British Pharmacopoeia 2013 Levonorgestrel左炔诺孕酮英国药典2013
[宝典]欧洲药典附录3.1.3.
![[宝典]欧洲药典附录3.1.3.](https://img.taocdn.com/s3/m/f3357cec760bf78a6529647d27284b73f2423679.png)
3.1.3. 聚烯烃定义聚烯烃是通过乙烯或丙烯的聚合而成,或是通过这些不超过25%的高同系物的物质或羧酸或酯的共聚作用获得。
某些材料可能是聚烯烃的混合物。
成品添加一定数量的添加剂到聚合物中是为了优化它们的化学性质,物理性质和机械性能,为了使它们适用于预定用途。
所有的这些添加剂都是选自附件列表,并指出了每一种产品中的最大允许含量。
产品中最多包含有三种抗氧化剂,一种或几种润滑剂或抗粘连剂以及当材料必须提供光照保护时,还要添加二氧化钛作为遮光剂。
–二叔丁基对甲酚(增塑剂07):限量:0.125%–四钛季戊四醇松香酸酯[3-(3,5-二叔丁基-4-羟苯基)丙酸酯](增塑剂09):限量:0.3%–1,3,5-三羟甲基氨基甲烷(3,5-二叔丁基-4-邻羟苄基 )- 三嗪-2,4,6(1H,3H,5H)-三酮, (增塑剂 13): 限量: 0.3%–二乙烯[3,3-二[3-(1,1-dimethylethyl)-4-羟苯基]丁酸甲酯] (增塑剂08):限量:0.3%–二(十八烷基)二硫化物(增塑剂15)限量:0.3%4,4′,4″-(2,4,6-三甲基苯-1,3,5-triyltrismethylene)–三羟甲基氨基甲烷[2,6-二(1,1-dimethylethyl)苯酚](增塑剂10)限量:0.3%2,2′-二(octadecyloxy)-5,5′-spirobi[1,3,2-dioxaphosphinane](增塑剂 14): 限量:0.3 %;–didodecyl 3,3′-硫代二丙酸(增塑剂16): 限量: 0.3 %;–dioctadecyl3,3′-硫代二丙酸(增塑剂 17): 限量:0.3 %;–三羟甲基氨基甲烷[2,4-二(1,1-dimethylethyl)苯基] 亚磷酸盐 (增塑剂 12): 限量:0.3 %;–增塑剂 18: 限量: 0.1%;–琥珀酸二甲酯和 (4-hydroxy-2,2,6,6-tetramethylpiperidin-1-yl)乙醇的共聚物 (增塑剂 22): 限量:0.3%上面列出的抗氧化添加剂总含量不超过0.3%。
英国国家处方集和其使用培训课件

文档仅供参考,不能作为科学依据,请勿模仿;如有不当之处,请联系网站或本人删除。
章节和单个药品举例
3 Respiratory system
3.1 Bronchodilators 3.1.1 Adrenoceptor agonists 3.1.1.1 Selective beta2 agonists 3.1.1.2 Other adrenoceptor agonists 3.1.2 Antimuscarinic bronchodilators 3.1.3 Theophylline 3.1.4 Compound bronchodilator preparations 3.1.5 Peak flow meters, inhaler devices and nebulisers
文档仅供参考,不能作为科学依据,请勿模仿;如有不当之处,请联系网站或本人删除。
How to use the BNF
Selecting the dose Selecting a suitable preparation Writing prescriptions Administering drugs Advising patients Monitoring drug treatment Identifying and reporting adverse drug reactions Finding significant changes in a new edition Nutrition Wound dressing ……
药学相关英文缩写

药学相关英文缩写API Active Pharmaceutical Ingredient 原料药(活性药物组分)BP British Pharmacopoeia 《英国药典》CAC Codex Alimentarius Committee (国际)食品法典委员会CBER FDA Center for Biologics Evaluation and Research (美国)FDA生物制品评价与研究中心CCD Certification Committee for Drugs(国家食品药品监督管理局)药品认证管理中心CDC Centers for Disease Control (美国)疾病控制中心CDE Center for Drug Evaluation (国家食品药品监督管理局)药品审评中心CDER FDA Center for Drug Evaluation and Research (美国)FDA药品评价与研究中心CDR Center for Drug Reevaluation (国家食品药品监督管理局)药品评价中心CDRH FDA Center for Devices and Radiological Health (美国)FDA医疗器械和辐射健康中心CFSAN FDA Center for Food Safety and Applied Nutrition (美国)FDA食品安全和应用营养中心CGMPs Current good manufacturing practices 现行良好制造规范;现行药品生产质量管理规范CMA Chinese Medical Association 中华医学会CNAO National Audit Office of the People's Republic of China 中华人民共和国审计署CNMA China Nonprescription Medicines Association 中国非处方药协会COS Certificate of Suitability (《欧洲药典》)适用性证书CP The Pharmacopoeia of the PRC 《中国药典》CPA China Pharmaceutical Association 中国药学会CPMA China Preventive Mediceine Association 中华预防医学会CVM FDA Center for Veterinary Medicine (美国)FDA兽药中心DE Drug evaluation 药品评价DEA Drug Enforcement Administration (美国)毒品强制执行管理局DIC Drug information center 药品信息中心DME Drug-metabolizing enzyme 药物代谢酶DMF Durg master file 药品主文件DUR Drug utilization review 药物利用评价DUR Drug use review 药物使用评论DHHS Department of Health and Human Services (美国)健康和人类服务部EFSA European Food Safety Authority 欧洲食品安全局ELA Establishment license application 机构许可申请EP European pharmacopoeia 《欧洲药典》EMEA European Agency for the Evaluation of Medicinal Products欧洲药品评价署EPA Environmental Protection Agency (美国)环境保护署EU European Union 欧盟FAO United Nations Food and Agricultural Organization 联合国粮农组织FD&C Act Federal Food, Drug and Cosmetic Act 《联邦食品、药品和化妆品法》FDA Food and Drug Administration (美国)食品药品管理局FSIS Food Safety Inspection Service (USDA) (美国农业部)食品安全检查服务局FTC Federal Trade Commission (美国)联邦贸易委员会GLP Good laboratory practice 良好实验室规范;药品非临床研究质量管理规范GMP Good manufacturing practices 药品生产和管理规范GPP Good pharmacy practice 良好药房规范;医疗机构制剂配制管理规范GPSP Good Post-marketing Surveillance Practice 良好上市后监督规范;上市药品监督规范GSP Good supplying practice 良好供应规范;药品经营质量管理规范HVAC Heating Ventilation Air Conditioning 空调净化系统ICH International Conference on Harmonization 国际(药品注册)协调会议IMIC International Medical Information Center 国际医学信息中心INCB International Narcotics Control Board 国际麻醉药品管制局IOM Institute of Medicine (美国国家科学院)医学研究所IPF International Pharmaceutical Federation 国际制药联合会IRC International Red Cross 国际红十字会IRCC International Red Cross Conference 国际红十字大会ISO International Standards Organization 国际标准化组织IVDC China Institute of Veterinary Drug Control 中国兽医药品监察所JIFSAN Joint Institute for Food Safety and Applied Nutrition (美国FDA)食品安全和应用营养联合研究所KFDA Korea Food and Drug Administration (韩国)食品药品管理局MHLW Ministry of Health, Labour and Welfare (日本)厚生劳动省MII China Ministry of Information Industry 中华人民共和国信息产业部MOF Ministry of Finance People's Republic of China 中华人民共和国财政部MoH Ministry of Health P.R.China 中华人民共和国卫生部MOST Ministry of Science and Technology of the People's Republic of China 中华人民共和国科学技术部NBS National Bureau of Statistics of China 国家统计局NCI National Cancer Institute (美国)国家癌症研究所NDA New drug application 新药申请NCTR FDA National Center for Toxicological Research (美国)FDA国家毒理学研究中心NIAID National Institute of Allergy and Infectious Diseases (美国)国家过敏症和传染病研究所NIDA National Institute on Drug Abuse (美国)国家药物滥用研究所NIH National Institute of Health (美国)国家健康研究所ORA FDA Office of Regulatory Affairs (美国)FDA监管事务办公室OTC Over the counter drug (Nonprescription drugs) 放在柜台上的药品(非处方药)PHS Public Health Service (美国)公众健康服务局Ph Gal Pharmacopoeia galisa 《法国药典》[拉]Ph J Pharmacopoeia japonica 《日本药典》PhI Pharmacopoeia internationalis 《国际药典》[拉]PLA Product license application 产品许可申请PRC People's Republic of China 中华人民共和国QA Quality assurance 质量保证QC Quality control 质量控制SAIC State Administration For Industry & Commerce 国家工商行政管理总局SAMHSA Substance Abuse and Mental Health Services Administration (美国)物质滥用和精神健康服务管理局SATCM State Administration of Traditional Chinese Medicine (中国)国家中医药管理局SETC State Economic and Trade Commission,PRC 中华人民共和国国家经济贸易委员会SFDA State Food and Drug Administration (中国)国家食品药品监督管理局SIPO State Intellectual Property Office of the People's Republic of China 国家知识产权局SOP Standard operating procedure 标准操作规程TFDA Thailand Food and Drug Administration (泰国)食品药品管理局TGA Therapeutic Goods Administration (澳大利亚)治疗产品管理局TQC Total quality control 全面质量控制UK United Kingdom (大不列颠)联合王国USP The united states pharmacopoeia 《美国药典》WHO United Nations World Health Organization (联合国)世界卫生组织WTO World Trade Organization 世界贸易组织ZDA Zhejiang Drug Administration 浙江省药品监督管理局。
1塞欧斯简介

塞欧斯简介
产品名:塞欧斯
成分:美洛昔康
规格:7.5mgХ6粒/板
适应症:
风湿性关节炎,类风湿性关节炎,骨关节炎,头痛,肌肉痛,痛经,牙痛,五官科疼痛
用法用量:
风湿、类风湿——15mg/天,根据治疗后反应,剂量可减至7.5mg/天,12周为一个疗程。
骨关节炎——7.5mg/天,假如需要,剂量可增至15mg/天
其他疼痛——7.5mg/天
禁忌症:对本品过敏者禁止使用
对于具有上消化道疾病史的病人和正在应用抗凝剂治疗的病人使用本药应该注意。
若出现消化道性溃疡或肠胃道出血应该停止使用本药。
产品特点:
1、新的高选择性环氧化酶—2(COX—2)抑制剂。
90年代初Needleman等人发现人体有两种不同的环氧化酶异构体,环氧化酶—1(COX—1)和环氧化酶—2(COX—2)。
COX—1是人体正常成分,它调节组织内生理性前列腺素合成,调节外周血管阻力,维持肾血流量,保护胃粘膜,调节血小板聚集等。
COX—2存在于炎症部位,如滑膜细胞,内皮细胞内,在外界刺激因子(IL —1)的作用下,促使炎症介质的释放,诱发炎症反应。
原有的抗炎镇痛药是通过抑制环氧化酶(COX)阻断花生四稀酸形成前列腺素,无选择的抑制了COX—1和COX—2,所以既有抗炎镇痛作用,也不可避免的出现胃肠道不良反应。
2、起效快——1小时即达到血浆药物峰值浓度,并且峰值浓度更高。
3、剂量小——7.5mg一粒(比较与其他镇痛药)
1 / 1。
各国药典比较

国际药典(Ph.Int) 国际药典(Ph.Int)
• 基本介绍
由联合国世界卫生组织主持编订。第一版于1951和 由联合国世界卫生组织主持编订。第一版于1951和 1955年分两卷用英、法、西班牙文出版,于1959出版增 1955年分两卷用英、法、西班牙文出版,于1959出版增 补本。第二版于1967年用英、法、俄、西班牙文出版。现 补本。第二版于1967年用英、法、俄、西班牙文出版。现 行版为第三版,于1979、1981、1988年、1994、2003分 行版为第三版,于1979、1981、1988年、1994、2003分 5卷出版,第1卷收载42项分析测试方法。第2、3两卷共 卷出版,第1卷收载42项分析测试方法。第2 收载药品383种。第4 收载药品383种。第4卷收载有关试验、方法的信息,以及 药品原料、赋形剂的一般要求和质量说明,以及剂型。第 5卷收载制剂通则以及药品原料和片剂的质量标准,这实 际上将涵盖目录上的有机合成药物以及一些抗疟疾药物及 其最广泛应用剂型的所有各论。
内容简介
• 美国药典正文药品名录分别按法定药名字
母顺序排列,各药品条目大都列有药名、 结构式、分子式、CAS登记号、成分和含量 结构式、分子式、CAS登记号、成分和含量 说明、包装和贮藏规格、鉴定方法、干燥 说明、包装和贮藏规格、鉴定方法、干燥 失重、炽灼残渣、检测方法等常规项目, 失重、炽灼残渣、检测方法等常规项目, 正文之后还有对各种药品进行测试的方法 和要求的通用章节及对各种药物的一般要 求的通则。可根据书后所附的USP和NF的 求的通则。可根据书后所附的USP和NF的 联合索引查阅本书。
图书版本
• • • • • •
最新版本: 最新版本: USP 33-NF 28重新发行版: 3328重新发行版 重新发行版: 2010年10月 日生效。 2010年10月1日生效。 增补版1 2010年 月出版,2010年10月 增补版1于2010年4月出版,2010年10月1日生效。 增补版2 2010年 月出版,2011年 增补版2于2010年6月出版,2011年1月1日生效。 U.S. Pharmacopeia / National Formulary《美国 Formulary《 药典/国家处方集》(简称USP/NF)。由美国政 药典/国家处方集》(简称USP/NF)。由美国政 府所属的美国药典委员会(The 府所属的美国药典委员会(The United States Pharmacopeial Convention)编辑出版。 Convention)编辑出版。
培美曲赛二钠英国药典

Pemetrexed Disodium HeptahydrateGeneral Notices(Ph. Eur. monograph 2637)C20H19N5Na2O6,7H2O 597.5 357166-29-1Action and useThymidylate synthetase inhibitor; cytostatic.Ph EurDEFINITIONDisodium(2S)-2-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentanedio ate heptahydrate.Content97.5 per cent to 102.0 per cent (anhydrous substance).CHARACTERSAppearanceWhite or almost white powder.SolubilityFreely soluble in water, very slightly soluble in anhydrous ethanol, practically insoluble in methylene chloride.IDENTIFICATIONCarry out either tests A, C, D, E or tests B, C, D, E.Results The 1H NMR spectrum obtained is qualitatively similar to the 1H NMR spectrum obtainedwith pemetrexed disodium heptahydrate CRS; disregard the peak located at approximately 5.0 ppm for the comparison.C. It gives reaction (a) of sodium (2.3.1).D. Enantiomeric purity (see Tests).E. Water (see Tests).TESTSSolution SDissolve 0.56 g in carbon dioxide-free water R and dilute to 10.0 mL with the same solvent.Appearance of solutionSolution S is not more opalescent than reference suspension II (2.2.1) and not more intensely coloured than reference solution GY4 or Y4(2.2.2, Method II).pH (2.2.3)7.5 to 8.4 for solution S.Enantiomeric purityLiquid chromatography (2.2.29). Prepare the solutions immediately before use or store them at 2-8 °C for not more than 24 h.Solution A Dissolve 8 g of β-cyclodextrin R in 900 mL of water for chromatography R. Add 15 mLof triethylamine R then 6 mL of phosphoric acid R and adjust to pH 6.0 with phosphoric acid R. Dilute to 1000 mL with water for chromatography R.Test solution Dissolve 12 mg of the substance to be examined in water for chromatography R and dilute to 50.0 mL with the same solvent.Reference solution (a) Dissolve 6 mg of pemetrexed for system suitability CRS (containing impurity E) in water for chromatography R and dilute to 25.0 mL with the same solvent.Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with water for chromatography R.Dilute 3.0 mL of this solution to 10.0 mL with water for chromatography R.Column:∙—size: l = 0.25 m, Ø = 4.6 mm;∙—stationary phase: octadecylsilyl silica gel for chromatography R (5 µm) with a pore size of 12 nm; ∙—temperature: 40 °C.Mobile phase acetonitrile R, solution A (5:95 V/V).Flow rate 1.0 mL/min.Detection Spectrophotometer at 230 nm.Injection 50 µL.Run time 1.5 times the retention time of pemetrexed.Relative retention With reference to pemetrexed (retention time = about 30 min):impurity E = about 0.94.System suitability:∙—symmetry factor: maximum 2.0 for the principal peak in the chromatogram obtained with reference solution (b);∙—peak-to-valley ratio: minimum 5.0, where H p = height above the baseline of the peak due to impurity E and H v = height above the baseline of the lowest point of the curve separating this peak from the peak due to pemetrexed in the chromatogram obtained with reference solution (a).Calculation of percentage contents:∙— for impurity E, use the concentration of pemetrexed disodium heptahydrate in reference solution (b).Limit:∙—impurity E: maximum 0.3 per cent.Column rinse The following program is given for information only.Use a gradient column rinse before column storage or after 30 sample injections to avoid build-up on the column. If a drifting baseline is observed, allow additional time for equilibration with the mobile phase. If a blank chromatogram exhibits broad humps, perform a gradient column rinse.Rinsing solution A water for chromatography R.Rinsing solution B acetonitrile R1.Related substancesLiquid chromatography (2.2.29). Prepare the solutions immediately before use or store them at 2-8 °C for not more than 24 h.Solution A 1.45 g/L solution of ammonium formate R in water for chromatography R, adjusted to pH 3.5 with anhydrous formic acid R.Test solution Dissolve 20 mg of the substance to be examined in water for chromatography R and dilute to 100.0 mL with the same solvent.Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with water for chromatography R.Dilute 1.0 mL of this solution to 10.0 mL with water for chromatography R.Reference solution (b) In order to prepare impurities B and C in situ, dissolve 30 mg of the substance to be examined in 10.0 mL of a 4.0 g/L solution of sodium hydroxide R, heat at 70 °C for 40 minutes and allow to cool. Dilute 1.0 mL of the solution to 10.0 mL with water for chromatography R.Reference solution (c) Dissolve the contents of a vial of pemetrexed impurity mixture CRS (impurities A and D) in 1.0 mL of water for chromatography R.Column:∙—size: l = 0.15 m, Ø = 4.6 mm;∙—stationary phase: base-deactivated octylsilyl silica gel for chromatography R (3.5 µm).Mobile phase:∙—mobile phase A: acetonitrile R, solution A (5:95 V/V);∙—mobile phase B: acetonitrile R, solution A (30:70 V/V);Flow rate 1.0 mL/min.Detection Spectrophotometer at 250 nm.Injection 20 µL.Identification of impurities Use the chromatogram supplied with pemetrexed impurity mixture CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A and D;use the chromatogram obtained with reference solution (b) to identify the peaks due to impurities B andC.Relative retention With reference to pemetrexed (retention time = about 26 min): impurity A = about 0.82;impurity B = about 0.87; impurity C = about 0.88; impurity D = about 0.90.System suitability Reference solution (b):∙—peak-to-valley ratio: minimum 1.5, where H p = height above the baseline of the peak due to impurity B and H v = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity C.Calculation of percentage contents:∙— for each impurity, use the concentration of pemetrexed disodium heptahydrate in reference solution(a).Limits:∙—impurities A, D: for each impurity, maximum 0.15 per cent;∙—unspecified impurities: for each impurity, maximum 0.10 per cent;∙—total: maximum 0.6 per cent;∙—reporting threshold: 0.05 per cent.Heavy metals (2.4.8)Maximum 20 ppm.Solvent mixture acetone R, water R (40:60 V/V).0.250 g complies with test H. Prepare the reference solution using 0.5 mL of lead standard solution (10ppm Pb) R.Water (2.5.12)19.5 per cent to 22.1 per cent, determined on 0.050 g.Bacterial endotoxins (2.6.14)Less than 0.17 IU/mg.ASSAYLiquid chromatography (2.2.29). Prepare the solutions immediately before use or store them at 2-8 °C for not more than 24 h.Acetate buffer Mix 1.7 mL of glacial acetic acid R and 900 mL of water for chromatography R, adjust to pH 5.3 with a 760 g/L solution of sodium hydroxide R in water for chromatography R and dilute to 1000 mL with water for chromatography R.Test solution Dissolve 30.0 mg of the substance to be examined in water for chromatography R and dilute to 200.0 mL with the same solvent.Reference solution Dissolve 30.0 mg of pemetrexed disodium heptahydrate CRS in water forchromatography R and dilute to 200.0 mL with the same solvent.Column:∙—size: l = 0.15 m, Ø = 4.6 mm;∙—stationary phase: base-deactivated octylsilyl silica gel for chromatography R (3.5 µm); ∙—temperature: 30 °C.Mobile phase acetonitrile R, acetate buffer (11:89 V/V).Flow rate 2.0 mL/min.Detection Spectrophotometer at 285 nm.Injection 20 µL.Run time Twice the retention time of pemetrexed (retention time = about 3 min).Calculate the percentage content of C20H19N5Na2O6 taking into account the assigned contentof pemetrexed disodium heptahydrate CRS.IMPURITIESSpecified impurities A, D, E.Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034).It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10.Control of impurities in substances for pharmaceutical use): B, C.A.(2S)-2-[[4-[2-(2-amino-1-methyl-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino ]-pentanedioic acid,B.(2S,2′S)-2,2′-[[(5R)-2,2′-diamino-4,4′,6-trioxo-1,4,4′,6,7,7′-hexahydro-1′H,5H-5,6′-bipyrrolo[2,3-d]pyrimi dine-5,5′-diyl]bis(ethylenebenzene-4,1-diylcarbonylimino)]dipentanedioic acid,C.(2S,2′S)-2,2′-[[(5S)-2,2′-diamino-4,4′,6-trioxo-1,4,4′,6,7,7′-hexahydro-1′H,5H-5,6′-bipyrrolo[2,3-d]pyrimi dine-5,5′-diyl]bis(ethylenebenzene-4,1-diylcarbonylimino)]dipentanedioic acid,D.(2S)-2-[[(4S)-4-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]-4-carboxybutanoyl]amino]pentanedioic acid,E.(2R)-2-[[4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]amino]pentane dioic acid.Ph Eur。
2013年6月FDA批准新药

BRISDELLE (NDA #204516) HYDROXYZINE 8 PAMOATE (ANDA #201507) MORPHINE SULFATE 9 (ANDA #202104) 编 药物名称 号 7
甲磺酸帕罗西汀 扑姆酸羟嗪 硫酸吗啡 有效成分
抗抑郁药 镇静,抗焦虑 镇痛药 适应症
批准 批准 批准
仿制
口服液
草酸依地普仑
IMIPRAMINE PAMOATE 仿制 (ANDA #202338) NORETHINDRONE (ANDA #200980) NORETHINDRONE (ANDA #201483) TESTOSTERONE CYPIONATE (ANDA #201720) TRANEXAMIC ACID (ANDA #201580) WARFARIN SODIUM (ANDA #200104) LAMOTRIGINE (ANDA #200694) LAMOTRIGINE (ANDA #202887) 仿制 仿制 仿制 仿制 仿制 仿制 仿制 仿制 仿制 仿制 仿制 类型
06/18/201 3 06/18/201 3 06/26/201 3 06/27/201 3 06/27/201 3 06/28/201 3
FLUDEOXYGLUCOSE 仿制 F18 (ANDA #203811) AMMONIA N 13 (ANDA 仿制 #203812) SODIUM FLUORIDE F仿制 18 (ANDA #203605)
仿制 仿制 仿制
片剂 片剂 注射剂
利鲁唑 利鲁唑 盐酸拓扑替康
本品用于治疗影响肌肉力量的神经系统 疾病:肌萎缩侧索硬化症。 本品用于治疗影响肌肉力量的神经系统 疾病:肌萎缩侧索硬化症。 用于治疗小细胞肺癌。
2013年7月FDA批准新药
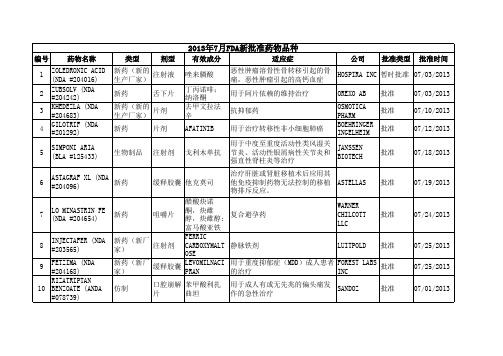
用于成人有或无先兆的偏头痛发 GLENMARK 作的急性治疗 GENERICS 用于成人有或无先兆的偏头痛发 APOTEX INC 作的急性治疗 用于成人有或无先兆的偏头痛发 AUROBINDO 作的急性治疗 PHARMA LTD 用于成人有或无先兆的偏头痛发 NATCO 作的急性治疗 PHARMA LTD 用于成人有或无先兆的偏头痛发 INVAGEN 作的急性治疗 PHARMS 用于偏头痛急性发作的治疗 APOTEX INC
42
仿制
胶囊
用于干燥综合征病人的口干症状 ROXANE 治疗。 用于治疗注意缺陷多动障碍 KUDCO IRELAND KUDCO IRELAND AUROLIFE PHARMA LLC CARACO
批准
07/08/2013
43
仿制
缓释片
批准
07/09/2013
44
仿制
缓释片
盐酸哌甲酯 硫酸右苯丙 胺 瑞格列奈 瑞格列奈 盐酸二甲双 胍
类型 仿制
剂型 片剂
有效成分 非那雄胺 去氧孕烯; 炔雌醇
适应症 用于治疗前列腺增生
公司 SUN PHARMA GLOBAL
批准类型 批准
批准时间 07/01/2013
31
仿制
片剂
避孕药
NOVAST LABS 批准 LTD WATSON LABS 批准 INC ALEMBIC PHARMS LTD ACTAVIS ELIZABETH TARO
6
ASTAGRAF XL (NDA 新药 #204096) LO MINASTRIN FE (NDA #204654) INJECTAFER (NDA #203565)
缓释胶囊 他克莫司
批准
07/19/2013
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
LevonorgestrelGeneral Notices(Ph. Eur. monograph 0926)C21H28O2 312.5 797-63-7Action and useProgestogen.PreparationsLevonorgestrel TabletsLevonorgestrel and Ethinylestradiol TabletsPh EurDEFINITION13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one.Content98.0 per cent to 102.0 per cent (dried substance).CHARACTERSAppearanceWhite or almost white, crystalline powder.SolubilityPractically insoluble in water, sparingly soluble in methylene chloride, slightly soluble in ethanol (96 per cent).IDENTIFICATIONA. Specific optical rotation (see Tests).B. Infrared absorption spectrophotometry (2.2.24).Comparison levonorgestrel CRS.TESTSSpecific optical rotation (2.2.7)-35 to -30.∙—mobile phase B: acetonitrile R1;Flow rate 0.7 mL/min.Detection Spectrophotometer at 215 nm and, for impurity O at 200 nm.Injection 50 µL.Identification of impurities Use the chromatograms supplied with levonorgestrel for system suitability CRS and the chromatograms obtained with reference solution (a) at 215 nm to identify the peaks due to impurities A, H, K, M and S, and at 200 nm to identify the peak due to impurity O; use the chromatogram obtained with reference solution (c) to identify the peak due to impurity B; use the chromatogramobtained with reference solution (d) to identify the peak due to impurity U.Relative retention With reference to levonorgestrel (retention time = about 20 min):impurity H = about 0.5; impurity U = about 0.8; impurity K = about 0.85; impurity A = about 0.91;impurity M = about 0.95; impurity O = about 1.16; impurity B = about 1.26; impurity S = about 1.9.System suitability:∙—signal-to-noise: minimum 60 for the principal peak in the chromatogram obtained with reference solution (b);∙—peak-to-valley ratio: minimum 3.0, where H p = height above the baseline of the peak due to impurity M and H v = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity A.Limits:∙—correction factors: for the calculation of content, multiply the peak areas of the following impurities by the corresponding correction factor: impurity A = 0.4; impurity M = 3.1; impurity O = 2.6; ∙—impurities A, K: for each impurity, not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);∙—impurity B: not more than 3 times the area of the corresponding peak in the chromatogram obtained with reference solution (c) (0.3 per cent);∙—impurity O at 200 nm: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);∙—impurities M, S: for each impurity, not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent);∙—impurity U: not more than twice the area of the corresponding peak in the chromatogram obtained with reference solution (d) (0.2 per cent);∙—impurity H: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);∙—unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);∙—sum of impurities other than O: maximum 1.0 per cent;∙—disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).Loss on drying (2.2.32)Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.Sulfated ash (2.4.14)Maximum 0.1 per cent, determined on 1.0 g.ASSAYDissolve 0.200 g in 45 mL of tetrahydrofuran R. Add 10 mL of a 100 g/L solution of silver nitrate R. After1 min, titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20). Carryout a blank titration.1 mL of 0.1 M sodium hydroxide is equivalent to 31.25 mg of C21H28O2.STORAGEProtected from light.IMPURITIESSpecified impurities A, B, H, K, M, O, S, U.Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use): C, D, G, I, J, L, N, P, Q, R, T.A. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregna-4,8(14)-dien-20-yn-3-one,B. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-5(10)-en-20-yn-3-one,C. 13-ethyl-3-ethynyl-18,19-dinor-17α-pregna-3,5-dien-20-yn-17-ol,D. 13-ethyl-18,19-dinor-17α-pregn-4-en-20-yn-17-ol (3-deoxolevonorgestrel),G. 13-ethyl-6α,17-dihydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one (6α-hydroxylevonorgestrel),H. 13-ethyl-6β,17-dihydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one (6β-hydroxylevonorgestrel),I. 13-ethyl-10,17-dihydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one (10-hydroxylevonorgestrel),J. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yne-3,6-dione (6-oxolevonorgestrel),K. 13-ethyl-17β-hydroxygon-4-en-3-one (18-methylnandrolone),L. 13-ethylgon-4-ene-3,17-dione (levodione),M. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregna-4,6-dien-20-yn-3-one (Δ6-levonorgestrel),N. 13-ethylgon-5(10)-ene-3,17-dione (Δ5(10)-levodione),O. 13-ethyl-17-hydroxy-5α-methoxy-18,19-dinor-17α-pregn-20-yn-3-one (4,5-dihydro-5α-methoxylevonorgestrel),P. 13-ethyl-17-hydroxy-18,19-dinor-17α-pregn-5-en-20-yn-3-one (Δ5-levonorgestrel),Q. 13-ethyl-3-methoxygona-2,5(10)-dien-17β-ol,R. 13-ethyl-3-methoxygona-2,5(10)-dien-17-one,S. 13-ethyl-3-methoxy-18,19-dinor-17α-pregna-3,5-dien-20-yn-17-ol,T. 13-ethyl-3-methoxy-18,19-dinor-17α-pregna-2,5(10)-dien-20-yn-17-ol,U. 17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one (norethisterone).。
