吡罗昔康贴片产品介绍
含有吡罗昔康的骨架型贴剂以及局部治疗急性和慢性疼痛及其相关炎

专利名称:含有吡罗昔康的骨架型贴剂以及局部治疗急性和慢性疼痛及其相关炎症的方法
专利类型:发明专利
发明人:王长金,埃里克·Y·许,南希·王
申请号:CN201180031485.5
申请日:20110526
公开号:CN102970986A
公开日:
20130313
专利内容由知识产权出版社提供
摘要:本发明涉及用于局部(即,经皮)递送吡罗昔康的骨架型贴剂以及用于治疗急性和慢性疼痛及其相关炎症(尤其是由需要施用镇痛剂和/或抗炎药物(例如,吡罗昔康)的运动损伤或其他肌肉疼痛或损伤引起的疼痛和炎症)的方法。
申请人:艾毕赛斯公司
地址:日本大阪
国籍:JP
代理机构:广州三环专利代理有限公司
更多信息请下载全文后查看。
吡罗昔康片

吡罗昔康片
Biluoxikang Pian
Piroxicam Tablets
书页号:中国药典2005版二部-247
[修订]
【检查】含量均匀度取本品1片(糖衣片除去包衣),置100ml量瓶中,加0.1mol/L盐酸甲醇溶液适量,超声20分钟使溶解,用0.1mol/L 盐酸甲醇溶液稀释至刻度,摇匀,滤过,精密量取续滤液5ml(10mg规格)或10ml(20mg规格)置100ml量瓶中,用0.1mol/L盐酸甲醇溶液稀释至刻度,摇匀。
照紫外-可见分光光度法(附录ⅣA),在334nm波长处测定
)为856计算含量,应符合规定(附吸光度,按C15H13N3O4S的吸收系数(E1%
1cm
录ⅩE)。
溶出度取本品,照溶出度测定法(附录XC第二法),以盐酸溶液(9→1000)900ml为溶出介质,转速为每分钟75转,依法操作,经40分钟时,取溶液适量,滤过,精密量取续滤液3ml,置5ml量瓶(10mg规格)或10ml量瓶(20mg规格)中,加溶出介质稀释至刻度,摇匀,作为供试品溶液;另取吡罗昔康对照品约12mg,精密称定,置200 ml量瓶中,加1mol/L盐酸溶液至刻度,摇匀,精密量取5ml,置50ml量瓶中,用水稀释至刻度,摇匀,制成每1ml中含6µg的对照品溶液。
取供试品溶液与对照品溶液,照紫外-可见分光光度法(附录Ⅳ A),在335nm的波长处分别测定吸光度,计算每片的溶出量。
限度为标示量的75%,应符合规定。
吡罗昔康贴片产品介绍

SK 集团生命科学领域的主要产品
Sales (US Million $)
45.00 40.00
主要产品的销售趋势图
GINEXIN-F 银杏叶提取制剂
35.00 30.00 25.00 20.00
TRAST
血液制品 疫苗产品
TRAST patch
TRAST 06年销售额 3400万美元
15.00 10.00
法人企业和63家分公司
TRAST patch
2007 销售额 : RMB 6,000亿 ($810亿)
能源化工 50%
信息通信 18%
贸易,服务 32%
TRAST patch
SK确立全球化的发展战略,将中国市场视为公司全球化发展战略的主要 目标,同时扩展东南亚、中南美、中东、中亚和俄罗斯等市场。
-第三次创业目标是 “全球的SK”
现在的 SK
在韩国内
• 韩国第三大集团(总资产规模) • 能源ㆍ化学领域 第1位
(1,500万 用户, M/S 38%)
• 信息通讯领域 第1位
(2,200万 用户, M/S 50.5%)
• 韩国 GDP 9% 比重
TRAST patch
在世界范围
• Fortune 500强企业(86位 ) • Asia 最优秀雇主 (SK电讯) • 全球 100 强 创新企业 91位 • 在全球38个国家拥有178个
分析方法: HPLC
2
结果:
1
Trast patch组在血药浓度很低的 情况下,滑液浓度却很高;与滑液
0
0.11 TRAST patch
口服吡罗昔康
浓度类似的同种成分口服剂型不同,
没有全身副作用,却能起到同等药
吡罗昔康 英文说明书

4′3′5′ C NH 6′ 2′N 3O 2 FELDENE®(piroxicam)CAPSULES 10 mg and 20 mgFor Oral Use Cardiovascular Risk• NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardialinfarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients withcardiovascular disease or risk factors for cardiovascular disease may be at greater risk (seeWARNINGS ).• FELDENE ® is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS ).Gastrointestinal Risk• NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (see WARNINGS).DESCRIPTION FELDENE® contains piroxicam which is a member of the oxicam group of nonsteroidalanti-inflammatory drugs (NSAIDs). Each maroon and blue capsule contains 10 mg piroxicam, each maroon capsule contains 20 mg piroxicam for oral administration. The chemical name for piroxicam is 4-hydroxyl-2-methyl-N -2-pyridinyl-2H -1,2,-benzothiazine-3-carboxamide 1,1-dioxide. Piroxicam occurs as a white crystalline solid, sparingly soluble in water, dilute acid, and most organic solvents. It is slightly soluble in alcohol and in aqueous solutions. It exhibits a weakly acidic 4-hydroxy proton (pKa 5.1) and a weakly basic pyridyl nitrogen (pKa 1.8). The molecular weight of piroxicam is 331.35. Its molecular formula is C 15H 13N 3O 4S and it has the following structural formula:The inactive ingredients in FELDENE capsules include: Blue 1, Red 3, lactose, magnesium stearate, sodium lauryl sulfate, starch.CLINICAL PHARMACOLOGYPharmacodynamicsFELDENE is a nonsteroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic, and antipyretic activities in animal models. The mechanism of action of FELDENE, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.PharmacokineticsAbsorption: FELDENE is well absorbed following oral administration. Drug plasma concentrations are proportional for 10 and 20 mg doses and generally peak within three to five hours after medication. The prolonged half-life (50 hours) results in the maintenance of relatively stable plasma concentrations throughout the day on once daily doses and to significant accumulation upon multiple dosing. A single 20 mg dose generally produces peak piroxicam plasma levels of 1.5 to 2 mcg/mL, while maximum drug plasma concentrations, after repeated daily ingestion of 20 mg FELDENE, usually stabilize at 3– 8 mcg/mL. Most patients approximate steady state plasma levels within 7–12 days. Higher levels, which approximate steady state at two to three weeks, have been observed in patients in whom longer plasma half-lives of piroxicam occurred.With food there is a slight delay in the rate but not the extent of absorption following oral administration. The concomitant administration of antacids (aluminum hydroxide or aluminum hydroxide with magnesium hydroxide) have been shown to have no effect on the plasma levels of orally administered piroxicam.Distribution: The apparent volume of distribution of piroxicam is approximately 0.14 L/kg.Ninety-nine percent of plasma piroxicam is bound to plasma proteins. Piroxicam is excreted into human milk. The presence in breast milk has been determined during initial and long-term conditions (52 days). Piroxicam appeared in breast milk at about 1% to 3% of the maternal concentration. No accumulation of piroxicam occurred in milk relative to that in plasma during treatment. Metabolism: Metabolism of piroxicam occurs by hydroxylation at the 5 position of the pyridyl side chain and conjugation of this product; by cyclodehydration; and by a sequence of reactions involving hydrolysis of the amide linkage, decarboxylation, ring contraction, and N-demethylation. In vitro studies indicate cytochrome P4502C9 (CYP2C9) as the main enzyme involved in the formation to the 5′-hydroxy-piroxicam, the major metabolite (see Pharmacogenetics, and Special Populations, Poor Metabolizers of CYP2C9 Substrates). The biotransformation products of piroxicam metabolism are reported to not have any anti-inflammatory activity.Higher systemic exposure of piroxicam has been noted in subjects with CYP2C9 polymorphisms compared to normal metabolizer type subjects (see Pharmacogenetics, and Special Populations, Poor Metabolizers of CYP2C9 Substrates).Excretion: FELDENE and its biotransformation products are excreted in urine and feces, with about twice as much appearing in the urine as in the feces. Approximately 5% of a FELDENE dose is excreted unchanged. The plasma half-life (T½) for piroxicam is approximately 50 hours. Pharmacogenetics: CYP2C9 activity is reduced in individuals with genetic polymorphisms, such as the CYP2C9*2 and CYP2C9*3 polymorphisms. Limited data from one published report that included nine subjects each with heterozygous CYP2C9*1/*2 and CYP2C9*1/*3 genotypes and one subject with the homozygous CYP2C9*3/*3 genotype showed piroxicam systemic levels that were 1.7-, 1.7- and 5.3-fold, respectively, higher compared to the 17 subjects with CYP2C9*1/*1 or normal metabolizer genotype. The pharmacokinetics of piroxicam have not been evaluated in subjects with other CYP2C9 polymorphisms, such as *5, *6, *9 and *11. It is estimated that the frequency of the homozygous *3/*3 genotype is 0.3% to 1.0% in various ethnic groups.Special PopulationsPediatric: FELDENE has not been investigated in pediatric patients.Race: Pharmacokinetic differences due to race have not been identified.Hepatic Insufficiency: The effects of hepatic disease on FELDENE pharmacokinetics have not been established. However, a substantial portion of FELDENE elimination occurs by hepatic metabolism. Consequently, patients with hepatic disease may require reduced doses of FELDENE as compared to patients with normal hepatic function.Poor Metabolizers of CYP2C9 Substrates: Patients who are known or suspected to be poor CYP2C9 metabolizers based on genotype or previous history/experience with other CYP2C9 substrates (such as warfarin and phenytoin) should be administered piroxicam with caution as they may have abnormally high plasma levels due to reduced metabolic clearance.Renal Insufficiency: FELDENE pharmacokinetics have been investigated in patients with renal insufficiency. Studies indicate patients with mild to moderate renal impairment may not require dosing adjustments. However, the pharmacokinetic properties of FELDENE in patients with severe renal insufficiency or those receiving hemodialysis are not known.Other InformationIn controlled clinical trials, the effectiveness of FELDENE has been established for both acute exacerbations and long-term management of rheumatoid arthritis and osteoarthritis.The therapeutic effects of FELDENE are evident early in the treatment of both diseases with a progressive increase in response over several (8–12) weeks. Efficacy is seen in terms of pain relief and, when present, subsidence of inflammation.Doses of 20 mg/day FELDENE display a therapeutic effect comparable to therapeutic doses of aspirin, with a lower incidence of minor gastrointestinal effects and tinnitus.FELDENE has been administered concomitantly with fixed doses of gold and corticosteroids. TheINDICATIONS AND USAGECarefully consider the potential benefits and risks of FELDENE and other treatment options before deciding to use FELDENE. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS).FELDENE is indicated:•For relief of the signs and symptoms of osteoarthritis.•For relief of the signs and symptoms of rheumatoid arthritis.CONTRAINDICATIONSFELDENE is contraindicated in patients with known hypersensitivity to piroxicam.FELDENE should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe, rarely fatal, anaphylactic-like reactions to NSAIDs have been reported in such patients (see WARNINGS: Anaphylactoid Reactions and PRECAUTIONS: Preexisting Asthma).FELDENE is contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS).WARNINGSCARDIOVASCULAR EFFECTSCardiovascular Thrombotic EventsClinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).Two large, controlled clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10–14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).Hypertensionthiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including FELDENE, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.Congestive Heart Failure and EdemaFluid retention and edema have been observed in some patients taking NSAIDs. FELDENE should be used with caution in patients with fluid retention or heart failure.Gastrointestinal Effects - Risk of Ulceration, Bleeding, and PerforationNSAIDs, including FELDENE, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3–6 months, and in about 2–4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase the risk of GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and, therefore, special care should be taken in treating this population.To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulcerations and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high-risk patients, alternate therapies that do not involve NSAIDs should be considered.Renal EffectsLong-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal antiinflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE-inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.Advanced Renal DiseaseNo information is available from controlled clinical studies regarding the use of FELDENE in patientspatients with advanced renal disease. If FELDENE therapy must be initiated, close monitoring of the patient’s renal function is advisable.Anaphylactoid ReactionsAs with other NSAIDs, anaphylactoid reactions may occur in patients without known prior exposure to FELDENE. FELDENE should not be given to patients with the aspirin triad. This symptom complex typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs (see CONTRAINDICATIONS and PRECAUTIONS: Preexisting Asthma). Emergency help should be sought in cases where an anaphylactoid reaction occurs.Skin ReactionsNSAIDs, including FELDENE, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.Other Hypersensitivity ReactionsA combination of dermatological and/or allergic signs and symptoms suggestive of serum sickness have occasionally occurred in conjunction with the use of FELDENE. These include arthralgias, pruritus, fever, fatigue, and rash including vesiculobullous reactions and exfoliative dermatitis. PregnancyIn late pregnancy, as with other NSAIDs, FELDENE should be avoided because it may cause premature closure of the ductus arteriosus.PRECAUTIONSGeneralFELDENE cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.The pharmacological activity of FELDENE in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.Hepatic EffectsBorderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs, including FELDENE. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.while on therapy with FELDENE. If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), FELDENE should be discontinued (see ADVERSE REACTIONS).Hematological EffectsAnemia is sometimes seen in patients receiving NSAIDs, including FELDENE. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including FELDENE, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving FELDENE who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored.Ophthalmologic EffectsBecause of reports of adverse eye findings with nonsteroidal anti-inflammatory agents, it is recommended that patients who develop visual complaints during treatment with FELDENE have ophthalmic evaluations.Preexisting AsthmaPatients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients withaspirin-sensitive asthma has been associated with severe bronchospasm which can be fatal. Since cross-reactivity, including bronchospasm, between aspirin and other nonsteroidal anti-inflammatory drugs has been reported in such aspirin-sensitive patients, FELDENE should not be administered to patients with this form of aspirin sensitivity and should be used with caution in patients with preexisting asthma.Information for PatientsPatients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.• FELDENE, like other NSAIDs, may cause CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur withoutwarning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative signs or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS, CARDIOVASCULAR EFFECTS).• FELDENE, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Althoughserious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medicaladvice when observing any indicative signs or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS, Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation).• FELDENE, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS and TEN, which may result in hospitalization and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever, or other signs of hypersensitivity such as itching, and shouldask for medical advice when observing any indicative signs or symptoms. Patients should beadvised to stop the drug immediately if they develop any type of rash and contact theirphysicians as soon as possible.• Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.• Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritus, jaundice, right upper quadrant tenderness, and “flu-like” symptoms).If these occur, patients should be instructed to stop therapy and seek immediate medicaltherapy.• Patients should be informed of the signs of an anaphylactoid reaction (e.g., difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS).• In late pregnancy, as with other NSAIDs, FELDENE should be avoided because it may cause premature closure of the ductus arteriosus.Laboratory TestsBecause serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs and symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g., eosinophilia, rash, etc.), or if abnormal liver tests persist or worsen, FELDENE should be discontinued.Drug InteractionsHighly Protein Bound Drugs: FELDENE is highly protein bound and, therefore, might be expected to displace other protein bound drugs. Physicians should closely monitor patients for a change in dosage requirements when administering FELDENE to patients on other highly protein bound drugs. Aspirin: When FELDENE is administered with aspirin, its protein binding is reduced, although the clearance of free FELDENE is not altered. Plasma levels of piroxicam are depressed to approximately 80% of their normal values when FELDENE is administered (20 mg/day) in conjunction with aspirin (3900 mg/day). The clinical significance of this interaction is not known; however, as with other NSAIDs, concomitant administration of piroxicam and aspirin is not generally recommended because of the potential for increased adverse effects.Methotrexate: NSAIDs have been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that they could enhance the toxicity of methotrexate. Caution should be used when NSAIDs are administered concomitantly with methotrexate.ACE-Inhibitors: Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE- inhibitors. This interaction should be given consideration in patients taking NSAIDs concomitantly with ACE-inhibitors.Diuretics: Clinical studies, as well as postmarketing observations, have shown that FELDENE can reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with NSAIDs, the patient should be observed closely for signs of renal failure (see WARNINGS: Renal Effects), as well as to assure diuretic efficacy.Lithium: NSAIDs have produced an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when NSAIDs and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.Warfarin: The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a risk of serious GI bleeding higher than users of either drug alone. Carcinogenesis, Mutagenesis, Impairment of FertilitySubacute, acute, and chronic toxicity studies have been carried out in rats, mice, dogs, and monkeys. The pathology most often seen was that characteristically associated with the animal toxicology of anti-inflammatory agents: renal papillary necrosis (see PRECAUTIONS) and gastrointestinal lesions. Reproductive studies revealed no impairment of fertility in animals.PregnancyTeratogenic Effects: Pregnancy Category CReproductive studies conducted in rats and rabbits have not demonstrated evidence of developmental abnormalities. However, animal reproduction studies are not always predictive of human response. There are no adequate and well-controlled studies in pregnant women. FELDENE is not recommended for use in pregnant women since safety has not been established in humans. FELDENE should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus.Nonteratogenic Effects:Because of the known effects of nonsteroidal anti-inflammatory drugs on the fetal cardiovascular system (closure of ductus arteriosus), use during pregnancy (particularly late pregnancy) should be avoided. In animal studies of FELDENE, gastrointestinal tract toxicity was increased in pregnant females in the last trimester of pregnancy compared to nonpregnant females or females in earlier trimesters of pregnancy.Labor and DeliveryIn rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of FELDENE on labor and delivery in pregnant women are unknown.Nursing MothersPiroxicam is excreted into human milk. The presence in breast milk has been determined during initial and long-term conditions (52 days). Piroxicam appeared in breast milk at about 1% to 3% of the maternal concentration. No accumulation of piroxicam occurred in milk relative to that in plasma during treatment. FELDENE is not recommended for use in nursing mothers.Pediatric UseSafety and effectiveness in pediatric patients have not been established.Geriatric UseAs with any NSAID, caution should be exercised in treating the elderly (65 years and older).Most spontaneous reports of fatal GI events with NSAIDs are in the elderly or debilitated patients and, therefore, care should be taken in treating this population. In addition to a past history of ulcer disease, older age and poor general health status (among other factors) may increase the risk for GI bleeding. To minimize the potential risk of an adverse GI event, the lowest effective dose should be used for the shortest possible duration (see WARNINGS, Gastrointestinal Effects – Risk of Ulceration, Bleeding, and Perforation).As with all other NSAIDs, there is a risk of developing renal toxicity in patients in which renal prostaglandins have a compensatory role in maintenance of renal perfusion. Discontinuation of nonsteroidal anti-inflammatory drug therapy is usually followed by recovery to the pretreatment state (see Warnings: Renal Effects).In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting a greater frequency of impaired drug elimination and of concomitant disease or other drug therapy.ADVERSE REACTIONSIn patients taking FELDENE or other NSAIDs, the most frequently reported adverse experiences occurring in approximately 1–10% of patients are:Cardiovascular System: Edema.Digestive System: Anorexia, abdominal pain, constipation, diarrhea, dyspepsia, elevated liver enzymes, flatulence, gross bleeding/perforation, heartburn, nausea, ulcers (gastric/duodenal), vomiting.Hemic and Lymphatic System: Anemia, increased bleeding time.Nervous System: Dizziness, headache.Skin and Appendages: Pruritus, rash.Special Senses: Tinnitus.Urogenital System: Abnormal renal function.Additional adverse experiences reported occasionally include:Body As a Whole: Fever, infection, sepsis.Cardiovascular System: Congestive heart failure, hypertension, tachycardia, syncope.Digestive System: Dry mouth, esophagitis, gastritis, glossitis, hematemesis, hepatitis, jaundice, melena, rectal bleeding, stomatitis.Hemic and Lymphatic System: Ecchymosis, eosinophilia, epistaxis, leukopenia, purpura, petechial rash, thrombocytopenia.Metabolic and Nutritional: Weight changes.Nervous System: Anxiety, asthenia, confusion, depression, dream abnormalities, drowsiness, insomnia, malaise, nervousness, paresthesia, somnolence, tremors, vertigo.Respiratory System: Asthma, dyspnea.Skin and Appendages: Alopecia, bruising, desquamation, erythema, photosensitivity, sweat.Special Senses: Blurred vision.Urogenital System: Cystitis, dysuria, hematuria, hyperkalemia, interstitial nephritis, nephrotic syndrome, oliguria/polyuria, proteinuria, renal failure.Other adverse reactions which occur rarely are:。
与好及施比较

一、产品基本信息比较
二、吡罗昔康贴片与好及施比较的优势
1、剂型更先进:吡罗昔康贴片的透皮控释的剂型,比巴布剂更为先进,并增加了控释系统,保证药物持续48小时以恒定速度释放,并有促进吸收剂可大大促进药物在局部的吸收率,保证药效更强、持久。
(临床研究证实:a.关节滑液内药物浓度与口服用药后相当;b.药物能有效释放48小时以上)
2、药物成份更强效:吡罗昔康是NSAIDs中较为强效的一种药物,具有明确的解热镇痛抗炎效果,且药效要强于阿斯比林、双氯芬酸等,更有别于中药成份。
这也保证了吡罗昔康贴片的局部治疗效果非常出色。
(临床研究证实:吡罗昔康贴片在治疗骨关节炎、急慢性疼痛的各项疗效指标明显优于双氯芬酸乳胶,具有统计学差异)
3、两天一贴,更方便: 不同于好及施等产品一天需要1-2贴,吡罗昔康贴片由于控释系统延长了给药时间,可以两天使用一贴,大大减少了更换的频率,方便患者使用。
4、局部粘贴牢固:好及施等一般产品是比较大、稍厚的方形贴片,贴在关节容易脱落。
而吡罗昔康贴片独有椭圆形专利设计,更加紧凑,轻巧,面积小,附着非常牢固,并且背衬材料有一定的弹性,贴敷非常舒适,不影响关节正常的活动。
5、皮肤刺激最小化:一般的贴片由于面积大、厚,皮肤透气性差容易导致接触过敏,而吡罗昔康贴片的背衬材料非常薄、透气排汗防水,把对皮肤的刺激降到最低,减少了处方者和患者的后顾之忧。
(临床试验证实:吡罗昔康贴片皮肤刺激与乳胶剂类似,无统计学差异)。
疼痛科门诊常用药
100
美洛昔康片
骨性关节炎
101
双醋瑞因胶囊
102
壮骨关节胶囊
103
兰索拉唑胶囊
18
加巴喷丁胶囊
19
甲钴胺
20
阿昔洛韦软膏
21
金乌骨通胶囊
22
依托考昔片
23
双醋瑞因胶囊
骨关节炎
24
壮骨关节胶囊
25
骨化三醇
26
吡罗昔康(片)
27
如意丸
三叉神经痛
28
甲钴胺
29
胰激肽原酶肠溶片
30
加巴喷丁胶囊
31
卡马西平(片)
三叉神经痛
32
如意丸
33
血脉通胶囊
腰椎间盘突出
34
美洛昔康(片/贴)
35
如意珍宝丸
36
脉血康胶囊
37
别嘌呤片
痛风
38
美洛昔康(片/贴)
39
脉血康胶囊
40
兰索拉唑片(胶囊)
41
正清风痛宁(缓释)片
滑膜炎
42
克痹骨泰片
43
艾瑞昔布片
44
兰索拉唑胶囊
枸盐酸莫沙必利
45
脉血康
46
盐酸多塞平片
骨质疏松?
47
骨化三醇
48
脉血康胶囊
49
阿莫西林克拉维酸钾
50
吡罗昔康(贴)
51
骨化三醇
肌筋膜炎
双侧肩关节压痛
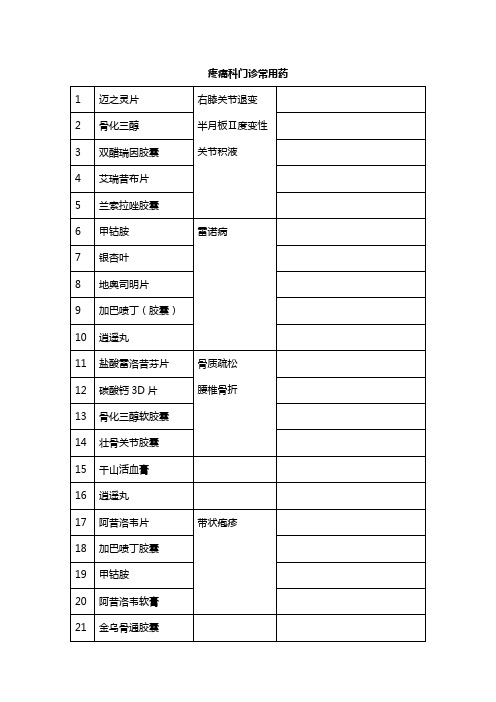
疼痛科门诊常用药
1
迈之灵片
右膝关节退变
半月板Ⅱ度变性
关节积液
2
骨化三醇
3
双醋瑞因胶囊
吡罗昔康透皮控释贴片

Con trolled2delivery pa tch of p irox icam
图 1 吡罗昔康的结构式
药理作用 本品为 NSA ID s,具有抗炎 、镇痛及解热作用 ,用
— 172 —
于缓解骨关节炎 、腱鞘炎 、肌痛 、骨关节痛 、外伤后及 骨折愈合后引起的疼痛 。作用机制为通过抑制环氧 酶 ,使组织局部前列腺素的合成减少 ,抑制白细胞的 趋化作用和溶酶体酶的释放 ,从而发挥较强的抗炎 、 镇痛及解热作用 。
对急 、慢性疼痛 (腱鞘炎 、肌痛 、骨关节痛 、外伤 引起的疼痛 、骨折பைடு நூலகம்疗引起的疼痛 )患者进行的 Ⅲ 期临床试验显示 ,在每隔 2 d使用本品 1次 , 4周后 , 本品能减少视觉模拟评分 (VAS)及各种疼痛参数如 主观疼痛 、压痛 、运动引起的疼痛及日常活动受限 , 对腱鞘炎的有效率分别为 77. 1%和 78. 1% ,对肌痛
[ Key words] p iroxicam; controlled2delivery patch; pharm acological action; pharm acokinetics; clinical e2 va lua tion
吡罗昔康为高效非甾体抗炎药 (NSA ID s) ,但长 期或大剂量口服常可导致胃肠道损伤 ,严重者并发 胃溃疡 、穿孔及出血 [ 1 ] 。近年对 NSA ID s的研发工 作主要分两方面进行 :一是开发新的化学实体 ,二是 对已上市的 NSA ID s进行剂型改造 ,开发新的药物 释放系统 ,实现靶向治疗 。韩国 SK Chem icals公司 研制的吡罗昔康透皮控释贴片 (商品名为特乐思 特 , Trast)即为剂型改造 , 化学名称为 22甲基 242羟 基 2N 2(22吡啶基 ) 22H21, 22苯并噻嗪 232甲酰胺 21, 12 二氧化物 ,分子式为 C15 H13 N3 O4 S,相对分子质量为 331. 35,结构式见图 1。
吡罗昔康透皮控释贴片治疗膝骨关节炎(OA)的疗效和安全性

计学 意义 ( <O 5 P . )。见表 1 0 。
表 1 治疗 前后 两组神 经功 能缺损 程度评 分 比较
部脑 血流量 的减少 ,使发病后第2天脑 中N A 8 A 含量较甘油对 照组明显
升高 。机理研究提示 ,依 达拉奉可清 除 自由基 ,抑制脂质过 氧化 ,从 而抑 制脑 细胞、血管内皮细胞、神经细胞的氧化损伤 。
i ii r i it c r rl e or a ei rt ]B a e,9 8 n bt s n nr e b a h m r g [ , ri R s 9 , h o a e h n aJ n 1 7 512: —0 9 (—) 37 . 6
【】 中华 神 经科学 会, 3 中华神 经 外科学 会 . 卒 中患者 临 床神 经功 能 脑
等 ,常 因用力 、情绪激动 等因素诱发 ,故 大多在活动 中突然 发病 ,临
床上脑 出血发病十分迅 速 ,主要表现为意 识障碍 、肢体偏瘫 、失语等 神经 系统 的损害 。它 起病急骤 、病情 凶险、病死率非 常高 ,是 目前 中
1 ・临床研究 ・ 4 4
10 L 0 m 生理盐水中静 脉滴注 ,3r 0 m内滴完 ,每 日 次 ,连用2 。 a 2 周 1 3疗效 评定 根 据 全 国第 四届 脑血 管 病 学 术 会议 制 定 的 神 经功 能 缺 损程 度 评 分标 准进 行 评分 。 临床疗 效判 定标 准 : ①基 本痊 愈 :功 能缺 损 ] 评 分减 少9 %~10 , 病残程 度为 0 ;②显 著进步 :功 能缺损评 1 0% 级
裂引起 的出血 ,最常见 的病 因是高血压 、脑 动脉硬化 、颅 内血管畸形
[】 陆磊 , 晓 红, 4 孙 张进, . 等 自由基 清 除剂在 急 性脑 出血 的应 用研 究
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
TRAST patch
产品特点和优势
TRAST patch
先进的透皮贴片patch 1. 剂型 强效药物吡罗昔康 2. 药物 持效48小时 3. 持效时间 关节部位附着牢固 4. 粘合力 皮肤刺激最小化 5. 皮肤过敏
独特 强效 长 强 少
TRAST patch
1. 剂型 patch(透皮控释贴片)
分析方法: HPLC
2
结果:
1
Trast patch组在血药浓度很低的 情况下,滑液浓度却很高;与滑液
0
0.11 TRAST patch
口服吡罗昔康
浓度类似的同种成分口服剂型不同,
没有全身副作用,却能起到同等药
Kor J of Clin. Pharmacology Therapeutics,
效。
Vol. 6, No. 1, 36~43(1998)
损害最大
疼痛治疗的风险
风险程度
TRAST patch
手术治疗
注射
口服药物
局部药物
心理疏导/理疗
损害最小
骨性关节炎目前的药物治疗
TRAST patch
对于轻度的骨性关节炎,
?
在中国目前主要选择口服药物治疗,尤其是长期口服NSAIDs
全球治疗骨性关节炎的用药趋势
各国NSAIDs口服剂型与外用剂型的使用比例
在中国,为了与SK社会奉献理念相结合,公益事业将作为一项长期的重要工作, 将SK在中国的成功回馈给社会,与民众共同分享幸福生活。
四川汶川大地震
SK集团支援
第一次 : 捐款 (1,020万元)
第二次 : 捐物 (约2,000万元的衣物和沥青)
社会责任
第三次 : 建希望小学校
TRAST patch
SK(北京)医药科技有限公司 是SK集团继SK爱康医 院和SK生物医药科技(上海)有限公司之后在中国独 立投资的第三家生命科学领域的独立法人企业。
TRAST patch
TRAST patch 产品介绍会
商务部 河清
SK集团简介
TRAST patch
在韩国首尔的SK大厦
SK集团的成长历程
TRAST patch
第1次飞跃
第2次飞跃
(1970’s ~ 1980’s)
(1953 ~ 1960’s)
从织物 到聚脂纤维
从15台纺机创业 到生产并出口 聚酯纤维
炎症部位 ▪ 巨噬细胞 ▪ 滑液纤维细胞
选择性COX-2抑制剂 • 选择性抑制COX-2发挥抗炎作用 • 心肾不良反应风险 • 减少GI不良反应
Adapted from Needleman P et al. J Rheumatol. 1997;24(Suppl 49):7.
非甾体抗炎药物种类
分类 COX-2选择性抑制剂
JOINS LEVOPRIDE OMED
5.00
0.00
'01
'02
'03
'04
'05
'06
TRAST patch
TRAST patch
吡罗昔康透皮控释贴片
✓ 国内独家产品 ✓ 世界首个关节炎治疗的patch剂型 ✓ 韩国外用抗炎镇痛类药物销量第一
TRAST patch
口服NSAIDs的风险---作用机理
不良反应降到最低。
TRAST patch
非甾体抗炎药处方药说明书修订内容
3.在使用所有非甾体抗炎药治疗过程中的任何时候,都可能出现胃肠道出血、 溃疡和穿孔的不良反应,其风险可能是致命的。这些不良反应可能伴有或不伴有警示 症状,也无论患者是否有胃肠道不良反应史或严重的胃肠事件病史。既往有胃肠道病 史(溃疡性大肠炎,克隆氏病)的患者应谨慎使用非甾体抗炎药,以免使病情恶化。 当患者服用该药发生胃肠道出血或溃疡时,应停药。老年患者使用非甾体抗炎药出现 不良反应的频率增加,尤其是胃肠道出血和穿孔,其风险可能是致命的。
欧洲
中国
在39个地区拥有 96个法人分公司
中东/非洲
在全球
38个 国家 拥有178 个法人
63 家分公司
东南亚
美洲
有重点的全球化战略
TRAST patch
SK与中国
在计划SK的发展与利益的同 时也考虑中国的需要和利益
为中国发展做出贡献的企业
“与中国一路同行”
“努力成为 中国的企业公民”
TRAST patch
诊断为 OA
确定疾 病状态
评估药 物治疗 风险
OA的 治疗
《OA诊治指南》(2007版)
TRAST patch
对于手和膝关节OA,在采用口服药前,建议首先选择局部药物治疗。 局部药物治疗包括各种NSAIDs乳胶剂、膏剂、贴剂和非NSAIDs擦剂。
轻/中度疼痛
中/重度疼痛
重度疼痛
局部药物治疗
局部药物与口 服NSAIDs联
合
口服NSAIDs
局部用NSAIDS药物可以做 为口服药物的局部增效剂
Байду номын сангаас
TRAST patch
重视NSAIDs外用药–各国指南对外用药物的推荐
法人企业和63家分公司
TRAST patch
2007 销售额 : RMB 6,000亿 ($810亿)
能源化工 50%
信息通信 18%
贸易,服务 32%
TRAST patch
SK确立全球化的发展战略,将中国市场视为公司全球化发展战略的主要 目标,同时扩展东南亚、中南美、中东、中亚和俄罗斯等市场。
试验机构: 三星生命科学研究所
研究对象: 需膝关节手术OA患者20名
给药方法:
6
– Trast patch两天1贴(48mg),
贴于骨关节两周(10名),
5
血浆内药物浓度 滑液内药物浓度
5.41
5.69 5.37
– 口服给药吡罗昔康1天1片
4
(20mg) ,口服 两周(10名)
3
样本收集:采集关节滑液及血液
TRAST patch
《OA诊治指南》(2007版)
骨关节治疗前需评估药物治疗风险
OA评估指标
影 像
疼痛评估1.总体评估 (无痛。轻度、中度 及重度)
合并疾病:
学 检 查
2.视觉模拟量表VAS (0-10分)3.功能 评估WOMAC、
AIMS
肥胖、营养 不良、糖尿 病等
胃肠道风险 心血管风险
病史、 特征
NSAIDs通过抑制环氧化酶的活性,从而减少前列腺素的生成,起到抗炎镇痛作用。
同时也会影响前列腺素的一系列正常生理作用如保护胃粘膜、维持肾脏生理功能以及抗凝的作用。
细胞膜磷脂
磷脂酶
花生四烯酸
环氧化酶(1,2)
前列腺素
NSAIDs
羧酸类
分类
丙酸类
乙酸类
昔康类 Pyrrolo-pyrrole Naphthylalkanone
TRAST patch
美国
日本
韩国
英国
中国
0%
20%
40%
60%
80%
100%
外用剂型 口服剂型
国外一些国家在骨性关节炎治疗中,外用剂型已成为一种较为普遍的使用方法!
产品概述
成分及含量: 吡罗昔康 48mg/贴
适应症: 骨关节炎,肌肉痛, 腱鞘炎,关节痛,
外伤后疼痛及骨折治愈后疼痛
用法用量: 两天一贴,贴于患处
完成从石油到纤维的 垂直系列化
-73年建立鲜京石油 -80年收购大韩石油
公社
第3次飞跃
(1990’s)
进入信息通讯 事业领域 94年收购
韩国移动通信
(2000’s)
加快进入全球市场的步 伐以及构筑下一代成长
基础
-公司治理结构进行 彻底改革
•以董事会为中心的 透明经营体制
•07年7月 向控股公司 转型
TRAST patch
COX-1 (基础酶)
TRAST patch
口服NSAIDs的风险---作用机理
花生四烯酸
非选择性NSAIDs • 抑制COX-2发挥抗炎作用
• 抑制COX-1引发不良反应
•
COX-2 (诱导酶)
增加胃肠道、心肾不良反应风险并引起血小板 功能障碍
胃肠道 肾脏 血小板
非选择NSAIDs抑制剂
例 塞来昔布:西乐葆 罗非昔布:万络 美洛昔康 尼美舒利
TRAST patch
NSAIDs药物的副作用
口服抗炎镇痛药
TRAST patch
过度过早口服药物干预OA 两种NSAIDs药合用
传统NSAIDs
特异性COX-2抑制剂
严重GI不良 反应
新的CVS不 良反应
CVS:心血管系统
口服NSAIDs——双刃剑
4.针对多种COX-2选择性或非选择性NSAIDs药物持续时间达3年的临床试验显示, 本品可能引起严重心血管血栓性不良事件、心肌梗塞和中风的风险增加,其风险可能 是致命的。所有的NSAIDs,包括COX-2选择性或非选择性药物,可能有相似的风险。 有心血管疾病或心血管疾病危险因素的患者,其风险更大。即使既往没有心血管症状, 医生和患者也应对此类事件的发生保持警惕。应告知患者严重心血管安全性的症状和/ 或体征以及如果发生应采取的步骤。
patch(透皮控释贴片)结构示意图
背衬层 粘合剂 药物层
皮肤
吡罗昔康(Piroxicam)
促进吸收剂
(Penetration Enhancer)
控释系统
(Realeasing Rate Controller)
✓ 促进吸收剂可大大提高吡罗昔康的经皮吸收率 ✓ 控释系统可使药物以恒定速率持续释放48小时
