国外对食品酶制剂的要求概要
饲料用酶制剂概况.doc

饲料用酶制剂概况概述:酶制剂是由微生物产生的生物制品。
自从1975年美国饲料工业首次把酶制剂作为添加剂应用于配合饲料中并取得显著效果后,饲用酶制剂日益受到世界养殖业的重视。
随着畜牧业的发展,抗生素、激素和药物类添加剂大量应用于饲料中,动物食品污染和有害物质残留日益加重,饲料安全问题日益突出。
目前,许多国家都在努力加大饲料添加剂的管理,西欧、日本、美国等国家相继颁布了一系列法律,在饲料中禁止或限制使用抗生素、激素和药物类添加剂。
“天然、绿色、无污染、无残留”成为21世纪世界畜牧业发展的主题。
酶制剂作为一类高效、无毒副作用和环保的“绿色”饲料添加剂在畜禽养殖业中具有广阔的应用前景,正在逐步替代常用药物类添加剂,实现添加剂“绿色化”。
一、饲用酶制剂的分类酶是一种由活细胞产生的具有生物催化反应能力的蛋白质,在动物体内消化与新陈代谢过程中起着重要的作用。
根据饲料中所含酶的种类,饲料用酶制剂主要可分为两类消化性酶和非消化性酶。
(1)消化性酶:饲料中常用的消化性酶制剂有α-淀粉酶、糖化酶、酸性蛋白酶和中性蛋白酶,主要辅助动物消化道酶系作用,降解淀粉和蛋白质成为易被吸收的小分子物质。
(2)非消化性酶:主要包括木聚糖酶、果胶酶、甘露聚糖酶、β-葡聚糖酶、纤维素酶等非淀粉多糖酶和植酸酶。
非淀粉多糖酶通过破坏植物细胞壁,分解纤维素、半纤维素和果胶等非淀粉多糖(NSP),既把这些不可利用的多糖分解成可被消化吸收的小分子糖类,又可以暴露细胞壁保护的淀粉、蛋白等,使其更充分吸收利用,同时降低因可溶NSP造成的粘稠食糜的粘度。
植酸酶催化植酸盐的水解反应,使其中的磷以无机磷的形式游离出来,提高饲料中磷和其它养分的利用率。
根据产品中所含酶的种类,饲用酶制剂一般分为饲用单一酶制剂和饲用复合酶制剂。
目前市场上的商品饲用酶制剂大多数以复合酶制剂的形式销售,如溢多酶、保安生等。
一般来说,复合酶制剂比单一酶制剂效果好,但并不意味着复合酶制剂中酶种类愈多愈好。
美国、欧盟、日本食品添加使用

美国、欧盟、日本食品添加剂的监管一、美国食品添加剂管理美国食品和药品管理法规第201款规定,食品添加剂是直接或间接进入食品并成为食品一部分的任何物质。
所谓直接食品添加剂,是指直接加入到食品中的物质。
所谓间接食品添加剂,是指包装材料或其他与食品接触的物质,在合理的预期下,转移到食品中的物质。
根据这个定义,食品配料也是食品添加剂的一部分,这是美国与大多数国家对食品添加剂定义的不同之处。
美国法律规定,由FDA(食品药物管理局)直接参与食品添加剂法规的制定和管理。
因肉类由美国农业部(USDA)管理,用于肉和家禽制品的添加剂需得到FDA和USDA双方的认证;而酒和烟由酒烟草税和贸易局(TTB)管理,用于酒、烟的食品添加剂也实行双重管理。
食品添加剂立法的基础工作往往由相应的协会承担。
如食品香精立法的基础工作由FEMA(美国食品香料和萃取物制造者协会)担任,其安全评价结果得到FDA认可后,以肯定的形式公布,并冠以GRAS (一般公认安全)的FEMA号码。
随着科技进步和毒理学资料的积累,以及现代分析技术的提高,每隔若干年后,食品添加剂的安全性会被重新评价和公布。
美国食品和药品管理法第402款规定,只有经过评价和公布的食品添加剂才能生产和应用,否则会被认定为不安全。
含有不安全食品添加剂的食品则“不宜食用”,不宜食用的食品禁止销售。
美国规定,食品中公认为可安全使用的物质不属于食品添加剂范畴,但对这类物质的使用也实行严格管理。
FDA已推行一项新的公认安全物质的通报系统,即由生产企业向FDA提交其产品,根据其用途属于公认安全物质的报告,FDA在一定时间内(通常为180天),向申请人发信确认或否认申请的物质的公认安全性。
二、欧盟食品添加剂管理由于欧盟是一个统一的市场,因此食品添加剂产品一旦进入某一欧盟成员国市场,原则上即可在其他成员国自由销售。
和其他国家相同,欧盟也有专项法规对食品添加剂进行管理。
欧盟食品添加剂的使用原则是食品中只能含有欧盟允许使用的食品添加剂和成员国允许使用的香料,即使用食品添加剂必须符合欧盟的相关规定和一般卫生法规的要求。
【世卫标准】美国FDA的食品添加剂使用卫生标准

[Code of Federal Regulations][Title 21, Volume 3][Revised as of April 1, 2006][CITE: 21CFR171]TITLE 21--FOOD AND DRUGSCHAPTER I--FOOD AND DRUG ADMINISTRATIONDEPARTMENT OF HEALTH AND HUMAN SERVICESSUBCHAPTER B--FOOD FOR HUMAN CONSUMPTION (CONTINUED) PART 171 FOOD ADDITIVE PETITIONSSubpart A--General ProvisionsSec. 171.1 Petitions.(a) Petitions to be filed with the Commissioner under the provisions of section 409(b) of the Federal Food, Drug, and Cosmetic Act (the act) shall be submitted in triplicate (quadruplicate, if intended uses include use in meat, meat food product, or poultry product). If any part of the material submitted is in a foreign language, it shall be accompanied by an accurate and complete English translation. The petition shall state petitioner's post office address to which published notices or orders issued or objections filed pursuant to section 409 of the Act may be sent.(b) Pertinent information may be incorporated in, and will be considered as part of, a petition on the basis of specific reference to such information submitted to and retained in the files of the Food and Drug Administration. However, any reference to unpublished information furnished by a person other than the applicant will not be considered unless use of such information is authorized in a written statement signed by the person who submitted it. Any reference to published information offered in support of a food additive petition should be accompanied by reprints or photostatic copies of such references.(c) Petitions shall include the following data and be submitted in the following form:(Date)Name of petitionerPost-office addressDateName of food additive and proposed use______________________________________________________________Petitions Control BranchFood and Drug AdministrationDepartment of Health and Human ServicesWashington, DC 20204.Dear Sirs:The undersigned, _____ submits this petition pursuant to section 409(b)(1) of the Federal Food, Drug, and Cosmetic Act with respect to _____(Name of the food additive and proposed use)Attached hereto, in triplicate (quadruplicate, if intended uses include use in meat, meat food product, or poultry product), and constituting a part of this petition are the following: A. The name and all pertinent information concerning the food additive, including chemical identity and composition of the food additive, its physical, chemical, and biological properties, and specifications prescribing the minimum content of the desired component(s) and identifying and limiting the reaction byproducts and other impurities. Where such information is not available, a statement as to the reasons why it is not should be submitted.When the chemical identity and composition of the food additive is not known, the petition shall contain information in sufficient detail to permit evaluation regarding the method of manufacture and the analytical controls used during the various stages of manufacturing, processing, or packing of the food additive which are relied upon to establish that it is a substance of reproducible composition. Alternative methods and controls and variations in methods and controls within reasonable limits that do not affect the characteristics of the substance or the reliability of the controls may be specified.If the food additive is a mixture of chemicals, the petition shall supply a list of all substances used in the synthesis, extraction, or other method of preparation, regardless of whether they undergo chemical change in the process. Each substance should be identified by its common English name and complete chemical name, using structural formulas when necessary for specific identification. If any proprietary preparation is used as a component, the proprietary name should be followed by a complete quantitative statement of composition. Reasonable alternatives for any listed substance may be specified.If the petitioner does not himself perform all the manufacturing, processing, and packing operations for a food additive, the petition shall identify each person who will perform a part of such operations and designate the part.The petition shall include stability data, and, if the data indicate that it is needed to insure the identity, strength, quality, or purity of the additive, the expiration date that will be employed.B. The amount of the food additive proposed for use and the purposes for which it is proposed, together with all directions, recommendations, and suggestions regarding the proposed use, as well as specimens of the labeling proposed for the food additive and any labeling that will be required by applicable provisions of the Federal Food, Drug, and Cosmetic Act on the finished food by reason of the use of the food additive. If the additive results or may reasonably be expected to result from the use of packaging material, the petitioner shall show how this may occur and what residues may reasonably be anticipated.(Typewritten or other draft-labeling copy will be accepted for consideration of the petition, provided a statement is made that final printed labeling identical in content to the draft copy will be submitted as soon as available and prior to the marketing of the food additive.)(If the food additive is one for which a tolerance limitation is required to assure its safety, the level of use proposed should be no higher than the amount reasonably required to accomplish the intended physical or other technical effect, even though the safety data may support a higher tolerance.)C. Data establishing that the food additive will have the intended physical or other technical effect or that it may reasonably be expected to become a component, or to affect the characteristics, directly or indirectly, of food and the amount necessary to accomplish this. These data should include information in sufficient detail to permit evaluation with control data.D. A description of practicable methods to determine the amount of the food additive in the raw, processed, and/or finished food and of any substance formed in or on such food because of its use. The test proposed shall be one that can be used for food-control purposes and that can be applied with consistent results by any properly equipped and trained laboratory personnel.E. Full reports of investigations made with respect to the safety of the food additive.(A petition may be regarded as incomplete unless it includes full reports of adequate tests reasonably applicable to show whether or not the food additive will be safe for its intended use. The reports ordinarily should include detailed data derived from appropriate animal and other biological experiments in which the methods used and the resultsobtained are clearly set forth. The petition shall not omit without explanation any reports of investigations that would bias an evaluation of the safety of the food additive.)F. Proposed tolerances for the food additive, if tolerances are required in order to insure its safety. A petitioner may include a proposed regulation.G. If submitting petition to modify an existing regulation issued pursuant to section409(c)(1)(A) of the Act, full information on each proposed change that is to be made in the original regulation must be submitted. The petition may omit statements made in the original petition concerning which no change is proposed. A supplemental petition must be submitted for any change beyond the variations provided for in the original petition and the regulation issued on the basis of the original petition.H. The petitioner is required to submit either a claim for categorical exclusion under25.30 or 25.32 of this chapter or an environmental assessment under 25.40 of this chapter. Yours very truly,PetitionerBy(Indicate authority)(d) The petitioner will be notified of the date on which his petition is filed; and an incomplete petition, or one that has not been submitted in triplicate, will usually be retained but not filed as a petition under section 409 of the Act. The petitioner will be notified in what respects his petition is incomplete.(e) The petition must be signed by the petitioner or by his attorney or agent, or (if a corporation) by an authorized official.(f) The data specified under the several lettered headings should be submitted on separate sheets or sets of sheets, suitably identified. If such data have already been submitted with an earlier application, the present petition may incorporate it by specific reference to the earlier. If part of the data have been submitted by the manufacturer of the food additive as a master file, the petitioner may refer to the master file if and to the extent he obtains the manufacturer's written permission to do so. The manufacturer may authorize specific reference to the data without disclosure to the petitioner. Nothing herein shall prevent reference to published data.(g) A petition shall be retained but shall not be filed if any of the data prescribed by section 409(b) of the Act are lacking or are not set forth so as to be readily understood. (h)(1) The following data and information in a food additive petition are available for public disclosure, unless extraordinary circumstances are shown, after the notice of filingof the petition is published in the Federal Register or, if the petition is not promptly filed because of deficiencies in it, after the petitioner is informed that it will not be filed because of the deficiencies involved:(i) All safety and functionality data and information submitted with or incorporated by reference in the petition.(ii) A protocol for a test or study, unless it is shown to fall within the exemption established for trade secrets and confidential commercial information in 20.61 of this chapter.(iii) Adverse reaction reports, product experience reports, consumer complaints, and other similar data and information, after deletion of:(a) Names and any information that would identify the person using the product.(b) Names and any information that would identify any third party involved with the report, such as a physician or hospital or other institution.(iv) A list of all ingredients contained in a food additive, whether or not it is in descending order of predominance. A particular ingredient or group of ingredients shall be deleted from any such list prior to public disclosure if it is shown to fall within the exemption established in 20.61 of this chapter, and a notation shall be made that any such ingredient list is incomplete.(v) An assay method or other analytical method, unless it serves no regulatory or compliance purpose and is shown to fall within the exemption established in 20.61 of this chapter.(2) The following data and information in a food additive petition are not available for public disclosure unless they have been previously disclosed to the public as defined in 20.81 of this chapter or they relate to a product or ingredient that has been abandoned and they no longer represent a trade secret or confidential commercial or financial information as defined in 20.61 of this chapter:(i) Manufacturing methods or processes, including quality control procedures.(ii) Production, sales, distribution, and similar data and information, except that any compilation of such data and information aggregated and prepared in a way that does not reveal data or information which is not available for public disclosure under this provision is available for public disclosure.(iii) Quantitative or semiquantitative formulas.(3) All correspondence and written summaries of oral discussions relating to a food additive petition are available for public disclosure in accordance with the provisions ofpart 20 of this chapter when the food additive regulation is published in the Federal Register.(4) For purposes of this regulation, safety and functionality data include all studies and tests of a food additive on animals and humans and all studies and tests on a food additive for identity, stability, purity, potency, performance, and usefulness.(i)(1)(i) Within 15 days after receipt, the Food and Drug Administration will notify the petitioner of the acceptance or nonacceptance of a petition, and if not accepted, the reasons therefor. If accepted, the petitioner will be sent a letter stating this and the date of the letter shall become the date of filing for the purposes of section 409(b)(5) of the act. In cases in which the Food and Drug Administration agrees that a premarket notification for a food contact substance (Food Contact Notification (FCN)) submitted under section 409(h) of the act may be converted to a petition, the withdrawal date for the FCN will be deemed the date of receipt for the petition.(ii) If the petitioner desires, he may supplement a deficient petition after being notified regarding deficiencies. If the supplementary material or explanation of the petition is deemed acceptable, the petitioner shall be notified. The date of such notification becomes the date of filing. If the petitioner does not wish to supplement or explain the petition and requests in writing that it be filed as submitted, the petition shall be filed and the petitioner so notified.(iii) Notwithstanding paragraph (i)(1)(ii) of this section, the petition shall not be filed if the Food and Drug Administration determines that the use identified in the petition should be the subject of an FCN under section 409(h) of the act rather than a petition. (2) The Commissioner will publish in the Federal Register within 30 days from the date of filing of such petition, a notice of the filing, the name of the petitioner, and a brief description of the proposal in general terms. In the case of a food additive which becomes a component of food by migration from packaging material, the notice shall include the name of the migratory substance, and where it is different from that of one of the original components, the name of the parent component, the maximum quantity of the migratory substance that is proposed for use in food, and the physical or other technical effect which the migratory substance or its parent component is intended to have in the packaging material. A copy of the notice will be mailed to the petitioner when the original is forwarded to the Federal Register for publication.(j) The Commissioner may request a full description of the methods used in, and the facilities and controls used for, the production of the food additive, or a sample of the food additive, articles used as components thereof, or of the food in which the additive is proposed to be used, at any time while a petition is under consideration. The Commissioner shall specify in the request for a sample of the food additive, or articles used as components thereof, or of the food in or on which the additive is proposed to be used, a quantity deemed adequate to permit tests of analytical methods to determine quantities of the food additive present in foods for which it is intended to be used oradequate for any study or investigation reasonably required with respect to the safety of the food additive or the physical or technical effect it produces. The date used for computing the 90-day limit for the purposes of section 409(c)(2) of the Act shall be moved forward 1 day for each day after the mailing date of the request taken by the petitioner to submit the sample. If the information or sample is requested a reasonable time in advance of the 180 days, but is not submitted within such 180 days after filing of the petition, the petition will be considered withdrawn without prejudice.(k) If nonclinical laboratory studies are involved, petitions filed with the Commissioner under section 409(b) of the act shall include, with respect to each nonclinical study contained in the petition, either a statement that the study has been, or will be, conducted in compliance with the good laboratory practice regulations as set forth in part 58 of this chapter, or, if any such study was not conducted in compliance with such regulations, a brief statement of the reason for the noncompliance.(l) [Reserved](m) If clinical investigations involving human subjects are involved, petitions filed with the Commissioner under section 409(b) of the Act shall include statements regarding each such clinical investigation relied upon in the petition that it either was conducted in compliance with the requirements for institutional review set forth in part 56 of this chapter, or was not subject to such requirements in accordance with 56.104 or 56.105, and that it was conducted in compliance with the requirements for informed consent set forth in part 50 of this chapter.(n)(1) If intended uses of the food additive include uses in meat, meat food product, or poultry product subject to regulation by the U.S. Department of Agriculture (USDA) under the Poultry Products Inspection Act (PPIA) (21 U.S.C. 451 et seq.) or the Federal Meat Inspection Act (FMIA) (21 U.S.C. 601 et seq.), FDA shall, upon filing of the petition, forward a copy of the petition or relevant portions thereof to the Food Safety and Inspection Service, USDA, for simultaneous review under the PPIA and FMIA.(2) FDA will ask USDA to advise whether the proposed meat and poultry uses comply with the FMIA and PPIA, or if not, whether use of the substance would be permitted in products under USDA jurisdiction under specified conditions or restrictions.[42 FR 14489, Mar. 15, 1977, as amended at 42 FR 15674, Mar. 22, 1977; 46 FR 8952, Jan. 27, 1981; 50 FR 7492, Feb. 22, 1985; 50 16668, Apr. 26, 1985; 62 FR 40599, July 29, 1997; 65 FR 51763, Aug. 25, 2000; 67 FR 35731, May 21, 2002]Effective Date Note:At 65 FR 51763, Aug. 25, 2000, 171.1 was amended in paragraph (a) by revising the first sentence, in paragraph (c) in the petition by revising the introductory paragraph preceding paragraph A., and by adding paragraph (n). The revised and added text containsinformation collection and recordkeeping requirements and will not become effective until approval has been given by the Office of Management and Budget.Sec. 171.6 Amendment of petition.After a petition has been filed, the petitioner may submit additional information or data in support thereof. In such cases, if the Commissioner determines that the additional information or data amount to a substantive amendment, the petition as amended will be given a new filing date, and the time limitation will begin to run anew. If nonclinical laboratory studies are involved, additional information and data submitted in support of filed petitions shall include, with respect to each nonclinical study, either a statement that the study was conducted in compliance with the requirements set forth in part 58 of this chapter, or, if the study was not conducted in compliance with such regulations, a brief statement of the reason for the noncompliance.[50 FR 7492, Feb. 22, 1985, as amended at 50 16668, Apr. 26, 1985]Sec. 171.7 Withdrawal of petition without prejudice.(a) In some cases the Commissioner will notify the petitioner that the petition, while technically complete, is inadequate to justify the establishment of a regulation or the regulation requested by petitioner. This may be due to the fact that the data are not sufficiently clear or complete. In such cases, the petitioner may withdraw the petition pending its clarification or the obtaining of additional data. This withdrawal will be without prejudice to a future filing. Upon refiling, the time limitation will begin to run anew from the date of refiling.(b) At any time before the order provided for in 171.100(a) has been forwarded to the Federal Register for publication, the petitioner may withdraw the petition without prejudice to a future filing. Upon refiling the time limitation will begin to run anew. (c) Any petitioner who has a food additive petition pending before the agency and who subsequently submits a premarket notification for a food contact substance (FCN) for a use or uses described in such petition shall be deemed to have withdrawn the petition for such use or uses without prejudice to a future filing on the date the FCN is received by the Food and Drug Administration.[42 FR 14489, Mar. 15, 1977, as amended at 67 FR 35731, May 21, 2002]Sec. 171.8 Threshold of regulation for substances used in food-contact articles. Substances used in food-contact articles (e.g., food-packaging or food-processing equipment) that migrate or that may be expected to migrate into food at negligible levels may be reviewed under 170.39 of this chapter. The Food and Drug Administration will exempt substances whose uses it determines meet the criteria in 170.39 of this chapter from regulation as food additives and, therefore, a food additive petition will not be required for the exempted use.[60 FR 36596, July 17, 1995]Subpart B--Administrative Actions on ApplicationsSec. 171.100 Regulation based on petition.(a) The Commissioner will forward for publication in the Federal Register, within 90 days after filing of the petition (or within 180 days if the time is extended as provided for in section 409(c)(2) of the Act), a regulation prescribing the conditions under which the food additive may be safely used (including, but not limited to, specifications as to the particular food or classes of food in or on which such additive may be used, the maximum quantity that may be used or permitted to remain in or on such food, the manner in which such additive may be added to or used in or on such food, and any directions or other labeling or packaging requirements for such additive deemed necessary by him to assure the safety of such use), and prior to the forwarding of the order to the Federal Register for publication shall notify the petitioner of such order and the reasons for such action; or by order deny the petition, and shall notify the petitioner of such order and of the reasons for such action.(b) The regulation shall describe the conditions under which the substance may be safely used in any meat product, meat food product, or poultry product subject to the Federal Meat Inspection Act (FMIA) (21 U.S.C. 601 et seq.) or the Poultry Products Inspection Act (PPIA) (21 U.S.C. 451 et seq.).(c) If the Commissioner determines that additional time is needed to study and investigate the petition, he shall by written notice to the petitioner extend the 90-day period for not more than 180 days after the filing of the petition.[42 FR 14489, Mar. 15, 1977, as amended at 65 FR 51763, Aug. 25, 2000]Sec. 171.102 Effective date of regulation.A regulation published in accordance with 171.100(a) shall become effective upon publication in the Federal Register.Sec. 171.110 Procedure for objections and hearings.Objections and hearings relating to food additive regulations under section 409 (c), (d), or(h) of the Act shall be governed by part 12 of this chapter.[42 FR 14491, Mar. 15, 1977, as amended at 42 FR 15674, Mar. 22, 1977]Sec. 171.130 Procedure for amending and repealing tolerances or exemptions from tolerances.(a) The Commissioner, on his own initiative or on the petition of any interested person, pursuant to part 10 of this chapter, may propose the issuance of a regulation amending or repealing a regulation pertaining to a food additive or granting or repealing an exception for such additive.(b) Any such petition shall include an assertion of facts, supported by data, showing that new information exists with respect to the food additive or that new uses have been developed or old uses abandoned, that new data are available as to toxicity of the chemical, or that experience with the existing regulation or exemption may justify its amendment or repeal. New data shall be furnished in the form specified in 171.1 and 171.100 for submitting petitions.[42 FR 14491, Mar. 15, 1977, as amended at 42 FR 15674, Mar. 22, 1977] Authority: 21 U.S.C. 321, 342, 348, 371.Source: 42 FR 14489, Mar. 15, 1977, unless otherwise noted.。
食品用酶制剂新品种
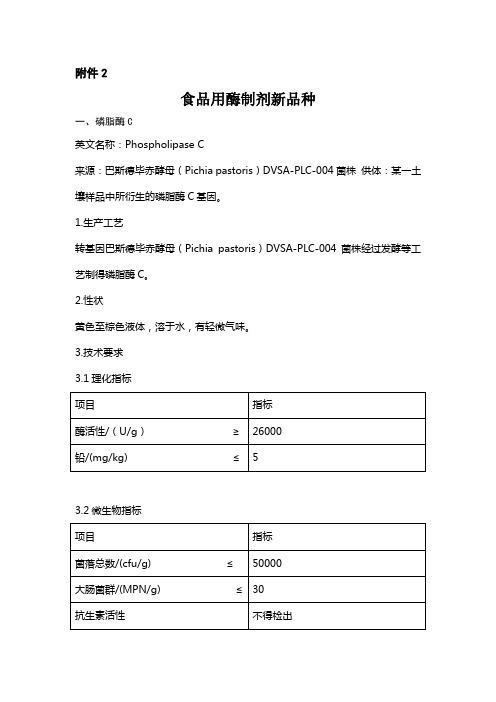
附件2食品用酶制剂新品种一、磷脂酶C英文名称:Phospholipase C来源:巴斯德毕赤酵母(Pichia pastoris)DVSA-PLC-004菌株供体:某一土壤样品中所衍生的磷脂酶C基因。
1.生产工艺转基因巴斯德毕赤酵母(Pichia pastoris)DVSA-PLC-004菌株经过发酵等工艺制得磷脂酶C。
2.性状黄色至棕色液体,溶于水,有轻微气味。
3.技术要求3.1理化指标二、谷氨酰胺酶英文名称:Glutaminase来源:解淀粉芽孢杆菌Bacillus amyloliquefaciens1.生产工艺解淀粉芽孢杆菌Bacillus amyloliquefaciens经培养,深层发酵、提取、多次过滤和纯化精制而成的谷氨酰胺酶。
2.性状淡棕色粉末,具有特有的气味、无异味。
在高温和酸性条件下稳定。
3.技术要求3.1理化指标三、天门冬酰胺酶英文名称:Asparaginase来源:黑曲霉Aspergillus niger,供体:黑曲霉Aspergillus niger1.生产工艺以筛选的重组黑曲霉在严格控制的条件下进行发酵制备而成的天门冬酰胺酶。
2.性状天门冬酰胺酶为浅黄色至褐色液体,或淡黄色至深黄色颗粒或粉末。
3.技术要求3.1理化指标备注:酶活性规格除以上规定外,还可按实际需要执行。
四、天门冬酰胺酶英文名称:Asparaginase来源:米曲霉Aspergillus oryzae;供体:米曲霉Aspergillus oryzae1.生产工艺以转基因米曲霉生产菌在严格控制的条件下进行液体深层发酵制备而成的天门冬酰胺酶。
2.性状浅褐色液体,产品颜色随批次不同略有不同。
3.技术要求3.1理化指标备注:酶活性规格除以上规定外,还可按实际需要执行。
五、果胶酯酶英文名称:Pectinesterase来源:米曲霉Aspergillus oryzae;供体:针尾曲霉Aspergillus aculeatus 1.生产工艺以转基因米曲霉生产菌在严格控制的条件下进行液体深层发酵制备而成的果胶酯酶。
国外对食品酶制剂的要求

美国
• 美国对食品用酶制剂的管理体现在其联邦 法规(Code Federal Regulation,CFR)、食品 化学法典(Food chemicals Codex,FCC)中。 • 在FCC第5版中对食品用酶制剂的活性、有 害物质的允许含量、实验方法等通用要求 作了规定,并要求食品用酶制剂的生产应遵 循GMP. • 美国FDA(Food and Drug Administration)负责 对食品用酶的安全性评价。
• 5 危险管理数据 • 食品安全风险评估,指对食品、食品添加 剂中生物性、化学性和物理性危害对人体 健康可能造成的不良影响所进行的科学评 估,包括危害识别、危害特征描述、暴露 评估、风险特征描述等。
• 风险评估四个步骤:
危害识别
危害特征描述
暴露评估
风险特征描述
• 1960年美国规定食品中不得加入致癌物, 进而提出零阙值理论,渐渐零阙值理论发 展成在一定概率条件下可接受风险的概念H J,后来衍变为食品中每日允许最大摄人量 (Acceptable Daily Intakes,ADI),当人接触 量低于或等于ADI值时认为是可接受风险. • 膳食暴露评估主要有3种模型:点评估模型、 简单分布模型、概率评估模
中 国
• 中国于1981年在原卫生部内部试行标 准的基础上正式公布了强制性国家标 准GB 2760-81《食品添加剂使用卫生 标准》,食品用酶制剂是其中的一部 分.
国际食品法典委员会(CAC)
• 按照CAC的相关定义,食品用酶制剂一般被 划为“加工助剂”的范畴。 • 加工助剂是指任何在原料、食品或食品配 料的加工过程中有意使用的以达到在食品 加工或者处理过程中的某种功能目的、但 其本身不作为食品配料食用的物质或原料。 • 加工助剂的使用可能会导致终产品中存在 无意的、不可避免的残留物或者衍生物。
酶制剂在食品中的应用现状与展望

河南质量工程职业学院毕业设计(论文)酶制剂在食品中的应用现状与展望系别:食品与化工系专业:农产品质量与检测班级: 11级农检学生姓名:***指导教师:***完成日期:2014-3-25摘要酶制剂在现代食品保鲜中取得了重要的地位,酶制剂在食品中越来越重要,在文章中对酶制剂的安全性和可发展性做了具体的讲解,酶制剂在低温肉制品中的保鲜和脱糖保鲜的应用,酶制剂可替代部分传统食品添加剂,同时也降低食品毒性。
关键词酶制剂;食品保鲜;发展前景;AbstractEnzymes made a important position in modern food preservation, enzymes in food more and more important, in the article to the safety of the enzyme preparation and evolvability, the interpretation of specific enzymes in the application of low temperature meat product in the preservation and sugar off, replacing part of the traditional food additive enzyme preparation at the same time improve food toxicity.Key words:Enzyme preparation;Food preservation;Prospects for development;目录前言 (1)1 酶的概念及功能特性 (1)1.1酶制剂的概念 (1)1.2 酶制剂用于食品保鲜的优点 (1)2 酶制剂在食品保鲜中的应用 (2)2.1 溶菌酶 (2)2.1.1 乳制品的保鲜与强化 (2)2.1.2在低温肉制品中的保鲜 (2)2.1.3 低浓度酿造酒的保鲜 (3)2.1.4 水产品的保鲜 (3)2.1.5其他食品的保鲜 (3)2.2木瓜蛋白酶在啤酒方面的应用 (3)2.3菠萝蛋白酶在食品中的的应用 (4)2.4 葡萄糖氧化酶 (4)2.4.1脱糖保鲜 (4)2.4.2 防止食品氧化 (4)3 酶制剂的发展前景 (5)3.1关于酶制剂的安全性 (5)3.2酶在食品加工中的应用前景 (6)3.2.1 取代部分传统食品添加剂 (6)3.2.2 降低食品毒性 (7)参考文献 (8)致谢 (9)前言酶是生物细胞原生质合成的具有高度催化活性的蛋白质,因其来源于生物,通常被称作“生物催化剂”。
食品中添加酶制剂的应用与研究
食品中添加酶制剂的应用与研究食品是人们日常生活中必不可少的一部分,而酶制剂作为食品加工中的一个重要组成部分,在食品加工和改良中起着至关重要的作用。
酶制剂的应用使得食品更容易消化吸收,提高食品的风味和质量。
在这篇文章中,我们将探讨食品中添加酶制剂的应用和研究进展。
首先,让我们了解什么是酶制剂。
酶制剂是一种通过生物技术手段获得的酶,它们能够在特定的条件下促进食品中特定的化学反应。
酶制剂可以提高食品加工的效率,减少生产成本。
在面包、啤酒、酸奶等食品的制作过程中,酶制剂可以发挥重要作用。
其次,酶制剂在食品加工中的应用主要体现在中性蛋白酶、淀粉酶和果胶酶等方面。
中性蛋白酶是一种能够降解食品中的蛋白质的酶制剂,它能够在食品加工中改善蛋白质的溶解度和可溶性。
淀粉酶则可以将淀粉分解为糖类,提高食品的甜味和可口度。
而果胶酶则可以分解果胶分子,使食品的口感更加细腻。
在食品加工过程中,酶制剂可以提高食品的品质和营养价值。
例如,酶制剂可以使面包更加松软可口。
在啤酒酿造中,酶制剂可以加速淀粉酶的降解,提高酿造效率。
此外,酶制剂还可以被用来改善奶制品的口感和质地。
通过添加酶制剂,奶制品可以更容易消化吸收,提供更多的营养给人体。
除了应用,酶制剂的研究也是食品科学领域的重要方向之一。
随着科技的发展,人们对酶制剂的研究已取得了一定的成果。
例如,近年来,研究人员在利用酶制剂改良食品质量的过程中,发现了一些新型酶制剂,如转化酶和修饰酶。
这些新型酶制剂具有更高的活性和更大的应用潜力,为食品加工和改良提供了新的思路。
此外,关于酶制剂的研究还涉及到酶的安全性和稳定性。
酶制剂在食品中的应用需要考虑其对人体健康的影响。
因此,研究人员对酶制剂的毒性、稳定性和合适的使用方法进行了深入研究和探讨。
他们通过实验和观察,不断优化酶制剂的配方和使用方式,以确保其在食品加工中的安全性和有效性。
食品中添加酶制剂的应用和研究虽然取得了一定的成果,但仍然面临一些挑战和限制。
酶制剂在食品工业中的应用 论文
酶制剂在食品工业中的应用摘要:酶制剂是一类特殊的食品添加剂,具有催化高效性,专一性等显著特点。
文章综述了食品工业中酶制剂利用及新动向,包括淀粉糖、油脂、蛋白质加工、面包、啤酒、饮料工业以及改善苦味的酶类的应用。
并介绍了酶与食品的关系、酶制剂在食品生产中用于保藏、改善质量和增加营养价值、增加品种种类、提高便捷性和提高食品生产效率等作用。
并对酶制剂在食品工业中的发展方向和安全问题进行了讨论。
关键词:酶制剂;食品工业;应用酶是一类具有专一性生物催化能力的蛋白质。
而从生物体中提取的具有酶活力的制品,称为酶制剂。
酶制剂主要用于食品加工和制造业方面,它在对提高食品生产效率和产量、改进产品风味和质量等方面有着其它催化剂所无法替代的作用。
另外,酶制剂在日化、纺织、环境保护和饲料等行业也有着较广泛的应用。
随着发酵工业的发展,酶制剂的主要来源已被微生物所取代,它具有不受季节、地区和数量等因素影响的特性,还具有种类多、繁殖快、质量稳定和成本低等特点。
随着微生物育种技术的发展,酶制剂的种类越来越多,分类也越来越细。
目前我国已工业化生产的、且用于食品工业的酶制剂主要有:淀粉酶、异淀粉酶、果胶酶和蛋白酶等,它们在食品加工中都起着十分重要的作用。
当然,尽管目前我国酶制剂行业的发展已有了长足进步,但与发达国家相比,还有很大差距。
为进一步加快酶制剂产业技术的进步,今后应注重在调整产品结构、增加新品种、提高产品质量和竞争力、实现规模化经营和拓宽应用领域等方面作深入的研究。
1.酶与食品的关系在食品生产加工中,为了保持食物原有的色、香、味和结构,就要尽量避免引起剧烈的化学反应。
酶是一类具有专一性生物催化能力的蛋白质,因此作用条件非常温和。
许多酶所催化的反应从动植物最初生长时就开始了,当它被作为食品时,其体内酶的催化作用仍然继续进行着。
如动物体死后,其合成代谢停止,而分解代谢加快,因此就会导致组织腐败,但这可能也会改善某些食品原料的风味。
食品中的酶制剂.
酶生物 传感器
2.在发酵工业的应用
(1) 利用酶制剂制备工业原料 以粗淀粉为原料,用淀粉酶发酵糖液,这种发酵糖液可代工 业用葡萄糖。酶制剂还用于发酵生产白酒、酒精,氨基酸和 抗生素等。味精发酵是以酸水解淀粉为原料,沈阳味精厂以 淀粉酶和糖化酶水解玉米淀粉为原料,用于50t罐进行味精发 酵,结果大幅度提高了原料的利用率和味精的产率。 (2) 酶法生产核苷酸 用桔青霉或产黄青霉产生的5’一磷酸二酯酶水解核糖核酸 (RNA)生产各种5’一核苷酸。用核苷磷酸化酶,可催化肌肝 进行磷酸化生成5’一肌苷酸,催化鸟苷可生产5’一鸟苷酸。 用腺苷酸脱氨酶水解AMP,脱氨基后生成肌苷酸(IMP) (3)其它产品 酶制剂还用于酶法生产有机酸、氨基酸,同时可用酶法制酱 及生产加酶饲料等.
4 、酶 制剂工 业发展 趋向垄 断化 3、 不 断拓宽 酶制剂 的新用 途
酶制剂市场竞争日趋激烈, 各大公司收购兼并重组,世界 上具有一定规模的酶制剂企 业已由20世纪80年代初80 多家减少到90年代中期的 20多家。
国外酶制剂公司研究开发经 费一般占产品销售额的10 2 、 %~15%。由于经费充足, 大力研 科研力量雄厚,早已把基因 制、开 工程、蛋白质工程等现代生 发了新 物技术用于产酶菌种的改良、 酶种 新型酶开发。 。 在工业酶制剂市场,长期以来水 解酶类一直处于主导地位,约占 市场销售总额的75%以上,而目 前也注意开发非水解酶类,特别 是氧化还原酶类,它们所占市场 份额不断扩大
3 .在医疗领域的应用
(1) 助消化剂 如胃酶、胰酶、纤维素酶、脂肪酶,可用作消化剂,治 疗消化不良、食欲不振等症状。 (2) 消炎剂 酸性蛋白酶、胰糜蛋白酶、菠萝蛋白酶等蛋白酶制剂, 可作为消炎剂治疗各种炎症,如血栓性静脉炎、慢性支气管 炎等。 (3) 溶茵酶 它具有抗菌、抗病毒和消炎等作用,与抗菌素合用能增强抗 菌素的疗效,如治疗支气管性肺炎等。
国内外常见酶制剂产品及生产方法
国内外常见酶制剂产品及生产方法酶制剂是一种由生物催化剂酶组成的产品,具有广泛的应用领域,包括食品工业、制药工业、农业和环境保护等。
以下是一些国内外常见的酶制剂产品以及它们的生产方法。
1.蛋白酶:蛋白酶是一种能够水解蛋白质的酶制剂。
目前市场上常见的蛋白酶产品包括胶原酶、淀粉酶和动物性胰蛋白酶等。
这些蛋白酶的生产通常使用微生物发酵的方法,例如利用大肠杆菌、酵母菌或者放线菌等进行发酵培养,然后通过离心、膜过滤和浓缩等工艺步骤得到精制的酶制剂。
2.脱氢酶:脱氢酶是一类能够催化氧化还原反应的酶制剂,包括脱氢酶、过氧化物酶和氧化酶等。
这些酶制剂的生产方法常常通过细胞工程技术来实现。
首先,将目标基因转入宿主细胞,并通过适当的培养条件和诱导剂来提高目标基因的表达水平。
然后,对发酵培养得到的细胞进行破碎,使用离心、超滤和柱层析等工艺步骤来提纯目标酶制剂。
3.混合酶:混合酶是一种含有多种酶活性的复合酶制剂,常用于饲料工业、肉类加工和农业等领域。
这些混合酶通常通过纯酶和非纯酶的混合物的方法生产。
首先,将多种单一酶制剂与适当的非酶助剂混合,并进行相应的活性检测和稳定性检测。
然后,通过进一步的加工工艺,如喷雾干燥和球磨研磨等,来提高混合酶的稳定性和活性。
4.反转录酶:反转录酶是一种能够将RNA逆转录为DNA的酶制剂,常用于分子生物学研究和基因工程应用。
这些反转录酶通常通过重组DNA技术生产。
首先,将反转录酶的基因序列克隆到适当的表达载体中,并转入宿主菌中进行表达。
然后,使用柱层析和膜过滤等工艺步骤来纯化反转录酶制剂。
5.脂肪酶:脂肪酶是一种能够催化脂肪水解的酶制剂,常用于乳品加工和食品加工。
这些脂肪酶通常通过酵母菌发酵的方法生产。
首先,将脂肪酶基因转入酵母菌中,并通过培养和诱导等步骤提高目标基因的表达。
然后,通过破细胞和加工工艺来提取和纯化脂肪酶制剂。
总而言之,常见的酶制剂产品包括蛋白酶、脱氢酶、混合酶、反转录酶和脂肪酶等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
• 5 危险管理数据 • 食品安全风险评估,指对食品、食品添加 剂中生物性、化学性和物理性危害对人体 健康可能造成的不良影响所进行的科学评 估,包括危害识别、危害特征描述、暴露 评估、风险特征描述等。
• 风险评估四个步骤:
危害识别
危害特征描述
暴露评估
风险特征描述
• CAC标准体系中的FAO/WH0食品添加剂联 合专家委员会(Joint FA0/WH0 Expert Committee on Food Additive,JECFA) 负责对食品用酶制剂的安全评估,截至 2005年,JECFA共对近70种不同来源(包括 基因修饰微生物)的酶制剂进行了安全性评 价.
• 1960年美国规定食品中不得加入致癌物, 进而提出零阙值理论,渐渐零阙值理论发 展成在一定概率条件下可接受风险的概念H J,后来衍变为食品中每日允许最大摄人量 (Acceptable Daily Intakes,ADI),当人 接触量低于或等于ADI值时认为是可接受风 险. • 膳食暴露评估主要有3种模型:点评估模型、 简单分布模型、概率评估模
• 4 毒理学数据 • 毒理试验的四个阶段和内容 • 1 第一阶段:急性毒性试验 • 经口急性毒性:LD50 ,联合急性毒性, 一次最大耐受量试验。 • 2 第二阶段:遗传毒性试验,30天喂 养试验,传统致畸试验
• 2.1 基因突变试验:鼠伤寒沙门氏菌/哺乳 动物微粒体酶试验(Ames试验)为首选, 其次考虑选用V79/HGPRT基因突变试验, 必要时可另选其它试验。 2.2 骨髓细胞微核试验或哺乳动物骨髓细胞 染色体畸变试验。 3 第三阶段:亚慢性毒性试验----90天喂养 试验、繁殖试验、代谢试验 4 第四阶段:慢性毒性试验(包括致癌试验)
• 食品酶制剂是专门用于食品加工的
植物
木瓜蛋白酶、菠萝蛋、酶、无花果蛋白酶、 麦芽中提取的β -淀粉酶
动物
胰蛋白酶、弹性蛋白酶、胃蛋白、凝乳酶、溶菌酶等
微生 酶、 物 果胶酶等等
а-淀粉酶、糖化酶、蛋白酶、葡萄糖;氧化酶、脂肪
• 目前已发现数千种生物酶,常用的生物酶 有30多种,包括蛋白酶、淀粉酶、纤维素 酶、脂肪酶等。 • 酶制剂级别:工业级、饲料级、食品级、 医药级 • 目前大多数食品工业微生物的生产菌仅限 于11种霉菌、8种细菌和4种酵母菌
美国
• 美国对食品用酶制剂的管理体现在其联邦 法规(Code Federal Regulation,CFR)、 食品化学法典(Food chemicals Codex, FCC)中。 • 在FCC第5版中对食品用酶制剂的活性、有 害物质的允许含量、实验方法等通用要求 作了规定,并要求食品用酶制剂的生产应遵 循GMP. • 美国FDA(Food and Drug Administration)负责对食品用酶的安全性 评价。
食品酶安全要求
• 必须是非致病性的,不产生毒素、抗菌素、 激素等生理活性物质,必须通过各种安全 性试验,证明安全可靠后,方可批准使用。 • 按照良好的食品制造技术生产酶制剂,根 据各种食品的微生物卫生标准,用酶制剂 加工的食品必须不引起微生物总量的增加, 用酶制剂加工的食品必须不带入或不增加 危害健康的杂质。
中 国
• 中国于1981年在原卫生部内部试行标 准的基础上正式公布了强制性国家标 准GB 2760-81《食品添加剂使用卫生 标准》,食品用酶制剂是其中的一部 分.
国际食品法典委员会(CAC)
• 按照CAC的相关定义,食品用酶制剂一般 被划为“加工助剂”的范畴。 • 加工助剂是指任何在原料、食品或食品配 料的加工过程中有意使用的以达到在食品 加工或者处理过程中的某种功能目的、但 其本身不作为食品配料食用的物质或原料。 • 加工助剂的使用可能会导致终产品中存在 无意的、不可避免的残留物或者衍生物。
欧盟食品酶注册
• 1 基本信息 • 产品名称,申请人信息,产品应用范围, 相似产品,不同来源菌种产品的批准情况 ,相同来源菌种不同产品的批准情况。
• 2 安全评估数据 • 2.1 技术数据 • 别名,化学组成,特性,指标,菌种来源, 生产过程与控制。
• 3 膳食暴露量 • 食品是最大的暴露来源。欧洲国家拥有从 农田到餐桌整条食品链全部环节的膳食暴露 风险评估法。 • 国际上最早研究膳食暴露风险评估的机构 主要是JMPR(Joint FAO/WHO Meeting Residue),该组织自1995年就已制定了急 性毒性物质的风险评估和急性毒性农药残 留摄入量的预测。 • 膳食暴露评估最早起源于化学品安全性评 价
欧盟
• 法国和丹麦设定了关于食品用酶的法规。 • 其中法国结合每种市售产品批准食品用酶 制剂的使用,在允许使用的食品用酶制剂 名单中包括酶制剂的来源、供体及使用范 围,并对列表中的酶在上市前进行公告和 批准。 • 在丹麦有针对食品用酶制剂批准使用的程 序。
加拿大
• 加拿大的食品药品法规(Food and Drug Regulations)将食品用酶制剂列 入可使用的食品添加剂名单,包括了酶 制剂的品种、来源、使用范围和使用量 (一般按照GMP使用).
