应用化学专业英语
应用化学专业英语-Lesson-2..

• Elements are composed of extremely small particles called atoms. All atoms of a given element are identical. The atoms of one element are different from the atoms of all other elements.
• Compounds are composed of atoms of more than one element.
• Chemical reactions involve only the rearrangement of atoms; atoms are not created or destroyed in chemical reactions.
• Protons and neutrons are located in the nucleus of the atom, which is small. Most of the mass of the atom is due to the nucleus.
• Electrons are located outside of the nucleus. Most of the volume of the atom is due to electrons.
Some Complex Ions
Name Carbonate Nitrate Phosphate Dihydrogen Phosphate Sulfate Sulfite Thiosulfate Perchlorate Chlorite Cyanide Chromate
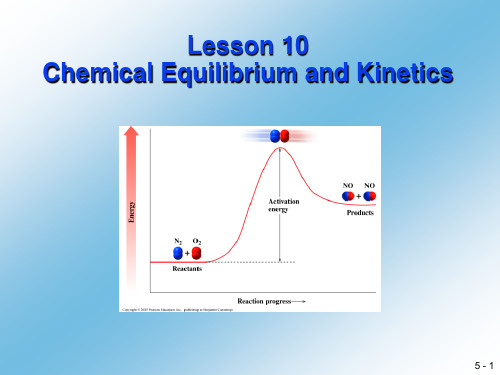
应用化学专业英语lesson10ChemicalEquilibriumandkinetics
NH3
Add more NH3?
Reaction shifts to the left [N2] and [H2] inc
5 - 35
Le Chatelier’s principle
Adding Pressure affects an equilibrium with gases
N2(g) + 3 H2(g)
N2(g) + 3 H2(g)
Keq =
[ NH3 ] 2 [ N2 ] [ H2 ] 3
2 NH3(g)
5 - 33
Le Chatelier’s principle
Stress causes shift in equilibrium Adding or removing reagent
N2(g) + 3 H2(g)
Temperature: 2. Higher Temperature:
Faster cars More collisions
More Energy More collisions
Reacting molecules move faster, providing colliding molecules w/ Eact.
At this point, equilibrium is achieved. Time
5 - 26
Figure 9.8
2SO2(g) + O2(g)
At Equilibium
2SO3(g)
SO2(g)+O2(g)
Initially
SO3(g)
Initially
5 - 27
Figure 9.9
2SO2(g) + O2(g)
应用化学专业英语介绍

应用化学专业报告学院:理学院专业:应用化学学号:*****************Applied chemistry specialityOne、applied chemistry speciality - main courses: Training target:This specialized raise has the chemical basic theory, basic knowledge in strong experimental skills, can in scientific research institutions, colleges and universities and enterprises and institutions, engaged in scientific research, teaching and management work of the senior specialized talents.Training requirements:Students of this specialty mainly study the basic knowledge of chemistry, the basic theory, basic skills and related engineering knowledge, is the basic research and applied basic research of scientific thought and scientific experiment training, has good scientific literacy, have use knowledge and experimental skills in applied research, technology development and technology management of the basic skills.Main courses:Main subject: chemicalMain course:Inorganic chemistry、, analytical chemistry (including instrument analysis), organic chemistry, physical chemistry (including structural chemistry, chemical engineering foundation and chemical mapping.The main practice teaching links include production practice, graduation thesis, general arrangement and a week of twenty.Length of schooling: four yearsawarded degree:physical or Bachelor'sSimilar professional:chemical applied chemistry chemical biology molecular science and engineering chemical engineering and technology Two、Four chemistry:(Inorganic chemistry, analytical chemistry, organic chemistry, physical chemistry)Inorganic chemistry:Inorganic chemistry relative to organic chemistry, the non carbon. However, some carbon compounds, such as carbon monoxide, carbon dioxide, carbon disulfide, carbonic acid compounds,cyanide and so on are still belongs to the category of inorganic chemistry. analytical chemistry:Analytical chemistry in chemistry basic theory and experiment technology as the foundation, and absorb the physical, biological, statistics, computer, automation and other aspects to enrich itself, so as to solve the content of science and technology proposed all sorts of analysis.The task of analytical chemistry(1) determining the material chemical composition, qualitative analysis(2) measurement of different components of content - quantitative analysis(3) characterization of physical chemical structure, form, energy state, structure analysis, the shape analysis, energy state analysis(4) representation composition, content, structure, form, energy state dynamics characteristics, dynamic analysisorganic chemistry:Organic chemistry is the study of organic compounds and a basic subject. It mainly includes the organic compound classification, structure, naming, property, preparation, chemical reaction and reaction mechanism of law. Organic chemistry is an important branch of chemistry, it is at the molecular level teaching carbon molecular structure and their mutual conversion mechanism, products and their separation, identification and application of basic science, chemistry, biology, pharmacy, medicine, agriculture, environment, materials science support discipline. Is to create new material is an important subject. physical chemistry:Physical chemistry is a physical principle and experiment technology as the foundation, study the properties and behavior of chemical system, found and the establishment of physical chemistry chemical system of the special law of discipline. With the rapid development of science and the mutual infiltration between subjects, physical chemistry and physics, inorganic chemistry, organic chemistry in the content are difficult to accurately demarcation line, so as to constantly create new branches, such as physical organic chemistry, biology, physics chemistry, chemical physics.Three、Applied chemistry specialty elective course: Organic synthesis, botany, colloid and surface chemistry, food chemistry, ecology, the university physics, management, professional English, plant chemistry, biological chemistry, polymer chemistry, and so on.Four、Professional teachers:Li yuqing、Li xianghong、Fuhui、Li huijuan、Liming、Liu shouqing、Liangkun、Chen yuhui、Leiran、and so on.Five、Applied chemistry employment direction:Applied chemistry professional graduates suitable to the petroleum chemical industry, environmental protection, commodity inspection, health and epidemic prevention, customs, medicine, fine chemical plant and other production, technical and administrative departments and the factories and mines enterprises engaged in applied research, technology development, production technology and management work; Can also to scientific research department and school engaged in scientific research and teaching work,。
应用化学专业英语复习资料

一单词短语1.Molecule 分子molecular 分子的2.chemical process 化学过程element 元素3.a t o m原子a t t r a c t i o n吸引力4.repulsion 排斥力distillation 蒸馏、n5.distill 蒸馏v rectification 精馏position 构成structure 结构7.property 性质mass 质量8.atomicweight 原子量atomic number 原子序数9.ionization energy 电离能period 周期10.g r o u p族f a m i l y族11.transition group 过渡族main group 主族12.i o n离子s u b s t i t u t i o n取代反应13.el i mi na ti on消除反应nucl eoph i l i c 亲核的14.nucleophilie 亲核试剂electrophilie亲电试剂15.alkyl 烷基的functional group 官能团16.halides 卤素的leaving group 离去基团17.transition state过渡态intermediate 中间体18.r e a c t a n t反应物p r o d u c t生成物19.concentration 浓度rate equation 速率方程20.c o n s t a n t常数e t h e r醚21.endothermic 吸热的substrate 反应底物22.mechanism 机理reagen 试剂23.alkene 烯烃exothermic 放热的24.A n i o n阴离子n i t r o g e n氮气25.Hydrocarbon 碳氢化合物carbonhydrate 碳水化合物26.Alkane 烷烃substituent 取代基27.Isomerism 同分异构现象isomer 同分异构28.V i n y l乙烯基d e r i v a t i v e s衍生物29.acid halides 酰卤acid anhydrides 酸酐30.e s t e r s酯a m i d e酰胺31.ammonia NH3 Acetic anhydride乙酸酐32.phenol 芬acid—base titration 酸碱滴定33.precipitation沉淀analyses 化学分析员34.IR 红外UV紫外MS质谱GC色相色谱HPLC高效液相色谱TLC薄层色谱X—rayX射线衍射二选词填空1、We can now easily account for many things,which were thought to be mysterious by theancients2、the acid acts on the metal and a gas is givenoff.3、you should adapt yourself to new ways oflooking at matters4、electrolytes have more pronounced effect oncolligative properties than do nonelectrolytes. 5、if water in these lakes evaporated at the samerate as fresh water ,both would nearly dryup in a matter of year.6、both laks evaporated very slow compared with afresh lake or even the ocean.7、a property that depends only on the relativeamounts of solute and solvent is know as acolligative property.8、for example ,both NaCl (ionic) and HCl (polarcovalent)are classified as electrolytes becausethey form ions in aqueous solution.9、when compounds such as NaCl and HCl aredissolved in water ,the effect is obvious.10、if the wires is cut ,the light goes out becausethe circuit is broken.11、when wires are attached to a charged batteryand then to a light bulb ,the light shinesbrightly.12、glass and wood as well as pure water areexamples or nonconductors of electricity.13、other substances resist the flow of electricityand are known as nonconductors orinsulators.14、it has long been known that the presence of asolute in water may affect its ability toconduct electricity.15、when the collection of papers was first broughtout,it was well received by the reviewers.16、in the same way the dozen or so mostcommon kinds of kinds of atoms can be put together in many millions of different ways tomake molecules .17、elements are made up of tiny fundamentalparticles called atoms. Fundamental, as it is usedhere ,means that they cannot be furtherdivided by any chemical metheods.18、each element has atoms that is different fromthe atoms of other elements.19、it would not be quite round; on the contraryit would consist of three parts represented byspheres.20、it is not to be summed up in a singleproduct or word ,but in an idea or basicconcept.21、the chemical symbol of an element may standthe element for.22、the rate of a chemical reaction is influencedby several factors such as temperature ,concentration of reagents , particle size ,light ,and catalyst.23、all forms of life in earth are very dependenton chemical reactions or chemical changes.24、a chemical reaction occurs when elements andcompounds react together to produce differentcompounds , or when compounds break down into simpler compounds or elements.三无机物的命名H Hydrogen Li Lithium Na Sodium K Potassium Mg Magnesium Ca CalciumMn manganese Cu copper Zn zinc Fe iron Hg mercury Ag silver Au gold C Carbon Si Silicon Pb Lead Al Aluminium F Fluorine Cl Chlorine Br Bromine I IodineO Oxygen S Sulfur N Nitrogen P Phosphorus1.直呼其名,即读其元素名称+ ion如:Na+ sodium ionK+ potassium ion2.对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous 表示低价,-ic 表示高价如:Cu+ copper (Ⅰ) ion 或cuprous ion Cu2+ copper (Ⅱ) ion 或cupric ionFe2+ iron (Ⅱ) ion 或ferrous ionFe3+ iron (Ⅲ) ion 或ferric ion3.含氢酸根:酸根中的H读做hydrogen,氢原子的个数用希腊前缀表示:mono- di - tri- tetra - penta- hexa-hepta- octa- nona- deca-举例:CO32-carbonate ionHCO3-hydrogen carbonate ionPO43- phosphate ionHPO42hydrogencarbonate ionH2PO4- dihydrogenphosphate ion4.结晶水读做hydrate ,结晶水的个数用希腊前缀表示:mono-di - tri- tetra - penta- hexa- hepta- octa- nona- deca-CuSO4·5H2O copper(Ⅱ) sulfate pentahydrateAlCl3 ·6H2O aluminum chloride hexahydrate5.测试Mg(OH)2magnesium hydroxide AlCl3aluminum chlorideFeBr2 iron(II) bromide CaSO4calcium sulfateZnCO3zinc carbonate HF hydrofluoric acidH3PO4phosphoric acid NO2nitrogen dioxideCuO copper(II) oxide Al2O3aluminum oxideNaHSO3sodium hydrogen sulfiteKMnO4potassium permanganateNaClO sodium hypochloride四有机物的命名1)命名正烷基时,只需把烷烃的词尾“-ane换成“-yl”,加在相应的烷烃的字首后2)字母规则:Butyl>Ethyl>Isopropyl>Methyl>Neopentyl>tert-Pentyl >Propyl3)环烷烃:只需在所对应的烷烃前加上cyclo-即可4)有些结构较复杂的烷基,需添加词头5)烯烃和炔烃命名时将相应的烷烃的词尾“烷”(ane)改为“烯”(ene)或“炔”(yne),后缀前加上不饱和键的编号即可。
应用化学专业英语(课后答案和课文翻译)

Unit 1 The Roots of ChemistryI. Comprehension.1.It can be inferred from this article which one of the following items is not mainly based on practical use C. Greek chemistry2. It was B. Empedocless who first introduced the idea that all things are not formed from just one element.3. In the development of Greek chemistry, D. Democritus was the first one definiting the ultimately constituents of matter?4. According to Plato, there are B. 4 ―elements‖ whose faces are constituted by regular polygons.5. In the last paragraph,authors think that experiment DD.can deal with the reactions by which one substance is converted into anotherII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerable difficulty, and itis necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematized knowledge and is also anactivity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistry observed on a singlemundane planet.4.People are made of molecules; some of the molecules in people are rather simple whereasothers are highly complex.5.Chemistry is ever present in our lives from birth to death because without chemistry there isneither life nor death.6.Mathematics appears to be almost as humankind and also permeates all aspects of human life,although many of us are not fully aware of this.III. Translation.1.(a)化学过程;(b)自然科学;(c)蒸馏技术(a) chemical process (b) natural science (c) the technique of distillation2.正是原子构成铁、水、氧等。
东华大学应用化学专业英语总结

东华大学应用化学专业英语总结专业英语重点总结单词Toxic chemicals:有毒化学品Chemical pollution:化学污染Physical property :物性 Isolate:分离Determine:测定 Synthesize:合成Fundamental principles:基本原理 Investigation:研究Utilize:利用 Catalyst 催化剂Enzyme 酶Biosphere 生物圈Heterogeneous catalyst 非均相催化剂Nanotechnology 纳米技术Carbon monoxide 一氧化碳Chemical formulas:化学式anion: 阴离子 Oxidation number:氧化值sulphate: 硫酸盐 Hydrides: 氢化物Sodium:钠 cation: 阳离子Covalent bond:共价键electroneutral: 电中性的Electronegative atom:电负性原子 trivial names:俗名Oxidation:氧化Peroxides:过氧化物Superoxide:超氧化物Periodic table:周期表Noble gases: 惰性气vacant orbital:空轨道Coordination (complex) compound: 配位化合物Unshared pair of electrons:未共用电子对oxidation state:氧化态 hydroxides:氢氧化物caustic soda solution:苛性钠溶液vacant orbital:空轨道Formula 分子式 Common name 俗名Derivative 衍生物 Acid salt 酸式盐Hydrate 水合物 Anhydrous 无水的Oxidizing agent 氧化剂Reducing agent 还原剂Oxidation reduction reaction氧化还原反应Electrochemistry 电化学 Electrolysis 电解Strong acid 强酸 Weak base 弱碱Acid-base indicator 酸碱指示剂Distilled water 蒸馏水Buffer solution 缓冲溶液Common ion effect 同离子效应Equivalencepoint 等效点 Neutralization 中和Dissociation 离解度 Anhydride 脱水物Periodic law: 元素周期率 periods (rows):周期group (columns):族 protons:质子Valence electrons:价电子 Halogens: 卤素Atomic radius: 原子半径alkaline earths:碱土金属attractive force: 吸引力electronegativity: 电负性electropositive:正电性univalent ion: 一价离子electron shell: 电子层 bonding force 结合力monatomic 单原子的 Neutrons:中子hydrogen bond 氢键conduct electricity 导电Electrically neutral 电中性的Electrostatic 静电的isomerism :异构现象Reversible :可逆的。
应用化学专业英语词汇
Toxic chemicals:有毒化学品Chemical pollution:化学污染Physical property :物性Natural changes: 自然变化Scientific fields:科学领域Isolate:分离Determine:测定Synthesize:合成Fundamental principles:基本原理Investigation:研究Utilize:利用化学式书写的基本规则如何写化学式命名化合物二元化合物:氧化物,盐,酸(1)阴离子元素加后缀–ide(2)多价态元素加前缀:mono-, di-, tri-, tetra-, penta-, hexa-(3)低价氧化态后缀–ous,高价氧化态后缀–ic氧化物盐酸:基础元素(前缀hydro-, 后缀-ic)+ acid氢氧化物(碱):金属元素(价态)+ hydroxide含氧酸及其盐(1)基本元素仅有一种氧化态酸:基础元素加后缀-ic + acid盐:阳离子元素+基础元素加后缀-ate(2)基本元素有二种氧化态酸:基础元素加后缀(-ous低价态,-ic高价态)+ acid盐:阳离子元素+ 基础元素加后缀(-ite低价态, -ate高价态)(3)基本元素有多种氧化态酸:最低氧化态基础元素(前缀hypo-, 后缀-ous)+ acid较低氧化态基础元素加后缀-ous+ acid较高氧化态基础元素加后缀-ic + acid最高氧化态基础元素(前缀per-, 后缀-ic)+ acid盐:最低氧化态阳离子元素+ 基础元素(前缀hypo-, 后缀-ite)较低氧化态阳离子元素+ 基础元素加后缀-ite较高氧化态阳离子元素+ 基础元素加后缀-ate最高氧化态阳离子元素+ 基础元素(前缀per-, 后缀-ate)不同水分子含量的酸较低水含量前缀meta-较高水含量前缀ortho-不同基本元素形成的酸前缀di-, pyro-含硫的酸:源于含氧酸中的氧被硫取代,使用前缀thio-含氢盐(酸式盐):源于含有1个以上氢原子酸中的氢原子被金属离子取代,形成酸式盐,氢原子以及金属离子使用前缀di-, (bi-),tri-配位化合物的命名阳离子+ [ 配体及中心原子] (氧化数)Chemical formulas:化学式anion: 阴离子Oxidation number:氧化值sulphate: 硫酸盐Sodium:钠cation: 阳离子Covalent bond:共价键electroneutral: 电中性的Electronegative atom:电负性原子mono-一Hydrogen: 氢di-二Hydrides: 氢化物tri-三Oxidation:氧化tetra- 四前缀Peroxides:过氧化物penta-五Superoxide:超氧化物hexa-六Positive:正oxidation state:氧化态Periodic table:周期表trivial names:俗名Noble gases: 惰性气sulphide: 硫化物Transition elements:过渡元素hydroxides:氢氧化物Ion: 离子caustic soda solution:苛性钠溶液Combining capacity:结合能力phosphorus: 磷Coordination (complex) compound: 配位化合物vacant orbital:空轨道Unshared pair of electrons:未共用电子对Ethene:乙烯Propene:丙烯butene :丁烯Single bond:单键double bond:双键triple bond:三键Benzene:苯Symmetrical:对称的Naphthalene: 萘functional group :官能团hydroxyl :羟基Carbohydrates: 碳水化合物,糖类Sucrose: 蔗糖glucose :葡萄糖alcohols:醇ether:醚ketone:酮aldehyde:醛fatty acids:脂肪酸esters:酯diethyl :二乙基hexagonal :六边的Formaldehyde: 甲醛methyl :甲基acetate :醋酸盐pentagonal :五边形的amines :胺类ammonia :氨amino acid :氨基酸methylamine :甲胺glycine :甘氨酸vitamin :维生素chlorophyll :叶绿素alkali :碱enzyme :酶-ane:-烷-ene:烯-yne:炔cyclo-:环-Meth-:甲eth-:乙propyl-:丙but-:丁Pent(a)-:戊hex-:己hepta-:庚oct-:辛non-:壬deca-:葵skeleton:骨架-yl: (烷)基methyl:甲基ethyl:乙基alkyl :烷基side chains: 侧链substituent:取代基cis and trans isomers :顺式和反式异构体enantiomers :对映异构体di-:二tri- : 三tetra- : 四penta-: 五straight-chain:直链iso-:异primary carbon:伯碳secondary carbon:仲碳tertiary carbon:叔碳allyl :烯丙基methylene :亚甲基ethylidene :亚乙基Ethylene:次乙基hybridization :杂化cycloalkane :环烷alkene :烯烃geometric isomer:几何异构体chiral carbon:手性碳clockwise direction:顺时针方向counterclockwise direction :逆时针方向Glyceraldehyde:甘油醛substitution reaction :取代反应nucleophilic substitution:亲核取代nucleophile :亲核试剂dissociate :离解carbocation :碳正离子Intermediate:中间体substrate :底物leaving group :离去基团electrophilic substitution:亲电取代carbanion :碳负离子addition reaction :加成反应addition product:加成物attacking reagent:进攻试剂free radical:自由基migration:移动elimination reaction:消去反应adjacent carbon:相邻碳rearrangement reaction:重排反应酸酐acid anhydride 酸性化物acid halide乙醇alcohol 乙醛aldehyde脂肪族aliphatic 烷烃alkane烯烃alkene 醇盐alkoxide烷基alkyl 炔烃alkyne烯丙基allyl 氨基化合物amide胺amine 氨基酸amino acid氨(络)合物ammine 芳烃arene芳香环aromatic ring 芳基aryl含氮的azo 羰基carbonyl羧酸carboxylic acid 胡萝卜素carotene螯合物chelate 手性chiral构象异构体conformer 配位数coordination number晶体场稳定化能crystal filed splitting energy右旋性的dextrorotary重氮化作用diazonium salt二氯甲烷dichloromethane 酯ester脂肪酸fatty acid 自由基free radical官能团functional group 甘油glycerol杂环的heterocyclic 高自旋配合物high spin complex 同系物homolog 烃hydrocarbon诱导效应inductive effect 酮ketone左旋的levorotatory 配体ligand低自旋配合物low spin complex 甲基methyl分子筛molecular sieve 单齿配位物monodentate辛烷octane 旋光性optical activity石蜡paraffin 苯酚phenol苯基Phenyl 多配位基的polydentate聚合物Polymer 丙烷propane外消旋的Racemic 共振效应resonance effect过氧化物superoxide互变异构体tautomer薄层分析法thin layer chromatography甘油三(酸)酯triglyceride不饱和化合物unsaturated compound水煤气water gas两性离子zwitterionbeaker 烧杯phenolphthalein indicator 酚酞指示剂Pipette 移液管acetic acid 醋酸pH meter pH计standard titration curve 标准滴定曲线benzoic acid 苯甲酸ethyl alcohol 乙醇qualitative organic analysis 定性有机分析Unambiguous 明确的characterize 表征structure determination 结构测定bunsen burners 煤气喷灯tripod supports 三角支架wash bottles 洗瓶dropper 滴管transfer pipette 移液管hot plate 轻便电炉wire gauzes 石棉网test tube brush 试管刷test tube rack 试管架ring stand with rings 带环环架filter paper 滤纸utility clamp 铁试管夹clamp holder 夹柄buret clamp 滴定管夹extension clamp 万能夹ring clamp 环形夹子pinchcock 弹簧夹pinch clamp 弹簧节流夹tubing clamp 管夹hose clamp 软管夹test tube clamp 试管夹cork borer set木塞钻孔器套件cork stopper 软木塞rubber stopper 橡胶塞laboratory jack 实验升降台spatulas 刮刀beaker tongs 烧杯钳crucible tongs 坩埚钳Forceps 医用钳tweezer 镊子watch glasses 表面皿goggles 护目镜carbon stirring rod 碳搅拌棒fume hoods 通风橱capillary melting point tube 毛细管熔点管Caliper 卡尺table balance 托盘天平analytical balance 分析天平top pan balance 市秤magnetic stirrers 电磁搅拌器Fahrenheit thermometer 华氏温度计celsius 摄氏度bulb pipettor 球形移液器magnetic stir bar 磁搅拌子Mortars 碾钵pestles 捣锤spotting plates 滴试板alcohol lamp 酒精灯Spectrophotometers 分光光度计connection tube 连接管rubber tube 橡皮管adapter 接合器Socket 套接口ball joint 球形接头Stopper 塞子adaptor 转接口splash heads 防溅头thermometer pocket温度计插孔air leak tube 空气渗漏管distill head 蒸馏头melting point tube 熔点管burettes 滴管Stopcock 活塞aspirating stopcock 吸气式移液管measuring cylinder 量筒volumetric flask 容量瓶centrifuge tube 离心管graduated 有刻度的chromatography colum 层析柱filter funnel 过滤漏斗pressure equalizing funnel 均压漏斗powder funnel 药粉漏斗separating funnel 分液漏斗dropping funnel 滴液漏斗rotary evaporator 旋转蒸发仪spectrophotometer 分光光度计crystallizing dishes with spout 带喷嘴的结晶皿petri dishes 培养皿evaporating dishes 蒸发皿drying tube 干燥管condenser 冷凝管Reflux 回流erlenmeyer flask 锥形烧瓶round bottom flask 圆底烧瓶flat bottom flask 平底烧瓶distillation flask 蒸馏烧瓶filtration flask 过滤瓶round bottom flask 圆底烧瓶weighing bottles 称量瓶reagent bottle 试剂瓶aspirator bottle 蒸馏水瓶BOD bottle 生化需氧量瓶dropping bottle 滴瓶specific gravity bottle 比重瓶glass desiccator 玻璃干燥器Beaker 烧杯extractor 萃取器dry tower 干燥塔vaccume dessicator 真空干燥器gas washing bottle 气泡吸收瓶distilling receiver 蒸馏接收器。
应用化学专业英语第一单元The-Roots-Of-Chemistry
The Roots Of ChemistryChemistry can be broadly defines as the science of molecules and their transformations.化学可以广泛地定义为科学的分子和他们的转换。
In contrast to mathematics,chemistry is older than people. 与数学不同,化学比人类更久远。
The appearance of life and people on our planet (earth) is most probably the end result of specific chemical processes .生命的出现和人类生活在我们地球上都最可能是特殊化学过程的结果。
Chemical processes have been present in the lives of people from the dawn of history until the present time .化学过程存从古至今存在人们的生活中。
Initially ,these processes were not under our control 最初,这些过程不受我们的控制,,for instance ,the fermentation of fruit juice,the rotting of meat and fish ,and the burning of wood .例如,果汁的发酵,肉和鱼的腐烂,木材的燃烧。
Later on we learned to control chemical processes and to use them to prepare a variety of different products such as food ,metals ,ceramics and leather .后来,我们学着控制化学过程,用它们来准备一系列不同的产品例如食物。
《应用化学专业英语》课程思政教学案例(一等奖)
《应用化学专业英语》课程思政教学案例(一等奖)一、课程简介《应用化学专业英语》课程的目标是培养学生对本专业科技文献的阅读能力、专业英语写作能力、以及听说能力。
通过课程的学习,可以提高学生阅读和理解专业文献的能力,满足其今后从事专业工作、科学研究和国际交流的要求。
然而,由于化学专业英语涉及到大量专业词汇,生僻而又难读;内容具有很强的专业性、逻辑性,深奥严谨而难以理解,对初涉专业英语学习的大学生而言,感到十分棘手的。
尤为重要的是,专业词汇和种类繁多的化合物名称的准确发音,这是一个在以往的教学中最容易被忽略的内容,也是目前专业英语教学中的一个薄弱环节。
为了解决这一重要环节,张秀凤老师在疫情期间克服重重困难、倾情录制完成《化学工程与工艺专业英语》专业音频课程,开辟了有声课堂的先例,从根本上改变了长期以来学习哑巴专业英语的局面,为学习化学专业英语打造了一套高质量、发音纯正地道的原汁原味的优质音频资料,填补了化学专业英语教学中音频课程缺失的历史空白,为专业英语课程的建设和改革提供了新思路及新探索。
二、教学目标(一)本讲的课程思政教学目标1.通过讲解化合物生产的来源以及全球资源现状,帮助学生树立可持续的科学发展观(思政目标),掌握专业英语词汇及文章翻译的技巧(教学目标);2.通过多媒体等多种形式的拓展教学,引导学生树立“绿色发展”理念(思政目标),坚定学习化学专业知识和从事化工行业的信心(教学目标)。
(二)案例如何体现课程思政教学目标1.结合案例中对塑料的回收再制造过程,即将塑料进行粉碎筛选、高温脱水、再生造粒等工艺,后续制成的再生塑料制品可用于汽车、玩具、文具、服装、建筑等多种领域。
将废弃塑料进行回收再生,大量减少了白色污染,降低了70%以上的碳排放,同时可拉动大量就业,实现了绿色全面发展。
2.现实中对塑料制品的随意丢弃,将全球严峻的资源现状进行拓展。
我国的资源现状是富煤、贫油、少气,因此如何高效率的利用这些不可再生资源是科学家工程师努力追求的目标,也体现了我国所倡导的可持续发展理念。
应用化学专业英语考试必背
3、 读 法
高温,高压
• 3.1 Nitrogen reacts with hydrogen to form ammonia at high temperature and pressure with the presence of a catalyst.
• 1 mol nitrogen reacts with 3 mol hydrogen to form 2 mol ammonia at high temperature and pressure with the presence of a catalyst.
pale yellow dark brown
2)state
solid
liquid
gas
gaseous
crystalline molten
oily
uncrystalline fused
3)smell
odourless
pungent
penetrating
choking
offensive
sour sweet bitter
CuO: copper(II) oxide或cupric oxide
2.化合物负电荷部分的读法:
2.1二元化合物 2.2 非金属氢化物 2.3 无氧酸 2.4 含氧酸与含氧酸根阴离子 2.5 盐
4.2.1 二元化合物
常见的二元化合物有卤化物,氧化物,硫化物,氮化物,磷化物,碳化 物,金属氢化物等,命名时需要使用后缀-ide, 如 : fluoride , chloride , bromide , iodide , oxide , sulfide , nitride, phosphide, carbide,hydride; OH -的名称也是用后缀-ide:hydroxide, 非金属氢化物不用此后缀,而是将其看成其它二元化合物(见4.2.2), 非最低价的二元化合物还要加前缀, 如O22-: peroxide O2-: superoxide 举例:NaF: sodium fluoride AlCl3: aluminium chloride Mg2N3 : magnesium nitride Ag2S: silver sulfide CaC2: calcium carbide Fe(OH)2:iron(II) hydroxide 有些物质常用俗称,如NO nitric oxide N2O nitrous oxide
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Names of Cations
阳离子名称
The names of monatomic cations are the same as the name of the element, with the addition of the word ion, as in sodium ion for Na+.
Names of Anions
阴离子名称
Monatomic anions are named by adding the suffix –ide and the word ion to the first part of the name of the element ( the “stem” of its name ).
大多数过渡金属都能形成超过一种的离子, 因此在它们化合物的名称中包含物料编号通 常是有必要的。
An older systFra bibliotekm of nomenclature is still in use.
旧的命名系统仍然在使用。
For example, some cations were once denoted by the endings –ous and –ic for the ions with lower and higher charges, respectively.
The Nomenclature of Inorganic Substance
无机化合物命名法
You will meet compounds in this text and will learn their name as you go along.
在本文中,你会遇到许多化合物,并且 当你阅读下去时你将获悉他们的名字。
However, it is useful from the outset to know something about how to form their names.
然而,从一开始了解一下化合物名字的形成 是有用的。
Many compounds were given common names before their compositions were known. Common names include water, salt, sugar, ammonia, and quartz.
单原子阳离子的名称与元素的名称相同, 外加离子一词,如Na+表示钠离子。
When an element can form more than one kind of cation, such as Cu+ and Cu2+ from copper, we use the Stock number, a Roman numeral equal to the charge of the cation.
ammonia [əˈməʊnɪə] 氨水
许多化合物在未了解其构成前给予他们俗称。 俗称包括水、盐、糖、氨和石英。
A systematic name, on the other hand, reveals which elements are present and, in some cases, how their atoms are arranged.
例如,一些阳离子曾经用-ous和-ic结尾分别 表示较低的和较高的电荷。
In this system, iron (Ⅱ) ions are called ferrous ions and iron (Ⅲ) ions are called ferric ions.
在这个系统中,二价铁离子被称作亚铁离子, 三价铁离子被称作铁离子。
当元素能形成超过一种的阳离子,如铜能形成 Cu+和Cu2+,我们使用物料编号,罗马数字等 于阳离子电荷,
Thus, Cu+ is a copper (Ⅰ) ion and Cu2+ is a copper (Ⅱ) ion. Similarly, Fe2+ is an iron (Ⅱ) ion and Fe3+ is an iron (Ⅲ) ion.
名称中不必给出电荷,因为大部分的元素形成 单原子阴离子只有一种形式。
单原子阴离子通过在元素名字(名字的词干) 的第一部分加后缀-ide和离子单词来命名。
There is no need to give the charge, because most elements that form monatomic anions form only one kind of ion.
另一方面,系统名称显示出其存在的元素, 在某些情况下可显示其原子是如何排列的。
The systematic name of table salt, for instance, is sodium chloride, which indicates at once that it is a compound of sodium and chlorine.
因此,Cu+是铜的一价离子,Cu2+铜的二价 离子。 类似的,Fe2+是铁的二价离子,Fe3+是铁的 三价离子。
Most transition metals form more than one kind of ion, so it is usually necessary to include a Stock number in the names of their compounds.
例如食盐的系统名称是氯化钠,这 立即表明它是一个钠和氯的化合物。
The systematic naming of compounds, which is called chemical nomenclature, follows a set of rules, so that the name of each compound need not be memorized, only the rules.
