头孢拉定胶囊英国药典BP2013 UpdatedB
头孢拉定胶囊10版196页

头孢拉定胶囊2010版二部196页(0.5g 、0.25g 、0.125g)【检查】有关物质供试品:50mg/规格*平均装量流 50ml (1mg/ml)对照溶液:取供试溶液5ml 流 100ml 5ml 流 50ml (5ug/ml)杂质对照:头孢氨苄、双氢苯甘氨酸、7-氨基去乙酰氧基头孢烷酸,加7.3%盐酸溶液4ml,用对照溶液稀释至每1ml含3种杂质对照各10ug混合溶液。
取杂质对照20ul,220nm进样。
R>1.5。
对照20ul,254nm进样调灵敏度。
供试、杂对、对照20ul,先254nm检测测定,再以220nm重新进样记录色谱图至主峰保修时间的2.5倍。
水分不得过7.0%溶出度第一法 100转/min 45min取样紫外法255nm限度80%介质:0.1mol/L盐酸溶液900ml 54ml盐酸水 6000ml供:0.5g/900ml 5ml 介质 100ml (28ug/ml)0.25g/900ml 5ml 介质 50ml (28ug/ml)0.125g/900ml 5ml 介质 25ml (28ug/ml)对照:平均装量介质 100ml 1ml 介质 100ml (28ug/ml)装量差异20粒精称【含量测定】 t=27min (柱:6554 t=42min t停止=50min )流动相: 水-甲醇-3.86%醋酸钠溶液-4%醋酸溶液(1564:400:30:6)(2346-600-45-9)1.7g醋酸钠水45ml 0.4ml醋酸水9ml波长:254nm 流速:0.7~0.9ml/min 进样量:10 ul R>1.5系统适用性试验:头孢拉定对照品溶液10份和头孢氨苄对照品贮备液1份头孢氨苄对照品:10mg 流 25ml(0.4mg/ml储备液) 5ml 流 50ml供试:70mg/规格*平均装量流+超声15min+振摇10min 100ml对照:头孢拉定对照品17.5mg 流 25ml。
头孢拉定

生产厂家
哈药集团制药总厂
主要生产青霉素类、 头孢菌素类抗生素, 共30多种原料药和18 种剂型的产品。其中 头孢噻肟钠原粉、头 孢唑啉钠原粉以及头 孢唑啉钠粉针的产量 和市场份额均居全国 首位
拥有国内外发明专 利35项,成功培 育出世界上唯一由 中国人自行研制、 拥有自主知识产权 并首先应用于临床 的半合成头孢菌 素——头孢硫脒, 获得国家技术发明 二等奖;自主研发 的头孢菌素类全新 化合物——头孢嗪 脒获美国专利授权 认证与国家科技部 重大新药创制专项 支持
水分 取本品,照水分测定法(附录Ⅷ M第一法A)测定, 含水分不得过6.0%。 炽灼残渣 取本品1.0g,依法检查(附录Ⅶ N),遗留残渣 不得过0.2%。 无菌 取本品,用2.6%无 菌碳酸钠溶液溶解后,转 移至不少于500ml的0.9% 无菌氯化钠溶液中,用薄 膜过滤法处理后,依法检 查(附录Ⅺ H),应符合 规定(供注射用)。 重金属
自主研发的头孢菌素类全新化合物头孢嗪脒获美国专利授权认证与国家科技部重大新药创制专项支持山东鲁抗医药股份有限公司山东鲁抗医药集团有限公司是中国重要的抗生素生产基地国有大型企业主要品种有青霉素类头孢类螺旋霉素大观霉素泰乐霉素以及他丁类等产品并已形成规模优势并出口远销欧洲北美洲和东南亚
头孢拉定
微生物11301 第四组
广州白云山制药股份
山东鲁抗医药股份有限公司
山东鲁抗医药集团有限公 司是中国重要的抗生素生 产基地,国有大型企业 主要品种有青霉素类、头 孢类、螺旋霉素、大观霉 素、泰乐霉素以及他丁类 等产品,并已形成规模优 势,,并出口远销欧洲、 北美洲和东南亚。
湖北贝克药业有限公司
BECK INTERNATIONAL SHARE LIMITED (贝克国际控股)是一家总部设于香港, 品质源于欧洲的企业集团。在药品研究、 药物制造、药品经营、植物药物筛选方面 具有雄厚的实力,尤其在儿童药物、儿童 用品、妇科产品应用方面已形成著名品牌, 包括贝克®(Beck®)等。新贝儿克® (Sunbeck)、爱婴思坦®(Einstone)是 贝克国际控股(BECK,HK)推出系列儿科 产品的总称。
头孢拉定的功能主治

头孢拉定的功能主治1. 头孢拉定的简介头孢拉定(Cefradine),属于头孢菌素类抗生素。
它是一种广谱抗生素,通过抑制细菌细胞壁合成来发挥杀菌作用。
头孢拉定广泛用于治疗多种感染疾病,特别是上呼吸道感染和泌尿系统感染等细菌感染。
2. 头孢拉定的功能•抗菌作用:头孢拉定具有广谱抗菌作用,能有效抑制多种细菌的生长和繁殖,包括革兰氏阳性菌和革兰氏阴性菌等。
•杀菌作用:头孢拉定能杀死敏感菌株,通过破坏细菌细胞壁合成从而导致菌体溶解,从而达到杀菌的效果。
•抗炎作用:除了杀菌作用外,头孢拉定还具有一定的抗炎作用。
它可以减轻炎症反应,减少相关炎症症状,有助于提高患者的自身免疫能力。
3. 头孢拉定的主治头孢拉定主要用于以下疾病的治疗:3.1 上呼吸道感染头孢拉定对于上呼吸道感染引起的咽喉炎、扁桃体炎、鼻窦炎等疾病具有较好的疗效。
常见感染致病菌包括链球菌、肺炎链球菌、流感嗜血杆菌等。
3.2 泌尿系统感染头孢拉定对于泌尿系统感染引起的膀胱炎、尿道感染、肾盂肾炎等疾病也具有良好的治疗效果。
常见感染致病菌包括大肠杆菌、铜绿假单胞菌、肺炎克雷伯菌等。
3.3 皮肤软组织感染头孢拉定在治疗轻中度的皮肤软组织感染方面也表现出一定的疗效。
常见的感染致病菌包括金黄色葡萄球菌、链球菌、葡萄球菌等。
3.4 妇科感染头孢拉定可用于治疗妇科感染引起的宫颈炎、阴道炎等疾病。
常见感染致病菌包括淋球菌、沙眼衣原体、念珠菌等。
3.5 呼吸道感染头孢拉定也适用于轻度上呼吸道感染引起的咳嗽、鼻塞、咽痛等症状。
常见感染致病菌包括流感嗜血杆菌、变异链球菌等。
3.6 骨关节感染头孢拉定在治疗轻中度骨关节感染方面也具有一定的效果。
常见感染致病菌包括金黄色葡萄球菌、链球菌等。
4. 头孢拉定的注意事项•头孢拉定过敏者禁用;•儿童、孕妇、哺乳期妇女应慎用;•肝肾功能不全者使用前应咨询医生并按照医嘱使用;•应避免与受酶诱导剂诱导的CYP450同步使用;•如出现过敏反应、消化道不适等不良反应应及时停药并就医。
头孢拉定胶囊的功效与作用

头孢拉定胶囊的功效与作用头孢拉定胶囊是一种广谱抗生素,主要成分是头孢克肟。
头孢拉定胶囊具有多种功效和作用,可用于治疗多种感染症状。
以下将详细介绍头孢拉定胶囊的功效与作用。
一、头孢拉定胶囊的抗菌作用头孢拉定胶囊属于头孢菌素类抗生素,具有广谱的抗菌作用。
它能够抑制许多常见的细菌,包括革兰阳性菌和革兰阴性菌。
革兰阳性菌如金黄色葡萄球菌、溶血性链球菌、肺炎链球菌等,革兰阴性菌如大肠杆菌、克雷伯菌、奇异变形杆菌等。
头孢拉定胶囊通过阻断细菌细胞壁的合成来抑制细菌的生长和繁殖,从而达到治疗感染的效果。
二、头孢拉定胶囊的适应症头孢拉定胶囊适用于各种感染症状,包括上呼吸道感染、下呼吸道感染、泌尿生殖系统感染、皮肤和软组织感染、骨和关节感染等。
具体的适应症包括咽炎、扁桃体炎、鼻窦炎、气管炎、肺炎、膀胱炎、尿道炎、盆腔炎、皮肤脓疱病等。
三、头孢拉定胶囊的药物动力学特性头孢拉定胶囊在人体内吸收迅速,口服后约1-2小时达到峰值浓度。
它在体内分布广泛,可以进入多种组织和体液。
头孢拉定胶囊在体内代谢较少,主要通过肾脏排泄。
对于肾功能受损的患者需要调整剂量,以防止药物在体内蓄积导致不良反应。
四、头孢拉定胶囊的药物不良反应头孢拉定胶囊的使用可能会引起一些不良反应。
常见的不良反应包括恶心、呕吐、腹泻、胃部不适、头晕、头痛、嗜睡等。
这些不良反应一般是轻微的,通常不会严重影响患者的健康。
但是对于部分患者可能出现过敏反应,如荨麻疹、瘙痒、皮疹、血管神经性水肿等。
对于出现严重不良反应和过敏症状的患者,应停止使用头孢拉定胶囊并及时就医处理。
五、头孢拉定胶囊的禁忌症头孢拉定胶囊在一些情况下是禁忌的,即不适合使用。
禁忌症包括对头孢菌素类抗生素过敏的患者、哮喘患者、荨麻疹和严重过敏病史的患者。
此外,头孢拉定胶囊不推荐给孕妇和哺乳期妇女,因为它可能对胎儿和婴儿有影响。
六、头孢拉定胶囊的使用注意事项1. 在使用头孢拉定胶囊之前,需要先检查患者的过敏史,以确定是否存在对该药物的过敏反应。
头孢拉定胶囊的作用与功效与作用

头孢拉定胶囊的作用与功效与作用头孢拉定胶囊是一种常用的抗生素药物,适用于治疗多种细菌感染。
它属于头孢菌素类抗生素,可以通过抑制细菌的细胞壁合成来杀灭细菌。
头孢拉定胶囊具有广谱抗菌作用,适用于治疗呼吸道感染、泌尿道感染、皮肤软组织感染等多种感染。
本文将详细介绍头孢拉定胶囊的作用、功效以及使用注意事项。
一、头孢拉定胶囊的作用与功效1. 杀菌作用:头孢拉定胶囊可以抑制细菌的细胞壁合成,导致细菌的死亡。
这是因为头孢拉定胶囊的主要成分是头孢菌素C,属于β-内酰胺类抗生素。
β-内酰胺类抗生素具有与细菌细胞壁的结合亲和力,能够与细菌酶特异性结合,从而阻碍细菌细胞壁的合成。
2. 广谱抗菌作用:头孢拉定胶囊具有广谱抗菌作用,可以对多种革兰氏阳性和阴性细菌产生杀菌作用。
常见的可敏感菌种有:大肠杆菌、肺炎杆菌、链球菌、葡萄球菌等。
3. 对呼吸道感染的治疗作用:头孢拉定胶囊适用于治疗上呼吸道感染,如急性咽喉炎、急性扁桃体炎、急性鼻窦炎等。
这些感染通常由于细菌感染引起,头孢拉定胶囊的抗菌作用可以快速杀灭感染的细菌,缩短疾病的持续时间。
4. 对泌尿道感染的治疗作用:头孢拉定胶囊也适用于治疗泌尿道感染,如膀胱炎、尿道炎等。
这些感染一般由细菌感染引起,头孢拉定胶囊可以通过杀灭细菌来治疗感染。
同时,由于头孢拉定胶囊在尿液中的浓度高,也可以有效抑制细菌在尿液中的增殖,提高治疗效果。
5. 对皮肤软组织感染的治疗作用:头孢拉定胶囊可以用于治疗皮肤软组织感染,如脓疱疮、红斑、蜂窝织炎等。
这些感染常常由于细菌感染引起,头孢拉定胶囊通过快速杀灭细菌,减轻炎症反应,帮助患者恢复健康。
除了上述作用与功效,头孢拉定胶囊还可以用于治疗其他感染,如中耳炎、妇科感染、骨与关节感染等。
然而,有关药物的使用应当根据医生的指导进行,并且使用药物时应当特别注意药物的剂量与用法。
二、头孢拉定胶囊的用法与用量1. 用法:头孢拉定胶囊为口服药物,可以直接吞服或者将胶囊打开后与食物一起服用。
BP2015英国药典索引
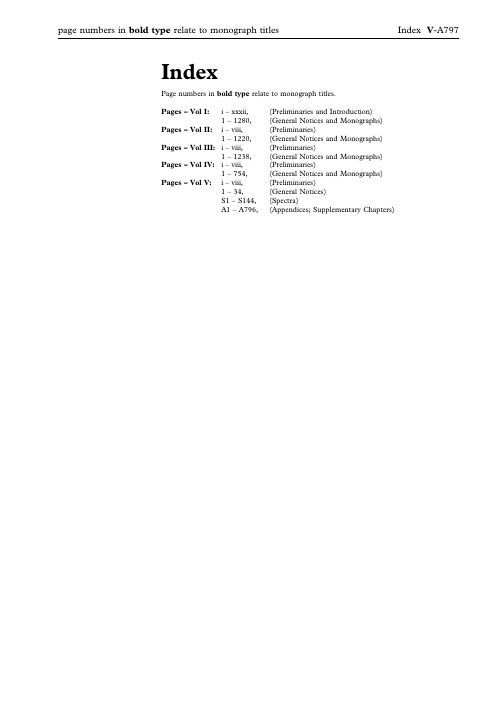
page numbers in bold type relate to monograph titles Index V-A797IndexPage numbers in bold type relate to monograph titles.Pages–Vol I:i–xxxii,(Preliminaries and Introduction)1–1280,(General Notices and Monographs)Pages–Vol II:i–viii,(Preliminaries)1–1220,(General Notices and Monographs)Pages–Vol III:i–viii,(Preliminaries)1–1238,(General Notices and Monographs)Pages–Vol IV:i–viii,(Preliminaries)1–754,(General Notices and Monographs)Pages–Vol V:i–viii,(Preliminaries)1–34,(General Notices)S1–S144,(Spectra)A1–A796,(Appendices;Supplementary Chapters)AAbacavir,V-S4Abacavir Oral Solution,III-85 Abacavir Sulfate,I-39Abacavir Tablets,III-86 Abbreviated,V-598Adjectives,V-598Anions,V-598Cations,V-598Preparations,V-598Titles of Monographs,V-598 Abbreviated Titles,Status of,I-7,II-7, III-7,IV-7,V-7Abbreviations and symbols,I-30,II-30, III-30,IV-30,V-30Abnormal Toxicity,Test for,V-409 About,definition of,I-5,II-5,III-5,IV-5,V-5Absence of Mycoplasmas,Test forV-487Absolute Ethanol,V-A61Absolute Ethanol R1,V-A62 Absorbent Cotton,IV-743Absorbent Viscose Wadding,IV-744 Absorption spectrophotometry,infrared, V-162Absorption Spectrophotometry, Ultraviolet and Visible,V-169 Acacia,I-41,V-A19Acacia Solution,V-A19Acacia Spray-dried,I-42 Acamprosate Calcium,I-43 Acanthopanax Bark,IV-49 Acarbose,I-44Accuracy,V-674Acebutolol Capsules,III-87 Acebutolol Hydrochloride,I-46,V-S5, V-A19Acebutolol Tablets,III-88 Aceclofenac,I-48Acemetacin,I-50Acenocoumarol,I-52,V-S5 Acenocoumarol Tablets,III-88 Acesulfame Potassium,I-52Acetal,V-A19Acetaldehyde,V-A19Acetaldehyde Ammonia Trimer Trihydrate,V-A20Acetaldehyde Standard Solution(100ppm C2H4O),V-A148 Acetaldehyde Standard Solution(100ppm C2H4O)R1,V-A148 Acetamide,V-A20Acetate Buffer pH2.8,V-A152 Acetate Buffer pH2.45,V-A152 Acetate Buffer pH3.4,V-A152 Acetate Buffer pH3.5,V-A152 Acetate Buffer pH3.7,V-A152 Acetate Buffer pH4.4,V-A152 Acetate Buffer pH4.6,V-A152 Acetate Buffer pH5.0,V-A152 Acetate Buffer pH6.0,V-A152 Acetate Buffer Solution pH4.7R1,V-A153Acetate Buffer Solution pH4.4,see Acetate Buffer pH4.4Acetate Buffer Solution pH4.6,see Acetate Buffer pH4.6Acetate Buffer Solution pH6.0,seeAcetate Buffer pH6.0Acetate Buffer Solution pH4.4,V-A152Acetate Buffer Solution pH4.5,V-A152Acetate Buffer Solution pH4.7,V-A152Acetate Buffer Solution pH5.0,V-A153Acetate Buffer Solution pH6.0,V-A153Acetate–edetate Buffer Solution pH5.5,V-A153Acetates,Reactions of,V-266Acetazolamide,I-54,V-S5Acetazolamide Oral Suspension,III-89Acetazolamide Tablets,III-90Acetic Acid,V-A20Acetic Acid(6per cent),I-56Acetic Acid(33per cent),I-56Acetic Acid,Anhydrous,V-A20Acetic Acid,Deuterated,V-A50Acetic Acid,Dilute,V-A20Acetic Acid,Dilute,see Acetic Acid(6per cent)Acetic Acid,Glacial,I-55,V-A20Acetic Acid in Synthetic Peptides,Determination of,V-299Acetic Acid VS,V-A142Acetic Acid,see Acetic Acid(33per cent)Acetic Anhydride,V-A20Acetic Anhydride Solution R1,V-A20Acetic Anhydride–Dioxan Solution,V-A20Acetic Anhydride–Sulfuric Acid Solution,V-A20Acetic Anhydride–Sulphuric AcidSolution,see Acetic Anhydride–SulfuricAcid SolutionAcetic Bromine Solution,V-A34Acetone,I-57,V-A20Acetone,Deuterated,V-A50Acetone Solution,Buffered,V-A153Acetone-dried Ox Brain,V-A98Acetonitrile,V-A20Acetonitrile for Chromatography,V-A20Acetonitrile R1,V-A20Acetoxyvalerenic Acid,V-A20Acetyl Chloride,V-A20Acetyl Groups,Reactions of,V-266Acetyl Salicylic Acid see AspirinAcetyl Value,Determination of,V-317Acetylacetamide,V-A20Acetylacetone,V-A20Acetylacetone Reagent R1,V-A20Acetylacetone Reagent R2,V-A204-Acetylbiphenyl,V-A20O-Acetyl Groups in PolysaccharideVaccines,V-467N-Acetyl-e-caprolactam,V-A20Acetylcholine Chloride,I-58,V-A20Acetylcysteine,I-59,V-S6Acetylcysteine Eye Drops,III-90Acetylcysteine Injection,III-91Acetyldigoxin,I-61b-Acetyldigoxin see AcetyldigoxinAcetyleugenol,V-A20N-Acetylglucosamine,V-A21Acetyl-11-keto-b-boswellic Acid,V-A21N-Acetyl-L-cysteine,V-A20N-Acetylneuraminic Acid,V-A21Acetylsalicylic Acid Tablets,see AspirinTabletsN-Acetyltryptophan,V-A21N-Acetyltryptophan see AcetyltryptophanAcetyltryptophan,I-63Acetyltyrosine,I-65N-Acetyltyrosine see AcetyltyrosineAcetyltyrosine Ethyl Ester,V-A21Acetyltyrosine Ethyl Ester,0.2M,V-A21Aciclovir,I-67Aciclovir Cream,III-93Aciclovir Eye Ointment,III-94Aciclovir Infusion,III-95Aciclovir Intravenous Infusion,seeAciclovir Infusion,Aciclovir Oral Suspension,III-97Aciclovir Sodium for Infusion,III-95Aciclovir Sodium for IntravenousInfusion,see Aciclovir Sodium forInfusion,Aciclovir Tablets,III-98Aciclovir Tablets,Dispersible,III-99Acid Blue92,V-A21Acid Blue92Solution,V-A21Acid Blue83,V-A21Acid Blue93Solution,V-A21Acid Blue90,V-A21Acid Gentian Mixture,IV-197Acid Gentian Oral Solution,IV-197Acid Potassium IodobismuthateSolution,V-A108Acid Value,V-317Acid/base Indicators,V-789Acid-base titrations,V-788Acidified Chloroform,V-A41Acidified Dichloromethane,V-A52Acidified Methanol,V-A85Acidified Methylene Chloride,seeAcidified DichloromethaneAcid-insoluble Ash,Determination of,V-336Acid-washed Diatomaceous Support,V-A51Acitretin,I-69Acitretin Capsules,III-100Acknowledgements,I-xxviiAcrylamide,V-A21Acrylamide/bisacrylamide(29:1)Solution,30per cent,V-A21Acrylamide/bisacrylamide(36.5:1)Solution,30per cent,V-A21Acrylic Acid,V-A21Actein,V-A21Acteoside,V-A21Action and Use Statement,Status of,I-17,II-17,III-17,IV-17,V-17Activated Acid Aluminium Oxide,V-A23Activated Attapulgite,I-220Activated Charcoal,I-496,V-A40Activated Zinc,V-A140Active Moiety,V-651Adamantane,V-A21Adapalene,I-71Adapalene Cream,III-101Adapalene Gel,III-103Additions,List of,I-xxviiiAdditions,List of Monographs,I-xxiiAdditives,Plastic,V-592Adenine,I-72,V-A21Adenosine,I-73,V-A21Adipic Acid,I-75,V-A21Adrenaline,V-A21Adrenaline/Epinephrine,I-76page numbers in bold type relate to monograph titles Index V-A799Adrenaline Acid Tartrate,V-A21 Adrenaline Acid Tartrate/Epinephrine Acid Tartrate,I-77Adrenaline and Cocaine Intranasal Solution,III-107Adrenaline(Epinephrine),V-S6 Adrenaline Eye Drops,Epinephrine Eye Drops,Neutral,III-104Adrenaline Eye Drops/Epinephrine Eye Drops,III-104Adrenaline Injection,Bupivacaine and, III-220Adrenaline Injection,Dilute(1in10,000),III-106Adrenaline Injection,Lidocaine and,III-751Adrenaline Injection/Epinephrine Injection,III-105Adrenaline Solution/Epinephrine Solution,III-106Adrenaline Tartrate see Adrenaline Acid TartrateAdrenaline TartrateInjection/Epinephrine Tartrate Injection,III-105Adrenaline TartrateSolution/Epinephrine Tartrate Solution,III-106Adrenalone Hydrochloride,V-A21 Adsorbed Diphtheria and Tetanus Vaccine,IV-537Adsorbed Diphtheria and Tetanus Vaccine for Adults and Adolescents, see Adsorbed Diphtheria and Tetanus Vaccine(adsorbed,Reduced Antigen(s) Content)Adsorbed Diphtheria,Tetanus and Pertussis(Acellular Component) Vaccine,IV-541Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Haemophilus Type b Conjugate Vaccine,IV-545Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Hepatitis B(rDNA)Vaccine,IV-547 Adsorbed Diphtheria,Tetanus,Pertussis (Acellular Component)and Inactivated Poliomyelitis Vaccine,IV-548Adsorbed Diphtheria Vaccine,IV-534 Adsorbed Diphtheria Vaccine for Adults and Adolescents,see Diphtheria Vaccine (Adsorbed,Reduced Antigen Content) Adsorbed Pertussis Vaccine(Acellular Component),IV-604Adsorbed Pertussis Vaccine(Acellular, Co-purified),IV-605Adsorbed Tetanus Vaccine,IV-633 Adsorbed Vaccines,Aluminium in,V-463Adsorbed Vaccines,Calcium in,V-464 Adsorption,Gas,Specific Surface Area By(2.9.26.)(5.8.),V-701Aescin,V-A22Aflatoxin B1,V-A22Aflatoxin B,in Herbal Drugs, Determination of,V-341Agar,I-79,V-A22Agarose for Chromatography,V-A22Agarose for Chromatography,Cross-linked,V-A22Agarose for Chromatography R1,Cross-linked,V-A22Agarose for Electrophoresis,V-A22Agarose/Cross-linked Polyacrylamide,V-A22Agarose-DEAE for Ion ExchangeChromatography,V-A22Agnus Castus Fruit,IV-50Agrimony,IV-52Air,Medical,I-78Air,Medicinal see Medical AirAir Permeability,Specific Surface Areaby,V-505Air,Synthetic,I-81Air,Synthetic Medicinal see Synthetic AirAlanine,I-83,V-A22ß-Alanine,see3-Aminopropionic AcidAlbendazole,I-84Albumin,Bovine,V-A22Albumin,Bovine R1,V-A22Albumin,Human,V-A22Albumin Solution,IV-467Albumin Solution,Human,V-A22Albumin Solution R1,Human,V-A22Alchemilla,IV-53Alcohol(20per cent),I-900Alcohol(25per cent),I-900Alcohol(45per cent),I-900Alcohol(50per cent),I-900Alcohol(60per cent),I-900Alcohol(70per cent),I-900Alcohol(80per cent),I-900Alcohol(90per cent),I-900Alcohol,Aldehyde-free,see Ethanol(96%),Aldehyde-freeAlcoholic Calcium Standard Solution(100ppm Ca),V-A149Alcoholic DimethylaminobenzaldehydeSolution,V-A55Alcoholic Hydroxylamine Solution,V-A74Alcoholic Iodine Solution,III-696,V-A75Alcoholic Potassium Hydroxide,2M,V-A108Alcoholic Potassium Hydroxide,seePotassium Hydroxide VS,EthanolicAlcoholic Potassium Hydroxide Solution,V-A108Alcoholic Potassium Hydroxide SolutionR1,V-A108Alcoholic Solution of Sulfuric Acid,V-A128Alcoholic Sulfuric Acid,0.25M,V-A128Alcoholimetric Tables,V-687Alcohol,see Ethanol(96%)Alcuronium Chloride,I-85Aldehyde Dehydrogenase,V-A22Aldehyde Dehydrogenase Solution,V-A22Aldehyde-free alcohol,see Ethanol(96%),Aldehyde-freeAldehyde-free Ethanol(96%),V-A62Aldehyde-free Methanol,V-A85Aldehydes,Determination of,V-321Aldrin,V-A22Alendronate Sodium Tablets,seeAlendronic Acid TabletsAlendronic Acid Tablets,III-109Aleuritic Acid,V-A22Alexandrian Senna Fruit,IV-362Alfacalcidol,I-87Alfadex,I-88Alfentanil Hydrochloride,I-89Alfuzosin,V-S7Alfuzosin Hydrochloride,I-91Alfuzosin Tablets,III-111Alfuzosin Tablets,Prolonged-release,III-112Alginate Antacid Oral Suspension,Compound,III-113Alginate Oral Suspension,Raft-forming,III-114Alginate Raft-forming Oral Suspension,III-114Alginic Acid,I-92Alimemazine,V-S7Alimemazine Oral Solution,Paediatric,III-115Alimemazine Tablets,III-116Alimemazine Tartrate,I-93Alizarin S,V-A22Alizarin S Solution,V-A22Alkaline Corallin Solution,V-A46Alkaline Eye Drops,see Hypromellose EyeDropsAlkaline Gentian Mixture,IV-198Alkaline Gentian Oral Solution,IV-198Alkaline Hydroxylamine Solution,V-A74Alkaline Hydroxylamine Solution R1,V-A74Alkaline Potassium Mercuri-iodideSolution,V-A109Alkaline Potassium TetraiodomercurateSolution,V-A109Alkaline Pyrogallol Solution,V-A111Alkaline Sodium Picrate Solution,V-A124Alkaline Tetrazolium Blue Solution,V-A132Alkali-washed Diatomaceous Support,V-A51Alkaloids,Complete Extraction of,V-335Alkaloids,Reactions of,V-266all--Alpha-Tocopherol,II-1051Allantoin,I-94,V-A23Allergen Products,I-95Allium Sativum for HomoeopathicPreparations,IV-427Allopurinol,I-98Allopurinol Oral Suspension,III-117Allopurinol Tablets,III-118all-rac-Alpha-Tocopheryl Acetate,II-1054all-rac-a-Tocopheryl see all-rac-Alpha-Tocopherylall-rac-Tocopheryl Acetate see all-rac-Alpha-Tocopheryl AcetateAlmagate,I-100Almond Oil Ear Drops,III-119Almond Oil,Refined,I-100Almond Oil,Virgin,I-99Almond Oil see Virgin Almond OilAloes,Barbados,IV-53Aloes,Cape,IV-54Alovudine,V-A23Alovudine(18F)Injection,IV-669V-A800IndexAloxiprin,I-102Aloxiprin Tablets,III-119Alpha Tocopheryl Acetate Concentrate (Powder Form),II-1057Alpha Tocopheryl Hydrogen Succinate, II-1058Alpha Tocopheryl Succinate Tablets,III-1164Alphacyclodextrin see Alfadex Alprazolam,I-103Alprenolol Hydrochloride,I-105 Alprostadil,I-107Alteplase for Injection,I-109 Alternative methods,I-20,II-20,III-20, IV-20,V-20Alternative Methods for Control of Microbiological Quality,V-745 Altizide,I-113Alum,I-114Aluminium,V-A23Aluminium Acetate Ear Drops,III-120 Aluminium Chloride,V-A23 Aluminium Chloride Hexahydrate,I-114 Aluminium Chloride Reagent,V-A23 Aluminium Chloride Solution,III-121, V-A23Aluminium Glycinate,I-115 Aluminium Hydroxide and Magnesium Trisilicate Tablets,Chewable,III-776 Aluminium Hydroxide,Dried,I-115 Aluminium Hydroxide Gel,V-A23 Aluminium Hydroxide,Hydrated for Adsorption,I-114Aluminium Hydroxide Oral Suspension, III-121Aluminium Hydroxide Oral Suspension, Magnesium Hydroxide and,III-401 Aluminium Hydroxide Tablets, Chewable,III-122Aluminium Hydroxide Tablets, Magnesium Hydroxide and,III-402 Aluminium Hydroxide Tablets see Chewable Aluminium Hydroxide Tablets,III-122Aluminium in Adsorbed Vaccines,V-463 Aluminium Magnesium Silicate,I-118 Aluminium Nitrate,V-A23Aluminium Oxide,Activated Acid,V-A23Aluminium Oxide,Anhydrous,V-A23 Aluminium Oxide,Basic,V-A23 Aluminium Oxide,Deactivated,V-A23 Aluminium Oxide G,V-A23 Aluminium Oxide,Neutral,V-A23 Aluminium Paste,Compound,III-120 Aluminium Phosphate Gel,I-120 Aluminium Phosphate,Hydrated see Dried Aluminium Phosphate Aluminium Potassium Sulfate,V-A23 Aluminium Potassium Sulphate,see Aluminium Potassium Sulfate Aluminium Powder,I-121Aluminium Salts,Reactions of,V-266 Aluminium Sodium Silicate,I-122 Aluminium Standard Solution(2ppm Al),V-A148Aluminium Standard Solution(10ppm Al),V-A148Aluminium Standard Solution(100ppm Al),V-A148Aluminium Standard Solution(200ppmAl),V-A148Aluminium Stearate,I-123Aluminium Sulfate,I-125,V-A23Aluminium Sulphate,see AluminiumSulfateAlverine Capsules,III-122Alverine Citrate,I-126,V-S7Amantadine Capsules,III-123Amantadine Hydrochloride,I-127Amantadine Oral Solution,III-124Amantidine,V-S8Amaranth S,V-A23Amaranth Solution,V-A23Ambroxol Hydrochloride,I-128Americium-243Spiking Solution,V-A23Amethocaine Eye Drops,see TetracaineEye DropsAmfetamine Sulfate,I-130Amfetamine Sulphote,see AmfetamineSulfate,I-130Amido Black10B Solution,V-A23Amidohexadecylsilyl Silica Gel forchromatography,V-A115Amidotrizoic Acid Dihydrate,I-130Amikacin,I-132Amikacin Injection,III-124Amikacin Sulfate,I-135Amiloride and Furosemide Tablets,seeCo-amilofruse TabletsAmiloride and Hydrochlorothiazide OralSolution,see Co-amilozide Oral SolutionAmiloride and HydrochlorothiazideTablets,see Co-amilozide TabletsAmiloride Hydrochloride,I-138Amiloride Tablets,III-125Amines,Primary Aromatic,Reactions of,V-266Amino Acid Analysis,V-221Amino Acid Analysis(2.2.56.)(5.8.),V-700Amino Acids,Use of Codes for,I-8,II-8,III-8,IV-8,V-8Aminoazobenzene,V-A23Aminobenzoic Acid,I-139,V-A23,V-A244-Aminobenzoic Acid Solution,V-A24(4-Aminobenzoyl)-L-glutamic Acid,V-A244-Aminobutanoic acid,see4-Amino-n-butyric Acid2-Aminobutan-1-ol,V-A24Aminocaproic Acid,I-1402-Amino-5-chlorobenzophenone,V-A24Aminochlorobenzophenone,see2-Amino-5-chlorbenzophenone4-Aminofolic Acid,V-A24Aminoglutethimide,I-141,V-S8Aminoglutethimide Tablets,III-126Aminohexadecylsilyl Silica Gel forChromatography,V-A1156-Aminohexanoic Acid,V-A24p-Aminohippuric Acid,V-A24Aminohippuric Acid Reagent,V-A244-Amino-3-hydroxynaphthalene-1-sulfonic Acid,V-A24Aminohydroxynaphthalenesulfonic AcidSolution,V-A24Aminohydroxynaphthalenesulfonic AcidSolution,Strong,V-A24AminohydroxynaphthalenesulphonicAcid Solution,Strong,seeAminohydroxynaphthalenesulfonic AcidSolution,StrongAminohydroxynaphthalenesulphonicAcid Solution,seeAminohydroxynaphthalenesulfonic AcidSolution4-Amino-3-hydroxynaphthalene-1-sulphonic Acid,see4-Amino-3-hydroxynaphthalene-1-sulfonic AcidAminohydroxynaphthalenesulphonic,Acid,see Aminonaphthalenesulfonic AcidSolution5-Aminoimidazole-4-carboxamideHydrochloride,V-A24cis-Aminoindanol,V-A24Aminomethylalizarindiacetic AcidReagent,V-A24Aminomethylalizarindiacetic AcidSolution,V-A253-Aminomethylalizarin-N,N-diaceticAcid,V-A244-Aminomethylbenzoic acid,V-A253-(Aminomethyl)pyridine,V-A258-Aminonaphthalene-2-sulfonic Acid,V-A25Aminonaphthalenesulfonic AcidSolution,V-A25Aminonaphthalenesulphonic AcidSolution,see AminonaphthalenesulfonicAcid Solution8-Aminonaphthalene-2-sulphonic Acid,see8-Aminonaphthalene-2-sulfonic Acid4-Amino-n-butyric Acid,V-A242-Amino-5-nitrobenzophenone,V-A25Aminonitrobenzophenone,see2-Amino-5-nitrobenzophenone4-Aminophenazone,V-A25Aminophenazone Solution,V-A253-Aminophenol,V-A254-Aminophenol-free Paracetamol,V-A99Aminophylline,I-143Aminophylline Hydrate,I-145Aminophylline Injection,III-128Aminophylline Tablets,III-128Aminophylline Tablets,Prolonged-release,III-129Aminopolyether,V-A253-Aminopropanol,V-A253-Aminopropionic Acid,V-A25Aminopropylmethylsilyl Silica Gel forChromatography,V-A115Aminopropylsilyl Silica Gel forChromatography,V-A115Aminopropylsilyl Silica Gel forChromatography R1,V-A115Aminopyrazolone,see4-AminophenazoneAminopyrazolone Solution,seeAminophenazone Solution3-Aminosalicylic Acid,V-A25Amiodarone,V-S8Amiodarone Concentrate,Sterile,III-130Amiodarone Hydrochloride,I-147Amiodarone Infusion,III-129Amiodarone Intravenous Infusion,seeAmiodarone Infusion,Amiodarone Oral Suspension,III-131Amiodarone Sterile Concentrate,III-130page numbers in bold type relate to monograph titles Index V-A801Amiodarone Tablets,III-132 Amisulpride,I-149,V-S9Amisulpride Oral Solution,III-133 Amisulpride Tablets,III-134 Amitriptyline Embonate,I-150 Amitriptyline Hydrochloride,I-151 Amitriptyline Tablets,III-135 Amlodipine Besilate,I-153 Ammonia,V-A25Ammonia(13N)Injection,IV-672 Ammonia Buffer pH9.5,see Ammonium Chloride Buffer Solution pH9.5 Ammonia Buffer pH10.9,V-A153 Ammonia Buffer pH10.9,Dilute,V-A153Ammonia Buffer pH10.0,V-A153 Ammonia,Chloride-free,V-A25 Ammonia,Concentrated,V-A25 Ammonia,Lead-free,V-A25 Ammonia,Methanolic,V-A25 Ammonia R1,Concentrated,V-A26 Ammonia R1,Dilute,V-A26 Ammonia R2,Dilute,V-A26 Ammonia R3,Dilute,V-A26 Ammonia Solution,Aromatic,III-136 Ammonia Solution,Concentrated see Strong Ammonia SolutionAmmonia Solution,Dilute,III-137 Ammonia Spirit,Aromatic,III-137 Ammoniacal Copper Oxide Solution,V-A46Ammoniacal Silver Nitrate Solution,V-A120Ammoniacal Solution of Copper Tetrammine,V-A46Ammonia-free Water,V-A139 Ammonio Methacrylate Copolymer (Type A),I-155Ammonio Methacrylate Copolymer (Type B),I-1560.5M Ammonium acetate buffer solution pH4.5,see Ammonium acetate buffer pH4.5,0.5M0.01M Ammonium and Cerium Nitrate, see Ammonium Cerium(IV)Nitrate VS 0.1M Ammonium and Cerium Sulfate, see Ammonium Cerium(IV)Sulfate VS Ammonium Acetate,V-A26 Ammonium acetate buffer pH4.5,0.5M, V-A153Ammonium Acetate Solution,V-A26 Ammonium Acetate Solution,Strong,III-137Ammonium and Cerium Nitrate,see Ammonium Cerium(IV)Nitrate Ammonium and Cerium Sulfate,see Ammonium Cerium(IV)Sulfate Ammonium and Cerium Sulphate,see Ammonium and Cerium Sulfate Ammonium Bicarbonate,I-157 Ammonium Bromide,I-158 Ammonium Carbamate,V-A26 Ammonium Carbonate,V-A26 Ammonium Carbonate Buffer Solution pH10.3,0.1M,V-A153Ammonium Carbonate Solution,V-A26 Ammonium Carbonate Solution,Dilute, V-A26Ammonium carbonate solution R1,V-A26Ammonium Cerium(IV)Nitrate,V-A26Ammonium Cerium(IV)Nitrate VS,V-A142Ammonium Cerium(IV)Sulfate,V-A26Ammonium Cerium(IV)Sulfate VS,V-A142Ammonium Cerium(IV)Sulphate VS,seeAmmonium Cerium(IV)Sulfate VSAmmonium Cerium(IV)Sulphate,seeAmmonium Cerium(iv)SulfateAmmonium Chloride,I-159,V-A26Ammonium Chloride Buffer SolutionpH10.0,see Ammonia Buffer pH10.0Ammonium Chloride Buffer SolutionpH10.4,V-A153Ammonium Chloride Buffer SolutionpH10.7,V-A153Ammonium Chloride Buffer SolutionpH9.5,V-A153Ammonium Chloride Buffer SolutionpH10.0,V-A153Ammonium Chloride Mixture,III-137Ammonium Chloride Oral Solution,III-137Ammonium Chloride Solution,V-A26Ammonium Citrate,V-A26Ammonium Citrate Solution,V-A26Ammonium CobaltothiocyanateSolution,V-A26Ammonium DihydrogenOrthophosphate,V-A26Ammonium Formate,V-A26Ammonium Glycyrrhizinate,I-160Ammonium Hexafluorogermanate,V-A26Ammonium Hydrogen Carbonate,V-A26Ammonium Hydrogen Carbonate seeAmmonium BicarbonateAmmonium Ichthosulphonate seeIchthammolAmmonium Iron(II)Sulfate,V-A26Ammonium Iron(II)Sulfate VS,V-A142Ammonium Iron(II)Sulphate VS,seeAmmonium Iron(II)Sulfate VSAmmonium Iron(II)Sulphate,seeAmmonium Iron(ii)SulfateAmmonium Iron(III)Citrate,V-A26Ammonium Iron(III)Sulfate,V-A26Ammonium Iron(III)Sulfate Solution R1,V-A26Ammonium Iron(III)Sulfate Solution R2,V-A26Ammonium Iron(III)Sulfate Solution R5,V-A26Ammonium Iron(III)Sulfate Solution R6,V-A27Ammonium Iron(III)Sulfate VS,V-A142Ammonium Iron(III)Sulphate SolutionR1,see Ammonium Iron(iii)SulfateSolution R1Ammonium Iron(III)Sulphate SolutionR2,see Ammonium Iron(iii)SulfateSolution R2Ammonium Iron(III)Sulphate SolutionR5,see Ammonium Iron(iii)SulfateSolution R5Ammonium Iron(III)Sulphate VS,seeAmmonium Iron(III)Sulfate VSAmmonium Iron(III)Sulphate,seeAmmonium Iron(iii)SulfateAmmonium Mercaptoacetate Solution,V-A27Ammonium Mercurithiocyanate Reagent,V-A27Ammonium Metavanadate,V-A27Ammonium Metavanadate Solution,V-A27Ammonium Molybdate,V-A27Ammonium Molybdate Reagent,V-A27Ammonium Molybdate Reagent R1,V-A27Ammonium Molybdate Reagent R2,V-A27Ammonium Molybdate Solution,V-A27Ammonium Molybdate Solution R2,V-A27Ammonium Molybdate Solution R3,V-A27Ammonium Molybdate Solution R4,V-A27Ammonium Molybdate Solution R5,V-A27Ammonium Molybdate Solution R6,V-A27Ammonium Molybdate-Sulfuric AcidSolution,V-A27Ammonium Molybdate-Sulphuric AcidSolution,see Ammonium Molybdate-Sulfuric Acid SolutionAmmonium Muriaticum,V-609Ammonium Nitrate,V-A27Ammonium Nitrate R1,V-A27Ammonium Oxalate,V-A27Ammonium Oxalate Solution,V-A27Ammonium Persulfate,V-A27Ammonium Persulphate,see AmmoniumPersulfateAmmonium Phosphate,see DiammoniumHydrogen OrthophosphateAmmonium Polysulfide Solution,V-A27Ammonium Polysulphide Solution,seeAmmonium Polysulfide SolutionAmmonium Pyrrolidinedithiocarbamate,V-A27Ammonium PyrrolidinedithiocarbamateSolution,V-A27Ammonium Reineckate,V-A27Ammonium Reineckate Solution,V-A28Ammonium Salts and Salts of VolatileBases,Reactions of,V-267Ammonium Salts Reactions of,V-266Ammonium Standard Solution(1ppmNH4),V-A148Ammonium Standard Solution(2.5ppmNH4),V-A148Ammonium Standard Solution(3ppmNH4),V-A148Ammonium Standard Solution(100ppmNH4),V-A148Ammonium Sulfamate,V-A28Ammonium Sulfate,V-A28Ammonium Sulfide Solution,V-A28Ammonium Sulphamate,see AmmoniumSulfamateAmmonium Sulphate,see AmmoniumSulfateAmmonium Sulphide Solution,seeAmmonium Sulfide SolutionV-A802IndexAmmonium Thiocyanate,V-A28 Ammonium Thiocyanate Solution,V-A28Ammonium Thiocyanate VS,V-A142 Ammonium Vanadate Solution,V-A28 Ammonium Vanadate,see Ammonium MetavanadateAmobarbital,I-161Amobarbital Sodium,I-162Amomum fruit,IV-56Amorphous Organosilica Polymer, Octadecylsilyl,V-A98Amoxicillin and Potassium Clavulanate Injection,see Co-amoxiclav Injection Amoxicillin and Potassium Clavulanate Oral Suspension,see Co-amoxiclav Oral SuspensionAmoxicillin and Potassium Clavulanate Tablets,Dispersible,see Dispersible Co-amoxiclav TabletsAmoxicillin and Potassium Clavulanate Tablets,see Co-amoxiclav Tablets Amoxicillin Capsules,III-138 Amoxicillin Injection,III-139 Amoxicillin Oral Suspension,III-141 Amoxicillin Sodium,I-163,V-S9 Amoxicillin Sodium for Injection,III-139Amoxicillin Trihydrate,I-165,V-S9,V-A28Ampere,Definition of,I-32,II-32,III-32,IV-32,V-32 Amperometric,Potentiometric and Voltametric Titrations,V-280 Amperometric Titration,V-280 Amphotericin,I-168Amphotericin B see Amphotericin Amphotericin for Infusion,III-142 Amphotericin Lozenges,I-xxix Amphotericin Oral Suspension,I-xxix Ampicillin,I-170Ampicillin Capsules,III-143Ampicillin Capsules,Flucloxacillin and, see Co-fluampicil CapsulesAmpicillin Injection,III-144Ampicillin Oral Suspension,III-146 Ampicillin Oral Suspension, Flucloxacillin and,see Co-fluampicil Oral SuspensionAmpicillin Sodium,I-172,V-S10 Ampicillin Sodium for Injection,III-144 Ampicillin Trihydrate,I-175,V-S10 Amyl Acetate,V-A28Amyl Alcohol,see Isoamyl Alcohola-Amylase,V-A28a-Amylase Solution,V-A28 Amylmetacresol,I-178,V-S10Amylose-derivative Silica Gel for Chromatography,V-A115b-Amyrin,V-A28Anacardium for Homoeopathic Preparations,IV-428Anaesthetic Ether,I-902Analytical Procedures,Validation of,V-673Analytical Sieving,Particle-size Distribution Estimation By,V-503 Anastrozole,I-179cis-Anethole,V-A28Anethum Graveolens L.Sowa Group,seeAnethum Graveolens Sowa Fruit,Anethum Graveolens Sowa Fruit,IV-58Angelica Archangelica Root,IV-59Angelica Dahurica Root,IV-60Angelica Pubescens Root,IV-62Angelica Sinensis Root,IV-63Angelica Sinensis Root,see ProcessedAngelica Sinensis RootAnhydrous Acetic Acid,V-A20Anhydrous Aluminium Oxide,V-A23Anhydrous Ampicilin see AmpicillinAnhydrous Azapropazone,V-S12Anhydrous Beclometasone Dipropionate,I-239Anhydrous Caffeine,see CaffeineAnhydrous Calcipotriol,I-353Anhydrous Calcium Acetate,see CalciumAcetateAnhydrous Calcium Chloride,V-A37Anhydrous Calcium Gluconate,I-372Anhydrous Calcium HydrogenPhosphate,I-377Anhydrous Calcium Lactate,I-378Anhydrous Chlorobutanol,I-518Anhydrous Citric Acid,I-569,V-A45Anhydrous Copper Sulfate,I-647Anhydrous Disodium HydrogenOrthophosphate,V-A59Anhydrous Disodium HydrogenPhosphate,I-788Anhydrous Docetaxel,I-796Anhydrous Ephedrine,I-849Anhydrous Formic Acid,V-A67Anhydrous Glucose,I-1083Anhydrous Iron(III)Chloride,V-A77Anhydrous Lactose,II-66Anhydrous Lithium Metaborate,V-A81Anhydrous Magnesium Citrate,II-166Anhydrous Methanol,V-A85Anhydrous Morphine,V-A91Anhydrous Nevirapine,II-358Anhydrous Niclosamide,II-362Anhydrous Paroxetine Hydrochloride,II-504Anhydrous Phloroglucinol,II-566Anhydrous Pyridine,V-A110Anhydrous Silica Gel,V-A114Anhydrous Silica,HydrophobicColloidal,II-807Anhydrous Sodium Acetate,V-A120Anhydrous Sodium Carbonate,II-830,V-A121,V-A141Anhydrous Sodium DihydrogenOrthophosphate,V-A122Anhydrous Sodium DihydrogenPhosphate,II-839,V-A122Anhydrous Sodium Sulfate,II-872,V-A124Anhydrous Sodium Sulfite,II-873,V-A124Anhydrous Sodium Sulphate seeAnhydrous Sodium SulfateAnhydrous Sodium Sulphite seeAnhydrous Sodium SulfiteAnhydrous Torasemide,II-1066Anhydrous Valaciclovir Hydrochloride,II-1134Aniline,V-A28Aniline Hydrochloride,V-A28Aniline Hydrochloride Solution,V-A28Animal Spongiform EncephalopathyAgents Via Human and VeterinaryMedicinal Products,Minimising theRisk of Transmitting,V-611Animals,Use of,I-15,II-15,III-15,IV-15,V-15Anion Exchange Resin,V-A28Anion Exchange Resin forChromatography,Strongly Basic,V-A28Anion Exchange Resin R1,V-A28Anion Exchange Resin R2,V-A28Anion exchange resin R3,V-A29Anion Exchange Resin,Strongly Basic,V-A28Anion Exchange Resin,Weak,V-A29Anion-exchange Resin forChromatography,Strongly Basic R1,V-A28Anionic Emulsifying Wax,see EmulsifyingWaxAnisaldehyde,V-A29Anisaldehyde Solution,V-A29Anisaldehyde Solution R1,V-A29Anise Ketone,V-A29Anise Oil,IV-71Anise Water,Concentrated,IV-73Aniseed,IV-66Aniseed Oil,see Anise Oilp-Anisidine,V-A29Anisidine Value,V-326Anolyte for Isoelectric Focusing pH3to5,V-A29Antazoline Hydrochloride,I-181Anthracene,V-A29Anthranilic Acid,see2-Aminobenzoic AcidAnthrax,see Anthrax Vaccine for HumanUse(Adsorbed,Prepared from CultureFiltrates)Anthrax Vaccine for Human Use(Adsorbed,Prepared from CultureFiltrates),IV-527Anthrone,V-A29Anthrone Reagent,V-A29Antibiotics,Microbiological Assay of,V-396,V-655Antibiotics,Potency of,I-14,II-14,III-14,IV-14,V-14Anticoagulant and Preservative Solutionsfor Blood,IV-461Anti-D Immunoglobulin for IntravenousUse,IV-497Anti-D(Rh0)Immunoglobulin,IV-496Antimicrobial Preservation,Efficacy of,V-494,V-653Antithrombin III ConcentrateAnticomplimentary activity ofimmunoglobulin,Test for V-427Anti-D immunoglobulin,human,Assayof V-429Anti-D antibodies in humanimmunoglobulin V-431Anti-A and anti-B haemogglutininsV-432Antimicrobial Preservatives,Definition ofSuitable,I-11,II-11,III-11,IV-11,V-11Antimony Compounds,Reactions of,V-267page numbers in bold type relate to monograph titles Index V-A803。
头孢拉定结构式

知识创造未来
头孢拉定结构式
头孢拉定是一种广泛应用于临床的β内酰胺类抗生素,是第三代
头孢菌素的代表之一。
其分子式为C16H17N5O7S2,属于无色晶体状物质。
头孢拉定的化学结构中包含内酰胺环以及两个硫基和一个羧基,
这使得其具备了抗菌作用。
头孢拉定主要通过抑制细菌细胞壁的合成而发挥其抗菌作用。
其
特殊的化学结构,使得其能够进入革兰氏阳性菌和大多数革兰氏阴性
菌的细胞壁,与PBP(penicillin binding protein)结合抑制其功能,从而导致菌体失去细胞壁的保护,最终导致菌体死亡。
头孢拉定广泛应用于多种感染的治疗,如呼吸道感染、泌尿系统
感染、皮肤软组织感染等。
对于一些对其他头孢菌素已产生耐药性的
细菌,头孢拉定也能表现出出色的抗菌作用。
在临床应用中,头孢拉
定可口服或注射,能够快速吸收并发挥其抗菌作用。
但值得注意的是,头孢拉定虽然具有出色的抗菌作用,但是如果
过度应用或长期应用,会对正常菌群产生影响,导致不良反应的发生。
因此,在使用头孢拉定时应该依照医生的指导合理使用,并严格遵循
用药方法和剂量的规定,以避免不必要的风险。
综上所述,头孢拉定是一种出色的抗生素,在临床上能够有效地
治疗多种感染。
但要充分认识和正确使用其药物作用,在使用上应尊
重药物的规律,严格按照医生指示用药,才能取得更好的治疗效果。
1 / 1。
注射用头孢拉定使用说明书

注射用头抱拉定使用说明书【药品名称】通用名:注射用头抱拉定商品名:英文名:Cefradine for Injection汉语拼音:Zhusheyong Toubao Lading本品主要成分为头抱拉定,其化学名为(6R,7R)-7[(R)-2-氨基-2-(1,4环己烯基)<>乙酰氨基卜3-甲基-8-氧代-5-硫杂-1-氮杂双环[4、2、0]辛-2-烯-2-羧酸。
其结构式为:分子式:G6H19N3O4S分子量:349.40【性状】本品为头抱拉定和精氨酸混合的无菌粉末,呈白色或类白色,易溶于水。
【药理毒理】本品为第一代头抱菌素,对不产青霉素酶和产青霉素酶金葡菌、凝固酶阴性葡萄球菌、A组溶血性链球菌、肺炎链球菌和草绿色链球菌等革兰阳性球菌的部分菌株具良好抗菌作用。
厌氧革兰阳性菌对本品多敏感,脆弱拟杆菌对本品呈现耐药。
耐甲氧西林葡萄球菌属、肠球菌属对本品耐药。
本品对革兰阳性菌与革兰阴性菌的作用与头抱氨苄相似。
本品对淋球菌有一定作用,对产酶淋球菌也具活性;对流感嗜血杆菌的活性较差。
【药代动力学】静脉滴注本品0.5g后5分钟后血药浓度为46mg/L,肌内注射0.5g后平均6mg/L 的血药峰浓度(Cmax于给药后1〜2小时到达。
肌内注射吸收较口服为差,但血药浓度维持较久。
血消除半衰期(t1/2b)为0.8〜1小时。
本品在组织体液中分布良好。
肝组织中的浓度与血清浓度相等。
在心肌、子宫、肺、前列腺和骨组织中皆可获有效浓度。
脑脊液中药物浓度仅为同期血药浓度的8%〜12%。
本品可透过血-胎盘屏障进入胎儿血循环,少量经乳汁排出。
血清蛋白结合率为6%〜10%。
静脉给药后6小时尿中累积排出量为给药量的90%以上;肌内注射后6小时尿中累积排出给药量的66%。
尿中浓度甚高,多可超过1000mg/L。
少量本品可自胆汁排泄,后者的浓度可为血清浓度的4 倍。
本品在体内很少代谢,能为血液透析和腹膜透析清除。
丙磺舒可减少本品经肾排泄。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
∙British Pharmacopoeia Volume III∙Formulated Preparations: Specific MonographsCefradine CapsulesGeneral NoticesAction and useCephalosporin antibacterial.DEFINITIONCefradine Capsules contain Cefradine.The capsules comply with the requirements stated under Capsules and with the following requirements. Content of cephalosporins, calculated as the sum of cefradine (C16H19N3O4S), cefalexin(C16H17N3O4S) and 4′,5′-dihydrocefradine (C16H21N3O4S)90.0 to 105.0% of the stated amount of Cefradine.IDENTIFICATIONCHROMATOGRAPHIC CONDITIONS(a) Use a TLC silica gel plate (Analtech plates are suitable). Impregnate the plate by placing it in a tankcontaining a shallow layer of a 5% v/v solution of n-tetradecane in n-hexane, allowing the impregnating solvent to ascend to the top, removing the plate from the tank and allowing the solvent to evaporate;use with the flow of the mobile phase in the same direction that the impregnation was carried out.(b) Use the mobile phase as described below.(c) Apply 5 µL of each solution.(d) Develop the plate to 15 cm.(e) After removal of the plate, heat at 90° for 2 to 3 minutes and spray the hot plate with a 0.1% w/vsolution of ninhydrin in the mobile phase. Heat at 90° for 15 minutes in a circulating air oven with the plates parallel to the airflow, cool for 15 minutes protected from light and examine in daylight.MOBILE PHASE3 volume of acetone, 80 volumes of 0.2M anhydrous disodium hydrogen orthophosphate and120 volumes of 0.1M citric acid.CONFIRMATIONThe principal spot in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).TESTSDissolutionComply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.TEST CONDITIONS(a) Use Apparatus 1, rotating the basket at 100 revolutions per minute.(b) Use 900 mL of 0.12M hydrochloric acid, at a temperature of 37°, as the medium.PROCEDUREDETERMINATION OF CONTENTCalculate the total content of cephalosporins, as the sum of the contents of C16H19N3O4S, C16H17N3O4S and C16H21N3O4S in the medium from the absorbance obtained and using the declared content of total cephalosporins in cefradine BPCRS.Related substancesCarry out the method for liquid chromatography, Appendix III D, using the following solutions.(1) Shake a quantity of the contents of the capsules containing 0.3 g of Cefradine in mobile phase A, add sufficient mobile phase A to produce 50 mL and filter through a 0.45-µm filter.(2) Dilute 1 volume of solution (1) to 100 volumes with mobile phase A.(3) 0.012% w/v of each of cefradine BPCRS and cefalexin BPCRS in mobile phase A.(4) 0.003% w/v of cyclohexa-1,4-dienylglycine EPCRS (impurity B) in mobile phase A.(5) Dissolve 6 mg of cefradine for peak identification EPCRS (containing impurities C, D and E) in 1 mL of mobile phase A.(6) Dissolve the contents of a vial of cefradine impurity mixture EPCRS (containing impurities A and G) in 1 mL of mobile phase A.CHROMATOGRAPHIC CONDITIONS(a) Use a stainless steel column (15 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Varian Chrompack Inertsil ODS-3 is suitable).(b) Use gradient elution and the mobile phase described below.(c) Use a flow rate of 1.0 mL per minute.(d) Use a column temperature of 30°.(e) Use a detection wavelength of 220 nm.(f) Inject 25 µL of each solution.MOBILE PHASEMobile phase A 0.272% w/v of potassium dihydrogen orthophosphate adjusted to pH 3.0 with dilute orthophosphoric acid.Mobile phase B methanol R2.When the chromatograms are recorded under the prescribed conditions the retention times relative to Cefradine (retention time = about 15 minutes) are: impurity A = about 0.27; impurity B = about 0.32; impurity C = about 0.53; impurity D = about 0.63; impurity E = about 0.80; impurity F = about 0.92; cefalexin = about 0.95; 4′,5′-dihydrocefradine = about 1.06; impurity G = about 1.32.SYSTEM SUITABILITYThe test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to cefalexin and cefradine is at least 4.0.LIMITSIdentify any peaks in the chromatogram obtained with solution (1) due to impurities C, D and E using the chromatogram obtained with solution (5) and the chromatogram supplied with cefradine for peak identification EPCRS. Identify any peaks in the chromatogram obtained with solution (1) due to impurities A and G using the chromatogram obtained with solution (6) and the chromatogram supplied with cefradine impurity mixture EPCRS.In the chromatogram obtained with solution (1):the area of any peak corresponding to impurity B is not greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (4) (0.25%);the area of any peak corresponding to impurity A, D, F or G is not greater than 0.25 times the area of the principal peak in the chromatogram obtained with solution (2) (0.25% for each);the area of any peak corresponding to impurity C is not greater than 0.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);the area of any peak corresponding to impurity E is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1%);the area of any other secondary peak is not greater than 0.25 times the area of the principal peak in the chromatogram obtained with solution (2) (0.25%);the sum of the areas of all the peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (2%).Disregard the peaks due to cefalexin, and 4′,5′-dihydrocefradine and any peak with an area less than 0.05 times the area of the principal peak in the chromatogram obtained with solution (2) (0.05%). CefalexinNot more than 10.0%, calculated as the percentage of C16H17N3O4S in the sum of C16H19N3O4S,C16H17N3O4S and C16H21N3O4S determined in the Assay.4′,5′-DihydrocefradineNot more than 2.0%, calculated as the percentage of C16H21N3O4S in the sum of C16H19N3O4S,C16H17N3O4S and C16H21N3O4S determined in the Assay.Loss on dryingThe contents of the capsules, when dried at 60° at a pressure not exceeding 0.7 kPa for 3 hours, lose not more than 7.0% of their weight. Use 1 g.ASSAYCarry out the method for liquid chromatography, Appendix III D, using the following solutions in a mixture of 3 volumes of 0.7M glacial acetic acid, 15 volumes of 0.5M sodium acetate, 200 volumes of methanol and 782 volumes of water (solution A).(1) Dissolve a quantity of the powdered mixed contents of 20 capsules to produce a solution containing 0.05% w/v of Cefradine.(2) 0.05% w/v of cefradine BPCRS.(3) 0.005% w/v of cefalexin BPCRS.(4) Dilute 1 volume of solution (2) to 10 volumes with solution A. Mix equal volumes of this solution and solution (3).CHROMATOGRAPHIC CONDITIONS(a) Use a stainless steel column (10 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Hypersil ODS is suitable).(b) Use isocratic elution and the mobile phase described below.(c) Use a flow rate of 1.5 mL per minute.(d) Use an ambient column temperature.(e) Use a detection wavelength of 254 nm.(f) Inject 5 µL of each solution.MOBILE PHASE25 volumes of methanol and 75 volumes of phosphate buffer solution pH 5.0.SYSTEM SUITABILITYThe Assay is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks corresponding to cefradine and cefalexin is at least 4.0.DETERMINATION OF CONTENTCalculate the content of cephalosporins in the capsules by determining the sum of the contents ofC16H19N3O4S, C16H17N3O4S and C16H21N3O4S. Calculate the content of C16H19N3O4S (cefradine) using the declared content of C16H19N3O4S in cefradine BPCRS. Calculate the content of C16H17N3O4S (cefalexin) using the declared content of C16H17N3O4S in cefalexin BPCRS. Calculate the content ofC16H21N3O4S (4′,5′-dihydrocefradine) using the declared content of C16H17N3O4S in cefalexin BPCRS and multiplying the area of the peak due to 4′,5′-dihydrocefradine by a correction factor of 1.6.。
