华工车辆工程热工学全英lecture3
动力工程及工程热物理专业英语课件

Engineering thermophysists contribute to the development of advanced energy conversion technologies like thermal power plants and heat engines, as well as energy storage systems such as thermal energy storage
Combustion and Pollution Control
• Combustion Fundamentals: The courseware should cover the fundamentals of combustion, including chemical reactions, flame stability, and combustion efficiency Students should learn about different types of communicators and how to design them for optimal performance
02
Professional Fundamentals
Fundamentals of Thermodynamics
01
Laws of Thermodynamics: The courseware should cover the three laws of thermodynamics, which are the foundation of power engineering and engineering thermophysics These laws explain the relationship between heat and work, and they govern the behavior of energy in all systems
lecture03
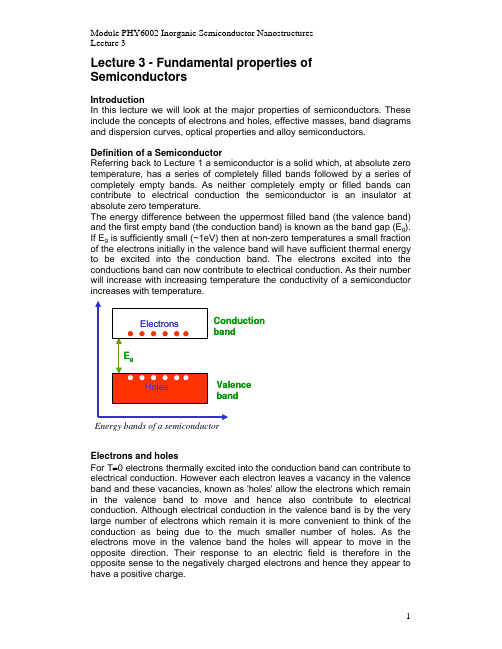
Lecture 3 - Fundamental properties of SemiconductorsIntroductionIn this lecture we will look at the major properties of semiconductors. These include the concepts of electrons and holes, effective masses, band diagrams and dispersion curves, optical properties and alloy semiconductors.Definition of a SemiconductorReferring back to Lecture 1 a semiconductor is a solid which, at absolute zero temperature, has a series of completely filled bands followed by a series of completely empty bands. As neither completely empty or filled bands can contribute to electrical conduction the semiconductor is an insulator at absolute zero temperature.The energy difference between the uppermost filled band (the valence band) and the first empty band (the conduction band) is known as the band gap (E g). If E g is sufficiently small (~1eV) then at non-zero temperatures a small fraction of the electrons initially in the valence band will have sufficient thermal energy to be excited into the conduction band. The electrons excited into the conductions band can now contribute to electrical conduction. As their number will increase with increasing temperature the conductivity of a semiconductorElectrons and holesFor T≠0 electrons thermally excited into the conduction band can contribute to electrical conduction. However each electron leaves a vacancy in the valence band and these vacancies, known as 'holes' allow the electrons which remain in the valence band to move and hence also contribute to electrical conduction. Although electrical conduction in the valence band is by the very large number of electrons which remain it is more convenient to think of the conduction as being due to the much smaller number of holes. As the electrons move in the valence band the holes will appear to move in the opposite direction. Their response to an electric field is therefore in the opposite sense to the negatively charged electrons and hence they appear to have a positive charge.Electrical conduction in a semiconductor therefore arises from the movement of electrons in the conduction band and holes in the valence band. The total current is sum of the individual currents due to the holes and the electrons. Because electrons and holes have opposite charges but move in opposite directions their contribution to the total current add.It was shown in Lecture 2 that the electrical conductivity, σ, is related to the mobility, µ, by the equationσµ=newhere e is the electronic charge and n is the density of free electrons. As electrons and holes generally have different mobilities and may have different densities, for the case where both electrons and holes contribute to the conductivity the above equation must be modified to the formσµµne pe=+e pwhere n and p are the densities of electrons and holes respectively and µe and µp are their mobilities.In the case considered so far n=p as each electron which is excited into the conduction band leaves one hole in the valence band. However we will see in the next lecture that it is possible to have cases where n≠p.Optical properties of SemiconductorsIlluminating a semiconductor with light may cause an increase in its conductivity. Light consists of a stream of particles, known of photons, each carrying an energy, E, which is related to the frequency of the light, f, via the relationship E=hf, where h is Planck's constant.When light passes through a semiconductor it may be absorbed (photons are destroyed) but for this to happen the energy carried by the photons must go somewhere. One possibility is for the energy to excite an electron from the valence band to the conduction band. This will increase the number of electrons and holes and hence will increase the conductivity of the semiconductor. However for this to happen the photon must have an energy equal to or greater than the band gap of the semiconductor. This leads to theimportant result that light consisting of photons having energy less than the band gap will simply pass through the semiconductor, as absorption is not possible. In contrast light consisting of photons of energy greater than the band gap will be strongly absorbed. If a sample of a semiconductor has a reasonable thickness then once the photon energy exceeds the band gap all of the light will be absorbed.If we measure the transmission spectrum of a semiconductor (this consists of measuring the fraction of the original light which passes through a suitable sample and plotting how this fraction varies as a function of the energy of the photons) we will obtain a result similar to that shown below. At the band gap the fraction of transmitted light rapidly switches from a value close to one to a value very close to zero. Recording a transmission spectrum provides a simple way to determine the band gap of a semiconductor.The fact that light may change the conductivity of a semiconductor forms the basis of the use of semiconductors to detect light.The opposite process to absorption occurs when an electron jumps back to the valence band from the conduction band. In this case an electron is lost from the conduction band and a hole is lost from the valence band - an electron-hole pair is said to be annihilated, this is known as electron-hole recombination. Again energy must be conserved and this can be achieved if a photon is emitted with an energy equal to that of the band gap. This process forms the basis of the production of light by a semiconductor in light emitting diodes (LEDs) and lasers.Carrier dispersion and effective massIf we add energy of amount E g to a semiconductor then we can excite one electron from the valence band to the conduction band. All the added energy is used to transfer the electron across the band gap, there is no energy remaining to give either the electron or hole any kinetic energy. The electron and hole are stationary. However if we add energy greater than E g then E g of this is used to excite the electron across the band gap, the remainder goes to give the electron and/or hole kinetic energy. In this case the electron and/or hole have a non-zero velocity.10T r a n s m i s s i o n Photon frequency (f )f=E g /h The transmission spectrum of a semiconductorThe dispersion relationship for a carrier describes how its energy depends upon its velocity (or related quantity – see below). For a particle of mass m travelling with a velocity v in free space the kinetic energy is given by122mvThis is a quadratic relationship – energy depends upon the square of the velocity.For an electron in a semiconductor we must add the band gap energy to the kinetic energy to give the electron’s total energy. In addition we must consider the dispersion relationship of the hole. Because the hole is just the absence of an electron the dispersion of a hole is similar to that of the electron but inverted (We can think of the hole as being similar to a bubble in a liquid. The energy of the bubble is increased as it is pushed deeper into the liquid).In terms of velocity the dispersion relationship (often referred to as dispersion curves) of a semiconductor will have the form shown in the figure below.For a number of reasons carrier dispersion curves are not generally plotted as a function of v but of a related quantity, the carrier wave vector k . If a particle has a wavelength λ then k =2π/λ. In addition, the momentum of a particle is given by p =mv and p is related to wavelength by the relationship p =h/λ (see Lecture 1). Hence we can writeKineticEnergy mv m p m p m h m h m k h k m k m==F H G I KJ =====12122222222222222222222λππ(/)()= Conduction band electronsValence band holes VelocityE n e r g yE g Dispersion curves for electrons and holes in a semiconductorwhere ==h /2π. The energy will again show a quadratic behaviour when plotted as a function of k .So far we have considered only the kinetic energy of the electrons and holes. However the carriers are not in free space but inside a solid. The negatively charged electrons interact with the positively charged atomic cores and this electrostatic interaction gives them an additional potential energy. Although it is mathematically very difficult to deal with this potential energy fully (there are a very large number of atomic cores in any solid) it turns out that, at least for small energies, the dispersion curves still have a quadratic form but their curvature is different to that obtained for carriers in free space. This is shown in the following figure.Because kinetic energy is given by =222k m /(), the fact that the curvature of the dispersion curves has changed from the free particle case implies that the electrons and holes behave as particles having masses different to that of a free electron. This new mass is known as the ‘effective mass’ and is denoted by the symbol m* (usually expressed as a fraction on the free electron mass). The dispersion curve is now described by the formula =222k m /()*.The concept of effective mass allows the complicated potential energy of a carrier in a solid to be approximated in a simple way by still treating the carrier as if it were free (kinetic energy only). The use of an effective mass combines the potential energy with the kinetic energy.In general holes and electrons have different effective masses, distinguishedas m e * and m h * respectively.kE n e r g y E gElectron and hole dispersion curves without potential energy (dashed lines) andwith the inclusion of potential energy (solid lines).For most semiconductors the effective masses are less than the free electron mass. For example the electron effective mass in GaAs is only 6.7% of thefree electron mass: m e *=0.067m 0, where m 0 is the free electron mass.Real band structuresThe band structures of real semiconductors are very complicated. For large energies the dispersion curves are no longer quadratic, there may be more than one valence band and, in addition, there is a dependence upon the direction of motion within the semiconductor crystal. Fortunately we are generally only concerned with small energies where the dispersion curves have a fairly simple form.The following figures show the calculated dispersion curves (also known as the band structure) for the two semiconductors Si and GaAs.Calculated band structures for Si (top) and GaAs (bottom)Direct and indirect band gap semiconductorsIn the above band structures zero energy corresponds to the top of the valence band and the point marked as Γ (the gamma point) corresponds to zero wave vector. In all common semiconductors the highest point (maxima) of the valence band (the lowest energy for holes) occurs at the gamma point (k=0).The conduction band has more than one minimum. One of these will always occur at Γ but there are other minima at positions corresponding to k≠0 (the X and L points in the above band structures).We can assign a band gap value to the difference in energy between the top of the valence band at k=0 (Γ-point) and each minima of the conduction band. For the conduction band minima at k=0, directly above the valence band maxima also at k=0, the band gap is known as the ‘direct band gap’. For conduction band minima at k≠0 the band gaps are known as ‘indirect band gaps’.The optical properties of a semiconductor are critically dependent upon where the very lowest point of the conduction band occurs. If this occurs at k=0 (the direct band gap is smaller than any of the indirect band gaps) then the semiconductor is said to be a ‘direct band gap semiconductor’. A typical example of a direct band gap semiconductor is GaAs. Alternatively if the very lowest point of the conduction band occurs at k≠0 (one of the indirect band gaps is smaller than the direct band gap) then the semiconductor is said to be an ‘indirect band gap semiconductor’. Si is an indirect band gap semiconductor because the lowest point in the conduction band occurs not atElectrons in the conduction band will tend to collect at the lowest energy point. If an electron in a direct band gap semiconductor now recombines with a hole it only has to change its energy. There is no change in its wave vector or momentum because both the electron and hole have the same wave vector. Although both energy and momentum (wave vector) must be conserved, in a direct band gap semiconductor the photon produced when the electron and hole recombine needs to only carry off the energy. Recombination of an electron and hole in a direct band gap semiconductor is therefore a one step process (see figure above). This occurs very rapidly and is very efficient.For an indirect band gap semiconductor the situation is very different. Now there is a large change in the wave vector of the electron as it recombines with the hole. This wave vector (momentum) must go somewhere. However the photon can only carry off a very small amount of momentum so an additional particle is required. In general this is a phonon. For an indirect band gap semiconductor the recombination of an electron and hole therefore produces a photon, which carries off most of the energy, and a phonon, which carries off most of the momentum. This is a two-step process which occurs relatively slowly. As there are other processes by which an electron and hole can recombine without producing a photon (so called non-radiative processes) these tend to be more likely to occur in an indirect band gap semiconductor compared to a direct gap one. The efficiency with which light (photons) can be produced is therefore much less in an indirect gap semiconductor compared to a direct one. This explains why although Si (an indirect band gap semiconductor) is the most important semiconductor for electronic applications it cannot be used to produce lasers or LEDs.Examples of real semiconductorsThe initially studied semiconductors are the elemental ones Si and Ge. However because these are both indirect band gap semiconductors, for opto-electronic applications other semiconductors have to be used. These are generally of the form of binary semiconductors, formed by combining elements from columns 3 and 5 of the periodic table (so called III-V semiconductors: GaAs, InAs, InSb, InP) or elements from columns 2 and 6 (so called II-VI semiconductors: CdTe, CdS, ZnSe). Each of these semiconductors has a particular size band gap and lattice constant (the distance between neighbouring atoms).However, even by combining all possible combinations of III-V or II-VI elements the number of different semiconductors is still not large. Additional semiconductors can be obtained by forming alloy compounds which consist of a mixture of two different semiconductors. For example Si and Ge can be mixed together to form the semiconductor Si x Ge1-x where x (0≤x≤1) indicates the relative amount of Si and Ge in the mixture. Similarly GaAs and AlAs can be mixed to form the ternary semiconductor Al x Ga1-x As (note that the total number of group III atoms (Al or Ga) equals the number of group V atoms (As)).When alloy semiconductors are formed it is found that many of their properties exhibit an average of those of the constituent semiconductors. For example the lattice constant of Si x Ge1-x varies continuously from that of Ge (for x=0) through to that of Si as x increases to 1. A similar variation of the band gap is found.The figures below show the composition dependence of the band gaps for the important ternary semiconductor Al x Ga1-x As. Note that both the direct and indirect band gaps vary with composition. GaAs is a direct band gap semiconductor. As Al is added to form Al x Ga1-x As both the direct and indirect band gaps increase. However because the former increases much more rapidly than the latter they cross over at a composition x≈0.4 and for higher compositions than this Al x Ga1-x As (and AlAs) is an indirect band gap semiconductor.Summary and ConclusionsIn this lecture we have looked at some specific properties of semiconductors. The majority of these will be needed as a basis for future lectures in this module so it is important that you understand them. You should now understand how electrical conduction in a semiconductor occurs by both electrons and holes, how a semiconductor can detect and produce light, what is meant by a dispersion curve, the importance of the concept of effective mass, the definition of direct and indirect band gaps and why the type of band gap is so important for the optical properties of a semiconductor. Finally you should have some knowledge of the different types of semiconductors (elemental, binary, ternary).Further reading•The structure of the valence bands of real semiconductors is relatively complex. Referring to the band structure of Si shown above there are two valence bands with the same maximum at k=0 (the Γ point). These bands are said to be degenerate for k=0. For k≠0 the bands split. Because they have different curvatures the effective masses for each band are different.One has a relatively high mass and is hence known as the 'heavy hole' band, the other has a lighter mass and is known as the 'light hole' band.Can you say which band is which on the figure? For GaAs there is an additional valence band (Γ7) which has a maximum just below the common maximum of the light and heavy hole bands (Γ8). The former band is known as the spin-split off band. The valence band structure in Si and III-V semiconductors is discussed in Yu and Cardona 'Fundamentals of Semiconductors'.•There are a number of processes by which an electron and a hole can recombine without producing a photon. The main ones are Auger recombination and recombination via deep levels. These processes are briefly discussed in Klingshirn ' Semiconductor Optics'.。
汽车专业英语教程多媒体教学课件Unit 3

德国是汽车的发源 地。1886年,卡 尔·本茨和哥德利 普·戴姆勒共享了 发明汽车带来的荣 耀。他们各自建立 了自己的汽车公司, 并于1926年合并组 建为戴姆勒—奔驰 汽车公司。如今, 梅塞德斯一奔驰这 个品牌已经蜚声国 际。而其无可争议 的德国竞争对手是 宝马公司,其公司 起源与其他公司不 同。宝马公司成立 于1916年,以生产 航空发动机开始创 业;1928年,宝马 公司转向生产汽车。
an enviable reputation as the maker of Mercedes-Benz cars . Its undisputed
German competitor is BMW (Bavarian Motor Works),a firm with very different origins . BMW , formed in 1916 , began by producing aero engines.and switched to motor cars in 1928.
investments in auto design and production.Each auto manufacturer has tried
to win the competition on the world
markets and therefore never stopped
making research for new models . Consequently , the world auto industry has been developed very fast.
1956年,第一 汽车制造厂在长 春建成,并生产 出我国第一批解 放牌汽车。在这 之后,其他几个 汽车制造厂相继 在南京、北京、 济南和四川建立。 20世纪80年代初 期,我国自行设 计和装备的第二 汽车制造厂投入 大规模生产。
汽车工程专业英语(汉化版)

2.3 The engine operation .. ……………………………………………………………................ .(26) 2.4 Basic engine systems .……………………………………………………………........... ...... (29) 2.5 Air induction System ..…………………………………………………………….......... ...... .. (31) 2.6 2.7 2.8 2.9 2.1 0 Gasoline fuel System ..…………………………………………….... ..……...................... .(32) Engine Cooling.………………………………………………………............... .. ............... (40) Engine Lubrication. …………………………………………………............................. .....(44) Diesel engine ..…………………………………………………·………………….......……….(46) Emission Control. ......... ....................................................................... ......... .. (53)
Review questions ................ .......................... .. ......................... ........................ ........ (61)
华工考研动力工程及工程热物理813工程热力学真题答案推荐书目

华工考研动力工程及工程热物理813工程热力学真题答案推荐书目一、资料简介《华南理工大学考研813工程热力学复习全析(含历年真题与答案)》由致远华工考研网依托多年丰富的教学与辅导经验,组织官方教学研发团队与华南理工大学电力学院的优秀研究生共同合作编写而成。
全书内容紧凑权威细致,编排结构科学合理,为参加华南理工大学考研的考生量身定做的必备专业课资料。
《华南理工大学考研813工程热力学复习全析(含历年真题与答案)》全书编排根据华工813工程热力学考研参考书目:沈维道《工程热力学》,高等教育出版社------2020华工官方规定的选读书目------《工程热力学》(第五版)沈维道童钧耕主编,高等教育出版社,2016年结合提供的往年华工考研真题内容,帮助报考华南理工大学考研的同学通过华工教材章节框架分解、配套的课后习题讲解及相关985、211名校考研真题与解答,帮助考生梳理指定教材的各章节内容,深入理解核心重难点知识,把握考试要求与考题命题特征。
通过研读演练本书,达到把握教材重点知识点、适应多样化的专业课考研命题方式、提高备考针对性、提升复习效率与答题技巧的目的。
同时,透过测试演练,以便查缺补漏,为初试高分奠定坚实基础。
为保障购书考生利益,本书仅对外出售80册。
因考研辅导资料的资源稀缺性,本书一旦出售,谢绝退货。
二、适用范围适用院系:机械与汽车工程学院:动力机械及工程电力学院:动力工程及工程热物理、动力工程(专硕)适用科目:813工程热力学三、内容详情本书包括以下几个部分内容:1、考试解读:part 1 学院专业考试概况①学院专业分析:含学院基本概况、考研专业课科目:813工程热力学的考试情况;②科目对应专业历年录取统计表:含华工动力工程相关专业的历年录取人数与分数线情况;③历年考研真题特点:含华南理工大学考研专业课813工程热力学各部分的命题规律及出题风格。
part 2 历年题型分析及对应解题技巧根据华工813工程热力学考试科目的考试题型(名词解释、简答题、论述题等),分析对应各类型题目的具体解题技巧,帮助考生提高针对性,提升答题效率,充分把握关键得分点。
华工汽车英语期末考试复习资料

汽车英语复习题一、汽车专业词组翻译成中文。
(10分)1、demonstrate success 显示/彰显成功2、medium-sized income 中等收入3、level of concern 关注程度4、lower emission 低排放量5、specific requirement 特定需求6、a big share of the auto market 占汽车市场很大份额P261、cargo space 后备箱容量2 、cargo versatility 多功能后备箱3、solid assembly 坚实的组装4、engine power 发动机功率5、split-folding rear seatbacks 可折叠后座椅6、crash test 碰撞试验(重点)7、antilock brakes(ABS) 防爆系统8、dual front airbags 前排双气囊P301、driver and front passenger air bags 前排双气囊2、side air bags 侧气囊/气帘3、seat belt 安全带4、anti-lock brake system(ABS) 防抱死制动系统5、parking aid system 泊车辅助系统6、electronic brake-force distribution 电子制动力分配系统7、electronic stability control(ESC) 电子稳定控制系统8、energy-absorbing steering column 吸能转向柱9、door crash beam 车门防撞梁10、collision buffer 碰撞缓冲区P50、1、front and rear suspensions 前后悬挂2、rear axles 后轴3、drive shaft 传动轴4、gear box 齿轮箱、传动箱5、floor pan 车底板6、left and right quarter panels 左右后侧围板(车身板)7、front and rear door pillars 前后门柱8、weather strip 密封条P741、vehicle recall 汽车召回2、Vehicle identification number(VIN) 汽车身份好码3、Statistical process control(SPC) 统计过程控制4、Tactile probe measurements 接触式探针测量5、3D geometry of components 零部件的三维尺寸6、Coordinate measurement machines(CMMS) 坐标系测量机7、Quality audit station 质量检验部门8、Validation process 验证过程P981、target consumer 目标客户2、Video clip 录像/视频剪辑3、rental program 租车项目4、auto dealer 汽车销售商5、Extended test drive 延时驾车体验6、Market share 市场份额7、Focus groups and clinics 焦点团体和讨论会8、Purchase accelerator 购买加速器P1441、wheel alignment 四轮定位2、Tire life 轮胎寿命3、Rear thrust line 后推力线4、Adjustable rear suspension 可调后悬挂5、Gas mileage 汽油里程6、Rolling resistance 车轮滚动二、翻译。
汽车专业英语教程多媒体教学课件Unit 4 section 2-3
Professors estimate that electric vehicle cannot replace fuel oil engine automobile less 10 years . The actuality compels engineers to think out a ways which makes the best of both worlds,i.e. to develop a Hybrid-Electric Vehicle (for short HEV).
从目前世界范 围内的整个形势 来看,日本是电 动汽车技术发展 速度最快的少数 几个国家之一, 特别是在发展混 合动力汽车方面, 日本居世界领先 地位。目前,世 界上能够批量产 销混合动力汽车 的企业,只有日 本的丰田和本田 两家汽车公司。
Three major companies of USA produce and market battery electric vehicle in limited quantities only,the production of hybrid power vehicle and fuel cell electric vehicle does not realize industrialization also . Each group of major auto company of China carries through research and development of Hybrid-Electric Vehicle are,majority is for main by hybrid power electric motor coach.
华工车辆工程热工学全英lecture3
-17.8
459.67
0
-273.15
-459.67
0
Temperature scale —
Ice point Celsius scale Fahrenheit scale 0 ℃ 32℉ Steam point 100 ℃ 212℉
9 ℉ =5 ℃
T(℃)= T(℉ )-32)×5/9
T(℉ )=1.8T(℃)+32 T(R)=1.8T(K)
l
11
Dimension Force Area Pressure
SI unit English Conversin Factor unit N lbf 1 lbf =4.44822 N m2 N/m2 Pa ft2 Lbf/ft2 psi
1 psi = 6.894757 kPa
!!
1 ft 2=0.30482 m2
Steam point (boiling point) the temperature at which water and water vapor is in equilibrium at 1atm pressure1大气压下水蒸 气和固态水的稳定状态 100 ℃
Celsius scale
参考温度 Reference temperature:
Analysis:
h1
Take the densities of water, oil, and mercury to be 1000, 850 and 13600 kg/m 3, respectively.
22
Eg. Measuring Pressure With a Multifluid Manometer
(p28,Fig1-49)
车辆工程专业英语词汇表
车辆工程专业英语词汇表摘要:本文根据车辆工程专业的主要课程,列出了每个课程所涉及的专业英语词汇,以帮助学习者掌握车辆工程相关的英语知识。
一、物理 Physics中文英文物理量physical quantity标量scalar矢量vector力force力矩torque功work能量energy动能kinetic energy势能potential energy动量momentum角动量angular momentum力学mechanics静力学statics动力学dynamics运动学kinematics牛顿定律Newton's laws欧拉定律Euler's laws拉格朗日方程Lagrange equation哈密顿方程Hamilton equation热力学thermodynamics热力学第一定律first law of thermodynamics热力学第二定律second law of thermodynamics熵entropy焓enthalpy自由能free energy卡诺循环Carnot cycle理想气体方程ideal gas equation流体力学fluid mechanics压强pressure流速velocity质量流率mass flow rate伯努利方程Bernoulli equation雷诺数Reynolds number粘性流体viscous fluid中文英文非牛顿流体non-Newtonian fluid流线型设计aerodynamic design升力系数lift coefficient阻力系数drag coefficient电磁学electromagnetism电场强度electric field intensity电势差potential difference电流强度electric current intensity二、高等数学 Advanced Mathematics 中文英文导数derivative积分integral微分方程differential equation偏导数partial derivative线性代数linear algebra矩阵matrix行列式determinant向量vector特征值eigenvalue特征向量eigenvector函数function极限limit连续性continuity三、机械制图 Mechanical Drawing中文英文图纸drawing视图view投影projection尺寸dimension标注annotation公差tolerance配合fit装配图assembly drawing零件图part drawing剖视图sectional view轴测图axonometric view透视图perspective view线条line中文英文符号symbol图例legend四、理论力学 Theoretical Mechanics中文英文质点particle刚体rigid body约束constraint平衡条件equilibrium condition平面运动planar motion空间运动spatial motion广义坐标generalized coordinate广义力generalized force虚功原理principle of virtual work达朗贝尔原理D'Alembert's principle动力学方程equation of motion小振动small oscillation阻尼damping五、工程力学 Engineering Mechanics中文英文应力stress应变strain弹性模量elastic modulus泊松比Poisson's ratio弹性区elastic region屈服区yield region断裂区fracture region材料试验material test弯曲bending剪切shear扭转torsion稳定性stability六、汽车机械基础 Automotive Mechanical Fundamentals 中文英文汽车结构automotive structure汽车底盘automotive chassis汽车车身automotive body汽车发动机automotive engine汽车变速箱automotive gearbox汽车传动系统automotive transmission system汽车制动系统automotive braking system汽车转向系统automotive steering system汽车悬架系统automotive suspension system汽车轮胎automotive tire汽车仪表盘automotive dashboard汽车点火系统automotive ignition system汽车燃油系统automotive fuel system汽车冷却系统automotive cooling system汽车润滑系统automotive lubrication system汽车排气系统automotive exhaust system汽车电气系统automotive electrical system汽车电子控制系统automotive electronic control system七、汽车英语 Automotive English中文英文汽车工程师automotive engineer汽车设计automotive design汽车制造automotive manufacturing汽车维修automotive maintenance汽车检测automotive testing汽车安全automotive safety汽车性能automotive performance汽车环保automotive environmental protection汽车节能automotive energy saving汽车智能化automotive intelligence八、电工与电子技术 Electrical and Electronic Technology中文英文电压voltage电流current电阻resistance电容capacitance电感inductance交流电alternating current (AC)直流电direct current (DC)半导体semiconductor集成电路integrated circuit (IC)微控制器microcontroller九、车辆技术评估与检测 Vehicle Technology Evaluation and Testing车辆技术评估vehicle technology evaluation车辆技术标准vehicle technology standard车辆技术规范vehicle technology specification车辆技术指标vehicle technology indicator车辆技术参数vehicle technology parameter车辆技术性能测试vehicle technology performance test车辆技术可靠性测试vehicle technology reliability test车辆技术安全性测试vehicle technology safety test车辆技术环境性测试vehicle technology environmental test车辆技术经济性测试vehicle technology economic test车辆技术评估报告vehicle technology evaluation report车辆技术检测仪器vehicle technology testing instrument车辆技术检测方法vehicle technology testing method车辆技术检测流程vehicle technology testing process 十、汽车构造 Automotive Construction 中文英文汽车框架automotive frame汽车梁automotive beam汽车柱automotive pillar汽车门automotive door汽车窗automotive window汽车座椅automotive seat汽车方向盘automotive steering wheel汽车刹车踏板automotive brake pedal汽车油门踏板automotive accelerator pedal汽车离合器踏板automotive clutch pedal。
2021华南理工大学车辆工程考研真题经验参考书
感谢曾经努力的自己,考研已经成功很多,大家都要加油。
英语:3月-7月中旬:①单词紧跟《一本单词》。
②真题阅读04——14年第一遍。
③真题翻译98——14年第一遍。
④木糖英语考研微信每日一句。
暑假(7、8月):①蛋核英语微信网课开启(因为我觉得不听网课真不行了),②04——14完型第一遍,这个工作其实我没做完,一直拖到十一结束才真正把完型的真题刷完一遍,听完型网课。
③英语单词第二遍,重点记第一遍不熟悉的,可惜的是我没做完,第二遍我只听到了二十多课,那个时候已经10月末了,我的重点也就不放在单词上了。
9-10月:①04-14年阅读真题第二遍,精读,每天1-2篇。
②翻译听蛋核英语微信老师的网课。
③十月中旬用一星期将04-14的新题型过了一遍,新题型一直是我比较放心的一块,所以没有花很多时间。
可以用《木糖英语真题手译》。
11月:①写作模块从11月的第一天正式开启,我觉得写作真的是需要积累,一定要自己动笔写,根据个人水平决定写作开始的时间。
我自己买了三本书但是发现跟我自己的写作思路不太适合,就只用了蛋核英语的写作书。
我是认认真真的把05-17的大小作文都写了一遍,并且个别文章还写了两三遍,写作就是要多写多修改多总结,我真的不主张背模板,我自己一篇都没有背,但是自己的写作套路一定要有。
②翻译第二遍。
12月:①写作继续按部就班进行。
②阅读真题第三遍,这一遍我的主要目的是提速,所以没有全做。
③新题型第二遍,两天搞定。
一定要留出近三年的真题给最后一个月模拟用。
政治:首先是思想政治理论,我是从10月中旬才开始复习的,这是很冒险的做法。
因为很多人早在暑假就已经把政治大纲看了一遍,这时候才复习已经落后于很多人。
别人说,政治看的太早容易忘,我觉得这个说法是很可笑的。
其实好的学习习惯加上好的复习计划,忘的可能性是基本不存在的,很多人都栽在政治里就是明证。
政治“得选择题者得天下。
”大纲最好看几遍,选择题要在暑假就开始刷,题目有多少做多少,当然不能太过,复习消化是最重要的。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
(page24-25)
Hydrostatic pressure
P Patm gh
Pgage gh
Pressure in a liquid at rest increases linearly with distance from the free surface.
In a room filled with a gas, the variation of pressure with height is negligible.
7
l
Express the temperature of 20 ℃ in R, K and ℉ . T(℉ = ℃ ×9/5 +32=68 ℉
T(R)=T(℉ )+459.67=527.67 R T(K)=T(℃)+273.15=293.15R
Comparison of magnitudes of various temperature units.
The water in a tank is pressurized by air, and the pressure is measured by a multifluid manometer as shown in Fig. The tank is located on a mountain at altitude of 1400m where the atmospheric pressure is 85.6kPa. Determine the air pressure in the tank if =0.1m, h2 =0.2m, and h3 =0.35m.
h1
Take the densities of water, oil, and mercury to be 1000, 850 and 13600 kg/m 3, respectively.
22
Eg. Measuring Pressure With a Multifluid Manometer
(p28,Fig1-49)
13
Pgage Pabs Patm Pvac Patm Pabs
(page23)
FIGURE 1-38
14
l
Compressive force per unit area, is the same at any point in fluid in all direction — a scalar quantity. 每单位面积的压力在流体在所有方向上的任何 点都是相同的 – 压强是标量 (p23)
The relation can be obtained by starting with point 1 , moving along the tube by adding or subtracting the terms until we reach point 2.
Eg. Measuring Pressure With a Multifluid Manometer (p28,Fig1-49)
-17.8
459.67
0
-273.15
-459.67
0
Temperature scale —
Ice point Celsius scale Fahrenheit scale 0 ℃ 32℉ Steam point 100 ℃ 212℉
9 ℉ =5 ℃
T(℃)= T(℉ )-32)×5/9
T(℉ )=1.8T(℃)+32 T(R)=1.8T(K)
10
1 atm
1 atm = 101,325 Pa l 1bar = 105 Pa
l
1 atm = 1.01325 bars l 1kgf/cm2=9.807×104 Pa=98.07kPa=0.9807bar
l
1kgf/cm2=10mH2O l 1 atm =760mmHg =10.33mH2O
l
H y d r a u l i c car jack P1=P2
F1 F2 A1 A 2 F2 A 2 F1 A 1
18
(page26)
1.10 The Manometer Manometers 压力计
The devices are used to measure pressure differences of a few hundred kilopascals (small and moderate). It mainly consists of a U-tube containing one or more fluids.
Kelvin scale 开尔文温标 l Rankine scale 摄氏温标
l
SI
(page1olute
Relative
Kelvin ,K
Celsius, ℃
Rankine, R 金氏温标
Fahrenheit, ℉ 华氏温标
T(K)=T(℃)+273.15
℃= (℉ -32)×5/9
Gage pressure—表压强
The difference between the absolute pressure and the local atmospheric pressure. 绝对压力和局部大 气压力之间的差值。
Vacuum pressure—真空压强
pressure below atmospheric pressure, measured by vacuum gages. 压力低于大气压,由真空计测 量。
Pressure in a fluid remains constant in the horizontal direction. 流体中的压力在水平方向 上保持恒定(p25)
l
l
Two point at the same elevation in a continuous fluid at rest are at the same pressure. 在静止的连续流体中 的相同高度处的两个点处于相同的压力 (p27)
The basic manometer.
P2 P1 Patm gh
19
(page27)
Eg. Measuring pressure with a manometer
P Patm ρgh
P 96 0.85 1000 9.81 0.55 / 1000
100.6kPa
Chapter 1
Introduction & Basic Concepts
1
Objectives
l l
Temperature, temperature scale
Pressure, absolute and gage pressure 全压力&表压力
2
1.8 Temperature & the zeroth law 温度第0定律
17
Pascal’s Law——
(page26)
The pressure applied to a confined fluid increases the pressure throughout by the same amount.
如果在静止均质流体的某一边界面上 施加某一压强,那么这一压强就会 均匀传遍整个流体,数值不变。
Steam point (boiling point) the temperature at which water and water vapor is in equilibrium at 1atm pressure1大气压下水蒸 气和固态水的稳定状态 100 ℃
Celsius scale
参考温度 Reference temperature:
3
Temperature scale —
Ice point & Steam point
Celsius scale: 1742年由瑞典天文学家摄尔 修斯(Anders Celsius) 选取标准大气压 下纯水的冰点为0℃,沸点 为100℃,中 间等分为100份。
Ice point (freezing point) definition the temperature at which water and ice is in equilibrium with air saturated with vapor at 1atm pressure饱和蒸汽 下固态水和冰的稳定状态 0 ℃
1 1 2 2 3 3
1
P1 1 g (a h ) 2 gh 1 ga P2
In stacked-up fluid layers, the pressure change across a fluid layer of density and height h is gh. 堆叠流体层中,分开每一层计算压强
T(R)=T(℉ )+459.67
T(R)=1.8T(K) T(℉ )=1.8T(℃)+32
5
常用温标之间的关系
绝对K 373.15 摄氏℃ 华氏F 朗肯R
100 水沸点
37.8
212
发烧 100
671.67
559.67 491.67
273.16 273.15
0.01 水三相点 冰熔点 0
32
盐水熔点 0
(page17)
The zeroth law— If two bodies are in thermal equilibrium with a third body, they are also in thermal equilibrium with each other. 如果两个物体与第三物体处于热平衡,它们也 彼此处于热平衡
1psi=1 lbf/1 in 2=4.44822N/(0.3048/12)2 m2=6894.76Pa
