台式眼压计产品介绍
拓普康CT80说明书-中英文

3
注意
图标
注意事项
页
为避免受伤,搬动仪器时请把持正确的位置。
13
为避免触电,不要用湿手拿电源插头。
14
不要把手放在测量头下。
*让病人知道这一点。
29
无意之中把手放在那里,有可能因挤压而受伤。
不要把手放在测量头下。
*让病人知道这一点。
33
无意之中把手放在那里,有可能因挤压而受伤。
不要在仪器附近使用喷雾清洁剂。
* 伤害包括损伤、烧伤、电击等。 * 设施损坏指的是对建筑物、设备和房内陈设的损坏。
图标的含义 图标
含义
它指的是必须执行的动作。 具体内容用紧贴图标的文字或图样表达。
它代表警告。 具体内容用紧贴图标的文字或图样表达。
它代表禁止。 具体内容用紧贴图标的文字或图样表达。
2
安全注意事项
警告
图标
注意事项
页
菜单开关…………………… 显示菜单屏。 自动、手动选择开关……… 在自动和手动方式间转换。 初始化开关………………. 测量头返回原始位置。 向下开关…………………… 选择菜单屏时,它可使光标向下移动(T)。 向上开关…………………… 选择菜单屏时,它可使光标向上移动(S)。
9
显示器屏幕
测量屏幕(自动方式,对准)
1.确认电源线已正确连接。 关于此连接请阅读第 14 页的“连接电源线”。
2.检查仪器的状况,打开电源开关(ON)。 仪器从寒冷的房间移动至温暖的房间,或室温
注意事项 突然升高时,可使仪器内产生露水,影响测量。
在这种情况下,把仪器放在那里大约 30 分钟, 直到它的温度和室温一样时再开机。 显示菜单屏 1.确认测量屏显示出来了。 2.按控制面板上的 钮。 菜单屏显示出来。 返回测量屏 1.按控制面板上的U、V钮,把光标移动到“EXIT”,再按测量键。
眼压测量与眼压计的介绍

眼压测量与眼压计的介绍眼压是诊断与治疗青光眼的一个必不可少的手段。
眼压测量方法有眼压计法、直接检测法(液体压力计)及指压法。
目前临床上常用的眼压计有Goldmann压平眼压计、Perkins手持眼压计、Tono-Pen眼压计、Proview眼压监测计与非接触式眼压计等。
一、Goldmann压平眼压计1.设计原理Goldmann压平眼压计是国际上用以测量眼压的“金标准”眼压计,它是利用测压头压平角膜来进行间接的眼内压测量。
根据Imbert-Fick原理:Pt(眼内压)=W(压平角膜的外力)/A(压平面积)而推算的。
Goldmann眼压计的直径为3.06mm,当测压头使角膜压平使7.35㎜2的环形面积所需的力即为眼压测量值。
若需1g的力量加在测压头上,达到7.35㎜2环形面积时,眼压为10mmHg(1克重≈1cm3H2o=1000mm3H2o ,Pt=W/A=W/πr2=1000mm3H2o/[3.14×(3.06/2)2]= 1000mm3H2o/7.35mm2=135.98mmH2o=135.98/13.6(mmHg)=10m mHg),依此类推。
2.Goldmann压平眼压计的结构(1)测压头:为一表面平滑的透明塑料柱,前端可直接接触角膜,作压平角膜用,压平面直径为3.06mm。
后端固定于测压杠杆末端的金属环内。
测压头内有两个基底相反的三棱镜,故能使与角膜接触处的环形物象移位成为两个半圆环形。
另在测压头前端侧面上有径线刻度,供测量高度散光时,作轴向定位用。
(2)测压装置:为一能前后移动的杠杆,其移动度受内部安装的弹簧控制,弹簧的张弛力可被一测压螺旋调整。
在测压螺旋表面有以克重量为单位的重力刻度,表示弹簧的张力(克重量),范围由0至8g(即相当于0至80mmHg)。
Goldmann 眼压计有“悬吊式”(T900型)与“座式”(R900型)两型。
操作时只需捻转测压螺旋,在裂隙灯显微镜观察下,当角膜压平面达3.06mm直径时,所需压力即眼压值。
Icare TA01i 眼压计说明书
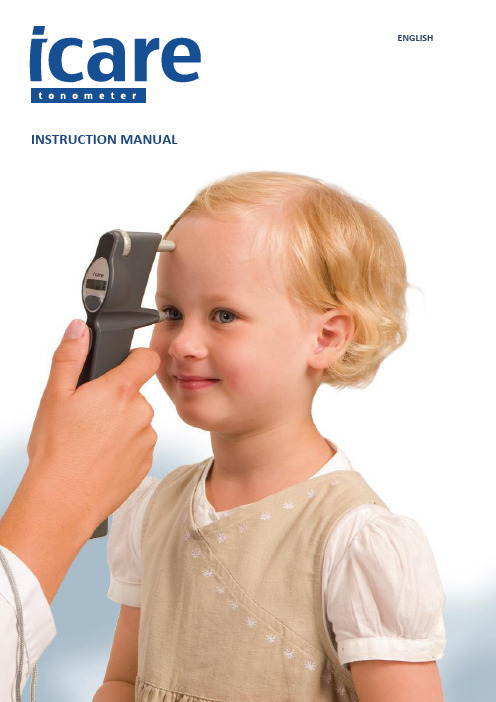
U S ER’S A N D M AI N TEN AN CE M A N U A L ENGLISHINSTRUCTION MANUAL 12TONOMETERIcare® TA01iINSTRUCTION MANUAL TA01i-001 EN-3.0The information in this document is subject to change without prior notice. In a conflict situation the English version prevails.0044This device complies with:Medical Device Directive 93/42/EEC Canadian Medical Device RegulationsCopyright © 2015 Icare Finland Oy Made in FinlandIcare Finland Oy/Tiolat OyÄyritie 22, FI-01510 Vantaa, FinlandTel. +358 9 8775 1150, Fax +358 9 728 6670 , *********************TABLE OF CONTENTSIndications for use ...................................................................................................................................................................................................................... 3 Introduction ............................................................................................................................................................................................................................... 3 Safety instructions ...................................................................................................................................................................................................................... 3 Parts of the tonometer ............................................................................................................................................................................................................... 4 Turning the tonometer on and loading the probe ...................................................................................................................................................................... 4 Load the probe in the following way: ................................................................................................................................................................................... 4 Measurement ............................................................................................................................................................................................................................. 4 Display after measurements ...................................................................................................................................................................................................... 5 Other functions .......................................................................................................................................................................................................................... 5 Accessing old measurement value ........................................................................................................................................................................................ 5 Turning the tonometer OFF .................................................................................................................................................................................................. 5 Error messages ..................................................................................................................................................................................................................... 5 Diagram of tonometer functions ................................................................................................................................................................................................ 6 Technical information ................................................................................................................................................................................................................ 6 Performance data....................................................................................................................................................................................................................... 7 Accessories ................................................................................................................................................................................................................................. 7 Maintenance .............................................................................................................................................................................................................................. 7 Replacing the probe base...................................................................................................................................................................................................... 8 Cleaning the probe base ....................................................................................................................................................................................................... 8 Cleaning the tonometer ........................................................................................................................................................................................................ 8 Replacing the batteries ......................................................................................................................................................................................................... 8 Returning the Icare tonometer for servicing /repair ............................................................................................................................................................. 9 Periodic Safety Checks .......................................................................................................................................................................................................... 9 Patents and copyrights ............................................................................................................................................................................................................... 9 Symbols ...................................................................................................................................................................................................................................... 9 Electromagnetic declaration (9)INDICATIONS FOR USEThe Icare tonometer TA01i is intended to be used for the measurement of intraocular pressure in the human eye.INTRODUCTIONThe Icare tonometer is used in the diagnosis, follow up and screening of glaucoma. It is based on a new, patented, induction-based rebound method, which allows intraocular pressure (IOP) to be measured accurately, rapidly and without an anesthetic.Since single-use probes are used for measurement, there is no risk of microbiological contamination. No part of the tonometer or probes are made with natural rubber latex. Intraocular pressure changes due to the effects of the pulse, breathing, eye movements and body position. Because measurements are taken using a handheld device in fractions of a second, several measurements are needed to obtain an accurate reading and there fore the software is pre-programmed for six measurements.SAFETY INSTRUCTIONSWARNINGThe tonometer must not come into contact with the patient’s eyes, except for the probes, which may do so for a fraction of a second during measurement. Do not bring the tonometer into contact with the eye or push it into the eye (the tip of the probe should be 4-8mm, or 1/6 – 1/3 inch, from the eye).WARNINGThe tonometer should only be opened by qualified service personnel. It contains no userserviceable parts, apart from the batteries and a probe base. The Icare tonometerrequires no routine servicing or calibration other than changing the batteries at least every 12 months or changing or cleaning the probe base. If servicing is necessary, contact qualified service personnel or your local Icare representative.WARNINGNever spray, pour or spill liquid onto the Icare tonometer, its accessories, connectors, switches or openings in the chassis. Dry any liquid on the surface of the tonometer immediately.WARNINGUse of any accessories and cables other than those specif ied in the manufacturer’s documentation, with the exception of cables sold by the manufacturer as replacement parts for internal components, may result in increased emissions or decreased immunity of the Icare TA01i tonometer.WARNINGUse of any accessory or cable with the Icare TA01i tonometer other than those specified may result in increased emissions or decreased immunity of the Icare TA01i tonometer.CAUTIONRead this manual carefully, since it contains important information on using and servicing the tonometer.Retain this manual for future use.When you have opened the package, check for any external damage or faults, particularly for damage to the case. If you suspect that there is something wrong with the tonometer, contact the manufacturer or distributor.Use the tonometer only for measuring intraocular pressure. Any other use is improper and the manufacturer cannot be held liable for any damage arising from improper use, or for the consequences thereof.Never open the casing of the tonometer, except for the battery compartment or to change the probe base.This manual contains instructions for replacing batteries and changing the probe base.Never use the tonometer in wet or damp conditions.The probe base, battery compartment cover, screws, collar and probes are so small that a child could swallow them. Keep the tonometer out of the reach of children.Do not use the device near inflammable substances, including inflammable anesthetic agents.Prior to each measurement, check that a new disposable probe from an intact package is being used.Be sure that the probe contains the small plastic round tip in front.Certain microbiological agents (e.g. bacteria) can be transmitted from the forehead support.To avoid this, the forehead support should be cleaned regularly with a disinfectant, e.g. an alcohol solution.The tonometer conforms to EMC requirements (IEC 60101-1-2: 2001), but interference may occur in it if used near (<1m) a device (such as a cellular phone) causing high-intensity elect romagnetic emissions. Although the tonometer’s own electromagnetic emissions are well below the levels permitted by the relevant standards, they may cause interference in other, nearby devices, e.g. sensitive sensors.If the device is not to be used for a long time, we recommend that you remove its AA batteries, since they may leak. Removing the batteries will not affect the subsequent functioning of the tonometer.Be sure to dispose of the single-use probes properly (e.g. in a container for disposable needles), because they may contain micro-organisms from the patient.Batteries, packaging materials and probe bases must be disposed of according to local regulations.To obtain firm support for the patient’sforehead, in order to obtain an accuratemeasurement at the right distance, you can adjust the forehead support by turning the forehead supportadjusting wheel.Open the probe tube by removing the cap and insert the probe into probe base as shown in the image. After the probe has been inserted, be careful not to point it down before activating the tonometer in order to prevent the probe from falling out. Activate by pressing the easurement button once and the tonometer will be ready for measurement when 00 appears on the display. After activating the probe is magnetized and will not fall out. CAUTIONFederal law (U.S.) restricts this device to sale by or on the order of a physician.PARTS OF THE TONOMETERTURNING THE TONOMETER ON AND LOADING THE PROBEPlace the wrist strap into the wrist strap attachment. Place the wrist strap around your wrist and secure it. The wrist strap protects the tonometer from dropping onto the floor accidentally. Insert batteries into the tonometer (page 9).Press the measurement button to turn the tonometer ON. The tonometer display will display all of the LCD segments (see the figure beside). Check that all of the segments are functional in the four-digit, sevensegment LCD display.Following a brief pause, the displa y will show “LoAd,” reminding the user to load the single use probe into the tonometer prior to measurement.Load the probe in the following way:MEASUREMENTSince local anesthetic may lower the tonometer reading, we recommend that you refrain from using an anesthetic when performing measurements.Ask the patient to relax and look straight ahead at a specific point. Bring the tonometer near the patient’s eye. The central groove should be in a horizontal position, and the distance from the eye to the front part of the collar shouldbe the length of the collar. In other words, the distance from the tip of the probe to the patient’s cornea (see picture) should be 4-8 mm (1/6-1/3 inch). 1. Forehead support2. Forehead support adjusting wheel3. Display4. Collar5. Selector button6. Measurement button7. Probe base8. Central grooveIf necessary, adjust the distance by turning the forehead support adjusting wheel. Press the measurement button lightly to perform the measurement, taking care not to shake the tonometer. The tip of the probe should make contact with the central cornea. Six measurements are made consecutively. After each successful measurement, you will hear a short beep. Once the six measurements have been performed, the IOP will be shown on the display after the ‘P’.If there is an erroneous measurement, the tonometer will beep twice and display an error message. Press the measurement button to clear the error message. If several erroneous measurements appear, see error messages (page 10).To obtain the most accurate reading, six measurements are required, but the result is also displayed after the first measurement, which can usually be considered valid. The measurement values displayed are average values for all previous measurements (1.-5.). Single measurement values are not shown. Should there be variation between the measurements, ‘P’ will flash on the display after the sixth measurement.Following the performance of the entire measurement, a new measurement series can be begun by pressing the measurement button. The tonometer will then be ready for the next measurement series (00 will show on the display, see page 8).If the user doubts the validity of the measurement (for example, if the probe made contact with the eyelid, or missed the central cornea etc.), we recommend that he/she make a new measurement. In addition, when encountering unusual values (for example over 22mmHg or below 8 mmHg) we recommend the performance of a new measurement to verify the result.*Badouin C, Gastaud P. Influence of topical anesthesia on tonometeric values of intraocular pressure. Ophthalmologica 1994;208:309-313 DISPLAY AFTER MEASUREMENTSBefore After the second measurement After the sixth measurement00 2.13 P 13After the sixth measurement, the letter P appears on the display, followed by the IOP (Intraocular pressure) reading.If the P is blinking, it means that the standard deviation of the measurements is greater than normal.P_ (line down) The standard deviation of the different measurements has a slightly greater value than normally, but the effect on the result is unlikely to be relevant.P-(line in the middle) The standard deviation of the different measurements is clearly greater than normal, but the effect on the result is probably irrelevant. A new measurement is recommended if the IOP is over 19 mmHg.P–(line up) The standard deviation of the different measurements is great and a new measurement is recommended.OTHER FUNCTIONSAccessing old measurement valueFrom the starting position, press the right or left selector buttonuntil ‘Old’ appears on the display. Then press the measurementbutton. You can now ‘scroll’ through the old values by pressingthe selector buttons (right=older, left=more recent, from 0-9).To exit the old values search, press the measurement button.The display will now show the word ‘Old’. Press either selector button to access other functions (00=measurement, End=turning OFF).Turning the tonometer OFFPress either selector butto n until the display shows ‘End‘. Pressthe measurement button for two seconds - the display will show‘byE’ and the tonometer will switch off. The used probe will bepartially ejected. Use the used package to remove it from thetonometer. Ensure that you dispose of the probe properly.Error messagesTo clear error messages, press the measurement button, after which the measurement can be repeated. The following messages may appear:DIAGRAM OF TONOMETER FUNCTIONSTECHNICAL INFORMATIONType: TA01i.The device conforms to CE regulations.Dimensions: 13 – 32 mm (W) * 45 – 80 mm (H) * 230 mm (L).Weight: 155 g (without batteries), 250 g (4 x AA batteries).Power supply: 4 x AA non-rechargeable batteries (e.g. alkaline).Measurement range: 7-50 mmHg, display range: 0-99 mmHg (IOP estimation beyond the measuring range). Accuracy (95 % tolerance interval relative to manometry): ±1.2 mmHg (≤20 mmHg) and ±2.2 mmHg (>20 mmHg). Repeatability (coefficient of variation): <8 %.Accuracy of display: 1.Display unit: Millimeter mercury (mmHg).The serial number is on the back of the battery compartment cover.There are no electrical connections from the tonometer to the patient.The device has B-type electric shock protection.Operation environment:Temperature: +10 °C to +35 °CRelative humidity: 30 % to 90 %Atmospheric pressure: 800 hPa-1,060 hPaStorage environment:Temperature: -10 °C to +55 °CRelative humidity: 10 % to 95 %Atmospheric pressure: 700 hPa-1,060 hPaTransport environment:Temperature: -40 °C to +70 °CRelative humidity: 10 % to 95 %Atmospheric pressure: 500 hPa-1,060 hPaMode of operation: continuous.PERFORMANCE DATAThe performance data is obtained from a clinical study, performed according to American National Standard ANSI Z80.10-2003 and International Standard ISO 8612.2 for tonometers. The study was performed ain the Department of Ophthalmology, Helsinki University Central Hospital. In the study, 158patients were measured. The mean paired difference and standard deviation (Goldmann-Icare) were -0.4 mmHg and 3.4 mmHg. A scattergram and Bland-Altman plot of the results is shown below.ACCESSORIESMAINTENANCEFollow local regulations and recycling instructions regarding the disposal or recycling of the Icare tonometerand accessories.WARNINGThe tonometer should only be opened by qualified service personnel. It contains no userserviceable parts, apart from the batteries and a probe base. The Icare tonometerrequires no routine servicing or calibration other than changing the batteries at least every 12 months or changing or cleaning the probe base. If servicing is necessary, contact qualified service personnel or your local Icare representative.Battery compartment coverReplacing the probe baseReplace the probe base every twelve months. Clean or replace the probe base if the error messages E01 or E03 are displayed.Instructions for replacing the probe base: • Turn off the tonometer.• Unscrew the probe base collar and put it in a safe place.• Remove the probe base by tilting the tonometer downwards and use your fingers to pull the probe base out of the tonometer.• Insert a new probe base into the tonometer. • Screw the collar in, to lock the probe base.Cleaning the probe baseYou can reuse the probe base after careful cleaning. Clean the probe base every six months. Clean or replace the probe base if the error messages E01 or E03 are displayed.Instructions for cleaning the probe base:• Fill the probe base cleaning container or other clean container with 100% isopropyl alcohol. • Turn the power off.• Unscrew the probe base collar.• Invert the probe base over the container, drop in the probe base into the container and l et soak for 5-30 minutes. • Remove the probe base from alcohol.• Dry the probe base by blowing clean canned or compressed air into the hole in the probe base. This will additionally remove possible residual dirt. • Insert the probe base into the tonometer. • Screw the collar in, to lock the probe base.Cleaning the tonometerWARNINGNever spray, pour or spill liquid onto the Icare tonometer, its accessories, connectors, switches or openings in the chassis. Dry any liquid on the surface of the tonometer immediately.Icare TA 01’s surfaces have been tested and found chemically resistan t to the following liquids: • 100 % 2-propanol • Mild soap solution • 95% Pursept solutionCleaning instructions for surfaces: • Turn the power off.• Dampen a soft cloth with one of the liquids mentioned above. • Lightly wipe the surfaces of the t onometer with the soft cloth. • Dry the surfaces with a dry soft cloth.Replacing the batteriesUnscrew the battery compartment locking screw with a screwdriver or a small coin.Remove the battery compartment cover. Remove the old batteries.Insert a new set of batteries (four AA batteries). Do not use rechargeable batteries, since they may not function properly (the inner resistance of some rechargeable batteries is too high). Insert the batteries in accordance with the diagrams inside the battery compartment, with the +terminals pointing downwards on the display side of the tonometer (the rear side), and the -terminals pointing downwards on the measurement side (the front side).Replace the battery compartment cover and secure it by screwing it in lightly using the coin or screwdriver. Take care not to use excessive force when screwing the cover into place.Returning the Icare tonometer for servicing /repairContact Icare Finland’s Technical Services Department (see ) or your local Icare representative for shipping instructions. Unless otherwise instructed by Icare Finland, there is no need to ship accessories along with the tonometer. Use a suitable carton with the appropriate packaging material to protect the device during shipment. Return the device using any shipping method that includes proof of delivery.Periodic Safety ChecksWe recommend that the following checks be performed every 24 months.Equipment inspection for mechanical and functional damage.Inspection of safety labels for legibility.Applicable in Germany only: Messtechnische Kontrolle nach MPG (Medizinproduktegesetz) alle 24 Monate.PATENTS AND COPYRIGHTSUS Patent No 6,093,147 and patents pending. The Icare tonometer is also protected by the applicable copyright laws.SYMBOLSELECTROMAGNETIC DECLARATIONWARNINGUse of any accessories and cables other than those specified in the manufacturer’s documentation, with the exception of cable s sold by the manufacturer as replacement parts for internal components, may result in increased emissions or decreased immunity of the Icare TA01i tonometer.WARNINGUse of any accessory or cable with the Icare TA01i tonometer other than those specified may result in increased emissions or decreased immunity of the Icare TA01i tonometer.Attention See instructionsSerial numberSingle use onlyB-type deviceLot numberManufacturing dateSterilized using radiationKeep dryStorage environmentTransport environmentManufacturerTA01i is class B equipment and needs special precautions regarding EMC and needs to be installed and put into service according to EMC information provided in user and maintenance manual.Compliance level Electromagnetic environment-GuidancePortable and mobile RF communications equipment should be usedno closer to any part of the IcareTA01i, including cables, than therecommended separation distance calculated from the equationapplicable to the frequency of the transmitter.Recommended separation distanced = 1 2 √Pd = 1.2 √P 80 MHz to 800 MHzd = 2.3 √P 800 MHz to 2 5 GHzwhere P is the maximum output power rating of the transmitter inwatts (W) according to the transmitter manufacturer and d is therecommended separation distance in metres (m).Field strengths from fixed RF transmitters, as determined by anelectromagnetic site survey should be less than the compliance levelin each frequency range.Interference may occur in the vicinity of equipment marked with thefollowing symbol:11。
电脑非接触眼压计对比表
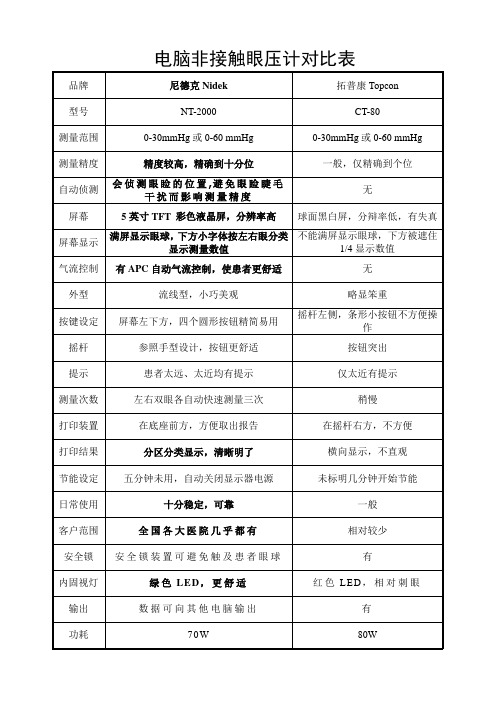
电脑非接触眼压计对比表产品介绍:1. NIDEK NT-2000利用自动对焦功能进行检测,即使是初次使用也可以轻松掌握操作。
2. NT-2000具有独特的软气流设计:NT-2000在第一次读数后将会自动启动APC功能,根据眼压情况调整喷气量的大小,从而在保证测量准确的同时尽可能让患者感觉更舒适。
3. NT-2000的安全锁装置可避免触及患者眼球。
4. 在检测前,NT-2000会侦测眼睑的位置,避免眼睑睫毛干扰而影响测量精度。
NIDEK眼压计进入中国市场已有十年,目前全国各大医院几乎都有NIDEK眼压计在使用中。
NIDEK眼压计稳定的性能已得到广大医务人员的认可。
功能参数:测量范围:1-60毫米贡柱(1毫米贡柱间隔)APC40.APC60工作距离;11毫米(喷头到角膜顶点)对位模式:一个对位光点和对焦显示内固视灯:绿色LED操纵柑工作范围:前/后36毫米左/右86毫米上/下28毫米对位模式:(对位)(对焦)(喷气)测量范围:1-60毫米贡柱(1毫米贡柱间隔)APC40.APC60工作距离;11毫米(喷头到角膜顶点)对位模式:一个对位光点和对焦显示内固视灯:绿色LED操纵柑工作范围:前/后36毫米左/右86毫米上/下28毫米对位模式:(对位)(对焦)(喷气)(自动) 自动手动自动(手动) 手动手动自动测量范围:1-60毫米贡柱(1毫米贡柱间隔)APC40.APC60工作距离;11毫米(喷头到角膜顶点)对位模式:一个对位光点和对焦显示内固视灯:绿色LED操纵柑工作范围:前/后36毫米左/右86毫米上/下28毫米对位模式: (对位)(对焦)(喷气)(自动) 自动手动自动(手动) 手动手动自动适用范围:测量范围:1-60毫米贡柱(1毫米贡柱间隔)眼科最基本视功能检查项目之一.适用于眼外伤,青光眼,眼底病,白内障,验光,以及原因不明性眼压升高.零配件:标准配件:保险丝打印纸颌托纸颌托纸固定针电源线防尘罩脉冲探测器顶盖。
眼压测量与眼压计的使用

眼压测量与眼压计的使用眼压是指眼球内眼房的液体对于眼球壁的压力。
眼压的正常范围为10-21毫米汞柱(mmHg),超过或低于这个范围都可能是眼部疾病的标志。
因此,眼压测量是一种常见的眼科检查方法,以检查眼压是否正常。
眼压计是一种用于测量眼压的专用仪器。
它的主要组成部分包括眼压计主机、显微镜和计量器。
下面将详细介绍眼压测量的步骤和眼压计的使用:1.准备工作:使用眼压计之前,需要进行一些准备工作。
首先,确保测量环境安静、明亮。
然后,确认仪器的电源已接通,然后等待其预热。
最后,预备好测量所需的消毒用品和眼压计笔尖。
2.患者准备:确保患者没有佩戴隐形眼镜,并进行眼部的简单清洁和消毒。
3.测量步骤:患者坐在测量椅上,保持眼睛的水平。
医生或技术人员戴上手套,并使用消毒液清洁手指。
然后,将消毒液放在眼压计笔尖上,在患者的眼上方放置显微镜。
医生使用另一只手的食指和中指,在患者下眼睑上施加轻微的压力使其下垂。
4.测量过程:医生使用眼压计笔尖轻轻触碰患者眼球的角膜中央。
然后医生通过显微镜观察角膜的形状和变化,并将结果读取到眼压计上的计量器上。
通常需要进行两次测量,以确保准确性。
5. 测量结果:眼压计的计量器会显示出测量结果,以毫米汞柱(mmHg)为单位。
正常的眼压范围是10-21mmHg,超过或低于这个范围可能表明患者存在眼部疾病。
1.统一标准:为了保证测量结果的准确性和可比性,需要遵循统一的测量标准。
医生或技术人员应接受专业培训,熟悉使用眼压计的方法和技巧。
2.注意清洁:眼压计使用之前需要进行清洁和消毒,以防止交叉感染。
同时,医生或技术人员需要注意手部清洁和消毒,避免引入细菌。
3.遵守操作规程:医生或技术人员在使用眼压计时需要遵守操作规程,确保测量过程的安全和有效。
比如,要保持手稳定,避免眼球受伤。
4.注意卫生习惯:医生或技术人员应注意个人卫生习惯,如佩戴手套、洗手等,以降低交叉感染的风险。
5.客观测试:为了得到准确的眼压测量结果,需要消除因素干扰。
眼压计市场分析报告

眼压计市场分析报告1.引言1.1 概述概述:眼压计是一种用于测量人眼内部压力的仪器,是眼科医生诊断和治疗青光眼的重要工具。
眼压计市场在近年来得到了迅速发展,随着人们对眼健康的关注度不断提升,对眼压计的需求也日益增加。
本报告将对眼压计市场进行深入分析,包括市场现状、发展趋势和竞争分析,旨在为行业相关企业和投资者提供全面的市场情报,并对市场未来的发展做出展望和建议。
1.2 文章结构文章结构:本报告共分为引言、正文和结论三个部分。
引言部分主要包括概述、文章结构、目的和总结等内容,用以引导读者对本报告的内容和结构有一个整体的把握。
正文部分将分为眼压计市场现状、眼压计市场发展趋势以及眼压计市场竞争分析三个小节,分析市场的现状和发展趋势,同时对市场的竞争情况进行深入剖析。
结论部分将对市场分析报告进行总结,并展望市场的发展前景,同时提出相关建议和展望。
整个报告将全面、系统地分析眼压计市场,为相关行业和企业提供可靠的参考和分析。
json"1.3 目的": {"content": "本报告的目的是对眼压计市场进行深入分析,了解市场现状、发展趋势和竞争情况。
通过对市场数据和趋势的分析,为相关企业、投资者和决策者提供有效的市场信息和参考,帮助他们制定正确的市场战略、投资决策和业务规划。
同时,通过对市场前景的展望,提供相关建议和展望,为市场未来发展提供指导和推动。
"}1.4 总结:在本篇市场分析报告中,我们对眼压计市场进行了深入分析,从现状、发展趋势到竞争分析,全面展现了眼压计市场的整体情况。
通过对市场现状的分析,我们发现眼压计市场正处于快速增长的阶段,需求持续增加,市场规模不断扩大。
同时,我们也分析了眼压计市场未来的发展趋势,包括技术创新、产品升级和市场需求的变化。
在竞争分析部分,我们对市场主要竞争对手进行了分析,并提出了一些市场策略和建议。
综合以上分析,我们认为眼压计市场有着广阔的发展前景,但也面临着激烈的市场竞争。
简述眼压计的工作原理

简述眼压计的工作原理一、引言眼压计是一种用于测量眼压的仪器,通常用于诊断青光眼等眼部疾病。
本文将详细介绍眼压计的工作原理。
二、眼压计的分类根据测量原理和使用场景,眼压计可以分为以下几种:1.接触式眼压计:需要接触到角膜表面进行测量;2.非接触式眼压计:无需接触角膜表面即可进行测量;3.便携式眼压计:体积小、重量轻,适合在户外或临床移动使用;4.台式眼压计:体积大、重量重,适合在医院或诊所等固定场所使用。
三、接触式眼压计的工作原理1.装置接触式眼压计由一个金属柄和一个圆锥形塑料头组成。
塑料头上覆盖着一个小球,该小球与角膜表面相接触。
2.测量原理当小球与角膜表面相接触时,会产生一个力。
这个力会传递到金属柄上,并通过一个弹簧传递到一个指针上。
指针的位置会随着力的大小而改变,因此可以测量出眼压。
3.注意事项接触式眼压计需要直接接触到角膜表面,因此需要消毒,并且需要由专业人员进行操作。
四、非接触式眼压计的工作原理1.装置非接触式眼压计通常由一个光源和一个探测器组成。
在使用时,光源会发射一束光线,探测器会检测这束光线经过角膜后的弯曲程度。
2.测量原理当光线经过角膜时,会受到折射和反射的影响,从而产生弯曲。
探测器会检测这种弯曲,并通过一个算法计算出眼压。
3.注意事项非接触式眼压计无需直接接触到角膜表面,因此不需要消毒,并且操作简单、方便。
五、便携式眼压计的工作原理1.装置便携式眼压计通常由一个小型电子仪器和一个小型气泵组成。
电子仪器用于控制气泵输出气体的流量和压力。
2.测量原理当气泵输出气体时,气体会通过一个小孔进入角膜表面下方的前房。
当压力达到一定值时,前房会变形,从而改变眼内压力。
电子仪器会测量这种压力变化,并通过一个算法计算出眼压。
3.注意事项便携式眼压计需要经常更换气泵中的电池,并且需要进行校准以保证测量精度。
六、台式眼压计的工作原理1.装置台式眼压计通常由一个大型仪器和一个附带支架的测量头组成。
测量头上覆盖着一个小球,该小球与角膜表面相接触。
眼压计及其选购

眼压计及其选购王英丽摘要:眼压计是定量测量眼压的专用仪器,未来会向便携性、非接触性、高精度性方面不断创新发展。
本文具体描述了目前几款常用眼压计的分类、测量原理并对比了各自优缺点。
同时,结合目前眼压计市场情况,通过分析其性能指标及参数,对其中应用最普遍的喷气式眼压计的选购方法进行了说明。
关键词:眼压计;分类;工作原理;选购方法眼睛是以光作为适宜刺激的视觉生物器官,是一种复合的光学系统。
在几何外形上,眼球可以近似认为是一个充满房液的密闭球体,眼球内部的压力叫做眼内压(IOP),简称眼压,是指包括房水、玻璃体、晶状体和血液在内的眼球内容物对于眼球壁所产生的压力。
眼球为了完成其正常视觉功能,必须维持内压高于大气压的一定水平才能保持形状的稳定。
现代眼科学已证实,人的眼压如同血压一样,是24小时波动的,掌握眼压的变化规律对于眼科疾病的诊断具有重要价值,是用于诊断眼科疾病、观察病情、估测预后、评价疗效等的重要手段和指标之一。
对于眼压过高或过低的病人,实施眼压监测是非常重要的。
图1 眼压计结构图眼压计(如图1)是定量测量眼压的专用仪器,它的工作原理是通过角膜形状变化或直接测量角膜血流脉动压力变化,换算获得眼内压。
到目前为止,已经有数十种眼压计相继问世,在众多的眼压计中,大致可分为压陷式眼压计、压平式眼压计两类,压平式眼压计又可分为接触式压平眼压计和非接触式压平眼压计两大类。
现常用的是非接触式压平眼压计,具有操作简便、测量迅速等优点。
1 眼压计的分类及对比1.1 压陷式眼压计压陷式眼压计起步于20世纪70年代,是测量人体眼压的一种专用仪器,Schiotz 眼压计(SIT)是压陷式眼压计的典型代表。
其工作原理是用一定重量的眼压测杆使角膜压成凹陷,在眼压计重量不变的条件下,压陷越深,其眼压越低,其测量值受眼球壁硬度的影响,智能型的压陷式眼压计通过光机电技术结合,可实现眼压的自动化测量。
压陷眼压计的优点是其结构简单、价格便宜、操作简便, 所以至今仍是临床上主要使用的眼压计之一。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
台式眼压计的产品组成
产品本体
充电适配器
台式眼压计调节开关作用
台式眼压计调节开关作用(续)
1.开/关键-首次按下开机,再次按下关机.(注:若设备处于待机状态,可以通过移 动面板唤醒设备,也可通过按下开/关键唤醒设备); 2.打印机外壳-打开外壳,可以更换打印纸; 3.电源输入连接/断开-通过电源接头的插入和拔出给设备供电或断电; B数据传输接口; 5.运动锁-旋紧锁定产品桌面,旋松释放产品桌面,使桌面可移动; 6.操纵杆-推动操纵杆,前后左右移动桌面.或顺时针/逆时针旋转操纵杆,升/降 头部组件; 7.简易模式键-按下可以进入简易模式,针对角膜条件不好患者使用,降低喷气要求, 使设备更容易喷气; 8.校准屏幕调整控制轮(处于操作者位置从左往右): a.颜色调节按钮; b.对比度调节按钮; c.亮度调节按钮
台式眼压计的操作方法(续)
1.接通电源后,仪器显示”待机”
2.当系统初始化时,仪器显示”开 始”
4.眼压大于25mmHg,仪器会自动加 强喷气压力;眼压大于50mmHg,仪器 会自动减弱喷气压力.
3.右眼测试数据,连同现时获取 的数据显示测量平均数.偏离值 较大的读数和虚假的数据会背自 动排除.
5.台式眼压计自动检测显示界面.
台式眼压计的操作方法(续)
在读取读数前,您应该: a.要求病人眨眼,确保有一层保护性泪膜. b.确保病人和产品的光学部件不处于直接的光照下(聚光灯或 阳光). c.确保病人的眼睛完全张开.这有助于防止挤压,即病人无意 识的绷紧眼睑,增加眼内压(IOP). d.在整个读数过程中,您应该: 允许病人不时的眨眼,以保持角膜泪膜. 3.获取读数: a.握住操纵杆,用另一只手移动台式眼压计的移动部分直至看 到患者需要测量的眼睛,并将患者的眼睛放置于校准显示屏的 中线上.
台式眼压计的操作方法(续)
2.为病人做好准备: a.在使用台式眼压计前,应该使患者保持轻松.确保他们处于最佳的读数位置,头 部最好有支撑.因为担心和紧张可能影响测试的结果.为了做到这一点,应遵循下 面列出的要点: a.确保病人处于舒适、放松的位置. b.如果病人佩戴了隐形眼镜,要求他们取下,并要求他们正常的眨眼和呼吸. c.为了让病人放心,你可以通过Demo按钮,在病人的手背上演示一下整个过程,获 取一个读数. d.把一次性的腮托纸放在腮托上.要求病人把下巴靠在腮托上. e.调整腮托高度以使患者外眼角与腮托上的刻度对齐.
台式眼压计故障汇总(续)
d.检查设备电源连接是否正常. 5.当上桌面滑动时设备无法从休眠状态中醒来: a.按要求将桌面结构从左向右滑动; b.按下仪器启动/停止按钮唤醒设备; 6.校准屏幕无图像,一直显示红色: a.检查运输用红色保护盖是否取下; 7.仪器校准屏幕显示微弱的交叉,但无法聚焦: a.调整校准屏幕对比度、颜色和亮度; 8.仪器校准屏幕图像质量差: a.调整校准屏幕对比度、颜色和亮度; 9.仪器无法喷气: a.确认仪器红色LED灯开启; b.检查仪器校准屏幕目标交叉点是否集中;
台式眼压计的操作方法(续)
b.小心的把眼压计向患者移动,直到眼睛外部的图像变成十字 叉校准图像. c.使用操纵杆对准十字叉校准目标,直到正确的位置触发眼压 计自动吹气. d.在获取每只眼睛四次读数后,保持吹气状态,直到台式眼压 计停止读数.如果台式眼压计获取了足够的读数,就会发出短 暂的哔哔声.例如:两个连续读数的偏差在+/-1mmHg之内后. e.如果读数被记录为无作用或无效,将听到一声长声报警. f.第一个读数是测试到的值,连续的读数将会显示运行的平均 眼内压.若测试的数据远离平均值或无效,计算机会自动删除 这些数据.
台式眼压计产品介绍
台式眼压计原理及产品应用
台式眼压计的原理: 台式眼压计使用喷气技术将一股经过校准的量子化空气喷射到角膜的中央部分,并 需要通过光学方法和角膜表面的反射探测到预先设定的角膜变形.喷气的过程中 ,一部分角膜由于机械刺激而收缩,而产生这种收缩所需要的喷射力或压力与眼内 压 力相关.
台式眼压计的应用: 台式眼压计不必接触到眼球表面就能精确的测量眼内压力(IOP).用来筛查青光 眼等病症.
台式眼压计调节开关作用(续)
9.校准屏幕-用于观察患者眼部,对准患者眼睛; 10.打印/菜单键 a.按动(短按)可以打印上次检查结果; b.按下(长按)可以进入仪器菜单选项; 11.清除/演示键 a.按动(短按)可以清除上次检查记录数据; b.按下(长按)可以测试喷气,产品由喷气口喷射气体; 12.开始/停止键 a.按下可使设备进入运行状态; b.再次按下可使设备进入待机状态; 13.测量显示屏-显示测量数据及各种提示信息; 14.腮托和腮托高度调节器-调节患者眼睛高度; 15.喷气口-喷射气体
台式眼压计的操作方法(续)
1.在任何情况下都可以按 下打印按钮来打印结果. 打印这些结果不会清空打 印数据的储存. [R]:xx,xx,xx,xx[xx.x] [L]:xx,xx,xx,xx[xx.x]
台式眼压计故障汇总
1.测量数据>25mmHg 测量显示屏显示:>25 READ AGAIN 解释:如果仪器喷气检测到角膜已被压平且读数大于25mmHg,屏幕将会显示该 信息.这种情况下,仪器会增大喷气压力,直到两次读数均小于20mmHg; 2.测量数据<5mmHg 测量显示屏显示:<5 READ AGAIN 解释:如果读数<5mmHg时屏幕将会显示这样的信息,这种情况下不会显示有效读 数(通过长低声音进行提醒) 3.测量数据>50mmHg 测量显示屏显示:>50 READ AGAIN 解释:如果读数>50mmHg时屏幕将会显示这样的信息,这种情况下不会显示有效读 数(通过长低声音进行提醒)-注意:该情况可能是由于患者在仪器喷气时闭上眼睛 导致; 4.仪器开启后不工作 a.检查电源是否已插到墙面插座,插座开关是否已开启; b.检查变压器上的绿色LED指示灯是否亮起; c.检查充电器接头是否插入仪器;
1.准备设备: a.将电源插在产品上.电源插口在产品右侧. b.使用位于仪器前面的开/关按钮启动设备.设备初始化之 后,便可备用. c.从吹气管上取下防尘罩. d.如果仪器被锁住,请旋开运动锁. e.使用操纵杆将台式眼压计的移动部分背对着您并且放 置在左边(以便先测量患者的右眼). f.在使用台式眼压计前,按压Clean/Demo(清理/演示)按钮 1秒钟以上,产品喷气,清除设备在闲置不用时聚集的极小尘 埃颗粒和水份.
