抗癌药物急性及亚急性毒性分级标准
一表总结:抗肿瘤药物常见不良反应分级
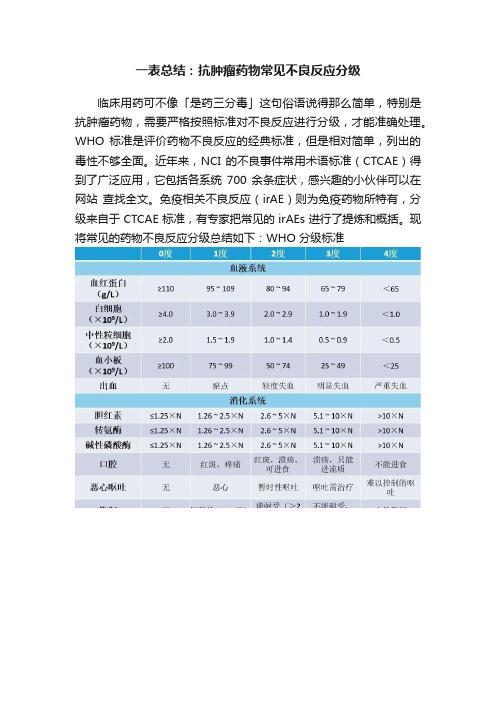
一表总结:抗肿瘤药物常见不良反应分级
临床用药可不像「是药三分毒」这句俗语说得那么简单,特别是抗肿瘤药物,需要严格按照标准对不良反应进行分级,才能准确处理。
WHO 标准是评价药物不良反应的经典标准,但是相对简单,列出的毒性不够全面。
近年来,NCI 的不良事件常用术语标准(CTCAE)得到了广泛应用,它包括各系统700 余条症状,感兴趣的小伙伴可以在网站查找全文。
免疫相关不良反应(irAE)则为免疫药物所特有,分级来自于 CTCAE 标准,有专家把常见的 irAEs 进行了提炼和概括。
现将常见的药物不良反应分级总结如下:WHO 分级标准
CTCAE 标准(4.0 版)
(5 级毒性为死亡,表中未列出)
LLN:正常下限;ULN:正常上限常见免疫相关不良反应。
高危药品的分级

高危药品的分级在医学领域中,药品的分类和管理是非常重要的一项工作。
其中,高危药品的分级更是至关重要,因为高危药品一旦使用不当,可能会对患者的健康造成重大危害。
因此,根据药品的毒性、副作用以及使用范围等因素,我们将高危药品分为三个等级:A级、B级和C级。
A级高危药品A级高危药品是指具有极高毒性和危险度的药品,误用或过量使用可能导致严重后果甚至危及生命。
这类药品一般用于治疗严重病症或手术中使用,必须在医生的指导下使用。
常见的A级高危药品包括强效止痛药、抗癌药物、麻醉药等。
在使用A级高危药品时,医务人员要格外谨慎,严格按照医嘱和用药要求来进行使用。
B级高危药品B级高危药品相对于A级来说毒性较低,但仍然具有一定的危险性,需要在专业人员的指导下合理使用。
这类药品可能会出现严重的副作用,对患者的健康造成损害。
常见的B级高危药品包括某些抗生素、激素类药物等。
在使用B级高危药品时,患者要注意严格遵守医嘱,避免自行停药或更改用药方案。
C级高危药品C级高危药品是相对安全性较高的一类药品,但在特定条件下使用时仍需谨慎。
这类药品可能会出现较轻的不良反应,但也有可能导致严重后果。
常见的C级高危药品包括一些抗生素、消炎药、镇痛药等。
在使用C级高危药品时,患者应严格按照药品说明书来使用,注意可能出现的不良反应,并及时向医生咨询。
综上所述,对高危药品进行科学合理的分级管理对于保障患者的安全和健康至关重要。
医务人员和患者在使用高危药品时都应当严格遵守相关规定,做到用药安全,最大限度地发挥药品的疗效,确保患者获得最佳治疗效果。
常见毒性标准(CTC)1999 英文版

COMMON TOXICITY CRITERIA (CTC)GradeAdverse Event01234ALLERGY/IMMUNOLOGYAllergic reaction/ hypersensitivity (including drug fever)none transient rash, drugfever <38°C (<100.4°F)urticaria, drug fever≥38°C (≥100.4°F),and/or asymptomaticbronchospasmsymptomaticbronchospasm,requiring parenteralmedication(s), with orwithout urticaria;allergy-relatededema/angioedemaanaphylaxisNote: Isolated urticaria, in the absence of other manifestations of an allergic or hypersensitivity reaction, is graded in the DERMATOLOGY/SKIN category.Allergic rhinitis (including sneezing, nasal stuffiness, postnasal drip)none mild, not requiringtreatmentmoderate, requiringtreatment--Autoimmune reaction none serologic or otherevidence ofautoimmune reactionbut patient isasymptomatic (e.g.,vitiligo), all organfunction is normal andno treatment is required evidence ofautoimmune reactioninvolving a non-essential organ orfunction (e.g.,hypothyroidism),requiring treatmentother thanimmunosuppressivedrugsreversible autoimmunereaction involvingfunction of a majororgan or other adverseevent (e.g., transientcolitis or anemia),requiring short-termimmunosuppressivetreatmentautoimmune reactioncausing major grade 4organ dysfunction;progressive andirreversible reaction;long-termadministration of high-dose immuno-suppressive therapyrequiredAlso consider Hypothyroidism, Colitis, Hemoglobin, Hemolysis.Serum sickness none--present-Urticaria is graded in the DERMATOLOGY/SKIN category if it occurs as an isolated symptom. If it occurs with other manifestations of allergic or hypersensitivity reaction, grade as Allergic reaction/hypersensitivity above.Vasculitis none mild, not requiringtreatment symptomatic, requiringmedicationrequiring steroids ischemic changes orrequiring amputationAllergy/Immunology - Other (Specify, __________)none mild moderate severe life-threatening ordisablingAUDITORY/HEARINGConductive hearing loss is graded as Middle ear/hearing in the AUDITORY/HEARING category. Earache is graded in the PAIN category.External auditory canal normal external otitis witherythema or drydesquamation external otitis withmoist desquamationexternal otitis withdischarge, mastoiditisnecrosis of the canalsoft tissue or boneNote: Changes associated with radiation to external ear (pinnae) are graded under Radiation dermatitis in the DERMATOLOGY/SKIN category.Adverse Event01234Inner ear/hearing normal hearing loss onaudiometry only tinnitus or hearing loss,not requiring hearingaid or treatmenttinnitus or hearing loss,correctable with hearingaid or treatmentsevere unilateral orbilateral hearing loss(deafness), notcorrectableMiddle ear/hearing normal serous otitis withoutsubjective decrease inhearing serous otitis or infectionrequiring medicalintervention; subjectivedecrease in hearing;rupture of tympanicmembrane withdischargeotitis with discharge,mastoiditis orconductive hearing lossnecrosis of the canalsoft tissue or boneAuditory/Hearing - Other (Specify, __________)normal mild moderate severe life-threatening ordisablingBLOOD/BONE MARROWBone marrow cellularity normal for age mildly hypocellular or≤25% reduction fromnormal cellularity forage moderately hypocellularor >25 - ≤50%reduction from normalcellularity for age or >2but <4 weeks torecovery of normalbone marrow cellularityseverely hypocellular or>50 - ≤75% reductionin cellularity for age or4 - 6 weeks to recoveryof normal bone marrowcellularityaplasia or >6 weeks torecovery of normalbone marrow cellularityNormal ranges:children (≤18 years)90% cellularityaverageyounger adults (19-59)60 - 70%cellularity averageolder adults (≥60 years)50% cellularityaverageNote: Grade Bone marrow cellularity only for changes related to treatment not disease.CD4 count WNL<LLN - 500/mm3200 - <500/mm350 - <200/mm3<50/mm3 Haptoglobin normal decreased-absent-Hemoglobin (Hgb)WNL<LLN - 10.0 g/dL<LLN - 100 g/L<LLN - 6.2 mmol/L 8.0 - <10.0 g/dL80 - <100 g/L4.9 - <6.2 mmol/L6.5 - <8.0 g/dL65 - <80 g/L4.0 - <4.9 mmol/L<6.5 g/dL<65 g/L<4.0 mmol/LFor leukemia studies or bone marrow infiltrative/ myelophthisic processes, if specified in the protocol.WNL10 - <25% decreasefrom pretreatment25 - <50% decreasefrom pretreatment50 - <75% decreasefrom pretreatment≥75% decrease frompretreatmentHemolysis (e.g., immune hemolytic anemia, drug-related hemolysis, other)none only laboratoryevidence of hemolysis[e.g., direct antiglobulintest (DAT, Coombs’)schistocytes]evidence of red celldestruction and ≥2gmdecrease in hemoglobin,no transfusionrequiring transfusionand/or medicalintervention (e.g.,steroids)catastrophicconsequences ofhemolysis (e.g., renalfailure, hypotension,bronchospasm,emergencysplenectomy)Also consider Haptoglobin, Hemoglobin.Adverse Event01234Leukocytes (total WBC)WNL<LLN - 3.0 x 109 /L<LLN - 3000/mm3≥2.0 - <3.0 x 109 /L≥2000 - <3000/mm3≥1.0 - <2.0 x 109 /L≥1000 - <2000/mm3<1.0 x 109 /L<1000/mm3For BMT studies, if specified in the protocol.WNL≥2.0 - <3.0 X 109/L≥2000 - <3000/mm3≥1.0 - <2.0 x 109 /L≥1000 - <2000/mm3≥0.5 - <1.0 x 109 /L≥500 - <1000/mm3<0.5 x 109 /L<500/mm3For pediatric BMT studies(using age, race and sexnormal values), if specifiedin the protocol.≥75 - <100% LLN≥50 - <75% LLN≥25 - 50% LLN<25% LLNLymphopenia WNL<LLN - 1.0 x 109 /L<LLN - 1000/mm3≥0.5 - <1.0 x 109 /L≥500 - <1000/mm3<0.5 x 109 /L<500/mm3-For pediatric BMT studies(using age, race and sexnormal values), if specifiedin the protocol.≥75 - <100%LLN≥50 - <75%LLN≥25 - <50%LLN<25%LLNNeutrophils/granulocytes (ANC/AGC)WNL≥1.5 - <2.0 x 109 /L≥1500 - <2000/mm3≥1.0 - <1.5 x 109 /L≥1000 - <1500/mm3≥0.5 - <1.0 x 109 /L≥500 - <1000/mm3<0.5 x 109 /L<500/mm3For BMT studies, if specified in the protocol.WNL≥1.0 - <1.5 x 109 /L≥1000 - <1500/mm3≥0.5 - <1.0 x 109 /L≥500 - <1000/mm3≥0.1 - <0.5 x 109 /L≥100 - <500/mm3<0.1 x 109 /L<100/mm3For leukemia studies or bone marrow infiltrative/ myelophthisic process, if specified in the protocol.WNL10 - <25% decreasefrom baseline25 - <50% decreasefrom baseline50 - <75% decreasefrom baseline≥75% decrease frombaselinePlatelets WNL<LLN - 75.0 x 109 /L<LLN - 75,000/mm3≥50.0 - <75.0 x 109 /L≥50,000 - <75,000/mm3≥10.0 - <50.0 x 109 /L≥10,000 - <50,000/mm3<10.0 x 109 /L<10,000/mm3For BMT studies, if specified in the protocol.WNL≥50.0 - <75.0 x 109 /L≥50,000 - <75,000/mm3≥20.0 - <50.0 x 109 /L≥20,000 - <50,000/mm3≥10.0 - <20.0 x 109 /L≥10,000 - <20,000/mm3<10.0 x 109 /L<10,000/mm3For leukemia studies or bone marrow infiltrative/ myelophthisic process, if specified in the protocol.WNL10 - <25% decreasefrom baseline25 - <50% decreasefrom baseline50 - <75% decreasefrom baseline≥75% decrease frombaselineTransfusion: Platelets none--yes platelet transfusions andother measures requiredto improve plateletincrement; platelettransfusionrefractoriness associatedwith life-threateningbleeding. (e.g., HLA orcross matched platelettransfusions)For BMT studies, if specified in the protocol.none 1 platelet transfusion in24 hours2 platelet transfusions in24 hours≥3 platelet transfusionsin 24 hoursplatelet transfusions andother measures requiredto improve plateletincrement; platelettransfusionrefractoriness associatedwith life-threateningbleeding. (e.g., HLA orcross matched platelettransfusions)Also consider Platelets.Adverse Event01234 Transfusion: pRBCs none--yes-For BMT studies, if specified in the protocol.none≤2 u pRBC in 24 hourselective or planned3 u pRBC in 24 hourselective or planned≥4 u pRBC in 24 hours hemorrhage orhemolysis associatedwith life-threateninganemia; medicalintervention required toimprove hemoglobinFor pediatric BMT studies, if specified in the protocol.none≤15mL/kg in 24 hourselective or planned>15 - ≤30mL/kg in 24hours elective orplanned>30mL/kg in 24 hours hemorrhage orhemolysis associatedwith life-threateninganemia; medicalintervention required toimprove hemoglobinAlso consider Hemoglobin.Blood/Bone Marrow - Other (Specify, __________)none mild moderate severe life-threatening ordisabling CARDIOVASCULAR (ARRHYTHMIA)Conduction abnormality/ Atrioventricular heart block none asymptomatic, notrequiring treatment(e.g., Mobitz type Isecond-degree AVblock, Wenckebach)symptomatic, but notrequiring treatmentsymptomatic andrequiring treatment(e.g., Mobitz type IIsecond-degree AVblock, third-degree AVblock)life-threatening (e.g.,arrhythmia associatedwith CHF, hypotension,syncope, shock)Nodal/junctional arrhythmia/dysrhythmia none asymptomatic, notrequiring treatmentsymptomatic, but notrequiring treatmentsymptomatic andrequiring treatmentlife-threatening (e.g.,arrhythmia associatedwith CHF, hypotension,syncope, shock)Palpitations none present---Note: Grade palpitations only in the absence of a documented arrhythmia.Prolonged QTc interval (QTc >0.48 seconds)none asymptomatic, notrequiring treatmentsymptomatic, but notrequiring treatmentsymptomatic andrequiring treatmentlife-threatening (e.g.,arrhythmia associatedwith CHF, hypotension,syncope, shock)Sinus bradycardia none asymptomatic, notrequiring treatment symptomatic, but notrequiring treatmentsymptomatic andrequiring treatmentlife-threatening (e.g.,arrhythmia associatedwith CHF, hypotension,syncope, shock)Sinus tachycardia none asymptomatic, notrequiring treatment symptomatic, but notrequiring treatmentsymptomatic andrequiring treatment ofunderlying cause-Supraventricular arrhythmias (SVT/atrial fibrillation/ flutter)none asymptomatic, notrequiring treatmentsymptomatic, but notrequiring treatmentsymptomatic andrequiring treatmentlife-threatening (e.g.,arrhythmia associatedwith CHF, hypotension,syncope, shock)Syncope (fainting) is graded in the NEUROLOGY category.Vasovagal episode none-present without loss ofconsciousness present with loss of consciousness-Adverse Event01234Ventricular arrhythmia (PVCs/bigeminy/trigeminy/ ventricular tachycardia)none asymptomatic, notrequiring treatmentsymptomatic, but notrequiring treatmentsymptomatic andrequiring treatmentlife-threatening (e.g.,arrhythmia associatedwith CHF, hypotension,syncope, shock)Cardiovascular/ Arrhythmia - Other (Specify, ___________)none asymptomatic, notrequiring treatmentsymptomatic, but notrequiring treatmentsymptomatic, andrequiring treatment ofunderlying causelife-threatening (e.g.,arrhythmia associatedwith CHF, hypotension,syncope, shock) CARDIOVASCULAR (GENERAL)Acute vascular leak syndrome absent-symptomatic, but notrequiring fluid supportrespiratory compromiseor requiring fluidslife-threatening;requiring pressorsupport and/orventilatory supportCardiac-ischemia/infarction none non-specific T - waveflattening or changes asymptomatic, ST - andT - wave changessuggesting ischemiaangina without evidenceof infarctionacute myocardialinfarctionCardiac left ventricular function normal asymptomatic declineof resting ejectionfraction of ≥10% but<20% of baseline value;shortening fraction≥24% but <30%asymptomatic butresting ejection fractionbelow LLN forlaboratory or decline ofresting ejection fraction≥20% of baseline value;<24% shorteningfractionCHF responsive totreatmentsevere or refractoryCHF or requiringintubationCNS cerebrovascular ischemia is graded in the NEUROLOGY category.Cardiac troponin I (cTnI)normal--levels consistent withunstable angina asdefined by themanufacturer levels consistent with myocardial infarction as defined by the manufacturerCardiac troponin T (cTnT)normal≥0.03 - <0.05 ng/mL≥0.05 - <0.1 ng/mL≥0.1 - <0.2 ng/mL≥0.2 ng/mLEdema none asymptomatic, notrequiring therapy symptomatic, requiringtherapysymptomatic edemalimiting function andunresponsive to therapyor requiring drugdiscontinuationanasarca (severegeneralized edema)Hypertension none asymptomatic, transientincrease by >20 mmHg(diastolic) or to>150/100* if previouslyWNL; not requiringtreatment recurrent or persistentor symptomatic increaseby >20 mmHg(diastolic) or to>150/100* if previouslyWNL; not requiringtreatmentrequiring therapy ormore intensive therapythan previouslyhypertensive crisis*Note: For pediatric patients, use age and sex appropriate normal values >95th percentile ULN.Adverse Event01234Hypotension none changes, but notrequiring therapy(including transientorthostatic hypotension)requiring brief fluidreplacement or othertherapy but nothospitalization; nophysiologicconsequencesrequiring therapy andsustained medicalattention, but resolveswithout persistingphysiologicconsequencesshock (associated withacidemia and impairingvital organ function dueto tissue hypoperfusion)Also consider Syncope (fainting).Notes:Angina or MI is graded as Cardiac-ischemia/infarction in the CARDIOVASCULAR (GENERAL) category.For pediatric patients, systolic BP 65 mmHg or less in infants up to 1 year old and 70 mmHg or less in children older than 1 year of age, use two successive or three measurements in 24 hours.Myocarditis none--CHF responsive totreatment severe or refractory CHFOperative injury of vein/artery none primary suture repairfor injury, but notrequiring transfusionprimary suture repairfor injury, requiringtransfusionvascular occlusionrequiring surgery orbypass for injurymyocardial infarction;resection of organ (e.g.,bowel, limb)Pericardial effusion/ pericarditis none asymptomatic effusion,not requiring treatmentpericarditis (rub, ECGchanges, and/or chestpain)with physiologicconsequencestamponade (drainage orpericardial windowrequired)Peripheral arterial ischemia none-brief episode ofischemia managed non-surgically and withoutpermanent deficit requiring surgicalinterventionlife-threatening or withpermanent functionaldeficit (e.g.,amputation)Phlebitis (superficial)none-present--Notes:Injection site reaction is graded in the DERMATOLOGY/SKIN category.Thrombosis/embolism is graded in the CARDIOVASCULAR (GENERAL) category.Syncope (fainting) is graded in the NEUROLOGY category.Thrombosis/embolism none-deep vein thrombosis,not requiringanticoagulant deep vein thrombosis,requiring anticoagulanttherapyembolic event includingpulmonary embolismVein/artery operative injury is graded as Operative injury of vein/artery in the CARDIOVASCULAR (GENERAL) category.Visceral arterial ischemia (non-myocardial)none-brief episode ofischemia managed non-surgically and withoutpermanent deficitrequiring surgicalinterventionlife-threatening or withpermanent functionaldeficit (e.g., resection ofileum)Cardiovascular/General - Other (Specify, ______________)none mild moderate severe life-threatening ordisablingAdverse Event01234COAGULATIONNote: See the HEMORRHAGE category for grading the severity of bleeding events.DIC(disseminated intravascular coagulation)absent--laboratory findingspresent with nobleedinglaboratory findings andbleedingAlso consider Platelets.Note: Must have increased fibrin split products or D-dimer in order to grade as DIC.Fibrinogen WNL≥0.75 - <1.0 x LLN≥0.5 - <0.75 x LLN≥0.25 - <0.5 x LLN<0.25 x LLNFor leukemia studies or bone marrow infiltrative/ myelophthisic process, if specified in the protocol.WNL<20% decrease frompretreatment value orLLN≥20 - <40% decreasefrom pretreatment valueor LLN≥40 - <70% decreasefrom pretreatment valueor LLN<50 mgPartial thromboplastin time(PTT)WNL>ULN - ≤1.5 x ULN>1.5 - ≤2 x ULN>2 x ULN-Phlebitis is graded in the CARDIOVASCULAR (GENERAL) category.Prothrombin time (PT)WNL>ULN - ≤1.5 x ULN>1.5 - ≤2 x ULN>2 x ULN-Thrombosis/embolism is graded in the CARDIOVASCULAR (GENERAL) category.Thrombotic microangiopathy (e.g., thrombotic thrombocytopenic purpura/TTP or hemolytic uremic syndrome/HUS)absent--laboratory findingspresent without clinicalconsequenceslaboratory findings andclinical consequences,(e.g., CNS hemorrhage/bleeding or thrombosis/embolism or renalfailure) requiringtherapeutic interventionFor BMT studies, if specified in the protocol.-evidence of RBCdestruction(schistocytosis) withoutclinical consequencesevidence of RBCdestruction withelevated creatinine (≤3x ULN)evidence of RBCdestruction withcreatinine (>3 x ULN)not requiring dialysisevidence of RBCdestruction with renalfailure requiringdialysis and/orencephalopathyAlso consider Hemoglobin, Platelets, Creatinine.Note: Must have microangiopathic changes on blood smear (e.g., schistocytes, helmet cells, red cell fragments).Coagulation - Other (Specify, __________)none mild moderate severe life-threatening ordisabling CONSTITUTIONAL SYMPTOMSFatigue(lethargy, malaise, asthenia)none increased fatigue overbaseline, but notaltering normalactivitiesmoderate (e.g., decreasein performance statusby 1 ECOG level or20% Karnofsky orLansky) or causingdifficulty performingsome activitiessevere (e.g., decrease inperformance status by≥2 ECOG levels or 40%Karnofsky or Lansky) orloss of ability toperform some activitiesbedridden or disablingNote: See Appendix III for performance status scales.Adverse Event01234Fever (in the absence of neutropenia, where neutropenia is defined as AGC <1.0 x 109/L)none38.0 - 39.0°C (100.4 -102.2°F)39.1 - 40.0°C (102.3 -104.0°F )>40.0°C (>104.0°F ) for<24hrs>40.0°C (>104.0°F ) for>24hrsAlso consider Allergic reaction/hypersensitivity.Note: The temperature measurements listed above are oral or tympanic. Hot flashes/flushes are graded in the ENDOCRINE category.Rigors, chills none mild, requiringsymptomatic treatment(e.g., blanket) or non-narcotic medication severe and/orprolonged, requiringnarcotic medicationnot responsive tonarcotic medication-Sweating(diaphoresis)normal mild and occasional frequent or drenching--Weight gain<5% 5 - <10%10 - <20%≥20%-Also consider Ascites, Edema, Pleural effusion (non-malignant).Weight gain associated with Veno-Occlusive Disease (VOD) for BMT studies, if specified in the protocol.<2%≥2 - <5%≥5 - <10%≥10% or as ascites≥10% or fluid retentionresulting in pulmonaryfailureAlso consider Ascites, Edema, Pleural effusion (non-malignant).Weight loss<5% 5 - <10%10 - <20%≥20%-Also consider Vomiting, Dehydration, Diarrhea.Constitutional Symptoms -Other(Specify, __________)none mild moderate severe life-threatening ordisablingDERMATOLOGY/SKINAlopecia normal mild hair loss pronounced hair loss--Bruising(in absence of grade 3 or 4 thrombocytopenia)none localized or independent areageneralized--Note:Bruising resulting from grade 3 or 4 thrombocytopenia is graded as Petechiae/purpura and Hemorrhage/bleeding with grade 3 or 4 thrombocytopenia in the HEMORRHAGE category, not in the DERMATOLOGY/SKIN category.Dry skin normal controlled withemollients not controlled withemollients--Erythema multiforme (e.g., Stevens-Johnson syndrome, toxic epidermal necrolysis)absent-scattered, but notgeneralized eruptionsevere or requiring IVfluids (e.g., generalizedrash or painfulstomatitis)life-threatening (e.g.,exfoliative or ulceratingdermatitis or requiringenteral or parenteralnutritional support)Flushing absent present---Hand-foot skin reaction none skin changes ordermatitis without pain(e.g., erythema, peeling)skin changes with pain,not interfering withfunctionskin changes with pain,interfering withfunction-Injection site reaction none pain or itching orerythema pain or swelling, withinflammation orphlebitisulceration or necrosisthat is severe orprolonged, or requiringsurgery-Adverse Event01234Nail changes normal discoloration or ridging(koilonychia) or pitting partial or complete lossof nail(s) or pain innailbeds--Petechiae is graded in the HEMORRHAGE category.Photosensitivity none painless erythema painful erythema erythema withdesquamation-Pigmentation changes (e.g., vitiligo)none localized pigmentationchangesgeneralizedpigmentation changes--Pruritus none mild or localized,relieved spontaneouslyor by local measures intense or widespread,relieved spontaneouslyor by systemic measuresintense or widespreadand poorly controlleddespite treatment-Purpura is graded in the HEMORRHAGE category.Radiation dermatitis none faint erythema or drydesquamation moderate to briskerythema or a patchymoist desquamation,mostly confined to skinfolds and creases;moderate edemaconfluent moistdesquamation ≥1.5 cmdiameter and notconfined to skin folds;pitting edemaskin necrosis orulceration of fullthickness dermis; mayinclude bleeding notinduced by minortrauma or abrasionNote: Pain associated with radiation dermatitis is graded separately in the PAIN category as Pain due to radiation.Radiation recall reaction (reaction following chemotherapy in the absence of additional radiation therapy that occurs in a previous radiation port)none faint erythema or drydesquamationmoderate to briskerythema or a patchymoist desquamation,mostly confined to skinfolds and creases;moderate edemaconfluent moistdesquamation ≥1.5 cmdiameter and notconfined to skin folds;pitting edemaskin necrosis orulceration of fullthickness dermis; mayinclude bleeding notinduced by minortrauma or abrasionRash/desquamation none macular or papulareruption or erythemawithout associatedsymptoms macular or papulareruption or erythemawith pruritus or otherassociated symptomscovering <50% of bodysurface or localizeddesquamation or otherlesions covering <50%of body surface areasymptomaticgeneralizederythroderma ormacular, papular orvesicular eruption ordesquamation covering≥50% of body surfaceareageneralized exfoliativedermatitis or ulcerativedermatitisAlso consider Allergic reaction/hypersensitivity.Note: Stevens-Johnson syndrome is graded separately as Erythema multiforme in the DERMATOLOGY/SKIN category.Rash/dermatitis associated with high-dose chemotherapy or BMT studies.none faint erythema or drydesquamationmoderate to briskerythema or a patchymoist desquamation,mostly confined to skinfolds and creases;moderate edemaconfluent moistdesquamation ≥1.5 cmdiameter and notconfined to skin folds;pitting edemaskin necrosis or ulcera-tion of full thicknessdermis; may includespontaneous bleedingnot induced by minortrauma or abrasionRash/desquamation associated with graft versus host disease (GVHD) for BMT studies, if specified in the protocol.None macular or papulareruption or erythemacovering <25% of bodysurface area withoutassociated symptomsmacular or papulareruption or erythemawith pruritus or otherassociated symptomscovering ≥25 - <50% ofbody surface orlocalized desquamationor other lesionscovering ≥25 - <50% ofbody surface areasymptomaticgeneralizederythroderma orsymptomatic macular,papular or vesiculareruption, with bullousformation, ordesquamation covering≥50% of body surfaceareageneralized exfoliativedermatitis or ulcerativedermatitis or bullousformationAlso consider Allergic reaction/hypersensitivity.Note: Stevens-Johnson syndrome is graded separately as Erythema multiforme in the DERMATOLOGY/SKIN category.Adverse Event01234Urticaria(hives, welts, wheals)none requiring no medication requiring PO or topicaltreatment or IVmedication or steroidsfor <24 hoursrequiring IV medicationor steroids for ≥24hours-Wound-infectious none cellulitis superficial infection infection requiring IVantibioticsnecrotizing fasciitisWound-non-infectious none incisional separation incisional hernia fascial disruptionwithout evisceration fascial disruption with eviscerationDermatology/Skin - Other (Specify, ________)none mild moderate severe life-threatening ordisablingENDOCRINECushingoid appearance (e.g.,moon face, buffalo hump,centripetal obesity,cutaneous striae)absent-present--Also consider Hyperglycemia, Hypokalemia.Feminization of male absent--present-Gynecomastia none mild pronounced or painful pronounced or painfuland requiring surgery-Hot flashes/flushes none mild or no more than 1per day moderate and greaterthan 1 per day--Hypothyroidism absent asymptomatic,TSHelevated, no therapygiven symptomatic or thyroidreplacement treatmentgivenpatient hospitalized formanifestations ofhypothyroidismmyxedema comaMasculinization of female absent--present-SIADH (syndrome ofinappropriate antidiuretichormone)absent--present-Endocrine - Other (Specify, __________)none mild moderate severe life-threatening ordisablingGASTROINTESTINALAmylase is graded in the METABOLIC/LABORATORY category.Anorexia none loss of appetite oral intake significantlydecreased requiring IV fluids requiring feeding tubeor parenteral nutritionAscites (non-malignant)none asymptomatic symptomatic, requiringdiuretics symptomatic, requiringtherapeutic paracentesislife-threateningphysiologicconsequencesColitis none-abdominal pain withmucus and/or blood instool abdominal pain, fever,change in bowel habitswith ileus or peritonealsigns, and radiographicor biopsydocumentationperforation or requiringsurgery or toxicmegacolonAlso consider Hemorrhage/bleeding with grade 3 or 4 thrombocytopenia, Hemorrhage/bleeding without grade 3 or 4 thrombocytopenia, Melena/GI bleeding, Rectal bleeding/hematochezia, Hypotension.Constipation none requiring stool softeneror dietary modification requiring laxatives obstipation requiringmanual evacuation orenemaobstruction or toxicmegacolon。
化疗不良反应分度

1
2
3
4
血红蛋白(g/L)
≥110
109-95
94-80
79-65
<65
白细胞(109/L)
≥4.0
3.9-3.0
2.9-2.0
1.9-1.0
<1.0
粒细胞(109/L)
≥2.0
1.9-1.5
1.4-1.0
0.9-0.5
<0.5
血小板(109/L)
≥100
99-75
74-50
49-25
<25
一般认为,粒细胞的减少通常开始于化疗停药后一周,至停药10-14日达到最低点,在低水平维持2~3天后缓慢回升,至第21~28天恢复正常,呈U型。血小板降低比粒细胞降低出现稍晚,也在两周左右下降到最低值,其下降迅速,在谷底停留时间较短即迅速回升,呈V型。红细胞下降出现的时间更晚。
萎缩、硬化
肾功能衰竭
亚硝脲类、顺铂
神经系统
前物碱
性腺
生殖细胞与Leydig细胞
再生停止或退化
不育症、乳房女性化
烷化剂、甲基苄肼
2.全身性
免疫功能抑制
烷化剂、甲基苄肼
畸形发生
烷化剂、抗代谢药
肿瘤(包括白血病)发生
烷化剂、甲基苄肼
关于单采血小板的使用:输注单采血小板能迅速提升血小板数量,从而防止在血小板最低阶段出血的发生。如果患者有3度血小板减少而且有出血倾向,则应输注单采血小板;如果患者为4度血小板减少,无论有无出血倾向,均应使用。一般而言,一单位单采血小板可提高血小板计数1~2万左右。然而,外源性血小板的寿命通常仅能维持72小时左右,而且反复输入后患者体内会产生抗体。因此,近年出现了一些新型药物,如重组人促血小板生成素。
nci常见毒性分级标准

nci常见毒性分级标准NCI常见毒性分级标准。
NCI(National Cancer Institute)常见毒性分级标准是临床试验中常用的一种评价药物毒性的标准,它能够帮助临床医生和研究人员对药物的毒性进行评估和监测,从而更好地保障患者的安全和药物的疗效。
NCI常见毒性分级标准主要包括对不同类型毒性的分级标准,如血液学毒性、消化道毒性、神经系统毒性等。
本文将对NCI常见毒性分级标准进行详细介绍,希望能为临床医生和研究人员提供一些帮助。
一、血液学毒性。
1. 白细胞减少,根据不同程度的白细胞减少,分为4个等级。
一般来说,一级白细胞减少是指白细胞计数低于正常下限,且大于细胞计数的50%。
二级白细胞减少是指白细胞计数低于细胞计数的50%,但高于1000/mm3。
三级白细胞减少是指白细胞计数低于1000/mm3。
四级白细胞减少是指白细胞计数低于500/mm3。
2. 血小板减少,根据不同程度的血小板减少,分为4个等级。
一级血小板减少是指血小板计数低于正常下限,但高于75,000/mm3。
二级血小板减少是指血小板计数低于75,000/mm3,但高于50,000/mm3。
三级血小板减少是指血小板计数低于50,000/mm3,但高于25,000/mm3。
四级血小板减少是指血小板计数低于25,000/mm3。
二、消化道毒性。
1. 恶心和呕吐,根据不同程度的恶心和呕吐,分为4个等级。
一级恶心和呕吐是指轻度的恶心或呕吐,不需要药物干预。
二级恶心和呕吐是指需要药物干预的恶心或呕吐,但对日常生活影响不大。
三级恶心和呕吐是指需要持续性药物干预的恶心或呕吐,对日常生活有较大影响。
四级恶心和呕吐是指需要紧急干预的恶心或呕吐,对生命有威胁。
2. 腹泻,根据不同程度的腹泻,分为4个等级。
一级腹泻是指轻度的腹泻,不需要药物干预。
二级腹泻是指需要药物干预的腹泻,但对日常生活影响不大。
三级腹泻是指需要持续性药物干预的腹泻,对日常生活有较大影响。
抗癌药物常见不良反应分级标准(who)表格

抗癌药物常见不良反应分级标准(who)表格随着癌症发病率的增加,抗癌药物的使用也越来越广泛。
然而,抗癌药物也会带来一系列不良反应,这给患者的治疗带来了一定的挑战。
了解抗癌药物的不良反应分级标准对于医生和患者都至关重要。
WHO(世界卫生组织)制定了一套抗癌药物常见不良反应的分级标准,这些标准帮助医生对患者的不良反应做出及时的评估和处理。
下面将介绍抗癌药物常见不良反应的分级标准。
1. 血液系统不良反应(1)贫血:Ⅰ级:Hb(血红蛋白)下降≥30 g/L,但≤80 g/LⅡ级:Hb下降>80 g/LⅢ级:需要输血或Hb下降≤60 g/L(2)白细胞减少:Ⅰ级:WBC(白细胞计数)下降<3.0×10^9/LⅡ级:WBC下降<2.0×10^9/LⅢ级:WBC下降<1.0×10^9/L,或伴有发热或症状2. 消化系统不良反应(1)恶心与呕吐:Ⅰ级:轻度,不影响日常生活Ⅱ级:需要药物治疗Ⅲ级:需要静脉输液治疗(2)腹泻:Ⅰ级:每天增加小于4次Ⅱ级:每天增加4-6次Ⅲ级:每天增加7次以上3. 皮肤及其附件不良反应(1)脱发:Ⅰ级:头发量减少Ⅱ级:需要使用帽子或假发Ⅲ级:持续脱发(2)皮疹:Ⅰ级:局部红斑、瘙痒等Ⅱ级:需要用药治疗Ⅲ级:需要住院治疗4. 肝功能不良反应(1)ALT(丙氨酸氨基转移酶)升高:Ⅰ级:ALT升高1-2倍正常上限Ⅱ级:ALT升高3-5倍正常上限Ⅲ级:ALT升高>5倍正常上限(2)黄疸:Ⅰ级:TB(总胆红素)升高1.25-2.5倍正常上限Ⅱ级:TB升高2.6-5倍正常上限Ⅲ级:TB升高>5倍正常上限5. 肾功能不良反应(1)肌酐升高:Ⅰ级:Cr(肌酐)升高1.5-2倍基础水平Ⅱ级:Cr升高2-3倍基础水平Ⅲ级:Cr升高>3倍基础水平(2)血尿素氮(BUN)升高:Ⅰ级:BUN升高1.5-2.5倍基础水平Ⅱ级:BUN升高2.6-5倍基础水平Ⅲ级:BUN升高>5倍基础水平以上就是抗癌药物常见不良反应分级标准(WHO)表格。
抗肿瘤药物的分级原则及划分标准

抗肿瘤药物的分级原则及划分标准下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!抗肿瘤药物的分级原则及划分标准在现代医学中,抗肿瘤药物是治疗肿瘤疾病的重要手段之一。
化疗药物的毒副作用分级及防治

1.胃肠道反应 食欲减退、恶心、呕吐,一般剂量多不严
重。偶见口腔黏膜炎或溃疡,腹部不适或腹 泻。 2.骨髓抑制
周围血白细胞减少常见(大多在疗程开始 后2~3周内达最低点,约在3~4周后恢复正 常),血小板减少罕见。 3.心脏毒性
心率加快,ST段抬高,心酶谱升高,可能为冠 状动脉痉挛导致心肌缺血缺氧,心肌受损。 4.长期应用可导致神经系统毒性
4、骨髓抑制:骨髓抑制(白细胞和/或血小 板下降)一般较轻,发生率与每疗程剂量有 关,若≤100mg/ m2,发生率约10~20%,若 剂量≥120mg/ m2,约40%,但亦与联合化疗 中其它抗癌药骨髓毒性的重叠有关。
氟尿嘧啶(5-FU)
适应症 消化系统肿瘤、乳腺癌、头颈部肿瘤
注意事项 1:一般溶于葡萄糖 2:静滴3小时以上 3:与亚叶酸钙联合在其后使用
2.肾毒性 :一般剂量每日超过90mg/m2即为肾 毒性的危险因素。主要为肾小管损伤。急性 损害一般见于用药后10~15天,血BUN、Cr 增高,肌酐清除率降低,多为可逆性,反复 高剂量治疗可致持久性轻至中度肾损害。目 前除水化外尚无有效预防本品所致的肾毒性 的手段;
3、神经毒性:神经损害如听神经损害所致耳 鸣、听力下降较常见。末梢神经毒性与累积 剂量增加有关,表现为不同程度的手、脚套 样感觉减弱或丧失,有时出现肢端麻痹、躯 干肌力下降等,一般难以恢复。
化疗药物的毒副作用分级及防治
文章内容来源于网络,如有侵权请联系我们删除。
化疗药物的毒副作用及其防 治
基本概念
一、在实施疗前必须确定 1、患者一般情况 2、病理诊断 3、TNM分期 4、PS评分 5、化疗同意书 6、化疗方案
基本概念
二、按化疗目的分为: 1、根治性化疗 2、姑息性化疗 3、辅助性化疗 4、新辅助性化疗 5、同步化疗
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
呕吐
无
1次/24小时
2-5次/24小时
6-10次/24小时
>10次/24小时需胃肠支持治疗
腹泻
无
大便次数增加2-3次/天
大便每天增加4-6次/天或夜间大便或中度腹痛
大便每天增加7-9次/天或大便失禁或严重腹痛
大便每天增加>10次/天或明显血性腹泻或需胃肠外支持治疗
口腔粘膜炎
无
无痛性溃疡,红斑或有轻度疼痛
肉眼血பைடு நூலகம்+血块
需输血
脱发
无
轻度
显著或完全脱发
-
-
肺
无
无症状,但有肺功能异常
用力活动后呼吸困难
一般活动后呼吸困难
休息时呼吸困难
疼痛
正常
轻度疼痛:不影响功能
中度疼痛:疼痛或用止痛药,影响功能,不影响日常活动
严重疼痛:疼痛或用止痛药,严重影响日常活动
病残
毒性
分数
0
1
2
3
4
心律失常
无
无症状,一过性不需治疗
经常发生或持久的,但不需治疗
严重(如KPS评分下降大于40%)或不能进行日常活动
卧床或病残
指、趾甲变化
无
变色或凹甲
部分或完全缺失或甲床疼痛
-
-
低钠血症
正常
<LLN-130mmol/L
-
120 -<130
mmol/L
<120mmol/L
低磷血症
正常
<LLN-0.8mmol/L
0.6 -<0.8 mmol/L
0.3 -<0.6
mmol/L
<0.3 mmol/L
疲劳
无
比化疗前加重但不影响正常活动
中度(如KPS评分下降大于20%)或致日常活动困难
需治疗
需监护,或低血压,或室性心动过速或颤动
心功能
无
无症状,静息时LVEF比化疗前降低<20%
无症状,静息时LVEF比化疗前降低≥20%
轻度慢性心功能衰竭治疗有效
严重或难治性慢性心功能衰竭
心肌缺血/心肌梗塞
无
非特异性T波变平
无症状,ST及T波改变提示缺血
心绞痛,但无心梗证据
急性心肌梗塞
高血压
无或无变化
>20.0×N
肝功能
(临床)
与疗前比
无变化
-
-
肝性昏迷前状态
肝性昏迷
肌酐
正常
<1.5×N
1.5-3.0×N
>3.1-6.0×N
>6.0×N
蛋白尿
无变化
+
或<0.3g%或<3g/L
++~+++
或0.3-1.0g%或3-10g/L
++++
或>1.0g%或>10g/L
肾病综合症
血尿
阴性
镜下血尿
肉眼血尿无血块
轻度无力,无明显功能障碍
检查肌无力伴功能障碍
麻痹
神经-皮质
无
轻度嗜睡或燥动
中度嗜睡或燥动
严重嗜睡,燥动定位障碍,幻觉
昏迷,发作性精神失常
神经-小脑
无
轻度共济运动失调或轮替运动障碍
意向性震颤,辩距障碍,口齿不清,眼球震颤
共济失调
小脑坏死
神经-情绪
轻度焦虑或抑郁
中度焦虑或抑郁
严重焦虑或抑郁
自杀意向
神经性头痛
无或无变化
血压降低不需治疗(包括一过性体位性低血压)
需扩容治疗或其它治疗,但不需住院
需治疗或住院但48小时内好转
停药后需治疗或住院>48小时
神经-感觉
无或无变化
轻度感觉异常,深腱反射消失
轻度或中度客观感觉消失或中度感觉异常
严重的客观感觉消失或感觉异常,影响功能
-
神经-运动
无或无变化
主观感觉异常但常规检查无异
≥5.1×N
高钙血症
正常
>ULN - 2.9
mmol/L
>2.9-3.1 mmol/L
>3.1-3.4 mmol/L
>3.4 mmol/L
低钙血症
正常
<LLN-2.0mmol/L
1.75-<2.0 mmol/L
1.5 -<1.75
mmol/L
<1.5 mmol/L
高钾血症
正常
>ULN-5.5mmol/L
血清病支气管痉挛需治疗(静脉)
过敏反应
非感染性发热
无
37.1-38.0℃
38.1-40.0℃
>40.0℃
不超过24小时
>40.0℃超过24小时或发热伴低血压
局部*
无
疼痛
疼痛、肿胀
静脉炎
溃疡
需整形术
体重增加/减轻
<5.0%
5.0-9.9%
10.0-19.9%
≥20.0%
-
高血糖
正常
>ULN**-8.9
NCI常见毒性分级标准(3)
毒性
分数
0
1
2
3
4
神经-视力
无或无变化
-
-
有症状,视力不全丧失
失明
皮肤
无或无变化
散在斑疹、丘疹、红斑,但无症状
散在斑疹、丘疹红斑,伴搔痒或其它相关症状
有症状的全身性斑疹、丘疹或疱疹
剥脱性皮炎或溃疡性皮炎
过敏(包括药物热)
无
一过性皮疹,药物性发热<38℃
荨麻疹,药物性发热≥38℃,轻度支气管痉挛
mmol/L
>8.9-13.9 mmol/L
>13.9-27.8 mmol/L
>27.8 mmol/L或酮症酸中毒
低血糖
正常
<LLN***- 3.0 mmol/L
2.2 -<3.0 mmol/L
1.7 -<2.2 mmol/L
<1.7 mmol/L
淀粉酶
正常
<1.5×N
1.5-2.0×N
2.1-5.0×N
>5.5 - 6.0 mmol/L
>6.0 - 7.0 mmol/L
>7.0 mmol/L
低钾血症
正常
<LLN-3.0mmol/L
-
2.5 -<3.0
mmol/L
<2.5 mmol/L
高钠血症
正常
>ULN-150mmol/L
>150–155 mmol/L
>155 - 160
mmol/L
>160mmol/L
无
轻度
中度或严重但一过性
严重且持续
-
神经-便秘
无或无变化
轻度
中度
严重
肠绞痛>96小时
神经-听力
无或无变化
无症状听力测定时有丧失
耳鸣
听力下降需助听器
耳聋,不可纠正
NCI(National Cancer Institute)常见毒性分级标准(2)
局部=注射部位的反应;** ULN =正常值高限;*** LLN =正常值低限
NCI(National Cancer Institute)常见毒性分级标准(Common Toxicity Criteria)(1)
毒性
分数
0
1
2
3
4
WBC
≥4.0
3.0-3.9
2.0-2.9
1.0-1.9
<1.0
PLT
正常范围内
75.0-正常
50.0-74.9
25.0-49.9
<25.0
HB
正常范围内
10.0-正常
8.0-9.9
6.5-7.9
<6.5
粒细胞
≥2.0
1.5-1.9
1.0-1.4
0.5-0.9
<0.5
出血
(临床)
无
轻度、无需输血
明显,每次需输血小板1-2单位
明显,每次需输血小板3-4单位
大量,每次需输血小板4单位
感染
无
轻度
中度
严重
危及生命
恶心
无
能吃,食欲正常
食欲明显下降但能进食
不能明显进食
无症状,舒张压一过性升高>20mmHg既往正常血压升至>150/100mmHg,不需治疗
经常出现或持续出现或有症状,舒张压升高>20mmHg或既往正常,血压>150/100mmHg,不需治疗
需治疗
高血压危象
心包炎
无
无症状性积液不需治疗
心包炎(肋骨、胸痛ECG改变)
有症状的积液需抽水
心包填塞需急抽液
低血压
疼痛性红斑水肿或溃疡,但能进食
疼痛性红斑水肿或溃疡,不能进食
需胃肠外或胃肠支持治疗
胆红素
正常
-
<1.5×N
1.5-3.0×N
>3.0×N
转氨酶
(AST/ALT)
正常
≤2.5×N
2.6-5.0×N
5.1-20.0×N
>20.0×N
AKP或5-核苷酸酶
正常
≤2.5×N
2.6-5.0×N
5.1-20.0×N
