.; Lindsley,C.W.J.Med.Chem.2013,56,5208.(b)Engers,D.W.; Blobaum,A.L.;Gogliotti,R.D.;Cheung,Y.;Salovich,J.M.;Garcia-Barrantes,P.M.;Daniels,J.S.;Morrison,R.;Jones,C.K.;Soars,M.G.;Zhuo,X.;Hurley,J.;Macor,J.E.;Bronson,J.J.;Conn,P.J.; Lindsley, C.W.;Niswender, C.M.;Hopkins, C.R.ACS Chem. Neurosci.2016,7,1192.(c)Bridges,T.M.;Rook,J.M.;Noetzel,M.J.; Morrison,R.D.;Zhou,Y.;Gogliotti,R.D.;Vinson,P.N.;Xiang,Z.; Jones,C.K.;Niswender,C.M.;Lindsley,C.W.;Stauffer,S.R.;Conn, P.J.;Daniels,J.S.Drug Metab.Dispos.2013,41,1703.(25)Human AChE inhibition assay by Eurofins using the method described by Ellman(Biochem.Pharmacol.1961,7,88).。
硼酸及其衍生物
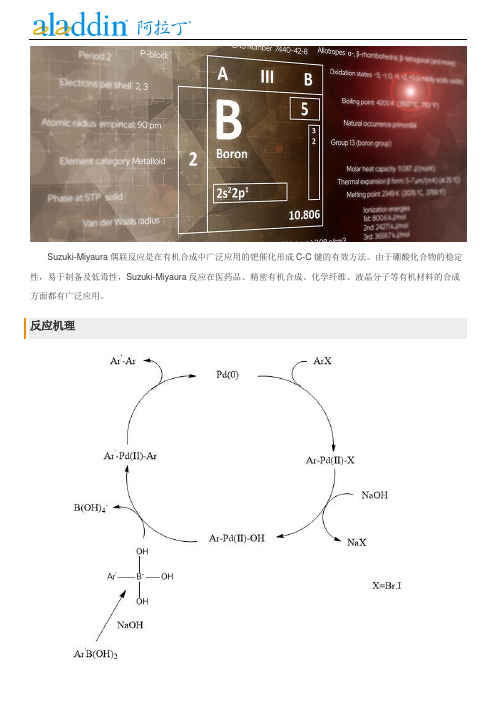
Suzuki-Miyaura偶联反应是在有机合成中广泛应用的钯催化形成C-C键的有效方法。
由于硼酸化合物的稳定性,易于制备及低毒性,Suzuki-Miyaura反应在医药品、精密有机合成、化学纤维、液晶分子等有机材料的合成方面都有广泛应用。
反应机理通常碳硼键的结合力较强(有机硼化物稳定),金属迁移不好发生。
加入碱,使生成更容易发生金属迁移的硼酸盐。
近年来随着催化剂和方法的发展,Suzuki偶联反应范围不再局限于硼酸化合物,硼酸酯、三氟硼酸钾盐和有机硼烷等都可以用来代替硼酸化合物。
硼酸√ 烷基硼酸4-三氟甲基苄基硼酸频哪醇酯475250-46-5√ 烯基硼酸√ 芳基硼酸(* 一元取代* 二元取代* 三元取代* 四元取代* 五元取代* 无取代)* 一元取代(部分):4-甲基-1-萘硼酸103986-53-4 * 二元取代(部分):4-乙氧羰基-2-硝基苯硼酸(含有数量不等的酸酐) 5785-70-6 * 三元取代(部分):2-溴-6-氟-3-丙氧基苯基硼酸849052-20-6 * 四元取代:* 五元取代:* 无取代:√ 杂环硼酸(部分)中文名称Cas硼酸酯(部分)硼酸酯参与的Suzuki-Miyaura偶联反应,可以解决与合成试剂不兼容的问题。
另外,也能避免芳基硼酸加热干燥过程中脱水形成酸酐降低产物得率的问题。
4-甲氧羰基苯硼酸频哪醇酯171364-80-0MIDA酯甲基亚氨二乙酸Methyliminodiacetic acid(MIDA)酯是可参与Suzuki-Miyaura偶联反应的一类新型试剂。
MIDA酯易于处理,在空气中是稳定的,并且可在温和的水碱性条件下容易脱保护。
MIDA酯中,sp3杂化的硼原子被两个五元环固定,极大地增加了硼酸的稳定性,利于合成复杂的分子。
中文名称Cas三氟硼酸钾盐在C-C键形成反应(如Suzuki-Miyaura)中,三氟硼酸钾盐(R-BF3K)是可有效替代有机硼酸盐的一类试剂。
笛柏试剂网:苯硼酸及其衍生物

苯硼酸苯硼酸及其衍生物及其衍生物及其衍生物取代基苯硼酸种类繁多,发展非常快。
各种取代基苯硼酸的合成方法主要有两种:有机锂试剂法和格氏试剂法。
苯硼酸及其衍生物被广泛应用于电子、化学、医药、生物等领域,例如液晶显示材料、化学发光增强剂、木制品防腐剂和酶的抑制剂,此外它还能选择性地促进葡萄糖通过脂双层的运输,目前被越来越多地用作分子识别单元,特别是,被用来设计和合成硼外源凝集素(糖蛋白)和糖类传感器。
苯硼酸及其衍生物的特性苯硼酸(phenylboronic acid)衍生物在水溶液中有带电荷与不带电荷两种形式,其中只有带电荷的形式可以与具有1,2-或1, 3-二醇基团的多羟基化合物形成可逆的五元或六元环酯。
这个过程是可逆的,而自然界中又有大量这样的多羟基化合物,例如多糖等物质,它们许多存在于生物体内,并且对于生物体的生命活动有重要影响。
NR OBOHN R OB OH OH N R OB OHO OpolyolO H OHOH-多羟基化合物存在时,苯硼酸在水溶液中的平衡状态苯硼酸及其衍生物的制备方法各种取代基苯硼酸的合成方法主要有以下两种: 有机锂试剂法和格氏试剂法。
有机锂试剂法:有机锂法是取代基溴苯先与烷基锂发生锂-溴交换反应得到取代基锂苯,然后与三烷基硼酸酯反应,水解得相应的取代基苯硼酸。
还可以直接将烷基锂试剂滴加到取代基溴苯与三烷基硼酸酯的混合液中得到苯硼酸的二烷基酯,然后水解得取代基苯硼酸。
Br LiR B(OR)B(OR)3H+/OH-B(OR)2有机锂法制备取代基苯硼酸格氏试剂法是由取代基溴苯与镁屑反应制得格氏试剂,然后与烷基硼酸酯反应,水解得取代基苯硼酸。
格氏试剂法适用范围较广,许多取代基苯硼酸如各种烷基/烷氧基苯硼酸都可用此法合成。
含有羟基等活泼基团的取代基苯硼酸借助保护基团和基团转化,也可用此法制备,苯环上连有硝基等致钝基团的取代基苯硼酸往往在苯硼酸合成后再硝化。
NO2MgCl2H+NO2B(OH)2R格式试剂法制备取代基苯硼酸苯硼酸及其衍生物的应用自然界中有大量的多羟基化合物,它们广泛存在于生物体内,并且对于生物体的生命活动有重要影响,因此利用苯硼酸除了可以对这些化合物进行检测、分离与提纯外,还可以将其对体内多羟基物质的识别功能用于自律式给药系统或调节某些生命活动。
化学名称缩写

ITBN端异氰酸酯基丁腈橡胶
ITPB端异氰酸酯基聚丁二烯
IV特性黏度
[K]
K乙醛苯胺缩合物
KPS过硫酸钾
[L]
LCR液体氯丁橡胶
LD50半致死剂量
LMP低聚物
LMW低相对分子质量
LNBR液体丁腈橡胶
LOI极限氧指数
L.P.石油醚
LPB液体聚丁二烯
LPO过氧化二月桂酰(引发剂B)
LP实验试剂
EAL乙醇
EB水性环氧丙烯酸酯
EC乙基纤维素
ECH环氧氯丙烷
EDA乙二胺
EDTA乙二胺四乙酸
EEP 3-乙氧基丙酸乙酯
EEW环氧当量
EG乙二醇
EGDA二丙烯酸乙二醇酯
EGDE乙二醇二缩水甘油醚(669稀释剂)EGDMA双甲基丙烯酸乙二醇酯
2-EI 2-乙基咪唑
Em乳化剂
EMA甲基丙烯酸乙酯
EMI-2,4 2-乙基-4-甲基咪唑
PIB聚异丁烯
PM丙二醇甲醚
PMA聚马来酸酐、丙二醇甲醚醋酸酯PMAA聚甲基丙烯酸
PMDA均苯四甲酸二酐
PMMA聚甲基丙烯酸甲酯
PMP丙二醇甲醚丙酸酯
PMS聚α-甲基苯乙烯
PN波兰国家标准
PNA苯基-β-萘胺
PNBR粉末丁腈橡胶
POE聚氧化乙烯
POP对辛基苯酚
PPA多聚磷酸
PPD六氢吡啶、对苯二胺
PPESK聚芳醚砜酮
CP氯化石蜡
CPDA环戊四酸二酐
CPP氯化聚丙烯
CPPD防老剂4010
CPVC氯化聚氯乙烯
CR氯丁橡胶
CRL氯丁胶乳
CS酪朊、烧碱、玉米淀粉
CSA加拿大标准
芳基硼酸类汇总

规格
98%
B119615
2-( 溴甲基 ) 苯硼酸 2-(Bromomethyl)phenylboronic acid
98%
B119995
3-( 溴甲基 ) 苯硼酸 3-(Bromomethyl)phenylboronic acid
97%
B120033
4-( 溴甲基 ) 苯硼酸 4-(Bromomethyl)phenylboronic acid
97%
C103544
4- 氯苯硼酸 4-Chlorophenylboronic acid
97%
C103581
2- 氯 -5-( 三氟甲基 ) 苯硼酸 2-Chloro-5-(trifluoromethyl)benzeneboronic acid
96%
C104194
3- 羧基 -4- 氟苯硼酸 3-Carboxy-4-fluorophenylboronic acid
3- 甲酰氨苯硼酸 3-Aminocarbonylphenylboronic acid
3- 氨基苯硼酸盐酸盐 3-Aminophenylboronic acid hydrochloride
4- 氨基甲酰苯硼酸 4-Aminocarbonylphenylboronic acid
二苯基硼酸 -2- 氨基乙酯 2-Aminoethyl diphenylborinate
品名
3- 氯 -4- 甲氧基苯硼酸 3-Chloro-4-methoxyphenylboronic acid
4- 氰基苯硼酸 4-Cyanophenylboronic acid
4- 羟基苯硼酸 4-Hydroxyphenylboronic acid
4- 羧基 -3- 氯苯硼酸 4-Carboxy-3-chlorobenzeneboronic acid
欧盟REACH法规 高关注物质144种简介

乙二醇乙醚醋
酸酯
22 第五批
203-839-2
2-ethoxyethyl
acetate
111-15-9
分子式:C6H12O3 分子量:132.16
1,2-苯二酸-二 (C7-11 支链与 直链)烷基(醇) 酯 23 第五批 1,2-Benzenedica 271-084-6 rboxylic acid, di-C7-11-branch ed and linear alkyl esters
蒽油 C14H10 178.22
黄绿色油状液体, 室温下有结
晶析出, 结晶为黄色、有蓝色荧
光, 能溶于乙醇和乙醚, 不溶
于水, 部分溶于热苯、氯苯等有
机溶剂,有强烈刺激性。遇高温
明火可燃,蒽的熔点 217℃, 密
CMRPBTvPv 制造涂料,木材,防 度 1.24g/ml,沸点 345℃, 自燃
B
腐油,杀虫剂等 点 540℃, 闪点 121.11℃(闭
641.69
构体)
(134237-51-7)
(134237-52-8)
毒害
主要用途
化学物理性质
PBTvPvB CMR PBT
按含氯量可分为:42%、48%、
橡胶、纺织品、密封 50-52%、65-70%四种。前三者淡
剂、黏合剂、涂料、黄色粘稠液体,后者为黄色粘稠
皮革中的涂层、纺织 液体。42%、48%、50~52%三种
117-81-7
C24H38O4390.56
邻苯二甲酸二 5 第一批 丁酯 Dibutyl 201-557-4
phthalate(DBP)
84-74-2
4,-二氨基二苯 甲烷 6 第一批 4,4'-Diaminodip 202-974-4 henylmethane (MDA)
对苯二甲酸MSDS
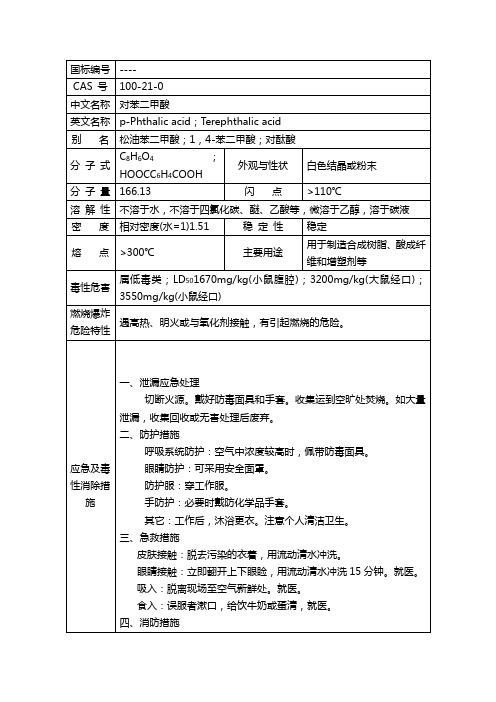
国标编号----CAS 号100-21-0中文名称对苯二甲酸英文名称p-Phthalic acid;Terephthalic acid 别名松油苯二甲酸;1,4-苯二甲酸;对酞酸分子式C8H6O4;HOOCC6H4COOH 外观与性状白色结晶或粉末分子量166.13 闪点>110℃溶解性不溶于水,不溶于四氯化碳、醚、乙酸等,微溶于乙醇,溶于碳液密度相对密度(水=1)1.51 稳定性稳定熔点>300℃主要用途用于制造合成树脂、酸成纤维和增塑剂等毒性危害属低毒类;LD501670mg/kg(小鼠腹腔);3200mg/kg(大鼠经口);3550mg/kg(小鼠经口)燃烧爆炸危险特性遇高热、明火或与氧化剂接触,有引起燃烧的危险。
应急及毒性消除措施一、泄漏应急处理切断火源。
戴好防毒面具和手套。
收集运到空旷处焚烧。
如大量泄漏,收集回收或无害处理后废弃。
二、防护措施呼吸系统防护:空气中浓度较高时,佩带防毒面具。
眼睛防护:可采用安全面罩。
防护服:穿工作服。
手防护:必要时戴防化学品手套。
其它:工作后,沐浴更衣。
注意个人清洁卫生。
三、急救措施皮肤接触:脱去污染的衣着,用流动清水冲洗。
眼睛接触:立即翻开上下眼睑,用流动清水冲洗15分钟。
就医。
吸入:脱离现场至空气新鲜处。
就医。
食入:误服者漱口,给饮牛奶或蛋清,就医。
四、消防措施灭火方法:雾状水、泡沫、二氧化碳、干粉、砂土。
对苯二甲酸100-21-0

12.1 毒性
对鱼类的毒性半静态试验LC50-青鳉鱼->18.6mg/l-96h方法:OECD测试导则203对水溞和其他 水生无脊椎动物的毒性活动抑制EC50-Daphniamagna(水溞)->20.1mg/l-48h方法:OECD测试 导则202对藻类的毒性生长抑制EC50-Pseudokirchneriellasubcapitata(绿藻)->19mg/l-72h 方法:OECD测试导则201细菌毒性呼吸抑制EC50-污泥处理-1,393mg/l-3h方法:OECD测试导则 209
化学品安全技术说明书
1 化学品及企业标识
1.1 产品标识符
化学品俗名或商品名: 对苯二甲酸 CAS No.: 100-21-0 别名: 1,4-苯二甲酸; p-苯二甲酸; 对酞酸; TPA; PTA
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
无数据资料
2 危险性概述
https:// 3/5
化学品安全技术说明书
无数据资料 特异性靶器官系统毒性(一次接触) 无数据资料 特异性靶器官系统毒性(反复接触) 无数据资料 潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。 吞咽 吞咽可能有害。 皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。 眼睛 可能引起眼睛刺激。 接触后的征兆和症状 据我们所知,此化学,物理和毒性性质尚未经完整的研究。 附加说明 化学物质毒性作用登记: WZ0875000
n) 溶解性 / 水溶性 不溶于水,不溶于四氯化碳、醚、乙酸,微溶于乙醇,溶于碱
液。
大约0.017 g/l 在 25 °C - 微溶
o) 辛醇/水分配系数的对数值 无数据资料
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
对苯二硼酸
Cas号:4612-26-4
分子式:C6H8B2O4
分子量:165.75
别名:苯基-1,4-二硼酸;对二苯硼酸;1,4-苯二硼酸
化学性质
熔点:>300℃
上海哈灵生物科技有限公司在组织免疫,基因重组,分子生物学,生物制药 , 生物医学等方面都有丰富的经验,并与各大院校、医院、科研单位都有合作并得到了他们的一致好评与认可。
公司本着客户至上的原则为客户提供高质量高品质的产品。
对苯二硼酸相关其它优质产品:
高车前苷 Homoplantaginin 17680-84-1 20mg 上海哈灵生物
大黄素甲醚 Emodin-3-methyl ether 521-61-9 20mg 上海哈灵生物
大黄酸 Rhein 478-43-3 20mg 上海哈灵生物
大黄酚 Chrysophanic acid 481-74-3 20mg 上海哈灵生物
大黄素 Emodin 518-82-1 20mg 上海哈灵生物
土大黄苷 Rhaponticin 155-58-8 20mg 上海哈灵生物
紫草酸 Lithospermic acid 28831-65-4 20mg 上海哈灵生物
地肤子皂苷Ic Momordin Ic 96990-18-0 20mg 上海哈灵生物
大豆苷 Daidzin 552-66-9 20mg 上海哈灵生物
大豆苷元 Daidzein 486-66-8 20mg 上海哈灵生物
黄豆黄苷 Glycitin 40246-10-4 20mg 上海哈灵生物
黄豆黄素 Glycitein 40957-83-3 20mg 上海哈灵生物
对甲氧基肉桂酸乙酯 ethylp-methoxycinnamate 24393-56-4 20mg 上海哈灵生物
去氢二异丁香酚 Dehydrodiisoeugenol 2680-81-1 20mg 上海哈灵生物
异丁香酚 Isoeugenol 97-54-1 20mg 上海哈灵生物
丁香油(UV98%) Clove oil 8000-34-8 20mg 上海哈灵生物
丁香酸 Syringic acid 530-57-4 20mg 上海哈灵生物
丁香酚 Eugenol 97-53-0 20mg 上海哈灵生物
原百部次碱 Protostemotinine 169534-85-4 5mg 上海哈灵生物
对叶百部碱 Tuberostemonine 6879-1-2 20mg 上海哈灵生物
地榆皂苷Ⅰ Ziyuglycoside I 35286-58-9 20mg 上海哈灵生物
地榆皂苷Ⅱ Ziyuglycoside II 35286-59-0 20mg 上海哈灵生物
大茴香醛 Anisic aldehyde 123-11-5 20mg 上海哈灵生物
党参炔苷 Lobetyolin 136085-37-5 10mg 上海哈灵生物
胆维他 Anethole trithione 532-11-6 20mg 上海哈灵生物
羟基茜草素(95%) Purpurin 81-54-9 20mg 上海哈灵生物
大叶茜草素 Swertiamarine 55481-88-4 20mg 上海哈灵生物。
