污水处理英文翻译
污水处理的英文文献翻译(1)

Nutrient removal in an A2O-MBR reactor with sludgereductionABSTRACTIn the present study, an advanced sewage treatment process has been developed by incorporating excess sludge reduction and phosphorous recovery in an A2O-MBR process. The A2O-MBR reactor was operated at a flux of 77 LMH over a period of 270 days. The designed flux was increased stepwise over a period of two weeks. The reactor was operated at two different MLSS range. Thermo chemical digestion of sludge was carried out at a fixed pH (11)and temperature (75℃) for 25% COD solubilisation. The released pbospborous was recovered by precipitation process and the organics was sent back to anoxic tank. The sludge digestion did not have any impact on COD and TP removal efficiency of the reactor. During the 270 days of reactor operation, the MBR maintained relatively constant transmembrane pressure. The results based on the study indicated that the proposed process configuration has potential to reduce the excess sludge production as well as it didn't detonated the treated water quality.Keywords: A2O reactor; MBR; Nutrient removal; TMP1. IntroductionExcess sludge reduction and nutrients removal are the two important problems associated with wastewater treatment plant. MBR process has been known as a process with relatively high decay rate and less sludge production due to much longer sludge age in the reactor (Wenet al., 2004). Sludge production in MBR is reduced by 28-68%, depending on the sludge age used (Xia et al.,2008). However, minimizing the sludge production by increasing sludge age is limited due to the potential adverse effect of high MLSS concentrations on membrane (Yoon et al., 2004). This problem can be solved by introducing sludge disintegration technique in MBR (Young et al., 2007). Sludge disintegration techniques have been reported to enhance the biodegradability of excess sludge (Vlyssides and Karlis, 2004). In overall, the basis for sludge reduction processes is effective combination of the methods for sludge disintegration and biodegradation of treated sludge. Advances in sludge disintegration techniques offer a few promising options including ultrasound (Guo et al., 2008), pulse power (Choi et al.,2006), ozone (Weemaes et al., 2000), thermal (Kim et al., 2003), alkaline (Li et al., 2008) acid (Kim et al., 2003) and thermo chemical(Vlyssides and Karlis, 2004). Among the various disintegration techniques, thermo chemical was reported to be simple and cost effective (Weemaes and Verstraete, 1998). In thermal-chemical hydrolysis, alkali sodium hydroxide was found to be the most effective agent in inducing cell lysis (Rocker et al., 1999). Conventionally, the nutrient removal was carried out in an A2O process. It has advantage of achieving, nutrient removal along with organic compound oxidation in a single sludge configuration using linked reactors in series (Tchobanoglous et al., 2003). The phosphoroes removal happens by subjecting phosphorous accumulating organisms (PAO) bacteria under aerobic and anaerobic conditions (Akin and Ugurlu, 2004). These operating procedures enhance predominance PAO, which are able to uptake phosphorous in excess. During the sludge pretreatment processes the bound phosphorous was solubilised and it increases the phosphorousconcentration in the effluent stream (Nishimura, 2001).So, it is necessary to remove the solubilised phosphorus before it enters into main stream. Besides, there is a growing demand for the sustainable phosphorous resources in the industrialized world. In many developed countries, researches are currently underway to recover the phosphoroes bound in the sludge's of enhanced biological phosphorus removal system (EBPR). The released phosphorous can be recovered in usable products using calcium salts precipitation method. Keeping this fact in mind, in the present study, a new advanced wastewater treatment process is developed by integrating three processes, which are: (a) thermo chemical pretreatment in MBR for excess sludge reduction (b) A2O process for biological nutrient removal (c) P recovery through calcium salt precipitation. The experimental data obtained were then used to evaluate the performance of this integrated system.2. Methods2.1. WastewaterThe synthetic domestic wastewater was used as the experimental influent. It was basically composed of a mixed carbon source, macro nutrients (N and P), an alkalinity control (NaHCO3) and a microelement solution. The composition contained (/L) 210 mg glucose, 200 mg NH4C1, 220 mg NaHCO3, 22一34 mg KH2PO4, microelement solution (0.19 mg MnCl2 4H20, 0.0018 mg ZnCl22H2O,0.022 mg CuCl22H2O, 5.6 mg MgSO47H2O, 0.88 mg FeCl36H2O,1.3 mg CaCl2·2H2O). The synthetic wastewater was prepared three times a week with concentrations of 210±1.5 mg/L chemical oxygen demand (COD), 40±1 mg/L total nitrogen (TN) and 5.5 mg/L total phosphorus (TP).2.2. A2O-MBRThe working volume of the A2O-MBR was 83.4 L. A baffle was placed inside the reactor to divide it into anaerobic (8.4 L) anoxic (25 L) and aerobic basin (50 L). The synthetic wastewater was feed into the reactor at a flow rate of 8.4 L/h (Q) using a feed pump. A liquid level sensor, planted in aerobic basin of A2O-MBR controlled the flow of influent. The HRT of anaerobic, anoxic and aerobic basins were 1, 3 and 6 h, respectively. In order to facilitate nutrient removal, the reactor was provided with two internal recycle (1R). IRl (Q= 1)connects anoxic and anaerobic and IR 2 (Q=3) was between aerobic and anoxic. Anaerobic and anoxic basins were provided with low speed mixer to keep the mixed liquid suspended solids (MLSS) in suspension. In the aerobic zone, diffusers were used to generate air bubbles for oxidation of organics and ammonia. Dissolved oxygen (DO) concentration in the aerobic basin was maintained at 3.5 mg/1 and was monitored continuously through online DO meter. The solid liquid separation happens inaerobic basin with the help of five flat sheet membranes having a pore size of 0.23 pm. The area of each membrane was 0.1 m2. They were connected together by a common tube. A peristaltic pumpwas connected in the common tube to generate suction pressure. In the common tube provision was made to accommodate pressure gauge to measure transmembrane pressure (TMP) during suction. The suction pump was operated in sequence of timing, which consists of 10 min switch on, and 2 min switch off.2.3. Thermo chemical digestion of sludgeMixed liquor from aerobic basin of MBR was withdrawn at the ratio of 1.5% of Q/day and subjected to thermo chemical digestion. Thermo chemical digestion was carried out at a fixed pH of 11(NaOH) and temperature of 75℃for 3 h. After thermo chemical digestion the supernatant and sludge were separated. The thermo-chemicallydigested sludge was amenable to further anaerobic bio-degradation (Vlyssides and Karlis, 2004), so it was sent to theanaerobic basin of the MBR2.4. Phosphorus recoveryLime was used as a precipitant to recover the phosphorous in the supernatant. After the recovery of precipitant the content was sent back to anoxic tank as a carbon source and alkalinity supelement for denitrification.2.5. Chemical analysisCOD, MLSS, TP, TN of the raw and treated wastewater were analyzed following methods detailed in (APHA, 2003). The influent and effluent ammonia concentration was measured using an ion-selective electrode (Thereto Orion, Model: 95一12). Nitrate in the sample was analyzed using cadmium reduction method (APHA, 2003).3. Results and discussionFig. 1 presents data of MLSS and yield observed during the operational period of the reactor. One of the advantages of MBR reactor was it can be operated in high MLSS concentration. The reactor was seeded with EBPR sludge from the Kiheung, sewage treatment plant, Korea. The reactor was startup with the MLSS concentration of 5700 mg/L. It starts to increase steadily with increase in period of reactor operation and reached a value of 8100 mg/L on day 38. From then onwards, MLSS concentration was maintained in the range of 7500 mg/L by withdrawing excess sludge produced and called run I. The observed yields (Yobs) for experiments without sludge digestion (run I) and with sludge digestion were calculated and given in Fig. 1. The Yobs for run I was found to be 0.12 gMLSS/g COD. It was comparatively lower than a value of 0.4 gMLSS/g CODreported for the conventional activated sludge processes (Tchoba-noglous et al., 2003). The difference in observed yield of these two systems is attributed to their working MLSS concentration. At high MLSS concentration the yield observed was found to be low (Visva-nathan et al., 2000). As a result of that MBR generated less sludge.The presently used MLSS ranges (7.5一10.5 g/L) are selected on the basis of the recommendation by Rosenberger et al. (2002). In their study, they reported that the general trend of MLSS increase on fouling in municipal applications seems to result in no impact at medium MLSS concentrations (7一12 g/L).It is evident from the data that the COD removal efficiency of A2O system remains unaffected before and after the introduction of sludge digestion practices. A test analysis showed that the differences between the period without sludge digestion (run I) and with sludge digestion (run II and III) are not statistically significant.However, it has been reported that, in wastewater treatment processes including disintegration-induced sludge degradation, the effluent water quality is slightly detonated due to the release of nondegradable substances such as soluble microbial products (Ya-sui and Shibata, 1994; Salcai et al., 1997; Yoon et al., 2004). During the study period, COD concentration in the aerobic basin of MBR was in the range of 18-38 mg/L and corresponding organic concentration in the effluent was varied from 4 to 12 mg/L. From this data it can be concluded that the membrane separation played an important role in providing the excellent and stable effluent quality.Phosphorus is the primary nutrient responsible for algal bloom and it is necessary to reduce the concentration of phosphorus in treated wastewater to prevent the algal bloom. Fortunately its growth can be inhibited at the levels of TP well below 1 mg/L (Mer-vat and Logan, 1996).Fig. 2 depicts TP removal efficiency of the A2O-MBR system during the period of study. It is clearly evident from the figure that the TP removal efficiency of A/O system was remains unaffected after the introduction of sludge reduction. In the present study, the solubilised phosphorous was recovered in the form of calcium phosphate before it enters into main stream. So, the possibility of phosphorus increase in the effluent due to sludge reduction practices has been eliminated. The influent TP concentration was in the range of 5.5 mg/L. During thefirst four weeks of operation the TP removal efficiency of the system was not efficient as the TP concentration in the effluent exceeds over 2.5 mg/L. The lower TP removal efficiency during the initial period was due to the slow growing nature of PAO organisms and other operational factors such as anaerobic condition and internal recycling. After the initial period, the TP removal efficiency in the effluent starts to increase with increase in period of operation. TP removal in A2O process is mainly through PAO organisms. These organisms are slow growing in nature and susceptible to various physicochemical factors (Carlos et al., 2008). During the study period TP removal efficiency of the system remains unaffected and was in the range of 74-82%.。
污水处理外文翻译带原文

Study on Disinfection and Anti –microbial Technologies for Drinking WaterZHU Kun, FU Xiao Yong(Dept. of Environmental Engineering, LAN Zhou Railway University, LAN Zhou 730070, China)Abstract: Disinfection by-products produced by the reaction between chlorine and dissolved organic compounds and other chemicals are considered as a worrying problem in the drinking water treatment process since a series of mutagenic carcinogen substances are formed including trihalomethanes (THMs). Among the tested disinfectants(chlorine , ozone , chlorine dioxide , potassium permanganate , chloramines and hydrogen peroxide etc. ) , chlorine dioxide has proved to be the most feasible and effective oxidant for drinking water treatment and removal of pathogens due to its oxidation efficiency , low cost and simple way of utilization. A series of experiments indicate that chlorine dioxide can significantly restrain production of trihalomethanes (THMs) and control bacteria growth particularly for Cryptosporidium oocysts. The experiments verified that both ozone and chlorine dioxide are absolutely vital to ensure thtion of water storage are destroyed. The paper discusses oxidation capacity of chlorine dioxide, especially for removing petroleum compounds, which is affected by reaction time, gas injection way, and pH of treated water.Key words: disinfection; oxidants; water treatment; pathogens; chlorine dioxideCLC number: X523 Document code: A1 IntroductionChemical and filtration processes are two main methods used in China for treating drinking water meanwhile UV radiation has been used successfully for water treatment with relatively low flow rate. On the individual family level, usually chemical treatment is a feasible alternative. The following guidelines exist for the selection of suitablal of contaminants should be done by decomposition, evaporation or precipitation etc, to eliminate or decrease the toxicity, oxidants or reactionby-products should not be harmful to human health, and the purification processes should be practical and economical. The objective of this paper is to evaluate and discuss available disinfectants for drinking water treatment. The different disinfectants are compared regarding purification efficiencies and application approaches.2 Comparison ofO3 > ClO2 > HOCl > OCl - > NHCl2 > NH2ClReferring to Fiessinger′s [2] suggestion, the properties of these disinfectants are compared in Tab. 1. Chlorine is shown to be an excellent disinfectant to prevent waterborne diseases such as typhoid fever over long periods. Chlorine reacts not only within oxidation, but also by electrophilic substitution to produce a variety of chlorinated organic by - products, particularly trihalomethanes (THMs) and other mutagens. Here THMs mainly refer to chloroform, bromoform, dibromochloromathane and bromodichloromathane etc. Since the 1970`s, the usage of Cl2 in drinking water disinfection has been questioned with ozone being substituted as the preferred disinfectant in the water supply plants. But , ozone could not be introduced to the rural farmer community due to its high costs and short half - life (15~20 min. ) . As with other disinfectants, ozonation also leads to formation of organic by - product s such as aldehyde, ketones, and carboxylic acids, and also mutagenicity may be induced if bromic anion exists.Tab. 1 Comparison of various oxidants- no effect ; + little effect ; + + effect ; + + + largest effectMany studies have pointed out that disinfection is absolutely vital to ensure that any microorganisms arising from fecal contamination of water storage are destroyed. The selection of the available disinfectant s must concern to reduce risk from microbial contamination of drinking water and the potential increase in risk from chemical contamination that result from using any of the disinfectant s. The biocidal efficiency of commonly used disinfectants - ozone, chlorine dioxide, chlorine and chloramines are ranked almost with the same order as the oxidizing capacity, but the stability of those are following the order as [3]:Chloramines > Chlorine dioxide > Chlorine > Ozone3 Purification of organic pollutants by chlorine dioxideAccording to WHO guideline for drinking water quality, much consideration should be paid to benzene homologous compounds; therefore, the study on purification effect s of chlorine dioxide is focused on petrochemical pollutants. A series of experiment s were carried out to simulate the oxidation processes of contaminated water. The polluted solutions were prepared in a dark barrel (10L capacity) of seven kinds of benzene homologous compounds-Benzene , toluene , ethyl benzene , p-phenylmethane, o-phenylmethane, m-phenylmethane and styrene. Samples were taken to determine the initial concentration of the compounds prior to the test s. Standard chlorine dioxide solution was produced from sodium chlorite reacted with HCl solution of 10% [4]. The GR - 16A Gas - chromatograph with FID detector Shenyang LZ-2000 was used for measurement of Cl2, ClO2, ClO-2 and ClO-3[5]. Oil concentrations were determined with an UV -120-20 spectrophotometer (Shimadzu) following the procedure described by APHA [4]. Organic compounds in the water samples were measured with a GC-MS (QP-1000A). ClO2and O3were standardized by iodimetric titration at pH7.For the purpose of chemical disinfection for drinking water, chlorine was instantaneously ignored due to the formation of THMs and other mutagenic substances. The results indicated that potassium permanganate and hydrogen peroxide did not have enough oxidation capability to decompose petroleum contaminant s achieving only 46 %, and 5.7% decomposition of styrene, respectively. Ozone could not be selected due to it s high cost, complex operation and short half-life although it is an excellent oxidant for water treatment. Chlorine dioxide was the next most successful alternative for disinfection. The benefit s include-effective oxidation capacity, algicidal effect and negligible formation of halogenated by-products. Based on economic and operational requirement, the mixing gas method is easily used. The results obtained suggest that disinfection of drinking water with ozone and or chlorine dioxide seems to be a suitable alternatives to the use of NaClO for cont rolling the formation of non-volatile mutagens[6].In the laboratory experiments, the oxidants ozone, chlorine dioxide, potassium permanganate and the mixing gas (mainly contained ClO2 and a certain amount of Cl2, O3 and H2O2) were tested for removal of the petroleum compounds, and results are shown in Tab. 2.Tab. 2 Comparison of oxidation capacity for the various oxidantsA study was conducted to elucidate the decay pathway of monochloramine in thepresence and absence of natural organic matter (NOM) [7]. It was found that natural organic matter acted primarily as a reductant rather than catalyst. This conclusion was verified using a redox balance, and much of oxidizing capacity of monochloramine goes towards NOM oxidation. Cleaning agents and disinfectants from house keeping, hospitals, kitchens are sources of absorbable halogenated organic compounds (AOX) in municipal wastewater. The amount of AOX generated strongly depends on the nature and concentrations of dissolved and solid organic compounds, the concentration of active substances, temperature, pH and reaction time [8] When the mixing gases react with water molecules and organic micro-pollutants, hypochlorous acid is formed by chlorine, chlorite and chlorate ions are produced from chlorine dioxide in a series of redox reactions. The principal reactions are summarized as follows:ClO2+ organic →ClO -² + oxidized organic (1)2ClO -² + Cl2 = 2ClO2 + 2Cl - (2)2ClO -²+ HOCl = 2ClO2 + 2Cl - + OH- (3)2ClO2 + HOCl + H2O = 2ClO - ³ + HCl + 2H+ (4)The rate of chlorate yield can be described by Equation (5):d [ClO3]/ d t = 2 k [ClO2] [HOCl] (5)in which k = 1.28 M/ min at 25 ℃ [9].The stoichiometry of the undesirable reactions that form chlorate in low concentration of chlorite or presents of excess chlorine is given as:ClO -² + Cl2 + H2O = ClO - ³ + 2Cl - + 2H+ (6)ClO - ² + HOCl = ClO - ³ + Cl - + H+ (7)At alkaline conditions:ClO -² + HOCl + OH- = ClO - ³ + Cl - + H2O (8)Typically, chlorine dioxide is used in drinking water treatment and the concentrations are ranging from 0.1 to 2.0 mg/L [10]. However, the relevant by - products of chlorine dioxide treatment-chlorite and chlorate have been found to induce methemoglobinemia in the human body when concentrations are more than 100 mg/L [11]. The oxidation results of the organic contaminants were affected byreaction time. The initial concentrations and removal rate at different times are listed in Tab. 3. It is shown that chlorine dioxide has a very strong oxidation capability including the break down of the benzene ring. There are no other commonly used oxidants to do like this except for ozone.Tab. 3 Removal rate of tested organic compounds at different operating time (at pH7)The injecting method for chlorine dioxide gas into the solution also has an apparent influence on the removal rate. With the indirect method, the gas firstly was dissolved in a certain amount of distilled water, and then added to the tested organic solutions, as a result, removal rates appear lower than for the direct blowing method. The main reason for the difference is due to the conversion and decomposition of chlorine dioxide in the dissolving process before the reaction. It is confirmed from Tab. 3 that the removal rate was proportional to operating time. Since chlorine dioxide showed very strong oxidation capability for organic chemicals but was reduced to chlorite anion according to Equation (4), and the removal rate initially appeared quite high. Then, chlorite keeps the oxidation capacity at a level, which allows decomposition of the organic compounds to continue even though the oxidation reaction gradually became weaker with reaction time. The experiment indicated that pH values significantly influenced the removal rate of the organic compounds. The differences of degradation rates in a variety of pH through indirect input way areshown in Tab. 4.Tab. 4 Degradation rate of benzene homologous compounds with indirect method at different pH (after 15 min)There are, however, some disadvantages with ClO2, such as easy loss from solution due to volatilization, and disproportionation above pH 10 into chlorate and chlorite ions that are of certain oxidation capacity, but reported to be harmful to health if the concentration is too high. Chlorine dioxide was unstable in the solution even though it has a stronger oxidation capability than chlorite and chlorate as the two resulted in anions being dominant in the oxidation processes. The actual concentration of chlorine dioxide depended on the existence of chlorine, chlorite and chlorate whose concentrations were determined by pH values of the solution according to Equations (6) and (8) respectively. Consequently, the pH is the critical controlling factor in the concentrations of chlorine dioxide, chlorite and chlorate. The latter two harmful ions can be removed quite quickly by treatment with a reducing agent such as sulfur dioxide - sulfite ion at pH values of 5~7[10 ,12]. Fe (II) can be used to eliminate chlorite from the water , and the redox reaction is kinetically more rapid at pH 5~7 as well[13]. It was evident that the decomposition in acidic conditions was much better than that in alkaline conditions because a disproportional amount of chlorine dioxide was consumed by the reactions under alkaline conditions. For drinking water treatment, it has been suggested that the mixture of chlorine 0.8 mg/L and chlorinedioxide 0.5 mg/L will achieve disinfection and control THMs formation in preference to use of pure chlorine dioxide[14]. According to USEPA drinking water standard, the residue of ClO2 is limited as 0.8 mg/L that tends to the goal of 0.4 mg/L.4 Control of pathogens with disinfectantsHuman pathogens that are transmitted by water including bacteria, viruses and protozoa. Organisms transmitted by water usually grow in the intestinal tract and leave the body in the feces. Thus, they are infections. Fecal pollution of water supplies may then occur, and if the water is not properly treated, the pathogens enter a new host when the water is consumed, therefore, it may be infectious even if it contains only a small number of pathogenic organisms. Most outbreaks of waterborne diseases are due to breakdowns in treatment systems or are a result of post contamination in pipelines.The microorganisms of concern are those which can cause human discomfort, illness or diseases. These microbes are comprised of numerous pathogenic bacteria, viruses, certain algae and protozoa etc. The disinfection efficiency is typically measured as a specific level of cyst inactivation. Protozoan cysts are the most difficult to destroy. Bacteria and viral inactivation are considered adequate if the requirement for cyst inactivation is met. Therefore, water quality standard for the disinfection of water have been set at microorganisms, usually take the protozoan cysts as indicator, so viruses will be adequately controlled under the same operation conditions required for inactivation of protozoan cysts. The widely found drinking water contamination is caused by protozoan that is a significant intestinal pathogens in diary cattle, likely a source of this outbreak.There are two of the most important protozoa - Cryptosporidium and Giardia cysts those are known to outbreak diseases, frequently are found in nature and drinking water storage ponds. Protozoa form protective stages like oocysts that allow them to survive for long periods in water while waiting to be ingested by a host. Protozoa cysts are not effectively removed by storing water because of their small size and density. Cryptosporidium oocysts have a setting velocity of 0.5 um/s. Therefore, if the water tank is 2 m deep, it will take the oocyst 46 days to settle to thebottom. Giardia cysts are much large and have a great settling velocity of 5.5um/s. It was evident that chlorine and chloramines were ineffective against Cryptosporidium oocysts, which was discovered to be amazingly resistant to chlorine, and only ozone and chlorine dioxide may be suitable disinfectants [15]. The investigations have verified that Cryptosporidium is highly resistant to chorine, even up 14 times as resistant as the chlorine resistant Giardia, therefore methods for removing it in past rely on sedimentation and filtration. Watson′s Law to study protozoan disinfection, reads as follows:K = Cηt (9)In the formula:K ——constant for a given microorganism exposed to a disinfectant under a fixed set of pH and temperature conditions;C ——disinfectant concentration (mg/ L);η——empirical coefficient of dilution ;t ——time required to achieve the fixed percentage inactivation.For the preoxidation and reduction of organic pollutants , the recommended dosages are between 0. 5~2. 0 mg/ L with contact time as 15~30 min depending on the pollutants characteristics in the water. In the case of post - disinfection , the safe dosages of ClO2 are 0. 2~0.4 mg/L. At these dosages, the potential by - products chlorite and chlorate do not constitute any health hazard [16]. The relation between disinfectant concentration and contact time can be established by using Ct products based on the experimental data. From this the effectiveness of disinfectants can be evaluated based on temperature, pH value and contact time. Since Cryptosporidium has become a focus of regulatory agencies in the United States and United Kingdom, the prospects of controlling this pathogen show more considerable. The comparison of the Ct values by using ozone , chlorine dioxide , chlorine and chloramines for Giardia and Cryptosporidium cyst s are listed in Tab. 5[17 ,18 ] , and for some microorganisms disinfection are displayed in Tab. 6[19 ] .Tab. 5 Ct values (mg·min/ L.) for disinfection of Giardia and Cryptosporidium cysts by using 4 disinfectantsTab. 6 Comparison of value intervals for the product Ct (mg·min/ L) for the inactivation of various microorganisms by using 4 disinfectantsThe mean Ct value for ClO2 at pH 7 and 5 ℃was 11. 9 mg·min/ L, and dropped to 5.2 at pH 7 and 25 ℃. High temperatures normally enhance the efficiency of disinfectants while lower temperatures have opposite effects requiring additional contact time or extra quantity of disinfectants. The best performance for ClO2 is at pH 9 and 25 ℃, which yields a Ct product of 2.8 mg·min/ L [20]. Chlorine dioxide appears to be more efficient for Cryptosporidium oocysts than either chlorine or monochloramine. Exposure of oocysts to 1.3 mg·min/ L at pH 7 reduces excystation from 87 % to 5 % in a hour at 25 ℃. Based on this result, Ct product of 78 mg·min/ L was calculated. However, the Ct product for ozone to do this work was examined as 5 - 10 mg·min/ L from observation that excystation decreased from 84 % to 0 % after 5 minutes with the ozone concentration of 1 mg/ L [15]. As with other disinfectants, increasing temperature decreased the Ct values and improved the cysticidal action. Increasing temperature unexpectedly reduced the Ct values from a high of 6.35 mg·min/ L at pH5 to a low of 2.91 mg·min/ L at pH 9[20]. It is generally the rule, that for protozoa ozone is the best cysticide, chlorine dioxide is superior to chlorine andiodine, but chlorine, in overall, is much superior to chloramines [21].Although disinfection efficiency of ozone is higher than chlorine dioxide, this difference can be compensated by the contact time. The experiment indicated that chlorine dioxide could reach the same results for disinfection of coliform bacteria as ozone did if time lasted long enough, which can be seen in Fig. 1. The added concentrations of both of ozone and chlorine dioxide were 2 mg/ L.Control of Cryptosporidium oocysts in potable water requires an integrated multiple barrier approach. Coagulation is critical in the effective control of Cryptosporidium by clarification and filtration. Dissolved air floatation can achieve oocysts removal of 3 logs compared to about 1 log by sedimentation. Dissolved air floatation and filtration provide two effective barriers to Cryptosporidium oocysts with cumulative log removal of 4 to 5 compared to log removals of 3 to 4 by sedimentation and filtration [22].Fig. 1 Comparison of disinfection efficiency between ozone and chlorine dioxide on coliform bacteria5 Tendency of disinfection for drinking waterIn the future, the burden of producing water with low pathogen level and low tastes and odor will be allocated to a combination of steps, including source water protection, coagulation - flocculation - sedimentation, filtration, floatation, membrane processes and adsorption. Some form of terminal treatment with chlorine, chlorine dioxide, ozone, UV, or other agents will also be required. No single step can or should be expected to shoulder the entire burden to controlling a given contaminant. With the development of techniques, new chemical and physical agents will meet tests of practicability for use in water treatment and will reduce pathogens. These may include electromagnetic fields and other forms of treatment with light or sonic energy [23].In light of availability, efficacy, operability and costs, the priority should be given to ultraviolet method among all of the currently utilized disinfection technologies, particularly in developing countries. The medium and low - pressure UV extends tremendous potential promise for adaptation into various scale water supply plants. The researches have validated that extremely low dosage of UV can behighly effective for inactivate oocysts [24]. Furthermore, comparison of medium and low - pressure lamps demonstrated no significant differences. By using low - pressure UV at the dosage of 3 , 6 and 9 mJ/ cm2 , oocyst inactivation levels were yielded between 3.4 and 3.7 log. In the trials of UV in water with turbidity of more than 1 NTU, the ability of medium –pressure was not affected, and high level of oocysts inactivation could still be achieved.6 ConclusionsTo purify drinking water, chlorine dioxide can be chosen instead of chlorine, ozone and other disinfectants because of it s advantages of high efficiency of disinfection, competent stability, low cost and simple utilizing way etc. Both ozone and ClO2 are absolutely vital to ensure that any microorganisms arising from fecal contamination of water storage are destroyed. The utilization of chlorine dioxide has been found to efficiently restrict protozoa growth, to disinfect from bacteria and viruses. Taking the protozoan cysts as indicator in which Cryptosporidium oocysts were solidly resistant to chlorine, but chlorine dioxide may be suitable disinfectants to mutilate. Thus, viruses will be adequately controlled by chlorine dioxide under the same operation conditions required for inactivation of protozoan cysts. The experiment indicated that chlorine dioxide could reach the same results for disinfection of coliform bacteria as ozone did if time lasted long enough although disinfection efficiency of ozone is higher than chlorine dioxide.It is an obvious preference for chlorine dioxide to pragmatically remove oil and benzene homologous compounds in water treatment meanwhile the formation of mutagenic and toxic substances is limited. The degradation rate was proportional to input amount of oxidants and increase of operating time. The dosage input , in overall , is suggested to range between 0. 5~2.0 mg/ L. The effective pH at which reactions occur is in the slightly acid range of 5 to 7 at which formation of chlorite and chlorate is minimized. The chlorine dioxide gas should be injected directly into the treated water body, so that high concentrations of ClO2 can be kept in the solution. Under these conditions, the elimination rate for organic pollutants will be much higher. For the storage system, input dosage of chlorine dioxide concentration should be higherthan that in laboratory studies due to complex pollutants in treated water. References:[1 ] Katz J . Ozone and chlorine dioxide technology for disinfection of drinking water [M]. Noyes New Jersey: Data Corporation, 1980.[2] Fiessinger F. Organic micropollutants in drinking water and health [M] . Publisher, N. Y., U. S. A: Elsevier Sci., 1985.[3 ] Hoff J C , Geldreich E E. Comparison of the biocidal efficiency of alternative disinfectants [C] . In Proceedings AWWA seminar, Atlanta, Georgia, 1980.[4 ] APHA , American Public Health Association. American Water Works Association and Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater. (16th Edition) [M]. Washington D. C., 1989.[5] Dietrich A M. Determination of chlorite and chlorate in chlorinated and chloraminated drinking water by flow injection analysis and ion chromatography[J ] .A nal. Chem., 1992, 64:496 - 502.[6] Monarca S. Mutagenicity of extracts of lake drinking water treated with different disinfectants in bacterial and plant tests[J ] . Water Res, 1998, (32):2 689 - 2 695.[7] Vikesland P , Ozekin K, Valentine R L. Effect of natural organic matter on monochloramine decomposition : pathway elucidation through the use of mass and redox balance[J ] . Envi ron. Sci. Tech., 1998, 32 (10):1 409 - 1 416.[8] Schulz S , Hahn H H. Generation of halogenated organic compounds in municipal wastewater [M] . Proc. 2nd Int. Assoc. Water Qual. Int. Conf. Sewer Phys. Chem. Bio. Reactor, Aalborg, Denmark, 1998.[9 ] Aieta E M. A review of chlorine dioxide in drinking water treatment [J]. J. A WWA, 1986, 78 (6): 62 - 72.[10 ] Gordon G Minimizing chlorine ion and chlorate ion in water treatment with chlorine dioxide[J ] . J. A WWA, 1990, 82 (4):160 - 165.[11] Kmorita J D , Snoeyink V L. Monochloramine removal from water by activated carbon[J ] . J. A WWA, 1985, (1):62 - 64.[12] Gordon G, Adam I , Bubnis B. Minimizing chlorate information[J ] . J. AWWA, 1995, 87, (6): 97 - 106.[13] Iatrou A. Removing chlorite by the addition of ferrous iron[J ] . J. A WWA, 1992, 84 (11): 63 - 68.[14 ] Schalekamp Maarten. Pre - and intermediate oxidation of drinking water with ozone, chlorine and chlorine dioxide [J]. J. Ozone Science and Engineering, 1986, 8: 151 - 186[15 ] Korich D G, Mead J R , Madore M S , et al . Effects of ozone, chlorine dioxide, chlorine and monochramine on Cryptosporidium parvum oosyst viability [J]. Applied and Environmental Microbiology, 1990, 56: 1 423 - 1 428.[16 ] AWWA Research Foundation. Chlorine dioxide; drinking water issues, 2nd International Symposium [R]. Houston, TX, 1992.[17] Lykins B W, Griese H G. Using chlorine dioxide for trihalomethane control[J ] . J, A WWA, 1986, 71 (6): 88 - 93.[18] Regli S. Chlorine dioxide , drinking water issues , 2nd International Symposium [ R ] . Houston, TX, AWWA Research Foundation, 1992.[19] Hoff J C. Inactivation of microbial agents by chemical disinfectants[J] . US EPA, 1986.[ 20 ] Rubin A , Evers D , Eyman C , et al . Interaction of gerbil - cultured Giardia lamblia cysts by free chlorine dioxide [J]. Applied and Envi ronmental Microbiology, 1989, 55: 2 592 - 2 594.[ 21 ] Rusell A D , Hugo WB , Ayliffe GA J . Principes and Practice of Disinfection [M]. Preservation and Sterilization. Blackwell Scientific Publications, Oxford, U K, 1992.[22 ] Edzwald J K, Kelley M B. Control of Cryptosporidium from reservoirs to clarifiers to filters [C] . Proc. 1st IAWQ –IWSA Joint Specialist Conf. Reservoir Manage. Water Supply, Prague, Czech, 1998.[23] Haas Charles N. Disinfection in the Twenty - first century[J ] . J. A WWA, 2000, 92 (2): 72 - 73.[24 ] Clancy L , Jenneifer , Bukhari Z , et al , Using UV to Inactivate Gryptosporidium[J ] . J. A WWA, 2000, 92: 97 - 104.饮用水的消毒及杀菌技术研究朱琨伏小勇(兰州铁道学院环境工程系, 甘肃兰州730070)摘要:饮用水处理消毒过程中可产生一系列致癌物质,主要是氯与水中的有机物和其它化学成分反应的结果,其中典型产物有三氯甲烷. 通过对常用消毒剂液氯,臭氧,二氧化氯,高锰酸钾,氯胺及过氧化氢的实验对比,证明二氧化氯是高效,方便,廉价的消毒剂. 它不仅对一般病原菌类有明显的抑制和杀菌作用,对清除难以灭杀的潜原性病毒也有理想的效果. 在净化水中石油类有机物时,二氧化氯的效果受到反应时间,注入方式和pH 值的影响.关键词:消毒;氧化剂;水处理;病原菌;二氧化氯中图分类号:X523 文献标识码:A中文译文:饮用水消毒和杀菌技术的研究朱琨伏小勇(兰州铁道学院环境工程系,甘肃兰州,730070 中国)在饮用水处理过程中,通过氯与溶解性有机物和其他化合物的反应所产生的消毒副产物被看作一个令人担忧的问题,因为一系列诱变致癌的物质组成包括总卤甲烷。
污水处理工艺中常用的名词术语及它们的英文缩写

污水处理工艺中常用的名词术语及它们的英文缩写污水处理工艺中常用的名词术语及它们的英文缩写给排水常用名词中英文对照1、给水工程 water supply engineering 原水的取集和处理以及成品水输配的工程.2、排水工程 sewerage ,wastewater engineering 收集、输送、处理和处置废水的工程.3、给水系统 water supply system 给水的取水、输水、水质处理和配水等设施以一定方式组合成的总体.4、排水系统sewerage system 排水的收集、输送、水质处理和排放等设施以一定方式组合成的总体.5、给水水源 water source 给水工程所取用的原水水体.6、原水raw water 由水源地取来的原料水.7、地表水surface water 存在于地壳表面,暴露于大气的水.8、地下水ground water 存在于地壳岩石裂缝或工壤空隙中的水.9、苦咸水(碱性水) brackish water ,alkaline water 碱度大于硬度的水,并含大量中性盐,PH值大于7.10、淡水fresh water 含盐量小于500mg/L的水.11、冷却水cooling water 用以降低被冷却对象温度的水.12、废水wastewater 居民活动过程中排出的水及径流雨水的总称.它包括生活污水、工业废水和初雨径流以及流入排水管渠的其它水.13、污水sewage ,wastewater 受一定污染的来自生活和生产的排出水.14、用水量 water consumption 用水对象实际使用的水量.15、污水量 wastewater flow ,sewage flow 排水对象排入污水系统的水量.16、用水定额 water flow norm 对不同的排水对象,在一定时期内制订相对合理的单位排水量的数值.17、排水定额 wastewater flow norm 对不同的排水对象,在一定时期内制订相对合理的单位排水量的数值.18、水质water quality 在给水排水工程中,水的物理、化学、生物学等方面的性质.19、渠道 channel ,conduit 天然、人工开凿、整治或砌筑的输水通道.20、泵站pumping house 设置水泵机组、电气设备和管道、闸阀等的房屋.21、泵站 pumping station 泵房及其配套设施的总称.22、给水处理 water treatment 对不符合用不对象水质要求的水.进行水质改善的过程.23、污水处理 sewage treatment ,wastewater treatment 为使污水达到排水某一水体或再次使用的水质要求,对其进行净化的过程.24、废水处理 wastewater disposal 对废水的最终安排.一般将废水排入地表水体、排放土地和再次使用等.25、格栅 bar screen 一种栅条形的隔污设备,用以拦截水中较大尺寸的漂浮物或其他杂物.26、曝气aeration 水与气体接触,进行溶氧或散除水中溶解性气体和挥发性物质的过程.27、沉淀 sedimentation 利用重力沉降作用去除水中杂物的过程.28、澄清clarification 通过与高浓度沉渣层的接触而去除水中杂物的过程.29、过滤filtration 借助粒状材料或多孔介质截除水中质物的过程.30、离子交换法ion exchange 采用离子交换剂去除水中某些盐类离子的过程.31、氯化chlorination 在水中投氯或含氯氧化物方法消灭病原体的过程.32、余氯residual chlorine 水中投氯,经一定时间接触后,在水中余留的游离性氯和结合性氯的总和.33、游离性余氯 free residual chlorine 水中以次氯酸和次氯酸盐形态存在的余氯.34、结合性余氯 combinative residual chlorine 水中以二氯胺和一氯胺形态存在的余氯.35、污泥sludge 在水处理过程中产生的,以及排水管渠中沉积的固体与水的混合物或胶体物.36、污泥处理sludge treatment 对污泥的最终安排.一般将污泥作农肥、制作建筑材料、填埋和投弃等.37、水头损失head loss 水流通过管渠、设备和构筑物等所引起的能量消耗.给水工程中系统和水量方面的术语1、直流水系统 once through system 水经过一次使用后即行排放或处理后排放的给水系统.2、复用水系统 water reuse system 水经重复利用后再行排放或处理后排放的给水系统.3、循环水系统 recirculation system 水经使用后不予排放而循环利用或处理后循环利用的给水系统.4、生活用水 domestic water 人类日常生活所需用的水.5、生产用水 process water 生产过程所需用的水.6、消防用水 fire demand 扑灭火灾所需用的水.7、浇洒道路用水 street flushing demand ,road watering 对城镇道路进行保养、清洗、降温和消尘等所需用水.8、绿化用水 green belt sprinkling ,green plot sprinkling 对市政绿地等所需用的水.给水工程取水构筑物的术语1、管井 deep well ,drilled well 井管从地面打到含水层,抽取地下水的井.2、管井滤水管 deep well screen 设置在管井动水位以下,用以从含水层中集水的有缝隙或孔隙的管段.3、管井沉淀管 grit compartment 位于管井最下部,用以容纳进入井内的沙粒和从水中析出的沉淀物的管段.4、大口井 dug well ,open well 由人工开挖或沉井法施工,设置井筒,以截取浅层地下水的构筑物.5、井群 batter of wells 数个井组成的群体.6、渗渠 infiltration gallery 壁上开孔,以集取浅层地下水的水平管渠.7、地下水取水构筑物反滤层 inverted layer 在大口井或渗渠进水处铺设的粒径沿水流方向由细到粗的级配砾层(简称反滤层)8、泉室 spring chamber 集取泉水的构筑物.9、进水间 intake chamber 连接取水管与吸水井、内设格栅或格网的构筑物.10、格网 screen 一种网状的用以拦截水中较大尺寸的漂浮物、水生动物或其他污染物的拦污设备.其网眼尺寸较格栅为小.11、吸水井 suction well 为水泵吸水管专门设置的构筑物.给水工程中净水构筑物的术语1、净水构筑物purification structure 以去除水中悬浮固体和胶体杂质等为主要目的的构筑物的总称.2、投药 chemical dosing 为进行水处理而向水中加一定剂量的化学药剂的过程.3、混合mixing 使投入的药剂迅速均匀地扩散于被处理水中以创造良好的凝聚反应条件的过程.4、凝聚 coagulation 为了消除胶体颗粒间的排斥力或破坏其亲水性,使颗粒易于相互接触而吸附的过程.5、絮凝flocculation A、完成凝聚的胶体在一定的外力扰动下相互碰撞、聚集以形成较大絮状颗粒的过程.曾用名反应. B、高分子絮凝剂在悬浮固体和胶体杂质之间吸附架桥的过程.6、自然沉淀 plain sedimentation 不加注任何凝聚剂的沉淀过程.7、凝聚沉淀coagulation sedimentation 加注凝聚剂的沉淀过程.8、凝聚剂 coagulant 在凝聚过程中所投加的药剂的统称.9、助凝剂 coagulant aid 在水的沉淀、澄清过程中,为改善絮凝效果,另设加的辅助药剂.10、药剂固定储备量standby reserve 为考虑非正常原因导致药剂供应中断,而在药剂仓库内设置的在一般情况下不准动用的储备量.简称固定储备量.11、药剂周转储备量 current reserve 考虑药剂消耗与供应时间之间差异所需的储备量.简称周转储备量.12、沉沙池(沉砂池)desilting basin ,grit chamber 去除水中自重很大、能自然沉降的较大粒径沙粒或杂粒的水池.13、预沉池pre-sedimentation tank 原水中泥沙颗粒较大或浓度较高时,在进行凝聚沉淀处理前设置的沉淀池.14、平流沉淀池 horizontal flow sedimentation tank 水沿水平方向流动的沉淀池.15、异向流斜管 (或斜板)沉淀池 tube(plate)settler 池内设置斜管(或斜板),水自下而上经斜管(或斜板)进行沉淀,沉泥沿斜管(或斜板)向下滑动的沉淀的池.16、同向流斜板沉淀池lamella 池内设置斜板,沉淀过程在斜板内进行,水流与沉泥均沿斜板向下流动的沉淀池.17、机械搅拌澄清池accelerator 利用机械使水提升和搅拌,促使泥渣循环,并使原水中固体杂质与己形成的泥渣接触絮凝而分离沉淀的水池.18、水力循环澄清池circulator clarifier 利用水力使水提升,促使泥渣循环,并使原水中固体杂质与己形成的泥渣接触絮凝而分离沉淀的水池.19、脉冲澄清池 pulsator 悬浮层不断产生固周期性的压缩和膨胀,促使原水中固体杂质与己形成的泥渣进行接触凝聚页分离沉淀的水池.20、悬浮澄清池 sludge blanket clarifier 加药后的原水由上通过处于悬浮状态的泥渣层,使水中杂质与泥渣悬浮层的颗粒碰撞凝聚而分离沉淀的水池.21、液面负荷 surface load 在沉淀池、澄清池等沉淀构筑物的净化部分中,单位液(水)面积所负担的出水流量.其计量单位通常以m3/(m2.h)表示.22、气浮池 floatation tank 运用絮凝和浮选原理使液体中的杂质分离上浮而去除的池子.23、气浮溶气罐dissolved air vessel 在气浮工艺中,水与空气在有压条件下相互溶合的密闭容器.简称溶气罐.24、清水池 clear-water reservoir 为贮存水厂中净化后的清水,以调节水厂制水量与供水量之间的差额,并为满足加氯接触时间而设置的水池.给水工程中输配水管网的术语1、配水管网 distribution system ,pipe system 将水送到分配管网以至用户的管系.2、环状管网pipe network 配水管网的一种置形式,管道纵横相互接通,形成环状.3、枝状管网branch system 配水管网的一种布置形式,干管和支管分明,形成树枝状.4、水管支墩 buttress ,anchorage 为防止由管内水压引起的水管配件接头移位而造成漏水,需在水管干线适当部位砌筑的墩座.简称支墩.排水工程中排水制度和管渠附属构筑物的术语及其涵义1、排水制度 sewer system 在一个地区内收集和输送废水的方式.它有合流制和分流制两种基本方式.2、合流制 combined system 用同一种管渠分别收集和输送废水的排水的方式.3、分流制 separate system 用不同管渠分别收集和输送各种污水、雨水和生产废水的排水的方式.4、检查井manhole 排水管渠上连接其他管渠以及供养护工人检查、清通和出入管渠的构筑物.5、跌水井 drop manhole 上下游管底跌差较大的检查井.6、事故排出口 emergency outlet 在排水系统发生故障时,把废水临时排放到天然水体或其它地点去的设施.7、曝雨溢流井 (截留井)storm overflow well ,intercepting well 合流制排水系统中,用来截留、控制合流水量的构筑物排水工程中水和水处理的术语及其涵义1、生活污水 domestic sewage ,domestic wastewater 居民中日常生活中排出的废水.2、工业废水 industrial wastewater 生产过程中排出的水.它包括生产废水和生产污水.3、生产污水polluted industrial wastewater 被污染的工业废水.还包括水温过高,排入后造成热污染的工业废水.4、生产废水 non-polluted industrial wastewater 未受污染或受轻微污染以及水温稍有升高的工业废水.5、城市污水 municipal sewage ,municipal wastewater 排入城镇污水系统的污水的统称.在合流制排水系统中,还包括生产废水和截留的雨水.6、旱流污水dry weather flow 合流制排水系统在晴天时输送的污水.7、水体自净 self-purification of water bodies 河流等水体在自然条件的生化作用下,有机物降解,溶解氧回升和水体生物群逐渐恢复正常的过程.8、一级处理 primary treatment 去除污水中的漂浮物和悬浮物的净化过程,主要为沉淀.9、二级处理secondary treatment 污水经一级处理后,用生物处理方法继续除去污水不胶体和溶解性有机物的净化过程.10、生物处理 biological treatment 利用微生物的作用,使污水中不稳定有机物降解和稳定的过程.11、活性污泥法 activated sludge process 污水生物处理的一种方法.该法是在人工充氧条件下,对污水和各微生物群体进行连续混和培养,形成活性污泥.利用活性污泥的生物凝聚、吸附和氧化作用,以分解去除污水中的有机污染物.然后使污泥与水分离,大部分污泥再回流到曝气池,多余部分则排出活性污泥系统.12、生物膜法 biomembrance process 污水生物处理的一种方法.该法采用各种不同载体,通过污水与载体的不断接触,在载体上繁殖生物膜,利用膜的生物吸附和氧化作用,以降解去除污水中的有机污染物,脱落下来的生物膜与水进行分离.13、双层沉淀池(隐化池) Imhoff tank 由上层沉淀槽和下层污泥消化室组成.14、初次沉淀池primary sedimentation tank 污水处理中第一次沉淀的构筑物,主要用以降低污水中的悬浮固体浓度.15、二次沉淀池secondary sedimentation tank 污水生物处理出水的沉淀构筑物,用以分离其中的污泥.16、生物滤池 biological filter ,trickling filter 由碎石或塑料制品填料构成的生物处理构筑物.污水与填料表面上生长的微生物膜间歇接触,使污水得到净化.17、生物接触氧化bio-contact oxidation 由浸没在污水中的填料和人工曝气系统构成的生物处理工艺.在有氧的条件下,污水与填表面的生物膜反复接触,使污水获得净化.18、曝气池aeration tank 利用活性污泥法进行污水生物处理的构筑物.池内提供一定污水停留时间,满足好氧微生物所需的氧量以及污水与活性污泥充分接触的混合条件.排水工程中污泥和污泥处理的术语及其涵义1、原污泥 raw sludge 未经污泥处理的初沉污泥、二沉剩余污或两者的混合污泥.2、初沉污泥 primary sludge 从初次沉淀池排出的沉淀物.3、二沉污泥 secondary sludge 从二次沉淀池排出的沉淀物.4、活性污泥activated sludge 曝气池中繁殖的含有各种好氧微生物群体的絮状体.5、消化污泥digested sludge 经过好氧消化或厌氧消化的污泥,所含有机物质浓度有一定程度的降低,并趋于稳定.6、回流污泥 returned sludge 由于次沉淀池(或沉淀区)分离出来,回流到曝气池的活性污泥.7、剩余污泥 excess activated sludge 活性污泥系统中从二次沉淀池(或沉淀区)排出系统外的活性污泥.8、污泥气 sludge gas 在污泥厌氧消化时,有机物分解所产生的气体.主要成分为甲烷和二氧化碳,并有少量的氢、氮和硫化氢.俗称沼气.9、污泥消化sludge digestion 在有氧或无氧条件下,利用微生物的作用,使污泥中有机物转化为较稳定物质的过程.10、好氧消化aerobic digestion 污泥经过较长时间的曝气,其中一部分有机物由好氧微生物进一步降解和稳定的过程.11、厌氧消化 anaerobic digestion 在无氧条件下,污泥中的有机物由厌氧微生物进行降解和稳定的过程.12、中温消化mesophilic digestion 污泥在温度为33℃-35℃时进行的厌氧消化工艺.13、高温消化thermophilic digestion 污泥在温度为53℃-55℃时进行的厌氧消化工艺.14、污泥浓缩sludge thickening 采用重力或气浮法降低污泥含水量,使污泥稠化的过程.15、污泥淘洗 elutriation of sludge 改善污泥脱水能的一种污泥预处理方法.用清水或废水淘洗污泥,降低水化污泥碱度,节省污泥处理投药量,提高污滤脱水效率.16、污泥脱水 sludge dewatering 对浓缩污泥进一步去除一部分含水量的过程,一般指机械脱水.17、污泥真空过滤 sludge vacuum filtration 利用真空使过滤介质一侧减压,介质的污泥脱水方法.18、污泥压滤 sludge pressure filtration 采用正压过滤,使污泥水强制滤过介质的污泥脱水方法.19、污泥干化 sludge drying 通过渗滤或蒸发等作用,从污泥中去除大部分含水量的过程,一般指采用污泥干化场(床)等自然蒸发设施.20、污泥焚烧sludge incineration 污泥处理的一种工艺.它利用焚烧炉将脱水污泥加温干燥,再用高温氧化污泥中的有机物,使污泥成为少量灰烬.排水工程中物理量的术语及其涵义1、生化需氧量 biochmical oxygen demand 水样在一定条件下,于一定期间内(一般采用5日、20℃)进行需氧化所消耗的溶解氧量.英文简称BOD.2、化学需氧量 chemical oxygen demand 水样中可氧化物从氧化剂重铬酸钾中所吸收的氧量.英文简称COD.3、耗氧量oxygen consumption 水样中氧化物从氧化剂高锰酸钾所吸收的氧量.英文简称OC或CODMn .4、悬浮固体suspended solid 水中呈悬浮状态的固体,一般指用滤纸过滤水样,将滤后截留物在105℃温度中干燥恒重后的固体重量.英文简称SS。
污水处理流程英文版

污水处理流程英文版English:The wastewater treatment process involves several stages to remove impurities and contaminants from the sewage before it is released back into the environment. The first stage is screening, where large objects like sticks, rags, and debris are removed from the sewage using bar screens or fine screens. Then, the sewage goes through the primary treatment stage, where solid materials are settled and removed from the water. After that, the sewage undergoes secondary treatment, which uses biological processes to break down organic matter and remove pathogens. The final stage is disinfection, where chemicals or physical methods like UV radiation are used tokill any remaining bacteria and microorganisms in the water. Oncethe wastewater has gone through all these stages, it can be safely discharged into rivers, lakes, or oceans.中文翻译:污水处理过程包括几个阶段,以在将其排放回环境之前从污水中去除杂质和污染物。
污水处理英文

污水sewage污水处理 sewage treatment一级处理 primary treatment二级处理 secondary treatment生物处理 biological treatment活性污泥法 activated sludge process曝气池 aeration tank曝气 aeration充氧oxygenation好氧消化 aerobic digestion厌氧消化 anaerobic digestion溶解氧dissolved oxygen沉淀 sedimentation搅拌agitation氯化 chlorination余氯 residual chlorine污泥 sludge泥龄sludge age回流污泥 returned sludge剩余污泥 surplus sludge消化污泥 digested sludge活性污泥 activated sludge污泥浓缩 sludge thickening污泥脱水 sludge dehydrating絮凝 flocculation水头Flood peak水头损失 head loss液面负荷 surface load、工艺参数类设计流量 Design flow泵流量pump flow栅前水深 water depth of ahead grille过栅流速Crosses the grille speed of flow栅条间隙Grille gap过栅水头损失head loss of crosses the grille 格栅倾角 Grille inclination angle过栅流量 flow of grille齿耙运行速度 rake speed有效水深Effective water depth水力停留时间HRT hydraulic residence time水力表面负荷Hydraulic load surface污泥浓度sludge concentration污泥回流比sludge reflux ratio机械设备类粗格栅 coarse screen回转格栅除污机grille decontaminating equipment 无轴螺旋输送机shaftless screw conveyor无轴螺旋压榨机 shaftless screw compressor潜污泵submersible sewage pump细格栅fine screen旋流沉砂器rotational sand processor砂水分离器 grit-water separator潜水搅拌器submersible agitator潜水推流器 submersible water impeller可调堰门adjustable weir曝气转刷aeration brushes吸刮泥机aspiration sludge scraper离心脱水机Decanter Centrifuge切碎机Macerator转鼓浓缩机Drum thickener絮凝剂投配单元polymer make-up &dosing unit圆形闸门Circular gate蝶阀 butterfly valve闸阀 gate valve球阀 Ball valve止回阀 Check Valve放空阀 Emptying valve微阻缓闭止回阀 Tiny Drag Slow Shut Check Valves 电磁阀 Mgnetic valve电动阀 Mortor operated valve法兰 Flange主轴承main bearing减速机gearbox超声波流量计supersonic flow meter电磁流量计electromagnetic flow meter阅读全文monitored control system 监察控制系统sewage treatment 污水处理Welcome to Danone monitored control system of sewage treatment。
污水专业英语翻译大全

污水处理专业英语翻译大全专有名词病毒细菌Campylobacteria Jejuni 弯曲菌Coliform-group bacteria 大肠菌群Corynebacterium 棒状杆菌属Cryptosporidium 隐孢子虫Escherichia Coli 大肠杆菌Dipterex 敌百虫Giardia 贾第鞭毛虫Legionella 军团菌Methanogenes 产甲烷细菌Norovirus 诺罗病毒Pathogen 病原菌(致病菌)Rydrogenes and acetic aid genes 产氢气乙酸菌自建字库A/A/O法 anaerobic-anoxic-oxic process (厌氧-缺氧-好氧法)A-A-O生物脱氮除磷工艺 A-A-O biological nitrogen and phosphorus removal process Activated sludge process 活性污泥法Adsorption 吸附Aeration 曝气Aerobinen bakteeri 需氧微生物A-O脱氮工艺 A-O nitrogen removal processA-O除磷工艺 A-O phosphorus removal processAB法Adsorption Biodegradation process (吸附生物降解法)ammonia-nitrogen 氨氮ammonium salt 铵盐amino acid 氨基酸Anaerobinen bakteeri 厌氧微生物A/O法(厌氧-好氧法) anaerobic-oxic processBackwater 回用水Biofilm 生物膜法Biological aerated 生物曝气BOD (Biochemical oxygen demand) 生物需氧量Campylobacteria Jejuni 弯曲菌Carrousel Oxidation ditch 卡鲁塞尔氧化沟(荷兰DHV1967研制)CAST回圈式活性污泥法(Cyclic Activated Sludge T echnology,简称CAST) Cesspool 污水池COD(chemical oxygen demand)化学需氧量Colloid 胶体Corrosion腐蚀Cryptosporidium 隐孢子虫Giardia 贾第鞭毛虫Escherichia Coli 大肠杆菌Eutrophication 富营养化Flocculation 絮凝Flotation 悬浮法= flotaatioHBR (high compound biological reactor)高效复合生物反应器Hydrogen sulfide 硫化氢= rikkivetykaasuICEAS间歇循环延时曝气系统(Intermittent Cycle Extended Aeration System)Ion Exchange 离子交换Legionella 军团菌Microbiology 微生物Nitragen氨化反应Norovirus 诺罗病毒Nutrients 营养物Orbal oxidation ditch奥贝尔(Orbal)型氧化沟ozone generator臭氧发生器ozonation process臭氧法ozone disinfection臭氧消毒Pathogen病原菌(致病菌)pathogen microorganism病原微生物primary treatment, 一级处理SBR序列间歇式活性污泥法(Sequencing Batch Reactor Activated Sludge Process)secondary treatment 二级处理Sedimentation tank 沉淀池Sewage 污水Sewage treatment 污水处理sewage treatment rate 污水处理率Suspended solids 悬浮物Tertiary treatment 三级处理Total a radioactivity总a放射线Unitank好氧生物污水处理法Wastewater 污水B巴登福脱氮除磷工艺Bardenpho nitrogen and phosphorus removal process白水(漂洗废水) white water(bleaching water)板框压滤 plate pressure filtration离心机 centrifugal machine半渗透膜 semi-permeable membrane棒状杆菌属 corynebacterium薄膜式淋水填料 film packing饱和常数(Ks) saturation constant暴雨公式 storm flow formula暴雨径流 storm runoff溢流井 overflow well苯 benzene苯胺 aniline总B放射性 Total B radioactivity泵型叶轮暴气器paddle impeller aerator泵站 pumping stationBMTS型一体化氧化沟 BMTS intrachannel clarifier oxidation ditch 闭路循环closed loop表面冲洗surface washing表面负荷surface load表面过滤surface filtration表面活性剂 surfactant表面活性物质 surface active additive agent表面曝气 surface aeration表面曝气器surface aerator表面淹灌 surface flood irrigation表面冲洗装置 surface washing facility丙烯酸acrylic acid丙烯腈acrylonitrile病毒virus病原菌(致病菌) pathogen病原微生物pathogen microorganismBOD-污泥负荷 BOD-sludge load补充水 make-up water布朗运动 Brownian movementBOD (Biochemical oxygen demand) 生物需氧量Campylobacteria Jejuni 弯曲菌Carrousel Oxidation ditch 卡鲁塞尔氧化沟(荷兰DHV1967研制)Cast活性污泥法之一Cintinuous Loop Reator,简称CLR连续环式反应池COD(chemicaloxygendemand)化学需氧量C财务评价 financial evaluation配水系统 distribution system侧渠型一体化氧化沟 integrated oxidation ditch with side ditch产氢气乙酸菌 Rydrogenes and acetic aid genes产甲烷细菌 methanogenes产率系数 yield coefficient常规给水处理工艺 conventional water treatment processes敞开式循环冷却水系统 opened recirculating cooling water system 超高纯水ultra-high-purify water超过滤 ultrafiltration超过滤膜法ultrafiltration membrane process沉淀 precipitation, sedimentation沉淀池 sedimentation tank沉砂池 grit chamber城市废水 municipal wastewater城市废水处理 municipal wastewater treatment澄清clalification可持续发展sustainable development充满度 degree of fullness重现期 exceedion interval, period of recurrence抽风式机械通风冷却塔 induced draft mechanical cooling tower臭氧发生器 ozone generator臭氧法 ozonation process臭氧消毒 ozone disinfection初次(级)沉淀池 primary clarifier, primary sedimentation tank除水器 drift eliminator除铁除锰 iron and manganese removal除盐水(脱盐水) desalted water,demineralized water除渣 desilication, silica removal除藻 algal removal除氟 algal fluorine穿透曲线 penetration curve活性污泥法 activated sludge process生物脱氮工艺 biological nitrogen removal process船型一体化氧化沟 Boat Type in intrachannel clarifier oxidation ditch 纯(富)氧曝气法 pure-oxygen aeration process磁凝聚 magnetic coagulation磁盘法 magnetic disk process磁过滤法 magnetic fierration process萃取extraction萃取剂 extractantD达西定律Darcy’s law大肠菌群 Coliform-group bacteria大气泡曝气装置 large bubble aerator代谢 metabolism带式过滤 belt press filtration]单级传统消化池 single-stage conventional digester单螺旋式曝气装置 single spiral aerator氮 nitrogen氮循环 nitrogen cycle蛋白质 protein倒虹管 inverted siphon低放射性废物 low-level radio active waste制浆废水 kraft mill wastewater敌百虫dipterex敌敌畏dichlorvos涤纶纤维 polyester fiber地表漫流系统 overland flow system(OF)地表水 surface water地面(表)水环境质量标准 environmental quality standard for surface water 地下滤场 underground filtration field地下渗漏 underground percolation地下渗滤系统 subsurface infiltration system地下水 groundwater人工湿地系统artificial(constructed) wetland再生水回流地下水质标准 water quality standard for recharging parified wastewater water into groundwater aquifer地下水位 underground water level淀粉生产废水starch producing wastewater点滴-薄膜式淋水填料splash-film packing点滴式淋水填料splash packing点污染源 point pollufion source电动电位 electromotance potential电镀废水 electroplating wastewater电极 electrode电解法 electrolytical process电流密度 eletronic density电渗析 electrodialysis电渗析器 electrodialyzer电晕放电 brush discharge动态年成本 dynamic annual cost动植物油 oil and grease对硫磷parathion多层床 multibed多环芳烃 polycyclic hydracarban多氯联苯 polychlorinated biphenyls(PCBs)Dat-Ita 活性污泥法之一Decant 排水E二次(级)沉淀池secondary clarifier, secondary sedimentation tank二级处理secondary tratamentEceas 活性污泥法之一Escherichia Coli 大肠杆菌F乏燃料spent fuel反冲洗black washing反渗透(逆渗透)reverse osmosis反渗透法reverse osmosis process反渗透膜reverse osmosis membrane反硝化,脱氮denitrification防止腐蚀corrosion prevention纺丝spining纺织废水textile wastewater放射性半衰期radioactive half-life放射性废水处理radioactive wastewater treatment放射性排出物radioactive effluent非点源污染(面源污染)non-point source pollution非离子氨non-ionic ammonia废水处理wastewater treatment废水中和neutralization of wastewaters分离separation分流制separate system分流排水系统separated sewer system酚phenol焚烧incineration风吹损失windage loss风筒式冷却塔chimmey cooling tower封闭循环系统closed recirculation system氟化物fluoride辐射流沉淀池radial flow sedimentation tank浮盖式消化池floating-cover digester气浮flotation福斯特利帕除磷工艺Phostrip phosphorus removal process福列德克斯脱氮除磷工艺Phoredox nitrogen and phosphorus removal process 腐蚀corrosion富营养化eutrophication富营养化湖泊、水库eutrophic lake,eutrophic reservoirFlocculant 絮凝Gr射线 gamma rays甘蔗废水 sugarcane wastewater干化 drying干化床 drying bed冷却塔 cooling tower钢铁工业废水 iron and steel mill wastewater高纯水 ultrapure water高放废物 high-level radio active wastes高分子电解质 polymolecular electrolye高分子絮凝剂 polymolecular floc高负荷活性污泥法 high-loading activated sludge method高负荷生物滤池 high loading biological filter高炉煤气洗涤水 wastewater produced from scrubbing blast furnace top gas 高锰酸盐指数 potassium permanganate index高速消化池 high-rate digester高梯度磁分离器(HGMS) high grade magnegic separator高浊度水 high turbidity water格栅 bar screen隔板反应池 baffle reaction tank隔板式混合槽 baffle mixer隔油池 oil separator镉cadmium铬chromium给水泵站 water pumping station给水处理 water treatment给水网管系统 water supply system工业水处理与循环系统industrial water treatment and recirculation system 工业废水 industrial wastewater汞 mercury鼓风曝气 blast aeration鼓风式机械通风冷却塔 forced draft mechanical cooling tower固定螺旋式曝气装置 fixed spiral aerator景观、娱乐水体landscape and recreation waterbody管道接口 conduit joint给水配水系统 water supply piping distribution system 网管平差 balancing netwok罐头生产废水 Cannery wastewater硅藻土 cilicious marH海水淡化 demineralization of sea water含酚废水 phenol contained wastewater含水量moisture content含盐量saline capacity含油废水 oily wastewater旱流污水量(DWF) dry-weather flow好氧生物处理 aerobic biological treatment好氧塘aerobic pond好氧稳定 aerobic stabilization合成洗涤剂 synthetic detergent合成纤维 synthetic fiber合成纤维废水 synthetic fiber wastewater合成橡胶 synthetic rubber合流城市废水 combined municipal wastewater合流制排水系统 combined sewer system水体功能分类 waterbody function classification核能工厂 nuclear power station核燃料循环 nuclear fuel cycle核素nuclide冶金工业废水 metallurgical industry wastewater黑液 black liquor黑液除硅 sillica-elimination from black liquid虹吸滤池 siphon filter化学处理 chemical treatment化学工业 chemical industry化学吸附 chemical adsorption化学纤维 chemical fiber化学需氧量 chemical oxygen demand (COD)环状管网系统 grid pipe network system缓蚀 corrosion inhibition缓蚀剂corrosion inhibitor磺化煤sulfonated coal挥发酚volatile phenol回流比recycle ratio回流污泥率 return sludge ratio汇水面积 catchment area, collection area混合 mixing混合床 miced bed混合液挥发性悬浮固体 mixed liquor volatile suspended solids(MLVSS)混合液悬浮固体 mixed liquor suspended solids(MLSS)混凝 coagulation混凝沉淀 coagulation-sedimentation混凝剂 coagulant浑浊度 tubidity活化产物 activation products硅酸钠 sodium silicate活性剂 activator活性染料 active dye活性炭 activated carbon活性炭的再生 re-generation of activated carbon活性炭吸附 active carbon adsorption活性污泥activated sludge活性污泥法 activated sludge process活性污泥负荷 activated sludge loading活性污泥驯化 acclimation of activated sludgeIdle 闲置Legionella 军团杆菌Oxidation ditch 氧化沟, 又名连续循环曝气池(Continuous loop reactor)Ritilä格栅?SBR(Sequencing Batch Reactor Activated Sludge Process) 序列间歇式活性污泥法Settle 沉淀Unitank活性污泥法之一污水处理技术知识目前,国内外城市污水处理厂处理工艺大都采用一级处理和二级处理。
污水处理中英文名词对照表
RO RS RSSS RSV SBR SDI
Reverse Osmosis Return Sludge Return Sludge Suspended Solid Return Sludge Volume Sequencing Batch Reactor Sludge Density Index
SRT SS SV SVI TNT TOC TOD TN TKN TP TS TPM UASB UBF UCT UF UNITANK UV UV-A UV-B UV-C VFA VIP VS VSS
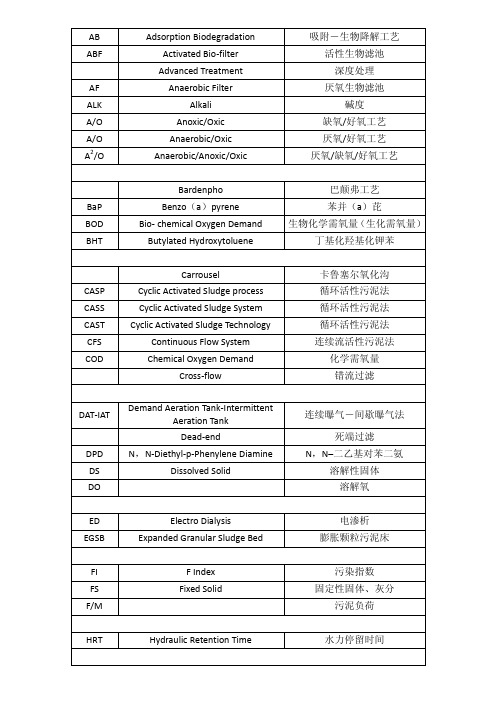
AB ABF AF ALK A/O A/O A2/O
Adsorption Biodegradation Activated Bio-filter Advanced Treatment Anaerobic Filter Alkali Anoxic/Oxic Anaerobic/Oxic Anaerobic/Anoxic/Oxic Bardenpho
Benzo(a)pyrene Bio- chemical Oxygen Demand Butylated Hydroxytoluene Carrousel
CASP CASS CAST CFS COD
Cyclic Activated Sludge process Cyclic Activated Sludge System Cyclic Activated Sludge Technology Continuous Flow System Chemical Oxygen Demand Cross-flow Demand Aeration Tank-Intermittent Aeration Tank Dead-end N,N-Diethyl-p-Phenylene Diamine Dissolved Solid
污水处理工艺工程中英文及缩写对照表

污水处理工艺工程中英文及缩写对照表缩写英文sewage treatmentsewage treatment structuresludge treatment structuretechnical pipelineWWTPCASFeCl3BFDPFDP&IDUIDO&MF/MWASSASRASSRTORPsewage treatment plant construction engineeringauxiliary engineeringAutocontrol and automated monitoring systemcup rabbetcofferdamconstruction drainage drainage by open channel drainage by well pointsconstruction jointpost-placed stripdeformation jointwater stopping band; water sealing band open caissonponding cisternoutlet structurewater (waste water) treatment structureadjusting structure watering testair tightness testWastewater treatment plantConventional Activated Sludgeferric chloridePermeate pump (suction filtration)block flow diagramProcess Flow DiagramPiping & Instrument DiagramUtility & Instrumentation DrawingsOperation and Maintenanceoperation and maintenance manualFood/microorganismwaste activated sludgesurplus?activated?sludgereturn?activated?sludgeSludge Retention Timeoxidation-reduction potentialABABFAFALKA/OA/OA2/ONH3-NBaPBODBHTCASPCASSCASTCFSCODDAT-IATDPDDSDOEDEGSBFIFSF/MHRTICEAScentrifugal pumpscrew pumpAdsorption?Biodegradation?Activated?Bio-filterAdvanced?Treatment?Anaerobic?Filter?AlkaliAnoxic/OxicAnaerobic/OxicAnaerobic/Anoxic/Oxicammonia-nitrogenammonium?saltamino??acidbackfillbackflowBardenphoBackwaterRclaimed?water;recycled?waterraw waterBiofilmBenzo(a)pyreneBio- chemical Oxygen DemandButylated HydroxytolueneCarrouselCyclic Activated Sludge processCyclic Activated Sludge SystemCyclic Activated Sludge TechnologyContinuous Flow SystemChemical Oxygen DemandCross-flowcolloidDemand Aeration Tank-Intermittent Aeration TankDead-endN,N-Diethyl-p-Phenylene?DiamineDissolved SolidElectro DialysisExpanded Granular Sludge BedF IndexFixed SolidHydraulic Retention TimeIntermittent Cyclic Extended SludgeIPFJTUMBRMFMLSSMLVSSMPNMSBRNFNTUOCOPFORPInorganic Polymer FlocculentJackson Turbidity UnitsMembrane BioreactorMicro-porous FiltrationMixed Liquor Suspended SolidMixed Liquor Volatile Suspended SolidMost Probable NumberModified Sequencing Bath ReactorNanometer-FiltrationNephelometric Turbidity UnitsOxygen ConsumedOrganic Polymer FlocculentOrbalOxygen Reduction PotentialPhostripPhoredoxPoly Aluminum ChloridePolycyclic Aromatic HydrocarbonPoly AcrylamidePoly Ferric SulfatePrepared Terephthalic AcidRelcained?Water,Recycled?WaterReverse OsmosisReturn SludgeReturn Sludge Suspended SolidReturn Sludge VolumeSequencing Batch ReactorSludge Density IndexSolids retention TimeSuspend SolidSettling VelocitySludge Volume IndexTrinitrotolueneTotal Organic CarbonTotal Oxygen DemandTotal NitrogenTotal PhosphorusTotal Productive MaintenanceUpflow Anaerobic Sludge BlanketUpflow Bio-filterUniversity of Cape TownPACPAHPAMPFSPTARORSRSSSRSVSBRSDISRTSSSVSVITNTTOCTODTNTKNTPTSTPMUASBUBFUCTUFUltra-FiltrationUp Graded Secondary TreatmentUNITANKUVUV-AUV-BUV-CVFAVIPVSVSSCaOCaSO4UltravioletUltraviolet-AUltraviolet-BUltraviolet-CVolatile Fatty AcidVolatile SolidVolatile Suspend Solidprimary treatmentsecondary treatmentTertiary treatmentfilter presscake of filter-pressbelt filter pressgrit chamberprimary clarifier, primary sedimentation tankblowerscreencoarse screenfine screensuper-fine screenbio-discbiofilterbuffer tankbulking of activated sludgecalcium lime (calcium oxide)calcium sulphatescumpH meterdefoaming agentdenitrificationdenitrifying bacteriadeodorizationdepositdischarge capacitydischarge permitdistributing troughemergency bypassequipment availabilityenclosurefacultativefeedfill and drawfillerfilterfilter screenfiltratefiltrationfinal clarifierfine gravelflow meterflow ratefluidized bedfoamgraphical symbollegendimpellerwater quality parameterwater temperaturecolor indextransparencyturbiditymunicipal sewagedomestic sewageindustrial wastewaterpollution discharge standardtotal amount of pollution discharge standardpriority monitoringintegrated prevention and control of pollutionenvironmental function zoningoxygen deficitreaerationsaturated dissolved oxygencombustionconvectioncomponentcomposite samplecompositionconsoleconsolidation tankconstant rate pumpproportional pumpmetering pumpcontact biological filterbiological contact oxidation感谢您的阅读,祝您生活愉快。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
河流污水处理的相关论述1 前言随着工业化和城市化的发展,水环境污染、水资源紧缺日益严重,水污染控制、水环境保护已刻不容缓。
我国现在新建城市或城区采用雨污分流制,但老城市或老城区大多仍然是雨污合流的排水体制。
许多合流污水是直接排放到水体。
而将旧合流制改为分流制,受现状条件限制大许多。
老城区建成年代较长,地下管线基本成型,地面建筑拥挤,路面狭窄,旧合流制改分流制难度较大。
合流污水的一大特点是旱季和雨季的水质、水量变化大,雨季污水BOD 浓度低,不利于生化处理。
国家提出2010 的我国城市污水处理率要求达到40%, 因此研究有效的合流污水处理方法对加快城市污水处理步伐具有重要的意义。
本文针对合流污水处理的有关情况,谈一些个人看法。
2 污水处理工艺要求我国目前不少城市,新城区与老城区并存,合流制与分流制并存。
因此,新建或扩建的污水处理厂,在满足城市总体规划和排水规划需要的同时,还应能达到如下要求:1. 具备接纳旧城区合流污水的能力,具有较强的适应冲击负荷的能力。
污水处理厂污水来源包括两部分,一是新城区分流污水,二是老城区合流污水。
与合流污水相比,分流污水水质、水量变化幅度小得多,对污水处理厂调节缓冲的要求小得多。
对于合流污水,降雨前期因雨水冲刷街区,合流污水较脏,但水量相对较小,降雨后期水量较大,但污水中有机物浓度相对较小。
因此,降雨前期合流污水,可考虑与分流污水一起经预处理后进入污水处理构筑物。
降雨后期合流污水,除一部分与分流污水一起经污水预处理构筑物进入污水处理构筑物外,另一部分可考虑通过雨污溢流构筑物进入雨污溢流沉定池后排入附近水体。
为了对进入污水处理构筑物的合流污水高峰流量、水质波动进行缓冲调节,污水处理构筑物前端可设缓冲调节池以均衡水质、储存水量。
2. 具有可靠的BOD、COD、SS 去除功能及氮磷去除功能,保证最终出水水质稳定。
通常情况下,城市污水中难降解有机物较少,BOD、COD 去除比较容易实现,而氮磷去除则较复杂。
我国现行的污水排放标准对污水处理厂出水氮磷指标有严格的要求,故城市污水处理都必须达到氮磷的有效去除。
在现行城市污水脱氮除磷工艺中,A2/0 采用较为广泛。
针对A2/0 工艺存在的问题目前出现了许多改进工艺,每种工艺又都存在各自的特点和局限。
由于合流污水引起的水质、水量波动较大,对污水厂各处理单元产生冲击,为了适应受纳水体的要求,为使BOD、COD 等指标进一步降低,进一步去除污水中的细菌及氮、磷等植物性营养物质,在污水厂与受纳水体之间可设氧化塘。
3. 具有灵活多变的运行方式,可根据收集的污水量、进水水质以及季节变化调整运行方式。
常规A2/0 工艺,很难做到灵活方便地调整运行方式。
但A2/0工艺从构成原理上讲,是在曝气段前加厌氧段和缺氧段。
这一原理用于氧化沟技术,便可形成各种适应不同水质、水量、季节变化的运行方式。
污水厂可根据实际情况设两个以上的氧化沟,每个沟设一定数量的水力推进器,池底均匀分布微孔爆气器。
通过调整氧化沟污水进水管阀门、曝气器的开及关的区域、内回流比大小、污泥回流比大小及水力推进器运行个数,便可形成串联、并联等若干种运行方式。
每种运行方式具有各自区域大小不同的厌氧段、缺氮段、曝气段。
当旱季污水量小则采用串联运行方式,雨季污水量大,则采用并联运行方式。
夏季温度高,硝化反应速度快,则采用具有较小曝气区域、较小硝化段的运行方式,相应反硝化区域增加、功能加强,冬季情况则正好相反。
如进水碳源浓度较低,则采取串联的、使后续反硝化段的碳源能得到补充的运行方式。
3 工艺流程选择和特点说明根据污水合流制与分流制并存的特点及处理后污水排放水体的要求: 来自新城区的分流污水,经格栅处理后进入后续污水处理构筑物; 来自老城区的合流污水平时直接进入污水处理系统。
降雨时,前期的较脏、水量较小的合流污水,与分流污水一起经格栅后进入后续污水处理构筑物。
降雨后期的合流污水水量较大主要含泥砂,一部分经雨污溢流构筑物,在沉淀池作短暂停留,去除部分泥砂后直接排放水体,另一部分则与分流污水一起经格栅后进入后续处理构筑物。
格栅用以去除污水中的大块悬浮物、漂浮物等污物,以消除大块污物对后续处理系统的不良影响。
曝气沉砂池用以去除较大砂粒及其他无机污染物颗粒,以提高污泥活性有机组分含量、减轻对管道设备的磨损、减轻后续沉淀池负荷、改善系统运行条件。
初沉池主要用以去除SS,在初沉池中,根据进水水质情况,可适时外加碳源和氨氮,以保证有足够量和适当比例的C、N、P 来源,为后续生化反应正常运行创造条件。
缓冲池主要作用是在合流污水高峰流量时均衡水质、储存水量。
氧化沟是一种简易、高效、经济的城市污水处理工艺近几十年发展迅速。
在流态上,它既是完全混合式,又具有推流式特征。
由于沟渠溶解氧浓度的递减变化规律,通过适当安排进水口、出水口、回流污泥入口位置,氧化沟可形成一个倒置A2/0 工艺。
根据硝化、反硝化、生物除磷及好氧活性污泥微生物的代谢特在缺氧段,主要功能是脱氮,回流污泥中反硝化菌以原水中有机物为碳源,以来自好氧段的硝化液中的硝酸盐为电子受体,将硝态氮NO-3-N 还原为气态氮N2。
在厌氧段,主要功能是释磷,回流污泥中聚磷菌分解释放体内聚磷酸盐,同时摄入污水中的有机物,以PHB 及糖原等形式储存于细胞内。
对于缺氧段与厌氧段的过渡过区域,既非严格的厌氧状态,而溶解氧浓度又低于缺氧段,脱氮与释磷过程都将存在,但都不易取得竞争优势。
在好氧段,功能有三: 1、好氧活性污泥中微生物,使污水中有机物得到降解、去除,好氧微生物本身得以增殖,活性污泥得以增长;2、在亚硝化菌和硝化菌作用下,将污水中氨氮NH+4-N 氧化成硝态氮,主要为NO-3-N; 3、聚磷菌体内PHB 氧化产生大量能量一部分用于从污水中过量吸收磷酸盐,并以聚磷的形式贮存于体内,一部分供给细菌合成和维持生命。
与A2/0工艺相比,前置缺氧段不仅可优先从污水中获得碳源,强化反硝化过程。
同时,因先经历反硝化过程,消除了硝酸盐的大量存在对聚磷菌厌氧释磷过程的不利影响。
通过对曝气器的控制,沟渠内可形成区域大小适宜的缺氧段、厌氧段、曝气段,在去除BOD 的同时进行生物脱氮除磷,能取得较好的氮磷去除效果。
特别是能够通过对曝气区域大小和进出水管阀门的控制,形成灵活多变的运行方式,适应污水水量、水质、季节性的变化,具有广阔的发展应用前景。
当水质波动幅度不大时,通过前述的预处理、生物处理后的污水,一般能排放水体,但由于各种不确定偶发因素的影响,这样考虑处理水排放存在不小风险。
当水质、水量大幅波动时这种情况更为突出。
由于水污染、生态破坏的严峻形势,城市污水处理厂必须从技术上严格把关,从工艺上确保处理水安全排放水体。
若在生物处理工艺之后设置熟化塘,不仅可在污水处理厂和受纳水体之间起缓冲作用,还能通过藻类-动物性浮游生物-鱼类等食物链和生态系统,使BOD、COD指标、细菌及氮磷等植物性营养物浓度进一步降低,具有良好稳定的处理效果。
特别是在熟化塘系统中,通过塘内生态系中多条食物链的物质迁移、转化和能量逐级传递、转化,在去除污染的同时,以水产资源形式达到物质、能量的回收,将污水处理与利用相结合,实现污水资源化。
4 结语1. 合流制污水水质、水量波动幅度大, 技术工艺必须满足缓和冲击负荷的要求,设置缓冲池均衡水质、储存水量比较适宜。
2. 通过多个氧化沟构成若干个串、并联运行方式,在适应进水水质、水量、季节性变化方面能够发挥重要作用。
3. 通过安排适当的进出水口位置、回流污泥入口位置,氧化沟可形式一个倒置A2/0 工艺,在去除BOD 的同时,能取得较好的氮磷去除效果4. 熟化塘的应用,为处理水安全排放水体,能够提供可靠的技术保证。
熟化塘投资省、运行费用低、管理维护方面、污水处理与利用相结合,在防治水污染、保护水环境及生态环境综合治理方面具有明显优势。
如果美化熟化塘表观,设置喷泉等设施,形成供人们休闲、游乐的人工景点,协调城市建设中土地资源的合理配置,那么熟化塘占地面积较大这一不足就不会成为突出的问题。
Combined sewage treatment related discussion1 PrefaceWith the industrialization and urbanization development, water pollution, worsening water shortage, water pollution control, environmental protection has urgent need of water. China is now adopting a new city or urban stormwater sewage diversion system, but the old city or the old city is still mostly combined sewage stormwater drainage system, a number of combined sewage is directly discharged into thewater body. The confluence of the old system to a triage system, the status quo conditions, and many of the old city built during the long, underground pipeline basic shape, the ground construction crowded, narrow roads, the old Combined triage system more difficult to change. Combined sewage is a major feature of the water quality of the dry and wet seasons, water changes, low BOD effluent concentration during the rainy season is not conducive to biochemical treatment. Countries, 2010 China's urban sewage treatment rate of 40 percent required, and therefore the study of the convergence of effective methods of sewage treatment, sewage treatment to accelerate the pace of the city is of great significance. In this paper, the convergence of the sewage treatment situation, some personal views.2 Requirements of the sewage treatment processMany cities in China, the new city with the coexistence of the old city, Combined with the streaming system co-exist. Therefore, new or expanded sewage treatment plant to meet the overall urban planning and drainage planning requirements should be able to meet the following requirements:(1) Have to accept merging the old city sewage capabilities, a strong ability to adapt to the impact load. Source of the sewage treatment plant effluent is composed of two parts: First, the new urban sewage diversion; Second, merging the old city sewage. Compared with the combinedsewage, sewage diversion of water quality, water, a much smaller range of sewage treatment plants require much less adjustment buffer. The confluence of sewage and rain red rain due to pre-rinse blocks, merging more dirty water, but water is relatively small; rain water, the larger the latter, but the concentration of organic compounds in water is relatively small. Therefore, pre-confluence rain water,could be considered together with the diversion of sewage by pre-treated sewage into the structure. Combined sewage late rainfall, with the exception of part of the sewage, together with the diversion of sewage by pre-treatment structures into the sewage treatment structures, and the other part to consider the adoption of stormwater pollution entering the storm sewage overflow structures Shen overflow pool set into the nearby water body after. In order to enter the structure of the combined sewage effluent peak flow, water quality buffer fluctuations conditioning, sewage treatment structures regulating the front-end buffer pool can be installed in order to balance water quality, storage of water.(2) Reliable BOD, COD, SS removal of nitrogen and phosphorus removal function and features to ensure the stability of the final effluent quality. Under normal circumstances, urban sewage in less refractory organic matter, BOD, COD removal is easier to achieve, rather than the complexity of nitrogen and phosphorus removal. The current effluent standards for sewage treatment plant effluent nitrogen and phosphorusindicators have strict requirements, it must be of urban sewage treatment to achieve the effective removal of nitrogen and phosphorus. Under the existing urban sewage nitrogen and phosphorus removal process, A2 / 0 used more widely. For the A2 / 0 process problems, the present situation of a number of improvements in technology, technology also exists for each of the characteristics and limitations of each. As a result of merging the water quality caused by sewage, water, volatile, wastewater treatment plant on the impact of the processing units, in order to adapt to the receiving water requirements, in order to BOD, COD and other indicators of a further drop low, further removal of sewage bacteria and nitrogen, phosphorus and other plant nutrients in the wastewater treatment plant and receiving water can be established between the oxidation pond.(3) With flexible operation mode, according to the quantity of sewage collection, water quality and seasonal variation of the adjustment operation. Conventional A2 / 0 process, it is difficult to do adjust the flexible operation mode. However, A2 / 0 process from the principle constitutes a sense, before the paragraph in the aeration and anoxic plus anaerobic paragraph. This principle for the oxidation ditch technology can be adapted to the formation of a variety of different water quality, quantity, seasonal changes in the operating mode. Wastewater treatment plant can be established according to the actual situation of the oxidation ditch more than two, each for a certain amount of ditch water propeller,bottom uniform microporous gas explosion device.3 Selection and characteristics of that processOxidation ditch is a simple, efficient and economical municipal wastewater treatment technology, has developed rapidly in recent decades. In the flow pattern, it is completely mixed, but also has push-flow characteristics. As a result of the decreasing concentration of dissolved oxygen ditches changes and appropriate arrangements through the inlet, outlet, back to the entrance of the location of the sludge, oxidation ditch can be inverted to form a A2 / 0 process:Combined sewage system in accordance with the characteristics of both a triage system, and treated water discharge requirements.Grille to remove the effluent suspended solids in the large, floating debris such as dirt, to remove large pieces of dirt on the follow-up to the adverse effects of treatment systems. Aerated grit chamber to remove large sand particles and other inorganic pollutants in order to improve Active organic component content in the sludge, reducing wear and tear on the plumbing, the follow-up sedimentation tanks to reduce load and improve the system operating conditions. Primary sedimentation tank for removal of the main SS, Shen pool in the beginning, according to the influent water quality can be a timely addition to carbon and nitrogen in order to ensure adequate and proper proportion of C, N, P sources, in order to follow-up biological and chemical response to create theconditions for normal operation. The main role of a buffer pool of sewage in the combined balance of the water quality peak flow, storage of water.And A2 / 0 process, the pre-hypoxic paragraph priority not only to obtain carbon from the wastewater, and strengthen the process of denitrification. At the same time, by first going through the process of denitrification, nitrate eliminated the existence of a large number of PAOs anaerobic phosphorus release the adverse effects of the process.City of diversion from new sewage treatment by the grating into the sewage treatment follow-up structures; from the merging of the old city sewage, usually directly into the sewage treatment system. Rainfall, the pre-than dirt, water, the merging of smaller sewage, together with the diversion of sewage into the follow-up by the grating structures sewage treatment; the merging of the late rains the sewage water, largely with silt, some through stormwater pollution overflow structures, in the sedimentation tank for a short stay, after the removal of some sediment, direct discharge of water bodies, the other part, together with the diversion of sewage into the follow-up by the grating structures to deal with.In accordance with nitrification, denitrification, biological phosphorus removal activated sludge and aerobic metabolism of microorganisms in the anoxic paragraph, the main function is to denitrification, sludge return to denitrifying bacteria as a carbon source oforganic matter in raw water, from the aerobic paragraph nitrification of nitrate solution for the electron acceptor, the nitrate (NO-3-N) back into gaseous nitrogen (N2). Anaerobic paragraph, the main function is to address the phosphorus, the return sludge in the decomposition of phosphate accumulating bacteria in vivo release of polyphosphate, while intake of organic matter in wastewater to the form of PHB and glycogen stored in the cells. Hypoxic anaerobic paragraph for paragraph and the transition over the region, neither strictly anaerobic state,And the concentration of dissolved oxygen above and below the anoxic, denitrification and phosphorus release process will exist, but are not easy to gain a competitive edge. In aerobic, functions There are three: first, Aerobic activated sludge micro-organisms, so that has been the degradation of organic matter in wastewater, removed themselves to the proliferation of aerobic micro-organisms, activated sludge to growth; Second, Nitrosation nitrification bacteria and fungi Under the effect of ammonia nitrogen in the wastewater (NH +4- N) oxidized to nitrate (mainly NO-3-N); III PAOs PHB in vivo oxidation of a large amount of energy, part of the sewage from excessive absorption of phosphorus salts, and the form of poly-P stored in the body, part of the supply and maintenance of bacterial synthesis4 ConclusionBy Aerator control ditches can be suitable for the formation of thesize of the hypoxic region paragraph, paragraph anaerobic, aeration paragraph, at the same time in the BOD removal of biological nitrogen and phosphorus removal to obtain a better removal of nitrogen and phosphorus effect. In particular to Aeration through the pipes into and out of the region and the size of the control valve to form a flexible operation mode, to adapt to water, sewage, water quality, seasonal changes, there are broad prospects for development of applications.。
