Chapter 2 Fundamentals of Materials_AMFM_2011
无损检测(射线,超声)
3.1.3 射线的产生
射线的性质,有利、有弊,应该科学地加以利 用和防范!射线学就是研究如何利用与防范射线 的科学。下面介绍产生x射线的主要设备: (1)x光管基本组成: • 阴极部件:灯丝(钨丝)——发射电子; 阴极罩——聚焦电子。 • 阳极部件:阳极靶——接收电子; 冷却介质——散热作用。 • 真空管——玻璃或金属陶瓷制作的真空外罩。
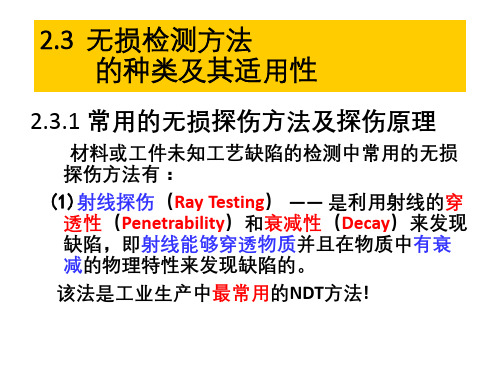
2.3.1 常用的无损探伤方法及探伤原理
(2)超声波探伤(Ultrasonic testing)—— 是利用超声波在 物质中传播(Propagation)、反射(Reflection)和 (Decay)等物理性质来发现缺陷的。 该法与射线探伤法形成优势互补. (3)磁力探伤(Magnetic testing)—— 是通过对铁磁材料 进行磁化所产生的漏磁场(Leakage magnetic field) 来发现其表面及近表面缺陷的。 在黑色金属( ferrous metal )的表面检测中应用广泛.
3.1.2 射线的性质 (1)不可见,直线传播—具有隐蔽性和指向性; (2)不带电,因而不受电磁场影响—电中性; (3)能穿透物质,但有衰减—具有穿透性和衰减性; 对同1种射线而言,功率越大,穿透性越强,衰减越慢; (4)能与某些物质产生光化作用,使荧光物质发光;可 使胶片感光—可成像; (5)能使某些气体电离—即产生电离辐射; (6)与光波一样,有反射、折射、干涉现象; (7)能产生生物效应,伤害和杀死生物细胞 —对人体有害。(此点非常重要)
2.3 无损检测方法 的种类及其适用性
2.3.1 常用的无损探伤方法及探伤原理
材料或工件未知工艺缺陷的检测中常用的无损 探伤方法有: (1)射线探伤(Ray Testing) —— 是利用射线的穿 透性(Penetrability)和衰减性(Decay)来发现 缺陷,即射线能够穿透物质并且在物质中有衰 减的物理特性来发现缺陷的。 该法是工业生产中最常用的NDT方法!
制造业生产计划管理英文书籍

制造业生产计划管理英文书籍Manufacturing Production Planning and Control.Introduction.Manufacturing Production Planning and Control is a crucial aspect of any manufacturing organization. It involves the coordination and management of resources, processes, and activities to ensure efficient and effective production. This book aims to provide a comprehensive guide to the principles, practices, and techniques used in manufacturing production planning and control.Chapter 1: Fundamentals of Manufacturing Production Planning.Manufacturing production planning is the process of planning, scheduling, and controlling the production of goods and services within a manufacturing organization. It involves the identification of production requirements,determination of the optimal production mix, allocation of resources, and monitoring and controlling the production process.The first step in manufacturing production planning is the identification of production requirements. This involves understanding the demand for products or services and translating it into production requirements. Production requirements can be derived from various sources such as customer orders, forecasted demand, and inventory levels.Once the production requirements are identified, the next step is to determine the optimal production mix. This involves deciding which products or services to produce, in what quantities, and when to produce them. The production mix should be optimized to maximize profitability, minimize costs, and meet customer demand.The allocation of resources is another crucial step in manufacturing production planning. Resources such as raw materials, equipment, labor, and capital need to be allocated efficiently to ensure smooth production. Resourceallocation should take into account the availability of resources, production requirements, and the capacity of the production system.Monitoring and controlling the production process is essential to ensure that production plans are being executed effectively. This involves tracking production performance, identifying bottlenecks and issues, and taking corrective actions to ensure that production remains on track.Chapter 2: Production Scheduling.Production scheduling is the process of developing a detailed plan for the production of goods and services based on the identified production requirements. It involves scheduling production activities, allocating resources, and ensuring that production deadlines are met.There are various types of production scheduling methods, including forward scheduling, backward scheduling, and constraint-based scheduling. Forward schedulinginvolves starting with the first available production slot and scheduling activities forward in time. Backward scheduling, on the other hand, starts with the final production deadline and schedules activities backward in time. Constraint-based scheduling focuses on identifying and managing the constraints that limit production capacity and scheduling activities around these constraints.Effective production scheduling requires the use of advanced planning and scheduling tools. These tools help manufacturers to analyze production requirements, identify bottlenecks, optimize resource allocation, and generate detailed production schedules.Chapter 3: Production Control.Production control is the process of monitoring and controlling the production process to ensure that production plans are being executed effectively. It involves tracking production performance, identifying bottlenecks and issues, and taking corrective actions to ensure that production remains on track.Production control systems are used to monitor and control the production process. These systems collect real-time data on production activities, analyze performance, and generate reports and alerts when issues arise. Manufacturers can use this information to identify bottlenecks, optimize production processes, and take corrective actions to improve production efficiency.In addition to production control systems, manufacturers also need to consider quality control and lean manufacturing principles. Quality control involves ensuring that products meet specified quality standards and customer requirements. Lean manufacturing, on the other hand, focuses on eliminating waste and improving production efficiency through continuous improvement and process optimization.Conclusion.Manufacturing Production Planning and Control is a critical aspect of any manufacturing organization. Itinvolves the coordination and management of resources, processes, and activities to ensure efficient and effective production. By understanding the fundamentals of manufacturing production planning, using advanced planning and scheduling tools, and implementing effective production control systems, manufacturers can optimize production processes, improve production efficiency, and meet customer demand.。
大学土木工程专业英语教材

大学土木工程专业英语教材[正文]Chapter 1: Introduction to Civil EngineeringIn this chapter, we will provide an overview of civil engineering as a discipline. Topics covered will include the history of civil engineering, its role in society, and the various sub-disciplines within the field.1.1 Definition of Civil EngineeringCivil engineering is a branch of engineering that deals with the design, construction, and maintenance of the built environment, including roads, bridges, buildings, and infrastructure. It plays a crucial role in ensuring the safety, functionality, and sustainability of our cities and communities.1.2 History of Civil EngineeringCivil engineering has a rich history dating back to ancient civilizations. From early engineering marvels such as the Pyramids of Egypt to the Roman aqueducts, significant advancements in construction techniques and materials have shaped the field over time.1.3 Role of Civil EngineersCivil engineers are responsible for planning, designing, and overseeing construction projects. They collaborate with architects, urban planners, and other professionals to ensure that infrastructure projects are safe, cost-effective, and environmentally sustainable.1.4 Sub-disciplines of Civil EngineeringCivil engineering encompasses various sub-disciplines, including structural engineering, geotechnical engineering, transportation engineering, environmental engineering, and water resources engineering. Each sub-discipline focuses on specific aspects of the built environment and requires specialized knowledge and skills.Chapter 2: Fundamentals of Civil Engineering MaterialsThis chapter will introduce students to the fundamental properties and behavior of materials commonly used in civil engineering projects. Topics covered will include the properties of concrete, steel, asphalt, and timber, as well as testing methods and quality control.2.1 Properties of ConcreteConcrete is a versatile and widely used construction material. In this section, students will learn about the composition of concrete, its strength and durability, and the factors that affect its performance.2.2 Properties of SteelSteel is essential in the construction industry due to its high strength and ductility. This section will cover the properties of structural steel, its various forms, and its applications in building and bridge construction.2.3 Properties of AsphaltAsphalt is commonly used in road construction due to its flexibility and durability. Students will study the properties of asphalt, including its viscosity, temperature susceptibility, and resistance to aging and deformation.2.4 Properties of TimberTimber has been used in construction for centuries. This section will explore the properties of timber, including its strength, moisture content, and susceptibility to decay, as well as its sustainability and potential environmental impact.2.5 Material Testing and Quality ControlEnsuring the quality and performance of construction materials is essential. Students will learn about various testing methods for assessing the properties of materials and the importance of quality control in civil engineering projects.Chapter 3: Structural Analysis and DesignStructural analysis and design are critical aspects of civil engineering. This chapter will introduce students to the principles and methods used in analyzing and designing structures, including beams, columns, and trusses.3.1 Statics and Mechanics of MaterialsStudents will first develop a solid understanding of statics and mechanics of materials, which form the foundation for structural analysis and design. Topics covered will include forces, moments, equilibrium, stress, and strain.3.2 Analysis of Determinate StructuresIn this section, students will learn how to analyze determinate structures, such as beams and frames, using methods such as the method of sections and the method of joint.3.3 Analysis of Indeterminate StructuresIndeterminate structures, which have more unknowns than available equations, require more advanced analysis methods. Students will be introduced to techniques such as the force method and the displacement method for analyzing indeterminate structures.3.4 Structural DesignThis section will cover the principles of structural design, including the selection of appropriate materials, load calculations, and the design of structural elements to ensure safety and stability.Chapter 4: Geotechnical EngineeringGeotechnical engineering focuses on the behavior of soils and rocks and their interaction with structures. This chapter will introduce students to geotechnical site investigations, soil mechanics, and foundation design principles.4.1 Geotechnical Site InvestigationsBefore any construction can take place, a thorough site investigation is necessary. Students will learn about various methods for assessing soil and rock conditions, including borehole logging, field testing, and laboratory testing.4.2 Soil MechanicsSoil mechanics is concerned with the behavior of soils under different loading conditions. This section will cover fundamental concepts such as soil classification, soil compaction, permeability, and consolidation.4.3 Foundation DesignThe design of foundations is critical for ensuring the stability and safety of structures. Students will be introduced to different types of foundations, including shallow foundations and deep foundations, and the design considerations for each.4.4 Slope Stability AnalysisSlope stability analysis is essential for evaluating the stability of natural and man-made slopes. This section will cover various methods for assessing slope stability and mitigating potential failures.Chapter 5: Transportation EngineeringTransportation engineering focuses on the planning, design, and operation of transportation systems. This chapter will cover topics such as traffic engineering, highway design, and public transportation planning.5.1 Traffic EngineeringTraffic engineering involves the analysis and management of traffic flow and the design of transportation facilities to ensure safe and efficient movement of vehicles and pedestrians. Students will learn about traffic studies, capacity analysis, and traffic signal design.5.2 Highway DesignHighway design encompasses the layout and geometric design of roadways to accommodate varying traffic volumes and vehicle types. This section will cover topics such as roadway alignment, intersection design, and pavement design.5.3 Public Transportation PlanningPublic transportation plays a crucial role in urban mobility. Students will be introduced to the principles of public transportation planning, including route planning, transit scheduling, and demand forecasting.Chapter 6: Environmental EngineeringEnvironmental engineering addresses the challenges of protecting and improving the environment. This chapter will cover topics such as water and wastewater treatment, air pollution control, and solid waste management.6.1 Water and Wastewater TreatmentAccess to clean water and proper wastewater treatment are essential for public health and environmental sustainability. Students will learn about different treatment processes and technologies used in water and wastewater treatment plants.6.2 Air Pollution ControlAir pollution poses significant risks to human health and the environment. This section will cover the sources and effects of air pollution, as well as various techniques for its control, including emission controls and air quality monitoring.6.3 Solid Waste ManagementProper solid waste management is necessary to minimize environmental impacts and promote recycling and resource recovery. Students will be introduced to waste collection, disposal, and recycling methods, as well as landfill design and management.ConclusionThis comprehensive English textbook for undergraduate civil engineering students covers a wide range of topics essential to the study of this discipline. From the fundamentals of civil engineering materials to structural analysis, geotechnical engineering, transportation engineering, and environmental engineering, students will gain a solid foundation in the core principles and practices of their field.。
本科课程《材料科学与工程基础》教学大纲 (1)

四川大学本科课程《材料科学与工程基础》教学大纲一、课程基本信息课程名称(中、英文):《材料科学与工程基础》(FUNDAMENTALS OF MATERIALS SCIENCE AND ENGINEERING)课程号(代码):30014530课程类别:专业基础课学时/学分:48 /3先修课程:大学化学、大学物理、物理化学适用专业:高分子材料与工程等二级学科材料类专业开课时间:大学二年级下期二、课程的目的及任务材料科学与工程是二十世纪六十年代初期创立的研究材料共性规律的一门学科,其研究内容涉及金属、无机非金属和有机高分子等材料的成分、结构、加工同材料性能及材料应用之间的相互关系。
材料科学、材料工业和高新技术的发展要求高分子材料与工程等二级学科材料类专业的学生必须同时具备“大材料”基础和“中材料”专业的宽厚知识结构。
本课程是材料类专业的学科基础课程,是联系基础课与专业课的桥梁。
本课程从材料科学与工程的“四要素”出发,采用“集成化”的模式,详细讲授金属材料、无机非金属材料、高分子材料、复合材料等各种材料的共性规律及个性特征。
使学生建立材料制备/加工——组成/结构——性能---应用关系的“大材料”整体概念,从原理上认识高分子材料等各种材料的基本属性,及其在材料领域中的地位和作用。
为以后二级学科“中材料”专业课程的学习、材料设计、以及材料的应用等奠定良好基础。
本课程采用中文教材与英文原版教材相结合,实施“双语”教学。
使学生通过本课程的学习,熟悉材料科学与工程领域的主要英文专业词汇,提高对英文教材的阅读理解能力。
三、课程的教学内容、要点及学时分配(以红字方式注明重点难点)第一章绪论(1学时)本章概要:简要介绍材料的定义及分类,材料科学与工程的基本内容。
使学生了解本课程的学习内容和学习方法。
讲授要点:材料的定义、分类材料科学与工程的定义、性质、重要性(举例)课程学习的目的、方法、要求第二章材料结构基础(15学时)本章概要:按照从微观到宏观、从内部到表面、从静态到动态、从单组分到多组分的顺序,阐述原子电子结构、原子间相互作用和结合方式,固体内部和表面原子的空间排列状态、聚集态结构的有序性、无序性和转变规律及相互关系。
《材料科学基础2》课程简介和教学大纲

《材料科学基础2》课程简介课程编号:02024036课程名称:材料科学基础2 [5E] /Fundamentals of MaterialsScience 2学分:2. 5学时:40适用专业:无机非金属材料建议修读学期:第5学期先修课程:物理化学,材料科学基础1 [无]考核方式与成绩评定标准:闭卷考试教材与主要参考书目:Ll]无机材料学基础,张其土,华东理工大学出版社[2]无机材料科学基础,陆佩文,武汉理工大学出版社[3]材料科学基础,张联盟,武汉理工大学出版社内容概述:本课程是无机非金属材料工程专业本科生的重要专业基础课,是一门理论性很强、涉及面广的课程,是本专业的专业课开设前所必须学的课程。
本课程是使学生掌握材料的组成、结构与性能之间的相互关系和变化规律,掌握材料的结构、物性和化学反应的规律及其相互的联系,为今后从事夏杂的技术工作和开发新型材料打下良好的基础。
The course of fUndamentals of materials science, which is highly theoretical, and almost involves all the sides of materials science, is an important fundamental one for the students majoring in inorganic materials science and engineering. Thus it is set to be taught before other specialized courses. It aims at allowing the students to master the relations between materials compositions, structures and properties, and to establish a good theoretical base for the research and development of new materials in the future.《材料科学基础2》[无]教学大纲课程编号:02024036课程名称:材料科学基础2 /Fundamentals of Materials Science 2学分:2. 5学时:40适用专业:无机非金属材料建议修读学期:第5学期先修课程:物理化学,材料科学基础1 [无]一、课程性质、目的与任务【课程性质】本课程是无机非金属材料工程专业(建材方向、陶瓷与耐火材料方向)本科生的重要专业基础课,是一门理论性很强、涉及面广的课程,是本专业的专业课开设前所必须学的课程。
Chemistry_coursebook_1_Ch_1_-9_answers

HKDSE CHEMISTRY – A Modern View(Chemistry)Coursebook 1Suggested answersChapter 1 The fundamentals of chemistry PageNumber •Class Practice1•Chapter Exercise 3Chapter2 The atmosphere•Class Practice6•Chapter Exercise7Chapter 3 Oceans•Chapter Exercise9Chapter4 Rocks and minerals•Class Practice12•Chapter Exercise13●Part Exercise15 Chapter 5 Atomic structure•Class Practice17•Chapter Exercise19Chapter6 The Periodic Table•Class Practice21•Chapter Exercise22Chapter 7 Chemical bonding: ionic bonding•Class Practice24•Chapter Exercise26 Chapter8 Chemical bonding: covalent bonding•Class Practice29•Chapter Exercise31 Chapter9 Structures and properties of substances•Class Practice34•Chapter Exercise35 ●Part Exercise37Chapter 1 Fundamentals of chemistryClass PracticeA1.1(b)Food : fertilizers, insecticides, food additives(c)Housing : metals, alloys, cement, glass, plastics(d)Transport : metals, alloys, fuels, glass, plastics(e)Medicines : drugs, antibiotics, artificial hormones(f)Amusement park facilities : metals, alloys, cement, glass, plastics,semi-conductorsA1.2Phosphorus and mercury are elements. The others are not.(Note: A substance with a name consisting of two words (e.g. sodium chloride) is not an element. A substance with a name of only one word (e.g. ammonia) may or may not be an element. The only sure way is to check the name against the Periodic Table.)A1.3Sodium - silvery grey solid;Chlorine - greenish yellow gas;Sodium chloride - white solid.A1.4(a) Hydrogen, oxygen, nitrogen, iron, sulphur(b) Water, carbon dioxide, carbon monoxide, sodium chloride, iron(II) sulphide(c) Air, sea water, town gas, sodium chloride solution, wine(Other answers may be given.)A1.5(a) Chemical change(b) Physical change(c) Physical change(d) Chemical change(b) and (c) are physical changes because no new substances are formed. (a) and (d) are chemical changes because new substances are formed.A1.6(a), (b) and (e).A1.8(a) Flat-bottomed flask (l) Crucible tongs (w) Reagent bottle(b) Round-bottomed flask (m) Spatula (x) Gas syringe(c) Clamp (n) Heat-resistant mat (y) Measuring cylinder(d) Retort stand (o) Pestle (z) Beaker(e) Conical flask (p) Mortar (aa) Funnel(f) Wire gauze (q) Desiccator (bb) Plastic washbottle(g) Evaporating basin (r) Test tube holder (cc) Teat pipette(h) Tripod (s) Test tube rack (dd) Thermometer(i) Crucible (t) Test tube (ee) Watch glass(j) Pipeclay triangle (u) Boiling tube (ff) Separating funnel (k) Bunsen burner (v) Dropping bottle (gg) Glass rodChapter 1 Fundamentals of chemistryChapter Exercise1. science, observations, experiments2. substances, compositions, structures, properties, changes3. Oxygen, atmosphere4. chemically combined together, hydrogen, oxygen.5. heating, electrolysis6. mixture7. chlorine, compound8. element, compound, mixture9. retains, different10. ppearance , dour , aste , ensity , elting11. chemical12. physical13. new14. A15. B16. D17. C18. C19. D20. B21. B22. A23. D24. A25. D26. C27. B28. D29. A30. (a) A = beaker, B = test tube, C = Bunsen burner, D = wire gauze, E = tripodstand, F = heat-proof mat, G = test tube holder, H = evaporating dish(evaporating basin)(b) (i) Test tube (B).(ii) Test tube(B), test tube holder (G), Bunsen burner (C) , heat-proof mat(F).(iii) Beaker (A), tripod stand (E), wire gauze (D), Bunsen burner (C),heat-proof mat (F).31. (a) Tasteless; no smell; colourless; liquid at room conditions(b) React with iron; react with sodium(c) Water changes into steam at 100o C. / Water changes into ice at 0o C.(d) It is because no new substance is formed.(e) Iron reacts with water to form iron rust. / Sodium reacts with water to formhydrogen gas.(f) New substance (e.g. rust or hydrogen gas) is formed.32. (a) Chlorine, hydrogen, iron, mercury , oxygen, sodium and sulphur(b) An element is a pure substance that cannot be broken down into anythingsimpler by chemical methods.(c) Ammonia, sodium chloride and water(d) A compound is a pure substance made up of two or more elementschemically combined together(e) A mixture consists of two or more pure substances (elements or compounds)which have not chemically combined together.(f) Sodium chloride solution is a mixture (because a solution is a homogeneousmixture).33. (a) No. Both oxygen and hydrogen are gases at room conditions while glucoseis a solid at room conditions. Carbon is black in colour while glucose iswhite.(b) Glucose solution is a mixture. It is because there is no chemical reactiontaking place between glucose and water.(c) Glucose + oxygen → carbon dioxide + water34. Compounds and mixtures are different in a number of ways. These include:(1) Compounds have fixed chemical composition while mixtures have variablechemical composition. Examples: water and air(2) During the formation of compounds, a chemical change occurs. Newsubstances are always formed. On the other hand, a mixture is obtainedwhen different substances are physically mixed. There is no chemicalchange. No new substance is formed and the change is seldom accompaniedby energy changes. Examples: formation of water from hydrogen andoxygen, mixing of sand and sugar(3) Properties of a compound are very different from that of its constituentelements. For example, water is colourless liquid while hydrogen and oxygenare colourless gases. On the other hand, each constituent substance retainsits own properties in mixtures. For example, nitrogen and oxygen are bothcolourless gases no matter whether they are isolated or present together inthe air.(4) Separation of the constituents of a compound requires a chemical process.For example, breaking water down into the elements hydrogen and oxygenrequires a chemical process called electrolysis. On the other hand,separation of a mixture requires a physical process only. For example,separation of iron powder from a mixture just requires the use of a magnet.35. -PHYSICAL PROPERTIES of a substance are those properties that can bedetermined without the substance changing into another substance.-Examples of physical properties of a substance include colour, odour (smell) and physical state. For example water is a colourless, odourless liquid under room conditions.-CHEMICAL PROPERTIES of a substance are the chemical reactions of the substance, and the respective conditions under which each reaction takes place.-Examples of chemical properties of a substance include how fast and vigorous it reacts (i.e., its reactivity) with another substance, the condition(s) needed for it to react with other substances and what products can be produced when it reacts with other substances. For example, hydrogen reacts vigorously with oxygen (or air) only when lit with a burning splint to form water.Class PracticeA2.1(a) People in ancient times had little scientific knowledge. In fact, any visibleportion of the Earth appeared more or less flat to the eyes.(b) Satellite photos clearly show that the Earth is roughly spherical.(Other answers may be given.)A2.2atmospherecrustmantleinner coreouter coreA2.31. (a) No. (7 planets have an atmosphere.)(b) Yes.2. There is no air on the Moon.A2.4Elements Compoundsnitrogen carbon dioxideoxygen water vapourheliumneonargonkryptonxenonA2.5Helium -269Neon -246Nitrogen -196Argon -186Oxygen -183Krypton -153Xenon -109Carbon dioxide -78Chapter Exercise1. crust, mantle , core, atmosphere2. atmosphere3. nitrogen, oxygen4. fractional distillation5. liquefied6. supporter7. glowing8. A9. A 10. B 11. B 12. B 13. D14. (a) Nitrogen (b) Carbon dioxide and water vapour (c) Oxygen, argon, neon, helium, krypton, xenon (any two)15. (a) The volumes of the three gases obtained i.e. argon, nitrogen and oxygen are930 litres, 78,000 litres and 21,000 litres respectively.(b) Fractional distillation (c) No. Oxygen is the most reactive gas in air, whereas nitrogen is unreactive,which serves the good purpose of ‘diluting ’ oxygen in air. If there were more oxygen in air, metals would be oxidized and corroded faster. Things would also burn easier, so there would be a greater hazard of fire.16. (a) Fractional distillation of liquid air (b) Oxidizing (c) Physical property: colourless, odourless Chemical property: it supports combustion (d) Put a glowing splint into a test tube containing the gas to be tested. If thegas is oxygen, the splint relights.17. (a) Nitrogen and oxygen (b) Oxygen (c) copper + oxygen → copper(II) oxide (d) 50 cm 3 - 33 cm 3 = 17 cm 3(e) 33cm50cm 17⨯ 100% = 34% (f) 21% (g) The percentage of oxygen in dissolved air (34 %) is much greater than thatin the atmosphere (21 %) because oxygen is more soluble in water than nitrogen.18. -Fractional distillation of liquid air is used to separate nitrogen and oxygenfrom air.-The air is first liquefied by repeated cooling and compression.-Then the liquid air is warmed up bit by bit very slowly.-Different gases in air boil at different temperatures, so they can be collected one by one.-The one boiling off first is nitrogen (boiling point -196 ︒C). The second one to be collected is argon(boiling point -186 ︒C) /noble gas. Then oxygen gas (boiling point -183 ︒C) is collected.Chapter 3 OceansChapter Exercise1. sodium chloride (common salt), sodium, chlorine2. evaporation3. filtration, crystallization4. saturated5. boiling, condensation6. distillate, residue7. distillation8. flame test9. brilliant golden yellow10. white11. water, white, blue, blue, pink12. Brine13. hydrogen, chlorine, sodium hydroxide14. B15. C16. B17. C18. D19. A20. (a) Filtration(b)(c) Distillation(d)(e) Test for sodium ions: Flame test. The sample gives a brilliant golden yellow flame in the flame test if sodiumions are present.Test for chloride ions: Silver nitrate testAdd silver nitrate solution to the sample, followed by excess dilute nitric acid. Theappearance of a white precipitate indicates the presence of chloride ions.21. His conclusion is not justified. He should add the white-powder to distilled waterand stir well, then filter and evaporate the filtrate to dryness by heating, and see if any solid is left.22. (a) This is because some metal ions can produce a characteristic coloured lightwhen they are heated strongly.(b) (1) Moisten a clean platinum wire with concentrated hydrochloric acid.(2) Dip the platinum wire into a crushed sample of the salt (or solution) tobe tested.(3) Heat the platinum wire with the sample strongly in a non-luminousflame.(4) Observe the colour of the flame at the wire and identify the metal ionspresent.(c) Potassium ions: lilac; calcium ions: brick red; copper (II) ions: bluish green.23. (a) It was not a suitable method because the liquid may be unclean, harmful oreven poisonous.(b) Flame test.(c) To show the presence of chloride ions, acidified silver nitrate solution isadded to the sample. If chloride ions are present, a white precipitate will be formed.(d) To show the presence of water, a few drops of the liquid are added toanhydrous copper(II) sulphate.The powder changes from white to blue if water is present.Alternatively, add a few drops of the liquid to dry cobalt chloride test paper. The paper changes from blue to pink if water is present. thermometerdelivery tubeclampboiling tube sodium chloridesolutionheatanti-bumping granulereceiver test tubewater pure water(e) He could not be sure that the liquid was sea water. Even if the tests showedthat sodium ions, chloride ions and water were present, the liquid might notnecessarily be sea water. For example, it might be just a sodium chloridesolution, without any other salts naturally present in sea water.24. (a) Electrolysis means ‘decomposition by electricity’.(b) Chlorine, hydrogen and sodium hydroxide.(c) Chlorine − water sterilization, manufacture of bleach, etc.Hydrogen − production of margarine, as rocket fuel, etc.Sodium hydroxide − manufacture of soap, extraction of aluminium, etc. 25. -Sea water is an important source of common salt (sodium chloride) which hasmany uses.-By the electrolysis of sea water, useful products, hydrogen, chlorine and sodium hydroxide are obtained. These products can be used to manufacture a lot of useful chemicals.-Hydrogen can be used to produce ammonia.- Chlorine can be used to produce bleach.- Sodium hydroxide can be used to produce soap.Class PracticeA4.1heat calcium oxide + carbon dioxideStep 1: calcium carbonate −−−−→Step 2: calcium oxide + water → calcium hydroxideStep 3: calcium hydroxide + water → calcium hydroxide solution (limewater) Step 4: calcium hydroxide solution (limewater) + carbon dioxide→ calcium carbonate + waterA4.2calcium carbonate + nitric acid → calcium nitrate + carbon dioxide + waterChapter Exercise1. mineral, a mixture of minerals2. crystalline, chemical3. extraction4. ore, aluminium5. haematite, carbon (or coke)6. chalk, marble7. Neutralizing, building material, cement (or other acceptable answers)8. Weathering9. Erosion10. PhysicalChemical11. uicklime, calcium oxide.12. acids, carbon dioxide13. milky14. iron + carbon dioxide15. aluminium + oxygen16. carbonic acid17. calcium hydroxide + heat18. calcium hydrogencarbonate19. calcium oxide + carbon dioxide20. calcium carbonate (white solid) + water21. calcium chloride + carbon dioxide + water22. B23. D24. D25. C26. A27. B28. D29. C30. C31. B32. (a) (1) Both react with acid to give out carbon dioxide.(2) Both are decomposed on strong heating.(b) (1) Neutralizing acidic soil and lakes affected by acid rain.(2) As a raw material to make glass by heating with sand and sodiumcarbonate.(3) As a raw material to make cement by heating with clay. (or any otherpossible answers)33. (a) Weathering is the slow process in which exposed rocks are broken downinto smaller pieces.(b) Physical weathering and chemical weathering.(c) It is because carbon dioxide in air dissolves slightly in rainwater, formingcarbonic acid. Carbonic acid can attack rocks.(d) Calcium hydrogencarbonate(e) calcium carbonate + carbonic acid → calcium hydrogencarbonate34. (a) Calcium carbonate(b) calcium carbonate −−−−→− heat strongcalcium oxide + carbon dioxide (c)(d) When the gas is passed through limewater for a few seconds, the limewater turns milky.35.(a) (i) Calcium oxide(ii) Calcium hydroxide(iii) Calcium hydroxide solution(b) (i) calcium carbonate −−→−heat calcium oxide + carbon dioxide(ii) calcium carbonate + hydrochloric acid → calcium chloride + carbon dioxide + water(iii) calcium oxide + water −→ calcium hydroxide(iv) carbon dioxide + calcium hydroxide solution−→ calcium carbonate + water (c) The rock fizzes (colourless gas is given out).heat limewaterPart I Planet earthPart Exercise1. C2. C3. A4. A5. C6. A7. B8. C9. A10. C11. D12. D13. C14. B15. A16. C17. B18. C19. B20. (a) Hydrogen ― as fuelOxygen ― in breathing aids (or any other possible answers)(b) No. Oxygen and hydrogen inside the container mix to form a gaseousmixture. All mixtures are impure substances.(c) Water(d) Yes. Water is a compound, and a single compound when existing alone is apure substance.21. (a) This conclusion is valid. The brick red colour in the flame test indicates thepresence of calcium, and the white precipitate formed when acidified silvernitrate solution is added indicates the presence of chloride.(b) This conclusion is invalid. The bubbles formed when acid was added maynot be carbon dioxide.(c) Conclusion (a) cannot be disproved. To test the validity of conclusion (b),pass the gas formed into limewater. If the limewater turns milky, the gas iscarbon dioxide, then the conclusion is valid. If the limewater doesn’t turnmilky, the conclusion is invalid.(d) The only validity of this statement is that the sample is a mixture containingcalcium chloride. Even carbonate is shown to be present, the tests carriedout are insufficient to rule out the possibility of other substances present inthe sample.22.(a) X : carbon dioxide; Y : water; Z : carbon dioxide.(b) calcium carbonate −−→−heat calcium oxide + carbon dioxideThe limestone cracks and makes a cracking noise.(c) calcium oxide + water → calcium hydroxide + heatA lot of heat is produced, with the possible production of some steamy vapour. The white solid turns into a paste.(d) calcium hydroxide + carbon dioxide → calcium carbonate + waterThe calcium hydroxide solution (limewater) turns milky.(e) This is the limewater test for carbon dioxide.23. (a) Refer to Coursebook 1 page 69.(b) Refer to Coursebook 1 page 70.(c) Frost action is a physical weathering process. This is because no newsubstances are formed during the process. Action of carbonic acid is a chemical weathering process. This is because carbonic acid changes calcium carbonate to a new substance, calcium hydrogencarbonate.(d) When excess of carbon dioxide is bubbled in, soluble calciumhydrogencarbonate is formed.Chapter 5 Atomic structureClass PracticeA5.1They are the only two liquid elements.A5.21. (a) Only an element can be classified as a metal or non-metal. Water is not anelement.(b) Non-metal.(c) Metal.2. (a) Mercury. All are metals. Mercury is a liquid, while the others are solids atroom conditions.(b) Sulphur. Sulphur is a non-metal, while the others are metals.(c) Iodine. All are non-metals. Iodine is a solid, while the others are gases atroom conditions.(d) Graphite. All are non-metals. Graphite conducts electricity, while the othersare non-conductors of electricity.A5.3(a) (i) Mg(ii)Ag(iii) Na(b)(i) Ar,(ii) He(iii) Ne(c) (i) fluorine(ii) bromine(iii) mercuryA5.4(a) 118(b) Br(c) N(d) The element copper or a copper atom.A5.5(a) The commonest type of hydrogen atom.(b) 91 electrons. Number of neutrons cannot be predicted.(c) It is not an atom. The numbers of protons and electrons are not equal.A5.6A magnesium atom would be changed to a chlorine atom.A5.71. (a) silver(b) silver(c) silver2. (a) Aluminium(b) Al1327(c) (i) 13(ii) 13(iii) 27 - 13 = 14A5.8(a) 3(b) O816(16O, or oxygen-16)A5.9(a) 37(b) 35(c) 4(d) 238(e) We cannot tell from the given data.(The mass number is not given.)A5.10(a) Relative atomic mass of sodium= mass number of the only type of sodium atom = 23(b) Relative atomic mass of neon=10010229020⨯+⨯= 20.2A5.11(a) (b)(c) (d)A5.12(a) 17(b) (i) 2,8,7(ii)Chapter 5 Atomic structureChapter Exercise1. physical2. bromine, mercury3. metals, non-metals4. metals, non-metals, graphite5. ymbol6. smallest part7. element8. atoms9. nucleus, neutrons, nucleus, electrons10. positively, negatively, neutral11. protons12. mass number13. same, different14. carbon-1215. weighted average, relative isotopic16. shells17. electronic arrangement (electronic configuration)18. B19. D20. D21. B22. C23. D24. C25. D26. (a) True. This is because there is no gaseous metal or semi-metal at roomconditions.(b) False. This is because mercury is a liquid metal at room conditions.(c) False. This is because carbon (graphite) is a non-metal which can conductelectricity. / This is because semi-metals cannot conduct electricity bythemselves.(d) False. This is because some metals (e.g. sodium) are soft.(e) True. This is because metals are silvery white, golden or brown in colour.No metal is red in colour.27. (a) The mass number of an atom is the sum of the number of protons andneutrons in the atom.(b) The atomic number of an atom is the number of protons in the atom.(c) Isotopes are different atoms of the same element, with the same number ofprotons (and electrons) but different numbers of neutrons.(d) Atom Number of protons Number of neutrons Electronic configuration105B 5 5 2, 3 115B 5 6 2, 3 (e) 10.810080112010=+⨯⨯ 28. (a) Q and R(b) Carbon(c) Carbon-13 and carbon-14(d) 135P , 136Q , 146R , 147S29. (a)AtomAtomic no. Mass no. Number of Electronic arrangement protons neutrons electrons (a)35Cl 17 35 17 18 17 2, 8, 7 (b)17O 8 17 8 9 8 2, 6 (c) 40Ar 18 40 18 22 18 2, 8, 8(b)(c) Neon(d) Argon is very unreactive.30. - Elements can be classified according to their physical states . For example, atroom temperature, hydrogen and oxygen are gases; bromine and mercury are liquids; carbon and iodine are solids.- Elements can also be classified into metals and non-metals. A few elementshave properties in between those of metals and non-metals. They are classified as semi-metals.- Examples of metals include sodium and mercury; examples of non-metalsinclude bromine and hydrogen; examples of semi-metals include boron and silicon.Class PracticeA6.1(a) Period 7, Group II; alkaline earth metals.(b) Radium.(c) Yes. Radium is a metal (all metals conduct electricity).A6.2Element X: MetalElement Y: Non-metalElement Z: We cannot tell from the given data as elements in Group IV can be a metal, non-metal or semi-metal.A6.3(a) 2, 8, 8, 2.(b) Yes, it is a metal.(c) (ii).A6.4(a) Yes. By knowing the chemical properties of familiar elements in the same groupand the group trend, predictions about the unfamiliar element can be made. (b) Astatine: D; strontium: AChapter Exercise1. electrons, outermost2. ascending, atomic numbers3. period, group, eight,4. period number, outermost5. metals, semi-metals, non-metals6. chemical7. 1, 1, increases8. 7, halogens, decreases9. 8, noble gases10. B11. B12. D13. C14. C15. C16. C17. D18.19. (a) 2(b) They all have two electrons in the outermost shell.(c) Increase down the Group.(d) (i) Beryllium reacts very slowly with water.(ii) Barium reacts vigorously with water.(e) Barium is more reactive than calcium. It should be stored under paraffin.20. (a) Magnesium, silicon, chlorine. They are in Period 3.(b) Lithium, rubidium, caesium. They are in Group I.(c) Iron, copper(d) Caesium(e) Fluorine(f) Silicon(g) Helium(h) Helium, fluorine, chlorine(i) Fluorine, chlorine21. (a) Group II(b) Alkaline earth metals(c) Strontium has 2 outermost shell electrons.(d) Strontium is a silvery white solid at room conditions.(e) Strontium reacts with cold water more readily than calcium does andcolourless gas bubbles are given off. This is because the reactivity of GroupII elements will increase down the group.22. - In the modern Periodic Table, elements are arranged in ascending order ofatomic number.- The elements are arranged in periods and groups of the Periodic Table.A horizontal row of elements is called a period while a vertical column ofelements is called group.- Period number = number of occupied electron shellsGroup number = number of electrons in outermost shell- Elements within the same group of the Periodic Table have similar chemical properties.- Across a period, the elements change from metals through semi-metals to non-metals.- Some of the groups have special names. Group I elements are named as alkali metals; Group II elements are named as alkaline earth metals; Group VII elements are named as halogens; Group 0 elements are named as noble gases.The elements in between Group II and Group III are called the transition elements.Chapter 7 Chemical bonding: ionic bondingClass PracticeA7.1(a) Delete ‘non-metals’.(b) Delete ‘metals’.A7.2(a) Colourless(b) Purple(c) Yellow(d) GreenA7.3(a) The cathode. Potassium ions are positively charged. They are thus attractedtowards the negative electrode (cathode).(b) No. Potassium ions are colourless.(c) A green patch would move towards the negative electrode (cathode).Chromium(III) ions are green in colour and positively charged. They are attracted towards the negative electrode.A7.4(a) (i) Aluminium atom: 2, 8, 3; aluminium ion: 2, 8(ii) Chlorine atom: 2, 8, 7; chloride ion: 2, 8, 8(b) Charge on aluminium ion = +3; charge on chloride ion = -1A7.5Simple ions: H+, H-, Mn2+Polyatomic ions: NH4+, NH2-, OH-A7.6(a)(ii) At-A7.7(a)(b)A7.8(a) CuCl2(b) CaS(c) Al(OH)3(d) (NH4)2CO3A7.9(a) Mg(OH)2(b) Na2O(c) PbSO4(d) K2Cr2O7A7.10(a) Calcium nitrate(b) Iron(III) chloride(c) Zinc sulphate-7-water(d) Copper(II) hydroxideChapter 7 Chemical bonding: ionic bondingChapter Exercise1. octet, duplet2. electrons, noble gas, ions3. simple, polyatomic4. cations, anions5. coloured6. electrolysis7. name, formula8. group9. minus10. ionic, ionic, calcium oxide, calcium, oxygen, Calcium (Ca2+), oxide (O2-), ionicbonds11. giant ionic structure12. B13. A14. D15. A16. C17. A18. C19. B20.21. (a) Calcium sulphate(b) Cation: calcium ion; anion: sulphate ion(c) Ionic bonding(d) CaSO4(e) The coagulant is white in colour.(g) Polyatomic ion22. (a) A: 2,5; B: 2, 8, 1; C: 2, 8, 2; D: 2, 8, 6(b) Elements A and D tend to gain electrons to attain an octet of electrons.(c) Elements B and C tend to lose electrons to attain an octet of electrons.(d) 4(e) B3A; B2D; C3A2; CD(f)B3A B2DC3A CD23. (a) Magnesium chloride: MgCl2; potassium chloride: KCl; sodium chloride:NaCl(b)MgCl2KClNaCl(c) Giant ionic structure24. -Consider the reaction between sodium and chlorine.A sodium atom Na has the electronic arrangement 2,8,1. It loses 1 electron toget the stable octet structure to form a Na+ ion.-A chlorine atom Cl has the electronic arrangement 2,8,7. It gains 1 electron to get the stable octet structure to form a Cl- ion.-When sodium atom reacts with a chlorine atom, the sodium atom loses 1 electron to the chlorine atom. By transfer of electron, two ions are formed.The electrostatic force between the ions is called ionic bonds and the compound is called ionic compound.electronsodium atom(Na) (loses one electron)sodium ion(Na+) chlorine ion(Cl-)transferchlorine atom(Cl)(gains one electron)。
国际金融英文版ChapterTwo

PPT文档演模板
国际金融英文版ChapterTwo
重要说明
偏差
PPT文档演模板
国际金融英文版ChapterTwo
此研究方法给我们的启发
³ 我国能源的短缺
PPT文档演模板
国际金融英文版ChapterTwo
结束句
谢谢
PPT文档演模板
国际金融英文版ChapterTwo
Fundamentals of BOP Accounting
New York bank deposits.
PPT文档演模板
国际金融英文版ChapterTwo
Fundamentals of BOP Accounting
3. How do we record transactions between residents and nonresidents
A set of accounts(p14-18)
³ Each transaction, involves two opposite flows of equal value. ³ For example: ³ The U.S. government sells $29 million worth of wheat to Russia, being paid
Debit: unilateral transfer $8 million
Credit: goods
$8 million
PPT文档演模板
国际金融英文版ChapterTwo
Account name
Debit
Current account
67
Goods
34(2)
Services
Income
25(4)
•Financia l account
材料科学基础专有名词英文翻译

Fundamentals of Materials Science 材料科学基础名词与术语第一章绪论metal: 金属ceramic:陶瓷polymer:聚合物Composites:复合材料Semiconductors: 半导体Biomaterials: 生物材料Processing:加工过程Structure: 组织结构Properties:性质Performance:使用性能Mechanical properties: 力学性能Electrical properties:电性能Thermal behavior:热性能Magnetic properties:磁性能Optical properties:光性能Deteriorative characteristics:老化特性第二章原子结构与原子键Atomic mass unit (amu):原子质量单位Atomic number:原子数Atomic weight:原子量Bohr atomic model: 波尔原子模型Bonding energy: 键能Coulombic force: 库仑力Covalent bond:共价键Dipole (electric):偶极子electronic configuration:电子构型electron state:电位Electronegative: 负电的Electropositive:正电的Ground state:基态Hydrogen bond: 氢键Ionic bond: 离子键Isotope: 同位素Metallic bond:金属键Mole:摩尔Molecule:分子Pauli exclusion principle:泡利不相容原理Periodic table:元素周期表Polar molecule:极性分子Primary bonding: 强键Quantum mechanics:量子力学Quantum number:量子数Secondary bonding:弱键valence electron:价电子van der waals bond:范德华键Wave—mechanical model: 波粒二象性模型第三章金属与陶瓷的结构Allotropy:同素异形现象Amorphous:无定形Anion: 阴离子Anisotropy: 各向异性atomic packing factor(APF): 原子堆积因数body—centered cubic (BCC): 体心立方结构Bragg’s law:布拉格定律Cation:阳离子coordination number:配位数crystal structure:晶体结构crystal system:晶系crystalline: 晶体的diffraction: 衍射face—centered cubic (FCC):面心立方结构第五章晶体缺陷Alloy:合金A metallic substance that is composed of two or more elements。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2.2.1.3 True stress and true strain
2.2.3 Hardness
布氏硬度
BHN
2P
D[ D D d ]
2 2
洛氏硬度 维氏硬度
R 100 500t
1.854 L VHN 2 D
charpy impact testing
(a) Charpy
冲击韧性
(tensile, compressive, shear) 抗拉, 耐压, 剪切
(Brinell, Rockwell, Vickers)
布氏, 洛氏, 维氏
延展性
(Charpy, Izod)
摆锤式,悬臂梁式
2.2 Engineering Properties
2.2.1 Tension
Chapter 2
Fundamentals of Materials
材料基础知识
contents
2.1 The Structure of Metals 2.2 Engineering Properties
2.2.1 Tension
2.2.2 Compression
2.2.3 Hardness
2.1 The Structure of Metals
the ratio of reduction in cross-sectional area to the original cross-section area
reduction in cross- sectionalarea original cross- sectionalarea
2.2.1.3 True stress and true strain
(b) Izod
Question
1.
2. 3.
What the structure of metals is?
What engineering properties of materials are?
Define strength. Explain the procedure for measuring the tensile strength of steels.
Space lattice
Unit cell
Crystalline solid
Face centred cubic: Body centred cubic:
FCC BCC HCP
Ca, Ni, Cu, Ag, Pt, Au, Pb, Al
Na, K, V, Mo, Ta, W
Hexagonal close packed:
original gage length l0, generally 50mm
cross-sectional area A0, diameter of 12.5mm
2.2.1 Tension
2.2.1 Tension
2.2.1.1 Stress-strain curves
应力
applied load stress area of crosssection opposingthe load
Be, Mg, Zn, Cd, Te
பைடு நூலகம்
2.1 The Structure of Metals
BCC
2.1 The Structure of Metals
FCC
2.1 The Structure of Metals
HCP
2.2 Engineering Properties
Strength
Hardness Ductility Toughness
Explain the behaviour of steels when they are tensile loaded.
4.
应变 strain changein dimension
original dimension
2.2.1.1 Stress-strain curves
2.2.1.2 Ductility
the ratio of elongation to the original length
elongation original length
