Ch2 Exercise Solutions
第二章:单官能团2019
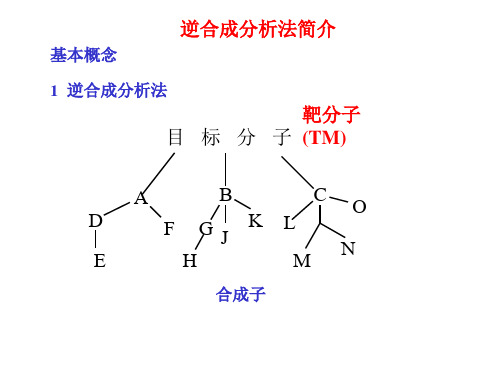
O
O
FGI
H
3
HC
O
O
HC
+ Cl
2 2
3
3
[合成]
HC
3
O
O
Cl
3
AlCl H C
3
CrO , Ac O TM HO
Exercise 合成下列两种常用药物分子
头痛、牙痛、 神经痛、肌肉痛及月经痛, 也用于感冒、流感等退热、消炎、抗风湿 预防暂时性脑缺血发作、心肌梗塞、心房 颤动、人工心脏瓣膜、动静脉瘘或其他手 术后的血栓形成
CH2Ph H3PO4
TM
(2) CH3COCH3 (3) H3O+
H3C OH
要点: 羟基添加的位置
Exercise
Ph
OAc Ph TM12
Cl O2N
3 Disconnection of alkene 烯烃的切断与合成
(1)Addition of H2O
O
FGI
HO
MgBr
FGI
HO
Useless
O Br
O CO2H
[分析]
1. 由卤代烃转变为增加一个碳原子的羧酸,可用腈化合物 水解法和Grignard试剂法。
2. 所给卤代烃虽然为伯卤代烃,但由于β-C上有两个支链, CNˉ 与其发生SN2反应位阻大,效果不好。
3. 由于卤代烃分子中含有羰基,格氏试剂会与羰基发生作用, 因此,我们只有将羰基保护起来,才能利用格氏试剂法。
CO2Et
Base
CH2
CO2Et CH CO2Et
Example:
Homework 4
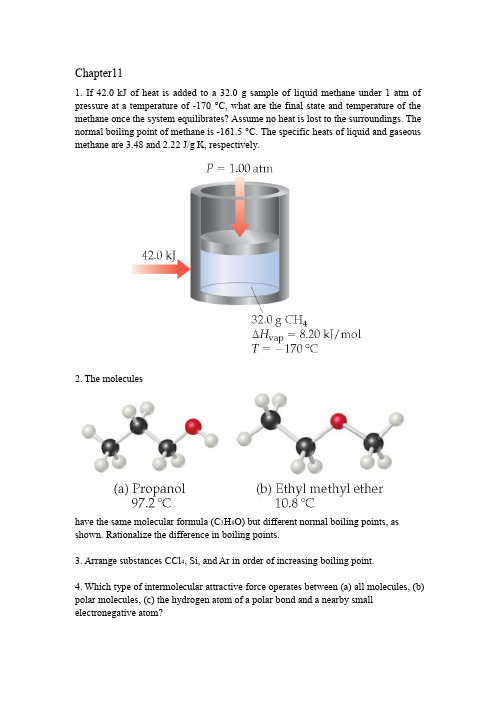
Chapter111.If 42.0 kJ of heat is added to a 32.0g sample of liquid methaneunder 1 atm of pressure at a temperature of -170 °C, what arethe final state and temperature of the methane once the systemequilibrates? Assume no heat is lost to the surroundings.The normal boiling point of methane is-161.5 °C. Thespecific heats of liquid and gaseous methane are3.48 and2.22 J/gK, respectively.2.The moleculeshave the same molecular formula (C3H8O) but different normalboiling points, as shown. Rationalize the difference inboiling points.3.Arrange substances CCl4, Si, and Ar in order of increasingboiling point.4.Which type of intermolecular attractive force operates between(a) all molecules, (b) polar molecules, (c) the hydrogenatom of a polar bond and a nearby smallelectronegativeatom?5.Describe the intermolecular forces that must be overcome toconvert these substances from a liquid to a gas: (a) SO2,(b) CH3COOH, (c) H2S.6.Which member in each pair has the larger dispersion forces:(a) H2O or H2S, (b) CO2 or CO, (c) SiH4 or GeH4?7.Ethylene glycol (HOCH2CH2OH), the major substance in antifreeze,has a normal boiling point of 198 °C. By comparison,ethyl alcohol (CH3CH2OH) boils at78 °C at atmospheric pressure.Ethylene glycol dimethyl ether (CH3OCH2CH2OCH3)has a normal boiling point of 83 °C, and ethyl methyl ether(CH3CH2OCH3) has a normal boiling point of 11 °C. (a) Explainwhy replacement of a hydrogen on the oxygen by a CH3group generally results in a lower boiling point. (b) What arethe major factors responsible for the difference in boilingpoints of the two ethers?8.Explain the following observations: (a) The surface tension ofCHBr3 is greater than that of CHCl3. (b) As temperature increases,oil flows faster through a narrow tube. (c) Raindropsthat collect on a waxed automobile hood take on a nearlyspherical shape. (d) Oil droplets that collect on a waxed automobilehood take on a flat shape. the phase transition in each of the following situationsand indicate whether it is exothermic or endothermic:(a)When ice is heated, it turns to water. (b)Wet clothes dry ona warm summer day. (c) Frost appears on a window on a coldwinter day. (d) Droplets of water appear on a cold glass ofbeer.10.For many years drinking water has been cooled in hot climatesby evaporating it from the surfaces of canvas bags or porousclay pots.How many grams of water can be cooled from35 °Cto20 °C by the evaporation of 60 g of water? (The heat of vaporizationof water in this temperature range is 2.4 kJ/g. Thespecific heat of water is 4.18 J/gK.)11.Ethanol (C2H5OH) melts at-114 °C and boils at 78 °C. Theenthalpy of fusion of ethanol is 5.02 kJ/mol, and its enthalpyof vaporization is 38.56 kJ/mol. The specific heats of solid andliquid ethanol are 0.97 J/gKand 2.3 J/gK, respectively.(a) How much heat is required to convert 42.0 g of ethanol at35 °C to the vapor phase at 78 °C? (b) How much heat isrequired to convert the same amount of ethanol at -155 °Ctothe vapor phase at 78 °C?12.(a) Place the following substances in order of increasing volatility:CH4, CBr4,CH2Cl2, CH3Cl, CHBr3, and CH2Br2. Explain.(b) How do the boiling points vary through this series?13.The phase diagram for neon isUse the phase diagram to answer the following questions. (a)What is the approximate value of the normal melting point?(b) Over what pressure range will solid neon sublime? (c) Atroom temperature (T = 25 °C) can neon be liquefied by compressingit?14.Chapter 131.The structures of vitamins E and B6 are shown below. Predictwhich is largely water soluble and which is largely fat soluble.Explain.2.The following diagram shows the vapor-pressure curves of avolatile solvent and a solution of that solvent containing anonvolatile solute. (a) Which line represents the solution? (b)What are the normal boiling points of the solvent and thesolution?3.Indicate the type of solute–solvent interaction that should be most important in each of the following solutions:(a) CCl4 in benzene (C6H6), (b) methanol (CH3OH) inwater,(c) KBr in water, (d) HCl in acetonitrile (CH3CN).mon laboratory solvents include acetone (CH3COCH3),methanol (CH3OH), toluene (C6H5CH3), and water.Which ofthese is the best solvent for nonpolar solutes? Explain.5.(a) Would you expect stearic acid, CH3(CH2)16COOH, to bemore soluble in water or in carbon tetrachloride? Explain.(b) Which would you expect to be more soluble in water,cyclohexane or dioxane? Explain.6.(a) Explain why carbonated beverages must be stored in sealedcontainers. (b) Once the beverage has been opened, why doesit maintain more carbonation when refrigerated than at roomtemperature?7.The Henry’s law constant for helium gas in water at30 °C is3.7 * 10-4 M/atm and the constant for N2 at30 °C is 6.0 * 10-4 M/atm. If the two gases are each present at1.5 atm pressure, calculate the solubility of each gas.8.(a) Calculate the mass percentage of Na2SO4 in a solutioncontaining 10.6 g Na2SO4 in 483 g water. (b) An ore contains2.86 g of silver per ton of ore. What is the concentration ofsilver in ppm?9.A solution is made containing 14.6 g of CH3OH in 184 g H2O.Calculate (a) the mole fraction of CH3OH, (b) the mass percentof CH3OH, (c) the molality of CH3OH.mercial aqueous nitric acid has a density of 1.42 g/mL andis 16 M. Calculate the percent HNO3 by mass in the solution.11.Consider two solutions, one formed by adding 10 g of glucose(C6H12O6) to 1 L of water and the other formed by adding10 g of sucrose (C12H22O11) to 1 L of water. Are the vaporpressures over the two solutions the same? Why or why not?12.At 63.5 °C the vapor pressure of H2O is 175 torr, and that ofethanol (C2H5OH) is 400 torr. A solution is made by mixingequal masses of H2O and C2H5OH. (a) What isthe mole fractionof ethanol in the solution? (b) Assuming ideal-solutionbehavior, what is the vapor pressure of the solution at 63.5 °C? (c)What is the mole fraction of ethanol in the vapor above thesolution?13.At20 °C the vapor pressure of benzene (C6H6) is 75 torr, andthat of toluene (C7H8) is 22 torr. Assume that benzene andtoluene form an ideal solution. (a)What is the composition inmole fractions of a solution that has a vapor pressure of35 torr at 20 °C?(b) What is the mole fraction of benzene inthe vapor above the solution described in part (a)?14.List the following aqueous solutions in order of increasingboiling point: 0.120 m glucose, 0.050 m LiBr, 0.050 mZn(NO3)2.15.What is the osmotic pressure formed by dissolving 44.2 mg ofaspirin (C9H8O4) in 0.358 L of water at 25 °C?16.Two beakers are placed in a sealed box at 25 °C. One beakercontains 30.0 mL of a 0.050 M aqueous solution of a nonvolatilenonelectrolyte. The other beaker contains 30.0 mL ofa 0.035 M aqueous solution of NaCl. The water vapor fromthe two solutions reaches equilibrium. (a) In which beakerdoes the solution level rise, and in which one does it fall? (b)What are the volumes in the two beakers when equilibriumis attained, assuming ideal behavior?17.At ordinary body temperature (37 °C) the solubility of N2 inwater in contact with air at ordinary atmospheric pressure(1.0 atm) is 0.015 g/L. Air is approximately 78 mol % N2.Calculate the number of moles of N2 dissolved per liter ofblood, which is essentially an aqueous solution.At a depth of100 ft in water, the pressure is 4.0 atm.What is the solubilityof N2from air in blood at this pressure? If a scuba diver suddenlysurfaces from this depth, how many milliliters of N2gas, in the form of tiny bubbles, are released into the bloodstreamfrom each liter of blood?。
FANUC FX-300 系列数字光纤传感器说明书

MARKERSPLCHUMAN MACHINEINTERFACESENERGYMANAGEMENTSOLUTIONSFA COMPONENTSMACHINE VISIONSYSTEMSUV CURINGSYSTEMSFX-311FX-301-F7/FX-301-FFX-11AFX2FX-CH2FX-CHFX-301FX-301-HS FX-305Large display 9999 FX-305Large display with 4 digits (9999). With a greater difference indigit value than previous models, threshold values can be setin units of 1 digit up to maximum 9999.Threshold setting can now be done more easily and accurately.(During STDF, LONG and U-LG modes)2.5 timespreviousmodels!* Cover opened state is shown.If the light receiving level becomes saturated during close-rangesensing or when sensing transparent or minute objects, youcan adjust the light emitting amount of the sensor to stabilizesensing without needing to change the response time. Sensingthat previously required the response time or fibers to bechanged can now be set much more easily using this function.Light-emitting amount selectionFX-301FX-301-HS FX-305In addition to a “four-chemical emitting element” which suppresseschanges in the light emitting element over time so that a stablelevel of light emission can be maintained over long periods, a“APC (Auto Power Control) circuit” has also been adopted afresh.The light emitting amount can be controlled in minute degreesso that even changes occurring over very short periods can behandled, allowing stable sensing performance by suppressingdeviations in light emitting amounts caused by changes in theambient environment that could not previously be suppressed.Stable sensing over long and short periodsDeviationLightemittingamountEven beginners can quickly learnhow to use the MODE NAVI All modelsMODE NAVI uses six indicators to display the amplifier’sbasic operations. The current operating mode can beconfirmed at a glance, so even a first time user caneasily operate the amplifier without becoming confused.Easy confirming of threshold value settingsFX-301FX-301-HS FX-305The threshold valuecan be confirmed byturning the jog switcheven during RUNmode.Jog switch is turnedRight: Output 2 forFX-305The thresholdLeft: FX-301(-HS)Output 1 for FX-305Digital Fiber SensorFX-300 SERIES150LASER SENSORS PHOTO-ELECTRIC SENSORS MICRO PHOTO-ELECTRIC SENSORS AREA SENSORSSAFETY LIGHT CURTAINS /SAFETY COMPONENTS PRESSURE / FLOW SENSORS INDUCTIVE PROXIMITY SENSORS PARTICULAR USE SENSORS SENSOR OPTIONS SIMPLE WIRE-SAVING UNITS WIRE-SAVING SYSTEMS MEASURE-MENT SENSORS STATIC CONTROL DEVICES LASER MARKERS PLC HUMAN MACHINE INTERFACESENERGY MANAGEMENT SOLUTIONS FACOMPONENTSMACHINE VISION SYSTEMS UVCURINGSYSTEMSFX-311FX-301-F7/FX-301-F FX-11A FX2FX-CH2FX-CH056 222 38 18*********************SEN TRONIC AG151Digital Fiber Sensor FX-300SERIESLASERSENSORSPHOTO-ELECTRICSENSORSMICROPHOTO-ELECTRICSENSORSAREASENSORSSAFETY LIGHTCURTAINS /SAFETYCOMPONENTSPRESSURE /FLOWSENSORSINDUCTIVEPROXIMITYSENSORSPARTICULARUSESENSORSSENSOROPTIONSSIMPLEWIRE-SAVINGUNITSWIRE-SAVINGSYSTEMSMEASURE-MENTSENSORSSTATICCONTROLDEVICESLASERMARKERSPLCHUMANMACHINEINTERFACESENERGYMANAGEMENTSOLUTIONSFACOMPONENTSMACHINEVISIONSYSTEMSUVCURINGSYSTEMSFX-311FX-301-F7/FX-301-FFX-11AFX2FX-CH2FX-CHQuick-connection cablesFor FX-305(P)End platesAmplifier mounting bracketFiber amplifier protection seal• FX-MB1056 222 38 18*********************SEN TRONICAGDigital Fiber SensorFX-300 SERIES152LASER SENSORS PHOTO-ELECTRIC SENSORSMICRO PHOTO-ELECTRIC SENSORS AREA SENSORSSAFETY LIGHT CURTAINS /SAFETY COMPONENTS PRESSURE / FLOW SENSORS INDUCTIVE PROXIMITY SENSORS PARTICULAR USE SENSORS SENSOR OPTIONS SIMPLE WIRE-SAVING UNITS WIRE-SAVING SYSTEMS MEASURE-MENT SENSORS STATIC CONTROL DEVICESLASERMARKERS PLCHUMAN MACHINE INTERFACES ENERGY MANAGEMENT SOLUTIONSFACOMPONENTS MACHINE VISION SYSTEMS UVCURINGSYSTEMSFX-311FX-301-F7/FX-301-F FX-11A FX2FX-CH2FX-CHNotes: 1) FX-305□: Output 1 operation indicator (Orange)2) FX-305□: Output 2 operation indicator (Orange)3) FX-301□: 3-pin, FX-305□: 4-pinNotes: 1) CN-74-C □ only2) CN-73-C □: 3-core Amplifier mounting bracket (Optional)MS-DIN-2Material:C old rolled carbon steel (SPCC) (Uni-chrome plated)2) CN-71-C □: 1-coreEnd plate (Optional)MS-DIN-E2.75Material: Polycarbonate056 222 38 18*********************SEN TRONIC AG。
NMR Chemical Shifts

/OrganometallicsPublished on Web 04/16/2010r 2010American Chemical Society2176Organometallics 2010,29,2176–2179DOI:10.1021/om100106eNMR Chemical Shifts of Trace Impurities:Common Laboratory Solvents,Organics,and Gases in DeuteratedSolvents Relevant to the OrganometallicChemistGregory R.Fulmer,*,†Alexander ler,‡Nathaniel H.Sherden,‡Hugo E.Gottlieb,§Abraham Nudelman,§Brian M.Stoltz,‡John E.Bercaw,‡andKaren I.Goldberg ††Department of Chemistry,University of Washington,Box 351700,Seattle,Washington 98195-1700,‡Arnold and Mabel Beckman Laboratories of Chemical Synthesis and Caltech Center for Catalysis andChemical Synthesis,Division of Chemistry and Chemical Engineering,California Institute of Technology,Pasadena,California 91125,and §Department of Chemistry,Bar Ilan University,Ramat Gan 52900,IsraelReceived February 11,2010Tables of 1H and 13C NMR chemical shifts have been compiled for common organic compounds often used as reagents or found as products or contaminants in deuterated organic solvents.Building upon the work of Gottlieb,Kotlyar,and Nudelman in the Journal of Organic Chemistry,signals for common impurities are now reported in additional NMR solvents (tetrahydrofuran-d 8,toluene-d 8,dichloromethane-d 2,chlorobenzene-d 5,and 2,2,2-trifluoroethanol-d 3)which are frequently used in organometallic laboratories.Chemical shifts for other organics which are often used as reagents or internal standards or are found as products in organometallic chemistry are also reported for all the listed solvents.Hanging above the desk of most every chemist whose work relies heavily on using NMR spectroscopy 1is NMR Chemi-cal Shifts of Common Laboratory Solvents as Trace Impu-rities by Gottlieb,Kotlyar,and Nudelman.2By compiling the chemical shifts of a large number of contaminants commonly encountered in synthetic chemistry,the publica-tion has become an essential reference,allowing for easy identification of known impurities in a variety of deuter-ated organic solvents.However,despite the utility of Gottlieb et al.’s work,3the chemical shifts of impurities in a number of NMR solvents often used by organometallic chemists were not included.Tetrahydrofuran-d 8(THF-d 8),toluene-d 8,dichloromethane-d 2(CD 2Cl 2),chlorobenzene-d 5(C 6D 5Cl),and 2,2,2-trifluoroethanol-d 3(TFE-d 3)are com-monplace in laboratories practicing inorganic syntheses.Therefore,we have expanded the spectral data compilation with the inclusion of chemical shifts of common impurities recorded in the deuterated solvents heavily employed in our organometallic laboratories.The chemical shifts of various gases (hydrogen,methane,ethane,propane,ethylene,propylene,and carbon dioxide)often encoun-tered as reagents or products in organometallic reactions,along with organic compounds relevant to organometallic chemists (allyl acetate,benzaldehyde,carbon disulfide,carbon tetrachloride,18-crown-6,cyclohexanone,diallyl carbonate,dimethyl carbonate,dimethyl malonate,furan,Apiezon H grease,hexamethylbenzene,hexamethyldisil-oxane,imidazole,pyrrole,and pyrrolidine),have also been added to this expanded list.Experimental SectionAll deuterated solvents were obtained commercially through Cambridge Isotope Laboratories,Inc.NMR spectra were recorded at 298K using 300,500,or 600MHz spectrometers (13C{1H}NMR frequencies of 75.5,126,or 151MHz,res-pectively).Adopting the previously reported strategy,2standard solutions of mixtures of specific impurities were used to reduce the number of necessary individual NMR experiments.The combinations of organic compounds were chosen in a way in which intermolecular interactions and resonance convolution would be minimized.Unless otherwise stated,the standard solutions were prepared with qualitatively equal molar amounts of the following compounds:(solution 1)acetone,dimethylform-amide,ethanol,toluene;(solution 2)benzene,dimethyl sulf-oxide,ethyl acetate,methanol;(solution 3)acetic acid,chloro-form,diethyl ether,2-propanol,tetrahydrofuran;(solution 4)acetonitrile,dichloromethane,1,4-dioxane,n -hexane,hexa-methylphosphoramide (HMPA);(solution 5)1,2-dichloroethane,n -pentane,pyridine,hexamethylbenzene;(solution 6)tert -butyl alcohol,2,6-di-tert -butyl-4-methylphenol (BHT),cyclohexane,*To whom correspondence should be addressed.E-mail:fulmerg@.(1)For general information on 1H and 13C{1H}NMR spectroscopy,see:Balc ı,M.Basic 1H-and 13C-NMR Spectroscopy ;Elsevier:Amsterdam,2005.(2)Gottlieb,H.E.;Kotlyar,V.;Nudelman,.Chem.1997,62,7512.(3)According to ACS Publications as of December 2009(/),Gottlieb et al.’s publication 2is the most downloaded Journal of Organic Chemistry article over the preceding 12months.Article Organometallics,Vol.29,No.9,20102177Table1.1H NMR Data aproton mult THF-d8CD2Cl2CDCl3toluene-d8C6D6C6D5Cl(CD3)2CO(CD3)2SO CD3CN TFE-d3CD3OD D2Osolvent residual signals 1.72 5.327.26 2.087.16 6.96 2.05 2.50 1.94 5.02 3.31 4.79 3.58 6.97 6.99 3.887.017.147.09water OH s 2.46 1.52 1.560.430.40 1.03 2.84b 3.33b 2.13 3.66 4.87acetic acid CH3s 1.89 2.06 2.10 1.57 1.52 1.76 1.96 1.91 1.96 2.06 1.99 2.08 acetone CH3s 2.05 2.12 2.17 1.57 1.55 1.77 2.09 2.09 2.08 2.19 2.15 2.22 acetonitrile CH3s 1.95 1.97 2.100.690.58 1.21 2.05 2.07 1.96 1.95 2.03 2.06 benzene CH s7.317.357.367.127.157.207.367.377.377.367.33tert-butyl alcohol CH3s 1.15 1.24 1.28 1.03 1.05 1.12 1.18 1.11 1.16 1.28 1.40 1.24 OH s c 3.160.580.63 1.30 4.19 2.18 2.20chloroform CH s7.897.327.26 6.10 6.15 6.748.028.327.587.337.9018-crown-6CH2s 3.57 3.59 3.67 3.36 3.39 3.41 3.59 3.51 3.51 3.64 3.64 3.80 cyclohexane CH2s 1.44 1.44 1.43 1.40 1.40 1.37 1.43 1.40 1.44 1.47 1.451,2-dichloroethane CH2s 3.77 3.76 3.73 2.91 2.90 3.26 3.87 3.90 3.81 3.71 3.78 dichloromethane CH2s 5.51 5.33 5.30 4.32 4.27 4.77 5.63 5.76 5.44 5.24 5.49diethyl ether CH3t,7 1.12 1.15 1.21 1.10 1.11 1.10 1.11 1.09 1.12 1.20 1.18 1.17 CH2q,7 3.38 3.43 3.48 3.25 3.26 3.31 3.41 3.38 3.42 3.58 3.49 3.56 diglyme CH2m 3.43 3.57 3.65 3.43 3.46 3.49 3.56 3.51 3.53 3.67 3.61 3.67 CH2m 3.53 3.50 3.57 3.31 3.34 3.37 3.47 3.38 3.45 3.62 3.58 3.61OCH3s 3.28 3.33 3.39 3.12 3.11 3.16 3.28 3.24 3.29 3.41 3.35 3.37 dimethylformamide CH s7.917.968.027.577.637.737.967.957.927.867.977.92 CH3s 2.88 2.91 2.96 2.37 2.36 2.51 2.94 2.89 2.89 2.98 2.99 3.01CH3s 2.76 2.82 2.88 1.96 1.86 2.30 2.78 2.73 2.77 2.88 2.86 2.85 1,4-dioxane CH2s 3.56 3.65 3.71 3.33 3.35 3.45 3.59 3.57 3.60 3.76 3.66 3.75 DME CH3s 3.28 3.34 3.40 3.12 3.12 3.17 3.28 3.24 3.28 3.40 3.35 3.37 CH2s 3.43 3.49 3.55 3.31 3.33 3.37 3.46 3.43 3.45 3.61 3.52 3.60 ethane CH3s0.850.850.870.810.800.790.830.820.850.850.850.82 ethanol CH3t,7 1.10 1.19 1.250.970.96 1.06 1.12 1.06 1.12 1.22 1.19 1.17 CH2q,7d 3.51 3.66 3.72 3.36 3.34 3.51 3.57 3.44 3.54 3.71 3.60 3.65OH s c,d 3.30 1.33 1.320.830.50 1.39 3.39 4.63 2.47ethyl acetate CH3CO s 1.94 2.00 2.05 1.69 1.65 1.78 1.97 1.99 1.97 2.03 2.01 2.07C H2CH3q,7 4.04 4.08 4.12 3.87 3.89 3.96 4.05 4.03 4.06 4.14 4.09 4.14CH2C H3t,7 1.19 1.23 1.260.940.92 1.04 1.20 1.17 1.20 1.26 1.24 1.24 ethylene CH2s 5.36 5.40 5.40 5.25 5.25 5.29 5.38 5.41 5.41 5.40 5.39 5.44 ethylene glycol CH2s e 3.48 3.66 3.76 3.36 3.41 3.58 3.28 3.34 3.51 3.72 3.59 3.65 H grease f CH3m0.85-0.910.84-0.900.84-0.870.89-0.960.90-0.980.86-0.920.900.82-0.880.88-0.940.86-0.93CH2br s 1.29 1.27 1.25 1.33 1.32 1.30 1.29 1.24 1.33 1.29 hexamethylbenzene CH3s 2.18 2.20 2.24 2.10 2.13 2.10 2.17 2.14 2.19 2.24 2.19n-hexane CH3t,70.890.890.880.880.890.850.880.860.890.910.90 CH2m 1.29 1.27 1.26 1.22 1.24 1.19 1.28 1.25 1.28 1.31 1.29 HMDSO CH3s0.070.070.070.100.120.100.070.060.070.080.070.28 HMPA CH3d,9.5 2.58 2.60 2.65 2.42 2.40 2.47 2.59 2.53 2.57 2.63 2.64 2.61 hydrogen H2s 4.55 4.59 4.62 4.50 4.47 4.49 4.54 4.61 4.57 4.53 4.56 imidazole CH(2)s7.487.637.677.307.337.537.627.637.577.617.677.78 CH(4,5)s 6.947.077.10 6.86 6.907.017.047.017.017.037.057.14 methane CH4s0.190.210.220.170.160.150.170.200.200.180.200.18 methanol CH3s g 3.27 3.42 3.49 3.03 3.07 3.25 3.31 3.16 3.28 3.44 3.34 3.34 OH s c,g 3.02 1.09 1.09 1.30 3.12 4.01 2.16nitromethane CH3s 4.31 4.31 4.33 3.01 2.94 3.59 4.43 4.42 4.31 4.28 4.34 4.40 n-pentane CH3t,70.890.890.880.870.870.840.880.860.890.900.90 CH2m 1.31 1.30 1.27 1.25 1.23 1.23 1.27 1.27 1.29 1.33 1.29 propane CH3t,7.30.900.900.900.890.860.840.880.870.900.900.910.88 CH2sept,7.3 1.33 1.32 1.32 1.32 1.26 1.26 1.31 1.29 1.33 1.33 1.34 1.30 2-propanol CH3d,6 1.08 1.17 1.220.950.95 1.04 1.10 1.04 1.09 1.20 1.50 1.17 CH sept,6 3.82 3.97 4.04 3.65 3.67 3.82 3.90 3.78 3.87 4.05 3.92 4.02 propylene CH3dt,6.4,1.5 1.69 1.71 1.73 1.55 1.55 1.58 1.68 1.68 1.70 1.70 1.70 1.70 CH2(1)dm,10 4.89 4.93 4.94 4.92 4.95 4.91 4.90 4.94 4.93 4.93 4.91 4.95CH2(2)dm,17 4.99 5.03 5.03 4.98 5.01 4.98 5.00 5.03 5.04 5.03 5.01 5.06CH m 5.79 5.84 5.83 5.70 5.72 5.72 5.81 5.80 5.85 5.87 5.82 5.90 pyridine CH(2,6)m8.548.598.628.478.538.518.588.588.578.458.538.52 CH(3,5)m7.257.287.29 6.67 6.66 6.907.357.397.337.407.447.45CH(4)m7.657.687.68 6.99 6.987.257.767.797.737.827.857.87 pyrrole NH br t9.968.698.407.717.808.6110.0210.759.27CH(2,5)m 6.66 6.79 6.83 6.43 6.48 6.62 6.77 6.73 6.75 6.84 6.72 6.93CH(3,4)m 6.02 6.19 6.26 6.27 6.37 6.27 6.07 6.01 6.10 6.24 6.08 6.26 pyrrolidine h CH2(2,5)m 2.75 2.82 2.87 2.54 2.54 2.64 2.67 2.75 3.11 2.80 3.07 CH2(3,4)m 1.59 1.67 1.68 1.36 1.33 1.43 1.55 1.61 1.93 1.72 1.87 silicone grease CH3s0.110.090.070.260.290.140.13-0.060.080.160.10 tetrahydrofuran CH2(2,5)m 3.62 3.69 3.76 3.54 3.57 3.59 3.63 3.60 3.64 3.78 3.71 3.74 CH2(3,4)m 1.79 1.82 1.85 1.43 1.40 1.55 1.79 1.76 1.80 1.91 1.87 1.88 toluene CH3s 2.31 2.34 2.36 2.11 2.11 2.16 2.32 2.30 2.33 2.33 2.32 CH(2,4,6)m7.107.157.17 6.96-7.017.027.01-7.087.10-7.207.187.10-7.307.10-7.307.16CH(3,5)m7.197.247.257.097.137.10-7.177.10-7.207.257.10-7.307.10-7.307.16 triethylamine CH3t,70.970.99 1.030.950.960.930.960.930.96 1.31 1.050.99 CH2q,7 2.46 2.48 2.53 2.39 2.40 2.39 2.45 2.43 2.45 3.12 2.58 2.57a Except for the compounds in solutions8-10,as well as the gas samples,hexamethylbenzene,and the corrected values mentioned in the Supporting Information,all data for the solvents CDCl3,C6D6,(CD3)2CO,(CD3)2SO,CD3CN,CD3OD,and D2O were previously reported in ref2.b A signal for HDO is also observed in(CD3)2SO(3.30ppm)and(CD3)2CO(2.81ppm),often seen as a1:1:1triplet(2J H,D=1Hz).c Not all OH signals were observable.d In some solvents,the coupling interaction between the CH2and the OH protons may be observed(J=5Hz).e In CD3CN,the OH proton was seen as a multiplet at2.69ppm,as well as extra coupling to the CH2resonance.f Apiezon brand H grease.g In some solvents,a coupling interaction between the CH3and the OH protons may be observed(J=5.5Hz).h Pyrrolidine was observed to react with(CD3)2CO.2178Organometallics,Vol.29,No.9,2010Fulmer et al.Table2.13C{1H}NMR Data acarbon THF-d8CD2Cl2CDCl3toluene-d8C6D6C6D5Cl(CD3)2CO(CD3)2SO CD3CN TFE-d3CD3OD D2O solvent signals67.2153.8477.16137.48128.06134.1929.8439.52 1.3261.5049.0025.31128.87129.26206.26118.26126.28127.96128.25125.13125.9620.43acetic acid CO171.69175.85175.99175.30175.82175.67172.31171.93173.21177.96175.11177.21 CH320.1320.9120.8120.2720.3720.4020.5120.9520.7320.9120.5621.03acetone CO204.19206.78207.07204.00204.43204.83205.87206.31207.4332.35209.67215.94 CH330.1731.0030.9230.0330.1430.1230.6030.5630.91214.9830.6730.89acetonitrile CN116.79116.92116.43115.76116.02115.93117.60117.91118.26118.95118.06119.68 CH30.45 2.03 1.890.030.200.63 1.12 1.03 1.79 1.000.85 1.47 benzene CH128.84128.68128.37128.57128.62128.38129.15128.30129.32129.84129.34tert-butyl alcohol(CH3)3C67.5069.1169.1568.1268.1968.1968.1366.8868.7472.3569.4070.36(C H3)3C30.5731.4631.2530.4930.4731.1330.7230.3830.6831.0730.9130.29carbon dioxide CO2125.69125.26124.99124.86124.76126.08125.81124.21125.89126.92126.31carbon disulfide CS2193.37192.95192.83192.71192.69192.49193.58192.63193.60196.26193.82197.25carbon tetrachloride CCl496.8996.5296.3496.5796.4496.3896.6595.4496.6897.7497.2196.73 chloroform CH79.2477.9977.3677.8977.7977.6779.1979.1679.1778.8379.4418-crown-6CH271.3470.4770.5570.8670.5970.5571.2569.8571.2270.8071.4770.14cyclohexane CH227.5827.3826.9427.3127.2326.9927.5126.3327.6328.3427.961,2-dichloroethane CH244.6444.3543.5043.4043.5943.6045.2545.0245.5445.2845.11dichloromethane CH254.6754.2453.5253.4753.4653.5454.9554.8455.3254.4654.78diethyl ether CH315.4915.4415.2015.4715.4615.3515.7815.1215.6315.3315.4614.77CH266.1466.1165.9165.9465.9465.7966.1262.0566.3267.5566.8866.42 diglyme CH358.7258.9559.0158.6258.6658.4258.7757.9858.9059.4059.0658.67CH271.1770.7070.5170.9270.8770.5671.0369.5470.9973.0571.3370.05CH272.7272.2571.9072.3972.3572.0772.6371.2572.6371.3372.9271.63 dimethylformamide CH161.96162.57162.62161.93162.13162.01162.79162.29163.31166.01164.73165.53CH335.6536.5636.5035.2235.2535.4536.1535.7336.5737.7636.8937.54CH330.7031.3931.4530.6430.7230.7131.0330.7331.3230.9631.6132.031,4-dioxane CH267.6567.4767.1467.1767.1666.9567.6066.3667.7268.5268.1167.19 DME CH358.7259.0259.0858.6358.6858.3158.4558.0358.8959.5259.0658.67CH272.5872.2471.8472.2572.2171.8172.4771.1772.4772.8772.7271.49 ethane CH3 6.79 6.91 6.89 6.94 6.96 6.91 6.88 6.61 6.997.01 6.98ethanol CH318.9018.6918.4118.7818.7218.5518.8918.5118.8018.1118.4017.47CH257.6058.5758.2857.8157.8657.6357.7256.0757.9659.6858.2658.05 ethyl acetate C H3CO20.4521.1521.0420.4620.5620.5020.8320.6821.1621.1820.8821.15CO170.32171.24171.36170.02170.44170.20170.96170.31171.68175.55172.89175.26CH260.3060.6360.4960.0860.2160.0660.5659.7460.9862.7061.5062.32CH314.3714.3714.1914.2314.1914.0714.5014.4014.5414.3614.4913.92 ethylene CH2123.09123.20123.13122.92122.96122.95123.47123.52123.69124.08123.46 ethylene glycol CH264.3564.0863.7964.2964.3464.0364.2662.7664.2264.8764.3063.17H grease b CH230.4530.1429.7130.3130.2230.11hexamethylbenzene C131.88132.09132.21131.72131.79131.54132.22131.10132.61134.04132.53CH316.7116.9316.9816.8416.9516.6816.8616.6016.9417.0416.90n-hexane CH314.2214.2814.1414.3414.3214.1814.3413.8814.4314.6314.45CH2(2,5)23.3323.0722.7023.1223.0422.8623.2822.0523.4024.0623.68CH2(3,4)32.3432.0131.6432.0631.9631.7732.3030.9532.3633.1732.73HMDSO CH3 1.83 1.96 1.97 1.99 2.05 1.92 2.01 1.96 2.07 2.09 1.99 2.31 HMPA c CH336.8936.9936.8736.8036.8836.6437.0436.4237.1037.2137.0036.46 imidazole CH(2)135.72135.76135.38135.57135.76135.50135.89135.15136.33136.58136.31136.65CH(4,5)122.20122.16122.00122.13122.16121.96122.31121.55122.78122.93122.60122.43 methane CH4-4.90-4.33-4.63-4.34-4.29-4.33-5.33-4.01-4.61-5.88-4.90 methanol CH349.6450.4550.4149.9049.9749.6649.7748.5949.9050.6749.8649.50d nitromethane CH362.4963.0362.5061.1461.1661.6863.2163.2863.6663.1763.0863.22n-pentane CH314.1814.2414.0814.2714.2514.1014.2913.2814.3714.5414.39 CH2(2,4)23.0022.7722.3822.7922.7222.5422.9821.7023.0823.7523.38CH2(3)34.8734.5734.1634.5434.4534.2634.8333.4834.8935.7635.30propane CH316.6016.6316.6316.6516.6616.5616.6816.3416.7316.9316.80 CH216.8216.6316.3716.6316.6016.4816.7815.6716.9117.4617.192-propanol CH325.7025.4325.1425.2425.1825.1425.6725.4325.5525.2125.2724.38 CH66.1464.6764.5064.1264.2364.1863.8564.9264.3066.6964.7164.88 propylene CH319.2719.4719.5019.3219.3819.3219.4219.2019.4819.6319.50 CH2115.74115.70115.74115.89115.92115.86116.03116.07116.12116.38116.04CH134.02134.21133.91133.61133.69133.57134.34133.55134.78136.00134.61pyridine CH(2,6)150.57150.27149.90150.25150.27149.93150.67149.58150.76149.76150.07149.18 CH(3,5)124.08124.06123.75123.46123.58123.49124.57123.84127.76126.27125.53125.12CH(4)135.99136.16135.96135.17135.28135.32136.56136.05136.89139.62138.35138.27 pyrrole CH(2,5)118.03117.93117.77117.61117.78117.65117.98117.32118.47119.61118.28119.06 CH(3,4)107.74108.02107.98108.15108.21108.03108.04107.07108.31108.85108.11107.83 pyrrolidine e CH2(2,5)45.8247.0246.9347.1246.8646.7546.5147.5747.4347.2346.83 CH2(3,4)26.1725.8325.5625.7525.6525.5925.2626.3425.7326.2925.86 silicone grease CH3 1.20 1.22 1.19 1.37 1.38 1.09 1.40 2.87 2.10 tetrahydrofuran CH2(2,5)68.0368.1667.9767.7567.8067.6468.0767.0368.3369.5368.8368.68 CH2(3,4)26.1925.9825.6225.7925.7225.6826.1525.1426.2726.6926.4825.67 toluene CH321.2921.5321.4621.3721.1021.2321.4620.9921.5021.6221.50 C(1)138.24138.36137.89137.84137.91137.65138.48137.35138.90139.92138.85CH(2,6)129.47129.35129.07129.33129.33129.12129.76128.88129.94130.58129.91CH(3,5)128.71128.54128.26128.51128.56128.31129.03128.18129.23129.79129.20CH(4)125.84125.62125.33125.66125.68125.43126.12125.29126.28126.82126.29 triethylamine CH312.5112.1211.6112.3912.3511.8712.4911.7412.389.5111.099.07 CH247.1846.7546.2546.8246.7746.3647.0745.7447.1048.4546.9647.19a Except for the compounds in solutions8-10,as well as the gas samples,hexamethylbenzene,and the corrected values mentioned in the Supporting Information,all data for the solvents CDCl3,C6D6,(CD3)2CO,(CD3)2SO,CD3CN,CD3OD,and D2O were previously reported in ref2.b Apiezon brand H grease.c Phosphorus coupling was observed(2J PC=3Hz).d Internal reference;see text.e Pyrrolidine was observed to react with(CD3)2CO.Article Organometallics,Vol.29,No.9,201021791,2-dimethoxyethane(DME),nitromethane,poly(dimethylsiloxane) (silicone grease),triethylamine;(solution7)diglyme,dimethyl-acetamide,ethylene glycol,ethyl methyl ketone;(solution8) allyl acetate,2,6-di-tert-butyl-4-methoxyphenol(BHA),long-chain,linear aliphatic hydrocarbons from pump oil;4(solu-tion9)benzaldehyde,carbon disulfide,carbon tetrachloride, cyclohexanone,dimethyl malonate,furan,Apiezon H grease (H grease);(solution10)18-crown-6,diallyl carbonate,dimethyl carbonate,hexamethyldisiloxane(HMDSO),imidazole,pyrrole, pyrrolidine.5In the case of TFE-d3,nitromethane was omitted from solution6and run separately,since the protons of nitro-methane exchange with deuterium from TFE-d3in the presence of triethylamine.In the case of(CD3)2CO,pyrrolidine was omitted from solution10,since the two compounds were observed to react with each other.The gases used in this study included hydrogen,methane,ethane,propane,ethylene,propylene,and carbon dioxide.Before examining the various standard contaminant solu-tions by1H NMR spectroscopy,solvent residual signals6and chemical shifts for H2O7for each NMR solvent were refer-enced against tetramethylsilane(TMS,δ0ppm)and reported. Before collecting13C{1H}NMR spectral data,solvent signals6 were recorded with reference to the signal of a TMS internal standard.For D2O,1H NMR spectra were referenced to the methyl signal(δ0ppm)of sodium3-(trimethylsilyl)propane-sulfonate,8,9and13C{1H}NMR spectra were referenced to the signal for the methyl group of methanol(one drop,added as an internal standard),which was set to49.50ppm.2In a typical experiment for collecting1H NMR spectral data,a 3μL sample of a standard contaminant solution was added to an NMR tube containing approximately0.4mL of a deuterated solvent.For13C{1H}NMR spectral data collection,an approxi-mately50μL sample of the standard contaminant solution was added.When there was any uncertainty in the assignment of a resonance,the solution was spiked with an additional1-2μL of the impurity in question to accurately identify its chemical shift.In cases where the chemical shifts of resonances were highly dependent on the concentration of the impurities pre-sent,ambiguous resonances were instead resolved via gradient-selected heteronuclear single-quantum coherence(gs-HSQC) and gradient-selected heteronuclear multiple-quantum coherence (gs-HMQC)NMR spectroscopies.For the experiments involving gases,a J.Young NMR tube containing approximately0.4mL of NMR solvent was first degassed with three freeze-pump-thaw ing a vacuum line equipped with a gas manifold,1atm of the desired gas was added to the tube.Each gas was run separately,degassing between each gas sample.Results and DiscussionChemical shifts for each of the impurities are reported in the tables:1H and13C{1H}NMR spectral data of all sub-strates are presented in Tables1and2,respectively.Notably, physically larger tables,containing all the data from Tables1 and2as well as the chemical shifts of additional organic compounds,are provided in the Supporting Information. Unless noted otherwise,coupling constants(reported in Hz) and resonance multiplicities(abbreviated as follows:s= singlet,d=doublet,t=triplet,q=quartet,p=pentet, sept=septet,m=multiplet,br=broad)were observed to be solvent-independent.It was noted that the amount of gas dissolved in solution gave1H NMR signal integrations that were qualitatively comparable to those for the solutions made with the3μL additions of the liquid or solid contaminants.However,typi-cally in order to observe signals for the gas samples by13C{1H} NMR spectroscopy,additional time for data collection was required.The solubility of each gas in D2O was extremely limited,making13C detection impractical.Of all the gases, methane required the most number of transients in order to obtain an observable signal by13C{1H}NMR spectroscopy. In most cases,the13C chemical shift of methane was acquired through the use of gs-HMQC NMR spectroscopy to provide enhanced sensitivity.In order to reflect what would be ob-served in typical NMR-scale experiments,13C detection was not pursued with isotopically enriched gases.A number of misreported values were discovered in the years since the original publication10and in the preparation of this paper. These are detailed in the Supporting Information,and the values are now correctly listed in Tables1and2. Acknowledgment.G.R.F.and K.I.G.thank the Depart-ment of Energy(Contract No.DE-FG02-06ER15765)for support.A.J.M.M.and J.E.B.thank the Moore Founda-tion for support.N.H.S.and B.M.S.thank Abbott Labora-tories,Amgen,Merck,Bristol-Myers Squibb,Boehringer Ingelheim,the Gordon and Betty Moore Foundation,and Caltech for financial support.Supporting Information Available:Large-format tables of the all the NMR data.This material is available free of charge via the Internet at .(4)VWR brand vacuum pump oil#19.(5)The components of solution10were stable together in dilute solution but unstable when neat mixtures were prepared.In general,it was observed that the nitrogen-containing compounds and possibly 18-crown-6catalyzed the hydrolysis of the carbonates,reacted directly with them,or both.Therefore,for the purpose of storage,the solution was partitioned into two subsolutions:(solution10A)18-crown-6, imidazole,pyrrole,pyrrolidine;(solution10B)diallyl carbonate,di-methyl carbonate,hexamethyldisiloxane.These subsolutions were stable for long periods as neat mixtures and were combined to form solution10by adding equal portions to an NMR tube containing the desired deuterated solvent.(6)For1H NMR spectra,the solvent residual signals arise from the proton of isotopomers containing one less deuterium atom than the perdeuterated solvent:e.g.,CDHCl2in CD2Cl2.For13C NMR spectra, the solvent signals arise from the13C atoms at natural abundance in the perdeuterated solvent.(7)The chemical shift for H2O can vary depending on the tempera-ture,[H2O],and the solutes present:e.g.,a downfield shift may be observed in the presence of any hydrogen bond acceptors.For more information see page75of ref1.(8)Harris,R.K.;Becker,E.D.;Cabral de Menezes,S.M.;Granger, P.;Hoffman,R.E.;Zilm,K.W.Pure Appl.Chem.2008,80,59.(9)For information on the temperature dependence of HDO chemi-cal shifts in D2O,see ref2.(10)The misreported value for acetonitrile in C6D6from the original paper2was also pointed out by Dr.Jongwook Choi,to whom we are grateful.。
dspch2

数字信号处理Digital Signal Processing 电子信息工程系韩建峰Tel:6575756 E-mail: hanjianfeng@KeywordsSections–Sinusoidal Signals–Complex Exponential Signal –PhasorSummaryReading assignments•Chapter 2,section 2-2,2-3,2-5•Appendix A: Complex NumbersKeywordsSinusoidal signals 正、余弦信号Function 函数Frequency vs. Period 频率、周期Phase shift vs. Time shift 相移、时移Complex exponential signals 复指数信号Phasor 相量Lecture Objectives•Write general formula for a “sinusoidal”waveform, or signal•From the formula, plot the sinusoid versus time •Define Sinusoid Formula from a plot•Relate TIME-SHIFT to PHASE•Introduce an ABSTRACTION:–Complex Numbers represent Sinusoids–Complex Exponential SignalSinusoids•A general class of signals.•Be called cosine signals or, sine signals .Collectively, sinusoidal signals .Concisely, sinusoids .A t cos()ωϕ+DEMO: Tuning fork A-440ms 3.285.515.8=-≈T Hz4353.2/1000/1≈==TfComplex numbers •To solve: z2= -1–z = j–Math and Physicsuse z = i •Complex number:z = x + j y•Cartesian form (rectangular form)•Relate (x,y) to (r,q)rqxy()xyyxr1222Tan-=+=qqqsincosryrx==Most calculators doPolar-Rectangular•Polar formComplex planeSinusoidal Signals•FREQUENCY–Radians/sec–Hertz (cycles/sec)•PHASE •AMPLITUDE –Magnitude •PERIOD (in sec)A t cos()ωϕ+ωA ϕωπ=()2fT f ==12πωExample of sinusoids •Given the Formula•How to make a plot?) 2.13.0cos(5ππ+t5,0.3, 1.2 Aωπϕπ===Example 2-1•Determine period:•Determine a peak location by solving•Zero crossing is T/4 before or after•Positive & Negative peaks spaced by T/23/203.0/2/2===ππωπT)2.13.0()(=+⇒=+ππϕωtt•PLOT the SINUSOID•Use T=20/3 and the peak location at t=-4)2.13.0cos(5ππ+tTime Shift•In a mathematical formula we can replace t with t-t m•Then the t=0 point moves to t=t m•Peak value of cos(ω(t-t m )) is now at t=t m ))(cos()(m m t t A t t x -=-ωPositive: delayedNegative: advancedIllustration of time-shiftingPhase Shift and Time Shift Equate the formulas:and we obtain:or, )cos())(cos(ϕωω+=-t A t t A m ϕω=-m t ωϕ-=m tTime-Shifted Sinusoid5cos(0.3(4))5cos(0.3((4))t t ππ+=--•Measure the period T–Between peaks or zero crossings–Compute frequency: ω= 2π/T•Measure time of a peak: tm–Compute phase: ϕ= -ωtm•Measure height of positive peak: A3 stepsππωϕ25.0))(200(=-=-=m m t t 1001period 1sec 01.0==T sec00125.0-=m t πωππ20001.022===TExercise 2.3The value of phase shift that falls between –πand +πis called the principal value of the phase shift.Review•Euler's formula &Inverse Euler's formula•To simplified the analysis and manipulation of sinusoidal signals greatly.•Use Euler's formula()00()cos()sin()j tz t AeA t jA tωϕωϕωϕ+==+++•It is an alternative representation for the realcosine signal.00()()cos(){}{}j t j tjx t A te Ae e Ae eωϕωϕωϕ+=+=ℜ=ℜ•DefinitionComplex addition26)53()24()52()34(213j j j j z z z +=+-++=++-=+=DEMO: Rotating Phasors DEMO: Phasor AdditionSummaryHomeworkP2.3, P2.14,P2.16, E2.1.1Review: Chapter 2Appendix A: Complex Numbers Preview:Chapter 3 Section 3-1, 3-2, 3-3, 3-4。
高分子链的近程结构

Projections of various helices
42= 21 51
52
61
62= 31 71
72 91
73 92
A ut:
81
83
Rotation necessary to go from lattice point t: turns to the next identical helix point to lattice point: = 2t / u u: periodicity (# of helical lattice points Translation necessary to go from lattice point per identical period) to lattice point: z = c / u c: distance to the next identical helix point
p( R)d 3 R
p(R) is the distribution function,
p ( R)d 3 R 1
p(R) is identical with the distribution function for the displacement of a Brownian particle, after Ns uncorrelated steps. It equals a Gaussian function:
Excluded volume effect (排除体积效应) A chain of monomers with non-vanishing sizes cannot occupy a given location twice.
Where does the ideal chain model satisfy?
苯乙烯装置产生聚合物的预防和解决办法
第49卷第7期 当 代 化 工 Vol.49,No.7 2020年7月 Contemporary Chemical Industry July ,2020收稿日期: 2020-03-12苯乙烯装置产生聚合物的预防和解决办法阚昊,于宸,金熙俊(中国石油集团东北炼化工程有限公司沈阳分公司,辽宁 沈阳 110167)摘 要:苯乙烯在化工生产中非常重要,它能够生产出聚苯乙烯、丁苯橡胶等合成的高分子材料,应用的领域也非常广泛,例如在纺织、涂料、医疗等领域。
大多数工业生产都是使用乙苯脱氢制苯乙烯工艺,苯乙烯在常温状冴下会发生聚合反应,这种情冴不利于生产的顺利迚行,所以需要对工作过程中产生的苯乙烯聚合问题迚行研究和分析。
提出设计加入阻聚剂系统、提高操作水平、更新填料等有针对性的预防和解决办法,保证整个石化企业的安全稳定生产。
关 键 词:苯乙烯;聚合物;产生;预防;解决办法中图分类号:TE626 文献标识码: A 文章编号: 1671-0460(2020)07-1488-04Prevention and Solution of Polymer Formation in Styrene UnitKAN Hao , YU Chen , JIN Xi-jun(CNPC Northeast Refining & Chemical Engineering Co., Ltd.,Shenyang Company, Shenyang Liaoning 110167, China )Abstract : Styrene is very important in chemical production. It can be used to produce synthetic polymer materials,such as polystyrene and styrene-butadiene rubber. Its application field is also very wide, for example the fields of textiles, coatings, medical and so on. Most of the production in the industry uses the ethylbenzene dehydrogenation to styrene process. Styrene easy polymerizes at normal temperature, which is not conducive to the smooth progress of production. Therefore, it is necessary to study and analyze the styrene polymerization problems generated during the work process. In this paper, prevention measures and solutions were proposed,such as adding a polymerization inhibitor system, improving the operation level and updating the filler, so as to ensure safe and stable production of the petrochemical enterprise.Key words : Styrene; Polymer; Formation; Prevention; Solution苯乙烯是应用十分广泛的有机化工原料,是一种聚合单体,化学性质比较活泼,容易发生聚合现象,在生产操作和储存过程中,它很容易受到储存环境如温度和氧气含量、阻止聚合剂含量以及设施的影响而产生质量的变化。
乙酸乙酯
纯化 与除 水方 法 水解 反应 实验 室制 取乙 酸乙 酯
0.894-0.898 ,相对蒸气密度(空气=1):3.04,有强烈的醚似的气味, 清灵、微带果香的酒香,易扩散,不持久。 微溶于水,溶于醇、酮、 醚、氯仿等多数有机溶剂。乙酸乙酯是一种用途广泛的精细化工产品, 具有优异的溶解性、快干性,用途广泛,是一种非常重要的有机化工原 料和极好的工业溶剂,被广泛用于醋酸纤维、乙基纤维、氯化橡胶、乙 烯树脂、乙酸纤维树酯、合成橡胶、涂料及油漆等的生产过程中。其主 要用途有:作为工业溶剂,用于涂料、粘合剂、乙基纤维素、人造革、 油毡着色剂、人造纤维等产品中;作为粘合剂,用于印刷油墨、人造珍 珠的生产;作为提取剂,用于医药、有机酸等产品的生产;作为香料原 料,用于菠萝、香蕉、草莓等水果香精和威士忌、奶油等香料的主要原 料。我们所说的陈酒很好喝,就是因为酒中含有乙酸乙酯。乙酸乙酯具 有果香味。因为酒中含有少量乙酸,和乙醇进行反应生成乙酸乙酯。因 为这是个可逆反应,所以要具有长时间,才会积累导致陈酒香气的乙酸 乙酯。 在一些发达国家, 它作为一种无毒、 无公害型溶剂正逐渐取代含苯溶剂、 甲基乙基酮等对人体和环境带来极大危害的溶剂。 现今乙酸乙酯的生产 路线分为酯化法、乙醛缩合法、乙烯加成法和乙醇脱氢法等。我公司采 用的乙醇脱氢法是二十世纪九十年代后期发展起来的新技术, 可以将乙 醇经一步脱氢转化为乙酸乙酯,同时生成副产品氢气。采用这种方法生 产的乙酸乙酯纯度高、含水和各种重金属等杂质低,质量超过国家质量 标准。 乙酸乙酯一般含量为 95%~98%, 含有少量水、乙醇和乙酸。可用下法 纯化:于 1000mL 乙酸乙酯中加入 100mL 乙酸酐,10 滴浓硫酸,加热 回流 4h,除去乙醇和水等杂质,然后进行蒸馏。馏液用 20~30g 无水 碳酸钾振荡,再蒸馏。产物沸点为 77℃,纯度可达以上 99%。 在酸的催化下,乙酸乙酯水解为乙酸和乙醇。 先加乙醇,再加浓硫酸(防止暴沸),最后加乙酸, 然后加热(可以 控制实验) 乙酸的酯化反应制乙酸乙酯的方程式: CH3COOH+CH3CH2OH===CH3COOC2H5+H2O (可逆反应、加热、浓 硫酸催化剂) 1:酯化反应是一个可逆反应。为了提高酯的产量,必须尽量使反应向 有利于生成酯的方向进行。一般是使反应物酸和醇中的一种过量。在实 验室里一般采用乙醇过量的办法。乙醇的质量分数要高,如能用无水乙 醇代替质量分数为 95%的乙醇效果会更好。催化作用使用的浓硫酸量 很少,一般只要使硫酸的质量达到乙醇质量的 3%就可完成催化作用, 但为了能除去反应中生成的水,应使浓硫酸的用量再稍多一些。 2:制备乙酸乙酯时反应温度不宜过高,要保持在 60 ℃~70 ℃左右, 温度过高时会产生乙醚和亚硫酸等杂质。液体加热至沸腾后,应改用小 火加热。事先可在试管中加入几片碎瓷片,以防止液体暴沸。 3导气管不要伸到 Na2CO3 溶液中去,防止由于加热不均匀,造成 Na2CO3 溶液倒吸入加热反应物的试管中。 3.1:浓硫酸既作催化剂,又做吸水剂。
电化学选择性氢化脱氯反应
2. 多氯吡啶甲酸的电化学选择性氢化脱氯反应
3. 其他多氯有机物的电化学选择性氢化脱氯反应
4. 总结和展望
Zhejiang University of Technology
2
1.银、钯催化的电化学选择性氢化脱氯反应简介
1.1. 主要用途及要求
有机合成:合成部分氯代 有机物作为药物中间体或 原料药; 要求:区域选择性脱氯。
Zhejiang University of Technology
10
Table 2.2 Effect of electrolyte composition on the ECH dechlorination of 0.25 M 3,6-D.a Entry 1 2 3 4 5 6d
a
Catholyte 1.25M NaOH 0.25 M NaOH 0.5 M KH2PO4 + 0.3 M NaOH 0.5 M KH2PO4 + 0.2 M NaOH 0.5 M KH2PO4 + 0.2 M
10
a
Ag(r)
10 mA
0.2
0
0
98.7
0
0
0
2 F· mol-1 of 3,5,6-T were consumed in the all 10 electrolysis experiments. b Current efficiency calculated with respect to the produced 3,5-D. c 3,5-D and 3,6-D selectivity calculated with respect to the converted 3,5,6-T.
Cl Cl N (3,5,6-T)
核磁常见溶剂峰
NMR Chemical Shifts of Common Laboratory Solvents as Trace Impurities Hugo E.Gottlieb,*Vadim Kotlyar,andAbraham Nudelman*Department of Chemistry,Bar-Ilan University,Ramat-Gan52900,IsraelReceived June27,1997In the course of the routine use of NMR as an aid for organic chemistry,a day-to-day problem is the identifica-tion of signals deriving from common contaminants (water,solvents,stabilizers,oils)in less-than-analyti-cally-pure samples.This data may be available in the literature,but the time involved in searching for it may be considerable.Another issue is the concentration dependence of chemical shifts(especially1H);results obtained two or three decades ago usually refer to much more concentrated samples,and run at lower magnetic fields,than today’s practice.We therefore decided to collect1H and13C chemical shifts of what are,in our experience,the most popular “extra peaks”in a variety of commonly used NMR solvents,in the hope that this will be of assistance to the practicing chemist.Experimental SectionNMR spectra were taken in a Bruker DPX-300instrument (300.1and75.5MHz for1H and13C,respectively).Unless otherwise indicated,all were run at room temperature(24(1°C).For the experiments in the last section of this paper,probe temperatures were measured with a calibrated Eurotherm840/T digital thermometer,connected to a thermocouple which was introduced into an NMR tube filled with mineral oil to ap-proximately the same level as a typical sample.At each temperature,the D2O samples were left to equilibrate for at least 10min before the data were collected.In order to avoid having to obtain hundreds of spectra,we prepared seven stock solutions containing approximately equal amounts of several of our entries,chosen in such a way as to prevent intermolecular interactions and possible ambiguities in assignment.Solution1:acetone,tert-butyl methyl ether,di-methylformamide,ethanol,toluene.Solution2:benzene,di-methyl sulfoxide,ethyl acetate,methanol.Solution3:acetic acid,chloroform,diethyl ether,2-propanol,tetrahydrofuran. Solution4:acetonitrile,dichloromethane,dioxane,n-hexane, HMPA.Solution5:1,2-dichloroethane,ethyl methyl ketone, n-pentane,pyridine.Solution6:tert-butyl alcohol,BHT,cyclo-hexane,1,2-dimethoxyethane,nitromethane,silicone grease, triethylamine.Solution7:diglyme,dimethylacetamide,ethyl-ene glycol,“grease”(engine oil).For D2O.Solution1:acetone, tert-butyl methyl ether,dimethylformamide,ethanol,2-propanol. Solution2:dimethyl sulfoxide,ethyl acetate,ethylene glycol, methanol.Solution3:acetonitrile,diglyme,dioxane,HMPA, pyridine.Solution4:1,2-dimethoxyethane,dimethylacetamide, ethyl methyl ketone,triethylamine.Solution5:acetic acid,tert-butyl alcohol,diethyl ether,tetrahydrofuran.In D2O and CD3OD nitromethane was run separately,as the protons exchanged with deuterium in presence of triethylamine.ResultsProton Spectra(Table1).A sample of0.6mL of the solvent,containing1µL of TMS,1was first run on its own.From this spectrum we determined the chemical shifts of the solvent residual peak2and the water peak. It should be noted that the latter is quite temperature-dependent(vide infra).Also,any potential hydrogen-bond acceptor will tend to shift the water signal down-field;this is particularly true for nonpolar solvents.In contrast,in e.g.DMSO the water is already strongly hydrogen-bonded to the solvent,and solutes have only a negligible effect on its chemical shift.This is also true for D2O;the chemical shift of the residual HDO is very temperature-dependent(vide infra)but,maybe counter-intuitively,remarkably solute(and pH)independent. We then added3µL of one of our stock solutions to the NMR tube.The chemical shifts were read and are presented in Table 1.Except where indicated,the coupling constants,and therefore the peak shapes,are essentially solvent-independent and are presented only once.For D2O as a solvent,the accepted reference peak(δ)0)is the methyl signal of the sodium salt of3-(trimeth-ylsilyl)propanesulfonic acid;one crystal of this was added to each NMR tube.This material has several disadvan-tages,however:it is not volatile,so it cannot be readily eliminated if the sample has to be recovered.In addition, unless one purchases it in the relatively expensive deuterated form,it adds three more signals to the spectrum(methylenes1,2,and3appear at2.91,1.76, and0.63ppm,respectively).We suggest that the re-sidual HDO peak be used as a secondary reference;we find that if the effects of temperature are taken into account(vide infra),this is very reproducible.For D2O, we used a different set of stock solutions,since many of the less polar substrates are not significantly water-soluble(see Table1).We also ran sodium acetate and sodium formate(chemical shifts: 1.90and8.44ppm, respectively).Carbon Spectra(Table2).To each tube,50µL of the stock solution and3µL of TMS1were added.The solvent chemical shifts3were obtained from the spectra containing the solutes,and the ranges of chemical shifts(1)For recommendations on the publication of NMR data,see: IUPAC Commission on Molecular Structure and Spectroscopy.Pure Appl.Chem.1972,29,627;1976,45,217.(2)I.e.,the signal of the proton for the isotopomer with one less deuterium than the perdeuterated material,e.g.,C H Cl3in CDCl3or C6D5H in C6D6.Except for CHCl3,the splitting due to J HD is typically observed(to a good approximation,it is1/6.5of the value of the corresponding J HH).For CHD2groups(deuterated acetone,DMSO, acetonitrile),this signal is a1:2:3:2:1quintet with a splitting of ca.2 Hz.(3)In contrast to what was said in note2,in the13C spectra the solvent signal is due to the perdeuterated isotopomer,and the one-bond couplings to deuterium are always observable(ca.20-30Hz). Figure1.Chemical shift of H DO as a function of tempera-ture..Chem.1997,62,7512-7515S0022-3263(97)01176-6CCC:$14.00©1997American Chemical Societyshow their degree of variability.Occasionally,in order to distinguish between peaks whose assignment was ambiguous,a further1-2µL of a specific substrate were added and the spectra run again.Table1.1H NMR Dataproton mult CDCl3(CD3)2CO(CD3)2SO C6D6CD3CN CD3OD D2O solvent residual peak7.26 2.05 2.507.16 1.94 3.31 4.79 H2O s 1.56 2.84a 3.33a0.40 2.13 4.87acetic acid CH3s 2.10 1.96 1.91 1.55 1.96 1.99 2.08 acetone CH3s 2.17 2.09 2.09 1.55 2.08 2.15 2.22 acetonitrile CH3s 2.10 2.05 2.07 1.55 1.96 2.03 2.06 benzene CH s7.367.367.377.157.377.33tert-butyl alcohol CH3s 1.28 1.18 1.11 1.05 1.16 1.40 1.24 OH c s 4.19 1.55 2.18tert-butyl methyl ether CCH3s 1.19 1.13 1.11 1.07 1.14 1.15 1.21 OCH3s 3.22 3.13 3.08 3.04 3.13 3.20 3.22 BHT b ArH s 6.98 6.96 6.877.05 6.97 6.92OH c s 5.01 6.65 4.79 5.20ArCH3s 2.27 2.22 2.18 2.24 2.22 2.21ArC(CH3)3s 1.43 1.41 1.36 1.38 1.39 1.40chloroform CH s7.268.028.32 6.157.587.90 cyclohexane CH2s 1.43 1.43 1.40 1.40 1.44 1.451,2-dichloroethane CH2s 3.73 3.87 3.90 2.90 3.81 3.78 dichloromethane CH2s 5.30 5.63 5.76 4.27 5.44 5.49diethyl ether CH3t,7 1.21 1.11 1.09 1.11 1.12 1.18 1.17 CH2q,7 3.48 3.41 3.38 3.26 3.42 3.49 3.56 diglyme CH2m 3.65 3.56 3.51 3.46 3.53 3.61 3.67 CH2m 3.57 3.47 3.38 3.34 3.45 3.58 3.61OCH3s 3.39 3.28 3.24 3.11 3.29 3.35 3.37 1,2-dimethoxyethane CH3s 3.40 3.28 3.24 3.12 3.28 3.35 3.37 CH2s 3.55 3.46 3.43 3.33 3.45 3.52 3.60 dimethylacetamide CH3CO s 2.09 1.97 1.96 1.60 1.97 2.07 2.08 NCH3s 3.02 3.00 2.94 2.57 2.96 3.31 3.06NCH3s 2.94 2.83 2.78 2.05 2.83 2.92 2.90 dimethylformamide CH s8.027.967.957.637.927.977.92 CH3s 2.96 2.94 2.89 2.36 2.89 2.99 3.01CH3s 2.88 2.78 2.73 1.86 2.77 2.86 2.85 dimethyl sulfoxide CH3s 2.62 2.52 2.54 1.68 2.50 2.65 2.71 dioxane CH2s 3.71 3.59 3.57 3.35 3.60 3.66 3.75 ethanol CH3t,7 1.25 1.12 1.060.96 1.12 1.19 1.17 CH2q,7d 3.72 3.57 3.44 3.34 3.54 3.60 3.65OH s c,d 1.32 3.39 4.63 2.47ethyl acetate CH3CO s 2.05 1.97 1.99 1.65 1.97 2.01 2.07C H2CH3q,7 4.12 4.05 4.03 3.89 4.06 4.09 4.14CH2C H3t,7 1.26 1.20 1.170.92 1.20 1.24 1.24 ethyl methyl ketone CH3CO s 2.14 2.07 2.07 1.58 2.06 2.12 2.19C H2CH3q,7 2.46 2.45 2.43 1.81 2.43 2.50 3.18CH2C H3t,7 1.060.960.910.850.96 1.01 1.26 ethylene glycol CH s e 3.76 3.28 3.34 3.41 3.51 3.59 3.65“grease”f CH3m0.860.870.920.860.88CH2br s 1.26 1.29 1.36 1.27 1.29n-hexane CH3t0.880.880.860.890.890.90CH2m 1.26 1.28 1.25 1.24 1.28 1.29HMPA g CH3d,9.5 2.65 2.59 2.53 2.40 2.57 2.64 2.61 methanol CH3s h 3.49 3.31 3.16 3.07 3.28 3.34 3.34 OH s c,h 1.09 3.12 4.01 2.16nitromethane CH3s 4.33 4.43 4.42 2.94 4.31 4.34 4.40 n-pentane CH3t,70.880.880.860.870.890.90CH2m 1.27 1.27 1.27 1.23 1.29 1.292-propanol CH3d,6 1.22 1.10 1.040.95 1.09 1.50 1.17 CH sep,6 4.04 3.90 3.78 3.67 3.87 3.92 4.02 pyridine CH(2)m8.628.588.588.538.578.538.52 CH(3)m7.297.357.39 6.667.337.447.45CH(4)m7.687.767.79 6.987.737.857.87 silicone grease i CH3s0.070.130.290.080.10 tetrahydrofuran CH2m 1.85 1.79 1.76 1.40 1.80 1.87 1.88 CH2O m 3.76 3.63 3.60 3.57 3.64 3.71 3.74 toluene CH3s 2.36 2.32 2.30 2.11 2.33 2.32CH(o/p)m7.177.1-7.27.187.027.1-7.37.16CH(m)m7.257.1-7.27.257.137.1-7.37.16 triethylamine CH3t,7 1.030.960.930.960.96 1.050.99 CH2q,7 2.53 2.45 2.43 2.40 2.45 2.58 2.57a In these solvents the intermolecular rate of exchange is slow enough that a peak due to HDO is usually also observed;it appears at2.81and3.30ppm in acetone and DMSO,respectively.In the former solvent,it is often seen as a1:1:1triplet,with2J H,D)1Hz. b2,6-Dimethyl-4-tert-butylphenol.c The signals from exchangeable protons were not always identified.d In some cases(see note a),the coupling interaction between the CH2and the OH protons may be observed(J)5Hz).e In CD3CN,the OH proton was seen as a multiplet atδ2.69,and extra coupling was also apparent on the methylene peak.f Long-chain,linear aliphatic hydrocarbons.Their solubility in DMSO was too low to give visible peaks.g Hexamethylphosphoramide.h In some cases(see notes a,d),the coupling interaction between the CH3and the OH protons may be observed(J)5.5Hz).i Poly(dimethylsiloxane).Its solubility in DMSO was too low to give visible peaks.Notes .Chem.,Vol.62,No.21,19977513.Chem.,Vol.62,No.21,1997NotesTable2.13C NMR Data aCDCl3(CD3)2CO(CD3)2SO C6D6CD3CN CD3OD D2O solvent signals77.16(0.0629.84(0.0139.52(0.06128.06(0.02 1.32(0.0249.00(0.01206.26(0.13118.26(0.02acetic acid CO175.99172.31171.93175.82173.21175.11177.21 CH320.8120.5120.9520.3720.7320.5621.03 acetone CO207.07205.87206.31204.43207.43209.67215.94 CH330.9230.6030.5630.1430.9130.6730.89 acetonitrile CN116.43117.60117.91116.02118.26118.06119.68 CH3 1.89 1.12 1.030.20 1.790.85 1.47 benzene CH128.37129.15128.30128.62129.32129.34tert-butyl alcohol C69.1568.1366.8868.1968.7469.4070.36 CH331.2530.7230.3830.4730.6830.9130.29 tert-butyl methyl ether OCH349.4549.3548.7049.1949.5249.6649.37 C72.8772.8172.0472.4073.1774.3275.62C C H326.9927.2426.7927.0927.2827.2226.60 BHT C(1)151.55152.51151.47152.05152.42152.85C(2)135.87138.19139.12136.08138.13139.09CH(3)125.55129.05127.97128.52129.61129.49C(4)128.27126.03124.85125.83126.38126.11CH3Ar21.2021.3120.9721.4021.2321.38C H3C30.3331.6131.2531.3431.5031.15C34.2535.0034.3334.3535.0535.36chloroform CH77.3679.1979.1677.7979.1779.44cyclohexane CH226.9427.5126.3327.2327.6327.961,2-dichloroethane CH243.5045.2545.0243.5945.5445.11 dichloromethane CH253.5254.9554.8453.4655.3254.78diethyl ether CH315.2015.7815.1215.4615.6315.4614.77 CH265.9166.1262.0565.9466.3266.8866.42 diglyme CH359.0158.7757.9858.6658.9059.0658.67 CH270.5171.0369.5470.8770.9971.3370.05CH271.9072.6371.2572.3572.6372.9271.63 1,2-dimethoxyethane CH359.0858.4558.0158.6858.8959.0658.67 CH271.8472.4717.0772.2172.4772.7271.49 dimethylacetamide CH321.5321.5121.2921.1621.7621.3221.09 CO171.07170.61169.54169.95171.31173.32174.57NCH335.2834.8937.3834.6735.1735.5035.03NCH338.1337.9234.4237.0338.2638.4338.76 dimethylformamide CH162.62162.79162.29162.13163.31164.73165.53 CH336.5036.1535.7335.2536.5736.8937.54CH331.4531.0330.7330.7231.3231.6132.03 dimethyl sulfoxide CH340.7641.2340.4540.0341.3140.4539.39 dioxane CH267.1467.6066.3667.1667.7268.1167.19 ethanol CH318.4118.8918.5118.7218.8018.4017.47 CH258.2857.7256.0757.8657.9658.2658.05 ethyl acetate C H3CO21.0420.8320.6820.5621.1620.8821.15 CO171.36170.96170.31170.44171.68172.89175.26CH260.4960.5659.7460.2160.9861.5062.32CH314.1914.5014.4014.1914.5414.4913.92 ethyl methyl ketone C H3CO29.4929.3029.2628.5629.6029.3929.49 CO209.56208.30208.72206.55209.88212.16218.43C H2CH336.8936.7535.8336.3637.0937.3437.27CH2C H37.868.037.617.918.148.097.87 ethylene glycol CH263.7964.2662.7664.3464.2264.3063.17“grease”CH229.7630.7329.2030.2130.8631.29n-hexane CH314.1414.3413.8814.3214.4314.45CH2(2)22.7023.2822.0523.0423.4023.68CH2(3)31.6432.3030.9531.9632.3632.73HMPA b CH336.8737.0436.4236.8837.1037.0036.46 methanol CH350.4149.7748.5949.9749.9049.8649.50c nitromethane CH362.5063.2163.2861.1663.6663.0863.22 n-pentane CH314.0814.2913.2814.2514.3714.39CH2(2)22.3822.9821.7022.7223.0823.38CH2(3)34.1634.8333.4834.4534.8935.302-propanol CH325.1425.6725.4325.1825.5525.2724.38 CH64.5063.8564.9264.2364.3064.7164.88 pyridine CH(2)149.90150.67149.58150.27150.76150.07149.18 CH(3)123.75124.57123.84123.58127.76125.53125.12CH(4)135.96136.56136.05135.28136.89138.35138.27 silicone grease CH3 1.04 1.40 1.38 2.10 tetrahydrofuran CH225.6226.1525.1425.7226.2726.4825.67 CH2O67.9768.0767.0367.8068.3368.8368.68 toluene CH321.4621.4620.9921.1021.5021.50C(i)137.89138.48137.35137.91138.90138.85CH(o)129.07129.76128.88129.33129.94129.91CH(m)128.26129.03128.18128.56129.23129.20CH(p)125.33126.12125.29125.68126.28126.29triethylamine CH311.6112.4911.7412.3512.3811.099.07 CH246.2547.0745.7446.7747.1046.9647.19a See footnotes for Table1.b2J PC)3Hz.c Reference material;see text.For D2O solutions there is no accepted reference for carbon chemical shifts.We suggest the addition of a drop of methanol,and the position of its signal to be defined as49.50ppm;on this basis,the entries in Table2were recorded.The chemical shifts thus obtained are,on the whole,very similar to those for the other solvents. Alternatively,we suggest the use of dioxane when the methanol peak is expected to fall in a crowded area of the spectrum.We also report the chemical shifts of sodium formate(171.67ppm),sodium acetate(182.02and 23.97ppm),sodium carbonate(168.88ppm),sodium bicarbonate(161.08ppm),and sodium3-(trimethylsilyl)-propanesulfonate[54.90,19.66,15.56(methylenes1,2, and3,respectively),and-2.04ppm(methyls)],in D2O. Temperature Dependence of HDO Chemical Shifts.We recorded the1H spectrum of a sample of D2O, containing a crystal of sodium3-(trimethylsilyl)propane-sulfonate as reference,as a function of temperature.The data are shown in Figure1.The solid line connecting the experimental points corresponds to the equation which reproduces the measured values to better than1 ppb.For the0-50o C range,the simplergives values correct to10ppb.For both equations,T is the temperature in°C.Acknowledgment.Generous support for this work by the Minerva Foundation and the Otto Mayerhoff Center for the Study of Drug-Receptor Interactions at Bar-Ilan University is gratefully acknowledged.JO971176Vδ)5.060-0.0122T+(2.11×10-5)T2(1)δ)5.051-0.0111T(2)Notes .Chem.,Vol.62,No.21,19977515。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Chapter 2, Exercise Solutions, Principles of Econometrics, 4e
27
Exercise 2.3 (Continued)
(d)
ˆi yi y ˆ i yi b1 b2 xi , are: The values of the least squares residuals, computed from e
Figure xr2.4(a) Sum of squares for 2
The minimum of this function is approximately 12 and occurs at approximately 2 1.95. The significance of this value is that it is the least-squares estimate. (d) To find the value of that minimizes S () we obtain
190 200 P Z 81 1 P Z 1.1111 0.8667
Chapter 2, Exercise Solutions, Principles of Econometrics, 4e
26
EXERCISE 2.3
(a) The observations on y and x and the estimated least-squares line are graphed in part (b). The line drawn for part (a) will depend on each student’s subjective choice about the position of the line. For this reason, it has been omitted. Preliminary calculations yield:
b1 y b2 x 5.5 1.514286 3.5 10.8
Figure xr2.3 Observations and fitted line
10 2 1 4 6 8
2 y
3 x
4 Fitted values
5
6
(c)
y yi N 33 6 5.5 x xi N 21 6 3.5
3
4
(f) (g) (h) (i)
See figure above. The fitted line passes through the point of the means, x 2,
y 2.4 .
Given b1 5, b2 1.3 and y b1 b2 x , we have y 2.4 b1 b2 x 5 1.3 2 2.4
ˆ y ˆ i N 12 5 2.4 y y
ˆ2
N 2
ˆi2 e
4.3 1.4333 3
ˆ2 1.4333 0.14333 10
(j)
var b2
xi x
2
Chapter 2, Exercise Solutions, Principles of Econometrics, 4e
=0
Chapter 2, Exercise Solutions, Principles of Econometrics, 4e
28
EXERCISE 2.4
(a) If 1 0, the simple linear regression model becomes
yi 2 xi ei
dS 2 xi yi 2 xi2 d
Setting this derivative equal to zero, we haveb xi2 xi yi
or
b
xi yi xi2
Thus, the least-squares estimate is
b2 176 1.9341 91
CHAPTER
2
Exercise Solutions
21
Chapter 2, Exercise Solutions, Principles of Econometrics, 4e
22
EXERCISE 2.1
(a)
x
0 1 2 3 4
y 6 2 3 1 0
xx
-2 -1 0 1 2 0
x x
xi
1 2 3 4 5 6
ˆi 0. e
yi
10 8 5 5 2 3
ˆi e
0.714286 0.228571 −1.257143 0.257143 −1.228571 1.285714
Their sum is (e)
ˆi 1 0.714286 2 0.228571 3 1.257143 4 0.257143 xi e 5 1.228571 6 1.285714
The predicted value for y at x x is
ˆ b1 b2 x 10.8 1.514286 3.5 5.5 y
ˆ b1 b2 x y . That is, the predicted value at the sample mean x is the We observe that y sample mean of the dependent variable y . This implies that the least-squares estimated line passes through the point ( x , y ) . This point is at the intersection of the two dashed lines plotted on the graph in part (b) .
0
x 2,
(b)
y 2.4
b2
x x y y 13 1.3 2 10 x x
b2 is the estimated slope of the fitted line.
b1 y b2 x 2.4 1.3 2 5
which agrees with the approximate value of 1.95 that we obtained geometrically.
215 200 180 200 P Z 100 100 P 2 Z 1.5 0.9104
.4
Figure xr2-2a
0
.1
f(z) .2
.3
-5
-2
0 z
1.5
5
(b)
X 190 y|x$2000 y|x $2000 P X 190 P 2 2 y| x $2000 y| x $2000
ˆi2 e
ˆi xi e
0 1 2 3 4 xi = 10
6 2 3 1 0 yi = 12
5 3.7 2.4 1.1 −0.2 ˆi = y 12
1 −1.7 0.6 −0.1 0.2 ˆi = e 0
1 2.89 0.36 0.01 0.04 ˆi2 = e 4.3
0 −1.7 1.2 −0.3 0.8 ˆi = xi e 0
b1 is the estimated value of E ( y ) when x 0 ; it is the intercept of the fitted line.
(c)
xi2 02 12 22 32 42 30
i 1
5
xi yi 0 6 1 2 2 3 3 1 4 0 11
(b)
xi 21
y 5.5
yi 33
x 3.5
xi x yi y 26.5
xi x
2
17.5
The least squares estimates are:
b2
x x y y 26.5 1.514286 2 17.5 x x
24
EXERCISE 2.2
(a)
180 X y|x$2000 215 y|x$2000 y|x $2000 P 180 X 215 P 2 2 2 y| x $2000 y| x $2000 y|x $2000
4 1 0 1 4
2
yy
3.6 −0.4 0.6 −1.4 −2.4
x x y y
−7.2 0.4 0 −1.4 −4.8 −13
xi =
10
yi
12
xi x xi x
10
2
y y x x y y
(c)
S () ( yi xi )2 ( yi2 2xi yi 2 xi2 ) yi2 2 xi yi 2 xi2
i 1 i 1
N
N
352 2 176 912 352 352 912
40 35 30 SUM_SQ 25 20 15 10 1.6 1.8 BETA 2.0 2.2 2.4
190 200 P Z 100 1 P Z 1 0.8413
Figure xr2-2b
0
.1
f(z) .2
.3
.4
-5
-1
0 z
5
Chapter 2, Exercise Solutions, Principles of Econometrics, 4e
