应用化学专业英语
应用化学专业英语 -化合物命名

醇
酚类命名法
硫醇和硫酚
酮的命名法Biblioteka 根基命名法:醚类命名
羧酸命名
CH3-CH2-CH(CH3)-CH3 2-methylbutane
2,2-dimethylpropane CH3CH(CH3)-CH2-CH(C2H5)-CH(CH3)-CH2-CH3 4-ethyl-2,5-dimethylheptane
除了系统命名法,有一些支链烷烃是可以采用普 通命名法来命名的
化合物的英语命名 Nomenclature of compounds
樊海梅
LOGO
有机化合物的命名
链 烃
饱和烃:烷烃 不饱和烃:烯烃,炔烃 脂环烃 芳香烃
烃
有 机 物
环 烃
卤代烃
烃 的 衍 生 物
醇
含 氧 衍 生 物
酚 醛 酮 羧酸
酯等
烷烃(alkanes) 直链烷烃:英文名称除了含1到4个碳原子以外,其余均用希腊
90 alkane:nonacontane
100 alkane:hectane
含支链烷烃和烷基 烷基:只需要把烷烃的后缀ane换成-yl加在相应烷烃的字 首后 如:CH3- methyl CH3-CH2- ethyl CH3-(CH2)9-CH2 undecyl
还有一些烷基也可以在相应的烃名前加iso-(异)、sec-仲、tert-叔、
CH3CH2-C(CH3)2-CH3:
烯烃(alkene)和炔烃(alkynes):将相应的烷烃的词尾(ane)改为
ene和yne,名称前加上不饱和键的编号, 如:乙烯 ethene 丁烯 butene 乙炔 HC≡CH ethyne 丁二炔 HC ≡C-C ≡CH butadiyne
应用化学专业英语-Lesson-2..

• Elements are composed of extremely small particles called atoms. All atoms of a given element are identical. The atoms of one element are different from the atoms of all other elements.
• Compounds are composed of atoms of more than one element.
• Chemical reactions involve only the rearrangement of atoms; atoms are not created or destroyed in chemical reactions.
• Protons and neutrons are located in the nucleus of the atom, which is small. Most of the mass of the atom is due to the nucleus.
• Electrons are located outside of the nucleus. Most of the volume of the atom is due to electrons.
Some Complex Ions
Name Carbonate Nitrate Phosphate Dihydrogen Phosphate Sulfate Sulfite Thiosulfate Perchlorate Chlorite Cyanide Chromate
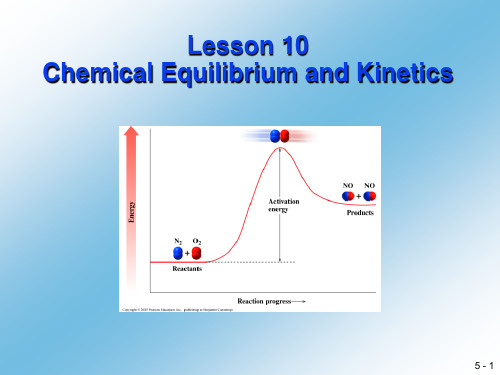
应用化学专业英语lesson10ChemicalEquilibriumandkinetics
NH3
Add more NH3?
Reaction shifts to the left [N2] and [H2] inc
5 - 35
Le Chatelier’s principle
Adding Pressure affects an equilibrium with gases
N2(g) + 3 H2(g)
N2(g) + 3 H2(g)
Keq =
[ NH3 ] 2 [ N2 ] [ H2 ] 3
2 NH3(g)
5 - 33
Le Chatelier’s principle
Stress causes shift in equilibrium Adding or removing reagent
N2(g) + 3 H2(g)
Temperature: 2. Higher Temperature:
Faster cars More collisions
More Energy More collisions
Reacting molecules move faster, providing colliding molecules w/ Eact.
At this point, equilibrium is achieved. Time
5 - 26
Figure 9.8
2SO2(g) + O2(g)
At Equilibium
2SO3(g)
SO2(g)+O2(g)
Initially
SO3(g)
Initially
5 - 27
Figure 9.9
2SO2(g) + O2(g)
应用化学专业英语 -无机化学命名

(3) 基本元素有多种价态 酸:最低氧化态(次酸) 基础元素(前缀 hypo-, 后缀 -ous) +acid 较低氧化态(亚酸) 基础元素加后缀-ous + acid 较高氧化态 (正酸) 基础元素加后缀-ic + acid 最高氧化态(高酸) 基础元素(前缀 per-, 后缀 -ic) +acid 盐:最低氧化态 阳离子元素 + 基础元素(前缀 hypo-, 后缀 -ite) 较低氧化态 阳离子元素 + 基础元素加后缀-ite 较高氧化态 阳离子元素 + 基础元素加后缀-ate 最高氧化态 阳离子元素 + 基础元素(前缀 per-, 后缀 -ate)
16. K4[Fe(CN)6]; 17. CuSO4· 5H2O 18. Cu2(OH)2CO3 19. NaNH4SO4
1. (NH4)2CO3: ammonium carbonate 2. N2O: nitrogen(Ⅰ) oxide; nitrous oxide ; laughing gas 3. H2SO4: sulphuric acid 4. P4O6 diphosphorus trioxide 5. Al2O3 Aluminum oxide 6. SnCl4 tin(Ⅳ) chloride; stannic chloride; tin terachloride 7. KHSO4 Potassium hydrogen sulfate 8. Cu2S copper(I) sulphide; dicopper sulphide 9. HClO4 perchloric acid
含氧酸及其盐:
(1) 基本元素仅有一种氧化态
酸:基本元素加后缀-ic +acid 例:H2CO3 carbonic acid 盐:阳离子元素+基础元素加后缀-ate 例:Na2CO3 sodium carbonate (2) 基本元素有两种氧化态 酸:基础元素加后缀(-ous 低价态,-ic 高价态) + acid HNO2:nitrous acid HNO3: nitric acid 盐:阳离子元素+基础元素加后缀( -ite低价态,-ate 高价态) NaNO2: Co(NO3)2: sodium nitrite cobalt(II) nitrate or cobaltous nitrate
应用化学专业英语复习资料

一单词短语1.Molecule 分子molecular 分子的2.chemical process 化学过程element 元素3.a t o m原子a t t r a c t i o n吸引力4.repulsion 排斥力distillation 蒸馏、n5.distill 蒸馏v rectification 精馏position 构成structure 结构7.property 性质mass 质量8.atomicweight 原子量atomic number 原子序数9.ionization energy 电离能period 周期10.g r o u p族f a m i l y族11.transition group 过渡族main group 主族12.i o n离子s u b s t i t u t i o n取代反应13.el i mi na ti on消除反应nucl eoph i l i c 亲核的14.nucleophilie 亲核试剂electrophilie亲电试剂15.alkyl 烷基的functional group 官能团16.halides 卤素的leaving group 离去基团17.transition state过渡态intermediate 中间体18.r e a c t a n t反应物p r o d u c t生成物19.concentration 浓度rate equation 速率方程20.c o n s t a n t常数e t h e r醚21.endothermic 吸热的substrate 反应底物22.mechanism 机理reagen 试剂23.alkene 烯烃exothermic 放热的24.A n i o n阴离子n i t r o g e n氮气25.Hydrocarbon 碳氢化合物carbonhydrate 碳水化合物26.Alkane 烷烃substituent 取代基27.Isomerism 同分异构现象isomer 同分异构28.V i n y l乙烯基d e r i v a t i v e s衍生物29.acid halides 酰卤acid anhydrides 酸酐30.e s t e r s酯a m i d e酰胺31.ammonia NH3 Acetic anhydride乙酸酐32.phenol 芬acid—base titration 酸碱滴定33.precipitation沉淀analyses 化学分析员34.IR 红外UV紫外MS质谱GC色相色谱HPLC高效液相色谱TLC薄层色谱X—rayX射线衍射二选词填空1、We can now easily account for many things,which were thought to be mysterious by theancients2、the acid acts on the metal and a gas is givenoff.3、you should adapt yourself to new ways oflooking at matters4、electrolytes have more pronounced effect oncolligative properties than do nonelectrolytes. 5、if water in these lakes evaporated at the samerate as fresh water ,both would nearly dryup in a matter of year.6、both laks evaporated very slow compared with afresh lake or even the ocean.7、a property that depends only on the relativeamounts of solute and solvent is know as acolligative property.8、for example ,both NaCl (ionic) and HCl (polarcovalent)are classified as electrolytes becausethey form ions in aqueous solution.9、when compounds such as NaCl and HCl aredissolved in water ,the effect is obvious.10、if the wires is cut ,the light goes out becausethe circuit is broken.11、when wires are attached to a charged batteryand then to a light bulb ,the light shinesbrightly.12、glass and wood as well as pure water areexamples or nonconductors of electricity.13、other substances resist the flow of electricityand are known as nonconductors orinsulators.14、it has long been known that the presence of asolute in water may affect its ability toconduct electricity.15、when the collection of papers was first broughtout,it was well received by the reviewers.16、in the same way the dozen or so mostcommon kinds of kinds of atoms can be put together in many millions of different ways tomake molecules .17、elements are made up of tiny fundamentalparticles called atoms. Fundamental, as it is usedhere ,means that they cannot be furtherdivided by any chemical metheods.18、each element has atoms that is different fromthe atoms of other elements.19、it would not be quite round; on the contraryit would consist of three parts represented byspheres.20、it is not to be summed up in a singleproduct or word ,but in an idea or basicconcept.21、the chemical symbol of an element may standthe element for.22、the rate of a chemical reaction is influencedby several factors such as temperature ,concentration of reagents , particle size ,light ,and catalyst.23、all forms of life in earth are very dependenton chemical reactions or chemical changes.24、a chemical reaction occurs when elements andcompounds react together to produce differentcompounds , or when compounds break down into simpler compounds or elements.三无机物的命名H Hydrogen Li Lithium Na Sodium K Potassium Mg Magnesium Ca CalciumMn manganese Cu copper Zn zinc Fe iron Hg mercury Ag silver Au gold C Carbon Si Silicon Pb Lead Al Aluminium F Fluorine Cl Chlorine Br Bromine I IodineO Oxygen S Sulfur N Nitrogen P Phosphorus1.直呼其名,即读其元素名称+ ion如:Na+ sodium ionK+ potassium ion2.对于有变价的金属元素,除了可用前缀来表示以外,更多采用罗马数字来表示金属的氧化态,或用后缀-ous 表示低价,-ic 表示高价如:Cu+ copper (Ⅰ) ion 或cuprous ion Cu2+ copper (Ⅱ) ion 或cupric ionFe2+ iron (Ⅱ) ion 或ferrous ionFe3+ iron (Ⅲ) ion 或ferric ion3.含氢酸根:酸根中的H读做hydrogen,氢原子的个数用希腊前缀表示:mono- di - tri- tetra - penta- hexa-hepta- octa- nona- deca-举例:CO32-carbonate ionHCO3-hydrogen carbonate ionPO43- phosphate ionHPO42hydrogencarbonate ionH2PO4- dihydrogenphosphate ion4.结晶水读做hydrate ,结晶水的个数用希腊前缀表示:mono-di - tri- tetra - penta- hexa- hepta- octa- nona- deca-CuSO4·5H2O copper(Ⅱ) sulfate pentahydrateAlCl3 ·6H2O aluminum chloride hexahydrate5.测试Mg(OH)2magnesium hydroxide AlCl3aluminum chlorideFeBr2 iron(II) bromide CaSO4calcium sulfateZnCO3zinc carbonate HF hydrofluoric acidH3PO4phosphoric acid NO2nitrogen dioxideCuO copper(II) oxide Al2O3aluminum oxideNaHSO3sodium hydrogen sulfiteKMnO4potassium permanganateNaClO sodium hypochloride四有机物的命名1)命名正烷基时,只需把烷烃的词尾“-ane换成“-yl”,加在相应的烷烃的字首后2)字母规则:Butyl>Ethyl>Isopropyl>Methyl>Neopentyl>tert-Pentyl >Propyl3)环烷烃:只需在所对应的烷烃前加上cyclo-即可4)有些结构较复杂的烷基,需添加词头5)烯烃和炔烃命名时将相应的烷烃的词尾“烷”(ane)改为“烯”(ene)或“炔”(yne),后缀前加上不饱和键的编号即可。
第二版应用化学专业英语课后答案

Unit 1 The Roots of ChemistryI. Comprehension.1.C2. B3. D4. C5. BII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerabledifficulty, and it is necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematizedknowledge and is also an activity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistryobserved on a single mundane planet.4.People are made of molecules; some of the molecules in people are rathersimple whereas others are highly complex.5.Chemistry is ever present in our lives from birth to death because withoutchemistry there is neither life nor death.6.Mathematics appears to be almost as humankind and also permeates allaspects of human life, although many of us are not fully aware of this.III. Translation.1.(a) chemical process (b) natural science (c) the technique of distillation2.It is the atoms that make up iron, water, oxygen and the like/and so on/andso forth/and otherwise.3.Chemistry has a very long history, in fact, human activity in chemistry goesback to prerecorded times/predating recorded times.4.According to/From the evaporation of water, people know/realized thatliquids can turn/be/change into gases under certain conditions/circumstance/environment.5.You must know the properties of the material before you use it.IV. Translation化学是三种基础自然科学之一,另外两种是物理和生物。
应用化学专业英语(课后答案和课文翻译)

Unit 1 The Roots of ChemistryI. Comprehension.1.It can be inferred from this article which one of the following items is not mainly based on practical use C. Greek chemistry2. It was B. Empedocless who first introduced the idea that all things are not formed from just one element.3. In the development of Greek chemistry, D. Democritus was the first one definiting the ultimately constituents of matter?4. According to Plato, there are B. 4 ―elements‖ whose faces are constituted by regular polygons.5. In the last paragraph,authors think that experiment DD.can deal with the reactions by which one substance is converted into anotherII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerable difficulty, and itis necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematized knowledge and is also anactivity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistry observed on a singlemundane planet.4.People are made of molecules; some of the molecules in people are rather simple whereasothers are highly complex.5.Chemistry is ever present in our lives from birth to death because without chemistry there isneither life nor death.6.Mathematics appears to be almost as humankind and also permeates all aspects of human life,although many of us are not fully aware of this.III. Translation.1.(a)化学过程;(b)自然科学;(c)蒸馏技术(a) chemical process (b) natural science (c) the technique of distillation2.正是原子构成铁、水、氧等。
应用化学专业英语(课后答案和课文翻译)

Unit 1 The Roots of ChemistryI. Comprehension.1.It can be inferred from this article which one of the following items is not mainly based on practical use C. Greek chemistry2. It was B. Empedocless who first introduced the idea that all things are not formed from just one element.3. In the development of Greek chemistry, D. Democritus was the first one definiting the ultimately constituents of matter?4. According to Plato, there are B. 4 ―elements‖ whose faces are constituted by regular polygons.5. In the last paragraph,authors think that experiment DD.can deal with the reactions by which one substance is converted into anotherII. Make a sentence out of each item by rearranging the words in brackets.1.The purification of an organic compound is usually a matter of considerable difficulty, and itis necessary to employ various methods for this purpose.2.Science is an ever-increasing body of accumulated and systematized knowledge and is also anactivity by which knowledge is generated.3.Life, after all, is only chemistry, in fact, a small example of chemistry observed on a singlemundane planet.4.People are made of molecules; some of the molecules in people are rather simple whereasothers are highly complex.5.Chemistry is ever present in our lives from birth to death because without chemistry there isneither life nor death.6.Mathematics appears to be almost as humankind and also permeates all aspects of human life,although many of us are not fully aware of this.III. Translation.1.(a)化学过程;(b)自然科学;(c)蒸馏技术(a) chemical process (b) natural science (c) the technique of distillation2.正是原子构成铁、水、氧等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
英译汉:1.First, electrons are added one at a time moving from left to right across aperiod……首先,从左向右横跨一个周期时每次增加一个电子。
当这种情况发生时,最外层电子将受到逐渐增强的核引力,所以电子将更接近原子核而受到其更紧密的束缚力。
其次,在周期表中从上向下移动一列,最外层电子受到核的束缚力将变弱。
这是因为主能级数(屏蔽最外层电子受到核的吸引)在每族向下移动时增加。
这些趋势解释了通过观察元素的原子半径、电离能、电子亲和力和电负性而得到的元素性质的周期性规律。
2.It is important to note that at equilibrium the rates of reaction,rate r and rate fare equilibrium mixture are usually not equal……值得注意的是,在化学平衡时的反应速率,正反应速率和你反应速率相等但反应物和生成物的摩尔浓度在平衡混合态时一般不相等。
但是,事实上每种反应物和生成物在平衡时其浓度为定值,因为每种物质在一个反应中的消耗速率与其在相应你反应正的生成速率相等。
在化学平衡提出之前,这种系统被称为动力学平衡状态。
3.This is a mathematical expression of the law of chemical equilibrium which maybe stated as follows: When a reversible…………这是化学平衡定律的数学表达式,它可以通过如下所述:当一个可逆反应在给定温度下达到平衡时,在方程式中箭头右边物质的摩尔浓度的积除以左边物质摩尔浓度的积(每种物质浓度的幂等于反应方程式中每种物质的分子数)为定值,4.Analytical chemistry,or the art of recognizing different substances anddetermining their constituents, takes a prominent position among分析化学或鉴定不同物质并测定其成分的技术,因为可以解决每当化学过程被用于科学的或技术性的目的是产生的问题,而在科学应用领域中占显著地位。
其极其高的重要性便得它在化学历史上的一个非常早的时期已经被辛勤耕耘了,其记载包含了分布在整个科学领域定量分析工作的一大部分。
定量分析的测量也在许多研究领域:化学、生物化学、地质学和其它科学中发挥重要作用。
5.The interaction of UV and visible radiation with matter can provide qualitativeidentification of molecules and polyatomic……………紫外可见光与物质相互作用时可以提供包含离子和复合物的分子和多原子的物种的定性鉴定。
分子和多原子物种,特别是有机分子的结构信息可以得到。
这种定性信息通常是通过研究紫外可见光谱获得(紫外可见过的吸收作为穿过分子的波长的函数)紫外可见光的吸收带的形状和强度与吸收物质的电子的结构有关。
分子通常是被溶解在溶剂中来获得光谱。
6.One of the most important features of fine chemicals manufacture is the greatvariety of …………………随着新产品持续不断地出现,精细化学品生产制造的一个最重要特征是产品的多样化。
因此,许多化学品都存在需求上的重大波动,如果每种产品都通过专用于某特定工序的车间来生产,投资和劳工费用将是巨大的。
结合需要的不断改变和假设机器设备一般在它们设定的最大容量一下正常运行,这将使制造业的花费很高。
因此,只有多产量的精细化学品或通过特殊的产法或纯度要求极高的化合物才可以用专门的车间来生产。
然后,大多数精细化学品都是在多目标的或多产品的生产线(车间)生产制造。
汉译英:7.Two conservation laws apply to all chemical reactions: Energy can neither becreated nor destroyed, and matter can neither be created nor destroyed.两个守恒定律的适用于所有的化学反应:能量既不能创造也不能被消灭,而物质既不能创造也不能被消灭。
8.We often hear about "toxic chemicals" or "chemical pollution" without hearingabout the absolutely central role that chemistry plays in human well-being.我们经常听到“有毒化学品”或“化学污染”没有听到关于绝对的核心作用,化学对人类福祉。
9.Some chemists investigate the natural world and try to understand it,while otherchemists create new substances and new ways to perform chemical changes that do not occur in nature.一些化学家的研究自然世界和理解它,而其他化学家创造新物质和新的方法来进行化学变化没有发生性质。
10.Based on the work of physicist Henry Mosteley, the periodic table was reorganizedon the basis of increasing atomic number rather than on atomic weight.根据物理学家亨mosteley,周期表被重组的基础上,原子序数的增加而不是原子重量。
11.Reactions in which the reactants and products coexist are considered to be inequilibrium.反应中,反应物和产物被认为是平衡共存。
12.The law of conservation of mass dictates the quantity of each element does notchange in a chemical reaction.守恒定律质量要求数量的每个元素不改变化学反应。
13.The zinc metal is oxidized to zinc ions and the copper ions are reduced to coppermetal.Zn 被氧化成Zn2+ ,Cu2+被还原14.Chemistry is the science that tries to understand the properties of substanceand the changes that substances undergo.化学科学试图了解物质的性质和变化的物质进行。
15.The accuracy of analytical methods has increased enormously in the past decadesand this has enabled detection of even almost negligible traces of impurities.该分析方法的准确度已大大增加,甚至可以检测几乎可以忽略的痕量杂制。
16.When chloride is added to be a silver solution ,solid silver chlorideprecipitates from solution .The resulting equilibrium is always written in the direction of the solid dissolving :AgCl(s)==Ag(aq)+Cl(s)氯离子加到银盐溶液,固态氯化银从溶液中沉淀出来。
结果平衡总是写成固体溶解。
17.Once these chemicals are isolated and their chemical structures aredetermined,the creativity of chemists takes over.一旦这些化学物质的分离和其化学结构决定的,创造性的化学家接管。
18.Syntheses are now normally designed using the fundamental principles thatchemists have discovered. As many as 30 or more predicted chemical steps are sometimes needed,in a sequence,to permit the synthesis of a complicated molecule from available simple chemicals.This could not be done without a clear understanding of chemical principles.合成现在通常使用而设计的基本原则,化学家们发现。
多达30个或更多的预测的化学步骤,有时是必要的,在一个序列,允许合成复杂分子从可用简单的化学品。
不能没有一个明确的了解化学原理。
19.In doing so, chemical engineer must also use principles of thermodynamics,reaction kinetics, fluid mechanics and transport phenomena.在这样做时,化学工程师还必须使用原则,热力学,反应动力学,流体力学和运输现象。
20.Both of these are combination reactions,and both can be reversed by heating theproducts.Metal hydroxides decompose on hearing to give the metal oxide and water.这些都是结合反应,都可以扭转的加热产品。
