Effect of polysaccharides from Angelica sinensis on gastric ulcer healing
参考文献1

参考文献[1] 姚磊,王静,赵海田.香菇多糖生物活性研究进展[J].中国甜菜糖业,2004,3:27-30.[2] 赵和平,张心团,马振声,等.抗肿瘤真菌药物的研究进展(综述)[J].安徽农业大学学报, 2003,30(4):462-465.[3] 唐省三,朱晓琴.复合真菌多糖的抗肿瘤及免疫增强作用初探[J].基础医学与临床,2004,24(5):599-600.[4] K.Noel Masihi, Katja Madaj, Henny Hintelmann,et al.Down-regulation of tumor necrosis factor-α, moderate reduction of interleukin-1β,but not interleukin-6 or interleukin-10,by glucan immunomodulators curdlan sulfate and lentinan[J].Int J Immunopharmac,1997,19(9/10):463-468.[5] Schepetkin IA, Faulkner C L, Nelson-Overton L K,et al. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum[J]. Int Immunopharmacol, 2005,5(13-14):1783-1799.[6] 龚非力.医学免疫学[M].北京:科学出版社医学出版分社,2004: 171.[7] 陈秀芳,金丽琴,吕建新,等.蝉拟青霉对腹腔及肺泡巨噬细胞的激活作用[ J].中国病理生理杂志, 2002, 18(6): 694-697.[8] 金丽琴,吕建新,袁谦,等.蝉拟青霉对大鼠免疫功能及血液生化指标的影响[J].温州医学院学报, 2001, 31(6): 344-346.[9] 陈秀芳,金丽琴,吕建新,等.蝉拟青霉减轻环磷酰胺所致免疫抑制效应的实验研究[ J].温州医学院学报,2002, 32(6): 351-353.[10] Schepetkin IA,Quinn MT.Botanical polysaccharides:macrophage immunomodulation and therapeuticpotential[J].IntImmunopharma-col,2006,6(3):317.[11] 段博文,李运,刘昕,等.柳茶多糖对小鼠免疫功能的影响[J].中国中医药杂志, 2010, 35(11): 1467-1468.[12] 沈新蛾,龚珊,蒋量红,等.复方虫草精华对小鼠免疫功能的影响.中国血液流变学杂志,2003,13(4):327-330.[13] 张劲松,贾薇,邢增涛,等.灵芝子实体和菌丝体的提取物及其各纯化组分生物活性的比较.菌物学报,2004,23(1):85一92.[14] Kim GY,Lee JY,Lee JO,et al.Partial characterization and im-munostimulatory effect of a novel polysaccharide-protein complex extracted from Phellinuslinteus[J].Biosci Biotechnol Biochem,2006, 70(5):1218.[15] Raveendran N,Sonia R,Reshma R,et al.Immune stimulatingprop-erties of a novelpolysaccharide from the medicinal plant Tinospora cordifolia[J].Int Immunopharmacol,2004,4(13):1645.[16] 董永杰,单铁英,岳峰,等.枸杞多糖对淋巴细胞功能的影响[J].现代中西医结合杂志,2010,19(19):2362.[17] Popov SV,Golovchenko VV,Ovodova RG,et al.Characterisation of the oral adjuvanteffect oflemnan,a pectic polysaccharide ofLemna minor L[J].Vaccine,2006,24(26):5413.[18] WangD,Li X,Xu L,et al.Immunologic synergismwith IL-2 and ef-fects of cCHMIs onmRNA expression of IL-2 and IFN-gamma in chickenperipheral Tlymphocyte[J].Vaccine,2006,24(49-50):7109.[19] 陈西广,张冀伸,李润秋.鬼毛针中水溶性多糖的研究[J].真菌学报,1990,9(2):155-160.[20] 徐秋萍.中药药理学[M].贵阳:贵州科技出版社,1994,10(2):280-331.[21] 朵存莲.浅淡中药在兽医临床的双向调节作用[J].中国畜牧兽医,2008,35(5):115-116.[22] 曲章义,郭彩玲,凌秋,等.香芪口服液增强非特异免疫和体液免疫作用的实验研究[J].中草药,2001,32(10): 908-910.[23] Sun JL,Hu YL,Wang DY,etal.Immunologic enhancement ofcom-pound Chinese herbalmedicinal ingredients and their efficacy comparison with compound Chinese herbal medicines[J].Vaccine,2006,24(13):2343.[24] Kong XF,Hu YL,Yin YL,et al.Chinese herbal ingredients are ef-fective immunestimulators for chickens infected with the Newcastle disease virus[J].PoultSci,2006,85(12):169.[25] WuY,SunH,QinF,etal.Effectofvariousextractsand a polysaccha-ride from theedible mycelia of Cordyceps sinensis on cellular and humoral immune response againstovalbumin in mice[J].Phytother Res,2006,20(8):646.[26] Siegell,LinTL,Gieicher.Theredeell immune system(J).Lancer,1981,2(8246):556.[27] 杨运高,陈先明,傅江南,等.大鼠红细胞免疫功能低下模型的研究[J].中国中医基础医学杂志,2002,8(3):44.[28] 应自忠,张慧,韩志红.壳多糖对荷瘤小鼠红细胞免疫功能的影响[J].中国公共卫生,2000,16(9):831.[29] 廉宜君,谷新利,李炳奇.从中药方剂中提取的复合多糖对小鼠免疫功能的影响[J].畜牧与兽医,2007,39(10):50-52.[30] 单俊杰,王顺春,刘涤,等.黄芪多糖的化学和药理研究进展[J].上海中医药大学学报,2000,14(3):61-65.[31] Cold spring harbor.Signal and gene expression in the immune sys-tem[M].NewYork:Cold spring harbor laboratory,1999(sympo- sium64):71-78.[32] 陆德源.现代免疫学[M].上海:上海科学技术出版社,1993,40(15):65.[33] YangT,Jia M,Meng J,et al.Immunomodulatory activity of polysac-charide isolated from Angelica sinensis[J].Int J Biol Macromol,2006,39(4-5):179.[34] Chen X,Hu ZP,Yang XX,et al.Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer[J].Int Immunopharmacol,2006,6(3):499.[35] Zhao L.Changchun Zhongyiyao Daxue Xuebao 2006;22(3):67-68赵雷.中药多糖类物质的应用研究与现状分析[J].长春中医药大学学报,2006,22(3):67-68.[36] Balachandran P,Pugh ND,Ma G,et al.Toll-like receptor 2-depen-dent activation of monocytes by Spirulina polysaccharide and its immune enhancing action in mice[J].Int Immunopharmacol,2006,6(12):18.[37] 徐军发,侯敢,黄迪南,等.酵母多糖对小鼠腹腔巨噬细胞产生一氧化氮和白细胞介素-1的影响[J].中国生化药物杂志, 2002, 23(2): 67-68.[38] Chen X,Hu ZP,Yang XX,etal.Monitoring of immune responses to a herbal immuno-modulator in patients with advanced colorectal cancer[J].Int Immunopharmacol,2006,6(308):499.[39] LimTS, Na K, Choi E M,etal. Immunomodulating activities of polysaccharides isolated fromPanax ginseng[J]. J Med Food,2004,7(1): 1-6.[40] Kim K A, Choi S K, Choi H S. Corn silk induces nitric oxide synthase in murine macrophages [J]. ExpMol Med, 2004,36(6):545-550.[41]Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential [J]. Int Immunopharmacol, 2006,6(3): 317-333.[42] Kim GY, Oh YH, Park YM. Acidic polysaccharide isolated from Phellinus linteus induces nitric oxide-mediated tumoricidal activity of macrophages through protein tyrosine kinase and protein kinase C[J].Biochem Biophys Res Commun,2003,309(2):339-407.[43]Chanq CL,Liao JC,Kuo L.Macrophage arginase promotes tumor cell growth and suppresses nitric oxide-mediated tumor cytotoxicity[J].Cancer Res,2001,61(3):1100-1106.[44] Soini Y,KalosK,Puhakka A,et al.Expression of inducible nitric ox-ide synthase in healthy pleura and in malignant mesothelioma[J].BrJ Cancer,2000,83(7):880-886. [45] GiYK, Gap SC, SangHL, eta.l Acidic polysaccharide i-solated from Phellinus linteus enhances through the up-regulation of nitric oxide and tumor necrosis factor-αfrom peritoneal macrophages [J]. J Ethnopharmaco,l 2004, 95(1): 69-76.[46] Kun YL,Young JJ. Macrophage activation by polysaccha-ride isolated from Astragalusmembranaceus [J]. Int Im-munopharmaco,l 2005, 5(7-8): 1225-1233.[47] Nose M,Terawaki K,Oguri K,et al.Activation of macrophages by crude polysaccharide fraction obtained from shoots of Glycyrrhiza glabra and hairyroots ofGlycyrrhiza uralensis in vivo[J].Biol Pharm Bull,1998,21(10):1110.[48] 张劲松,李卫军.肿瘤免疫抑制与治疗[J].国外医学:免疫学分册,2001,24(1):50-53.[49] 米蕊芳,张宏冰.雷帕霉素在免疫抑制和肿瘤抑制中的双重作用[J].基础医学与临床.2008,28(12):1329-1331.[50] 张艳,梁华平.黄芪多糖对烧伤小鼠细胞免疫功能的作用及机理探讨[J].中国实验临床免疫学杂志,1995,7(2):41.[51] 石焱,弓小雪,那婕.牛大力多糖对免疫抑制小鼠的免疫调节作用[J].临床军医杂志,2008,36(4):530-532.[52] 许爱华,任莉,郑媛媛,等.银杏外种皮多糖对环磷酰胺诱导的免疫抑制小鼠免疫反应的调节作用[J].中国药理学与毒理学杂志,2008,22(1):69-72.[53] 王惠珍.盐酸左旋咪唑搽剂可安全方便地提高机体免疫力[J].中国社区医师,2007,23(13):16.[54] 董兰凤,刘京生,苗智慧,等.附子多糖对H22和S180荷瘤小鼠的抗肿瘤作用研究[J].中国中医基础医学杂志,2003,9(9):14-17.[55] 刘建东.赤雹果乙醇提取物的抗炎作用的实验研究[J].中国医药指南, 2009, 7(2): 40.[56] XU L,CHEN H,XU H,et al.Anti-tumour and immuno-modulation effects of triptolide-loaded polymeric micelles[J].European Journal of Pharmaceutics and Biopharmaceutics,2008,70(3):741-748.[57] 陈秀芳,金丽琴,吕建新,等.蝉拟青霉减轻环磷酰胺所致免疫抑制效应的实验研究[ J].温州医学院学报,2002, 32(6): 351-353.[58] 邵树军,刘彩玉,刘雄伯,等.牛膝多糖对小鼠免疫功能影响的研究[J].肿瘤防治杂志,2002,9(1):57.[59] 宋春华,金明,陈京,等.保元汤醇提取物不同途径给药对小鼠体重及免疫器官的影[J].中国实验临床免疫学杂志, 1998,10(3):39-41.[60] 于丽萍,邹碧珍,李新玉,等.真菌多糖对小鼠腹腔巨噬细胞免疫功能的影响[J].生物技术,1998,8(2):38-40.[61] 吴小丽,蔡云清,赵岩,等.蒲公英提取物对小鼠免疫功能的调节作用[J].南京医科大学学报:自然科学版,2005,25(3):163-165.[62] 王莉,何赟绵,姚全胜.毛蚶多糖免疫调节作用的实验研究[J].华西药学杂志,2009,24(4):340-342.[63] 张艳,梁华平.黄芪多糖对烧伤小鼠细胞免疫功能的作用及机理探讨[J].中国实验临床免疫学杂志,1995,7(2):41.[64] 吴宪瑞,孔令员、淦洪.黑木耳多糖的医疗保健价值.林业科技,1996,21(3):32-33.[65] 甘勇,吕作丹.阿魏蘑多糖理化性质及免疫活性研究.菌物系统,200l,20(2):228-232.[66] 于敏,沈业寿,梅一德,等.蜜环菌菌索多糖的免疫增强作用研究.生物学杂志,2001,18(4):16-19.[67] 贺新怀,席孝贤.中医药免疫学[M].北京:人民军医出版社,2002,25(9)406-409.[68] 龚非力.医学免疫学[M].北京:科学出版社,2002:77-88.[69] 赵玉珍,陶上乘,王英华,等.小叶黑柴胡药理作用的进一步研究[J].中药材, 1998,21(6):307.[70] 李映丽,吴万兴, 鲍德虎,等 .魔芋甘露聚糖免疫作用的研究[J].陕西林业科技,1999,28(4):68-70.[71] 徐月清,曹志然,李立萍,等.灵芝对小鼠免疫功能调节作用的研究[J].实验动物科学与管理,1999,16(1):18-20.。
蒙药斯日西防治实验性胃溃疡作用及机制研究

CHINA MEDICAL HERALD Vol.17No.12April 2020[基金项目]内蒙古自治区蒙医药研究所蒙药新药研发及药物实验室的建设项目(2016YJS06);中共内蒙古自治区蒙医药研究所蒙药新药研发及产业化创新新团队项目(内组通字[2015]56号)。
[作者简介]敖登其木格(1987.11-),女,硕士,主要从事蒙药药理及相关新药研发工作。
消化性溃疡是消化系统最常见的慢性疾病,在蒙医医学中属于“胃宝日病”范畴[1-2]。
蒙药斯日西是传统蒙医药中的一个经典药物,其功能主治为祛“宝日”病,“宝日”病初,中期嗳气吞酸、胸背作痛、气滞血瘀、血热陷中、潜伏于脏等病症[3-4]。
本研究通过观察斯日西散对溃疡模型中胃黏膜、髓过氧化物酶(MPO)活性、总谷胱甘肽(GSH)水平、外周血T 淋巴细胞亚群,蒙药斯日西防治实验性胃溃疡作用及机制研究敖登其木格王海荣乌汉其木格特格喜白音内蒙古自治区国际蒙医医院创新蒙药制剂工程实验室,内蒙古呼和浩特010065[摘要]目的研究蒙药斯日西对胃溃疡的防治作用及其可能的机制。
方法选取25只Wistar 大鼠,采用吲哚美辛诱导的急性胃溃疡模型,苏木精-伊红染色观察胃黏膜变化;选取25只昆明小鼠,采用乙醇/HCL 诱导的急性胃溃疡模型,观察胃组织中髓过氧化物酶(MPO)活性,总谷胱甘肽(GSH)水平以及外周血T 淋巴细胞水平的变化;选取20只Wistar 大鼠,采用乙酸烧灼性慢性胃溃疡模型,酶联免疫吸附测定法检测胃组织中炎性因子肿瘤坏死因子-α(TNF-α)、细胞间黏附分子1(ICAM-1)表达水平。
结果与模型组比较,斯日西散组胃黏膜病变减轻;与模型组比较,斯日西散组胃组织MPO 活性、大鼠胃黏膜TNF-α、ICAM-1表达水平降低,GSH 表达水平升高,差异均有高度统计学意义(均P <0.01);与模型组比较,斯日西散组胸腺、脾脏重量指数、外周血CD4+细胞表达水平升高,差异均有统计学意义(P <0.05或P <0.01),而两组CD3+、CD4+/CD8+、CD8+细胞表达水平比较,差异无统计学意义(P >0.05)。
基于JAK/STAT信号通路探究黄连素对溃疡性结肠炎小鼠结肠皮细胞凋亡作用

EffectsoflentinanondendriticcellmetabolismLIULi1,ZHANGBao chen1,LIXiu yun1,WUEn hui1,SHIZhong feng1,DENGXiang liang1,2(1.SchoolofChineseMateriaMedica,GuangdongPharmaceuticalUniversity,Guangzhou 510006,China;2.SchoolofChineseMedicine,GuangdongPharmaceuticalUniversity,Yunfu,Guangdong 527300,China)Abstract:Aim Tostudytheeffectsoflentinan(LNT)onthemetabolismofdendriticcells(DCs)bymetabonomics,anduncoverthepotentialmechanismofitsregulationofDCfunction.Methods DC2 4cellswereco incubatedwithLNTfor24h,andtheactivityofthecellswasdetectedbythiazolylbluetetrazoliumbromide(MTT)assay.Thecontentsofinterleukin 6(IL 6),tumornecrosisfactor α(TNF α)andinter leukin 12(IL 12)insupernatantweredetectedbyen zyme linkedimmunosorbentassay(ELISA).Themet abolicgeneralchangesofDC2 4cellsweredetectedbyUltraperformanceliquidchromatography quadrupoletime of flightmassspectrometry(UPLC Q TOF/MS),andthedifferentialmetaboliteswereanalyzedbymulti distancecovariatesandbioinformatics,partialleastsquares discriminantanalysis(PLS DA).Finally,metabolicpathwayanalysiswasperformedbyMetabo Analyst5 0.Results LNTdidnotsignificantlyinhib ittheactivityofDC2 4cellsatthedoseof25~100mg·L-1.LNT(100mg·L-1)couldsignificantlystimulatethesecretionofIL 6,TNF αandIL 12inDC2 4cells.20differentialmetaboliteswereidentifiedinDC2 4cellsafterbeingstimulatedbyLNT(100mg·L-1),whichinvolved25metabolicpathwaysinclu dingureacycle,arginineandprolinemetabolism.Conclusion TheregulationofLNTonDCfunctioninvolvesavarietyofaminoacidmetabolism.Keywords:lentinan;dendriticcells;metabolomics;differentialmetabolites;ureacycle;argininemetabo lism网络出版时间:2023-05-1508:37:59 网络出版地址:https://kns.cnki.net/kcms/detail/34.1086.r.20230511.1719.006.html基于JAK/STAT信号通路探究黄连素对溃疡性结肠炎小鼠结肠皮细胞凋亡作用李春霖1,李时超2,贾英田2,李 建3,赵鲲鹏4,税丕先1(1.西南医科大学,2.西南医科大学附属中医医院肛肠科、3.药学部,四川泸州 646000;4.甘肃中医药大学临床学院,甘肃兰州 734000)doi:10.12360/CPB202210070文献标志码:A文章编号:1001-1978(2023)05-0938-08中国图书分类号:R 332;R284 1;R322 45;R329 25;R574 62摘要:目的 探讨黄连素调控JAK/STAT信号通路对溃疡性结肠炎(ulcerativecolitis,UC)小鼠结肠上皮细胞和外周血中性粒细胞(peripheralbloodneutrophils,PMN)凋亡的影响。
黄芪对蛋鸡生产性能和抗氧化情况的影响

D ESCRIPTION OF PROBLEMR eactive oxygen species (ROS), including superoxide, hydroxyl radicals, hydrogen per-oxide, and nitric oxide, are continuously gener-ated inside cells by several oxidases and by the dismutation of the superoxide anion formed by electron leakage during mitochondrial respira-tion [1]. Oxidative stress, which is induced by the imbalance between the production and re-moval of ROS, is regarded as a primary factor in various degenerative diseases, such as cancer [2], atherosclerosis [3], gastric ulcers [4], and aging [5]. As a result, there is a growing interest in using natural antioxidants because of the po-tential negative effect of synthetic antioxidants on human health [6]. Singh et al. [7] reported that both the essential oil and an acetone extract of Myristica fragrans Houtt. (aril part) exhib-ited a broad spectrum of antimicrobial activity against the tested microorganisms and were ef-fective in preventing rapeseed oil from oxidiz-ing. Jang et al. [8] demonstrated that inclusion of a medicinal herb extract in the diet increased© 2012 Poultry Science Association, Inc.E ffects of Astragalus membranaceus on laying performance and antioxidant status of laying hensZ. Y. Z uo ,* W. R. Y ang ,*1Y. W ang ,† Z. B. Y ang ,* S. Z. J iang ,* and G. G. Z hang * *D epartment of Animal Sciences and Technology, Shandong Agricultural University, Tai-an, Shandong, 271018, P. R. China; and †A griculture and Agri-Food Canada, Lethbridge Research Centre, PO Box 3000, Lethbridge, Alberta, T1J 4B1, CanadaPrimary Audience: Nutritionists, Researchers, Egg ProducersS UMMARYI nterest in the use of natural feed additives in the animal and poultry industries is growing. In the present study, we assessed the effects of dietary supplementation of Astragalus mem-branaceus root powder (AMP) on the laying performance and serum and egg yolk antioxidant status of laying hens. Four groups (135 each) of laying hens fed a corn- and soybean meal-based diet were supplemented with 0, 5, 10, or 15 g/kg of diet of AMP for 10 wk, and their layingperformance and serum and egg yolk antioxidant status were measured. Supplementation of AMP did not affect ADFI or average egg weight but did linearly increase egg mass of the lay-ing hens. Antioxidant status of the serum and egg yolk was improved, as judged by an increase in antioxidant enzymatic activities and a decrease in concentrations of oxidized products, with supplementation of AMP in a dose-dependent manner. In conclusion, supplementing a corn- and soybean meal-based laying hen diet with AMP at levels of 5 to 10 g/kg of diet had the potential to improve the antioxidant status of laying hens and improve laying performance.K ey words:a ntioxidant status ,A stragalus membranaceus,l aying hen ,l aying performance2012 J. Appl. Poult. Res. 21 :243–250/ 10.3382/japr.2011-00351 1Corresponding author: w ryang@ at Poultry Science Association Member on March 20, 2014/ Downloaded from244JAPR: Research Reportthe dietary antioxidative potential. Zhang et al. [9] also reported that supplementing ginger powder at a level of 5 g/kg improved the antioxi-dant status of broilers and that its efficacy in en-hancing the antioxidant capacity was enhanced as the particle size decreased from 300 to 37 μm. The dried roots of Astragalus membranaceus (AM), also known as Huangqi, is a traditional medicinal herb that originated in Northern Chi-na. In traditional Chinese medicine, AM is an important “qi tonifying” or adaptogenic herb and is often used in combination with other herbs, such as angelica, Paeonia lactiflora, and ginseng, to improve overall well-being [10]. Astragalus membranaceus has been reported to contain various bioactive compounds, including astragalosides, flavonoids, isoflavones, isofla-van, saponins, kumatakenin, choline, betaine, polysaccharides, and glucuronic acid [11–13], and to possess antinociceptive [14], anti-aging [15], anti-infarction [16], hepatoprotective [17], immunomodulating [18], anti-inflammatory [19], and antitumor effects [20]. The increas-ing availability of AM because of the improved technology in Huangqi cultivation and produc-tion has made it possible to extend its use as a feed supplement to benefit animal and poultry health and production. Research conducted in our laboratory showed that dietary supplemen-tation of AM improved growth performance and enhanced the antioxidant status and carcass quality of broiler chickens [21]. However, in-formation on the effect of AM on laying hens is lacking. The objective of this study was to assess the effects of supplementing AM at dif-ferent levels on the production performance and the serum and egg yolk antioxidant status of lay-ing hens.MATERIALS AND METHODS Preparation of the AM Root PowderOne batch of fresh AM roots [22] was cleaned by rinsing with tap water to remove soil, dried at 65°C, and then ground to pass through a 300-μm screen to yield an AM powder (AMP). The AMP was stored in covered containers at ambi-ent temperature (21 to 24°C) before being mixed into the diets.Experimental Design, Birds, and ManagementA 10-wk feeding experiment was conductedat the Research Station of Shandong Agricul-tural University, Tai-an, China. The animal careand use protocol was approved by the Shan-dong Agricultural University Animal Nutrition Research Institute. The experiment was a com-pletely randomized design, with different levelsof AMP supplementation as the treatments.A total of 540 laying hens (Hy-Line Brown,27 wk old) were randomly allocated into 20 feeding units (each with 9 cages in 3 levels, with3 birds/cage) that were then randomly assignedto 4 dietary treatments (5 units/treatment). Eachmetal wire cage (46 × 50 × 44 cm) was equippedwith an independent feeder and 2 nipple drink-ers. Feeding units were located randomly insidea ventilated house. The house was maintained ata temperature of 22 ± 2°C and in a daily photo-period of 16L:8D during the entire experimental period.A corn- and soybean meal-based diet (Table1) that was formulated to meet or slightly ex-ceed nutrient requirements [23] was left un-modified (control) or was supplemented with 5,10, or 15 g/kg of diet above the prepared AMP (treatments denoted as AMP5, AMP10, andAMP15, respectively) by replacing equivalent amounts of wheat bran in the diet formulation.The AMP was first mixed with a premix, whichwas subsequently mixed with other dietary in-gredients and then stored in covered containersbefore feeding. Experimental diets were madeevery 2 wk. The experiment lasted for a periodof 10 wk and commenced after an adaptationperiod of 1 wk. All feeding conditions were thesame between the adaptation and experimental periods. The diet was offered to the laying henstwice daily for ad libitum intake, and all henshad free access to water. Mortalities and healthstatus of the experimental laying hens were vi-sually observed and recorded daily throughoutthe entire experimental period.Laying PerformanceThe feed residue in each feeding unit was weighed at the end of each feeding week to ob-tain the ADFI. The number of eggs from each feeding unit was recorded, and the weights of in-at Poultry Science Association Member on March 20, 2014/Downloaded from245 ZUO ET AL.: ASTRAGALUS MEMBRANACEUS AND LAYING HENSdividual eggs were measured daily to determine the daily egg mass (g/d per hen) and laying rate.Assay of Antioxidant Status in the Egg Yolk and SerumAt wk 5 and 10 of the experiment (32 and 37 wk of the age), 15 eggs (3 per feed unit) were randomly chosen from each treatment to deter-mine antioxidant activity of the egg yolk. On the same dates, 10 laying hens (2 per feed unit) were also randomly selected from each treat-ment, and a blood sample (5.0 mL) was taken from the wing vein of each hen into an non-heparinized tube for subsequent determination of antioxidant activity of the serum. The blood samples were allowed to clot at 37°C for 2 h and subsequently centrifuged [24] at 1,500 × g and 4°C for 10 min to obtain the serum, which was then stored at −20°C for analysis of the activities of total superoxide dismutase (T-SOD), gluta-thione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), and concentration of malon-dialdehyde (MDA). Yolks of 3 eggs from each unit in each treatment were pooled and homog-enized for 5 min with ice-cold isotonic physi-ological saline (0.154 mol/L; pH 7.4) at a ratio of 1:9. The homogenates were then centrifuged at 1,500 × g and 4°C for 10 min, and the su-pernatant was subjected to analysis for activity of T-SOD and for concentrations of protein and MDA.All these determinations used spectropho-tometric methods and followed the analytical instructions of kits [25], as described by Zhang et al. [9]. Enzyme activities and MDA content were expressed as units per milligram of protein for egg yolk and units per milliliter for serum.at Poultry Science Association Member on March 20, 2014/ Downloaded from246JAPR: Research ReportData Calculation and Statistical Analyses Average daily feed intake, egg mass, and FE (ADFI:egg mass) were calculated as the mean value of each unit over the 10-wk experimental period. Antioxidant parameters of egg yolk and serum on 2 sampling dates were calculated, and the mean was used for each sample. Data were analyzed statistically by one-way ANOV A using the GLM procedures of SAS [26], with dietary concentration of AMP as the main treatment effect and the individual unit (5) as the statisti-cal unit. The significance of difference among treatments was tested using LSMEANS with the PDIFF option of SAS [26]. Orthogonal polyno-mial contrasts were used to determine linear and quadratic responses of laying hens to AMP lev-els [26]. Significance and trends of significance were declared if P < 0.05 and P ≤ 0.1, respec-tively.RESULTS AND DISCUSSION Laying PerformanceAll laying hens were healthy, and no mortal-ity was observed during the entire experimental period (data not shown). Average egg weight and ADFI over the 10-wk feeding period were similar among treatments. In contrast, laying rate and egg mass of birds were linearly in-creased (P = 0.044 and 0.019, respectively) and the ADFI:egg mass ratio tended (P = 0.097) to be linearly decreased as the levels of AMP in-creased from 5 to 15 g/kg of diet (Table 2). The increased egg mass with AMP supple-mentation was due to the increased laying rate rather than the individual egg weight. Likewise, the tendency for improvement in feed conver-sion was mainly due to the increased egg mass rather than the effect on feed intake. However, it was not clear why inclusion of AMP led to a linear increase in laying rate.Although AMP and its extract have been used as an immunomodulating agent in both humans and experimental animals and poultry for many years, little information is available on the ef-fects of AMP as a feed additive on the laying performance of hens. Wang et al. [21] reported that AMP supplemented at a level of 10 g/kg hada growth-promoting effect on grower broilers(4 to 6 wk) but not for the entire experimental period, and the effect was dose-dependent. For piglets, Hu et al. [27] also reported the same growth-promoting effect of AMP prepared by using different comminution techniques. On the contrary, Ma et al. [28] reported that a diet con-taining 10 g/kg of AMP did not affect the growth rate of chickens. Therefore, the effects of AMP on animal and poultry growth performance may vary depending on the dietary concentra-tion, particle size, and animal or bird species. Furthermore, supplementing the diets of laying hens with AMP up to a level of 15 g/kg of diet improved laying performance by improving egg mass and potentially improving FE.Serum Antioxidant Status Supplementing AMP up to a level of 15 g/kg of diet quadratically increased T-SOD (P < 0.01) and GSH-Px (P < 0.05) activities in the serum at wk 5, but not at wk 10 of the experiment (Tableat Poultry Science Association Member on March 20, 2014/ Downloaded from247 ZUO ET AL.: ASTRAGALUS MEMBRANACEUS AND LAYING HENS3). At wk 5, laying hens consuming diets AMP5 and AMP10 had higher (P < 0.05) serum T-SOD activities than laying hens consuming the con-trol and AMP15 diets, whereas a difference in GSH-Px activities was observed only between the control and AMP10 diets (P < 0.05). Serum T-AOC was quadratically increased (P < 0.01) but the concentration of MDA was quadratically reduced (P < 0.05) with AMP supplementation at both wk 5 and 10 of the experiment. Com-pared with laying hens fed the control diet, all hens supplemented with AMP had a higher (P < 0.01) serum T-AOC activities at both wk 5 and 10 but a lower (P < 0.05) MDA concentration at wk 5.The increased activities of T-SOD, GSH-Px, and T-AOC but reduced MDA concentration in the serum with AMP supplementation in this study indicated that AMP enhanced the antioxi-dant status of laying hen serum. This is consis-tent with the observation of Wang et al. [21], who also reported increased activities of T-AOC (in both liver and serum) and GSH-Px (in liver) and a reduced MDA concentration in the serum of broilers resulting from dietary AMP supple-mentation at levels of 5, 10, and 15 g/kg. On the basis of these 2 studies, the optimal dietary level of AMP for improving the antioxidant status of serum would be between 5 and 10 g/kg of diet for both laying hens and broilers. It is well acknowl-edged that GSH-Px, superoxide dismutase, and catalase are 3 endogenous antioxidant enzymes constituting the antioxidant cellular enzymatic system [29]. Malondialdehyde is one of the end products of lipid peroxidation, and it can endog-enously reflect the extent of lipid peroxidation [30]. Therefore, the higher activities of T-SOD and GSH-Px in the AMP-supplemented groups in this study may have resulted in a greater ca-pacity of laying hens to scavenge free radicals and ROS and reduce the MDA concentration, as indicated by the lower extent of lipid peroxida-tion. In addition, on the basis of the increased level of T-AOC in the serum of AMP-supple-mented hens, the increase in nonenzymatic an-tioxidant defenses also contributed to reducing endogenous lipid peroxidation and oxidation. The enhanced serum antioxidant status with AMP supplementation in a dose-dependent manner was due to the combined action of an-tioxidant compounds in AMP. Astragalus mem-branaceus has been reported to contain varieties of naturally occurring compounds, such as poly-saccharides, saponins, and flavonoids [31–33]. Astragalus polysaccharides have been reported to possess strong antioxidant and antitumor activities [34]. Yan et al. [35] reported that ad-ministration of AM polysaccharides at a rate of 40, 80, and 160 mg/kg of BW significantly increased serum and liver antioxidant enzymeat Poultry Science Association Member on March 20, 2014/ Downloaded from248JAPR: Research Reportactivities in a dose-dependent manner and de-creased lipid peroxidation levels in mice. It has also been demonstrated that flavonoids and sa-ponins, which are bioactive compounds found in AMP, increased the antioxidant status by increasing endogenous antioxidants and scav-enging free radicals [36, 37]. Further research is needed to determine the interaction of these AMP compounds in improving the antioxidant status of animals and poultry fed different diets. Egg Yolk Antioxidant StatusActivity of T-SOD in the egg yolk was not affected by AMP supplemented at either wk 5 or 10, but concentration of MDA in the egg yolk was linearly and quadratically reduced (P < 0.001) by AMP at both wk 5 and 10 of the experiment (Table 4). Egg yolks from all AMP-supplemented laying hens had lower (P < 0.001) concentrations of MDA at both wk 5 and 10 as compared with the control. This is consistent with the increase in serum antioxidant status with AMP observed in this study. Our results were also in agreement with the report of Sa-hin et al. [38], who observed that egg yolk MDA concentrations of quail decreased linearly in response to an increase in the level of dietary resveratrol (another naturally occurring plant antioxidant compound). In numerous studies, a dose-dependent increase in egg yolk antioxi-dant content was observed in response to dietary antioxidants in poultry [39, 40]. Therefore, the enhanced antioxidant status of egg yolk in the AMP-supplemented groups can likely be attrib-uted to the antioxidant compounds in AMP, as discussed above. Further studies are needed to elucidate the antioxidant mechanisms of AMP and to investigate the carryover effects of di-etary AMP supplementation on product quality with respect to shelf life and its nutritive value for humans as a functional food.CONCLUSIONS AND APPLICATIONS1. Supplementing the diets of laying henswith AMP at levels up to 15 g/kg linearlyincreased laying rate and egg productionbut tended (P ≤ 0.1) to linearly improveFE without affecting ADFI and averageegg weight during the 10-wk period ofexperiment.2. Supplementing the diets of laying henswith AMP enhanced antioxidant enzymeactivities and retarded lipid oxidation inthe serum and egg yolk in a dose-depen-dent manner, with the optimal dietaryAMP level being 5 to 10 g/kg of diet.3. Therefore, AMP is a natural feed addi-tive that has the potential to increase thelaying performance of laying hens.REFERENCES AND NOTES1. Fridovich, I. 1978. The biology of oxygen radicals. Science 201:875–880.2. Muramatsu, H., K. Kogawa, M. Tanaka, K. Okumura, Y. Nishihori, K. Koike, T. Kuga, and Y. Niitsu. 1995. Super-oxide dismutase in SAS human tongue carcinoma cell line is a factor defining invasiveness and cell motility. Cancer Res. 55:6210–6214.at Poultry Science Association Member on March 20, 2014/ Downloaded from249 ZUO ET AL.: ASTRAGALUS MEMBRANACEUS AND LAYING HENS3. Kris-Etherton, P. M., M. Lefevre, G. R. Beecher, M.D. Gross, C. L. Keen, and T. D. Etherton. 2004. Bioactive compounds in nutrition and health-research methodologies for establishing biological function: The antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu. Rev. Nutr. 24:511–538.4. Adesina, O. A., M. O. Japhet, E. Donbraye, T. E. Ku-mapayi, and A. Kudoro. 2009. Anti hepatitis E virus antibod-ies in sick and healthy individuals in Ekiti State, Nigeria. Afr. J. Microbiol. Res. 3:533–536.5. Oliver, C. N., B. Ahn, E. J. Moerman, S. Goldstein, and E. R. Stadtmaan. 1987. Age-related changes in oxidized protein. J. Biol. Chem. 262:5488–5491.6. Dragland, S., H. Senoo, K. Wake, K. Holte, and R. Blomhoff. 2003. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 133:1286–1290.7. Singh, G., P. Marimuthu, C. S. de Heluani, and C. Catalan. 2005. Antimicrobial and antioxidant potentials of essential oil and acetone extract of Myristica fragrans Houtt. (aril part). J. Food Sci. 70:M141–M148.8. Jang, A., X. D. Liu, M. H. Shin, B. D. Lee, S. K. Lee, J. H. Lee, and C. Jo. 2008. Antioxidative potential of raw breast meat from broiler chicks fed a dietary medicinal herb extract mix. Poult. Sci. 87:2382–2389.9. Zhang, G. F., Z. B. Yang, Y. Wang, W. R. Yang, S. Z. Jiang, and G. S. Gai. 2009. Effects of ginger root (Zingiber officinale) processed to different particle sizes on growth performance, antioxidant status, and serum metabolites of broiler chickens. Poult. Sci. 88:2159–2166.10. Sun, W. Y., W. Wei, L. Wu, S. Y. Gui, and H. Wang. 2007. Effects and mechanisms of extract from Paeonia lac-tiflora and Astragalus membranaceus on liver fibrosis in-duced by carbon tetrachloride in rats. J. Ethnopharmacol. 112:514–523.11. Ma, X. Q., Q. Shi, J. A. Duan, T. T. Dong, and K. W. Tsim. 2002. Chemical analysis of Radix astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 50:4861–4866.12. Ma, X., P. Tu, Y. Chen, T. Zhang, Y. Wei, and Y. Ito. 2004. Preparative isolation and purification of isoflavan and pterocarpan glycosides from Astragalus membranaceus Bge. var. mongholicus (Bge.) Hsiao by high-speed counter-current chromatography. J. Chromatogr. A 1023:311–315.13. Liu, W., J. Chen, W. J. Zuo, X. Li, and J. H. Wang. 2007. A new isoflavane from processed Astragalus membra-naceus. Chin. Chem. Lett. 18:1092–1094.14. Yang, Q., J. T. Lu, A. W. Zhou, B. Wang, G. W. He, and M. Z. Chen. 2001. Antinociceptive effect of astragalo-sides and its mechanism of action. Acta Pharmacol. Sin. 22:809–812.15. Lei, H., B. Wang, W. P. Li, Y. Yang, A. W. Zhou, and M. Z. Chen. 2003. Anti-aging effect of astragalosides and its mechanism of action. Acta Pharmacol. Sin. 24:230–234. 16. Luo, Y., Z. Qin, Z. Hong, X. Zhang, D. Ding, J.-H. Fu, W.-D. Zhang, and J. Chen. 2004. Astragaloside IV pro-tects against ischemic brain injury in a murine model of tran-sient focal ischemia. Neurosci. Lett. 363:218–223.17. Gui, S. Y., W. Wei, H. Wang, L. Wu, W. Y. Sun, W. B. Chen, and C. Y. Wu. 2006. Effects and mechanisms of crude astragalosides fraction on liver fibrosis in rats. J. Ethnophar-macol. 103:154–159.18. Cho, W. C. S., and K. N. Leung. 2007. In vitro and in vivo immunomodulating and immunorestorative effects of Astragalus membranaceus. J. Ethnopharmacol. 113:132–141.19. Ko, J. K., and C. W. Chik. 2009. The protective actionof radix Astragalus membranaceus against hapten-induced colitis through modulation of cytokines. Cytokine 47:85–90.20. Ionkova, I., G. Momekov, and P. Proksch. 2010. Ef-fects of cycloartane saponins from hairy roots of Astragalus membranaceus Bge., on human tumor cell targets. Fitotera-pia 81:447–451.21. Wang, H. F., W. R. Yang, H. W. Yang, Y. Wang, Z.B. Yang, S. Z. Jiang, and G. G. Zhang. 2010. Effects of As-tragalus membranaceus on growth performance, carcass characteristics, and antioxidant status of broiler chickens. Acta Agric. Scand. A Animal Sci. 60:151–158.22. Bozhou Medicine Co., Bozhou, Anhui, China.23. NRC. 1994. Nutrient Requirements of Poultry. 9th rev. ed. Natl. Acad. Press, Washington, DC.24. D-37520 Osterode, Kendro Laboratory Products, Heraeus Instruments, Germany.25. Nanjing Jiancheng Bioengineering Institute, Nanjing, China.26. SAS Institute. 2000. User’s Guide. Release 8.1 ed. SAS Inst. Inc., Cary, NC.27. Hu, Y. L., C. L. Xu, Y. Z. Wang, Y. J. Li, J. X. Liu,and J. Feng. 2006. Effect of dried roots of Astragalus mem-branaceus in the diets of young growing pigs on growth performance and immune function. J. Anim. Feed Sci. 15:599–607.28. Ma, D. Y., A. S. Shan, and Q. D. Li. 2004. Effects of Chinese medical herbs on chicken growth and immuniza-tion. Acta Zoonutrimenta Sin. 16:36–40.29. Blokhina, O., E. Virolainen, and K. V. Fagerstedt. 2003. Antioxidants, oxidative damage and oxygen depriva-tion stress: A review. Ann. Bot. 91:179–194.30. Sumida, S., K. Tanaka, H. Kitao, and F. Nakadomo. 1989. Exercise-induced lipid peroxidation and leakage of enzyme before and after vitamin E supplementation. Int. J. Biochem. 21:835–838.31. Ma, X., P. Tu, Y. Chen, T. Zhang, Y. Wei, and Y. Ito. 2003. Preparative isolation and purification of two isofla-vones from Astragalus membranaceus Bge. var. mongholi-cus (Bge.) Hsiao by high-speed counter-current chromatog-raphy. J. Chromatogr. A 992:193–197.32. Zhang, W. D., C. Zhang, R. H. Liu, H. L. Li, J. T. Zhang, C. Mao, S. Moran, and C. L. Chen. 2006. Preclinical pharmacokinetics and tissue distribution of a natural cardio-protective agent astragaloside IV in rats and dogs. Life Sci. 79:808–815.33. Yu, Q. T., P. Li, Z. M. Bi, J. Luo, and X. D. Gao. 2007. Two new saponins from the aerial part of Astraga-lus membranaceus var. mongholicus. Chin. Chem. Lett. 18:554–556.34. Li, R., W. C. Chen, W. P. Wang, W. Y. Tian, and X.G. Zhang. 2010. Antioxidant activity of Astragalus polysac-charides and antitumour activity of the polysaccharides and siRNA. Carbohydr. Polym. 82:240–244.35. Yan, H., Y. P. Xie, S. G. Sun, X. D. Sun, F. X. Ren,Q. R. Shi, S. H. Wang, W. D. Zhang, X. M. Li, and J. Zhang. 2010. Chemical analysis of Astragalus mongholicus poly-saccharides and antioxidant activity of the polysaccharides. Carbohydr. Polym. 82:636–640.at Poultry Science Association Member on March 20, 2014/Downloaded from250JAPR: Research Report36. Yu, D. H., Y. M. Bao, C. L. Wei, and L. J. An. 2005. Studies of chemical constituents and their antioxidant activi-ties from Astragalus mongholicus Bunge. Biomed. Environ. Sci. 18:297–301.37. Sheng, B. W., X. F. Chen, J. Zhao, D. F. He, and X. Y. Nan. 2005. Astragalus membranaceus reduces free radical-mediated injury to renal tubules in rabbits receiving high-energy shock waves. Chin. Med. J. (Engl.) 118:43–49. 38. Sahin, K., F. Akdemir, C. Orhan, M. Tuzcu, A. Hayirli, and N. Sahin. 2010. Effects of dietary resveratrol supplementation on egg production and antioxidant status.Poult. Sci. 89:1190–1198.39. Meluzzi, A., F. Sirri, G. Manfreda, N. Tallarico, andA. Franchini. 2000. Effect of dietary vitamin E on the qual-ity of table eggs enriched with n-3 long chain fatty acids.Poult. Sci. 79:539–545.40. Akdemir, F., and K. Sahin. 2009. Genistein supple-mentation to the quail: Effects on egg production and eggyolk genistein, daidzein, and lipid peroxidation levels.Poult. Sci. 88:2125–2131.at Poultry Science Association Member on March 20, 2014/Downloaded from。
奥美拉唑联合L-谷氨酰胺呱仑酸钠治疗老年非甾体类抗炎药相关溃疡临床研究
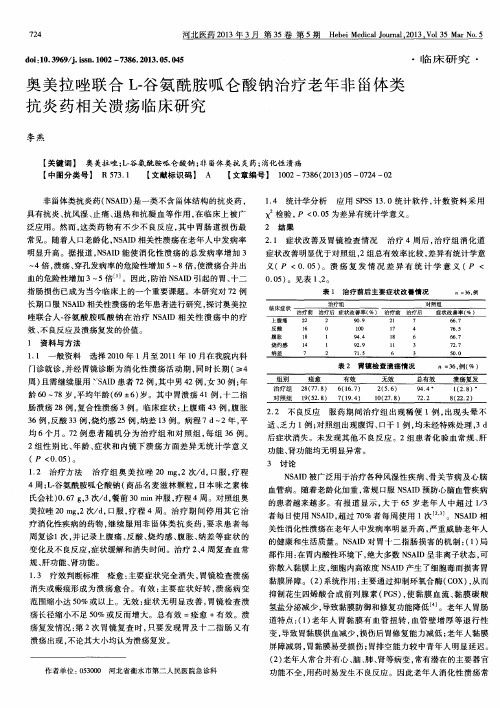
1 资 料 与 方 法
症 状丽
1 . 1 一般资料 选 择 2 0 1 0年 1 月至 2 0 1 1 年1 0月在我 院内科
门诊就诊 , 并经 胃镜诊断 为消化性 溃疡活 动期 , 同时长 期 ( >4 I
周) 且需继续服用 S A I D患者 7 2例 , 其中男 4 2例 , 女3 0例 ; 年
龄6 0— 7 8岁 , 平均年龄( 6 9± 6 ) 岁 。其 中 胃溃疡 4 1例 , 十二 指 肠溃疡 2 8例 , 复合性溃疡 3例 。临床症 状 : 上腹痛 4 3例 , 腹 胀 3 6例 , 反酸 3 3例 , 烧灼感 2 5例 , 纳差 1 3例。病程 7 d~ 2年 , 平 均 6个月 。7 2例患 者随 机分 为治疗 组 和对 照组 , 每组 3 6例。
义( P <0 . 0 5 ) 。溃 疡 复 发 情 况 差 异 有 统 计 学 意 义 ( P <
0 . 0 5 ) 。见 表 1 、 2 。
表 1 治 疗 前 后 主 要 症 状 改 善情 况 : 3 6 , 例
4倍 , 溃疡、 穿孔发病率的危险性增加 5~ 8倍 , 使溃疡合并 出
4周 ; L - 谷氨酰胺呱仑酸钠 ( 商品名麦滋 林颗粒 , 日本 味之素株 氏会社 ) 0 . 6 7 g , 3次/ d , 餐前 3 0 mi n冲服 , 疗程 4周 。对 照组 奥 美拉唑 2 0 m g , 2次/ d , 口服 , 疗 程 4周。治疗 期 间停用 其它 治 疗消化性疾病的药物 , 继续 服用非 甾体类抗 炎药 , 要求 患者 每 周复诊 1 次, 并记录上腹 痛、 反 酸、 烧灼 感、 腹 胀、 纳 差等症状 的 变化及不 良反应 , 症状缓解 和消失时 间。治疗 2 、 4周复 查血常 规、 肝功能、 肾功能 。 1 . 3 疗效判 断标准 痊愈 : 主要症状完全 消失 , 胃镜 检查溃疡 消失或瘢痕形成为溃 疡愈合 。有 效 : 主要症 状好 转 , 溃 疡病 变 范围缩小达 5 0 %或以上 。无效 : 症状无 明显改善 , 胃镜 检查 溃 疡长径缩小不足 5 0 %或反 而增大 。总有效 =痊愈 +有效 。溃
几丁聚糖口腔溃疡喷雾剂治疗复发性口腔溃疡的临床疗效观察

海药临床几丁聚糖口腔溃疡喷雾剂治疗复发性口腔溃疡的临床疗效观察许晓燕1,2,邓婧2,宋文斌2,于文功1(1.中国海洋大学医药学院,山东青岛266003;2.青岛大学医学院附属医院口腔内科,山东青岛266003)摘 要:目的观察几丁聚糖溃疡喷雾剂治疗复发性口腔溃疡的临床疗效。
方法将确诊为复发性口腔溃疡的60例患者随机分为治疗组和对照组,分别使用几丁聚糖口腔溃疡喷雾剂和锡类散治疗,根据中华医学会口腔黏膜病专业委员会公布的 复发性阿弗他溃疡评价试行标准 ,评价其治疗效果。
结果治疗组的平均治疗期较对照组明显缩短;治疗组较对照组疼痛指数明显减小。
结论几丁聚糖口腔溃疡喷雾剂治疗复发性口腔溃疡的疗效优于锡类散。
关键词:复发性口腔溃疡;几丁聚糖;中药;疗效中图分类号:R931.77,R988.2 文献标识码:A 文章编号:1002 3461(2008)03 0048 03 Clinical application of chitosan oral ulcer spray in treatment of recurrent aphthous ulcerXU Xiao yan1,2,DENG Jing2,SONG Wen bin2,YU Wen gong1(1.Colledge of med icine and p har macy,Ocean Univ ersity of China,Qingd ao266003, China;2.T he A f f iliated H osp ital of Qingdao Univ er sity Medical Colleg e,Qingd ao 266003,China)Abstract:Objective To observe the therapeutic effect of chitosan or al ulcer spray in treatm ent of recurrent aphthous ulcer(RAU).Methods Sixty patients suffered w ith RAU w ere random ly divided into tw o gr oups.The test g roup w as treated w ith chitosan oral ulcer spray,w hile the contr ol g roup w as given a tr aditional Chinese m edicine named Xileisan.To evaluate the efficacy acco rding to a specialized ev aluate standard.Results The disease cour se of test gro up w as sho rter than that of the co ntro l gr oup.The test gr oup w as more effectiv e in m itigating the pain of RAU than that of the contr ol gr oup.Conclusion T he efficacy of chito san oral ulcer spray in treatment o f RAU is superior to Xileisan.Key words:RAU;chitosan;Chinese materia medica;efficacy复发性口腔溃疡(recur rent aphtho us ulcer,RAU)又称 复发性阿弗他溃疡 ,是临床上比较常见的口腔黏膜疾病,在人群中的患病率高达20%左右,据推测,RAU的发生可能是多种因素综合作用的结果[1],因此,临床上对该病主要是对症治疗,常用喷剂或散剂施于溃疡处,以消除炎症、减轻患处疼痛、缩短病程。
人参胃康片防治胃溃疡药理学研究
笔者实验观察人参胃康片防治胃溃疡、保护胃黏膜、 促进胃排空、镇痛等作用。 l材料与方法
1.1材料
1.1.1动物NIH小鼠雌雄兼有,体重18~22
g
广州中医药大学实验动物中心提供,许可证号 SCXK(粤)2003-0001。SD大鼠,雌雄各半,体重
2.1各组对盐酸乙醇致大鼠胃黏膜损伤影响的比较
结果见表1。 表1各组对盐酸乙醇致大鼠胃黏膜损伤的比较
量士s
ml/只,缝合腹腔,常规饲养。术后次日随机分
为5组:空白对照组,雷尼替丁组,人参胃康片大、
中、小剂量组,每组8只,分别灌胃给药,每日1次,
连续7 d,末次给药24 h后处死大鼠,摘取胃,沿胃 大弯剪开,观察溃疡情况,用溃疡的最长径和最短径 的均值(ram)表示溃疡指数,愈合者溃疡指数为0。 另外将每只大鼠溃疡灶周围的组织剪下,置于一
与空白对照组比较”P<0.05,∞P<0.01
・184・
中国中西医结合消化杂志2008年6月第16卷第3期
2.2各组对冰乙酸致大鼠胃溃疡指数和lVII的,,SOD影响的比较
表6各组小鼠冰乙酸致痛扭体次数的比较
z士s
结果见表2。
2.3各组对消炎痛致大鼠胃黏膜损伤影响的比较
结果见表3。 表3 各组对消炎痛致大鼠胃黏膜损伤影响的比较
干净,观察胃黏膜损伤情况,以条索状损伤的长度计 算溃疡指数,1 mm计1分,<1 mm计0.5分。计
算每只大鼠损伤指数总和。 1.2.2对冰乙酸诱发大鼠胃小弯溃疡的作用及 MPO、SOD的影响 40只大鼠,乙醚麻醉下开腹, 暴露胃体,在胃前壁小弯侧膜下注射40%冰乙酸
杜仲雄花粉总黄酮抗衰老作用
J
ou
r
na
lo
fHenan Un
i
ve
r
s
i
t
i
c
a
lSc
i
enc
e 2023 42 5
y Med
·药学研究·
杜仲雄花粉总黄酮抗衰老作用
高梦珂,代俊俊,许世伟,李钦✉
河南大学 药学院,河南 开封 475000
摘要
河南大学学报 医学版
︵
︶
目的:探讨杜仲雄花粉总黄酮(
FEF)对 D-半乳糖致衰老小鼠的抗衰老作 用。 方 法:将 60 只 昆 明 小 鼠 随 机 分 为 6
节血压、抗菌抗病毒等作用。黄酮类化合物为杜仲
的主要活性成分之一,具有抗氧化、消除自由基、抑
菌、
抗过敏等作用[4-7]。目前对杜仲的研究主要聚焦
阅文献可知,杜仲雄花粉含有总黄酮、蛋白质、维生
素等有效成分,在药用方面及保健品方面具有较高
的利用价值[8]。为了更好地开发利用这一资源,本
实验利用 D
-半乳糖建立衰老模型,探讨杜仲雄花粉
l
ef
l
owe
r powde
ro
f Euc
ommi
au
lmo
i
de
s Thesuba
cu
t
e ag
i
ng mode
lo
f mi
c
e wa
se
s
t
ab
l
i
shed by subcu
t
ane
ous
i
n
e
c
t
i
ono
fDl
石见穿多糖抑制人胃癌细胞系MGC-803细胞迁移及其机制的初步研究
石见穿多糖抑制人胃癌细胞系MGC-803细胞迁移及其机制的初步研究朱红岩;孙玉国;曲杰;卞永生【摘要】Objective To investigate the effect of Salvia chinensisBenth.Polysaechandes (SBP) on the metastasis ability of human gastric cancer cell line (MGC-803 cells) in vitro, and to explore the action mechanisms of SBP. Methods SBP prepared by extraction of water, the effect of the different concentration of SBP on the experimental metastasis ability of MGC-803 cells was observed by scratch test and Transwell metastasis experiments. The level of IL-8 protein in MGC-803 cells were detected by Western Blotting. Results Compared with control group (no drug), SBP (50 and 100 g/L) inhibited the MGC-803 cells wound, MG-63 cells scratches healed was significantly weakened, number of cells passing through filter membrane would decrease. The level of IL-8 protein in MG-63 cells decreased significantly after treated with SBP and correlation with the concentration of SBP. Conclusion SBP ia able to inhibit the metastasis ability of MGC-803 cells. The action mechanisms of SBP are related to the inhibition of IL-8 protein levels in MGC-803 cells.%目的观察石见穿多糖对人胃癌细胞系MGC-803细胞迁移能力的影响,并探讨其作用机制.方法利用水萃取法制备石见穿多糖,通过划痕实验、Transwell实验观察不同浓度对石见穿多糖同样条件培养下胃癌MGC-803细胞迁移能力的影响,并采用Western blot方法检测不同浓度石见穿多糖对MGC-803细胞内白介素8(IL-8)蛋白表达水平的影响.结果同未加药物对照组相比,经高浓度石见穿多糖(50、100 g/L)处理后,MG-63细胞的划痕愈合能力明显减弱,穿膜细胞的数量显著减少;同时,细胞内IL-8蛋白的表达水平明显降低,并与石见穿多糖的浓度有关.结论石见穿多糖抑制了胃癌MGC-803细胞的迁移能力,其作用与抑制MGC-803细胞内的IL-8蛋白表达水平有关.【期刊名称】《中国医药导报》【年(卷),期】2012(009)032【总页数】3页(P5-7)【关键词】石见穿多糖;MGC-803细胞;白介素8;迁移【作者】朱红岩;孙玉国;曲杰;卞永生【作者单位】承德医学院,河北承德,067000;承德医学院,河北承德,067000;承德医学院附属医院,河北承德,067000;河北省保定市第一医院,河北保定,071000【正文语种】中文【中图分类】R730.52石见穿为唇形科植物华鼠尾Salvia chinensis Benth.的全草,味苦、辛,性平,具有清热解毒、活血镇痛的功能。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
EffectofpolysaccharidesfromAngelicasinensisongastriculcerhealing
Y.N.Yea,b,H.L.Soa,E.S.L.Liua,V.Y.Shina,C.H.Choa,*
aDepartmentofPharmacology,L02-55,2/F,LaboratoryBlock,FacultyofMedicineBuilding,
21SassoonRoad,UniversityofHongKong,HongKong,PRChinabDepartmentofPharmacology,ZhejiangUniversity,Hangzhou,PRChina
Received15April2002;accepted6September2002
AbstractOurpreviousstudyshowedthatacrudeextractfromAngelicasinensis(ASCE),whichmainlyconsistedofpolysaccharides,significantlypromotedmigrationandproliferationofnormalgastricepithelialcells.TheseresultsstronglysuggestthatASCEhasadirectwoundhealingeffectongastricmucosa.However,thereisnoreportconcerningtheeffectofASCEongastriculcerhealinginanimalmodels.Inthisstudy,wefoundthatASCEpromotedulcerhealing.Theareaoftheulcerwasreduced.Thiswasaccompaniedwithasignificantincreaseinmucussynthesiswhencomparedwiththecontrol.AngiogenesiswasinhibitedbythetreatmentofASCE.Cellproliferation,ODCandEGFRproteinexpressionwasnotaffectedinthisprocess.Thus,themechanismofhowASCEacceleratesulcerhealinginadditiontoitseffectonmucussynthesisremainstobeinvestigated.D2002ElsevierScienceInc.Allrightsreserved.
Keywords:Angelicasinensis;Polysaccharides;Gastriculcer;Mucus;Angiogenesis
IntroductionAnulcerisadeepnecroticlesionpenetratingintogastrointestinalmucosaandmuscularismucosae.Ulcerhealingisacomplexandtightlyregulatedprocessoffillingthemucosaldefectwithproliferatingandmigratingepithelialandconnectivetissuecells.Thisprocessincludesthere-establishmentofthecontinuoussurfaceepitheliallayer,glandularepithelialstructures,microvesselsandconnectivetissuewithinthescar.Epithelialcellsinthemucosaoftheulcermarginproliferateandmigrateontothegranulationtissuetore-epithelializetheulceratedarea[1].
0024-3205/02/$-seefrontmatterD2002ElsevierScienceInc.Allrightsreserved.PII:S0024-3205(02)02332-9
*Correspondingauthor.Tel.:+852-2819-9252;fax:+852-2817-0859.E-mailaddress:chcho@hkusua.hku.hk(C.H.Cho).
www.elsevier.com/locate/lifescieLifeSciences72(2003)925–932Intherecentyears,awidespreadsearchhasbeenlaunchedtoidentifynewanti-ulcerdrugsfromnaturalsources.Anumberofspices,suchasgingerandturmeric,havebeenshowntopossesssignificantgastroprotectiveactivity[2,3].OurpreviousstudyshowedthatacrudeextractfromAngelicasinensis(ASCE),whichmainlyconsistedofpolysaccharides,preventedethanol-orindomethacin-inducedgastricmucosaldamage[4].InvitrostudiesshowedthatASCEsignificantlypromotedthemigrationofgastricepithelialcells(RGM-1)overanartificialwound.Thisextractalsostimulated[3H]-thymidineincorpo-
rationinRGM-1cellsinaconcentration-dependentmanner.TheseresultsstronglysuggestthatASCEhasadirectwoundhealingeffectongastricmucosa[5].So,theaimofthisstudyistoinvestigatetheeffectofASCEongastriculcerhealinginratsandthepossiblemechanismsunderlined.
MethodsExtractionofpolysaccharidesfromAngelicasinensisTherootsofAngelicasinensis(Oliv.)Diels,Danggui,werepurchasedfromMinxianCounty,GansuProvince,China.PolysaccharideswereisolatedaccordingtothemethoddescribedbyChoetal.[4].
AnimalsandinductionofgastrickissingulcersThestudywasapprovedbytheCommitteeontheUseofLiveAnimalsforTeachingandResearchintheUniversityofHongKong.MaleSprague-Dawleyrats(weighing180–200g)wererearedonastandardlaboratorydiet(RalstonPurina,Chicago,Illinois,USA)andgiventapwater.Theywerekeptinaroomwheretemperature(22F1jC),humidity(65–70%),andday/nightcycle(12hours/12hours)werecontrolled.Ratsweredeprivedoffoodbuthadfreeaccesstotapwater24hoursbeforeulcerinduction.Gastrickissingulcerswereproducedbyluminalapplicationofanaceticacidsolution,asdescribedbyTsukimiandOkabe[6]withsomemodifications[7].Thereafter,animalswerefedastandarddietoflaboratorychowandgiventapwateradlibitum.TheratsweregivenwaterorASCEsolutiononcedailybyP.O.startingfromonedayafterulcerformation.After3daystreatment,aportionofratswaskilledandanotherportionwasleftforanother4daysbeforesacrifice.Ratswerekilledbyetheranesthesiafollowedbycuttingofftheabdominalaorticartery.Thestomachwasremovedrapidly,openedalongthegreatercurvature,andrinsedwithnormalsalinethoroughly.Afterrecordingtheulcersproducedinthestomach,alongitudinalsectionofthegastrictissuewastakenfromtheanteriorpartofthestomachandfixedina10%formalinsolution.After24hoursoffixationandbeingembeddedinaparaffinblock,itwascutintosectionsof5Amandthentransferredontoaglassslide.Thesesectionswereusedlaterforthehistologicalassessmentofthegastricmucosa.Theremainingpartofthestomachwasscrapedwithaglassslideonaglassdishonice.Theywerewrappedbyapieceofaluminumfoilandimmediatelyfrozeninliquidnitrogen.ThesesampleswerekeptunderÀ70jCuntilassaysfordifferentcytokines.
