cochrane纳入的RCT文献质量评价英文原版
cochrane评分表

Risk of biasItem Authors'judgementDescriptionAdequate sequence generation? Yes Prinicipal author stated thatcomputer generatedallocation was usedAllocation concealment? Yes Prinicipal author stated thatallocation was concealedBlinding? Unclear No mention of study personnelor participants being blindto treatment groupIncomplete outcome data addressed? Yes All participants accountedfor, one 'drop out' recordedbut included by us in analysisFree of other bias? Unclear Possible uneven distributionof complete and incompleteparalysis at start of studybetween the two treatmentgroupsCochrane RCT质量评价标准:①随机方法是否正确。
②是否隐蔽分组。
③盲法的使用情况。
④失访或退出描述情况,有无采用意向性(ITT)分析。
以上质量标准中,如所有标准均为“充分”,则发生各种偏倚的可能性很小;如其中一条为不清楚,则有发生相应偏倚的中等度可能性;如其中一条为“不充分”或“未采用”,则有发生相应偏倚的高度可能性。
可参见:RCT的质量评价标准选择总结/bbs/topic/18137535?tpg=1&age=-1Quality assessmentThe quality of the trials was assessed and graded independently by two authors according to the criteria described in The Cochrane Handbook 4.2.6 (Higgins 2006). Gradings were compared and any inconsistencies between the authors in the interpretation of inclusion criteria and their significance to the selected study were discussed and resolved.The selected study was assessed for the following characteristics:1. The adequacy of the randomisation process (possible selection bias). Adequate randomisation includes any one of the following methods: computer generated or table of random numbers, drawing of lots, coin-toss, shuffling cards or throw of a dice. Inadequate methods of randomisation include the following: case record number, date of birth or alternate numbers.2. The adequacy of the allocation concealment (possible selection bias). Adequate methods of allocation concealment include either central randomisation (i.e. separate to other aspects of trial administration) or sequentially numbered sealed opaque envelopes. Inadequate concealment means an open allocation sequence in which either participants or trialists were able to foresee the upcoming assignment.3. The blinding of outcome assessors (i.e. whether the persons assessing the outcome of care were aware of which treatment the participant had received - possible performance bias).4. The extent and handling of losses to follow up (possible attrition bias). Adequate handling of losses to follow up involves a clear description and explanation being given of any significant difference between the losses of the intervention groups. An unacceptable loss in any one intervention group was considered to be loss greater than 20%.Study gradings A, B or C were employed for overall quality as follows.A: Minimisation of bias in all four categories above: i.e. adequate randomisation, few losses to follow up and intention-to-treat analysis, blinding of outcome assessors, high quality outcome assessment;B: Each of the criteria in A partially met;C: One or more of the criteria in A not met.Risk of bias in included studiesWe classified this study as grade C because of the uncertainty about blinding. The possibility of an uneven distribution of complete and incomplete palsies between the two groups is another potential source of bias and we conclude overall that this is a low quality study.Table 8.5.a: The Cochrane Collaboration’s tool for assessi ng risk of biasTable 8.5.c: Criteria for judging risk of bias in the ‘Risk of bias’ assessment toolFigure 8.6.a: Example of a ‘Risk of bias’ table for a single study (fictional)Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies。
cochrane纳入的RCT文献质量评价中文版教学内容

不全结局数据的数量,性质,处理方式导致失访偏倚
发表偏倚
Selective reporting.
说明如何审查选择性报道结局的可能性,以及审查结果
选择性报道结局导致发表偏倚
无缺失数据
缺失数据的产生不大可能与真实结局相关(对于生存数据,删失不大可能引入偏倚)
缺失数据的数目在各干预组相当,且各组缺失原因类似
对二分类变量,与观察事件的发生风险相比,缺失比例不足以影响预估的干预效应
对连续性结局数据,缺失数据的合理效应规模(均数差或标准均数差)不会大到影响观察的效应规模;
缺失的数据用合适的方法进行估算
无盲法或盲法不充分,但系统评价员判断结局很可能受到缺乏盲法的影响
尝试对关键的参与者和实施者行盲法,但盲法很可能被打破,结局很可能受到缺乏盲法的影响
风险未知
任何如下标准:
没有足够信息判断为低风险或高风险
研究未描述此情况
结局数据不完整
不全结局数据的数量,性质,处理方式导致失访偏倚
偏倚低风险标准
任何如下标准:
通过奇偶或出生日期产生序列
通过入院日期产生序列
通过类似住院号或门诊号产生序列
相对于上面提到的系统方法,其它非随机的方法少见的多,也更明显。通常包括对参与者进行判断或非随机的方法,例如:
临床医生判断如何分配
参与者判断如何分配
基于实验室检查或系列测试的结果分配
基于干预的可获取性进行分配
偏倚风险不清楚的判断标准
高风险判断标准
任何如下标准:
缺失数据的产生很大可能与真实结局相关,缺失数据的数目及缺失原因在各干预组相差较大
(完整版)Cochrane协作网研究质量评价工具

(完整版)Cochrane协作网研究质量评价工具Cochrane协作网是一个致力于提供高质量临床和流行病学研究的组织。
为了保证研究的可靠性和准确性,Cochrane协作网开发了一套研究质量评价工具,以帮助研究人员和读者判断研究的科学性和可靠性。
以下是Cochrane协作网研究质量评价工具的完整版:1. 研究设计的选择和描述:研究应明确阐述其研究设计,包括目的、研究问题、研究范围和参与对象等。
合适的研究设计能提供可靠的数据和结论。
研究设计的选择和描述:研究应明确阐述其研究设计,包括目的、研究问题、研究范围和参与对象等。
合适的研究设计能提供可靠的数据和结论。
2. 样本选择的合理性:研究应对样本选择的方法进行详细描述,包括样本的来源、选择标准和样本量的计算等。
样本的合理选择能提高研究结果的可靠性和普遍适用性。
样本选择的合理性:研究应对样本选择的方法进行详细描述,包括样本的来源、选择标准和样本量的计算等。
样本的合理选择能提高研究结果的可靠性和普遍适用性。
3. 数据收集和测量工具的可靠性和有效性:研究应清楚描述用于数据收集的方法和工具,并验证其可靠性和有效性。
合适的数据收集工具能提供准确和可比较的数据。
数据收集和测量工具的可靠性和有效性:研究应清楚描述用于数据收集的方法和工具,并验证其可靠性和有效性。
合适的数据收集工具能提供准确和可比较的数据。
4. 数据分析的方法和结果的解释:研究应明确描述所使用的数据分析方法,并对结果进行准确的解释。
合理的数据分析方法能提供可靠的研究结论。
数据分析的方法和结果的解释:研究应明确描述所使用的数据分析方法,并对结果进行准确的解释。
合理的数据分析方法能提供可靠的研究结论。
5. 偏倚的评估:研究应对偏倚的可能性进行评估,并采取适当的措施减少偏倚的影响。
合理的偏倚评估能提高研究的可靠性和可信度。
偏倚的评估:研究应对偏倚的可能性进行评估,并采取适当的措施减少偏倚的影响。
合理的偏倚评估能提高研究的可靠性和可信度。
cochrane纳入的RCT文献质量评价(风险偏倚评估工具)中英文对照版

交替或循环
出生日期
病历号
其它明确的非隐藏过程
风险未知
没有足够信息判断为低风险或高风险。通常因分配隐藏的方法未描述或描述不充分。例如描述为使用信封分配,但为描述信封是否透明?密封?顺序编号?
对参与者和实施者的盲法
因参与者和实施者了解干预情况而导致实施偏倚
低风险判断标准
参与者以及纳入参与者的研究者因以下掩盖分配的方法或相当的方法,事先不了解分配情况
中心分配(包括电话,网络,药房控制随机)
相同外形的顺序编号的药物容器;
顺序编号、不透明、密封的信封
高风险判断标准
参与者以及纳入参与者的研究者可能事先知道分配,因而引入选择偏倚,譬如基于如下方法的分配:
使用摊开的随机分配表(如随机序列清单)
Selectionbias(biasedallocationtointerventions)duetoinadequateconcealmentofallocationspriortoassignment.
Performancebias.
BlindingofparticipantsandpersonnelAssessmentsshouldbemadeforeachmainoutcome(orclassofoutcomes).?
可能存在偏倚风险,但存在以下两种中的一种
没有足够信息评估是否存在其它重要的偏倚风险
没有足够的证据认为发现的问题会引入偏倚
summaryassessmentsoftheriskofbiasforeachimportantoutcome(acrossdomains)withinandacrossstudies
文献质量评估

文献质量评估
各纳入RCT的方法学质量评价采用Cochrane5.1手册推荐的简单评估法[9],评价的关键指标:①随机方法是否正确;②是否做到分配隐藏,分配方法是否正确;③是否实施盲法;
④是否报告失访和退出情况;⑤基线是否可比。
对于分配隐藏,将试验评为A(完全隐藏)、B(不清楚是否隐藏)、C(隐藏不充分)和D(没有使用隐藏)4个等级。
在其他方面将试验评为A(是)、B(不清楚)、C(否)三级。
如各评价条目均为A级,则为低度偏倚,发生各种偏倚的可能性最小,质量评为A级;若有一条目或多个条目为B,则该试验有发生相应偏倚的中等度可能性,质量评为B级;如其中有一条目或多个条目为C,则该试验有发生相应偏倚的高度可能性,质量评为C级。
文献质量评价

•文献介绍
目的 对危重症患者实施早期主动 活动,探讨其能否改善患者的营养指标 研究设计 前瞻性随机对照研究
分组方法 依据患者入院时的BMI (BMI≥28、18.5<BMI<27.9/BMI≤18.5), 采用分层随机抽样的方法,依次分为试验组 及对照组。
1
•文献介绍
干预措施 对照组给予常规护理及康复锻 炼,试验组在常规护理的基础上制定早期 主动活动标准,拟定早期主动活动方案
结局指标 分别在患者入院时、入院 3 天、 7 天、14 天,测量患者的前白蛋白、白蛋 白、视黄醇结合蛋白,比较两组患者的营 养指标、肌力、机械通气时间及深静脉血 栓发生率、ICU 住院时间。
•Cochrane循证医学中心对 RCT的评价原则
Cochrane协作网关于干预 性研究系统评价手册5.1.0 版(2011年)
17a
ቤተ መጻሕፍቲ ባይዱ危害
19
报告各组主要 / 次要结局指标的结果, 23-30 效应估计值及精度(95%可信区间) 对于二分类结局指标,建议同时提供相 对效应值和绝对效应值 报告所做的其他分析,如亚组分析、调 整分析,指出哪些是预先设定的,哪些 是探索性的分析 报告各组出现的所有不良事件或非预期 28 效应
3.报告清单
分对 配随 隐机 藏方 案 的 偏倚 风险低
不清楚 高
使用研究者或研究对象能预见到的分配顺 序,例如:公开的随机分配表或透明的信 封等。
随机方案隐藏
偏移风 险低
2.评价原则
作者提及对研究对象及研究 信息不充分,无法 人员采取盲法,且不易识破; 判断。 或未采取但不影响结果。
实对 施研 者究 采对 取象 盲及 法干 预 偏倚 风险低
感谢在座各位聆听
cochrane纳入的RCT文献质量评价英文原版

If particular questions/entries were pre-specified in the review’s protocol, responses should be provided for each question/entry.
Bias due to problems not covered elsewhere in the table.
Domain
Support for judgement
Review authors’ judgement
Selection bias.
Random sequence generation.
Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Detection bias due to knowledge of the allocated interventions by outcome assessors.
Attrition bias.
cochrane纳入的RCT文献质量评价(风险偏倚评估工具)中英文对照版
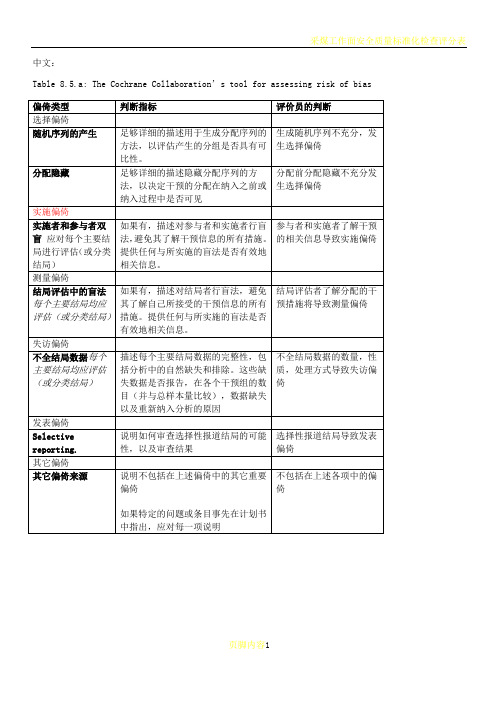
中文:Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of biasTable 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment tool研究者描述随机序列产生过程譬如:参考随机数字表使用计算机随机数字生成器扔硬币洗牌的卡片和信封掷骰子抽签最小化*最小化,可实现无随机元素,被认为相当于是随机的。
研究者描述序列的产生使用的是非随机的方法。
通常是系统的非随机方法,例如:通过奇偶或出生日期产生序列通过入院日期产生序列通过类似住院号或门诊号产生序列相对于上面提到的系统方法,其它非随机的方法少见的多,也更明显。
通常包括对参与者进行判断或非随机的方法,例如:临床医生判断如何分配参与者判断如何分配基于实验室检查或系列测试的结果分配基于干预的可获取性进行分配中心分配(包括电话,网络,药房控制随机)相同外形的顺序编号的药物容器;顺序编号、不透明、密封的信封参与者以及纳入参与者的研究者可能事先知道分配,因而引入选择偏倚,譬如基于如下方法的分配:使用摊开的随机分配表(如随机序列清单)分发信封但没有合适的安全保障(如透明、非密封、非顺序编号)交替或循环出生日期病历号其它明确的非隐藏过程任何如下标准:无盲法或盲法不充分,但系统评价员判断结局不太可能受到缺乏盲法的影响参与者和主要实施者均实施可靠的盲法,且盲法不太可能被打破任何如下标准:无盲法或盲法不充分,但系统评价员判断结局很可能受到缺乏盲法的影响尝试对关键的参与者和实施者行盲法,但盲法很可能被打破,结局很可能受到缺乏盲法的影响任何如下标准:没有足够信息判断为低风险或高风险研究未描述此情况任何如下标准:无盲法或盲法不充分,但系统评价员判断结局不太可能受到缺乏盲法的影响参与者和主要实施者均实施可靠的盲法,且盲法不太可能被打破任何如下标准:无盲法或盲法不充分,但系统评价员判断结局很可能受到缺乏盲法的影响尝试对关键的参与者和实施者行盲法,但盲法很可能被打破,结局很可能受到缺乏盲法的影响任何如下标准:没有足够信息判断为低风险或高风险研究未描述此情况任何如下标准:无缺失数据缺失数据的产生不大可能与真实结局相关(对于生存数据,删失不大可能引入偏倚)缺失数据的数目在各干预组相当,且各组缺失原因类似对二分类变量,与观察事件的发生风险相比,缺失比例不足以影响预估的干预效应对连续性结局数据,缺失数据的合理效应规模(均数差或标准均数差)不会大到影响观察的效应规模;缺失的数据用合适的方法进行估算任何如下标准:缺失数据的产生很大可能与真实结局相关, 缺失数据的数目及缺失原因在各干预组相差较大对二分类变量,与观察事件的发生风险相比,缺失比例足以影响预估的干预效应对连续性结局数据,缺失数据的合理效应规模(均数差或标准均数差)足以影响观察的效应规模;意向治疗分析中存在实际干预措施与随机分配的干预相违背的情况对缺失数据进行简单的不合适的估算任何如下标准:没有报道缺失或排除的情况,无法判断高风险或低风险(如未说明随机的数量,未提供数据缺失的原因)研究未描述此情况任何如下标准:实验的计划书可获取,系统评价感兴趣的所有首要或次要结局均按计划书预先说明的方式报道实验计划书不可得,但很明显发表的报告包括所有的结局,包括预先说明的结局(这种性质的有说服力的文字可能少见)任何如下标准:不是所有的预先说明的首要结局均被报道一个或多个首要结局为采用预先说明的测量方法、分析方法或数据子集来报道系统评价感兴趣的一个或多个首要结局报道不全,以至于不能纳入meta分析研究未报道此研究应当包含的主要关键结局具有与特殊试验设计相关的潜在偏倚来源或被指欺诈或其它问题可能存在偏倚风险,但存在以下两种中的一种没有足够信息评估是否存在其它重要的偏倚风险没有足够的证据认为发现的问题会引入偏倚Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies英文:Table 8.5.a: The Cochrane Collaboration’s tool for assessing risk of biasTable 8.5.d: Criteria for judging risk of bias in the ‘Risk of bias’ assessment toolprocess such as:Referring to a random number table;Using a computer random number generator;Coin tossing;Shuffling cards or envelopes;Throwing dice;Drawing of lots;Minimization*.*Minimization may be implemented without a random element, and this isconsidered to be equivalent to being random.judgement The investigators describe a non-random component in the sequence generation process. Usually, the description would involve somesystematic, non-random approach, for example:Sequence generated by odd or even date of birth;Sequence generated by some rule based on date (or day) of admission;Sequence generated by some rule based on hospital or clinic recordnumber.Other non-random approaches happen much less frequently than thesystematic approaches mentioned above and tend to be obvious. Theyusually involve judgement or some method of non-random categorization ofparticipants, for example:Allocation by judgement of the clinician;Allocation by preference of the participant;Allocation based on the results of a laboratory test or a seriesof tests;Allocation by availability of the intervention.Criteria for a judgement Participants and investigators enrolling participants could not foreseeassignment because one of the following, or an equivalent method, was usedto conceal allocation:Central allocation (including telephone, web-based andpharmacy-controlled randomization);Sequentially numbered drug containers of identical appearance;Sequentially numbered, opaque, sealed envelopes.judgement Participants or investigators enrolling participants could possiblyforesee assignments and thus introduce selection bias, such as allocationbased on:Using an open random allocation schedule (e.g. a list of randomnumbers);Assignment envelopes were used without appropriate safeguards(e.g. if envelopes were unsealed or nonopaque or not sequentiallynumbered);Alternation or rotation;Date of birth;Case record number;Any other explicitly unconcealed procedure.Criteria for a judgement Any one of the following:No blinding or incomplete blinding, but the review authors judgethat the outcome is not likely to be influenced by lack of blinding;Blinding of participants and key study personnel ensured, andunlikely that the blinding could have been broken.judgementAny one of the following:No blinding or incomplete blinding, and the outcome is likely tobe influenced by lack of blinding;Blinding of key study participants and personnel attempted, butlikely that the blinding could have been broken, and the outcomeis likely to be influenced by lack of blinding.judgement ‘Unclear risk’ ofAny one of the following:Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’;The study did not address this outcome.Criteria for a judgement Any one of the following:No blinding of outcome assessment, but the review authors judge thatthe outcome measurement is not likely to be influenced by lack ofblinding;Blinding of outcome assessment ensured, and unlikely that theblinding could have been broken.judgementAny one of the following:No blinding of outcome assessment, and the outcome measurement islikely to be influenced by lack of blinding;Blinding of outcome assessment, but likely that the blinding couldhave been broken, and the outcome measurement is likely to beinfluenced by lack of blinding.judgement ‘Unclear risk’ ofAny one of the following:Insufficient information to permit judgement of ‘Low risk’ or‘High risk’;The study did not address this outcome.Criteria for a judgement Any one of the following:No missing outcome data;Reasons for missing outcome data unlikely to be related to trueoutcome (for survival data, censoring unlikely to be introducingbias);Missing outcome data balanced in numbers across interventiongroups, with similar reasons for missing data across groups;For dichotomous outcome data, the proportion of missing outcomescompared with observed event risk not enough to have a clinicallyrelevant impact on the intervention effect estimate;For continuous outcome data, plausible effect size (difference inmeans or standardized difference in means) among missing outcomesnot enough to have a clinically relevant impact on observed effectsize;Missing data have been imputed using appropriate methods.judgement Any one of the following:Reason for missing outcome data likely to be related to trueoutcome, with either imbalance in numbers or reasons for missingdata across intervention groups;For dichotomous outcome data, the proportion of missing outcomescompared with observed event risk enough to induce clinicallyrelevant bias in intervention effect estimate;For continuous outcome data, plausible effect size (difference inmeans or standardized difference in means) among missing outcomesenough to induce clinically relevant bias in observed effect size;‘As-treated’ analysis done with substantial departure of theintervention received from that assigned at randomization;Potentially inappropriate application of simple imputation.judgement ‘Unclear risk’ ofAny one of the following:Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number ran domized not stated,no reasons for missing data provided);The study did not address this outcome.Criteria for a judgement Any of the following:The study p rotocol is available and all of the study’spre-specified (primary and secondary) outcomes that are of interestin the review have been reported in the pre-specified way;The study protocol is not available but it is clear that thepublished reports include all expected outcomes, including thosethat were pre-specified (convincing text of this nature may beuncommon).judgementAny one of the following:Not all of the study’s pre -specified primary outcomes have beenreported;One or more primary outcomes is reported using measurements,analysis methods or subsets of the data (e.g. subscales) that werenot pre-specified;One or more reported primary outcomes were not pre-specified(unless clear justification for their reporting is provided, suchas an unexpected adverse effect);One or more outcomes of interest in the review are reportedincompletely so that they cannot be entered in a meta-analysis;The study report fails to include results for a key outcome thatwould be expected to have been reported for such a study.judgementThere is at least one important risk of bias. For example, the study: Had a potential source of bias related to the specific study designused; orHas been claimed to have been fraudulent; orHad some other problem.judgement ‘Unclear risk’ ofThere may be a risk of bias, but there is either:Insufficient information to assess whether an important risk of bias exists; orInsufficient rationale or evidence that an identified problem willintroduce bias.Table 8.7.a: Possible approach for summary assessments of the risk of bias for each important outcome (across domains) within and across studies。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Performance bias.
Blinding of participants and personnelAssessments should be made for each main outcome (or class of outcomes).
Using an open random allocation schedule . a list of random numbers);
Allocation by availability of the intervention.
Criteria for the judgement of ‘Unclear risk’ of bias.
Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’.
cochrane纳入的RCT文献质量评价英文原版
Domain
Support for judgement
Review authors’ judgement
Selection bias.
Random sequence generation.
Describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation:
RANDOM SEQUENCE GENERATION
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence.
Criteria for a judgement of ‘Low risk’ of bias.
Central allocation (including telephone, web-based and pharmacy-controlled randomization);
Sequentially numbered drug containers of identical appearance;
Reporting bias due to selective outcome reporting.
Other bias.
Other sources of bias.
State any important concerns about bias not addressed in the other domains in the tool.
Detection bias due to knowledge of the allocated interventions by outcome assessors.
Attrition bias.
Incomplete outcome dataAssessments should be made for each main outcome (or class ofoutcomes).
Describe all measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective.
Describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each interventiongroup (compared with total randomized participants), reasons for attrition/exclusions where reported, and any re-inclusions in analyses performed by the review authors.
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence.
Allocation concealment.
Describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Performance bias due to knowledge of the allocated interventions by participants and personnel during the study.
Detection bias.
Blinding of outcome a each main outcome (or class of outcomes).
ALLOCATION CONCEALMENT
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment.
Criteria for a judgement of ‘Low risk’ of bias.
If particular questions/entries were pre-specified in the review’s protocol, responses should be provided for each question/entry.
Bias due to problems not covered elsewhere in the table.
Sequentially numbered, opaque, sealed envelopes.
Criteria for the judgement of ‘High risk’ of bias.
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on:
Attrition bias due to amount, nature or handling of incomplete outcome data.
Reporting bias.
Selective reporting.
State how the possibility of selective outcome reporting was examined by the review authors, and what was found.
Describe all measures used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Provide any information relating to whether the intended blinding was effective.
Coin tossing;
Shuffling cards or envelopes;
Throwing dice;
Drawing of lots;
Minimization*.
*Minimization may be implemented without a random element, and this is considered to be equivalent to being random.
Criteria for the judgement of ‘High risk’ of bias.
The investigators describe a non-random component in the sequence generation process. Usually, the description would involve some systematic, non-random approach, for example:
