质量验厂审核清单
最新的SQP验厂审核清单checklist及SQP评估标准

Document No.: SQP-D02 Issue Date: 25 Nov 2011 Issue No.: 00 Page 1 of 1©2011 Intertek, All Rights ReservedThe Intertek Group is the owner of the copyright in the material and intellectual know-how presented. No parts in this material maybe reproduced, adapted or distributed outside of your company without the written consent of the Intertek Group other than to the extent necessary to view the material.SQP Document List1. Organization chart2. Responsibility and/or job description,3. Quality System Procedures (e.g., quality policy, objectives, manual and procedures for theQuality Management System and other processes)4. Management review records5. Internal audit documents (audit plan, report, etc.)6. Supplier Control documents (supplier approval procedure / criteria, list of approval supplier list, supplier evaluation records, on-going performance monitoring, etc.)7. Document control procedure and records (including record keeping)8. Product specifications/requirements9. Inspection Instructions, acceptance criteria and inspection & testing reports (including thestages of IQC, In-process and Final inspection)10. Work instructions / workmanship standards for each manufacturing process11. Production schedules/records12. Procedure for defining and reporting of “incident”13. Product recall procedure14. Customer complaints records15. Corrective action reports (related to incident, internal audit, complaint, etc)16. Test records on Traceability system17. Equipment maintenance documents (plan, procedure, record, etc)18. Calibration of monitoring & measuring devices (plan, procedures, records, etc)19. Cleaning schedule and procedure20. List of Approved Chemicals with Corresponding Brands / Manufacturers21. Pest control documents (list of trained pest control staff, contract with external pest controlagency, pest control inspection record, bait documentation, etc)22. Record / plan for “Risk Assessment” of the entire manufacturing processes23. Risk assessment records of final product24. Product testing procedure/program25. Laboratory test reports (including lead and heavy metals content in paints, coatings and non-paint components, hardware, labels, final product, etc).26. Monitoring records of foreign body detectors (e.g. metal detection records, daily sensitivitychecking records of metal detectors…etc)27. Broken needle procedure & records (if applicable)28. Pre-production meetings records29. Process Control Plan30. Training (procedure, training needs & records)SQP文件清单1. 组织架构图2. 责任和 / 或职责描述3. 质量体系程序 (包括:质量政策、目标、质量管理体系手册和程序,以及其它流程)4. 管理层审查记录5. 内部审核文件 (审核计划、报告等)6. 供应商监管文件 (供应商核准程序 / 标准、已核准的供应商清单、供应商评估记录、持续表现监督等)7. 文件监管程序和记录 (包括记录保管)8. 产品规格 / 要求9. 检验要求说明、可接受的标准、检验和测试报告 (包括IQC的阶段、过程中和最终检验)10. 工作要求说明 / 每项生产工序的工艺技术标准11. 生产日程安排 / 记录12. “事故”的界定和报告程序13. 产品召回程序14. 客户投诉记录15. 整改行动报告 (关于事故、内部审核、投诉等)16. 追溯系统中的测试报告17. 设备维护文件 (计划、程序、记录等)18. 监督和测试设备的校准 (计划、程序、记录等)19. 清理日程安排和程序20. 已核准的化学品清单,附带相应的品牌 / 生产商21. 有害物管控文件 (受过培训的管控人员的名单、外部有害物管控机构的联系方式、有害物管控检查记录、投饵记录,等)22. 整个生产流程的“风险评估”记录 / 计划23. 最终产品的风险评估记录24. 产品测试步骤 / 程序25. 实验室测试报告 (包括涂料、涂层和非涂料部件中的铅和重金属、硬件、标签、最终产品,等)26. 夹杂物监控记录 (如:金属探测记录、金属探测器的日常敏感物检查记录,等)27. 断针处理程序 (如适用的话)28. 生产前会议记录29. 程序控制计划30. 培训 (程序、培训需求和记录)Supplier Qualification Program (SQP) Assessment CriteriaSection 1 - Management Commitment and Continual ImprovementAssesses the degree to which a company’s management is committed to providing adequate assessment resources, effective communication, systems of review that identify actions taken and opportunities for improvement.Section 2 - Risk Management SystemsThe company shall have management systems for assuring product safety, legality and quality. (Applies basic risk assessment principles)♦Legislative and Safety Requirements - the company must be aware of and make reference to up-to-date legislation, product standards, codes of practice and developments in science or technology that may impact the risk concerning their products and packaging in the countries of intended sales.♦Risk Assessment - the company shall have risk management plan for product and production processes, based on a risk assessment system which is systematic, comprehensive, thorough, fully implemented and maintained.♦Risk Assessment Verification - the company shall conduct the verification of risk assessment by competent person. Section 3 - Quality Management SystemsThe company shall develop, document and implement an effective quality management system, and address the following areas: ♦Policy Statement♦Control of Document - All documents, records and data impacting the management of product safety, legality and quality are present and effectively controlled♦Control of Records♦Specifications♦Responsibility and Authority - clearly defined and documented organizational structure♦Internal Audit♦Purchasing, Supplier & Sub-contractor Approval and Performance Monitoring♦Customer Property - customer property (including intellectual property) should be subject to controls♦Corrective and Preventive Action - procedures to record, investigate, analyse and correct cause(s) of non-conforming products or failure(s) to meet standards, specifications and procedures♦Identification & Traceability - a system to identify and trace product lots including raw materials, components and packaging materials for all phases of the production process (receipt of materials to product dispatch) ♦Incident, Product Withdrawal and Product Recall - a plan and system to effectively manage product withdrawal and product recall processes♦Business Continuity Planning - plan for identifying methods that ensure business continuity in the event of major incidents/threats to a business.Supplier Qualification Program (SQP) Assessment Criteria♦Customer Focus♦Complaint HandlingSection 4 - Site and Facilities ManagementThe site and the facilities must be maintained and managed so as to prevent or minimize contamination and assure the production of safe and legal finished products. Areas of focus include:♦Site Location and Perimeter♦Factory Layout, Product Flow and Segregation♦Staff Facilities - such facilities must be designed and operated so that they sufficiently minimise all risk of product contamination♦Cleaning and Hygiene Practices♦Waste/Waste Disposal - systems for the collection, collation and disposal of waste material♦Pest Control - controls and practices for minimizing the risk of pest infestationSection 5 – Product ControlThe company shall demonstrate effective control of its products to ensure safety, legality and quality including the following areas: ♦Reference Samples (pre-production and production) - procedures in place for the selection, handling, storage, approval and use of reference samples♦Chemical Control - chemical composition of products and chemicals used in the manufacture or processing of products shall be identified, monitored and recorded as required by legislation in the country of sale and / or manufacture ♦Product Packaging Materials♦Control of non-conforming materials - non-conforming materials, components and products shall be clearly identified, labelled, quarantined, investigated and documented♦Special Handling - handling requirements shall be in place for specific materials♦Product Transport, Storage and Distribution♦Stock Control and Product Release – procedures shall be in place to prevent release of finished product unless all agreed procedures have been followedSection 6 – Product Testing and Product Claims♦Product Testing – the company shall have a suitable, sufficient and validated testing program to ensure the safe, legal production of products that meet required quality standards.♦Product Claims – the company shall validate any declared product information or claims made regarding its products and monitor compliance with such claims necessary.Supplier Qualification Program (SQP) Assessment CriteriaSection 7 – Process ControlThe company shall demonstrate effective control of all operations undertaken, to ensure product safety, legality and quality – as well as ensure that the processes and equipment employed are capable of producing consistently safe and legal product with the desired quality characteristics. The following areas shall be addressed:Generic Hardline♦Control of Operations - ensure processes and equipment employed are capable of producing consistently safe and legal product with the desired quality characteristics♦Control of Incoming Components and Raw Materials♦In-Process and Final Inspections - to assure delivery of safe, legal product of the required quality♦Foreign Body Detection and Control♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standard♦Equipment & Tooling Maintenance♦Final Product Packing and ControlGarments♦Sample Preparation, Pattern & Marker♦Pre-production Activity♦Control of Incoming Components and Raw Materials♦Spreading, Cutting and Bundling♦Knitting♦Embroidery / Appliqué♦Printing♦Fusing♦Sewing♦Linking♦Washing♦Mending and Stitching♦Attachment♦Finishing and Pressing♦Final Inspections - to assure delivery of safe, legal product of the required quality♦Metal Detection and Control♦Final Packing♦Final Audit♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standardSupplier Qualification Program (SQP) Assessment CriteriaToys♦Pre-production Activity♦Control of Incoming Components and Raw Materials♦Molding (Injection molding, Blow molding, Insert molding, Roto cast molding, Diecast molding, Vacuum Forming, etc) ♦Die Cutting for Fabric/Rigid Plastic/PVC Sheet or Laminates, etc.♦Forming and Stamping♦Decoration (Spray Decoration, Coating, Tempo, Hand Painting, Printing)♦Sonic Welding Process♦Gluing Process♦Assembly (Manual/Automated)♦Cutting♦Sewing / Hair Rooting♦Attachment (e.g., eyes, noses, buttons, snaps or other metal press fasteners)♦Stuffing♦Metal Detection and Control♦Final Inspections - to assure delivery of safe, legal product of the required quality♦Final Product Packing and Control♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standardFootwear♦Footwear Manufacturing - Sample Development Activity♦Pre-production Activity (Footwear)♦Shoe Sole Bonding Test Process♦Wear Test Process♦Control of Incoming Components and Raw Materials (Footwear)♦Cutting♦Preparation / Secondary Processing♦Stitching♦Injection Molding♦Bottoming♦Assembly Operation – Lasting♦Autoclave Process (Vulcanizing)♦Finishing♦Final Inspections (Footwear)Supplier Qualification Program (SQP) Assessment Criteria♦Metal Detection and Control♦Final Packing♦Storage♦'Lasts' Management♦Equipment & Tooling Maintenance (Footwear)♦Calibration and Control of Measuring and Monitoring Devices (for purposes of monitoring product safety, quality and legality) - shall be identified and calibrated to a recognized national or international standardSection 8 – Personnel Training and CompetencyThe company shall ensure that personnel performing work affecting product safety, legality and quality are demonstrably competent to carry out their activity, as a result of training, work experience and / or qualification.Americas Asia EMEAElma Isakovic Samuel Lau Catherine BeareTel: +1 732 394 5367 Tel: +852 3760 6334 Tel: +44 78 7237 9094elma.isakovic@ u@ catherine.beare@。
SMETA-4P验厂审核文件清单

SMETA-4P验厂审核文件清单经常有客户会问及smeta和sedex什么区别?对此,我们再次阐述一下他们的关系,其次,我们会提供一份国内smeta-4P验厂审核文件清单供大家参考。
ETI的英文全称:Ethical Trading Initiative,中文名为:英国道德贸易组织。
SEDEX的英文全称:Supplier Ethical Data Exchange,中文名为:供货商道德数据交换。
SMETA 全称:Sedex Members Ethical Trade Audit,中文名为:SEDEX贸易道德审计。
SEDEX是一个欧洲的网络数据库,只要做了SEDEX的验厂,工厂的验厂报告都会上传到这个数据库,买家可以看到。
SEDEX验厂是社会责任验厂,其审核内容和BSCI基本类似。
如果工厂有BSCI的报告,有些SEDEX的成员也是认可的,不需要重复审核。
SEDEX验厂主要由英国的买家发起,需要一个第三方公证行对工厂进行审核,最后出具报告,发给客人。
SMETA验厂-4P审核所需的文件清单审核程序包括:审核前会议,设施巡察,查阅文件,员工面谈及总结会议。
Audit procedures includes: Opening Meeting, Facility Tour, Documents review, Employee Interview and Closing Meeting.请准备以下文件的正本予以审核,并恳请允许复印样本,谢谢!Please prepare the original documentation listed below for verification and sample photocopying, thanks!1. 工商营业执照(副本)Business Registration (Official Duplicate)2. 工卡或考勤记录(过去十二个月),包括在职与离职人员。
Sedex质量验厂清单

SEDEX质量验厂文件资料清单1.营业执照2.质量体系认证证书3.组织架构图4.质量手册5.程序文件;6.质量体系内审计划;7.质量体系内审记录 :1 内审员资格证书;2、首末次会议 3检查表4不符合项报告8.内审报告9.质量体系管理评审计划10.质量体系管理评审记录11.管理评审会议记录;12.管理评审报告3)决议事项的跟进记录13.主要生产设备清单14.设备保养计划15.设备保养记录、检验仪器清单16.仪器校准计划17.仪器校准记录;1)外校 2)内校人员资格证3)内校规程;4)内校报告18.年度培训计划19.培训记录: 1签到表、测试卷20.品管人员岗前资质认定资料(培训及测试记录;21.新产品设计开发资料1产品规格书;2)BOM表 3)安规认证证书; 4样品检测报告5)试产记录6)试产评估报告7)作业指导书8)检验标准9)FMEA分析资料 10)产品质量控制计划(QC工程图。
22.订单评审记录;新供应商资格评定报告23.现有供应商质量、交期、价格及服务定期评分表24.原材料采购订单25.原材料规格承认书26.进料检验作业指导书27.进料检验标准28.进料检验样板清单及定期评估记录29.进料检验记录30.不合格来料处理记录(含供应商纠正预防措施报告;31.原材料保存周期规定32.原材料过期重检记录控制图表及超限处理记录33.CPK应用指引34.CPK测量记录及制程能力不足时的改进记录35.生产作业指导书36.制程检验作业指导书;37..制程检验标准38.制程检验记录:1)首件检验记录2巡检记录 3抽检记录39.制程不合格品的处理记录(含纠正预防措施报告)40.制程检验不良统计报表(周报月报,柏拉图)41.停线管理规定及记录42.成品检验作业指导书;43.成品检验标准、44.成品检验记录45.不合格成品处理记录1)返工、返修记录2重检记录3纠正预防措施报告46.成品入库单47.产品可靠性及环境测试计划及记录48.数据分析程序49.质量目标统计资料50.客户沟通资料51.客户投诉处理程序52.客户投诉处理记录。
供应商验厂文件清单(Excel可编辑)
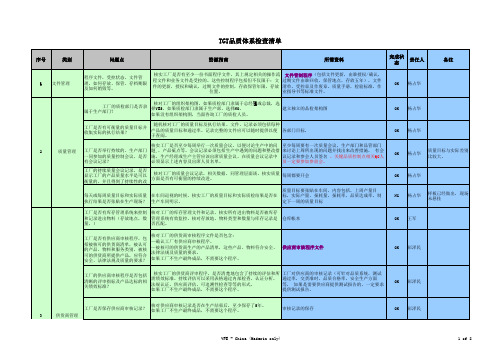
18 近三个月的“生产计划达成率统计表”
生 21 近三个月的ECR/ECN(“工程变更申请/通知单”)和近一个月的材料承认书 产 22 生产设备维护保养计划及相应的设备维修、保养记录(近一个月)
23 物质的控制与管理稽查计划(供应商、内部)与相关记录
24 与我司所采购产品相关之规格书、检验标准(来料、制程、最终)、作业指导书 其 25 危险废弃物处置协议 他
26 与我司所采购产品相关材料的采购记录(协议、订单) 27 与我司所采购产品相关的第三方检测报告 28 应急演练计划及记录 说明:验厂小组正式对供应商审核的前,由采购部将此清和可靠性之试验标准或试验计划、记录
12 检测设备和试验设备之校验与维护(包括台帐、标识、校准和检定、存放)记录
13 不合格品处理的“纠正和预防措施报告书 14 检测设备和试验设备之校验与维护计划
15 常用物料安全库存管理规定、报价分析管理规定
采 16 近一月的“生产计划安排表”(周、日)及对应的“生产日报表”记录 购 17 对供应商管理审核的计划及相应记录
4 管理手册、程序文件、作业指引(质量、职业健康安全环保方面)
5 品质控制流程图
6 品质目标(包括产品出货不良率)统计表 7 公司年度培训计划及各类特殊工种和质量控制人员的培训、考核记录
8 品质改善会议记录
品 9 客户报怨或客户投诉或客户退货处理规定、报告
质 控
10 近一个月的IQC进料检验记录、PQC过程检验记录、OQC出货检验记录
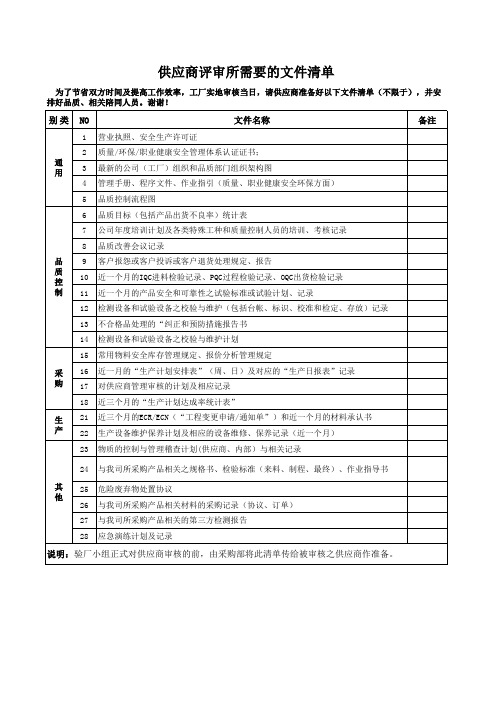
供应商评审所需要的文件清单
为了节省双方时间及提高工作效率,工厂实地审核当日,请供应商准备好以下文件清单(不限于),并安 排好品质、相关陪同人员。谢谢!
别 类 NO
文件名称
验厂清单
OK
宋丰辰
MCS张小姐已经修改 很多次,已经给出最 终版本。
核对工厂的产前会议记录中是否有讨论产品测试要求和产前测 试结果的记录。
OK
宋丰辰/ 有测试记录 杨占华
核对工厂的产前会议记录中是否有确认产品的关键质量要求的记 录。
OK
宋丰辰/ 杨占华
工厂是否将产前会中发现的 核实工厂是否有程序文件要求将产前会的会议记录,相关解决 问题及会议记录传达到相关的管 方案及时传达到相关人员。 理人员?
OK
杨占华
工厂的最终随机抽检报告是否包 括包装和纸箱的检验?
核对工厂的最终随机抽检记录。 包装和纸箱的检验结果必须记录在工厂内部的最终随机抽检报告 上。
最终随机抽检记录要包括对包装和纸箱的检查(即记 录上有包装和纸箱检查项)
OK
杨占华
工厂是否有内部产前会议的流 程?
核对工厂的产前会议的程序文件是否列明会议何时召开,参加会 议的人员。 会议讨论的议题必须有样品的确认,生产工艺单的核对,包装, 商标,测试的要求和生产计划。
建立颜色检查程序(须有标准对色灯箱)
OK
杨占华
每离窗户50CM 以上,不可直接放地上, 原材料仓, 成品仓都需要有温度测湿计,并有相关记录
OK
王军
工厂是否有明确的生产流程图 表,其上标明产品的质量控制 点?
核实工厂的生产程序文件,察看生产流程图表是否符合工厂的实 际生产流程。生产程序文件必须使用生产工人可以理解的语言。 生产质量控制计划( 生产程序和工序要标明,并指 必须将各个部门的生产程序和操作工序分门别类列明。核实质量 出质量控制重点), 工艺流程图 控制点是否也标注在生产流程图表上。
OK
宋丰辰
VFE - China (Madarin only)
翠丰验厂审核文件清单 ver

DOCUMENT REQUISITION LIST文件清单TECHNICAL AUDIT技术审核Date: 13 Nov 2013 (Version 3)Note: Please prepare following documents/records for document review during factory audit. If you have any query, please do not hesitate to contact us.注意: 请准备以下清单中的文件/记录以备审核员在审核过程中核查.如有任何疑问,请与我们联系.Document Types 文件For Auditor Use Only 只供审核员填寫 ▼Copy Required Remarks 备注栏Yes No NA A1 ISO9001:2008 Certificate ISO9001:2008证书 A2 Quality Manual 品质手册A3 Quality Procedures / Instructions 程序文件 / 指导书 A4 Management Review Records 管理评审记录 A5 Internal Audit Records 内部审核记录 A6 Production Flow Chart 生产流程图A7 Products Related Legislations/standards 产品相关法规/标准 A8 Records of Patent Management 专利管理记录 A9 Customer Complaint Records 客户投诉记录A10 R&D Records / Technical Files 设计开发记录/技术文档 A11 Inspection Instructions and Records测试指导书及检验记录(来料, 制程及最终检验)A12 Engineering Change Records 工程变更记录 A13 Equipment list of Internal lab 内部实验室设备清单 A14 Measuring/Testing Equipment List 测试仪器清单 A15 Calibration Plan 仪器校正计划A16 Internal & External Calibration Records 仪器内部/外部校正记录 A17 Qualification of Internal Calibration Operator 内校人员资格 A18 Internal Calibration Instructions 内部校正指导书 A19 Purchasing Order 采购单A20 Approved Vendor List 合格供应商名单A21 On-site Vendor Audit Reports 供应商现场评估报告A22 Vendor Performance Evaluation Records 供应商绩效考评记录 A23 Certification for wood management (FSC, PEFC, TFT, etc.) 木材管理体系证书 (FSC, PEFC, TFT, etc.)A24 Wood supply chain and relevant records (Invoice, delivery documents, etc.) 木材供应链及相关资料(发票,运输证等) A25 Crisis Management Procedure 危机管理程序A26C-TPAT/AEO procedures/records 安保程序/记录。
阿迪达斯验厂审核清单
审核范围和时间安排及相关文件资料要求Audit scope, Time arrangement and Relevant documentation list预计预计时间安排时间安排 (Tentative Time Arrangement):时间 Time 内容 Content 备注 Remark10:00-10:30 准备会议, 自我介绍,工厂管理层的会面,及工厂基本信息了解, opening meeting, factory management interview,解释审核程序,安排, explainaudit procedure 10:30-12:00 工厂现场查看, Factory tour 拍照只关注于HSE 方面,不会涉及产品客户信息, 需要工厂提供厂区平面图和相关熟悉工厂生产人员陪同, only take HSE issue photos, factory should provide floor plan and guider12:00-13:00 简单午餐, Lunch Break 13:00-15:30 文件资料审查, Documentation review 分成LABOR 和HSE 两方面,需要工厂相关负责人员陪同并解释文件, 并复制相关文件,Document review and copy file if needed.15:30-16:30 员工面谈,employee interview 地点可以车间或者单独的房间,工厂管理人员需要回避,interview can be conducted in workshop or independent room without factory management16:30-17:00 内部讨论, internal discussion 17:00-18:00 结束总结会议, close meeting 总结审核的不符合项,并与工厂一起协商改正计划, explain all the violations and complete the AP with management文件清单,documentation list:劳工部分 (Labor) :1) 工厂营业执照及税务登记, Business license and Tax registration certificate2)工厂竣工验收和消防验收资料, 环境三同时验收Fire safety inspection certificate and Building construction inspection certificate, Environmental inspection certificate3) 工厂的组织机构图及平面图, Organization chart and Floor plan4)政府最新文件. 如:最低工资,社会保险的规定, 综合计时申批记录等, Relevant law documents (Local minimum wage standard, Social insurance regulation, Comprehensive Working Hours System, etc.)5)员工手册 (包括厂规,厂纪 如: 对员工表现的评估系统,工厂的奖惩制度,工资制度等; 员工福利, 解除合同的程序等) Employee manual (Factory rule, Employee assessmentsystem, Punishment and reward policy, Wage calculation policy, Employee benefitspolicy, Resign procedure, etc.)6)招工广告, 招工过程描述 Recruit post and hiring policy, procedure7)员工的培训程序, 培训需求表, 培训计划, 培训记录 Employee training need assessment,Training procedure, training plan, training records.8)员工资料:新员工申请表, 员工身份证复印件及支撑文件, 各种请假申请表, 奖惩记录.Employee files ( Fresh employee application form, I.D. copy, background documents,leave records, punishment and reward records)9)员工月流动率, 离职名单, 离职报告 (employee monthly flow rate, employee resignrecords and application )10)劳动合同 Labor contracts11)青少年员工的记录, 例如: 青少年工人清单, 岗位, 体检记录等 Juvenile workers list,Juvenile workers physical examination records.12)考勤及工资记录, 工资单 Attendance records and Payroll records, Pay slip加盖银行印章的工资转帐明细(适用于通过银行转帐方式给付工资的供应商)hardcopy ofpayment transactions with bank stamp(applicable for suppliers who pay the wagethough electronic bank transfer)13)工人志愿加班记录/文件 Workers volufinteer overtime records14)社会保险, 工伤, 医疗等保险记录 Social insurance payment records, (work relatedinjury, medical etc.)职业卫生及安全 (HSE):15)最新健康安全法规 (Updated Relevant HSE regulation)16)工厂内部健康安全政策 (Internal HSE policy)17)注册安全员证书 (Safety officer registration)18)健康安全协调员及其文件化的工作职责描述 (Documented Safety coordinator jobresponsibility)19)急救培训证书或相关培训记录 (First aid training certificate and training records)20)消防及疏散演习计划及相关记录 (Fire drill records and fire evacuation plan)21)工伤及意外事故记录 (Work related injury records and accident records)22)全厂各区域的疏散平面图 (Fire evacuation plan for each workshop)23)灭火器月检查记录 (Fire equipments monthly inspection records)24)电梯,锅炉, 压力容器, 发电机等使用证书及定期检验记录 (Registration records andannual inspection certificate for all special appliances)25)餐厅卫生许可证, 餐厅人员的健康证 (Canteen hygiene certificate, kitchen staffphysical examination records)26)特殊岗位员工上岗证. 如:电工,锅炉操作工,叉车工,电梯操作工等 ( Certificate forspecial appliances operators and specialist)27)有毒有害环境工作人员的职业健康体检报告, (occupational health physicalexamination report for workers in hazard working condition)28)工厂使用的化学品清单及其物质安全资料表 (chemicals inventory, MSDS/CSDS)*以上信息只是用作参考, 具体安排和要求将依据工厂地点,人数规模,审核范围而改变,实际情况由SEA人员现场自行调整. Above arrangement only for reference, SEA staff will make adjustment depend on actual situation.*所有可追溯性资料必须提供至少3个月, 如果有需要, 审核员也可以挑选之前任意月的相关资料(p.s. some relevant documents should be available at least for 3 months, e.g. payroll and attendance records. Auditor can select any previous documents depend on actual situation.)。
质量验厂(FCCA)记录清单
化学品控制程序
产品召回控制程序
首件检验控制程序
温湿度霉变控制程序
成品出货放行控制程序
20 FCCA补充程序 质量事故控制程序
产前会议控制程序
产前试做控制程序
老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 辅导老师 辅导老师 辅导老师 辅导老师 辅导老师 辅导老师 辅导老师 辅导老师 辅导老师 辅导老师 辅导老师
品)
包装首巡检记录
产品全检记录
首件确认单
不良品处理记录
出货抽箱检验(成品验货报告)
序号 文件内容
支持的记录表格
化学品清单
16
化学品
化学品领用记录
相关化学品MSDS、化学品测试报告
老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录)
老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录) 老师提供表单(工厂填写记录)
生产工艺(指令单)
包装指示单(验厂当天产品)
供应商名录
供应商调查评估表
供应商的产品测试报告(第三方)
布料、塑料袋、印刷品的油墨
年度评审表
老师提供表单(工厂填写记录)
月供货统计(供应商考核表)
原材料采购清单)
PB质量验厂清单
PB工厂技术评审
评审需提供的资料(记录部分须提供三个月, 如工厂允许, *画线部分要复印):
1 质量手册及年度评审记录
2 所有程序文件:
(如生产机器/设备管理, 货仓物料管理, 采购控制, 供应商评估, 物料进出控制, 客供物料控制, 设计控制, 来料/制程/成品品质控制, 不合格品控制, 质量记录控制, 利器/针控制, 检测仪器控制)
3 *工厂组织架构图
4 厂房/车间/设备分布图
5 基础设施(灯/电梯/电源/电脑/供水/窗户/墙)保养计划及保养记录
6 * 生产机器/设备清单, 保养指引及保养记录
7 生产用工模/夹具/机架清单及收发记录
8 物料采购单及供应商来料质量/数量/交货期历史记录
9 货仓物料收发货记录, 出入帐本, 物料定期盘点记录及客供物料沟通记录
10 生产总计划, 各工序生产计划, 生产日报, 生产周报及生产周会记录
11 出货装货柜指引及出货单据(包装清单, 提单, 发票等)
12 产品设计会议, 设计输入, 设计输出, 设计评审, 设计确认及设计更改记录
13 *产品生产流程图, 作业指导书, 试产后(产前)评审记录及生产绩效分析记录
14 *来料/过程/最终检验指引及报告,来料/过程紧急放行及成品仓定期巡查记录
15 不良品记录或检验报告, 品质部停产记录及不良品处理记录
16 纠正及预防措施记录
17 *利器/针收发记录, 断利器/针记录, 金属探测机检测及测货记录(如要求)
18 * 检验/测量/测试仪器清单, 计量证书/内较记录及实验室环境记录
19 *安全标准(美国, 欧洲, 日本, 加拿大等) (如要求)
20 *产品产前测试报告及成品批验测试报告
21 * ISO证书及外培训/内培训记录。
Sedex验厂审核文件清单
Sedex验厂审核文件清单1.1公司简介---人员\产量\产值\品牌\市场分布1.2公司组织结构1.3公司平面图1.4供应商评审记录1.5营业执照2.1员工手册2.2厂纪\厂规\奖惩记录2.3宿舍规章制度3.1招工程序3.2人事记录\员工登记表(所有员工)3.3劳动合同3.4体检记录3.5未成年工登记证及工作安排3.6综合计时(加班)批准3.7社保(工伤\养老\医疗保险)等缴费记录凭证3.8请假单3.9离职记录3.10警告单4.1工卡/考勤表4.2计件工生产(台班)记录4.3当地最低工资文件4.4工资表(一年)4.5有工人签名的工资条4.6工资扣除/罚款记录5.1厂房建筑结构安全合格5.2厂房消防合格证6.1公司的健康安全政策6.2(消防卫生)安全主任资格证(任命书\培训结业证书)6.3消防上岗证/急救人员资格证书6.4消防演习/消防逃生程序/急救计划及记录7.1电梯起重设备登记准用/验收/年检合格证7.2特种作业人员(电梯\行车\电工\韩工)资格证书7.3设备维修工\技工资格证书7.4锅炉\压力容器使用登记证/年检合格证7.5司炉上岗证8.1化学危险品许可/储存物空记录/安全应急措施/安全数据表8.2化学危险品仓库管理人员上岗培训证书8.3车间有毒有害作业环境检测报告8.4发电机房噪音测试报告8.5排污许可/环境检测报告9.1食堂卫生许可及食堂员工健康证9.2医疗人员资格资格证书9.3工伤医疗事故处理记录/纠正预防措施9.4定期除虫记录10保安守则\条例\上岗证11工会组织会议记录/工会代表程序及职责诞生背景:SEDEX是一家总部设在英国伦敦的非赢利组织,世界上任何地点的公司都可以申请会员资格。
SEDEX已获得了许多大型零售商和生产商的青睐,许多零售商、超市、品牌商、供应商和其它组织都要求与之合作的农场、工厂和制造商参加SEDEX成员道德经营审核(SMETA),以确保其经营符合相关道德标准的要求,审核结果可以得到所有SEDEX会员的认可并被他们共享,所以供应商接受SEDEX验厂可以省去很多来自客户的重复审核。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
List of documents to be reviewed during audit – for reference
1. Quality Manual (including quality policy, objectives, organization chart, job
description, reference to / procedures for the Quality Management System)
品质手册(包括质量方针,质量目标,组织架构图,工作职责描述,及与品质管理体系相关的程序)
2. Management Review (procedure, agenda, report)
管理评审(程序,议题,报告)
3. Internal audit documents (procedure, plan, audit report)
内审文件(程序文件,计划,评审报告)
4. Supplier Control documents (supplier approval control procedure / criteria, list of
approval supplier list, supplier evaluation records)
供应商管控文件(供应商评估控制程序/标准,合格供应商明细,供应商评估报告)5. Document Control procedure (including that for record keeping)
文件控制程序(包括记录文件)
6. Inspection Specification / Instruction (including IQC, In-process, Final)
检验标准/指导(包括来料检验,制成检验及终检)
7. Work Instruction for each manufacturing process
作业指导:所有生产工序的指导
8. Procedure for definition and reporting of “incident”
事故定义及报告程序
9. Product recall procedure
产品招回程序
10. Customer complaints procedure and complaint records
客户投诉程序及记录
11. Corrective Action reports (related to incident, internal audit, complaint, etc)
改善措施报告(与事故,内审及投诉等相关的)
12. Test records on Traceability system
追溯体系的测试记录
13. Equipment maintenance documents (plan, procedure, record)
设备保养文件(保养计划,保养程序及保养记录)
14. Calibration of monitoring & measuring devices (plan, procedures, records)
监控及测量仪器的校验(计划,程序文件,记录)
15. Written procedure for handling glass and hard clear plastic breakages Cleaning
schedule and procedure
书面玻璃及利器管控程序和清理安排
16. Waste handling / storage procedure or record
废品控制/存储程序及记录
17. Pest control documents (list of trained pest control staff, contract with external pest
control agency, pest control inspection record, bait documentation)
虫害控制文件(专业虫害控制人员名单,与外部虫害控制公司的合同书,虫害控制记录等)
18. Process flow documentation
工艺流程文件
19. Record / plan for “Hazard Analysis” of the entire production processes
全部流程的风险评估计划及记录
20. Hazards Assessment records during product design and development
产品设计及开发过程中的风险评估记录
21. Shelf life test / reliability trail documents (procedure, records)
寿命测试,可靠性试运行的文件记录
22. Monitoring records of foreign body detectors (e.g. daily sensitivity records of metal
detectors)
外来物品探测器(比如:每日金属探测记录)
23. Broken needle procedure & records (if applicable)
断针控制程序及记录(如果适用)
24. Sharp tool control procedure & records (if applicable)
利器控制程序及记录(如果适用)
25. Nonconforming product control procedure
不良品控制程序。
