有机化学方面的专业英语.pdf
(完整word版)有机化学专业英语

(完整word版)有机化学专业英语烷基Alkyl [ˈælkil ]芳基aryl [ˈæril ]甲基methyl [ˈmeθil]亚甲基methylene [ˈmeθili:n ]乙基ethyl [ˈeθil,ˈeθəl]丙基propyl [ˈprəupil]异丙基isopropyl [ˌaisəuˈprəupil]丁基butyl [ˈbju:til]戊基pentyl [ˈpentil]己基hexyl [ˈheksil]庚基heptyl [ˈheptil]辛基octyl [ˈɔktəl]壬基nonyl [ˈnɔnil]奎基decyl [’desəl][di:’s i l]叔丁基tert-butyl异丁基iso-butyl环戊基cyclopentyl []环己基cyclohexyl []甲氧基methoxyl [’metɒksɪl]乙氧基ethoxyl [eˈθɔksil]丁氧基butoxyl酰基 acyl [ˈæsil]甲酰基formyl [ˈfɔ:mil]乙酰基acetyl [ˈæsitil]乙烯基vinyl [ˈvaɪnəl]或ethenyl丁烯基butenyl [ˈbjutənil]己烯基hexenyl庚烯基heptenyl [ˈheptəˌnil]烯丙基allyl [ˈælil]乙炔基ethinyl [eˈθainil]或alkynyl硝基nitro [ˈnaitrəu]亚硝基nitroso [naiˈtrəusəu]氨基amino [əˈmi:nəʊ,ˈæməˌnəʊ] 二氨基diamino亚氨基imino [ˈiminəu,iˈmi:nəu]重氮基diazo [daiˈæzəu]苯基phenyl [ˈfenəl,ˈfi:nəl,ˈfi:nil] 苄基benzyl [ˈbenzil]或phenmethyl [ˌfinˈmeθil]苯乙基phenethyl [fenˈeθəl]乙氧苯基ethoxyphenyl 苯胺基anilino [ˈænili:n]羰基carbonyl [ˈkɑ:bənil]羧基carboxyl [kɑ:ˈbɔksil]联苯基biphenyl [baiˈfenl]甲酰基formyl [ˈfɔ:mil]苯酰,苯甲酰benzoyl [’benzəʊɪl]脒基guanyl [il]羟基hydroxyl [haiˈdrɔksil]烷氧基alkoxy [ælˈkɔksi]或alkoxyl group芳基 aryl group二芳基diaryl group [daiˈæril]吡啶基pyridyl [ˈpiridil]三苯甲基trityl[’traɪtl]二苯甲基benzhydryl [benaɪd’raɪl]氨基甲酰基carbamoyl[kɑ:'bæməɪl]三甲基硅基trimethylsilyl炔丙基propargyl [prəʊ’pɑ:dʒɪl]丙酮基(乙酰甲基)acetonyl ['æsɪtənɪl]正n,normal异iso邻位ortho—[ˈɔ:θəu]间位meta- ['mɛtə]对位para—[ˈpɑ:rə]伯Primary [’praimәri]仲Secondary [’sekәndәri]叔Tertiary ['tә:ʃәri] tert-季碳quaternary [kwəˈtə:nəri] carbon一,单mono-二di-,双bis ,bi(化学中只有碳酸氢根才用bi,如bicarbonate [baiˈkɑ:bənit])三tri-,tris四tetra- 四quadric-五penta—五quinque—六hexa—七hepta—七septi八octa-九nona—十deca- [’dɛkə]十一undeca ,hendeca-十二dodeca-十三trideca-十四tetradeca(完整word版)有机化学专业英语十五pentadeca-十六hexadeca—十七heptadeca-顺式,cis—同,共syn反式trans有机化合物类名Aliphatic compound 脂肪族化合物[]Hydrocarbon 碳氢化合物[ˌhaɪdr əˈkɑ:b ən ]Alkane 烷[]Wax 蜡[]Paraffin wax 石蜡arene 芳烃[]Alkene 烯[]Alkyne 炔[ˈælkain]Acetylide 炔化物[]Active hydrogen compounds 活泼氢化合物acid [ˈæsid]Carbon acid 碳氢酸Super acid 超酸Diene 双烯[ˈdaii:n]Triene 三烯[ˈtraii:n ]Allene 丙二烯[ˈæli:n]Propylene丙烯[]cumulene 累积多烯[]Enyne 烯炔[eˈni:n]Diyne 二炔Alkyl halide 卤代烷[ˈælkil ˈhælaid]Alcohol 醇[]Homoallylic alcohol 高烯丙醇Ether 醚[ˈi:θə]Ester 酯[ˈestə]Ketone 酮Aldehyde 醛[ˈældihaid]Epoxide 环氧化物[eˈpɔksaid]Sulfone 砜[ˈs ʌlf əun]Sulfoxide 亚砜Sulfonic acid 磺酸Carboxylic acid 羧酸Cellosolve 溶纤剂Crown ether 冠醚Nitro compound 硝基化合物Amine 胺[]Quaternaryammonium compound 季铵化合物[][]Amine oxide 氧化胺Diazoalkane 重氮烷[daɪ,æzəʊ’ælkeɪn]Mercaptan 硫醇[]Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛[ˌhemiˈæsitæl]Acetal 缩醛acetal [化]乙缩醛, 乙缩醛二乙醇[ˈæsitæl] Ketal 缩酮[ˈki :tæl]thiazole 噻唑[ˈθai əˌzəul]Dithiane 二噻烷[daiˈθai ən]Aminal 缩醛胺;动物imine 亚胺[]Aldimine 醛亚胺Oxime 肟[]nitroso compound 亚硝基化合物aldoxime 醛肟,乙醛肟[ælˈdɔksi:m]Hydrazone 腙[ˈhaidrəˌzəun]Azine 嗪[ˈæzi:n]Semicarbazone 缩氯基脲Cyanohydrin 羟腈, 氰醇[ˌsaiənəuˈhaidrin] Pinacol 频哪醇Enol 烯醇[ˈi:nɔl]Enol ether 烯醇醚Enol ester 烯醇酯[ˈi:nɔl][ˈest ə] Enamine 烯胺[i ˈn æmin]Ynamine 炔胺Mannich base 曼尼希碱orthoester 原酸酯Acyl halide 酰卤[ˈæsil]Acyl fluoride 酰氟[] Acyl chloride 酰氯Acyl bromide 酰溴Acyl iodide 酰碘[ˈaiədaid]Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮[ˈki:ti:n]Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈[ˈnaitrail](完整word版)有机化学专业英语acetonitrile 乙腈[ˌæsitəuˈnaitril]或met hyl cyanide [ˈsaɪəˌnaɪd]Nitrile oxide 氧化腈Isonitrile 异腈,异氰化物Amide 酰胺[ˈæmaid]Imide 二酰亚胺[ˈimaid]N—bromo compound N—溴化物Hydrazide 酰肼[]Azide 叠氮化物[ˈæzaid,ˈeizid]Acyl azide 酰基叠氮[ˈæsil][ˈæzaid,ˈe izid]Amidine 脒[ˈæmiˌdi:n]Keto ester 酮酸酯Acyl cyanide 酰腈[ˈæsil][ˈsaɪəˌnaɪd]Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯[ˈkɑ:bəmeit]Urea 脲,尿素[]Cyanamide 氨腈[saiˈænəmaid]Carbodiimide 碳二亚胺[,kɑ:bədai'imaid] Allophanate 脲基甲酸酯Thioester 硫代酸酯[ˌθaiəuˈestə]Thiol acid 硫羰酸[ˈθaiəu]Lactone 内酯[ˈlæktəun]Lactol 内半缩醛[ˈlæktəl]Macrolide 大环内酯[ˈmækrəlaid]Amino acid 氨基酸Zwitterion两性离子[ˈtsvitəraiən]Inner salt 内盐Betaine 甜菜碱[ˈbi:təi:n]Lactam 内酰胺[ˈlæktæm]Hydantoin 或glycolylurea 乙内酰脲[haiˈdæntəwin]Hydration水合,水合作用[haɪ'dreʃən] Peptide 肽[ˈpepˌtaɪd]Glycol 乙二醇[]Aldol 羟醛[ˈældəul]Acyloin 偶姻,酮醇[əˈsiləuin]acyloin condensation 酮醇缩合Carbohydrate 碳水化合物Aldose 醛糖[ˈældəus]Ketose 酮糖[ˈki:təus]Furanose 呋喃糖[ˈfjuərəˌnəus]Pyranose 吡喃糖[ˈpaiərənəus]Glycoside 糖苷[ˈɡlaikəˌsaid]Glucoside 葡[萄]糖苷Aglycon 苷元[əˈɡlaikɔn]Saccharide 糖类[ˈsækəraid]Oligosaccharide 寡糖[ˌɔliɡəuˈsækəraid] Polysaccharide 多糖[pɔliˈsækəraid]Alditol 糖醇[ˈælditɔl]Osazone 脎[ˈəusəˌzəun]Alicyclic compound 脂环化合物[æliˈsiklik]Cycloalkane 环烷Cycloalkene 环烯Spirane 螺烷[ˈspaiərein]Cage compound 笼型化合物Propellane 螺桨烷Rotazane 轮烷Catenane 索烃[ˈkætnein ]Fused ring 稠环[fju:zd riŋ]化学专业英语词汇常用前后缀—acetal 醛缩醇acetal—乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 丙烯基'alkoxy- 烷氧基Methoxy甲氧基的-amide 酰胺[]amino- 氨基的[əˈmi:nəʊ,ˈæməˌnəʊ]-amidine 脒[ˈæmiˌdi:n]—amine 胺—ane 烷anhydride 酐[ænˈhaidraid]anilino- 苯胺基[ˈænili:n]aquo—含水aqueous水的,水成的[ˈeikwiəs]-ase 酶—ate 含氧酸的盐、酯-atriyne 三炔azo- 偶氮[ˈæzəu]azoxy—氧化偶氮—N=N(O)—(完整word版)有机化学专业英语hydrazo—氢化偶氮 -NH-NH—benzene 苯[ˈbenˌzi:n, benˈzi:n] bi —在盐类前表示酸式盐bis- 双-borane 硼烷[ˈbəurein]bromo—溴butyl 丁基.—carbinol 甲醇carbonyl 羰基-caboxylic acid 羧酸centi- 10-2chloro—氯代cis—顺式condensed 缩合的、冷凝的cyclo- 环deca—十deci 10—1di二-dine 啶dodeca- 十二—ene 烯epi—表epoxy- 环氧[]-ester 酯—ether 醚ethoxy- 乙氧基[]ethyl 乙基fluoro—或fluor—氟代—form 仿—glycol 二醇hemi- 半hendeca—十一hepta- 七heptadeca- 十七hexa—六hexadeca—十六-hydrin 醇hydro—氢或水hydroxyl 羟基hypo—低级的,次-ic 酸的,高价金属-ide 无氧酸的盐,酰替胺,酐-il 偶酰—imine 亚胺/iodine 碘[] iodo—碘代iso—异,等,同-ite 亚酸盐keto—酮ketone 酮—lactone 内酯mega —106meta- 间,偏methoxy—甲氧基methyl 甲基micro—10-6milli- 10-3mono—( mon—) 一,单nano- 10-9nitro- 硝基nitroso—亚硝基nona- 九nonadeca—十九octa- 八octadeca —十八-oic 酸的-ol 醇9 a $f! Q, H: [5 n& G—one 酮ortho—邻,正,原—ous 亚酸的,低价金属oxa- 氧杂—oxide 氧化合物-oxime 肟[]oxo- 酮[]oxy- 氧化[]-oyl 酰para—对位,仲penta- 五pentadeca- 十五per- 高,过petro- 石油phenol 苯酚[ˈfi:nəl]phenyl 苯基[]pico—10—12poly—聚,多(完整word版)有机化学专业英语quadri- 四quinque- 五semi- 半septi- 七sesqui 一个半sulfa—磺胺[]sym- 对称syn —顺式,同,共ter—三—tetra- 四tetradeca—十四tetrakis—四个thio- 硫代[]trans- 反式,超,跨tri- 三trans- 反式,超,跨tri- 三trideca- 十三tris- 三个undeca- 十一。
有机化学方面的专业英语

Paramagnetic ring current 顺磁环电流 Diamagnetic ring cruuent 抗磁环电流 Homoaromaticity 同芳香性 Antiaromaticity 反芳香性 Alternant hydrocarbon 交替烃 Non-alternant hydrocarbon 非交替烷 Pericyclic reaction 周环反应 Electrocyclic rearrangement 电环[化]重排 Conrotatory 顺旋 Disroatatory 对旋 Cycloaddition 环加成 Symmetry forbidden-reaction 对称禁阻反应 Synfacial reaction 同面反应 Antarafacial reaction 异面反应 Mobius system 默比乌斯体系 Leois structure 路易斯结构 Coordinate-covalent bond 配位共价键 Banana bond 香蕉键 Pauling electronegativity scale 鲍林电负性标度 Polarizability 可极化性 Inductive effect 诱导效应 Field effect 场效应 Electrical effect 电场效应 tautomerism 互变异构 Tautomerization 互变异构化 Keto-enol tautomerism 酮-烯醇互变异构 Phenol-keto tautomerism 酚-酮互变异构 Imine-enamine atutomerism 亚胺-烯胺互变异构 Ring-chain tautomerism 环-链互变异构 Valence tautomerism 价互变异构 Ambident 两可[的] Solvent effect 溶剂效应 Acid-base catalyxed reaction 酸性溶剂 Basic solvent 碱性溶剂 Dielectric constant 介电常数 Solvated electron 溶剂化电子 Acid-base catalyzed reaction 酸碱催化反应
有机化学专业词汇-英语

有机化学专业词汇Route [`raut]【路线】Strategy [’strætidʒi]【策略】Rearrangement [ˏri:ә'reindʒmәnt]【重排】Asymmetric ['eisi'metrik]【不对称】Migration [mai'greiʃәn]【迁移】Mechanism [’mekәnizәm]【机理】Substrate [’sʌbstreit]【底物、培养基】Synthesis [’sinθisis]【合成】Labile [’leibail]【不稳定的】Azeotropic [әˏzi:ә’trɔpik]【共沸的】Scheme [ski:m]【图示】Derivative [di’rivәtiv]【衍生物】Analogous [ә'nælәgәs]【类似物】Primary ['praimәri]【伯—】Secondary ['sekәndәri]【仲-】Tertiary ['tә:ʃәri]【叔-】Functional ['fʌŋkʃәnl] group【官能团】Catalyst [`kætәlist] 【催化剂】Catalysis [kә`tælisis]【催化(作用)】Catalytical [kætә`litikәl] 【催化的】Hydrogenolysis [ˏhaidrәudʒә'nɔlәsis]【氢化】Hydrogenous [hai’drɔdʒinәs] 【含氢的】pericyclic [peri’saiklik] reaction【周环反应】transition [træn'ziʒәn] state 【过渡态】homolysis [hɔ'mɔlisis]【均裂】heterolysis [ˏhetә'rɔlisis] 【异裂】homogeneous [ˏhɔmәu'dʒi:njәs]【均相的】heterogeneous [ˏhetәrәu'dʒi:niәs] 【非均相的】Steric [’sterik] hindrance[`hindrәns] 【立体位阻】Lipophilic [ˏlipә`filik] 【亲脂性的】chiral [`kairәl]【手性的】electrophile [i`lektrәfail]【亲电试剂】nucleophilic [ˏnju:kliәu'filik]【亲核的】hydrolysis [hai`drɔlisis] 【水解(作用)】acidolysis [ˏæsi`dɔlisis] 【酸解(作用)】displacement [dis’pleismәnt]【取代】formalin ['fɔ:mәlin]【甲醛溶液】dilute [dai'lju:t]【稀释】dehydrate [di:'haidreit] 【脱水】dilation [dai’leiʃәn]【膨胀】saponification [sәpɔnifi’keiʃәn]【皂化】emulsification [iˏmʌlsifi'keiʃәn] 【乳化】reactant [ri:’æktәnt]【反应物】adduct [ә'dʌkt]【加合物】dissolve [di'zɔlv]【溶解】degradation [ˏdegrә'deiʃәn]【降解】mild [maild]【(反应)温和的】harsh [hɑ:ʃ]【(反应)苛刻的】racemic [rә’si:mik]【消旋的】substituent [sʌb'stitjuәnt]【取代基】conjugation [ˏkɔndʒu'geiʃәn]【偶和】equivalent [i'kwivәlәnt]【当量的】volatile [’vɔlәtail]【易挥发的】susceptible [sә'septәbl]【易受影响的】in large scale【大量】protease [’prәutieis]【蛋白酶】side chain【侧链】Impuritiy [im'pjuәriti]【杂质】Component [kәm'pәunәnt]【组分】Tare [tєә]【去皮】Syringe ['sirindʒ]【注射器】Concentrate [’kɔnsentreit]【浓缩】Aqueous [’eikwiәs]【水相(的)】Methodology [meθә'dɔlәdʒi]【方法学】Precursor [pri(:)'kә:sә] 【前体】Separation [sepә'reiʃәn]【分离】Purification [ˏpjuәrifi'keiʃәn]【纯化】Recrystallization [’rikristәlai'zeiʃәn]【重结晶】Crystalline [’kristәlain]【晶体】Distillation [ˏdisti'leiʃәn]【蒸馏】Sublimation [ˏsʌbli'meiʃәn]【升华】Microwave ['maikrәuweiv]【微波】Infrared ['infrә’red]【红外】ultraviolet [’ʌltrә’vaiәlit] active【紫外显色】isotope ['aisәutәup]【同位素】isomer ['aisɔmә]【同分异构体】Isomerization [aiˏsɔmәrai'zeiʃәn]【异构化】Transformation [ˏtrænsfә'meiʃәn]【转化】Stability [stә’biliti]【稳定性】Poisoned [’pɔiznd]【中毒的】Filter ['filtә]【过滤器】Filtration [fil'treiʃәn]【过滤】Filtrate ['filtreit]【滤液】Filter Cake【滤饼】Eluent [’eljuәnt]【淋洗液】Thin—layer-chromatography [ˏkrәumә'tɔgrәfi] TLC【薄层色谱】Preparative [pri'pærәtiv] TLC【制备薄层色谱】HPLC【高效液相色谱】LC—MS【液质联用仪】Protic [’prәutik]【质子的】Aprotic[ә'prәutik]【非质子的】Decompose [ˏdi:kәm'pәuz]【分解】Removal [ri'mu:vәl]【除去】Reduced [ri’dju:st] pressure 【减压】Vacuum ['vækjuәm] 【真空】Elimination [iˏlimi’neiʃәn]【消除】Addition [ә'diʃәn]【添加、加成】Ligand ['laigәnd]【配体】Stopper ['stɔpә]【塞子】Separatory/ addition/ Büchner funnel ['fʌnәl]【分液/加料/布什】column chromatography【柱层析】Run column【用柱层析分离】Stopcock ['stɔpkɔk] 【旋塞阀】Condenser [kәn'densә]【冷凝管】Chamber ['tʃeimbә]【(薄层用)展缸】Bench [bentʃ] 【实验台】Hood [hud]【通风橱】Rotavapor [ˏrәutә'veipә]【旋蒸】Evaporation [iˏvæpә'reiʃәn] 【蒸发】vacuum hose [hәuz]【真空管】silicon [’silikәn] oil bath 【(硅)油浴】sink [siŋk]【水槽】neutralize ['nju:trәlaiz] 【中和】stir bar 【搅拌子】Dry ice trap【干冰冷阱】Glovebox [glʌvә’bɔks]【手套箱】vacuum pump [pʌmp]【真空泵】spatula [’spætjulә]【刮刀】miscible ['misibl]【混溶的】Quench [kwentʃ]【淬灭】Purging [’pә:dʒiŋ]【鼓气】Titration [tai’treiʃәn]【滴定】Trituration [tritju’reiʃәn]【研磨】Dessicator ['desikeitә:]【干燥剂】Hygroscopic [ˏhaigrәu’skɔpik] 【吸湿的】Lachrymator [’lækriˏmeitә]【催泪剂】DI (de-ion) water【去离子水】Stoichiometric [ˏstɔikiәuˋmєtrik] 【化学量的】Volumetric [vɔlju’metrik]【测体积的】Weighing ['weiiŋ] paper【称量纸】Mechanical [mi’kænikl] stirring apparatus [ˏæpә’reitәs]【机械搅拌装置】heating mental ['mentl]【加热套】Eclipse [i’klips] 【使失色】Anomeric [’ænәˏmerik] Effect 【端基异构效应】Equatorial [ˏekwә’tɔ:riәl]【平伏(键)的】Axial [’æksiәl]【直立(键)的】Gauche [gәuʃ] 【构象】Thermodynamic ['θә:mәudai’næmik]【热力学的】Kinetic [kai’netik]【动力学的】Enantioselective [eˏnæntiәsi’lektiv]【对映选择性的】Diastereotopic [ˏdaiәstiәriә'tɔpik] 【非对映的】Chemoselectivity 【化学选择性】。
有机化学方面的专业英语

Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic 烯丙型[的]Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium ring 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical cation 自由基正离子Radical anion 自由基负离子Isomerism 异构[现象]Aci form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象] Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的] Heterotopic 异位[的]Enantiotopic 对映异位Diastereotopic 非对映异位[的] Configuration 构型Absolute configuration 绝对构型Chirality 手性Chiral 手性[的]Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的] Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L命名体系R-S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体Diastereomer 非对映[异构]体Epimer 差向异构体Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S基团Re face Re面Si face Si面Racemic mixture 外消旋混合物Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体Conformation 构象Conformational 构象分析Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skew con-formation 邻位交叉构象Staggered conformation 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropismer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram’s rube 克拉姆规则Prelog’rule普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Leois structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donof-acceptor complex, EDAcomplex 电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力F strain 前张力 B strain 后张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[的]Mechanism 机理Unimolecular nucleophilic 单分子亲核取代Bimolecular nucleophilic sub-stitution 双分子亲核取代Bimolecular nucleophilic substi-tution(with allylic rearrange-ment) 双分子亲核取代(含烯丙型重排)Internal nucleophilic substiru-tion 分子内亲核取代Aromatic nucleophilic substitu-tion 芳香亲核取代Unimolecular electrophilic sub-stitution 单分子亲电取代Bimolecular electrophilic substi-tution 双分子亲电取代Nucleophile-assisted unimolecu-lar electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugate base 单分子共轭碱消除Bimolecular elimination through the conjugate base 双分子共轭碱消除Bimolecular elimination with for-mation of a carbonyl group 双分子羰基形成消除Unimolecular acid-catalyzed acyl-oxygen cleavage 单分子酸催化酰氧断裂Bimolecular base-catalyzed acyl-oxygen cleavage 双分子碱催化酰氧断裂Unimolecular acid-catalyzed alkyl-oxygen cleavage 单分子酸催化烷氧断裂Bimllecular base-catalyzed al- kyl-oxygen cleavage 双分子碱催化烷氧断裂π-allyl complex mechanism π烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair 离子对Carbocation 碳正离子Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov’s rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromatic substitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性α-effect α-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proup assistance,anchimeric assistance 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化02.3有机化学反应Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化Decarboxylative nitration 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer 重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross-coupling reaction 交叉偶联反应1,4-addition 1,4-加成Conjugate addition 共轭加成Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入Carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成- elimination -消除Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应] Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应]Ring expansion,ring enlargement 扩环[反应] -ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2.4 有机化合物类名Aliphatic compound 脂肪族化合物Hpdrocarbon 碳氢化合物Alkane 烷Wax 蜡Paraffin wax 石蜡Alkene 烯Alkyen 炔Acetylide 炔化物Active hydrogen compounds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene 双烯Triene 三烯Allene 丙二烯Ccumulene 累积多烯Enyne 烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide 环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound 硝基化合物Amine 胺Quaternaryammonium com-pound 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfonic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛Detone 酮Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛Acetal 缩醛acetal [化]乙缩醛, 乙缩醛二乙醇Ketal 缩酮Dithiane 二噻烷Aminal 缩醛胺imine 亚胺Aldimine 醛亚胺Oxime 肟Aldimine 醛肟Oxime 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol 频哪醇Enol 烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Ynamine 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl rtomide 酰溴Acyl iodide 酰碘Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Isonitrile 异腈Amide 酰胺Imide 二酰亚胺N-bromo compound N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯Urea 脲Cyanamide 氨腈Carbodiimide 碳二亚胺Allophanate 脲基甲酸酯Thioester 硫代酸酯Thiol acid 硫羰酸Lactone 内酯Lactol 内半缩醛Macrolide 大环内酯Amino acid 氨基酸Zwitterions 两性离子Inner salt 内盐Betaine 甜菜碱Lactam 内酰胺Hydantion 乙内酰脲Peptide 肽Glycol 二醇Aldol 羟醛Acyloin 偶姻Carbohydrate 碳水化合物Aldose 醛糖Ketose 酮糖Furanose 呋喃糖Pyranose 吡喃糖Glycoside 糖苷Glucoside 葡[萄]糖苷Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 脎Alicyclic compound 脂环化合物Cycloalkene 环烷Spirane 环烯Cage compound 螺烷Propellane 笼型化合物Rotazane 螺桨烷Catenane 轮烷Rused ring 索烃活化剂的中英文名称Bis(2-ethylhexyl) sebacate (癸二酸二仲辛酯;癸二酸二2-乙基己酯)Zinc stearate (硬脂酸锌)Suberic acid (辛二酸)Adipic acid, (己二酸)Hexanedioic acid, (己二酸)Sebacic acid, dibutyl ester (癸二酸二丁酯)Abietic acid (松香酸)Lactic acid (乳酸)Poly(ethylene glycol) (聚乙二醇)Glycerol stearate (硬脂酸甘油酯)Imidazoline (咪唑啉,间二氮杂环戊烯)-Pinene (β-蒎烯,β-松油二环烯)βAdipic acid (脂肪酸)Butyl acetate (乙酸丁酯)Ethylene glycol butyl ether (乙二醇丁醚)Sebacic acid, (癸二酸)Decanedioic acid, (癸二酸)Ethylene glycol ethyl ether (乙二醇乙醚)2-Butenedioic acid (E)-, (2-丁烯二酸)Succinic acid, (琥珀酸,丁二酸)Ethylene glycol methyl ether (乙二醇甲醚)Acetyl acetate (乙酸乙酰脂)1H-Benzotriazole (1-H-笨并三唑)-Pinene (α-蒎烯,α-松香二环烯)αSalicylic acid (水杨酸)Iso-Propanol (异丙醇) Benzoic acid (苯甲酸)Ethanol (乙醇) Lysine (赖氨酸)Glutamic acid (谷氨酸,2-氨基戊二酸) Glyceroyl, (甘油酰)N,N,N',N'-Tetrakis-(2-hydroxypropyl)-ethylene-diamine(N,N,N,N-四(2-羟基丙基)乙烯二氨)Isoleucine, (异亮氨酸)Decamethylenedicarboxylic acid, disalicyloylhydrazide (Tris(2,3-dibromopropyl)isocyanurate(3(2,3-2溴丙基)异氰尿酸盐)3-(N-Salicyloyl)amino-1,2,4-triazole (3-(N-水杨酰)氨-1,2,4-三唑)Isocyanuric acid (异氰尿酸)Salicylamide (水杨酰胺)Polyethylene glycol (聚乙二醇)Diethylene glycol diethyl ether (二甘醇二乙醚,(一缩)二乙二醇二乙醚)Butyl carbitol (丁基卡必醇)Ethyl carbitol (乙基卡必醇)Methyl carbitol (甲基卡必醇)Ethylene glycol monobutyl ether (乙二醇单丁醚)Glutaric acid (戊二酸,谷酸)Succinic acid (琥珀酸,丁二酸)Citric acid (柠檬酸)Salicylic acid (水杨酸)Lactic acid (2-羟基丙酸,乳酸)Glycerin monostearate (甘油一硬脂酸)Pentaerythritol (季戊四醇)-(3,5-di-tert-butyl-4-hydroxy-phenyl)propionate] 四[β-(3,5-二叔丁基-4-羟基-苯基)丙酸酯βtetrakis[Dioctyl sebacate (癸二酸二辛酯)N-Methyl pyrrolidone, (N-甲基吡咯烷酮)Diethylene glycol ethyl ether (二甘醇乙醚)Propylene glycol (丙二醇)Octanedioic acid (辛二酸)Oleamide (油酸酰胺)[olamine 乙醇胺]2-Mercapto benzothiazole (2-巯基-苯并噻唑)Nonanedioic acid, (壬二酸)cis-9-Octadecenoic acid, (顺式-9-十八炭烯酸,油酸)Sebacic acid, uses (癸二酸)12-Hydroxy stearic acid (十二羟基硬脂酸)Phthalic acid (苯二甲酸)1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylphenyl)butane(1,1,3-三(2-甲基-4-羟基-5-叔丁基苯基)丁烷)1,3,5-Trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)benzene(1,3,5-三甲基-2,4,6-三(3,5-二叔丁基-4-羟基苯基)苯)1-Methyl-2-pyrrolidone, (1-甲基-2-吡咯烷酮)Carbonic acid, (碳酸)Phthalic acid, (苯二甲酸)Malic acid (苹果酸,羟基丁二酸)2,3-Dibromo-2-butene-1,4-diol (2,3-二溴-2-丙烯-1,4-二醇)Cetylpyridinium bromide (溴代十六烷基吡啶)Pentanedioic acid (戊二酸) pentanediol (戊二醇)pentanoic acid ( 戊酸) pentanol (戊醇) Butanedioic acid, (丁二酸)1,2-Dibromoethylbenzene (1,2-二溴乙基苯)Salicylic acid, (水杨酸)Stearic acid (硬脂酸)Pentanedioic acid (戊二酸)Maleic acid, (马来酸,失水苹果酸)Phthalic acid, (苯二甲酸)Tartaric acid, (酒石酸)Acetic acid, (乙酸)Polyoxyethylene octylphenol ether (聚氧乙烯辛基酚醚,聚氧化亚乙基辛基分醚)Ethanedioic acid, (乙二酸)Polyethylene glycol (聚乙二醇)Diethylene glycol butyl ether (二甘醇丁醚)Diethylene glycol monoethyl ether (二甘醇单乙醚)Ethylene glycol monobutyl ether (乙二醇单丁醚)Pentaerythritol, (季戊四醇)Diglycol, (二甘醇,一缩二乙二醇)Hexylene glycol (己二醇)Ethylene glycol, (乙二醇)Glycerol, (甘油,丙三醇)Cyclobutanediamine (环丁烷二胺)Dibromobutenediol (二溴丁二醇)Cyclohexanediamine (环己烷二胺)Succinamide (琥珀酰胺,丁二酸胺)Ethylenediamine, (乙二胺)Triethanolamine, (三乙醇胺)5-Aminoisophthalic acid (5-氨基间苯二甲酸)p-tert-Butylbenzoic acid (对叔丁基苯甲酸)Propionic acid, (丙酸)Benzoic acid, (安息香酸, 苯(甲)酸)Salicylamide (水杨酰胺)Aniline, (苯胺)Palmitic acid, (棕榈酸, 十六酸, 软脂酸)Glutamic acid, (谷氨酸) Glutaric acid (谷酸,戊二酸)Glycine, (甘氨酸,氨基乙酸)Malic acid (苹果酸,羟基丁二酸)Adipic acid, (己二酸)Diethanolamine (二乙醇胺)Triethylamine, (三乙胺)Malic acid (苹果酸)Oxalic acid, (草酸)Oleic acid, (油酸)Glutaric acid (谷氨酸)Sorbic acid (山梨酸) sorbic alcohol (山梨醇)=sorbit。
有机化学专业英语

烷基Alkyl [??lkil]芳基aryl [??ril]甲基methyl [?meθil]亚甲基methylene[?meθili:n]乙基ethyl [?eθil,?eθ?l]丙基propyl [?pr?upil]异丙基isopropyl [?ais?u?pr?upil]丁基butyl [?bju:til]戊基pentyl [?pentil]己基hexyl [?heksil]庚基heptyl [?heptil]辛基octyl [??kt?l]壬基nonyl [?n?nil]奎基decyl ['des?l]?[di:'s i l]?叔丁基tert-butyl异丁基iso-butyl环戊基cyclopentyl []环己基cyclohexyl []甲氧基methoxyl ['met?ks?l]?乙氧基ethoxyl [e?θ?ksil]丁氧基butoxyl 酰基 acyl[??sil]甲酰基formyl [?f?:mil]乙酰基acetyl [??sitil]乙烯基vinyl [?va?n?l]或ethenyl丁烯基butenyl [?bjut?nil]己烯基hexenyl庚烯基heptenyl [?hept??nil]烯丙基allyl [??lil]乙炔基ethinyl [e?θainil] 或alkynyl 硝基nitro [?naitr?u]亚硝基nitroso [nai?tr?us?u]氨基amino [??mi:n??, ??m??n??]二氨基diamino亚氨基imino [?imin?u,i?mi:n?u]重氮基diazo [dai??z?u]苯基phenyl [?fen?l,?fi:n?l,?fi:nil]苄基benzyl [?benzil] 或phenmethyl [?fin?meθil]苯乙基phenethyl [fen?eθ?l]乙氧苯基ethoxyphenyl苯胺基anilino [??nili:n]羰基carbonyl [?kɑ:b?nil]羧基carboxyl [kɑ:?b?ksil]联苯基biphenyl [bai?fenl]甲酰基formyl [?f?:mil]苯酰,苯甲酰benzoyl ['benz???l]脒基guanyl [il]羟基hydroxyl [hai?dr?ksil]烷氧基alkoxy [?l?k?ksi]或alkoxyl group芳基 aryl group二芳基diaryl group [dai??ril]吡啶基pyridyl[?piridil]三苯甲基trityl['tra?tl]二苯甲基benzhydryl [bena?d'ra?l]氨基甲酰基carbamoyl[kɑ:'b?m??l]三甲基硅基trimethylsilyl炔丙基propargyl [pr??'pɑ:d??l]丙酮基(乙酰甲基)acetonyl['?s?t?n?l]正n,normal异iso邻位ortho- [??:θ?u]间位meta- ['m?t?]对位para- [?pɑ:r?]伯Primary ['praim?ri]仲Secondary ['sek?nd?ri]叔Tertiary ['t?:??ri] tert-季碳quaternary [kw??t?:n?ri] carbon一,单mono-二di-,双bis ,bi(化学中只有碳酸氢根才用bi,如bicarbonate [bai?kɑ:b?nit])三tri-,tris四tetra- 四quadric-五penta- 五quinque-六hexa-七hepta- 七septi八octa-九nona-十deca- ['d?k?]十一undeca ,hendeca-十二dodeca-十三trideca-十四tetradeca十五pentadeca-十六hexadeca-十七heptadeca-顺式,cis-同,共syn反式trans有机化合物类名Aliphatic?compound?脂肪族化合物[]Hydrocarbon?碳氢化合物[?ha?dr??kɑ:b?n] Alkane?烷[]Wax?蜡[]Paraffin?wax?石蜡arene芳烃[]Alkene?烯[]Alkyne?炔[??lkain]Acetylide?炔化物[] Active?hydrogen?compounds?活泼氢化合物acid [??sid]Carbon?acid?碳氢酸Super?acid?超酸Diene?双烯[?daii:n]Triene?三烯[?traii:n]Allene?丙二烯[??li:n]Propylene丙烯[] cumulene?累积多烯[] Enyne?烯炔[e?ni:n]Diyne?二炔Alkyl?halide?卤代烷[??lkil ?h?laid]Alcohol?醇[]Homoallylic?alcohol?高烯丙醇Ether?醚[?i:θ?]Ester?酯[?est?]Ketone?酮Aldehyde?醛[??ldihaid]Epoxide?环氧化物[e?p?ksaid]Sulfone?砜[?s?lf?un]Sulfoxide?亚砜Sulfonic?acid?磺酸Carboxylic?acid?羧酸Cellosolve?溶纤剂Crown?ether?冠醚Nitro?compound?硝基化合物Amine?胺[] Quaternaryammonium?compound?季铵化合物[] [] Amine?oxide?氧化胺Diazoalkane?重氮烷[da?,?z??'?lke?n] Mercaptan?硫醇[] Aldehyde?hydrate?醛水合物Ketone?hydrate?酮水合物Hemiacetal?半缩醛[?hemi??sit?l]Acetal?缩醛acetal [化]乙缩醛,?乙缩醛二乙醇[??sit?l] Ketal?缩酮[?ki:t?l]thiazole噻唑[?θai??z?ul]Dithiane?二噻烷[dai?θai?n]Aminal?缩醛胺;动物imine?亚胺[]Aldimine?醛亚胺Oxime?肟[]nitroso compound?亚硝基化合物aldoxime?醛肟,乙醛肟[?l?d?ksi:m] Hydrazone?腙[?haidr??z?un]Azine?嗪[??zi:n]Semicarbazone?缩氯基脲Cyanohydrin?羟腈,氰醇[?sai?n?u?haidrin] Pinacol?频哪醇Enol?烯醇[?i:n?l]Enol?ether?烯醇醚Enol?ester?烯醇酯[?i:n?l][?est?]Enamine?烯胺[i?n?min]Ynamine?炔胺Mannich?base?曼尼希碱orthoester?原酸酯Acyl?halide?酰卤[??sil]Acyl?fluoride?酰氟[] Acyl?chloride?酰氯Acyl?bromide?酰溴Acyl?iodide?酰碘[?ai?daid]Carbobenzoxy?chloride?苄氧甲酰氯Acyl?tosylate?酰基对甲苯磺酸酐Ketene?乙烯酮[?ki:ti:n]Peracid?过酸Perester?过酸酯Acyl?peroxide?酰基过氧化物Nitrile?腈[?naitrail]acetonitrile 乙腈[??sit?u?naitril] 或methyl cyani de [?sa???na?d]Nitrile?oxide?氧化腈Isonitrile?异腈,异氰化物Amide?酰胺[??maid]Imide?二酰亚胺[?imaid]N-bromo?compound?N-溴化物Hydrazide?酰肼[]Azide 叠氮化物[??zaid,?eizid]Acyl?azide?酰基叠氮[??sil] [??zaid,?eizid]Amidine?脒[??mi?di:n]Keto?ester?酮酸酯Acyl?cyanide?酰腈[??sil] [?sa???na?d]Carbon?suboxide?二氧化三碳Glycidic?acid?环氧丙酸Carbammic?acid?氨基甲酸Carbamate?氨基甲酸酯[?kɑ:b?meit]Urea?脲,尿素[] Cyanamide?氨腈[sai??n?maid]Carbodiimide?碳二亚胺[,kɑ:b?dai'imaid] Allophanate?脲基甲酸酯Thioester?硫代酸酯[?θai?u?est?] Thiol?acid?硫羰酸[?θai?u]Lactone?内酯[?l?kt?un]Lactol?内半缩醛[?l?kt?l]Macrolide?大环内酯[?m?kr?laid] Amino?acid?氨基酸Zwitterion两性离子[?tsvit?rai?n] Inner?salt?内盐Betaine?甜菜碱[?bi:t?i:n]Lactam?内酰胺[?l?kt?m]Hydantoin?或glycolylurea 乙内酰脲[hai?d?nt?win]Hydration水合,水合作用[ha?'dre??n] Peptide?肽[?pep?ta?d]Glycol?乙二醇[] Aldol?羟醛[??ld?ul]Acyloin?偶姻,酮醇[??sil?uin]acyloin condensation 酮醇缩合Carbohydrate?碳水化合物Aldose?醛糖[??ld?us]Ketose?酮糖[?ki:t?us]Furanose?呋喃糖[?fju?r??n?us] Pyranose?吡喃糖[?pai?r?n?us]Glycoside?糖苷[?ɡlaik??said] Glucoside?葡[萄]糖苷Aglycon?苷元[??ɡlaik?n]Saccharide?糖类[?s?k?raid] Oligosaccharide?寡糖[??liɡ?u?s?k?raid] Polysaccharide?多糖[p?li?s?k?raid] Alditol?糖醇[??ldit?l]Osazone?脎[??us??z?un]Alicyclic?compound?脂环化合物[?li?siklik] Cycloalkane?环烷Cycloalkene?环烯Spirane? 螺烷[?spai?rein]Cage?compound?笼型化合物Propellane?螺桨烷Rotazane?轮烷Catenane?索烃[?k?tnein]Fused?ring?稠环[fju:zd ri?]化学专业英语词汇常用前后缀-acetal?醛缩醇?acetal-?乙酰?acid?酸?-al?醛?alcohol?醇?-aldehyde?醛?alkali-?碱?allyl?丙烯基?'?alkoxy-?烷氧基Methoxy甲氧基的?-amide?酰胺?[]amino-?氨基的[??mi:n??, ??m??n??]-amidine?脒?[??mi?di:n]-amine?胺?-ane?烷?anhydride?酐?[?n?haidraid]anilino-?苯胺基[??nili:n]aquo-?含水aqueous水的,水成的?[?eikwi?s] -ase?酶?-ate?含氧酸的盐、酯?-atriyne?三炔?azo-?偶氮?[??z?u]azoxy-氧化偶氮-N=N(O)-hydrazo-氢化偶氮-NH-NH-benzene?苯?[?ben?zi:n, ben?zi:n]bi-?在盐类前表示酸式盐bis-?双?-borane?硼烷?[?b?urein]bromo-?溴?butyl?丁基?.-carbinol?甲醇? carbonyl?羰基?-caboxylic?acid?羧酸?centi-?10-2?chloro-?氯代?cis-?顺式?condensed?缩合的、冷凝的? cyclo-?环?deca-?十deci?10-1?di二?-dine?啶?dodeca-?十二?-ene?烯?epi-?表epoxy-?环氧?[]-ester?酯-ether?醚?ethoxy-?乙氧基? [] ethyl?乙基?fluoro-或fluor-?氟代?-form?仿?-glycol?二醇?hemi-?半?hendeca-?十一?hepta-?七?heptadeca-?十七?hexa-?六?hexadeca-?十六?-hydrin?醇?hydro-?氢或水?hydroxyl?羟基?hypo-?低级的,次?-ic?酸的,高价金属?-ide?无氧酸的盐,酰替?胺,酐-il?偶酰?-imine?亚胺?/iodine?碘[]iodo-?碘代?iso-?异,等,同?-ite?亚酸盐?keto-?酮?ketone?酮?-lactone?内酯?mega-?106?meta-?间,偏?methoxy-?甲氧基?methyl?甲基micro-?10-6?milli-?10-3mono-?(?mon-)?一,单? nano-?10-9?nitro-?硝基?nitroso-?亚硝基?nona-?九?nonadeca-?十九?octa-?八?octadeca-?十八?-oic?酸的?-ol?醇9?a$?f!?Q,?H:?[5?n&?G -one?酮?ortho-?邻,正,原?-ous?亚酸的,低价金属?oxa-?氧杂?-oxide?氧化合物?-oxime?肟?[] oxo-?酮?[]oxy-?氧化?[]-oyl?酰?para-?对位,仲?penta-?五?pentadeca-?十五?per-?高,过?petro-?石油?phenol?苯酚?[?fi:n?l]phenyl?苯基?[]pico-?10-12?poly-?聚,多?quadri-?四?quinque-?五?semi-?半?septi-?七?sesqui?一个半?sulfa-?磺胺?[] sym-?对称?syn-?顺式,同,共?ter-?三?-tetra-?四?tetradeca-?十四?tetrakis-?四个?thio-?硫代[] trans-?反式,超,跨?tri-?三?trans-?反式,超,跨? tri-?三?trideca-?十三?tris-?三个?undeca-?十一?.Alkylation?烷基化C-?alkylation?C-烷基化O-?alkylation?O-烷基化N-alkylation?N-烷基化Silylation?硅烷[基]化Exhaustive?methylation?彻底甲基化Seco?alkylation?断裂烷基化Demethylation?脱甲基化Ethylation?乙基化Arylation?芳基化Acylation?酰化Formylation?甲酰化Carbalkoxylation?烷氧羰基化Carboamidation?氨羰基化Carboxylation?羧基化Amination?氨基化Bisamination?双氨基化Cine?substitution?移位取代Transamination?氨基交换Hydroxylation?羟基化acyloxyation?酰氧基化Decarboxylative?nitration?脱羧卤化Allylic?halogenation?烯丙型卤化Dehalogenation?脱卤Nitration?硝化Decarboxylative?nitration?脱羧硝化Nitrosation?亚硝化Sulfonation?磺化Chlorosulfonation?氯磺酰化Desulfonation?脱磺酸基Sulfenylation?亚磺酰化Sulfonylation?磺酰化Chlorosulfenation?氯亚磺酰化Chlorocarbonylation?氯羰基化Diazotization?重氮化[dai??z?tai?zei??n] Diazo?transfer?重氮基转移Coupling?reaction?偶联反应uni-?单,一?unsym-?不对称的,偏位?-yl?基?-ylene?撑(二价基,价在不同原子上)? -yne?炔Diazonium?coupling?重氮偶联[?dai??z?uni?m]Cross-coupling?reaction?交叉偶联反应1,4-addition?1,4-加成C-C Pi-bond C-C π键Conjugate?addition?共轭加成[?k?nd???ge?t] Dimerization?二聚Trimefization?三聚Additive?dimerization?加成二聚Sulfurize 使硫化[]sulfurization?硫化Selenylation?硒化Hydroboration?硼氢化[?haidr?u?b?:?rei??n] Oxyamination?羟氨基化Insertion?插入carbonylation?羧基化Hydroformylation?加氢甲酰基化Hydroacylation?加氢酰化Oxo?process?羰基合成Decarbonylation?脱羰[di:?kɑ:b?n??l ei??n] Hydrocarboxylation?氢羧基化Homologization?同系化Cyanoethylation?氰乙基化Decyanoethylation?脱氰乙基Ring?closure?环合,闭环Diene?synthesis?双烯合成Dienophile?亲双烯体Endo?addition?内型加成Exo?addition?外型加成Diels-Alder?reaction?第尔斯-尔德反应Retro?Diels-Alder?reaction?逆第尔斯-阿尔德反应Ene?synthesis?单烯合成Anionic?cycloaddition?负离子环加成Dipolar?addition?偶极加成Dehydrohalogenation?脱卤化氢Deamination?脱氨基Pyrolytic?elimination?热解消除Elimination-addition?消除-加成Decarboxylation?脱羧Decarboxamidation?脱酰胺Decyanation?脱氰基Alkylolysis,alkyl?cleavage?烷基裂解Acylolysis,acyl?cleavage?酰基裂解Flash?pyrolysis?闪热裂Fragmentation?碎裂Chiletropic?reaction?螯键反应Chelation?螯环化Esterification?酯化Transesterification?酯交换Saponification?皂化Alcoholysis?醇解Ethanolysis?乙醇解Cyanomethylation?氰甲基化Aminomethylation?氨甲基化Hydroxymethylation?羟甲基化Hydroxyalkylation?羟烷基化Cholromethylation?氯甲基化Haloalkylation?卤烷基化Transacetalation?缩醛交换Enolization?烯醇化Haloform?reaction?卤仿反应Condensation?缩合Aldol?condensation?羟醛缩合Cross?aldol?condensation?交叉羟醛缩合Retrograde?aldol?condensation?逆羟醛缩合Acyloin?condensation?偶姻缩合Cyclization?环化Annulation,annelation?增环反应Spiroannulation?螺增环Autoxidation?自氧化Allylic?hydroperoxylation?烯丙型氢过氧化Epoxidation?环氧化Oxonolysis?臭氧解Electrochemical?oxidation?电化学氧化Oxidative?decarboxylation?氧化脱羧Aromatization?芳构化Catalytic?hydrogenation?催化氢化Heterogeneous?hydrogenation?多相氢化Homogeneous?hydrogenation?均相氢化Catalytic?dehydrogenation?催化脱氢Transfer?hydrogenation?转移氢化Hydrogenolysis?氢解Dissolving?metal?reduction?溶解金属还原Single?electron?transfer?单电子转移Bimolecular?reduction?双分子还原Electrochemical?reduction?电化学还原Reductive?alkylation?还原烷基化Reductive?acylation?还原酰化Reductive?dimerization?还原二聚Deoxygenation?脱氧Desulfurization?脱硫Deselenization?脱硒Mitallation?金属化Lithiation?锂化Hydrometallation?氢金属化Mercuration?汞化Oxymercuration?羟汞化Aminomercuration?氨汞化Abstraction?夺取[反应]Internal?abstraction?内夺取[反应] Rearrangement?重排Prototropic?rearrangement?质了转移重排Double?bond?migration?双键移位Allylic?migration?烯丙型重排Allylic?migration?烯丙型迁移Ring?contraction?环缩小[反应]Ring?expansion,ring?enlargement?扩环[反应] -ketol?rearrangement?-酮醇重排Pinacol?rearrangement?频哪醇重排Retropinacol?rearrangement?逆频哪醇重排Semipinacol?rearrangement?半频哪醇重排Benzilic?rearrangement?二苯乙醇酸重排Acyl?rearrangement?酰基重排Migratory?aptitude?迁移倾向Transannular?insertion?跨环插入Transannular?rearrangement?跨环重排Migration?迁移Prototropy?质子转移Cationotropic?rearrangement?正离子转移重排Anionotropy?负离子转移Anionotropic?rearrangement?负离子转移重Sigmatropic?rearrangement?-迁移重排Homosigmatropic?rearrangement?同迁移重排Electrophilic?rearrangement?亲电重排Photosensitization?光敏化Forbidden?transition?禁阻跃迁photooxidation?光氧化Photoisomerization?光异构化Photochemical?rearrangement?光化学重排Angular?methyl?group?角甲基Alkylidene?group?亚烷基[?l?kil??di:n] Methylene 亚甲基[?meθili:n]Allyl?group?烯丙基Allylic?烯丙型[的] [??l??lik]Phenyl?group?苯基[?fen?l,?fi:n?l,?fi:nil] Aryl?group?芳基Benzyl?group?苄基Benzylic?苄型[的]Activating?group?活化基团Chromophore?生色团[?kr?um?f?:] Auxochrome?助色团[??:ks?kr?um]Magnetically?anisotropic?group?磁各向异性基团[??nais?u?tr?pik]Smally?ring?小环Common?ring?普通环Medium?ring?中环[?mi:dj?m]Large?ring?大环Bridged-ring?system?桥环体系Spiro?compound?螺环化合物Helical?molecule?螺旋型分子Octahedral?compound?八面体化合物Conjugation?共轭[]Conjugated-system?共轭体系Acyl?cation?酰[基]正离子Benzylic?cation?苄[基]正离子[?k?tai?n]Arenium?ion?芳[基]正离子或aryl cationKetyl?radical?羰自由基Radical?ion?自由基离子[?ai?n]Radical?cation?自由基正离子Radical?anion?自由基负离子[??nai?n]Isomerism?异构[现象]Acid?form?酸式Fluxional?structure?循变结构Stereochemistry?立体化学Optical?activity?光学活性,旋光性Dextro?isomer?右旋异构体[]Laevo?isomer?左旋异构体[]Tetrahedral?configuration?四面体构型Stereoisomerism?立体异构[现象]Asymmetric?atom?不对称原子Asymmetric?carbon?不对称碳Pseudoasymmetric?carbon?假不对称碳Phantom?atom?虚拟原子[]Homotopic?等位[的]Heterotopic?异位[的]Enantiotopic?对映[异构体]的Diastereotopic?非对映异构体[的] [?dai?sti?ri??t?p ik]Configuration?构型[k?n?fiɡju?rei??n]Absolute?configuration?绝对构型Chirality?手性Chiral?手性[的] [英] [?t?ir?l][美] [?ka?r?l]Chiral?center?手性中心Chiral?molecule?手性分子Achiral?非手性[的] [ei'kair?l]Fischer?projection?费歇尔投影式Neoman?projection?纽曼投影式D-L?system?of?nomenclature?D-L命名体系R-S?syytem?of?nomenclature?R-S命名体系Cahn-Ingold-Prelon?sequence?顺序规则Symmetry?factor?对称因素Plane?of?symmetry?对称面Mirror?symmetry?镜面对称Enantiomer?对映[异构]体[]Diastereomer?非对映[异构]体[]Epimer?差向异构体[] Anomer?端基[差向]异构体Erythro?configuration?赤型构型Erythro?isomer?赤型异构体Threo?configuration?苏型构型Threo?isomer?苏型异构体Trigonal?carbon?三角型碳Cis-trans?isomerism?顺反异构E?isomer?E异构体Z?isomer?Z异构体Endo?isomer?内型异构体Exo?isomer?外型异构体Prochirality?前手性Pro-R?group?前R基团Pro-S?proup?前S基团Re?face?Re面Si?face?Si面Racemic?mixture?外消旋混合物[]Racemic?compound?外消旋化合物Racemic?solid?solution?外消旋固体溶液Meso?compound?内消旋化合物Quasi?recemate?准外消旋体[?kwɑ:zi(:),?kweisai] Conformation?构象Conformational?构象的Torsion?angle?扭转角Rotamer?旋转异构体Anti?conformation?反式构象Bisecting?conformation?等分构象Anti?periplanar?conformation?反叠构象Synperiplanar?conformation?顺叠构象Synclinal?conformation?反错构象Synclinal?conformation?顺错构象Eclipsed?conformation?重叠构象Gauche?conformation,?skew?con-formation?邻位交叉构象Staggered?conformation?对位交叉构象Steric?effect?空间效应[?sti?rik,?sterik]Steric?hindrance?位阻Atropismer?阻转异构体Puckered?ring?折叠环Conformational?inversion?构象反转Chair?conformation?椅型构象Boat?conformation?船型构象Twist?conformation?扭型构象Skew?boat?conformation?扭船型构象Half-chair?conformation?半椅型构象Pseudorotation?假旋转Envelope?conformation?信封[型]构象Axial?bond?直[立]键[??ksi:?l]Equatorial?bond?平[伏]键[?i:kw??t?:ri:?l, -?t??r-, ?ekw?-]Cisoid?conformation?顺向构象Transoid?conformation?反向构象Retention?of?configuration?构型保持Regioselectivity?区域选择性Regiospecificity?区域专一性Stereocelectivity?立体选择性Stereospecificty?立体专一性Conformer?构象异构体Conformational?effect?构象效应Cram’s?rube?克拉姆规则Prelog’rule?普雷洛格规则Stereochemical?orientation?立体[化学]取向Conformational?transmission?构象传递Homolog?同系物Ipso?position?本位Ortho?position?邻位Meta?position?间位Para?position?对位Amphi?position?远位Peri?position?近位Trigonal?hybridization?三角杂化Molecular?orbiral?method?分子轨道法Valence?bond?method?价键法Delocalezed?bond?离域键Cross?conjugation?交叉共轭Vinylog?插烯物Mesomeric?effect?中介效应Resonance?共振[?rez?n?ns] Resonance?effect?共振效应Hyperconjugation?超共轭Isovalent?hyperconjugation?等价超共轭No-bond?resonance?无键共振Aromaticity?芳香性Aromatic?sexter?芳香六隅Huckel’rule?休克尔规则Paramagnetic?ring?current?顺磁环电流Diamagnetic?ring?cruuent?抗磁环电流Homoaromaticity?同芳香性Antiaromaticity?反芳香性Alternant?hydrocarbon?交替烃Non-alternant?hydrocarbon?非交替烷Pericyclic?reaction?周环反应Electrocyclic?rearrangement?电环[化]重排Conrotatory?顺旋Disroatatory?对旋Cycloaddition?环加成Symmetry?forbidden-reaction?对称禁阻反应Synfacial?reaction?同面反应Antarafacial?reaction?异面反应Mobius?system?默比乌斯体系Leois?structure?路易斯结构Coordinate-covalent?bond?配位共价键Banana?bond?香蕉键Pauling?electronegativity?scale?鲍林电负性标度Polarizability?可极化性Inductive?effect?诱导效应Field?effect?场效应Electrical?effect?电场效应tautomerism?互变异构Tautomerization?互变异构化Keto-enol?tautomerism?酮-烯醇互变异构Phenol-keto?tautomerism?酚-酮互变异构Imine-enamine?atutomerism?亚胺-烯胺互变异构Ring-chain?tautomerism?环-链互变异构Valence?tautomerism?价互变异构Ambident?两可[的]Solvent?effect?溶剂效应Acid-base?catalyxed?reaction?酸性溶剂Basic?solvent?碱性溶剂Dielectric?constant?介电常数Solvated?electron?溶剂化电子Acid-base?catalyzed?reaction?酸碱催化反应Conjugate?base?共轭酸Conjugate?base?共轭碱Therm?odynamic?acidity?热力学酸度Kinetic?acidity?动力学酸度Electron?donof-acceptor?complex,EDAcomplex?电子给[体]受体络合物Host?主体Guest?客体Primary?isotope?effect?一级同位素效应Secondary?isotope?effect?二级同位数效应Inverse?isotope?effect?逆同位素效应Kinetic?control?动力学控制Thermodynamic?control?热力学控制Substrate?底物Intermediate?中间体Reactive?intermediate?活泼中间体Microscopic?reversibility?微观可逆性Hammond?postulate?哈蒙德假说Linear?free?energy?线性自由能Non-bonded?interaction?非键相互作用Torsional?effect?扭转效应Pitzer?strain?皮策张力Restricted?rotation?阻碍旋转Eclipsing?effect?重叠效应Eclipsing?strain?重叠张力Small-angle?strain?小角张力Large?angle?strain?大角张力Transannular?interaction?跨环相互作用Transannular?strain?跨环张力I?strain?内张力F?strain?前张力B?strain?后张力Anomeric?effect?端基异构效应Walden?inversion?瓦尔登反转Racemization?外消旋化Isoinversion?等反转Isoracemization?等消旋Homochiral?纯手性[的]Mechanism?机理Unimolecular?nucleophilic?单分子亲核取代Bimolecular?nucleophilic?sub-stitution?双分子亲核取代Bimolecular?nucleophilic?substi-tution(with?allylic?r earrange-ment)?双分子亲核取代(含烯丙型重排)Internal?nucleophilic?substiru-tion?分子内亲核取代Aromatic?nucleophilic?substitu-tion?芳香亲核取代Unimolecular?electrophilic?sub-stitution?单分子亲电取代Bimolecular?electrophilic?substi-tution?双分子亲电取代Nucleophile-assisted?unimolecu-lar?electrophilic?sub stitution?亲核体协助单分子亲电取代Unimolecular?elimination?单分子消除Bimolecular?elimination?双分子消除Unimolecular?elimination?through?the?conjugate?bas e?单分子共轭碱消除Bimolecular?elimination?through?the?conjugate?base?双分子共轭碱消除Bimolecular?elimination?with?for-mation?of?a?carbo nyl?group?双分子羰基形成消除Unimolecular?acid-catalyzed?acyl-oxygen?cleavage?单分子酸催化酰氧断裂Bimolecular?base-catalyzed?acyl-oxygen?cleavage?双分子碱催化酰氧断裂Unimolecular?acid-catalyzed?alkyl-oxygen?cleavage?单分子酸催化烷氧断裂Bimllecular?base-catalyzed?alkyl-oxygen?cleavage?双分子碱催化烷氧断裂π-allyl?complex?mechanism?π烯丙型络合机理Borderline?mechanism?边理机理Homolysis?均裂Heterolysis?异裂Heterolytic?michanism?异裂机理Counrer[gegen]ion?反荷离子Ion?pair?离子对Carbocation?碳正离子[?kɑ:b??kei??n]Nonclassical?carbocation?非经典碳正离子Carbanion?碳负离子[?kɑ:b?nai?n]Masked?carbanion?掩蔽碳负离子Carbenoid?卡宾体Carbene?卡宾[]Nitrene?氮宾[a] Carbine?碳炔[] Electrophilic?addition?亲电加成Electrophile?亲电体Diaxial?addition?双直键加成Markovnikov’s?rube?马尔科夫尼科规则Anti-Markovnikov?addition?反马氏加成Michael?addition?迈克尔加成Substitution?取代Electrophilic?substitution?亲电取代Addition-elimination?mechanism?加成消除机理[?mek?niz?m]Electrophilic?aromatic?substitu-tion?亲电芳香取代Electron?transfer?电子转移Electron-donating?group?给电子基团Electron-Withdrawing?group?吸电子基团Deactivating?group?钝化基团Orinentation?取向Ortho-para?directing?group?邻对位定位基Meta?directing?group?间位定位基Ortho?effect?邻位效应Partial?rate?factor?分速度系数Nucleophilic?reaction?亲核反应Internal?return?内返Nucleophilicity?亲核体Nucleophilicity?亲核性α-effect?α-效应Backside?attack?背面进攻Inversion?反转Umbrella?effect?伞效应Push-pull?effect?推拉效应Leaving?group?离去基团Electrofuge?离电体Nucleofuge?离核体Phase-transfer?catalysis?相转移催化Neighboring?group?participation?邻基基参与Neighboring?proup?assistance,anchimeric?assistance?邻助作用Neighboring?group?effect?邻基效应Apofacial?reaction?反面反应Briddgehead?displacement?桥头取代Aryl?action?芳正离子Benzyne?苯炔Zaitsev?rule?札依采夫规则Anti-Zaitsev?orientation?反札依采夫定向Hofmann’s?rule?霍夫曼规则Bredt?rule?布雷特规则Initiation?引发Anionic?cleavage?负离子裂解Partial?bond?fixation?键[的]部分固定化exothermic发热的,放出热量的[?eks?u?θ?:mik]各种反应类型Halogenations reaction卤化反应Hydrogenation reaction氢化反应Alkylation reaction烷基化(烃化)反应Hydrocarbylation 烃基化反应Oxidation and reduction reaction氧化还原反Reductive amination还原胺化反应Cross-coupling reaction交叉耦合反应Cycloaddition reaction环加成反应Rearrangement reaction重排反应Acylation reaction酰化反应Acetylization reaction乙酰化反应Amide reaction酰胺反应Sulfonylation磺酰化反应Nitration reaction硝化反应Esterification酯化反应Anhydride reaction酸酐反应Oximation reaction肟化反应Coupled(或coupling)reaction偶联反应1,3-dipolar cycloaddition 1,3-偶极环加成Pericyclic reaction周环反应Hydrolysis reaction 水解反应Ester hydrolysis 酯水解反应Hydrolytic-polymeric reaction水解聚合反应Dehydrogenation reaction脱氢反应Dehydrohalogenation reaction 脱卤化氢反应Dehydration reaction脱水反应Decarboxylation reaction脱羧反应Addition reaction加成反应Substitution reaction取代反应Cracking reaction 裂化反应Elimination reaction消除反应And metal response reaction与活泼金属反应Phase transfer catalytic reaction相转移催化反应Acid-base catalyzed reaction酸碱催化反应Polymerization reaction聚合反应Polycondensation reaction缩聚反应Condensation reaction 缩合反应Silver mirror reaction银镜反应Nucleophilic reaction亲核反应Electrophilic reaction亲电反应Nucleophilic cycloaddition reaction亲核环加成反应Nucleophilic substitution亲核取代反应Electrophilic substitution亲电取代反应Unimolecular electrophilic substitution单分子亲电取代反应Bimolecular electrophilic substitution双分子亲电取代反应Unimolecular elimination reaction单分子消除反应Bimolecular elimination reaction双分子消除反应Unimolecular nucleophilic substitution单分子亲核取代Bimolecular nucleophilic substitution双分子亲核取代反应Internal nucleophilic substitution分子内亲核取代Aromatic nucleophilic substitution reaction芳香亲核取代反应活化剂的中英文名称Bis(2-ethylhexyl)?sebacate?(癸二酸二仲辛酯;癸二酸二2-乙基己酯)Zinc?stearate?(硬脂酸锌)Suberic?acid?(辛二酸)Adipic?acid,?(己二酸)?Hexanedioic?acid,?(己二酸)Sebacic?acid,?dibutyl?ester?(癸二酸二丁酯)Abietic?acid?(松香酸)Lactic?acid?(乳酸)Poly(ethylene?glycol)?(聚乙二醇)Glycerol?stearate?(硬脂酸甘油酯)Imidazoline?(咪唑啉,间二氮杂环戊烯)-Pinene?(β-蒎烯,β-松油二环烯)Adipic?acid?(脂肪酸)Butyl?acetate?(乙酸丁酯)Ethylene?glycol?butyl?ether?(乙二醇丁醚)Sebacic?acid,?(癸二酸)Decanedioic?acid,?(癸二酸)Ethylene?glycol?ethyl?ether?(乙二醇乙醚)2-Butenedioic?acid?(E)-,?(2-丁烯二酸)Succinic?acid,?(琥珀酸,丁二酸)Ethylene?glycol?methyl?ether?(乙二醇甲醚)Acetyl?acetate?(乙酸乙酰脂)1H-Benzotriazole?(1-H-笨并三唑)?-Pinene?(α-蒎烯,α-松香二环烯)Salicylic?acid?(水杨酸)Iso-Propanol?(异丙醇)Benzoic?acid?(苯甲酸)Ethanol?(乙醇)Lysine?(赖氨酸)Glutamic?acid?(谷氨酸,2-氨基戊二酸)Glyceroyl,?(甘油酰)N,N,N',N'-Tetrakis-(2-hydroxypropyl)-ethylene-diami ne?(N,N,N,N-四(2-羟基丙基)乙烯二氨)Isoleucine,?(异亮氨酸)Decamethylenedicarboxylic?acid,?disalicyloylhydrazi de?(Tris(2,3-dibromopropyl)isocyanurate(3(2,3-2溴丙基)异氰尿酸盐)3-(N-Salicyloyl)amino-1,2,4-triazole?(3-(N-水杨酰)氨-1,2,4-三唑)Isocyanuric?acid?(异氰尿酸)Salicylamide?(水杨酰胺)Polyethylene?glycol?(聚乙二醇)Diethylene?glycol?diethyl?ether?(二甘醇二乙醚,(一缩)二乙二醇二乙醚)Butyl?carbitol?(丁基卡必醇)Ethyl?carbitol?(乙基卡必醇)Methyl?carbitol?(甲基卡必醇)Ethylene?glycol?monobutyl?ether?(乙二醇单丁醚)Glutaric?acid?(?戊二酸,谷酸)Succinic?acid?(琥珀酸,丁二酸)Citric?acid?(柠檬酸)Salicylic?acid?(水杨酸)Lactic?acid?(2-羟基丙酸,乳酸)Glycerin?monostearate?(甘油一硬脂酸)Pentaerythritol?(季戊四醇)tetrakis[?-(3,5-di-tert-butyl-4-hydroxy-phenyl)propion ate]?四[β-(3,5-二叔丁基-4-羟基-苯基)丙酸酯Dioctyl?sebacate?(癸二酸二辛酯)N-Methyl?pyrrolidone,?(N-甲基吡咯烷酮)Diethylene?glycol?ethyl?ether?(二甘醇乙醚)Propylene?glycol?(丙二醇?)Octanedioic?acid?(辛二酸?)Oleamide?(油酸酰胺)[olamine?乙醇胺]2-Mercapto?benzothiazole?(2-巯基-苯并噻唑)Nonanedioic?acid,?(壬二酸)cis-9-Octadecenoic?acid,?(顺式-9-十八炭烯酸,油酸)Sebacic?acid,?uses?(癸二酸)12-Hydroxy?stearic?acid?(十二羟基硬脂酸)Phthalic?acid?(苯二甲酸?)1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylphenyl)but ane(1,1,3-三(2-甲基-4-羟基-5-叔丁基苯基)丁烷)1,3,5-Trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxy benzyl)benzene(1,3,5-三甲基-2,4,6-三(3,5-二叔丁基-4-羟基苯基)苯)1-Methyl-2-pyrrolidone,?(1-甲基-2-吡咯烷酮)Carbonic?acid,?(碳酸)Phthalic?acid,?(苯二甲酸)Malic?acid?(苹果酸,羟基丁二酸)2,3-Dibromo-2-butene-1,4-diol?(2,3-二溴-2-丙烯-1,4-二醇)Cetylpyridinium?bromide?(溴代十六烷基吡啶)Pentanedioic?acid?(戊二酸)?pentanediol?(戊二醇)?pentanoic?acid?(?戊酸)?pentanol?(戊醇)Butanedioic?acid,?(丁二酸)1,2-Dibromoethylbenzene?(1,2-二溴乙基苯)Salicylic?acid,?(水杨酸)Stearic?acid?(硬脂酸)Pentanedioic?acid?(戊二酸)Maleic?acid,?(马来酸,失水苹果酸)Phthalic?acid,?(苯二甲酸)Tartaric?acid,?(酒石酸)Acetic?acid,?(乙酸)Polyoxyethylene?octylphenol?ether?(聚氧乙烯辛基酚醚,聚氧化亚乙基辛基分醚)Ethanedioic?acid,?(乙二酸)Polyethylene?glycol?(聚乙二醇)Diethylene?glycol?butyl?ether?(二甘醇丁醚)Diethylene?glycol?monoethyl?ether?(二甘醇单乙醚)Ethylene?glycol?monobutyl?ether?(乙二醇单丁醚)Pentaerythritol,?(季戊四醇)Diglycol,?(二甘醇,一缩二乙二醇)Hexylene?glycol?(己二醇)Ethylene?glycol,?(乙二醇)Glycerol,?(甘油,丙三醇)Cyclobutanediamine?(环丁烷二胺)Dibromobutenediol?(二溴丁二醇)Cyclohexanediamine?(环己烷二胺)Succinamide?(琥珀酰胺,丁二酸胺)Ethylenediamine,?(乙二胺)Triethanolamine,?(三乙醇胺)5-Aminoisophthalic?acid?(5-氨基间苯二甲酸)p-tert-Butylbenzoic?acid?(对叔丁基苯甲酸)Propionic?acid,?(丙酸)Benzoic?acid,?(安息香酸,?苯(甲)酸)Salicylamide?(水杨酰胺)Aniline,?(苯胺)Palmitic?acid,?(棕榈酸,?十六酸,?软脂酸)Glutamic?acid,?(谷氨酸)?Glutaric?acid?(谷酸,戊二酸)Glycine,?(甘氨酸,氨基乙酸)Malic?acid?(苹果酸,羟基丁二酸)Adipic?acid,?(己二酸)Diethanolamine?(二乙醇胺)Triethylamine,?(三乙胺)Malic?acid?(苹果酸)Oxalic?acid,?(草酸)Oleic?acid,?(油酸)Glutaric?acid?(谷氨酸)Sorbic?acid?(山梨酸)?sorbic?alcohol?(山梨醇)=sorbit?Reactant 反应物nProduct 产物nCatalyst 催化剂catalytic agent,catalyzerDegree Celsius摄氏度Sodium Borohydride硼氢化钠Lithium Aluminum Hydride (LAH)氢化铝锂Lithiumtrit-Butoxyaluminohydride?LiAlH(O t-C4H9)3叔丁氧基氢化铝锂Diisobutylaluminum Hydride AlH[CH2CH(CH3)2]2二异丙基氢化铝Diborane B2H6硼烷Reactive Metals 活泼金属如?Na, or Li, or K?,Mg or Al or Zn or Fe Potassium carbonate碳酸钾Sodium carbonate 碳酸钠Sodium bicarbonate 碳酸氢钠[]Sodium chloride氯化钠Sodium acetate 乙酸钠Sodium cyanide[?sa???na?d]氰化钠Sodium methoxide或Sodium methylate甲醇钠,甲氧基钠Sodium ethoxide[?s??di:?m i:?θ?ksaid]或Sodium ethylate[?s??di:?m ?eθileit]乙醇钠Sodium sulfate 硫酸钠Magnesium sulfate [m?g?ni:zi:?m ]硫酸镁Acetic acid乙酸Formic acid甲酸Ammonium format甲酸铵Formamide[f?:?m?mid]或formyl amide 甲酰胺formaldehyde甲醛[f?:?m?ld??ha?d]Methyl iodide碘甲烷Potassium iodide碘化钾[] Potassium chloride氯化钾Potassium cyanide[?sa???na?d]氰化钾Dimethyl sulfate硫酸二甲酯Palladium –carbon 钯碳Pd/C [p??leidi?m]Palladium chloride氯化钯Palladium diacetate醋酸钯Chloroacetone 氯丙酮Tetrabutyl ammonium bromide四丁基溴化铵Tetrabutyl ammonium fluoride四丁基氟化铵容器类:量杯 measuring cup烧杯 beaker 不锈钢杯stainless-steel beaker量筒 measuring flask/measuring cylinder 量筒graduated flask/measuring cylinder坩埚crucible 坩埚钳crucible clamp 坩埚crucible pot, melting pot试管 test tube 试管架 test tube holder漏斗 funnel 分液漏斗 separatory funnel烧瓶 flask 锥形瓶 conical flask塞子 stopper洗瓶 plastic wash bottle滴定管 burette玻璃活塞 stopcock冷凝器 condenser试剂瓶 reagent bottles玻棒 glass rod 搅拌棒stirring rod蒸馏烧瓶 distilling flask碘量瓶 iodine flask表面皿 watch glass蒸发皿 evaporating dish容量瓶 volumetric flask/measuring flask移液管 (one-mark) pipette 刻度移液管 graduated pipettes称量瓶weighing bottle吸液管pipette滤管filter天平 balance/scale分析天平 analytical balance台秤 platform balance游码 crossbeams and sliding weights酒精灯alcohol burner酒精喷灯blast alcohol burner搅拌装置 stirring device洗耳球 rubber suction bulb研磨钵 mortar 研磨棒 pestle 玛瑙研钵agate mortar瓷器 porcelain白细口瓶flint glass solution bottle with stopper滴瓶 dropping bottle 小滴管 dropper蒸馏装置distilling apparatus蒸发器 evaporator试验用器材:升降台lab jack铁架台iron support万能夹extension clamp蝴蝶夹double-buret clamp双顶丝clamp regular holder止水夹flatjaw pinchcock圆形漏斗架cast-iron ring移液管架pipet rack试管架tube rack沸石boiling stone橡胶管rubber tubing药匙lab spoon镊子forceps坩埚钳crucible tong剪刀scissor打孔器stopper borer石棉网asbestos-free wire gauze电炉丝wire coil for heater脱脂棉absorbent cottonphph试纸 universal ph indicator paper滤纸 filter paper称量纸weighing paper擦镜纸wiper for lens秒表stopwatch量杯glass graduates with scale白滴定管(酸) flint glass burette with glass stopcock棕色滴定管(酸) brown glass burette with glass stopcock白滴定管(碱) flint glass burette for alkali 棕色滴定管(碱) brown glass burette for alkali比重瓶specific gravity bottle水银温度计mercury-filled thermometerph计ph meter折光仪refractometer真空泵vacuum pump冷、热浴bath离心机centrifuge口罩respirator防毒面具respirator、gasmask磁力搅拌器magnetic stirrer电动搅拌器power basic stirrer烘箱oven闪点仪flash point tester马弗炉furnace电炉 heater微波炉电热套heating mantleBunsen burner 本生灯product 化学反应产物apparatus 设备PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂matrass 卵形瓶litmus 石蕊litmus paper 石蕊试纸burette 滴定管。
(完整版)有机化学专业英语词汇(精)

有机化学专业英语词汇(精)acetal 醛缩醇acetal- 乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 烯丙基 [propenyl(丙烯基)] alkoxy- 烷氧基-amide 酰胺amino- 氨基的-amidine 脒-amine 胺-ane 烷anhydride 酐anilino- 苯胺基aquo- 含水的-ase 酶-ate 含氧酸的盐、酯-atriyne 三炔azo- 偶氮benzene 苯bi- 在盐类前表示酸式盐bis- 双-borane 硼烷bromo- 溴butyl 丁基-carbinol 甲醇carbonyl 羰基-carboxylic acid 羧酸centi- 10-2chloro- 氯代cis- 顺式condensed 缩合的、冷凝的cyclo- 环deca- 十deci 10-1-dine 啶dodeca- 十二-ene 烯epi- 表epoxy- 环氧-ester 酯-ether 醚ethoxy- 乙氧基ethyl 乙基fluoro- 氟代form 仿-glycol 二醇hemi- 半hendeca- 十一hepta- 七heptadeca- 十七hexa- 六hexadeca- 十六-hydrin 醇hydro- 氢或水hydroxyl 羟基hypo- 低级的,次hyper- 高级的,高-ic 酸的,高价金属-ide 无氧酸的盐,酰替胺,酐-il 偶酰-imine 亚胺iodine 碘iodo- 碘代iso- 异,等,同-ite 亚酸盐keto- 酮ketone 酮-lactone 内酯mega- 106meta- 间,偏methoxy- 甲氧基methyl 甲基micro- 10-6milli- 10-3mono- ( mon-) 一,单nano- 10-9nitro- 硝基nitroso- 亚硝基nona- 九nonadeca- 十九octa- 八octadeca- 十八-oic 酸的-ol 醇-one 酮ortho- 邻,正,原-ous 亚酸的,低价金属oxa- 氧杂-oxide 氧化合物-oxime 肟oxo- 酮oxy- 氧化-oyl 酰para- 对位,仲penta- 五pentadeca- 十五per- 高,过petro- 石油phenol 苯酚phenyl 苯基pico- 10-12poly- 聚,多quadri- 四quinque- 五semi- 半septi- 七sesqui 一个半sulfa- 磺胺sym- 对称syn- 顺式,同,共ter- 三tetra- 四tetradeca- 十四tetrakis- 四个thio- 硫代trans- 反式,超,跨thio- 硫代tri- 三trideca- 十三tris- 三个undeca- 十一uni- 单,一unsym- 不对称的,偏位-yl 基-ylene 撑(二价基,价在不同原子上) -yne 炔organic compounds 有机化合物compounds of carbon 碳化合物hydrocarbons and their derivatives 碳氢化合物及其衍生物organic chemistry 有机化学structure of molecule 分子结构chemical bond 化学键covalent bond 共价键hybrid orbital 杂化轨道bond length 键长bond angle 键角bond energy键能polarity 极性dissociation energy 离解能constitution构造contiguration构型conformation构象stereochemistry立体化学tetrahedral正四面体cis-顺trans-反isomerism同分异构现象isomer异构体stereoisomer立体异构constitutional isomer构造异构structural formula 结构式octet八隅体perspective 透视式eclipsed conformation重叠式构象staggered conformation交叉式构象newman projection纽曼投影式functional group 官能团chain compoud 链状化合物carbocyclic compound碳环化合物heterocyclic compound杂环化合物dipole-dipole interactions 偶极-偶极相互作用van der Waals forces 范德华力hydrogen bonding 氢键dipole moment偶极矩electronegativity 电负性physical property物理性质melting point熔点boiling point 沸点reaction mechanism反应机理homolysis均裂free redical自由基heterolysis异裂ionic type离子型electrophilic reagent亲电试剂electrophilic reaction亲电反应nucleophilic reagent亲核试剂nucleophilic reaction 亲核反应英文名汉文名Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic 烯丙型[的]Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium rimg 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical cation 自由基正离子Radical anion 自由基负离子Isomerism 异构[现象]Aci form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象] Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的]Heterotopic 异位[的] Enantiotopic 对映异位[的] Diastereotopic 非对映异位[的] Configuration 构型Absolute configuration 绝对构型Chirality 手性Chiral 手性[的]Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的]Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L命名体系R-S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体Diastereomer 非对映[异构]体Epimer 差向异构体Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S基团Re face Re面Si face Si面Racemic mixture 外消旋混合物Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体Conformation 构象Conformational 构象分析Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skewcon-formation 邻位交叉构象Staggered conformation 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropismer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram’s rube 克拉姆规则Prelog’rule 普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule 休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Leois structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donof-acceptor complex,EDAcomplex 电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力F strain 前张力B strain 后张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[的] Mechanism 机理Unimolecular nucleophilic 单分子亲核取代Bimolecular nucleophilicsub-stitution 双分子亲核取代Bimolecular nucleophilicsubsti-tution(with allylic rearrange-ment) 双分子亲核取代(含烯丙型重排)Internal nucleophilicsubstiru-tion 分子内亲核取代Aromatic nucleophilicsubstitu-tion 芳香亲核取代Unimolecular electrophilicsub-stitution 单分子亲电取代Bimolecular electrophilicsubsti-tution 双分子亲电取代Nucleophile-assistedunimolecu-lar electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugate base 单分子共轭碱消除Bimolecular elimination through the conjugate base 双分子共轭碱消除Bimolecular elimination withfor-mation of a carbonyl group 双分子羰基形成消除Unimolecular acid-catalyzedacyl-oxygen cleavage 单分子酸催化酰氧断裂Bimolecular base-catalyzedacyl-oxygen cleavage 双分子碱催化酰氧断裂Unimolecular acid-catalyzedalkyl-oxygen cleavage 单分子酸催化烷氧断裂Bimllecular base-catalyzed al- kyl-oxygen cleavage 双分子碱催化烷氧断裂π-allyl complex mechanism π烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair 离子对Carbocation 碳正离子Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov’s rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromaticsubstitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性α-effect α-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proupassistance,anchimeric assistance 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化02.3有机化学反应Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化Decarboxylative nitration 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer 重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross-coupling reaction 交叉偶联反应1,4-addition 1,4-加成Conjugate addition 共轭加成Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成- elimination -消除- elimination -消除- elimination -消除-elimination -消除Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应]Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应] Ring expansion,ring enlargement 扩环[反应]-ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2.4 有机化合物类名Aliphatic compound 脂肪族化合物Hpdrocarbon 碳氢化合物Alkane 烷Wax 蜡Paraffin wax 石蜡Alkene 烯Alkyen 炔Acetylide 炔化物Active hydrogen compounds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene 双烯Triene 三烯Allene 丙二烯Ccumulene 累积多烯Enyne 烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide 环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound 硝基化合物Amine 胺Quaternaryammonium com-pound 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfonic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛Detone 酮Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛Acetal 缩醛Ketal 缩酮Dithiane 二噻烷Aminal 缩醛胺imine 亚胺Aldimine 醛亚胺Oxime 肟Aldimine 醛肟Oxime 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol 频哪醇Enol 烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Ynamine 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl rtomide 酰溴Acyl iodide 酰碘Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Isonitrile 异腈Amide 酰胺Imide 二酰亚胺N-bromo compound N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯Urea 脲Cyanamide 氨腈Carbodiimide 碳二亚胺Allophanate 脲基甲酸酯Thioester 硫代酸酯Thiol acid 硫羰酸Lactone 内酯Lactol 内半缩醛Macrolide 大环内酯Amino acid 氨基酸Zwitterions 两性离子Inner salt 内盐Betaine 甜菜碱Lactam 内酰胺Hydantion 乙内酰脲Peptide 肽Glycol 二醇Aldol 羟醛Acyloin 偶姻Carbohydrate 碳水化合物Aldose 醛糖Ketose 酮糖Furanose 呋喃糖Pyranose 吡喃糖Glycoside 糖苷Glucoside 葡[萄]糖苷Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 脎Alicyclic compound 脂环化合物Cycloalkene 环烷Spirane 环烯Cage compound 螺烷Propellane 笼型化合物Rotazane 螺桨烷Catenane 轮烷Rused ring 索烃11。
化学专业英语之有机金属化合物——金属配合物
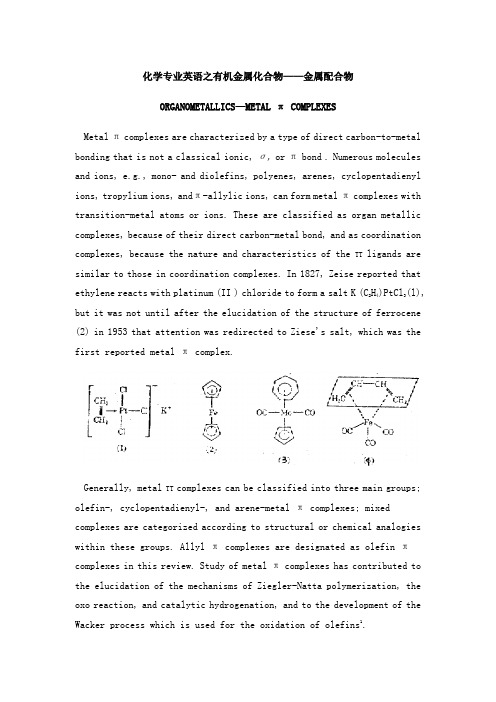
化学专业英语之有机金属化合物——金属配合物ORGANOMETALLICS—METAL π COMPLEXESMetal π complexes are characterized by a type of direct carbon-to-metal bonding that is not a classical ionic, σ, or πbond . Numerous molecules and ions, e.g., mono- and diolefins, polyenes, arenes, cyclopentadienyl ions, tropylium ions, andπ-allylic ions, can form metal πcomplexes with transition-metal atoms or ions. These are classified as organ metallic complexes, because of their direct carbon-metal bond, and as coordination complexes, because the nature and characteristics of the TT ligands are similar to those in coordination complexes. In 1827, Zeise reported thatethylene reacts with platinum (II ) chloride to form a salt K (C2H4)PtCl3(l),but it was not until after the elucidation of the structure of ferrocene (2) in 1953 that attention was redirected to Ziese's salt, which was the first reported metal π complex.Generally, metal TT complexes can be classified into three main groups; olefin-, cyclopentadienyl-, and arene-metal π complexes; mixed complexes are categorized according to structural or chemical analogies within these groups. Allyl π complexes are designated as olefin πcomplexes in this review. Study of metal πcomplexes has contributed to the elucidation of the mechanisms of Ziegler-Natta polymerization, the oxo reaction, and catalytic hydrogenation, and to the development of the Wacker process which is used for the oxidation of olefins1.The following nomenclature for metal it complexes is used:(1) Organic πligands precede the metal atom. (2)Organic πligands precede inorganic 7t ligands. (3)Inorganic π ligands, e.g., carbonyl or nitrosyls, generally follow the metal atom; halides also follow the metal but precede carbonyls or nitrosyls. (4)A prefix, e.g., di, is preferred rather than bis in describing sandwich-typeπ complexes, e.g., dibenzenechromium.(5) The symbol π can be used preceding a ligand in order to distinguish π-complex bonding from a, ionic, or other bonding. The symbol η(eta or hapto)precedes a ligand and indicates the number of C—M bonds in the ligand.Monoolefins , dienes, polyolefins, and acetylenes serve as ligands to transition metals and form olefin πcomplexes. Typical examples of olefin πcomplexes are monoolefin ligands, e.g., potassium η2-ethyleneplatinum trichloride (1); and cyclopentadienylium. –η3-cycloheptatrienylium molybdenum dicarbonyl (3); diene ligands, eg, η4-butadieneiron tricarbonyl(4 ).Certain of the delocalized π-electron ring systems of aromaticmolecules overlap with dxy and dy3metal orbitals as do the π electronsof alkenes with metal d orbitals2. The following aromatic rings can form π complexes;The C5H5- ,C6H6,and C8HSarenes are the most common in arene K complexesthat are characterized by π-bonded rings alone or π-bonded rings that are associated with one ring and other ligands, eg, halogens, CO, RNC, and R3P. Typical examples are the di-η5-cyclopentadienyl complexes , ie, metallocenes , eg , di-η5-cyclopentadienyliron (2 ). Indi-η4-5-cyclopentadienyliron ,ie, ferrocene, the 6-π-electron system ofthe C5H5- ion is bonded to the metal. Other aromatic ring systems aremono-η5-cyclopentadienylmetal nitrosyl and carbonyl complexes.PropertiesThe π-Complex Bond.Metal πcomplexes are among those that are least satisfactorily described by crystal-field theory (CFT) or valence-bond theory (VBT). The nature of the bonding can be treated more completely and quantitatively by molecular-orbital theory (MOT) or ligand-field theory (LFT). The ligand-field theory originally was advanced as a corrected CFT. The LFT relies on the use of molecular orbitals and often is used interchangeably with the MOT. The usual approach is to use the linear combination of atomic orbitals (LCAO) method. It is assumed that when an electron in a molecule is near a particular nucleus, the molecular wave function is approximately an atomic orbital that is centered at the nucleus. The molecular orbitals are formed by adding or subtracting the appropriate atomic orbitals. For transition metals .the "3d, 4s, and 4p orbitals are the atomic orbitals of interest. The ligands may have σ-and π-valence orbitals. Once the appropriate atomic orbitals have been selected for the metal and ligands, the proper linear combination of valence atomic orbitals is determined for the molecular orbitals. The determination of orbital overlaps that are possible, ie, meet inherent symmetry requirements, is done by application of the principles of group theory. At this point, the procedure becomes arbitrary in that approximate wave functions must be selected for use in the calculations of the overlap integrals and coulomb integrals3. Finally, an arbitrary charge distribution is chosen and the orbital energies and interaction energies are calculated, and a solution of the secular equation for the energies and coefficients of the atomic wave functions can be determined. A new initial charge distribution is repeated until consistent values are obtained.ReactionsMetal πcomplexes react with a wide range of chemical reagents. However, the reactions of the π-olefin-, π-cyclopentadienyl-, andit-arene-metal complexes are distinctly characteristic of each group, πCyclopentadienyl complexes, ie, metallocenes ,exhibit a high degree of aromaticity and undergo many typical aromatic substitution reactions. However, the π arene complexes do not exhibit a discernible degree of aromaticity.Although most physical properties, particularly the structure of metal TT complexes, are interpreted by use of the basic principles of coordination chemistry, these established principles do not explain suitably some reaction anomalies of the different groups of metal π complexes.Olefin πComplexes. Reactions involving olefin x. complexes similarly are characteristic of uncomplexed and complexed olefinic functions. Generally, reactions involving the former are not very different from those observed for free olefins. However, reactions of the latter are altered significantly by π-complex formation. Among the reactions of interest are addition, elimination, and substitution.Cyclopentadienyl πComplexes. The most significant feature of the reactions of π-cyclopentadienyl complexes in general and ferrocene in particular involves their aromatic nature. The resonance stabilization energy for ferrocene is 210 kj/mol(50 kcal/mol). Ferrocene undergoes a large number of typical ionic aromatic substitution reactions, eg, Friedel-Crafts acylation, alkylation, metalation, sulfonation, and aminomethylation.Friedel-Crafts Acylation. The acylation of metallocenes proceeds easily. The equimolar reaction of ferrocene and acetyl chloride in the presence of aluminum chloride yields monoacetylferrocene almostexclusively. When an excess of acetyl chloride and aluminum chloride is used, a mixture of two isomeric diacetylferrocenes is produced. The heteroannular disubstituted derivative 1,1'-diacetylferrocene and the homoannular isomer 1,2-diacetylferrocene are obtained in a ratio of 60:1. The first acetyl group deactivates the π-cyclopentadienyl ligand toward further electrophilic substitution. Thus, the second acetyl group enters the other ring.Sulfonation. Ferrocene can be sulfonated readily by sulfuric acid or cholrosulfonic acid in acetic anhydride to form ferrocenesulfonic acid and heteroannular disulfonic acid, π-Cyclopentadienylrhenium tricarbonyl can be sulfonated with concentrated sulfuric acid in acetic anhydride; the product is isolated as the p-toluidine salt. Formylation. Ferrocene is formylated with N-methylformanilide in the presence of phosphorus oxychloride. This reaction also is characteristic of highly reactive aromatic rings.Arylation. The most significant radical substitution reaction of ferrocene is its reaction with aryl diazonium salts giving an arylation product.Arene-Metal πComplexes.Generally, arene πcomplexes do not undergo the reactions that are characteristic of benzene and its derivatives. However, arene π complexes do undergo a limited number of substitution .addition .expansion, and condensation reactions.UsesCatalysis Involving Metal 7t-Complex Intermediates. Manymetal-catalyzed reactions proceed by way of a substrate metal π-complex intermediate. Commercially, the most-significant of these include the polymerization of ethylene,the hydroformylation of olefins yieldingaldehydes , ie , the oxo process (qv ), and the air oxidation of ethylene-producing acetaldehyde(qv) ,ie ,the Wacker process. Polymerization of Olefins. Ziegler-Natta Process. During the 1950s, ethylene was polymerized using a Ziegler-Natta catalyst, ie, a mixture of transition metal halides, eg, titanium halides, and trialkylaluminum (triethylaluminum commonly is used). The use of trialkylaluminum stimulated research into the use of organ metallic compounds in general. It has been determined that the Ziegler-Natta process involves a metal π-complex intermediate. A plausible mechanism for the polymerization can be formulated by applying typical organometallic and coordination reactions.Oxidation of Olefins. Wacker Process. The oxidation of ethylene exclusively to ace-taldehyde and of other straight-chain olefins to ketones is achieved by the catalytic reaction of ethylene in an aqueous solution by palladium (II) or by oxygen in the presence of palladium( II ) chloride, copper (II)chloride,or iron(III)chloride. Generally, the oxidation of olefins by other metal ions ,eg ,Hg(II) ,Th(III) ,andPb( IV ) ,yields glycol derivatives as well as carbonyl products. The mechanism for the oxidation is postulated to include n-o rearrangements. Addition of Carbon Monoxide. Oxo Reaction. The oxo process has been developed extensively to produce primary alcohols by the reduction of the aldehydes which are formed in the process.Health and Safety FactorsSome metal π complexes are air-sensitive and, therefore, their preparation requires an air-free reaction system. Their toxicity usually is based on the metal; however, organometallic compounds generally exhibit greater toxicities than their corresponding inorganic salts. The alkyl derivatives tend to be more toxic than the aryl complexes, which exhibit toxicities similar to those of the corresponding inorganic compounds.。
化学专业英语之有机
Organic ChemistryBasic scientific EnglishIntroduction to chemistryInorganic chemistryOrganic chemistryPhysical chemistryAnalytical chemistryResearch methods and related equipments Academic communicationMiscellaneous (green chemistry, bio…video)Nomenclature of Simple Organic Compounds 芳香族Aromatics胺Amine酰胺Amide酯Esters羧酸Carboxylic acid酮Ketone醛Aldehyde醚Ether [i Өə]醇Alcohol卤代物Organic Halides炔烃Alkyne烯烃Alkene烷烃AlkaneHydrocarbons:Aliphatic hydrocarbons Aromatic hydrocarbonsAlkanes are a hydrocarbon family that with carbon atoms that are only bonded to each other with single bonds. Alkanes have the general molecular formula, C n H 2n+2, where n = number of carbon atoms in the alkane molecule. A normal hydrocarbon alkane is one where all the carbon atoms in the molecule are in a "continuous" chain. A continuous chain does not necessarily mean that thecarbons are in a straight line. The carbons can be in a "zig zag" chain as long as the chain is connected by a carbon atom. The simplest alkane is methane, CH 4. It is made from one carbon atom bonded to four hydrogen atoms.©SL 2014AlkanesAlkanes CnH2n+2 saturated hydrocarbons174.0-29.710CH3-(CH2)8-CH3dec ane 150.8-53.59CH3-(CH2)7-CH3Non ane125.7-56.88CH3-(CH2)6-CH3Oct ane98.4-90.67CH3-(CH2)5-CH3Hept ane68.7-95.36CH3-(CH2)4-CH3Hex ane36.1-129.85CH3-(CH2)3-CH3Pent ane-0.5-138.34CH3-(CH2)2-CH3But ane-42.1-189.73CH3-CH2-CH3Prop ane-88.6-183.32CH3-CH3Eth ane-161.6-182.51CH4Meth aneBoiling Point Melting Point Number of Carbon Atoms Molecular Formula Name ofhydrocarbon©SL 2014Alkyl GroupsAlkyl GroupsAn alkyl is basically an alkane minus one of its hydrogen atoms. Alkyl names are used to name branched alkanes. Any branch in an alkane would be named as the number of carbons + "yl".©SL 2014Rules for naming Alkanes1.First, identify the longest continuous chain in the link of carbonatoms, also known as the parent chain. The parent chain does not have to be connected in a straight line, it could be in "zigzag" lines only if it proves to have the most amount of carbon atoms in its chain. Number the carbons in the parent chainstarting from the end closest to the branch(es) so that thesubstituents will have the smallest possible numbers.©SL 20142. Next, find each alkyl branch and assign it a numberaccording to which carbon atom it is attached to. The name for the alkyl branch is followed by "-yl" toindicate the number of carbon atoms are in a branch.3. List the substituents(with their carbon number) in alphabeticalorder followed by the name of the parent alkane. In some cases when there are more than one of a given substituent use theprefixes di-, tri-, tetra-. Prefixes are not used to determinealphabetical order. The structural format should look like this: (number of location)-(branch name)(name of parent chain)4. Use commas between numbers and dashes between numbers and words in naming the IUPAC formulas. Do not leave spaces in the name.© SL 2014© SL 2014Cycloalkanes Cycloalkanes are alkanes in which a bond is formed between the two terminal carbons in the chain to for a cyclical or ring structure. The prefix cyclo- is written before the name designating the carbon number in the ring (e.g. cyclohexane). 1. Substituents are named similarly to straight-chained alkanes. The carbons in the ring are numbered so that the substituents have the smallest numbers. 2. Add the prefix cyclo- to the alkane name© SL 2014Common Alkyl Groupsname methyl Ethyl n-propyl n-butyl isopropyl t-butylformula -CH3 -CH2CH3 -CH2-CH2-CH3 -CH2-CH2-CH2-CH3 -CH(CH3)2 -C(CH3)3Secondary butyl or sec-Butyl: CH3–CH2–CH(CH3)– (fully systematic name: 1-methylpropyl) Isobutyl: (CH3)2CH–CH2– (fully systematic name: 2-methylpropyl) Tertiary butyl, tert-Butyl or t-butyl: (CH3)3C– (fully systematic name: 1,1-dimethylethyl)© SL 2014AlkenesAlkenes are hydrocarbons that contain one or more carbon-carbon double bonds. Alkenes with only one double bond have the general formula CnH2n Naming alkenes has the same format as naming alkanes, but be sure to change the -ane ending on the parent name to -ene ending. This shows that the compound is an alkene.© SL 20141. Determine the longest continuous chain of carbons that have the double bond between two of its carbons. The parent chain must contain the double bond. 2. Number the carbons in the chain so that the double bond would be between the carbons with the lowest designated number. Numbering could start either from the left end or right end of the chain. The location of the double bond, not the location of the branches are used for numbering the alkene. 3. Identify the various branching groups attached to this continuous chain of carbons by name. 4. The format is as follows: (location of branch)-(branch name)(parent chain)CH3CH2CH=CH2 4 3 2 1 1-butene CH3CH3CH=CHCH3 1 2 3 4 2-butene CH3CH3CH2CHCH2CH=CCH3 7 6 5 4 3 2 1 2,5-dimethyl-2-heptene© SL 2014Geometric Isomers In alkanes, rotational freedom is possible but for alkenes, due to the carbon-carbon double bond, it is impossible to rotate the bond without breaking it. Geometric isomers are isomers that differ from each other based on the position of the attached groups relative to the double bond. Two substituents that are on the same side of the double bond is called cis, meaning "same", two substituents that are on the same side of the double bond is called trans, meaning "across".© SL 2014AlkynesAlkynes are hydrocarbons that have at least one triple covalent bond between two carbon atoms. The general formula for the Alkyne CnH2n-2. Similarly, alkynes are named just like alkenes only with the -yne ending instead of the -ene ending in naming compounds.© SL 20141. Determine the longest continuous chain of carbons that have the triple bond between two of its carbon atoms. 2. Number the carbons in the chain so that the triple bond would be between the carbons with the lowest designated number. This means that you have to decide whether to number beginning on the right end or left end of the chain in order to obtain the smallest possible numbers. 3. Identify the various branching groups attached to this continuous chain of carbons by name. 4. The format is as follows: (location of branch)-(branch© SL 2014name)(parent chain) propyne C-HH-CAromaticsAromatics are carbon compounds that have benzene-like properties. The term "aromatic" came to be because earlier compounds found with the rings had pleasant fragrances, but it turns out that the ring has nothing to do with smell. Benzene, back then, had special properties and fascinated scientists for many years. At last, a structural model was proposed and accepted as the benzene ring. Aromatics consists of a benzene ring located anywhere within the molecular structure.If an alkyl group substitutes a hydrogen atom on the benzene ring, the structure is similarly named like an branched alkane.1.Obtain the smallest possible numbers to indicate the location ofthe branched alkanes. In order to obtain this, numbering starts at one of the substituents and continues either clockwise orcounterclockwise to get the smallest possible numbers. If there is only one substituent, numbering is not required.2.Identify the various branching groups attached to thiscontinuous chain of carbons by name.3.The format is as follows: (location of branch)-(branchname)(parent chain)If a benzene ring is considered a branch(in larger molecules), then the benzene ring is called a phenyl group. In this case, the alkane becomes the parent chain and the phenyl thus becomes a branch of the alkane structure.Organic HalidesOrganic halides are organic compounds in which one or more hydrogen atoms have been substituted by a halogen atom. The IUPAC name for halides is the same as branched chain hydrocarbons. The branch is named by shortening the halogen name to fluoro-, chloro-, bromo-, or iodo-.The halide(s) are treated as branched groups and are located on the continuous chain of carbons as you would locate and name any alkyl branch.1.Determine the name of the parent chain.2.Numbering could start either from the left end or right end of the chain.3.Identify the various branching groups attached to this continuous chain of carbons by name, if any.4.The format for naming halides is:(location of halides)-(halogen prefix)(parent chain)CH 3-C-C-CH 31,21,2--difluorobutaneH HF FAlcohols All alcohols contain the hydroxyl functional group, -O-H, attached to single bonded hydrocarbons (alkanes). Alcohol have the general formula R-OH where R represents any chain of carbon and hydrogen atoms. The four most common alcohols are:CH 3OHmethanolCH 3CH 2OHethanolCH 3CH 2CH 2OH1-propanolCH 3CH(OH)CH 3Alcohols use the same formats as alkanes. To name alcohols,1.Determine the parent chain. The parent chain must be the longestthat includes the carbon holding the OH group.2.Number according to the end closest to the -OH group regardlessof where alkyl substituents are.3.The format is as follows: (location of branch)-(branch name)-(location of OH group)-(parent chain)4.Change the parent chain -e ending and replace it with an -ol.Alchoholscontaining more than one hydroxyl group are also called polyalcohols. Polyalcohols are named similarly toalcohols, with the exception of the prefix di-, tri-, etc before the -ol ending.H-C-C-H1,21,2--ethanediolH HOHHOEthersEthers are compounds with two alkyl groups bonded to anoxygen atom. Ethers have the general formula R1-O-R2whereR 1and R2are hydrocarbon groups. The difference betweenalcohol and ether are that in an alcohol, one hydrogen atom is replaced by an alkyl group. In an ether, however, both hydrogen atoms are replaced by alkyl groups.Simple ethers can be named by naming the alkyl groups alphabetically followed by the word "ether". A more efficient way of naming ethers would be by adding -oxy- to the prefix for the smaller hydrocarbon group and joining it to the alkane name of the larger hydrocarbon group. For example, CH3-O-CH2-CH3 would be called using this common name approach as Ethyl Methyl Ether and another way to name it would be methoxyethane. If just two alkyl groups are identical in its molecular formula, use the prefix di-, tri-, tetra-, etc to signify these branches. e.g. diethyl ether Example: CH3CH2-O-CH2CH3 ethoxyethaneAldehydes [ai] Aldehydes are a family of organic compounds that contain the carbonyl functional group, -CO-, and in which the carbonyl group is bonded to at least one hydrogen. The general formula for an aldehyde is RCHO, where R is hydrogen or an alkyl group.© SL 2014When naming aldehydes: 1. Identify the longest continuous chain of carbons with the carbonyl carbon as part of the chain. 2. Number the carbon chain so that the carbonyl carbon is always number one. 3. Locate and identify alphabetically the branched groups by prefixing the carbon number it is attached to. If more than one of the same type of branched group is involved use the prefixes di for 2, tri for three, etc. 4. After identifying the name, number and location of each branched group, use the alkane name that represents the number of carbons in the continuous chain. 5. Change the "e" ending and replace it with -al.分子式 HCHO CH3CHO普通命名 甲醛 formaldehyde 乙醛 acetaldehyde系统命名 methanal ethanalCH3CH3CHO 丙醛 propionaldehyde propanalKetonesBoth aldehydes and ketones are often referred to as carbonyl compounds. The difference between aldehydes and ketones, however, lies in the atoms or groups to which the carbon of the carbonyl group is bonded. In aldehydes, at least one of the bonds is connected to a hydrogen atom, while in ketones neither bond is connected directly to hydrogen, but both are connected to carbon atoms.© SL 2014In naming ketones, 1. Identify the parent chain, it must include the carbonyl group 2. Number the parent chain starting from the end closest to the carbonyl location. 3. Identify the various branching groups attached to this continuous chain of carbons by name. 4. Change the "e" ending and replace it with -one. CH3 C=O CH3 propanone (acetone) CH3CH C=O CH2CH2CH3 3-hexanoneCarboxylic AcidsCarboxylic acids is a family of organic compounds that contains the carboxyl group, -COOH. which consists of a carbon atom joined to an oxygen atom by a double bond and to a hydroxyl group, OH, by a single bond.Carboxyl group© SL 20141. Determine the parent chain. The parent chain must include the carboxyl carbon. 2. Number starting at the end closest to the carboxyl group. 3. The name of the alkane (parent chain) is changed by replacing the e with -oic followed by the word "acid". Example: HCOOH methanoic acid CH3COOH ethanoic acid (acetic acid) CH3CH2CH2COOH butanoic acidEstersEsters are a group of organic compounds with a general formula R1CO2R2 (where R1 and R2 are alkyl groups) that are formed, along with water, by the reaction of acids and alcohols. Natural occurring esters of organic acids in fruits and flowers give them their distinctive odors. It also used for food aroma and taste, perfumes, synthetic fibers, and solvents.© SL 2014To name esters, 1. Identify the alkyl group that is attached to the oxygen atom 2. Number according to the end closest to the -CO- group regardless of where alkyl substituents are. 3. Determine the alkane that links the carbon atoms together. If there is a separation of a continuous link of carbon atoms due to the oxygen atom, individually name the two alkanes before and after the oxygen atom. The longer structural alkane is the one that should contain the carbonyl atom. 4. The format is as follows: (alkane further from carbonyl) (alkane closest to carbonyl)(parent chain) 5. Change the parent chain -e ending and replace it with an -oate. Example: CH3COOC7H14CH3 octyl ethanoateAmidesAmide is a group of organic compounds containing the carbonyl group that is bonded to a nitrogen atom, CONH2 and, usually, a hydrocarbon. It is formed by the reaction of an ester with an amine. All amides are solids at room temperature and are neutral compounds, neither acidic nor basic since they are formed in a neutralization.To name amides,1.Determine the name of the alkane that indicates the samenumber of carbon atoms.2.Simply change the carboxylic acid reactant -oic acidending and replace it with -amide.Example:CH3CONH2ethanamideAminesAmines are organic derivatives of ammonia, meaning that one, two, or three hydrocarbon groups have replaced the hydrogens. Amines have an extremely high solubility and have disagreeable odors (fish smell). Amines could be classified as primary, secondary, and tertiary depending on the amount of hydrogen that has been replaced by a hydrocarbon group. Amines could also be found in plants which are called alkaloids and affect the central nervous system of many creatures.To name amines,1.Identify the names of the alkyl groups bonded to the nitrogen atom2.Simply replace the alkane -e ending with -amine.3.The format is as follows: (alkyl name)(-amine)H-N-H ammoniaH CH3-N-H methylamineH CH3-N-CH3 dimethylamineHCH3-N-CH3 trimethylamine orethylmethylamineCH3Names of Common Substituent GroupsVinyl-CH=CH2Nitro -NO2iodo -I bromo -Br Chloro -Cl Fluoro -F Amino -NH2name Functional groupWhat is Oil?•A mixture of hydrocarbons from C1–C60.C 1= methane CH4C 3= propane C3H8C 7= heptane C7H16C 10= decane C10H22C 12= hexadecane C12H26Just a fewexamples!!How do we separate these?•Boiling point:temp. at which liquid →gas.•Compounds with different molecular weights (molar masses) have different boiling points.Petroleum Fractional DistillationBoilerCrude OilBottoms: waxes, asphalt, paraffin, etc.C1 –C4, petroleum gases (methane, ethane,propane, butane) < 40°COil vapors (~370°C)D i s t i l l a t i o n C o l u m n1) Hot vapors rise through column 2) Vapors cool and condense3) Condensed hydrocarbons drawn offC15 –C18 Heating oils, diesel fuel, cracking C16 –C20 Lubricating OilsC12 –C16 diesel fuel, kerosene, cracking C5 –C12, Gasoline, motor oil, solvents CoolerPetroleum Refining•We can refine petroleum distillates to produce whatever hydrocarbon mix we want!!•Catalytic Cracking–Chemical processes that use a catalyst to break heavier hydrocarbons down to lighter ones.•Catalytic combination–Chemical processes that use a catalyst to combine lighter hydrocarbons to form heavier ones.C 16H 34+ catalyst →C 8H 18+ C 8H 16+ catalyst 4C 2H 4+ catalyst →C 8H 16+ catalystCarboxylic Acids•The functional group of carboxylic acids consists of a C=O with -OH bonded to the same carbon.•Carboxyl group is usually written -COOH.•Aliphatic acids have an alkyl group bonded to -COOH.•Aromatic acids have an aryl group.•Fatty acids are long-chain aliphatic acids.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic 烯丙型[的]Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的]Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium ring 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical cation 自由基正离子Radical anion 自由基负离子Isomerism 异构[现象]Aci form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象]Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的]Heterotopic 异位[的]Enantiotopic 对映异位Diastereotopic 非对映异位[的] Configuration 构型Absolute configuration 绝对构型Chirality 手性Chiral 手性[的]Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的]Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L命名体系R-S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体Diastereomer 非对映[异构]体Epimer 差向异构体Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S基团Re face Re面Si face Si面Racemic mixture 外消旋混合物Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体Conformation 构象Conformational 构象分析Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skew con-formation 邻位交叉构象Staggered conformation 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropismer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram’s rube 克拉姆规则Prelog’rule普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Leois structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donof-acceptor complex,EDAcomplex 电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力F strain 前张力B strain 后张力Anomeric effect 端基异构效应Walden inversion 瓦尔登反转Racemization 外消旋化Isoinversion 等反转Isoracemization 等消旋Homochiral 纯手性[的]Mechanism 机理Unimolecular nucleophilic 单分子亲核取代Bimolecular nucleophilic sub-stitution 双分子亲核取代Bimolecular nucleophilic substi-tution(with allylic rearrange-men t) 双分子亲核取代(含烯丙型重排)Internal nucleophilic substiru-tion 分子内亲核取代Aromatic nucleophilic substitu-tion 芳香亲核取代Unimolecular electrophilic sub-stitution 单分子亲电取代Bimolecular electrophilic substi-tution 双分子亲电取代Nucleophile-assisted unimolecu-lar electrophilic substitution 亲核体协助单分子亲电取代Unimolecular elimination 单分子消除Bimolecular elimination 双分子消除Unimolecular elimination through the conjugate base 单分子共轭碱消除Bimolecular elimination through the conjugate base 双分子共轭碱消除Bimolecular elimination with for-mation of a carbonyl group 双分子羰基形成消除Unimolecular acid-catalyzed acyl-oxygen cleavage 单分子酸催化酰氧断裂Bimolecular base-catalyzed acyl-oxygen cleavage 双分子碱催化酰氧断裂Unimolecular acid-catalyzed alkyl-oxygen cleavage 单分子酸催化烷氧断裂Bimllecular base-catalyzed al- kyl-oxygen cleavage 双分子碱催化烷氧断裂π-allyl complex mechanism π烯丙型络合机理Borderline mechanism 边理机理Homolysis 均裂Heterolysis 异裂Heterolytic michanism 异裂机理Counrer[gegen]ion 反荷离子Ion pair 离子对Carbocation 碳正离子Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov’s rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromatic substitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性α-effect α-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与Neighboring proup assistance,anchimeric assistance 邻助作用Neighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化02.3有机化学反应Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化Decarboxylative nitration 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer 重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross-coupling reaction 交叉偶联反应1,4-addition 1,4-加成Conjugate addition 共轭加成Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成- elimination -消除- elimination -消除- elimination -消除-elimination -消除Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应]Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应]Ring expansion,ring enlargement 扩环[反应] -ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2.4 有机化合物类名Aliphatic compound 脂肪族化合物Hpdrocarbon 碳氢化合物Alkane 烷Wax 蜡Paraffin wax 石蜡Alkene 烯Alkyen 炔Acetylide 炔化物Active hydrogen compounds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene 双烯Triene 三烯Allene 丙二烯Ccumulene 累积多烯Enyne 烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide 环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound 硝基化合物Amine 胺Quaternaryammonium com-pound 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfonic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛Detone 酮Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛Acetal 缩醛acetal[化]乙缩醛, 乙缩醛二乙醇Ketal 缩酮Dithiane 二噻烷Aminal 缩醛胺imine 亚胺Aldimine 醛亚胺Oxime 肟Aldimine 醛肟Oxime 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol 频哪醇Enol 烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Ynamine 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl rtomide 酰溴Acyl iodide 酰碘Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Isonitrile 异腈Amide 酰胺Imide 二酰亚胺N-bromo compound N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯Urea 脲Cyanamide 氨腈Carbodiimide 碳二亚胺Allophanate 脲基甲酸酯Thioester 硫代酸酯Thiol acid 硫羰酸Lactone 内酯Lactol 内半缩醛Macrolide 大环内酯Amino acid 氨基酸Zwitterions 两性离子Inner salt 内盐Betaine 甜菜碱Lactam 内酰胺Hydantion 乙内酰脲Peptide 肽Glycol 二醇Aldol 羟醛Acyloin 偶姻Carbohydrate 碳水化合物Aldose 醛糖Ketose 酮糖Furanose 呋喃糖Pyranose 吡喃糖Glycoside 糖苷Glucoside 葡[萄]糖苷Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 脎Alicyclic compound 脂环化合物Cycloalkene 环烷Spirane 环烯Cage compound 螺烷Propellane 笼型化合物Rotazane 螺桨烷Catenane 轮烷Rused ring 索烃化学专业英语词汇常用前后缀-acetal 醛缩醇acetal- 乙酰acid 酸-al 醛alcohol 醇-aldehyde 醛alkali- 碱allyl 丙烯基alkoxy- 烷氧基-amide 酰胺amino- 氨基的-amidine 脒-amine 胺-ane 烷anhydride 酐anilino- 苯胺基aquo- 含水的-ase 酶-ate 含氧酸的盐、酯-atriyne 三炔azo- 偶氮benzene 苯bi- 在盐类前表示酸式盐bis- 双-borane 硼烷bromo- 溴butyl 丁基-carbinol 甲醇carbonyl 羰基-caboxylic acid 羧酸centi- 10-2chloro- 氯代cis- 顺式condensed 缩合的、冷凝的cyclo- 环deca- 十deci 10-1二-dine 啶dodeca- 十二-ene 烯epoxy- 环氧-ester 酯-ether 醚ethoxy- 乙氧基ethyl 乙基fluoro- 氟代-glycol 二醇hemi- 半hendeca- 十一hepta- 七heptadeca- 十七hexa- 六hexadeca- 十六-hydrin 醇hydro- 氢或水hydroxyl 羟基hypo- 低级的,次-ic 酸的,高价金属-ide 无氧酸的盐,酰替胺,酐-il 偶酰-imine 亚胺iodine 碘iodo- 碘代iso- 异,等,同-ite 亚酸盐keto- 酮ketone 酮-lactone 内酯meta- 间,偏methoxy- 甲氧基methyl 甲基mono- ( mon-) 一,单nitro- 硝基nitroso- 亚硝基nona- 九nonadeca- 十九octa- 八octadeca- 十八-oic 酸的-ol 醇-one 酮ortho- 邻,正,原-ous 亚酸的,低价金属oxa- 氧杂-oxide 氧化合物-oxime 肟oxo- 酮oxy- 氧化-oyl 酰para- 对位,仲penta- 五pentadeca- 十五per- 高,过petro- 石油phenol 苯酚phenyl 苯基pico- 10-12poly- 聚,多quadri- 四quinque- 五semi- 半septi- 七sesqui 一个半sulfa- 磺胺sym- 对称syn- 顺式,同,共ter- 三tetra- 四tetradeca- 十四tetrakis- 四个thio- 硫代trans- 反式,超,跨tri- 三thio- 硫代trans- 反式,超,跨tri- 三trideca- 十三tris- 三个undeca- 十一uni- 单,一unsym- 不对称的,偏位-ylene 撑(二价基,价在不同原子上)-yne 炔活化剂的中英文名称Bis(2-ethylhexyl) sebacate (癸二酸二仲辛酯;癸二酸二2-乙基己酯)Zinc stearate (硬脂酸锌)Suberic acid (辛二酸)Adipic acid, (己二酸)Hexanedioic acid, (己二酸)Sebacic acid, dibutyl ester (癸二酸二丁酯)Abietic acid (松香酸)Lactic acid (乳酸)Poly(ethylene glycol) (聚乙二醇)Glycerol stearate (硬脂酸甘油酯)Imidazoline (咪唑啉,间二氮杂环戊烯)-Pinene (β-蒎烯,β-松油二环烯)βAdipic acid (脂肪酸)Butyl acetate (乙酸丁酯)Ethylene glycol butyl ether (乙二醇丁醚)Sebacic acid, (癸二酸)Decanedioic acid, (癸二酸)Ethylene glycol ethyl ether (乙二醇乙醚)2-Butenedioic acid (E)-, (2-丁烯二酸)Succinic acid, (琥珀酸,丁二酸)Ethylene glycol methyl ether (乙二醇甲醚)Acetyl acetate (乙酸乙酰脂)1H-Benzotriazole (1-H-笨并三唑)-Pinene (α-蒎烯,α-松香二环烯)αSalicylic acid (水杨酸)Iso-Propanol (异丙醇)Benzoic acid (苯甲酸)Ethanol (乙醇)Lysine (赖氨酸)Glutamic acid (谷氨酸,2-氨基戊二酸)Glyceroyl, (甘油酰)N,N,N',N'-Tetrakis-(2-hydroxypropyl)-ethylene-diamine(N,N,N,N-四(2-羟基丙基)乙烯二氨)Isoleucine, (异亮氨酸)Decamethylenedicarboxylic acid, disalicyloylhydrazide (Tris(2,3-dibromopropyl)isocyanurate(3(2,3-2溴丙基)异氰尿酸盐)3-(N-Salicyloyl)amino-1,2,4-triazole (3-(N-水杨酰)氨-1,2,4-三唑)Isocyanuric acid (异氰尿酸)Salicylamide (水杨酰胺)Polyethylene glycol (聚乙二醇)Diethylene glycol diethyl ether (二甘醇二乙醚,(一缩)二乙二醇二乙醚)Butyl carbitol (丁基卡必醇)Ethyl carbitol (乙基卡必醇)Methyl carbitol (甲基卡必醇)Ethylene glycol monobutyl ether (乙二醇单丁醚)Glutaric acid (戊二酸,谷酸)Succinic acid (琥珀酸,丁二酸)Citric acid (柠檬酸)Salicylic acid (水杨酸)Lactic acid (2-羟基丙酸,乳酸)Glycerin monostearate (甘油一硬脂酸)Pentaerythritol (季戊四醇)-(3,5-di-tert-butyl-4-hydroxy-phenyl)propionate] 四[β-(3,5-二叔丁基-4-羟基-苯基)丙酸酯 tetrakis[Dioctyl sebacate (癸二酸二辛酯)N-Methyl pyrrolidone, (N-甲基吡咯烷酮)Diethylene glycol ethyl ether (二甘醇乙醚)Propylene glycol (丙二醇)Octanedioic acid (辛二酸)Oleamide (油酸酰胺)[olamine 乙醇胺]2-Mercapto benzothiazole (2-巯基-苯并噻唑)Nonanedioic acid, (壬二酸)cis-9-Octadecenoic acid, (顺式-9-十八炭烯酸,油酸)Sebacic acid, uses (癸二酸)12-Hydroxy stearic acid (十二羟基硬脂酸)Phthalic acid (苯二甲酸)1,1,3-Tris(2-methyl-4-hydroxy-5-tert-butylphenyl)butane(1,1,3-三(2-甲基-4-羟基-5-叔丁基苯基)丁烷)1,3,5-Trimethyl-2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)benzene (1,3,5-三甲基-2,4,6-三(3,5-二叔丁基-4-羟基苯基)苯)1-Methyl-2-pyrrolidone, (1-甲基-2-吡咯烷酮)Carbonic acid, (碳酸)Phthalic acid, (苯二甲酸)Malic acid (苹果酸,羟基丁二酸)2,3-Dibromo-2-butene-1,4-diol (2,3-二溴-2-丙烯-1,4-二醇)Cetylpyridinium bromide (溴代十六烷基吡啶)Pentanedioic acid (戊二酸)pentanediol (戊二醇)pentanoic acid ( 戊酸) pentanol (戊醇)Butanedioic acid, (丁二酸)1,2-Dibromoethylbenzene (1,2-二溴乙基苯)Salicylic acid, (水杨酸)Stearic acid (硬脂酸)Pentanedioic acid (戊二酸)Maleic acid, (马来酸,失水苹果酸)Phthalic acid, (苯二甲酸)Tartaric acid, (酒石酸)Acetic acid, (乙酸)Polyoxyethylene octylphenol ether (聚氧乙烯辛基酚醚,聚氧化亚乙基辛基分醚)Ethanedioic acid, (乙二酸)Polyethylene glycol (聚乙二醇)Diethylene glycol butyl ether (二甘醇丁醚)Diethylene glycol monoethyl ether (二甘醇单乙醚)Ethylene glycol monobutyl ether (乙二醇单丁醚)Pentaerythritol, (季戊四醇)Diglycol, (二甘醇,一缩二乙二醇)Hexylene glycol (己二醇)Ethylene glycol, (乙二醇)Glycerol, (甘油,丙三醇)Cyclobutanediamine (环丁烷二胺)Dibromobutenediol (二溴丁二醇)Cyclohexanediamine (环己烷二胺)Succinamide (琥珀酰胺,丁二酸胺)Ethylenediamine, (乙二胺)Triethanolamine, (三乙醇胺)5-Aminoisophthalic acid (5-氨基间苯二甲酸)p-tert-Butylbenzoic acid (对叔丁基苯甲酸)Propionic acid, (丙酸)Benzoic acid, (安息香酸, 苯(甲)酸)Salicylamide (水杨酰胺)Aniline, (苯胺)Palmitic acid, (棕榈酸, 十六酸, 软脂酸)Glutamic acid, (谷氨酸)Glutaric acid (谷酸,戊二酸) Glycine, (甘氨酸,氨基乙酸)Malic acid (苹果酸,羟基丁二酸)Adipic acid, (己二酸)Diethanolamine (二乙醇胺)Triethylamine, (三乙胺)Malic acid (苹果酸)Oxalic acid, (草酸)Oleic acid, (油酸)Glutaric acid (谷氨酸)Sorbic acid (山梨酸)sorbic alcohol (山梨醇)=sorbit。
