应用化学专业英语经典题
应用化学专业英语及答案

黄冈师范学院2009—2010学年度第一学期期末试卷考试课程:专业英语考核类型:考试A卷考试形式:闭卷出卷教师:杨一思考试专业:化学考试班级:应用化学200601 一、Translate the following into English(20 points)1.过滤2.浓缩3.结晶化4.吸附5. 蒸馏6.超临界的7.二氯甲烷8.热力学平衡9.亲电性10.表面张力11.共轭的12.酮13.平衡常数14.丙基15.丁基16.亚甲基18.环己酮19.同位素20.标准熵二、Translate the following into Chinese(20 points)1. methyl propanoate2. rate constant3. ethyl methyl ketone4. free energy5. radical intermediate6. isobutyl methyl ether7. 3-chloropropene8. primary radical9. n-propyl bromide10. bond energy 11. circulating electrons12. local magnetic fields13. tetramethylsilane14. mass to charge ratios15 phenylamine16 amide17. amine18. nucleophile19. perchlorate20. carbocation三、Translation the following into chinese (40 points)A卷【第1页共 3 页】1. We can see why benzene is stable: according to resonance theory, the more resonance forms a substance has, the more stable it is. Benzene, with two resonance forms of equal energy, is therefore more stable and less reactive than a typical alkene.2. Membranes can be defined essentially as barrier, which separates two phases and restricts transport of various chemicals in a selective manner. A membrane can be homogenous or heterogeneous, symmetric or asymmetric in structure, solid or liquid, can carry a positive or negative charge or be neutral or bipolar. Transport through a membrane can be effected by convection or by diffusion of individual molecules, induced by an electric field or concentration, pressure or temperature gradient. The membrane thickness may vary from as small as 100 micron to several mms.3. The most common industrial adsorbents are activated carbon, silica gel, and alumina, because they present enormous surface areas per unit weight.A surface already heavily contaminated by adsorbates is not likely to have much capacity for additional binding, but further heating will drive off these compounds to produce a surface with high adsorptive capacity.Temperature effects on adsorption are profound, and measurements are usually at a constant temperature. Graphs of the data are called isotherms. Most steps using adsorbents have little variation in temperature.A卷【第2页共 3 页】4. In the absence of peroxides, hydrogen bromide adds to peopene via the Markovnikov pathway to yield isopropyl bromide. In the presence of peroxides, however, the order of addition is reversed, and the product is n-propyl bromide; the addition in this case is said to be anti-Markovnikov. This is interpreted in terms of initiation of the addition reaction by bromine atom, rather than by a proton, as is the case for electrophilic addition.四、Translate the following paragraphs into Chinese(20 points)1.Benzene and its derivatives can be nitrated using a mixture of concentrated nitric and sulphuric acid. The temperature must be controlled to prevent more than one nitro-group going in.2. Benzene can be made to react with halogen derivatives using aluminium chloride as a catalyst. This is called a Friedel-Crafts reaction.can be sulphonated by reacting it with fuming sulphuric acid(oleum). The benzene reacts with sulphur trioxide in the oleum.benzene is converted into ethylbenzene by reacting it with ethene. The ethylbenzene (also called styrene) is used to make polystyrene.黄冈师范学院2009—2010学年度第一学期期末试卷参考答案及评分标准考试课程:专业英语考核类型:考试A卷考试形式:闭卷出卷教师:杨一思考试专业:化学考试班级:应用化学200601 一、Translate the following into English(20 points)2. concentrate 4. adsorption chlorideequilibriumtensionconstant14. propylmagneticresonanceentropy二、Translate the following into Chinese(20 points)1. 丙酸甲酯2. 速率常数3. 甲乙酮4. 自有能5. 自由基中间体6. 异丁基甲醚7. 3-氯丙烯8. 伯自由基9. 正丙基溴化10. 键能11.循环电子12. 局部电磁场13. 四甲基硅烷14. 质荷比15.苯胺16.氨基化合物17.胺18亲核试剂19.高氯酸盐20.碳正离子三、Translation the following into chinese (50 points)1.依据共振理论,物质具有的共振式越多就越稳定。
应用化学专业英语

应用化学专业英语Cu—copper Fe—iron Hg—mercury Na—sodium K—potassium Ag—silverNaOH—sodium hydroxide KOH—potassium hydroxide Fe(OH)2—iron(Ⅱ)hydroxide Fe(OH)3—iron(Ⅲ)hydroxide NH4OH—ammonium hydroxideK3[Fe(CN)6]—potassium hexacyanoferrate(Ⅲ)K4[Fe(CN)6]—potassium hexacyanoferrate(Ⅱ)Ⅰ.Name the following1.(NH4)2CO3Ammonium carbonate2.N2O Nitrous Oxide3.H2SO4Sulfuric acid4.P4O6Phosphorus(Ⅲ)trioxide5.Al2O3Aluminium oxide6.SnCl4Tin(IV)chloride7.KHSO4Potassium hydrogen sulfate8.Cu2S Copper(Ⅰ)sulfide9.HClO4Perchloric acid10.BaCl2Barium chloride11.P4O10Phosphorus(Ⅴ)pentoxide12.NaH Sodium hydride13.Ca(MnO4)2Calcium permanganate14.PF5Phosphorus pentafluoride15.(NH4)2HPO4Diammonium hydrogen phosphateⅡ.Give formulas for the following1.ammonium sulfate(NH4)2SO42.barium iodide BaI23.iron(Ⅱ)sulfate Fe2SO44.potassium permanganate KMnO45.copper(Ⅱ)oxide CuO6.carbonic acid H2CO3Melting point 熔点boiling point 沸点1.Which particles play the most active role in chemical bonding?(a)electrons (b)neutrons (c)protons (d)valence electrons2.An ionic bond is formed when electrons are:(a)completely destroyed (b)compeltely transferred (c)divied (d)equally shared3.Due to the that Ionic compounds have strong intermolecular forces they are at room temperature.(a)bonded covalently (b)gases (c)liquids(d)solids 1-butene trans -2-butenecis -2-butene iso -butene (E )-2-butene (Z )-butene 2-methylpropene1.Draw structure that correspond to the following names.(a)2,2-dimethylpentane (b)4-isobutyl-2,5-dimethylheptane (c)(Z)-3-menthyl-2-octene (d)(2R,3S)-2,3-pentanediol2.Give the IUPAC name for each of the following structures.(e)(f)(E)-1-methyl-4-ethylcyclohexane(g)(h)(S)-2-chloro-butyraldehyde (2R,3R)-2,3-dichlorobutyric acid补充:(E)-2-chloro-3-methyl-2-octene Nucleophile亲核试剂carbocation碳阳离子Compressible可压缩的incompressible不可压缩的1.A chemical system can be studied from either a or a(n)viewpiont.(A)physical...chenical(B)molecual...atomic (C)Microscopic...macroscopic(D)Mechanic...kinetic2.Is a macroscopic science that studies the interrelationships between the various equilibrium properties of a stystem.(A)Kinetics(B)Thermodynamics (C)Statistical mechanics(D)Quantum chenistry3.In,the molecular and macroscopic levels are related to each other.(A)quantum(B)statistical(C)thermodynamics(D)kinetics4.thermodynamics studies.(A)heat,work,energy,and the changes they produce in the states of systems(B)The relationships between the molecules of a system(C)heat,work,temperature,and the energy they produce in the states of systems(D)heat,energy,and work5.For a(n)system,neither matter nor energy can be transferred between system and surroundings.(A)closed(B)open(C)isolated(D)none of the aboveⅠ.Translate the following from English into Chinese.(1)pollution of the atmosphere(2)nondegradable pollutant大气污染不可降解污染物(3)harmless pollutant(4)interacting chemicals无害污染物相互作用的化学物质(5)threshold level(6)sound pressure level限定值,阈值声压水平(7)speech interference(8)transmission path 语音干扰传输途径Translate the following from Chinese into English.(1)定性分析qualitative analysis (2)分析物analyte (3)准确度accuracy (5)反应速率reaction-rate (5)解吸附作用deserption (6)吸附absorption conduction 热传导convection 对流radiation 辐射Balance and classify each of the following chemical equations as a (1)combination reactions ,(2)decomposition reaction ,(3)displacement reaction ,or (4)partner-exchange reaction.(a))()(2243l O H s Fe H O Fe +→+)(4)(342243l O H s Fe H O Fe +→+displacement reaction 置换反应(b))()()(23g O s KCl s KClO +→)(3)(2)(223g O s KCl s KClO +→decomposition reaction 分解反应(c)steam and hot carbon react to form gasecous hyfrogen and gaseous carbon monoxide.)()()()(22g CO g H s C l O H +→+displacement reaction 置换反应(d))()()(4272aq HClO g O H g O Cl →+)(2)()(4272aq HClO g O H g O Cl →+combination reactions 化合反应(e))()()(22aq HBrO aq HBr O H l Br +→+)()()(22aq HBrO aq HBr O H l Br +→+decomposition reaction 分解反应(f))()()()()(43442243aq PO H s CaSO aq SO H s PO Ca +→+)(2)(3)(3)()(43442243aq PO H s CaSO aq SO H s PO Ca +→+partner-exchange reaction 复分解反应(g)Potassium reacts with water to give aqueous potassium hydroxide and gaseous hydroxide.)()(2)(2)(222g H aq KOH l O H s K +→+displacement reaction 置换反应(h)Solid magnesium carbonate decomposes to form solid magnesium oxide and gaseous carbon monoxide.)()()(23g CO s MgO s MgCO +→decomposition reaction 分解反应Abstract 摘要Results and discussion 结果与讨论Experimental实验References参考文献E-factor影响因素Journal of the American Chemical Society美国化学会志Journal of the Chemical Society化学会志Journal of Organic Chemistry有机化学杂志Tetrahedron四面体'\.._/ ( Wb川ache mical reaction?Acherr山al react i on occurs when subs'孟忘"(tlie reactants) collide (碰撞) with enough energy to rearrange to form different compounds (由e produc时. η1e change in energy由at occurs when a reaction take place is described by thermodynamics (热力学) and the rate or speed at which a reaction occ u rs is described by kfaetics (动力学) . Reactions in which the reactants and produc臼coexist are considered to be in equ山brium (处于平衡). A chemical equation consists of the chemical formula (化学式) of the reactants,且目the chemical formula of the products. The two are separated by an 一一- usually read as ”yielas·’and each chemical formula is separated from others by a plus sign (加号) . Sometimes a triangle is drawn over the arrow symbol to denote energy must be added to the substances for the reaction to begin. Each chemical formula may be preceded by a scalar (数量的) coefficient ind i cating the proportion (比例) of that substance necessary to produce the reaction in formula. For instance, the formula for the burning of methane (C比+ 202 →C02 + 2H20) indicates that twice as much 02 as C比is needed, and when they react, twice as much H20 as C02 will be produced.η1is is because during the reaction, each atom of carbon needs exactly two atoms of oxygen to combine with, to produce the C02, and every two atoms of hydrogen need an atom of oxygen to combine with to produce the H20. If the proportions of t he reactants are not respected, when they are forced to react, either not all of the substanc e used will participate in the react i on, or the react i on that will take p l ace will be different from the one noted in the equation.。
应用化学专业英语lesson10ChemicalEquilibriumandkinetics
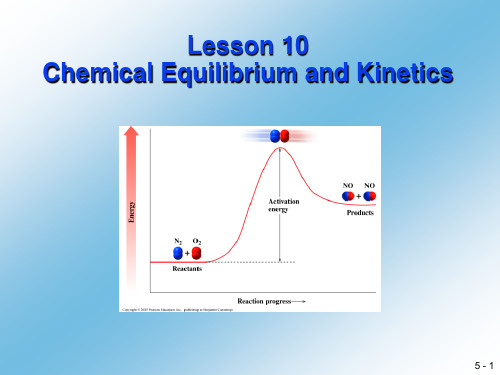
NH3
Add more NH3?
Reaction shifts to the left [N2] and [H2] inc
5 - 35
Le Chatelier’s principle
Adding Pressure affects an equilibrium with gases
N2(g) + 3 H2(g)
N2(g) + 3 H2(g)
Keq =
[ NH3 ] 2 [ N2 ] [ H2 ] 3
2 NH3(g)
5 - 33
Le Chatelier’s principle
Stress causes shift in equilibrium Adding or removing reagent
N2(g) + 3 H2(g)
Temperature: 2. Higher Temperature:
Faster cars More collisions
More Energy More collisions
Reacting molecules move faster, providing colliding molecules w/ Eact.
At this point, equilibrium is achieved. Time
5 - 26
Figure 9.8
2SO2(g) + O2(g)
At Equilibium
2SO3(g)
SO2(g)+O2(g)
Initially
SO3(g)
Initially
5 - 27
Figure 9.9
2SO2(g) + O2(g)
应用化学专业英语课后练习题含答案

5.A heterogeneous mixture is one in which the components are evenly distributed throughout.
Answer: False.
Short answer
4.What is the difference between an acid and a base?
Answer: An enzyme is a protein that acts as a catalyst in a biological reaction, lowering the activation energy required for the reaction to occur.
6.What is a heterogeneous mixture?
4.What is the definition of a chemical reaction?
Answer: A chemical reaction is a process in which one or more substances are transformed into different substances through the breaking and forming of chemical bonds.
Chapter 2
True or False
3.The pH scale is a measure of the concentration of hydrogen ions in a solution.
Answer: True.
4.A catalyst is a substance that changes the rate of a chemical reaction without being consumed in the reaction itself.
化学专业英语练习题

Final Examination PaperⅠⅠ. Monochoice questions酸碱滴定1. Which species cannot be titrated accurately with solution of strong base of 0.1mol/L HCl? ( ).A. Na3PO4(Ka1=7.5×10-3, Ka2=6.23×10-8, Ka3=2.2×10-13)B. Na3BO3(Ka=7.3×10-10 )C. NaCN(Ka=4.93×10-10 )D. NH4OH(Kb=1.75×10-8)2. Which answer is right in arithmetic setup of 38.91× (6.81-6.73)? ( )A. 3.113B. 3.11C. 3.1D. 33. In quantitative analysis, which method listed below can not be used to decrease the system error?( )A. instrument correctionB. increase the mensurationC. change the reagentD. temperature correction4. Use potassium acid phthalate (KHC8H4O4) as a primary standard substance to standardize theconcentration of the NaOH solution. How about the concentration of the NaOH solution standardized when there are a small quantity of neutral impurity in KHC8H4O4?()A. Its concentration standardized will be on the high side.B. Its concentration standardized will be on the low side.C. Its concentration standardized will not be affected.D. the influence on its concentration standardized is uncertain.5. Assuming the error is ±0.0001g when we use the analytical balance to weigh the sample, so how much is the relative error when we use the analytical balance to weigh 0.1000g sample in direct way.( )A. ±0.01%B. ±0.02%C. ±0.1%D. ±0.2%6. If we want to determine the concentration of Na2HPO4, Which standard solution as titrant listedbelow is reasonable?( )A. solution of 0.1 mol·L-1 H3PO4.B. solution of 0.1 mol·L-1 HCl.C. solution of 0.1 mol·L-1 NaOHD. solution of 0.1 mol·L-1 NH3.7. Which of the following is 5 for the number of significant figures ( )?.A. 1.200 ×108B. pH=12.245C. 0.00605D. 12.708%8. Which substance can use as indicator to show the end point for the titrations that range of titration jump is 5.7~6.5? ( )A. bromophenol blue(3.1~4.6)B. methyl red.C. phenolphthalein.D. bromthymol blue(6.0~7.6).9. Assuming the error is ±0.0001g when we use the analytical balance to weigh the sample. so how much is the relative error when we use the analytical balance to weigh 0.1000g sample in direct way.( )A. ±0.01%B. ±0.02%C. ±0.1%D. ±0.2%10. Which choice listed below is wrong when we talk about range of titration jump. ( )A. The range of titration jump depend on concentration of solution.B. The range of titration jump depend on strength of acid.C. The range of titration jump depend on strength of base.D. The range of titration jump depend on range of color change of indicator.依数性6. The red blood cell will be shrinkable in which solution listed below?()A. 10.0 g·L-1CaCl2·2H2O(Mr=147)B. 12.5g·L-1NaHCO3(Mr=84.0)C. 1.00 g·L-1NaClD. 224g·L-1C3H5O3Na(Mr=112)11. There are four water solutions of the equal volume in which there are equal mass of glucose, CaCl2, NaHCO3and sucrose respectively. Then whose freezing-point is the lowest? ( )[Mr(glucose)180( HAc)60(Na2CO3)106 (CaCl2) 111]A. GlucoseB. HAcC. CaCl2D. Na2CO312. Which choice listed below is isotonic solution? ( )A. 5% glucose solution and 5% sucrose solutionB. 1 mol·L-1 glucose solution and 0.5 mol·L-1 sucrose solutionC. 0.5 mOsmol·L-1 urea solution and 0.5 mOsmol·L-1 NaCl solutionD. 0.5 mol·L-1 MgSO4solution and 0.5 mol·L-1 CaCl2 solution电解质9. We learn that the K a of HF is 3.53×10-4 and the K b of NH3·H2O is 1.79×10-5, then which option following is true?( )A. NH4+is a stronger acid than HFB. NH4+is a weaker acid than HFC. the acidic strength of NH4+and HF are equal.D. can not do the compare13. A solution was prepared by mixing equal volume of 0.10mol·kg-1NH4Cl and 0.10mol·kg-1NH·H2O, its ionic strength ( I ) is ( ) mol·kg-1.3A. 0.05B. 0.075C. 0.10D. 0.1514. 0.10mol NaOH and 0.10mol HAc are dissolved into 1.0L distilled water together, please calculate the pH of this solution( ) (Ka(HAc)=1.74×10-5)(A) 10.28 B. 11.28 C. 8.88 D. 12.2815. There is 1L 0.1mol·L-1H2CO3 solution with the addition of 0.5ml 0.1mol·L-1 HCl solution. Which choice listed below is true? ( )A. pH decrease, dissociation degree of H2CO3 decrease.B. pH decrease, dissociation degree of H2CO3 increase.C. pH increase, dissociation degree of H2CO3 increase.D. pH increase, dissociation degree of H2CO3 decrease.16. A solution containing the equal concentrations of Cl-,I- and CrO42- ions. Add the AgNO3 to the solution drop by drop, then the sequence of the ions precipitating out of the solution is ( ).(KspAgCl=1×10-10,KspAg2CrO4=4×10-12,KspAgI=9×10-17)A. CrO42->I->Cl--B. CrO42-<I-<Cl--C. I->Cl-> CrO42D. I-> CrO42-> Cl-17. The solubility of Mg(OH)2 is maximum when it was dissolved in ( ).A. 1mol·L-1NH4Cl solutionB. 2mol·L-1MgCl2 solutionC. pure waterD. 1mol·L-1NaOH solution18. The concentration of every solutions listed below is 0.10mol·L-1, the pH of which is greater than 7?( )A. NH4ClB. Na2CO3C. NH4AcD. MgCl2缓冲溶液16.The buffer range of a buffer solution prepared by mixing 500ml 0.4mol·L-1 H2CO3 solution and 200ml 0.4mol·L-1 NaOH is about ( ). (pK a1=6.37; pK a2=10.25 )A. 1.12~3.12B. 6.21~8.21C. 11.32~13.32D. 5.37~7.37.19. If you mix two solutions of equal volume in each option listed below, which option has no buffer action. ()A. 0.2mol·L-1HCl和0.2mol·L-1KClB. 0.02mol·L-1HCl和0.04mol·L-1NH3·H2OC. 0.01mol·L-1KH2PO4和0.2mol·L-1Na2HPO4D. 0.01mol·L-1NaOH和0.02mol·L-1HAc20. The most important ACID resistant in the plasma of human being is ()A. H2PO4-B. HPO42-C. HCO3-D. H2CO321. The color of the solution is orange with the addition of methyl orange indicator. In order tokeep the pH of the solution stable, which buffer system listed below is the best? ( )A. 0.1mol·L-1 HAc — 0.1mol·L-1 NaAc (K a = 1.8×10-5)B. 0.1mol·L-1 NH3·H2O — 0.1mol·L-1 NH4Cl (K b = 1.8×10-5)C. 0.1mol·L-1 NaH2PO4 — 0.02mol·L-1 Na2HPO4(K a = 6.2×10-8)D. 0.1mol·L-1 HCN — 0.02mol·L-1 NaCN (Ka = 4.9×10-10)Ⅱ.Fill the blank. (Please fill your answers into the blanks following).1. When the HCl standard solution is used to titrate a sample solution which containing NaHCO3 and Na2CO3, the indicator is(1) in the first step of the titration,the indicator is (2)in the second step of the titration (pKa1=6.35;pKa2=10.33)2.A 2.05 g sample of white phosphorus was dissolved in 25.0g of carbon disulfide,CS2. The of the carbon disulfide solution was found to be 1.59℃. The molecular weight of the phosphorus is (3) g·mol-1 in solution? The formula of molecular phosphorus is (4) mol·L-1 (boiling-point elevation constant K b of CS2=2.4; Mr(P)=31)Ⅲ.Calculation1. A sample of 0.1276g of an unknown monoprotic acid was dissolved in 25.00 mL of water andtitrated with 0.0633 M NaOH solution. The volume of base required to reach the equivalence point was 18.4 mL. (a) Calculate the molar mass of the acid. (b) After 10.00 mL of base had been added in the titration, the pH was determined to be 5.87. What is the K a of the unknown acid?Final Examination PaperⅡⅠ. Monochoice questions依数性6. The osmotic pressure of a solution prepared by the equal volume of 8.4%(g/ml)NaHCO3 and18%(g/ml)glucose (C6H12O6) is equal to the osmotic pressure of ( ). [Mr(glucose)180 (NaHCO3)84]A. 5.85%(g/ml)NaCl solutionB. 1.5mol·L-1sucrose solutionC. 1mol·L-1glucose solutionD. 1 mol·L-1 CaCl2 solution7. Which of the following statements is a logical deduction ( )A. if a nonvolatile solute is added to water, the boiling point of the solution will be 100℃.B. the addition of a volatile solute will change the boiling point of the water.C. atmospheric pressure will affect the composition of the aqueous solution.D. if a nonvolatile solute is added to water, the freezing point of the solution will be lower than that of water.电解质8. Which species is the strongest acid that can exist in aqueous solution? ( )A. NaOHB. Na2CO3C. OH-D. KOH9. A 0.1 mol·L-1 solution of potassium acetate, KC2H3O2, has a lower pH than a 0.1 mol·L-1 solution of potassium cyanide, KCN. From this, you can correctly conclude that ( )A. hydrocyanic acid, HCN, is a weaker acid than acetic acid, HC2H3O2.B. hydrocyanic acid, HCN, is less soluble in water than acetic acid, HC2H3O2.C. the cyanide ion, CN–, is a weaker base than the acetate ion, C2H3O2–.D. acetate ion, C2H3O2, partially dissociates to form hydronium ion, H3O+.10. The factor that does not affect on the activity coefficient has ( )A. ionic concentrationB. charge on the ionC. ionic strengthD. K a or K b11.The pH of mixed solution by 0.10mol·L-1NH3 and 0.10mol/L NaOH is about ( ).(K b=1.8×10-5)A. 9B. 1C. 6D. 1312. The solubility of BaSO4 is not changed when it was dissolved in ( ).A. 1mol·L-1KCl solutionB. 2mol·L-1 Na2SO4 solutionC. pure waterD. no answer13. There is 1L 0.4mol·L-1Na2CO3 solution with the addition of 1.0 ml 0.1mol·L-1 HCl solution. Which choice listed below is true? ( )A. pH decrease, dissociation degree of CO32- decrease.B. pH decrease, dissociation degree of CO32- increase.C. pH increase, dissociation degree of CO32- increase.D. pH increase, dissociation degree of CO32- decrease.14. 25℃, the Ksp of Ag2CrO4is 1.12×10-12, so the concentration of Ag+ions in the saturate solution of Ag2CrO4 is ( )A. 6.54×10-5mol·L-1B. 1.21×10-5mol·L-1C. 1.21×10-4mol·L-1D. 6.54×10-4mol·L-1缓冲溶液15.The buffer range of a buffer solution in which there are the same concentrations of Na2HPO4 and NaH2PO4 is about. (pK a1=2.12; pK a2=7.21 pK a3=12.32)Which one is wrong? ( ).A. 1.12~3.12B. 6.21~8.21C. 11.32~13.32D. all the choice above16. To determine the content of Mg2+ and Ca2+ ions in tap water, in order to keep the pH =10,which buffer system listed below is the best? ( )A. 0.1mol·L-1 HAc — 0.1mol·L-1 NaAc (K a = 1.8×10-5)B. 0.1mol·L-1 NH3·H2O — 0.1mol·L-1 NH4Cl (K b = 1.8×10-5)C. 0.1mol·L-1 NaH2PO4 — 0.02mol·L-1 Na2HPO4(K a = 6.23×10-8)D. 0.1mol·L-1 H2CO3— 0.02mol·L-1 NaHCO3(Ka = 4.3×10-7)17. The most important ACID resistant in the plasma of human being is ()A. H2PO4-B. HPO42-C. HCO3-D. H2CO318. If you mix two solutions of equal volume in each option listed below, which option has no buffer action. ()A. 0.2mol·L-1HCl and 0.2mol·L-1KClB. 0.02mol·L-1HCl and0.04mol·L-1NH3·H2OC. 0.01mol·L-1KH2PO4and 0.2mol·L-1Na2HPO4D. 0.01mol·L-1NaOH and 0.02mol·L-1HAcⅡ.Fill the blank. (Please fill your answers into the blanks following)1. the theoretical range of color change of a weak basic indicator is (1) which K b is 1.0×10-42. A 1.0 g sample of the protein hemoglobin is dissolved in enough water to make 1 L (kg) of solution. The osmotic pressure of the solution is measured at 25 ℃and found to be 0.1 kPa. The molecular mass of hemoglobin is (2) and the molality of the hemoglobin solution is (3) .Final Examination PaperⅢⅠ. Monochoice questions滴定分析1.What is the result of calculation of (20.83--20.43)/0.4000? ( )A. 0.10B. 1.0C. 1.000D. 0.10002.If the K In of a weak basic indicator is 1.0×10-5, what is the color transition pH range of this indicator? ( )A. 4-6B. 6-8C. 7-9D. 8-103.What result would be if NaOH solution was standardized against potassium hydrogen phthalate, the measured concentrations of the NaOH solution in the cases that the initial buret reading of the NaOH solution should be recorded as 1.00 mL, but was recorded as 0.10 mL by mistake. ( )A. highB. lowC. unchangeD. uncertain4.Weigh 1.3350 g of analytical reagent Na2CO3to prepare 250.00 mL of primary standard solution, and use it to titrate approximate 0.1 mol·L-1HCl solution. If 25.00 mL is required to neutralize 24.50 mL of HCl completely and methyl orange is used to indicate end point, report the molarity of HCl solution. ( ) [Mr(Na2CO3)=106]A. 0.1028 mol·L-1B.0.2056 mol·L-1C. 0.05140 mol·L-1D. 0.4112 mol·L-15.依数性How much is the normal freezing points of the solution in which 21.0g NaCl is dissolved in 135mLof water ? [K f=1.86 K·kg·mol-1 , Mr(NaCl)=58.5] ( )A. -9.89℃B. 19.89℃C. 9.89℃D. 19.89℃6.If you want to have osmosis between two dilute solutions separated by semipermeable membrane, which choice listed below is wrong.()A.Both of the two osmotic pressure are not equal.B. Both of the two osmolarity are not equal.C.Both of the two solutions are not isotonic.D. Both of the two molality are not equal.7.In 500mL normal saline water, the osmolarity of the Cl-ions is ( ) mOsmol·L-1 [Mr(Cl)=35.5]A. 77B. 196C. 154D. 3088.The minimum mass of NaCl that would have to be added to 1.200×103 g H2O so the resulting solution would not freeze outside on a cold day(-10℃) is ( ) (K f=1.86 K·kg·mol-1 , Mr[NaCl]=58.5)A.94.3gB. 188.6gC.282.9gD.377.2g9.电解质There is 1L 0.1mol·L-1 HAc solution with the addition of 0.5mL 0.1mol·L-1 NaCl solution. Which choice listed below is true? ( )A. pH decrease, dissociation degree of HAc decrease.B. pH decrease, dissociation degree of HAc increase.C. pH increase, dissociation degree of HAc increase.D. pH increase, dissociation degree of HAc decrease.10.What is ionic strength ( I ) for the solution that contains 0.10 mol·kg-1NaCN and 0.10 mol·kg-1HCN ( ).A. 0.025mol·kg-1B. 0.050mol·kg-1C. 0.20mol·kg-1D. 0.10mol·kg-111.K sp for SrSO4 is 4.0×10-8 at certain temperature. How much is the solubility of SrSO4 in H2O. ( )A. 4.0×10-8 mol·L-1B. 2.0×10-4 mol·L-1C. 8.0×10-8 mol·L-1D. 1.0×10-4 mol·L-112.Which substance can use as ampholyte in different solvent?( )A. Na NO3B. HAcC. NaClD. Na OH13. A solution is 0.15 mol·L-1 in Pb2+ and 0.20 mol·L-1 in Ag+. If a solid of Na2SO4 is added slowly to this solution, which option of the following is true? ( ) K sp for PbSO4 = 2.53×10-8, Ag2SO4 =1.20×10-5A. PbSO4 will precipitate out of solution firstB. Ag2SO4will precipitate out of solution firstC. PbSO4 and Ag2SO4 will precipitate out of solution simultaneouslyD. no precipitate14.pH of the solution in which 0.2 mol·L-1 H3A solution and 0.6 mol·L-1 NaOH solution are mixed in the same volume is ( ) (Ka1= 1.0×10-5 , Ka2= 1.0×10-7 , Ka3= 1.0×10-9)A. 5.0B. 6.5C. 11.0D. 1.015.缓冲溶液Which option of the following determines the capacity of a buffer ( )A. Conjugate acid-base pairB. Buffer-component ratioC. Buffer rangerD. p K a of the acid component16.The buffer range of a buffer solution prepared by mixing 100mL 0.2mol·L-1 H2A solution and 100mL 0.3mol·L-1 NaOH is about ( ). (pK a1=4.00; pK a2=9.00 )A. 3.00~5.00B. 5.00~7.00C. 8.00~10.00D. 9.00~11.0017.If two solutions are mixed in equal volume in each option listed below, which option has no buffer action? ()A. 0.2 mol·L-1 NaOH and 0.2 mol·L-1 KClB. 0.2 mol·L-1 HCl and 0.4 mol·L-1 NH3·H2OC. 0.1 mol·L-1 H3PO4 and 0.1 mol·L-1 Na2HPO4D. 0.1 mol·L-1 NaOH and 0.2 mol·L-1 HAc18.To prepare a buffer of pH 9, which buffer system listed below is the best? ( )A. 0.1 mol·L-1 HAc — 0.1 mol·L-1 NaAc (p K a = 4.75)B. 0.1 mol·L-1 NH3·H2O — 0.1 mol·L-1 NH4Cl (p K b = 4.75)C. 0.1 mol·L-1 H2CO3— 0.15 mol·L-1 NaOH(pK a1=6.37; pK a2=10.25 )D. 0.1 mol·L-1 HCN — 0.02 mol·L-1 NaCN (p K a = 9.5)Ⅱ. Simple answer question1.酸碱滴定Can 0.1000 mol·L-1 formic acid (HCOOH) of 20.00 mL be titrated by 0.1000 mol·L-1 NaOH standard solution directly? Please give reasons. What indicator can be used to signal endpoint (p K a = 3.75)?(5 marks)2.电解质溶液Pivaic acid is a monoprotic weak acid. A 0.100 mol·L-1 solution of pivalic acid has a pH=3.00. What is the pH of 0.100 mol·L-1 sodium pivalate at the same temperature?3. How many significant figures are there in each of the following numbers (assume that each number is a measured value)?3.25 0.0025 0.0203 2.3% 0.900 0.2530 1.3830 2.0 105 pH=3.21Final Examination PaperⅣⅠ. Monochoice questions1.酸碱滴定If you had to do the calculation of (22.83--21.43)/1.4000, what would be the correctresult of significant figure? ( )A. 0.10B. 0.100C. 1.00D. 1.0002.If the K HIn of a weak acidic indicator is 1.0×10-5, what is the color transition pH range of thisindicator? ( )A. 4-6B. 6-8C. 7-9D. 8-103.What result would be if NaOH solution was standardized against potassium hydrogen phthalate, the measured concentrations of the NaOH solution in the cases that the mass of potassium hydrogen phthalate should be 0.3510 g, but was recorded as 0.3570 g by mistake? ( )A. highB. lowC. unchangeD. uncertain4.How many grams of potassium hydrogen phthalate (KHC8H4O4) primary standard substance are required to standardize about 25 mL of 0.1 mol·L-1 NaOH solution? ( ) [Mr(KHC8H4O4)=204 g·mol-1]A. 0.2550gB.0.5100gC. 0.05100gD. 1.0200g5.依数性How much is the normal freezing points of the solution in which 15.4g of urea is dissolved in 66.7 mL of water ? [K f=1.86 K·kg·mol-1 , Mr(CON2H4)=60.0] ( )A. -7.16℃B. 0℃C.-0.25℃D. 1.11℃6.The osmolarity of 1000 mL officinal solution in which it contains NaHCO3 of 6.45g·L -1 andKCl of 5.79 g·L -1 is ( ) mOsmol·L-1. [Mr(NaHCO3)=84, Mr(KCl)=74.5]A. 76.3B. 152.7C. 309D. 6107. A hemoglobin (Hb) solution of 1L is prepared by dissolving 35.0g of Hb into water. If the osmotic pressure of the solution is found to be 1.33kPa at 25℃. The molar mass of hemoglobin is ( ) .A. 537B. 5.37×10-4C. 6.52×104D. 1008.电解质8,9,10,11,12,13,14There is 1L 0.1mol·L-1NaHCO3solution with the addition of0.1mol NaCl solid. Which choice listed below is true? ( )A. pH unchanged, dissociation degree of HCO3 unchange.B. pH decrease, dissociation degree of HCO3 increase.C. pH increase, dissociation degree of HCO3 increase.D. pH increase, dissociation degree of HCO3 decrease.9.what is ionic strength ( I ) for 0.10 mol·kg-1 NaCl solution ( ) mol·kg-1.A. 0.025 mol·kg-1B. 0.050mol·kg-1C. 0.20mol·kg-1D. 0.10mol·kg-1PO ion is 3.3×10-7 10.In a saturated solution of calcium phosphate, the concentration of 34mol·L-1. the K sp of Ca3(PO4)2 is ( )A. 3.3×10-7B. 1.65×10-7C. 9.9×10-21D. 1.3×10-3211.Which substance can use as strong base in glacial acetic acid ?( )A. HAcB. NH3C. H2OD. H3PO412.A solution is 0.15 mol·L-1in Pb2+and 0.20 mol·L-1in Ag+. If a solid of Na2SO4is added slowly to this solution until the Ag+ starts to precipitate as the sulfate. What is SO42- concentration reached at least at this point? ( ) K sp for PbSO4 = 2.53×10-8, Ag2SO4 =1.20×10-5.A. 1.7×10-8B. 2.53×10-8C. 3.0×10-4D. 1.20×10-513.pH of solution in which 0.2 mol·L-1H3PO4solution and 0.2 mol·L-1Na3PO4solution are mixed in the same volume is ( D ) (Ka1= 7.5×10-3 , Ka2= 6.3×10-8 , Ka3= 2.2×10-13)A. 12.8B. 1.32C. 2.12D. 7.2114.125.0 mL of 0.40 mol·L-1 propanic acid, HPr, is diluted to 500.0 mL. What will the final pH of the solution be? (K a=1.0×10-5) ( )A. 3B. 11C. 5D. 9缓冲溶液15.The buffer range of a buffer solution prepared by mixing 100ml 0.2mol·L-1 H3PO4 solution and 100ml 0.5mol·L-1 NaOH is about ( ). (pK a1=2.16; pK a2=7.21; pK a3=12.32 )A. 1.16~3.16B. 6.21~8.21C. 8.00~10.00D. 11.32~13.3216.The color of the solution is yellow with the addition of methyl orange indicator and red with the addition of methyl red. In order to keep the pH of the solution stable, which buffer system listed below is the best? ( )A. 0.1mol·L-1 HAc — 0.1mol·L-1 NaAc (p K a = 4.75)B. 0.1mol·L-1 NH3·H2O — 0.1mol·L-1 NH4Cl (p K b = 4.75)C. 0.1mol·L-1 NaH2PO4 — 0.1mol·L-1 Na2HPO4(pK a2=7.21 )D. 0.1mol·L-1 HCN — 0.1mol·L-1 NaCN (p K a = 9.5)17.Which option has largest increase of pH when add 0.5mL of 0.1 mol·L-1NaOH in the following solution? ( )A. 0.1 mol·L-1 HAc — 0.1 mol·L-1 NaAc (p K a = 4.75)B. 0.1 mol·L -1 NH 3·H 2O — 0.1 mol·L -1 NH 4Cl (p K b = 4.75)C. 0.1 mol·L -1 H 2CO 3 — 0.15 mol·L -1 NaOH (pK a1=6.37; pK a2=10.25 )D. 0.1 mol·L -1 HCN — 0.02 mol·L -1 NaCN (p K a = 9.5)18. To prepare a buffer of pH 10.5, which buffer system listed below is the best? ( )A. CH 3NH 2·HCl —CH 3NH 2 (p K a =10.65)B. NH 3·H 2O —NH 4Cl (p K a =9.25)C. Na 3PO 4 —Na 2HPO 4 (pK a3 = 12.32)D. H 2CO 3—NaHCO 3 (pK a1=6.37)Ⅱ.Calculation1. 电解质Ethylamine, CH 3CH 2NH 2, has a strong, pungent odor similar to that ammonia. Likeammonia, it is a base. A 0.10 mol·L -1 solution has a pH of 11.86. Calculate the K b for theethylamine, and find K a for its conjugate acid, 323NH CH CH .2. Calculate the osmotic pressure of 0.020mol·L-1 NaCl solution at 25 ℃.3. (1) 0.1mol·L-1 HAc solution 。
2020-2021某大学《化学专业英语》期末课程考试试卷(含答案)

《化学专业英语》期末课程考试试卷考试所需时间:120分钟适用专业:应用化学总分:100分PartⅠ、Choice(28×1.5=42)1 How many neutrons are present in an atom of tin that has the atomic number 50 and a mass number of 119?A. 50B.69C. 119D. 1692. Isotopes of an element differ in their ____.A. atomic numbersB. electron configurationsC. number of protonsD. masses3. Atomic masses for elements shown on the periodic table are not expressed as whole numbers because __A. the number of protons in an atom of an element variesB. atoms may gain or lose electrons during a chemical reactionC. they represent weighted averages of the isotopes of that atomD. scientists cannot measure the masses of atoms with great precision4. Group 17 elements, the halogens, are the most reactive of the nonmetals because they ____.A. are the farthest to the right of the periodic tableB. require only one electron to form the stable configurations of the noble gasesC. have the largest atomic radiiD. have the greatest ionization energies5.In the modem periodic table, elements are ordered ac-cording to ____.A. decreasing atomic massB. Mendeleev's original designC. increasing atomic numberD. when they were discovered6.The energy it takes to remove an electron from an atom as you move left to right across the period, from Na through to Cl, ___.A. generally increasesB. generally decreasesC. does not changeD. varies unpredictably7.Which of the following is the correct formula for iron (III) sulfate? ____.A. Fe3SO4C. Fe3(SO4)2B. Fe2(SO4)3D. 3FeSO48. The electroneutrality principle ____.A. states that the number of cations equals the number of anionsB. is demonstrated in any polyatomic ionC. states that the net charge on a binary ionic com-pound is zeroD. all of the above9. The correct name for NH4NO3is ____.A. ammonium carbonateB. ammonium hydroxideC. ammonium acetateD. ammonium nitrate10. When an acid reacts with a metal, ____A.the hydronium ion concentration increasesB.the metal forms anionsC.water is producedD.the pH value decreases11.Which of the following is a binary acid ?A. H2O B. H3PO4C. H2SO4D. HCl12.Which of the following solutions would have a pH value greater than 7 ?A.[OH-]=2.4×10-2MB.[H3O+]=1.53×10-4MC.0.0001M HClD.[OH-]=4.41×10-11M13.If the empirical formula of a compound is known, thenA. its true formula is also knownB. its percentage composition can be calculatedC. the arrangement of its atoms is also knownD. the percentage water in the compound can be determined14.The units for molar mass are ___A. g/mol C. g/atomsB. atoms/mol D. mol/g15.Which of the following compounds has the highest percentage composition of oxygen?A. CH4O C. H2OB CO2 D. Na2CO316.Pressure can be measured in ___A. grams C. pascalsB. meters D. liters17.A sample of oxygen gas has a volume of 150 mL when its pressure is 0.947 atm. If the pressure is increased to 0. 987 atm and the temperature remains constant, the new gas volume will be ___A. 140 mL C. 200 mLB. 160 mL D. 240 mL18.A sample of neon gas occupies a volume of 752 mL at 25℃. What volume will the gas occupy at 500℃ if the pressure remains constant? ___A. 694 mL C. 815 mLB. 752 mL D. 955 mL19.Potatoes will cook faster at sea level than at higher altitudes because the water used to cook them willA. be boiling more rapidlyB. boil at a lower temperatureC. increase in temperature while boilingD. boil at a higher temperature20.If the temperature outside is 26℃,then the temperature would be ____ kelvins.A. 26 C. 299B. 273 D. -24721. If the empirical formula of a compound is known, thenA. its true formula is also knownB. its percentage composition can be calculatedC. the arrangement of its atoms is also knownD. the percentage water in the compound can be determined22.Examine the following skeletal structure:OHOThe correct chemical formula for this compound isA. C2H4O2C. C5H8O2B. C5H4O2D. CHO23.Identify the following reactions as either reduction or oxidation. Indicate whether they occur at the cathode or anode. A. Ra(s) →Ra2+(aq) + 2e-B. Hg22+(aq) + 2e-→2Hg(l)C. Pb(s) + SO42-(aq) →PbSO4(s) + 2e-D. O2(g) + 2H2O(l) + 4e-→4OH-(aq)24.In the following reaction, which species is being reduced?2K+Br2→2K++2Br-A. K only C. both K and Br2B. Br2only D. neither K nor Br225.The electrode at which reduction occurs is ____.A. always the anodeB. always the cathodeC. either the anode or the cathodeD. always the half-cell26. Sulfuric acid, H2SO4, or a similar substance is added to water that is to be electrolyzed in order to ____.A. react with the waterB. keep the electrode cleanC. provide adequate conductivityD. supply energy27.If an exothermic reaction has reached equilibrium, increasing the temperature willA. favor the forward reactionB. favor the reverse reactionC. favor both the forward and reverse reactionD. have no effect on the equilibrium28 Consider the following reaction:COBr2(g) →CO(g) + Br2(g)At 73°C , the Keqvalue for this reaction is 0.190. This Keqvalue indicates that ____.A. the reverse reaction is favoredB. the forward reaction is favoredC. the reaction has reached equilibriumD. the concentrations of CO(g) and Br2(g) are greater than theconcentration of Br2(Part Ⅱ common skill (20×0.5=10)1.write out the English speaking of the following symbol s(1).Mg(OH)2(2)↓→+++3223CaCOCaCO (3).log n x(4).nX(5).−−→−∆,Cu (6). X -8 (7). 1235(8). 3:2 (9). ± (10).100℃2.write out the chemical Chinese meaning of the followingabbreviation(1).alc. (2).amt. (3).A ·P (4).app. (5).contg. (6).C ·P (7).detn. (8).fig. (9).L-R (10).resp.Part Ⅲ Write out IUPAC naming of the following organic matter in English ( 1.5×8=12)CH 3CH 3CH 3CH 3⑴ C H 3CH 3CH 3CH 3⑵CH 2CH 3⑶C H 3CH 3H 3OH ⑷CH 3O CH 3⑸CH 3CH 3O⑹CH 3OHO ⑺CH 32O⑻Part Ⅳ write out English name of the following chemical elements(1×10)H B C N O F Si S K ClPart Ⅴ Translate the following passages into Chinese (2×8+1×10=26)Passage one : Certain membranes made of an animal bladder, a slice ofvegetable tissue, or a piece of parchment, act as a barrier between twosolutions, and simultaneously allow specific types of molecules. These are called semipermeable membranes. Semipermeable membranes that allow passage of solvent molecules but do not allow passage of solute molecules or ions are called osmotic membranes. If a NaCl solution is separated from pure water by an osmotic membrane, H 2O molecules spontaneously penetrate the membrane from both directions; however, passage across the membrane from the pure water side is faster than passage across the membrane from the solution side. The net result is exactly like that illustrated already previously and involves a net transfer of H 2O from the pure water side of the membrane to the solution side of the membrane. The passage of solvent molecules from a region with little or no dissolved solute, through an osmotic membrane, to a region with more dissolved solute is called osmosis.Passage two: As in ionic bonding and covalent bonding, outer shell electrons are responsible foe bonding between metal atoms. However, it is unreasonable to assume that ionic bonds occur between metal atoms since all the atoms are alike and no single atom would give up electrons to another atom. Covalent bonding between metal atoms is almost as unreasonable because not enough outer shell electrons are available for as many shared-pair bonds as each metal atom seems to form. Instead, a metallic lattice consists of a regular array of positive ions immersed in a cloud of highly mobile outer shell electrons. Metals have relatively low ionization energies or relatively loose holds on their outer shell electrons. These electrons are free to move throughout the metallic lattice. Metallic bonding results from attraction between the positive ions and the cloud of negative electrons. Such attractive forces are weaker than ionic or covalent bonding forces. Thus, many metals are soft and fairly low melting. Potassium is soft enough to be cut with a knife and melt at 68.7℃. On the other hand, some of the transition metals, where significant covalent character is superimposed on the metallic lattice, are hard and high melting.Tungsten is very hard and melts at about 3410℃Passage three: Major branches of Chemistry .The body of knowledge about chemicals and chemical reactions is so vast that for convenience chemists have divided the study of chemistry into several major branches: l. Analytical chemistry: The study of what types of elements and compounds are present in a sample of matter — called qualitative analysis — and how much of each element and compound is present in a sample of matter — called quantitative analysis.2. Physical chemistry: The study of the scientific laws and theories that attempt to describe and explain the structure of matter, the chemical bonds that hold matter together, the changes that matter undergoes, and the energy involved in these changes.3. Organic chemistry: The study of the properties and reactions of hydrocarbons, compounds containing only the elements carbon and hydrogen, and other compounds derived from hydrocarbons that contain one or more other elements such as oxygen, nitrogen, sulfur, phosphorus, and chlorine. About4.9 million of the 5 million officially identified compounds are classified as organic compounds—explaining why an entire branch of chemistry is devoted to studying these compounds4. Inorganic chemistry: The study of all elements and the properties and reactions of the compounds not classified as organic compounds.5. Biochemistry: The study of the properties and reactions of compounds found in living organisms and those that are important to living organisms.These branches make it easier to study chemistry. Real chemistry, however, almost always involves a blend of information and ideas from most — if not all — of these branches. This book is concerned with general chemistry — a survey and introduction to all the major branches of chemistry except biochemistry.2020-2021《化学专业英语》期末课程考试试卷答案PartⅠ、Choice(28×1.5=42 points)(按顺序填入答案)1—5题 ADCAC 6—10题 ABBCB11—15题 CABAC 16—20题 CACDC 21—25题 CACBA 26—28题 CBA Part Ⅱ1.(1) Magnesium hydroxide(2) Nitrogen reacts with hydrogen to form ammonia at high temperatureand pressure with the presence of a catalyst.(3) Log x to the base n.(4) The nth root of x.(5) Calcium carbonate when heated produces calcium oxide and carbondioxide.(6) X to the minus eighth (power).(7) Five over one hundred and twenty_three.(8) The ratio of two to three.(9) Plus and minus.(10) One (a) hundred degrees Centigrade.2. (1)醇(2)量(3)分析纯(4)装置(5)含有。
第二版应用化学专业英语课后答案
Unit 1 The Roots of ChemistryI. Comprehension.1.C2. B3. D4. C5. BII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerabledifficulty, and it is necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematizedknowledge and is also an activity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistryobserved on a single mundane planet.4.People are made of molecules; some of the molecules in people are rathersimple whereas others are highly complex.5.Chemistry is ever present in our lives from birth to death because withoutchemistry there is neither life nor death.6.Mathematics appears to be almost as humankind and also permeates allaspects of human life, although many of us are not fully aware of this.III. Translation.1.(a) chemical process (b) natural science (c) the technique of distillation2.It is the atoms that make up iron, water, oxygen and the like/and so on/andso forth/and otherwise.3.Chemistry has a very long history, in fact, human activity in chemistry goesback to prerecorded times/predating recorded times.4.According to/From the evaporation of water, people know/realized thatliquids can turn/be/change into gases under certain conditions/circumstance/environment.5.You must know the properties of the material before you use it.IV. Translation化学是三种基础自然科学之一,另外两种是物理和生物。
化学专业英语试卷A答案
2012—2013学年度第一学期应用化学专业《专业英语》课程试卷(A )注意事项:1. 考生务必将自己姓名、学号、专业名称写在指定位置;2. 密封线和装订线内不准答题。
一、词汇填空 (写出下列每个词汇对应的英汉单词)(共20小题,每空1分,共20分)1、化学性质 (chemical property )2、物理性质 (physical property )3、溶解度 (solubility )4、密度 (density )5、沸点 (boiling point )6、熔点 (melting point )7、反应 (reaction )8、无机的 (inorganic )9、有机的 (organic )10、化合物 (c ompound )11、烷烃 (alkane )12、乙醇 (ethanol )13、烯烃 (alkene )14、炔烃 (alkyne )15、ester ( 酯 )16、ether ( 醚 )17、acetone( 丙酮 )18、formaldehyde ( 甲醛 )19、ammonia ( 氨 )20、benzene ( 苯 )二、给下列无机化合物的英语名称(共10小题, 每小题2分,共20分)1、CaO calcium oxide2、HClO 4 perchloric acid3、CuSO 4 copper sulfate4、NaBrsodium bromide 5、NaCl sodium chloride6、HNO 3 nitric acid7、HNO 2 nitrous acid8、Al 2O 3 aluminum oxide9、KNO 3 potassium nitrate10、FeBr 3 ferric bromide三、给下列有机化合物的英语名称(共5小题, 每小题4分,共20分)1.辛烷octane2.CH 2=CHCH 2CH 31-butene3.CH 3CH 2CH 2CH 2OHbutanol4.CH 3CH 2OCH 3ethyl methyl ether5.CH3(CH2)4CO2Hhexanoic acid四、英译汉(共10小题, 每小题4分,共40分)Array1、The properties of substances are their characteristic qualities. Thephysical properties are those properties of a substance that can be observed without changing the substance into other substances.物质的性质是它们的特别品质。
最新应用化学专业英语第二版万有志主编版(课后答案和课文翻译)资料
Unit 1 The Roots of ChemistryI. Comprehension.1.C2. B3. D4. C5. BII. Make a sentence out of each item by rearranging the words in brackets.1. The purification of an organic compound is usually a matter of considerable difficulty, and it is necessary to employ various methods for this purpose.2. Science is an ever-increasing body of accumulated and systematized knowledge and is also an activity by which knowledge is generated.3. Life, after all, is only chemistry, in fact, a small example of chemistry observed on a single mundane planet.4. People are made of molecules; some of the molecules in people are rather simple whereas others are highly complex.5. Chemistry is ever present in our lives from birth to death because without chemistry there is neither life nor death.6. Mathematics appears to be almost as humankind and also permeates all aspects of human life, although many of us are not fully aware of this.III. Translation.1. (a) chemical process (b) natural science (c) the technique of distillation2. It is the atoms that make up iron, water, oxygen and the like/and so on/andso forth/and otherwise.3. Chemistry has a very long history, in fact, human activity in chemistry goesback to prerecorded times/predating recorded times.4. According to/From the evaporation of water, people know/realized thatliquids can turn/be/change into gases under certain conditions/circumstance/environment.5. You must know the properties of the material before you use it.IV . Translation化学是三种基础自然科学之一,另外两种是物理和生物。
应用化学专业英语经典题
1. Hydrogen is prepared by means of _D___ reaction in lab.(a) substitution (b) decomposition (c) addition (d) displacement2. Benzene reaction with Cl2 belongs to __A__a) substitution (b) decomposition (c) addition (d) displacement3. The __C__ elements from monoatomic molecules due to their filled valent electronic configurations.(a) representative (b) transition element (c) inert gas (d) p-block4. Alkenes are produced in __B__ reaction as described below(a) addition (b) elimination (c) displacement (d) substitution5. The __C__ structure of CH4 has been verified by electron diffracxtion because of sp3 hybridization.(a) flat square (b) parellel tetragonal (c) tetrahedral (d) tetragonal6. The octet rile simoly states that an atom tends to gain or lose electrons until there are __B__ electrons in its valent shell(a) 12 (b) 8 (c) 6 (d) 127. When water combines with pure CuSO4, a __B__ forms(a) hydrogenate (b) hydrate (c) hydrocarbon (d) hydride8. Lyophobic colloid generally have _A___ identity between their molecules and solvent molecules.(a) attraction (b) electrostatic force (c) repulsion (d) electronegativity9. Which of the following has minimum ionization energy D(a) Li (b) Na (c) K (d) Rb10. The seawater is saline solution. To desalinate seawater is the process to _D___ salt component(a) add (b) dilute (c) concentrate (d) remove11. There are(is) __A_ paired electrons in hydrogen(a) 1 (b) 2 (c) 3 (d)412. The valent electronic configuration for fluorine is __C___(a) 1s2 (b) 2s2 (c) (d) is2. 2s2, 2p513. The octet means that the number of electrons in _C___ shell has to be eight.(a) innermost (b) the whole (c) outermost (d) no14. Polysaccarides like cellulose and starch must first be subjected to _A__ to form simpler sugar, such as monosaccarides.(a) dehydration (b) composition (c) hydrolysiis (d) comparsion15. Which of the following belongs to transition metal D(a) S (b) Na (c) He (d) V16 Addition of AgNO3 in a solution generates white AgCl __C__ that does not dissolve in HNO3(a) solvent (b)silica-gel (c) precipitate (d) solvent1. The nature of organic species present depends on the degree of biochemical change of the original plant, C(a) pattern (b) fractions (c) substances (d) ceramics2. I(py)2+ appears to be present in solution C(a) be made (b) be dissolved (c) be detected (d) occurM-F-M bridge bonds invariably have greater M-F distances than corresponding terminal M-F bonds. C(a) seldom (b) strongly (c) usually (d) never4. The chemical potential decreases and both the effect, ., solubility and normal temperature effect. B(a) for example (b) that is to say (c) through (d) approximatelyM-F-M bridge bonds invariably have greater M-F distances than corresponding terminal M-F bonds. A(a) end (b) head (c) initial (d) intermediate6. The corresponding bromine-and chlorine-species are less stable. B(a) compound (b) substances (c) supports (d) elementsH2O2, hydrogen peroxide Na2O2, sodium peroxide Se, selenium F , fluorine Br2, brominePCl3, phosphorus trichloride HI, hydrogen iodide AgI, silver iodide I2, iodine I-, iodideICl3, iodine trichloride Br-, bromide NF3, nitrogen trifluoride ClF7, chlorine heptafluoride CCl4, carbon tetrachloride PF3, phosphorus trifluoride Cl2O7 dichlorine heptoxideHydration--combination with water molecules.1. The _A___ ion can be considered s hydrated proton, H3O+.(a) hydrogen (b) hydroxyl (c) hydronium (d) hydroxo2. Chlorine is bubbled into aqueous sodium bromide, __A__ is liberated(a) bromine (b) dibromide (c) bromide (d) bromide ion3. Which of the following is diatomic molecule D(a) He (b) Ne (c) Ar (d) H24. Which of the following is oxoanion C(a) S2-, (b) HS-, (c) SO42-, (d) H2S1. In addition, for first order model the intercept of the straight line plots of log(qe-qt)against t B(a) therefore (b) Furthermore (c) But (d) Actually2. Most fluorine compounds are obtained from fluorspar via HF B(a) in (b) through (c) of (d) containingSnCl4, tin(IV) chloride FeCl3, iron(III) chloride PbCl4, lead(IV) chloride Sn2+,tin(II) ionCaCl2, calcium chloride I3-, triiodide ion CdCl2, cadmium chloride S2-,sulfide ionHgCl2, mercury(II) chloride FeO, iron(II) oxide Cu2O, copper(I) oxide O2-,oxide ionAlCl3, aluminium chloride CBr4, carbon tetrabromide CdS, cadmium sulfide S22-,disulfide ionZnO, zinc oxide V5+, vanadium(V) ion Cr3+, chromium(III) ion SiO2, silicon dioxideSnCl2, tin(II) chloride H+, hydrogen ion Mg2+, magnesium ion O22-, peroxide ionK+, potassium ion Be2+, beryllium ion Tl+, thallium(I) ion Ti4+, titanium(IV) ionPb2+, lead(II) ion Br-, bromide ion Se2-, selenide ion Ca2+, calcium ion Fe2+, iron(II) ionSn4+, tin(IV) ion Hg2Cl2, mercury(I) dichloride Al3+, aluminium ion1. The diversity of carbon compound is duo to formation of very long chain of carbon atom by the strong carbon-carbon __D__ bond(a) ionic (b) metallic (c) hydrogen (d) covalent2. An inportant quantity, particular for first-order reaction, is _B___ , the length of time required for the concentration of reactant to be decreases to half of its initial value(a) filled (b) half-life (c) half-filled (d) partiallly-filled3. In alkane of CH3CH2CH3, there are _D___ substituents.(a) 2 (b) 3 (c) 8 (d) no4. In alkane CH3Ch2CH3, there are __B__ carbons in parent chain(a) 2 (b) 3 (c) 8 (d) 85. Environment preotection is a global process which has also included pollution of aquasphere, ., __B__ is polluted.(a) soil (b) water (c) chemical reagent (d) atmosphere3. Reaction of sodium carbonate with dilute sulfuric acid will generate __A__.(a) a gas (b) a precipitate (c) aqueous solute (d) emulsionCH2BrCH=C(CH3)CH2CH2CH3, 1-bromo-3-methyl-2-hexene CH3(CH2)10CH3, dodecaneCH3CH=CHCH3, 2-butene CH3(CH2)16CH3, octadecane CCl2=CCl2 tetrachloroetheneCH3CH=CHCH2CH3 2-pentene CH3CH2CH3 propane CH2=CH2 ethenetrans-3-hexene3-ethyl-4-methylhexanezinc chloride hexahydrate zinc sulfate heptahydrateRbMgCl3 magnesium rubidium chloride calcium sulfate dihydrateH4P2O7 diphosphoric acid cadmium chloride hexahydrateCu2(OH)2CO3 copper(II) hydroxide carbonate CsCo(SO4)2 cesium cobalt sulfateH2S2O7 disulfuric acid iron(II) sulfide heptahydrate(NH4)2Fe(SO4)2 ammonium iron(II) sulfate K2Cr2O7 potassium dichromatesodium sulfate decahydrate KMnO4, potassium permanganateK2MnO4, potassium manganate MnO4-, permanganate ion MnO2 manganese(IV) oxideCrO3, chromium(VI) oxide CrO42-, chromate ion K2CrO4, potassium chromateCr2O3, chromium(III) oxide H2SO4. Sulfuric acid H2SO3. Sulfurous acid H2S(aq), hydrosulfuric acid As2S3, diarsenic trisulfide H2S(as a gas), hydrogen sulgideSF6, sulfur hexfluoride NaHSO3, sodium hydrogen sulfite HCl(a gas), hydrogen chlorideNaHSO4, sodium hydrogen sulfate ClO3-. Chlorate ion HClO3, chloric acid HCl(aq), hydrochloric acid KVO3, potassium vanadate HVO3, vanadic acid V2O5, divanadium pentoxide VCl3, vanadium(III) chloride Al(NO3)3, aluminium nitrateNaAlO2,sodium alumate Al(OH)3, aluminium hydroxide Fe(NO3)3, iron(III) isopropylcyclohexanetert-butylcyclohexane2-ethyl-1-isobutylcyclohexanecyclopentanecyclopropanenitrateCa(BrO2)2, calcium bromite Na2S, sodium sulfide I-, iodide ion IO2-, ioditeionH2MnO4, manganic acid CaCl2, calcium chloride Na2SO3, sodium sulfiteKI, potassium iodide KIO2, potassium iodite HBrO, hypobromous acidNaBrO, sodium hypobromite NaBr, sodium bromide NaBrO3, sodium bromateKPO3,potassium phosphorate HPO3, metaphosphoric acid K3PO4, potassium phosphorate H3PO4 phosphoric acidm=chlorofluorobenzeneP-bromochlorobenzenem-nitroiodobenzenemethylenecyclohexanem-methylphenol(1-menthylvinyl)benzene 2-methyl-1-penten-3-y2-chloro-1,3-butadi2-propanol 2,4-hexadiyne-1,6-diol2,4-hexadiene-1,6-diol4-hexen-2-olcis-1,2-dimethyl-1,2-cyclopentatrans-1,2-dimethyl-1,2-cyclopent2-propynol1,3-cyclohexadiene(1-methylvinyl)cyclopentanebenzylcyclohexanephenylcyclohexane diphenylmethane1-penten-3-yne 2,5-heptadiyne 1,3-diphenylpropaneCyclohexane-1,2-diol2,5-cyclohexadiene-1,44-hexene-1,2-diolallylbenzene vinylcyclohexane3-butynal ethanenitrilcyclohexanecarboxylic acid cyclohexanedicarboxylic1,2-cyclohexanedinitrile ethanamide ethanyl chloridesec-butylethanoate4-methoxyphenol 2,5-hexanedione dimethyl1. Alkenes and alkynes are classified into __B_ compound(a) cyclic (b) unsaturated (c) aromatic (d) straight chain2. The general formula R-C=N-O is called cyanate, while R-N=C=O is called _B__(a)cyano (b) isocyanate (c)nitrile (d) cyanide3. Reaction between __C__ chloride and sodium hydroxide will release a pungent gas.(a) amino (b) amine (c) ammonium (d) ammonia4. Which of the following belons to triprotic acid D(a) HNO3 (b) H2SO4 (c) HNO2 (d) H3PO45. Animal and vegetable oil and fat are a more specialized source of a limit number of __C__ compounds, including fatty acid such as CH3(CH2)16COOH and a long chain alcohol as CH3(CH2)11OH(a) petroleum (b) inorganic (c) aliphatic (d) aromatic6. The general term for species that is active in the interface medium between phase is ___A__.(a) a surfactant (b) a nucleophile (c) an electrolyte (d) an electrophile--------------------------------------------------------------------------1,4-butanedithioldimethyl ethanedioatetetrabutylammonium hydroxidemethanal2-chloro-1,3-butadiene1,3-propanediol2-propanol2-aminophenol3-methylphenolN-methyl-1-aminomethanethanedioic acid1-pentyn-4-one1,2-methoxycyclobutanethyl ethanoate2-butanol-3-one4-cyclopentene-1,2-dinitri。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1. Hydrogen is prepared by means of _D___ reaction in lab.(a) substitution (b) decomposition (c) addition (d) displacement2. Benzene reaction with Cl2 belongs to __A__a) substitution (b) decomposition (c) addition (d) displacement3. The __C__ elements from monoatomic molecules due to their filled valent electronic configurations.(a) representative (b) transition element (c) inert gas (d) p-block4. Alkenes are produced in __B__ reaction as described below(a) addition (b) elimination (c) displacement (d) substitution5. The __C__ structure of CH4 has been verified by electron diffracxtion because of sp3 hybridization.(a) flat square (b) parellel tetragonal (c) tetrahedral (d) tetragonal6. The octet rile simoly states that an atom tends to gain or lose electrons until there are __B__ electrons in its valent shell(a) 12 (b) 8 (c) 6 (d) 127. When water combines with pure CuSO4, a __B__ CuSO4.5H2O forms(a) hydrogenate (b) hydrate (c) hydrocarbon (d) hydride8. Lyophobic colloid generally have _A___ identity between their molecules and solvent molecules.(a) attraction (b) electrostatic force (c) repulsion (d) electronegativity9. Which of the following has minimum ionization energy? D(a) Li (b) Na (c) K (d) Rb10. The seawater is saline solution. To desalinate seawater is the process to _D___ salt component(a) add (b) dilute (c) concentrate (d) remove11. There are(is) __A_ paired electrons in hydrogen(a) 1 (b) 2 (c) 3 (d)412. The valent electronic configuration for fluorine is __C___(a) 1s2 (b) 2s2 (c) 2s2.2p5 (d) is2. 2s2, 2p513. The octet means that the number of electrons in _C___ shell has to be eight.(a) innermost (b) the whole (c) outermost (d) no14. Polysaccarides like cellulose and starch must first be subjected to _A__ to form simpler sugar, such as monosaccarides.(a) dehydration (b) composition (c) hydrolysiis (d) comparsion15. Which of the following belongs to transition metal? D(a) S (b) Na (c) He (d) V16 Addition of AgNO3 in a solution generates white AgCl __C__ that does not dissolve in HNO3(a) solvent (b)silica-gel (c) precipitate (d) solvent1. The nature of organic species present depends on the degree of biochemical change of the original plant, C(a) pattern (b) fractions (c) substances (d) ceramics2. I(py)2+ appears to be present in solution C(a) be made (b) be dissolved (c) be detected (d) occur3.The M-F-M bridge bonds invariably have greater M-F distances than corresponding terminal M-F bonds. C(a) seldom (b) strongly (c) usually (d) never4. The chemical potential decreases and both the effect, i.e., solubility and normal temperatureeffect. B(a) for example (b) that is to say (c) through (d) approximately5.The M-F-M bridge bonds invariably have greater M-F distances than corresponding terminal M-Fbonds. A(a) end (b) head (c) initial (d) intermediate6. The corresponding bromine-and chlorine-species are less stable. B(a) compound (b) substances (c) supports (d) elementsH2O2, hydrogen peroxide Na2O2, sodium peroxide Se, selenium F , fluorine Br2,brominePCl3, phosphorus trichloride HI, hydrogen iodide AgI, silver iodide I2, iodineI-, iodideICl3, iodine trichloride Br-, bromide NF3, nitrogen trifluoride ClF7, chlorine heptafluoride CCl4, carbon tetrachloride PF3, phosphorus trifluoride Cl2O7 dichlorine heptoxideHydration--combination with water molecules.1. The _A___ ion can be considered s hydrated proton, H3O+.(a) hydrogen (b) hydroxyl (c) hydronium (d) hydroxo2. Chlorine is bubbled into aqueous sodium bromide, __A__ is liberated(a) bromine (b) dibromide (c) bromide (d) bromide ion3. Which of the following is diatomic molecule? D(a) He (b) Ne (c) Ar (d) H24. Which of the following is oxoanion? C(a) S2-, (b) HS-, (c) SO42-, (d) H2S1. In addition, for first order model the intercept of the straight line plots of log(qe-qt)against t B(a) therefore (b) Furthermore (c) But (d) Actually2. Most fluorine compounds are obtained from fluorspar via HF B(a) in (b) through (c) of (d) containingSnCl4, tin(IV) chloride FeCl3, iron(III) chloride PbCl4, lead(IV) chloride Sn2+,tin(II) ionCaCl2, calcium chloride I3-, triiodide ion CdCl2, cadmium chloride S2-,sulfide ionHgCl2, mercury(II) chloride FeO, iron(II) oxide Cu2O, copper(I) oxide O2-,oxide ionAlCl3, aluminium chloride CBr4, carbon tetrabromide CdS, cadmium sulfide S22-,disulfide ionZnO, zinc oxide V5+, vanadium(V) ion Cr3+, chromium(III) ion SiO2, silicon dioxideSnCl2, tin(II) chloride H+, hydrogen ion Mg2+, magnesium ion O22-, peroxide ionK+, potassium ion Be2+, beryllium ion Tl+, thallium(I) ion Ti4+, titanium(IV) ionPb2+, lead(II) ion Br-, bromide ion Se2-, selenide ion Ca2+, calcium ion Fe2+,iron(II) ionSn4+, tin(IV) ion Hg2Cl2, mercury(I) dichloride Al3+, aluminium ion1. The diversity of carbon compound is duo to formation of very long chain of carbon atom bythe strong carbon-carbon __D__ bond(a) ionic (b) metallic (c) hydrogen (d) covalent2. An inportant quantity, particular for first-order reaction, is _B ___ , the length of time required for the concentration of reactant to be decreases to half of its initial value(a) filled (b) half-life (c) half-filled (d) partiallly-filled3. In alkane of CH3CH2CH3, there are _D ___ substituents.(a) 2 (b) 3 (c) 8 (d) no4. In alkane CH3Ch2CH3, there are __B__ carbons in parent chain(a) 2 (b) 3 (c) 8 (d) 85. Environment preotection is a global process which has also included pollution of aquasphere, i.e., __B __ is polluted.(a) soil (b) water (c) chemical reagent (d) atmosphere3. Reaction of sodium carbonate with dilute sulfuric acid will generate __A__.(a) a gas (b) a precipitate (c) aqueous solute (d) emulsionCH2BrCH=C(CH3)CH2CH2CH3, 1-bromo-3-methyl-2-hexene CH3(CH2)10CH3, dodecaneCH3CH=CHCH3, 2-butene CH3(CH2)16CH3, octadecane CCl2=CCl2 tetrachloroetheneCH3CH=CHCH2CH3 2-pentene CH3CH2CH3 propane CH2=CH2 etheneZnCl2.6H2O zinc chloride hexahydrate ZnSO4.7H2O zinc sulfate heptahydrateRbMgCl3 magnesium rubidium chloride CaSO4.2H2O calcium sulfate dihydrateH4P2O7 diphosphoric acid CdCl2.6H2O cadmium chloride hexahydrate Cu2(OH)2CO3 copper(II) hydroxide carbonate CsCo(SO4)2 cesium cobalt sulfateH2S2O7 disulfuric acid FeSO4.7H2O iron(II) sulfide heptahydrate (NH4)2Fe(SO4)2 ammonium iron(II) sulfate K2Cr2O7 potassium dichromate isopropylcyclohexanetert-butylcyclohexane2-ethyl-1-isobutylcyclohexanecyclopentanecyclohexane cyclopentene 3-ethyl-4-methylhexanecyclopropane trans-3-hexeneNa2SO4.10H2O sodium sulfate decahydrate KMnO4, potassium permanganateK2MnO4, potassium manganate MnO4-, permanganate ion MnO2 manganese(IV) oxideCrO3, chromium(VI) oxide CrO42-, chromate ion K2CrO4, potassium chromateCr2O3, chromium(III) oxide H2SO4. Sulfuric acid H2SO3. Sulfurous acidH2S(aq), hydrosulfuric acid As2S3, diarsenic trisulfide H2S(as a gas), hydrogensulgideSF6, sulfur hexfluoride NaHSO3, sodium hydrogen sulfite HCl(a gas), hydrogenchlorideNaHSO4, sodium hydrogen sulfate ClO3-. Chlorate ion HClO3, chloric acidHCl(aq), hydrochloric acid KVO3, potassium vanadate HVO3, vanadic acidV2O5, divanadium pentoxide VCl3, vanadium(III) chloride Al(NO3)3, aluminiumnitrateNaAlO2,sodium alumate Al(OH)3, aluminium hydroxide Fe(NO3)3, iron(III)nitrateCa(BrO2)2, calcium bromite Na2S, sodium sulfide I-, iodide ion IO2-, ioditeionH2MnO4, manganic acid CaCl2, calcium chloride Na2SO3, sodium sulfiteKI, potassium iodide KIO2, potassium iodite HBrO, hypobromous acidNaBrO, sodium hypobromite NaBr, sodium bromide NaBrO3, sodium bromateKPO3,potassium phosphorate HPO3, metaphosphoric acid K3PO4, potassium phosphorate H3PO4 phosphoric acidm=chlorofluorobenzeneP-bromochlorobenzenem-nitroiodobenzenemethylenecyclohexanem-methylphenol(1-menthylvinyl)benzene 2-methyl-1-penten-3-yne2-chloro-1,3-butadiene2-propanol 2,4-hexadiyne-1,6-diol 2,4-hexadiene-1,6-diol4-hexen-2-olcis-1,2-dimethyl-1,2-cyclopentanedioltrans-1,2-dimethyl-1,2-cyclopentanediol2-propynol(1-methylvinyl)cyclopentane(1-propenyl)cyclopentanebenzylcyclohexanephenylcyclohexane diphenylmethane1-penten-3-yne 2,5-heptadiyne1,3-diphenylpropane Cyclohexane-1,2-diol2,5-cyclohexadiene-1,4-dio 4-hexene-1,2-diolallylbenzene vinylcyclohexane 3-butynalethanenitrile cyclohexanecarboxylic acid cyclohexanedicarboxylic anhydride 1,2-cyclohexanedinitrileethanamide ethanyl chloridesec-butylethanoate 4-methoxyphenol2,5-hexanedione dimethyl sulfate1. Alkenes and alkynes are classified into __B_ compound(a) cyclic (b) unsaturated (c) aromatic (d) straight chain2. The general formula R-C=N-O is called cyanate, while R-N=C=O is called _B__(a)cyano (b) isocyanate (c)nitrile (d) cyanide3. Reaction between __C__ chloride and sodium hydroxide will release a pungent gas.(a) amino (b) amine (c) ammonium (d) ammonia4. Which of the following belons to triprotic acid? D(a) HNO3 (b) H2SO4 (c) HNO2 (d) H3PO45. Animal and vegetable oil and fat are a more specialized source of a limit number of __C__compounds, including fatty acid such as CH3(CH2)16COOH and a long chain alcohol as CH3(CH2)11OH(a) petroleum (b) inorganic (c) aliphatic (d) aromatic6. The general term for species that is active in the interface medium between phase is ___A__.(a) a surfactant (b) a nucleophile (c) an electrolyte (d) an electrophile--------------------------------------------------------------------------1,4-butanedithioldimethyl ethanedioatetetrabutylammonium hydroxidemethanal2-chloro-1,3-butadiene1,3-propanediol 2-propanol2-aminophenol3-methylphenolN-methyl-1-aminomethaneethanedioic acid 1-pentyn-4-one1,2-methoxycyclobutaneethyl ethanoate2-butanol-3-one4-cyclopentene-1,2-dinitrile。
