Response to Activation of the DNA Integrity Checkpoint
胆道系统恶性肿瘤免疫治疗的研究进展及展望

胆道系统恶性肿瘤免疫治疗的研究进展及展望①甄自力沈哲民孙培龙(复旦大学附属金山医院普外科,上海201508)中图分类号R735.8R392文献标志码A文章编号1000-484X(2021)22-2766-05[摘要]胆道系统恶性肿瘤(BTC)是与化疗耐药和不良预后相关的侵袭性肿瘤,现有的干预方法无法有效改善预后,因此需要探寻新的治疗方法。
免疫治疗近年来有了突破性的成就,其利用患者自身免疫系统攻击肿瘤,为BTC的治疗提供了新的方向。
初步临床研究表明,BTC的免疫疗法可有效增强肿瘤免疫反应,延长患者中位生存期,改善预后。
主要方法有免疫检查点阻断、基于肽和树突状细胞的靶向肿瘤抗原疫苗和过继T细胞转移。
随着BTC免疫治疗的深入研究,该方法的抗瘤功效将得到进一步明确,最终将成为BTC的常规治疗手段。
[关键词]胆道;恶性肿瘤;免疫检查点阻断;疫苗;过继T细胞转移Research progress and prospects of immunotherapy for biliary tract cancers ZHEN Zi-Li,SHEN Zhe-Min,SUN Pei-Long.Department of General Surgery,Jinshan Hospital,Fudan Univer⁃sity,Shanghai201508,China[Abstract]Biliary tract cancers(BTC)are aggressive tumors related to chemotherapy resistance and poor prognosis.Existing intervention methods cannot effectively improve the prognosis,so new treatment methods need to be explored.Immunotherapy has made breakthrough achievements in recent years.It uses the patient's own immune system to attack tumors,providing a new direction for treatment of BTC.Preliminary clinical studies have shown that BTC immunotherapy can effectively enhance the anti-tumor immune response,prolong the median survival of patients,and improve prognosis.The main methods are immune checkpoint blockade,targeted tumor antigen therapy via peptide and dendritic cell-based vaccines and adoptive T cell transfer.With the in-depth study of BTC immunotherapy,the anti-tumor efficacy of immunotherapy will be further clarified,and will eventually become the conventional treat‐ment of BTC.[Key words]Biliary tract;Cancers;Immune checkpoint blockage;Vaccines;Adoptive T cell therapy胆道系统恶性肿瘤(biliary tract cancers,BTC)是起源于胆管上皮细胞的恶性肿瘤,可发生于肝内胆管、肝外胆管等不同部位,主要包括肝内胆管细胞癌(intrahepatic cholangiocarcinoma,ICC)和胆管癌(cholangiocarcinoma,CCA)。
oral tolerance
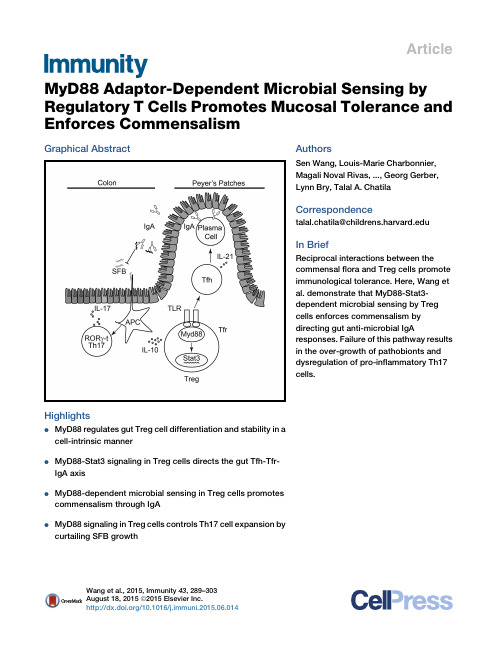
Article MyD88Adaptor-Dependent Microbial Sensing by Regulatory T Cells Promotes Mucosal Tolerance and Enforces CommensalismGraphical AbstractHighlightsd MyD88regulates gut Treg cell differentiation and stability in acell-intrinsic mannerd MyD88-Stat3signaling in Treg cells directs the gut Tfh-Tfr-IgA axisd MyD88-dependent microbial sensing in Treg cells promotescommensalism through IgAd MyD88signaling in Treg cells controls Th17cell expansion bycurtailing SFB growth AuthorsSen Wang,Louis-Marie Charbonnier, Magali Noval Rivas,...,Georg Gerber, Lynn Bry,Talal A.ChatilaCorrespondencetalal.chatila@In BriefReciprocal interactions between the commensalflora and Treg cells promote immunological tolerance.Here,Wang et al.demonstrate that MyD88-Stat3-dependent microbial sensing by Treg cells enforces commensalism by directing gut anti-microbial IgA responses.Failure of this pathway results in the over-growth of pathobionts and dysregulation of pro-inflammatory Th17cells. Wang et al.,2015,Immunity43,289–303August18,2015ª2015Elsevier Inc./10.1016/j.immuni.2015.06.014ImmunityArticleMyD88Adaptor-Dependent Microbial Sensingby Regulatory T Cells Promotes Mucosal Tolerance and Enforces CommensalismSen Wang,1,2Louis-Marie Charbonnier,1,2Magali Noval Rivas,1,2Peter Georgiev,1,2Ning Li,3Georg Gerber,3Lynn Bry,3 and Talal A.Chatila1,2,*1Division of Immunology,Boston Children’s Hospital,Boston,MA02115,USA2Department of Pediatrics,Harvard Medical School,Boston,MA02115,USA3Center for Clinical and Translational Metagenomics,Department of Pathology,Brigham&Women’s Hospital,Harvard Medical School, Boston,MA02115,USA*Correspondence:talal.chatila@/10.1016/j.immuni.2015.06.014SUMMARYCommensal microbiota promote mucosal tolerance in part by engaging regulatory T(Treg)cells via Toll-like receptors(TLRs).We report that Treg-cell-specific deletion of the TLR adaptor MyD88resulted in deficiency of intestinal Treg cells,a reciprocal increase in T helper17(Th17)cells and heightened interleukin-17(IL-17)-dependent inflammation in experimental colitis.It also precipitated dysbiosis with overgrowth of segmentedfilamentous bacteria (SFB)and increased microbial loads in deep tissues. The Th17cell dysregulation and bacterial dysbiosis were linked to impaired anti-microbial intestinal IgA responses,related to defective MyD88adaptor-and Stat3transcription factor-dependent T follicular reg-ulatory and helper cell differentiation in the Peyer’s patches.Thesefindings establish an essential role for MyD88-dependent microbial sensing by Treg cells in enforcing mucosal tolerance and maintaining commensalism by promoting intestinal Treg cell for-mation and anti-commensal IgA responses. INTRODUCTIONThe gastrointestinal commensal microbiota play a critical role in shaping host immune and metabolic responses(Ba¨ckhed et al., 2005;Chu and Mazmanian,2013;Lee and Mazmanian,2010; Round and Mazmanian,2009).Although pathogenic bacteria trigger inflammation and symbiotic bacteria promote tolerance, both sets of responses involve the activation of host pattern recognition receptors(PRRs),including Toll-like receptors (TLRs)(Hooper et al.,2012;Palm and Medzhitov,2009).In the case of commensal bacteria,PRR signaling in the absence of tis-sue damage channels the immune response toward tolerance (reviewed in Chu and Mazmanian,2013).T regulatory(Treg)cells expressing the transcription factor Foxp3play a critical role in this process(Josefowicz et al.,2012;Nutsch and Hsieh,2012; Round and Mazmanian,2009).How Treg cells sense microbial signals and translate them into a tolerogenic response remains incompletely understood.Both natural Treg(nTreg)and induced Treg(iTreg)cells contribute to gastrointestinal tolerance(Haribhai et al.,2009, 2011).The former are a distinct thymus-derived lineage that express a T cell antigen receptor(TCR)repertoire biased toward self antigens(Hsieh et al.,2004).The latter are induced de novo from conventional CD4+Foxp3–T cells upon encountering anti-gens in the presence of transforming growth factor-b(TGF-b), interleukin-2(IL-2),and retinoic acid(Coombes et al.,2007; Mucida et al.,2005,2007;Sun et al.,2007).iTreg cells carry a distinct TCR repertoire that is biased toward recognition of foreign antigens including the microbiota,reflective of their deri-vation from conventional T(Tconv)cells(Haribhai et al.,2011; Lathrop et al.,2008,2011).Both nTreg and iTreg cells are required for optimal peripheral tolerance and prevention of intes-tinal inflammation(Haribhai et al.,2009,2011).In their absence, the microbiota drive intestinal inflammation in a TLR-and MyD88-dependent manner(Izcue et al.,2009;Rivas et al.,2012). Commensal bacteria favor iTreg cell differentiation in the gut (Atarashi et al.,2011;Geuking et al.,2011;Lathrop et al.,2011; Round et al.,2011;Round and Mazmanian,2010).Promotion by the gut microbiota of Treg cell generation involves TLR signaling,evidenced by the failure to expand colonic lamina propria(cLP)Treg cells in germ-free(GF)mice doubly deficient in the TLR adaptor molecules MyD88and Trif when colonized with altered Schaedlerflora(Geuking et al.,2011).TLR2and TLR4signaling promotes Treg cell proliferation and survival(Car-amalho et al.,2003;Chen et al.,2009;Liu et al.,2006;Sutmuller et al.,2006).Polysaccharide A of Bacteroides fragilis signals directly via TLR2receptors on T cells to promote iTreg cell differ-entiation and IL-10and TGF-b production,to suppress Th17cell differentiation,and to establish colonization of Bacteroides fragi-lis at the mucosal interface(Round et al.,2011;Wang et al., 2006).Collectively,these studies indicate that Treg cells might directly respond to microbial signals and that this response is important for tolerance acquisition.To further elucidate the role TLR-MyD88signaling in Treg cells in promoting mucosal tolerance,we examined the conse-quences of Treg cell lineage-specific Myd88deletion.We identi-fied an essential role for MyD88in the induction and stability of mucosal Treg cells and the differentiation of T follicularregulatory Immunity43,289–303,August18,2015ª2015Elsevier Inc.289(Tfr)and helper(Tfh)cells in the Peyer’s patches(PPs).Further-more,MyD88signaling in Treg cells acts via a Stat3-dependent mechanism to promote healthy commensalism by supporting anti-microbial IgA antibody responses,thereby suppressing overgrowth of segmentedfilamentous bacteria(SFB)and re-straining Th17cell responses.RESULTSTreg-Cell-Specific MyD88Deletion Results in Treg Cell Deficiency and Th17Cell Dysregulation in the Gut MucosaTo analyze the role of TLR signaling in Treg cells in maintaining peripheral tolerance,we generated mice with Treg-cell-specific MyD88deficiency by crossing mice harboring a Cre recombi-nase and an EGFP reporter under the control of the Foxp3pro-moter(Foxp3EGFPcre)with others,that carried afloxed Myd88 allele(Figure S1A;Hou et al.,2011;Zhou et al.,2009).The resul-tant mice,termed Foxp3EGFPcre Myd88D/D,expressed MyD88in different tissues examined and in immune cells,including con-ventional CD4+T cells,with the exception of CD4+Foxp3+Treg cells,where transcripts encoding MyD88were absent in the face of normal expression of Foxp3transcripts(Figure S1B). These results indicate that Foxp3EGFPcre was fully effective in specifically deleting Myd88in Treg cells while sparing CD4+ Tconv cells.Mice with the Myd88lineage-specific deletion were no different in weight and gross phenotype from their MyD88-sufficient littermates(data not shown).The frequency and absolute numbers of Treg cells of MyD88-sufficient (Myd88fl/fl)and-deficient(Foxp3EGFPcre Myd88D/D)littermate mice were similar across most lymphoid tissues,including the thymus,spleen,and peripheral lymph nodes.However,the Foxp3EGFPcre Myd88D/D mice exhibited a selective decrease in the Treg cells found in the small and large intestinal lamina prop-ria(sLP and cLP,respectively)as well as those in the PPs(Fig-ures1A and1B).This decrease was not reproduced by deletion of MyD88-coupled cytokine receptor pathways,including IL-1R1and IL-18R.It was,however,recapitulated by antibiotic treatment of MyD88-sufficient mice(Figures S1C and S1D). Comparison of MyD88-sufficient and-deficient cLP Treg cells revealed that expression of most key Treg cell markers,including Foxp3,CD25,CTLA-4,CD103,and Helios,was not different between the two populations,and that their in vitro suppressive capacity was similar(Figures1C and1D and data not shown). However,expression of Nrp1,associated with nTreg cells,was increased in the cLP of Foxp3EGFPcre Myd88D/D mice,suggesting a deficiency in iTreg cells.To establish the presence of a defect in iTreg cell formation in Foxp3EGFPcre Myd88D/D mice,we compared the capacity of naive CD4+T cells from Foxp3EGFPcre and Foxp3EGFPcre Myd88D/D mice to differentiate into antigen-specific iTreg cells in vivo.Naive CD45.2+CD4+T cells expressing the OT-II TCR transgene, specific for the OVA323-339peptide in the context of I-A b,were isolated from either Foxp3EGFPcre or Foxp3EGFPcre Myd88D/D and transferred into CD45.1+recipient mice.The latter were sub-sequently exposed to OVA via the drinking water and examined for the presence of CD45.2+CD4+EGFP+Treg cells in the cLP. Treg-cell-specific MyD88deficiency resulted in a profound reduction of OVA-induced iTreg cell formation in cLP and mesenteric lymph nodes(MLNs),indicating that decreased iTreg cell formation is a key contributor to Treg cell deficiency in intes-tinal mucosa of Foxp3EGFPcre Myd88D/D mice(Figures1E–1G). This deficiency could not be attributed to diminution of intestinal Treg cell proliferation,as revealed by staining with the prolif-eration marker Ki-67or by labeling with5-bromodeoxyuridine (BrdU),nor to increased apoptosis as detected by AnnexinV staining(Figures S1E–S1G).Also,MyD88-deficient T cells retained the capacity to differentiate to iTreg cells in vitro in response to treatment with anti-CD3and CD28mAb and TGF-b(Figure S1H).Analysis of the conserved non-coding sequence2(CNS2)at the Foxp3locus,whose epigenetic deme-thylation is necessary for stable Foxp3expression,revealed decreased CNS2demethylation in Foxp3EGFPcre Myd88D/D Treg cells(Figures1H and1I).Together,these results indicate that MyD88signaling plays a critical role in mucosal iTreg cell induc-tion and epigenetic stability.Analysis of cLP Treg cells of Foxp3EGFPcre Myd88D/D mice un-covered several abnormalities.Flow cytometric and real-time PCR analysis revealed that MyD88-deficient cLP Treg cells ex-hibited increased expression of the transcription factor ROR g-t (Figures2A–2C).In contrast,expression of the transcription factor GATA-3,implicated in Treg cell stability,was decreased (Figures2D–2F;Wang et al.,2011;Wohlfert et al.,2011).IL-10 expression and Il10transcripts were decreased in MyD88-defi-cient as compared to MyD88-sufficient cLP Treg cells(Figures 2G–2I).In parallel with the contraction of the cLP Treg cell population in Foxp3EGFPcre Myd88D/D mice,there was expansion of IL-17-expressing cLP CD4+Tconv cells(Figures2J and2K).The frequency and numbers of cLP CD4+T cells expressing ROR g-t was also increased(Figures2L and2M).In contrast,interferon-g (IFN-g)secretion(Figures2J and2K)and expression of the T helper1(Th1)cell master regulator T-bet by cLP CD4+Tconv cells was unchanged(Figure2N).Similarly,the frequency of IL-22-expressing cLP CD4+Tconv cells was unchanged,as was the expression of Il22transcripts in colonic tissues(data not shown).Treg-Cell-Specific MyD88Deficiency Aggravates the Severity of Experimental ColitisTo determine the functional significance of MyD88deficiency in Treg cells,we examined the outcome of dextran sodium sulfate(DSS)-induced colitis in Foxp3EGFPcre Myd88D/D mice as compared to control littermate Myd88fl/flmice.Mice were exposed to DSS at3.5%–5%in drinking water for5days,then placed on regular water and followed up for another week. Treg-cell-specific MyD88deficiency resulted in aggravated DSS-induced weight loss and mortality associated with increased mucositis as revealed by colon length and histolog-ical scoring(Figures3A–3C).This phenotype was rescued by treatment with MyD88-sufficient but not-deficient Treg cells, consistent with impaired function of the latter as the underlying mechanism of disease severity.MyD88deficiency was associ-ated with decreased IL-10production by Treg cells and increased IL-17but not IFN-g production by CD4+Tconv cells (Figures3D–3I).To analyze the role of dysregulated IL-17production in disease severity,we examined the impact of treatment with a290Immunity43,289–303,August18,2015ª2015Elsevier Inc.Figure1.Treg-Cell-Specific MyD88Deletion Results in Gut Mucosa Treg Cell Deficiency(A)Flow cytometric analysis of CD4+Foxp3+Treg cells in the cLP of Myd88fl/fland Foxp3EGFPcre Myd88D/D mice.(B)Frequencies and absolute numbers of Treg cells in different tissues of Myd88fl/fland Foxp3EGFPcre Myd88D/D mice.(C and D)Flow cytometric analysis(C)and meanfluorescence intensity(MFI)(D)of CD25,CTLA-4,Helios,Nrp1,CD103,and Foxp3cLP Treg cells of Myd88fl/fland Foxp3EGFPcre Myd88D/D mice.(E)Flow cytometric analysis of Foxp3expression in cLP CD45.2+CD4+T cells in CD45.1+WT mice that received naive CD45.2+CD4+Foxp3–T cells from OT-II+ Foxp3EGFPcre or OT-II+Foxp3EGFPcre Myd88D/D mice and were then fed OVA in the drinking water for1week.(F)Frequencies(left)and absolute numbers(right)of donor CD45.2+CD4+EGFP+iTreg cells in the cLP of recipient mice from(E).(G)Absolute numbers of donor CD45.2+CD4+Foxp3EGFP+iTreg cells in the MLN of recipient mice.(H)Methylation status of individual CpG motifs within the Foxp3CNS2in cLP Treg cells of Foxp3EGFPcre and Foxp3EGFPcre Myd88D/D mice.Individual CpG motifs are numbered with reference to the transcription initiation site of Foxp3.(I)Global methylation status of Foxp3CNS2in splenic and cLP Treg cells shown in(H).Results are representative of four independent experiments;n=3–8mice/group.*p<0.05;**p<0.01;***p<0.001by Student’s unpaired two-tailed t test and one-way ANOVA.Error bars represent SEM.See also Figure S1.Immunity43,289–303,August18,2015ª2015Elsevier Inc.291Figure2.Treg-Cell-Specific MyD88Deletion Results in Colonic Th17Cell Skewing(A and B)Flow cytometric analysis(A)and enumerated cell frequencies and numbers(B)of cLP ROR g-t+CD4+Foxp3+Treg cells in Myd88fl/fland Foxp3EGFPcre Myd88D/D mice.(C)Real-time PCR analysis of Rorc expression in cell-sorted cLP CD4+Foxp3+Treg cells.(legend continued on next page)292Immunity43,289–303,August18,2015ª2015Elsevier Inc.neutralizing IL-17antibody on disease pathogenesis.Results revealed that the anti-IL-17antibody treatment rescued disease severity in Foxp3EGFPcre Myd88D/D but not Myd88fl/flcontrol mice (Figures3J–3M).This rescue was associated with decreased frequency and cell number of Th17but not Th1cells in Foxp3EGFPcre Myd88D/D mice(Figure3M).Defective function of MyD88-deficient Treg cells in suppress-ing inflammation was further verified in a second lymphopenia-induced model of colitis.MyD88-deficient Treg cells were ineffective in controlling the colitic response as evidenced by the weight loss,pathological response,and dysregulated Th17 and Th1effector cell responses(Figures S2A–S2E).Importantly, MyD88-deficient donor Treg cells were prone to lose Foxp3 expression and become pathogenic ex-Treg cells that secreted IFN-g and IL-17,indicative of increased instability(Figures S2F and S2G).Treg-Cell-Specific MyD88Deficiency Impairs Intestinal IgA Production and Increases Commensal Bacterial Translocation to Deep TissuesAs noted earlier,the defect in mucosal Treg cell populations extended to the PP(Figure1B).PP Tfr cells have previously been shown to promote luminal IgA production either directly and/or through differentiation into Tfh cells(Cong et al.,2009; Kawamoto et al.,2014;Tsuji et al.,2009).As compared to WT Tfh cells,those of Foxp3EGFPcre Myd88D/D mice were decreased in frequencies and numbers and exhibited decreased expres-sion of the cytokine IL-21,important for B cell germinal center response(Figures4A and4B).Tfr cells were more severely compromised(Figures4C)and they exhibited decreased expression of canonical Tfr cell markers including BCL-6, ICOS,and CD40L(Figures S3A and S3B).In contrast,both cell populations were not altered in the mesenteric lymph nodes (data not shown).We examined Foxp3EGFPcre Myd88D/D and con-trol Myd88fl/fllittermate mice for their frequencies of IgA-positive B cells in the PP.Results revealed decreased frequencies of switched IgA+B220+B cells and IgA+B220–B cells(the latter composed mostly of CD138+plasma cells)in the PP of Foxp3EGFPcre Myd88D/D mice(Figure4D and data not shown). The intestinal luminal,but not serum,IgA concentrations were decreased also in Foxp3EGFPcre Myd88D/D as compared to Myd88fl/flmice,and it remained so in the context of mice with DSS-induced colitis(Figure4E).In contrast,serum and intestinal luminal concentrations of IgG,and of IgG1and IgG2a sub-classes,were similar in the two mouse strains(Figure4E). Antibiotic treatment of WT mice reproduced the effects of Treg-cell-specific MyD88deficiency in terms of decreased Tfh and Tfr cell frequencies in the PP and depressed luminal but not serum IgA concentrations,indicating that the adverse effects of MyD88deficiency on gut IgA responses resulted from the interruption of microbial sensing by Treg cells(Figures S3C–S3E).We further investigated the impact of MyD88Treg cell deficiency on the differentiation of antigen-specific Tfr and Tfh cells in the PP by means of adoptive transfer experiments.First, we examined the capacity of sorted splenic Foxp3EGFPcre and Foxp3EGFPcre Myd88D/D Treg cells transferred into Tcra–/–mice to differentiate into Tfr and Tfh cells in the PP and support luminal IgA production.Results showed that whereas the total numbers of T cells in the PP were similar in the two transfer groups (data not shown),the frequencies and absolute numbers of Foxp3EGFPcre Myd88D/D-derived PP Tfh cells were decreased as compared to Foxp3EGFPcre-derived cells(Figure4F).In particular, Foxp3EGFPcre Myd88D/D Tfr cells were profoundly decreased both in frequencies and cell numbers as compared to those derived from Foxp3EGFPcre cells(Figure4G).In contrast,the fre-quencies and numbers of Foxp3EGFPcre Myd88D/D Treg cells in the spleens of recipient mice were similar to those of trans-ferred Foxp3EGFPcre Treg cells(data not shown).The percentage of IgA+B220+B cells and the concentrations of luminal IgA were all markedly decreased in Tcra–/–mice receiving Foxp3EGFPcre Myd88D/D as compared to Foxp3EGFPcre Treg cells. In contrast,luminal IgG1concentrations were similar between the two groups(Figures4H and4I).To rule out a role for changes in Treg cell TCR repertoire induced by MyD88deficiency in the observed results,we car-ried out transfer studies with OT-II+TCR transgenic cells,spe-cific for OVA323-339peptide.Naive CD4+Foxp3–T cells isolated from OT-II+Foxp3EGFPcre or OT-II+Foxp3EGFPcre Myd88D/D mice were transferred into Tcra–/–mice.The recipients were fed OVA in the drinking water for1week,then examined for the differentiation of the donor T cells in the PP into Tfr and Tfh cells.Results revealed that there was no difference in the frequency of donor cells in the spleens of recipient mice (data not shown).In the PP,the frequencies of OT-II+Tfh and Tfr cells were markedly reduced in Tcra–/–recipients of naive CD4+T cells from Foxp3EGFPcre Myd88D/D as compared to Foxp3EGFPcre mice(Figures S4A and S4B).The frequencies of PP IgA+B220+B cells,concentrations of luminal IgA,and titers of luminal OVA-specific IgA antibodies were similarly reduced(Figures S4C and S4D).The reduced capacity of naive OT-II+CD4+T cells of OT-II+Foxp3EGFPcre Myd88D/D mice to support Tfh and Tfr cell differentiation in PP,luminal IgA production,and luminal OVA-specific IgA responses was reproduced when OT-II+Foxp3EGFPcre Myd88D/D Treg cells were isolated and transferred into Tcra–/–mice(Figures S4E–S4H).These results indicate that the antigen-driven differenti-ation of Tfr and Tfh cells in the PP from both naive CD4+T cell precursors and preformed Treg cells was dependent on Treg-cell-intrinsic MyD88signaling.(D–I)Analysis of GATA3expression and Gata3mRNA(D–F)and IL-10expression and Il10mRNA(G–I)in cLP CD4+Foxp3+Treg cells of the respective mouse strain,performed as in(A)–(C).(J and K)Expression of IL-17and IFN-g in cLP CD4+Foxp3–T cells of Myd88fl/fland Foxp3EGFPcre Myd88D/D mice.Flow cytometric analysis(J)and frequencies and absolute numbers(K)of cytokine-expressing cells.(L and M)Flow cytometric analysis(L)and frequencies and absolute numbers(M)of cLP CD4+Foxp3–ROR g-t+T cells of the respective mouse strain.(N)Frequencies(left)and total cell number(right)of cLP CD4+Foxp3ÀT-bet+of Foxp3EGFPcre Myd88D/D mice compared with Myd88fl/flcontrol mice.Results are representative of four independent experiments;n=4–7mice/group.*p<0.05;**p<0.01;***p<0.001by Student’s unpaired two-tailed t test.Error bars represent SEM.Immunity43,289–303,August18,2015ª2015Elsevier Inc.293Intestinal IgA production controls pathobionts and main-tains commensalism (Cong et al.,2009;Fagarasan et al.,2010;Peterson et al.,2007).Significantly,analysis revealed decreased IgA bound to bacteria in fecal pellets of Foxp3EGFPcre Myd88D /D as compared to Myd88fl/flmice,indic-ative of decreased IgA anti-bacterial responses (Figure 5A).The functional implications of luminal IgA deficiency inFoxp3EGFPcre Myd88D /D mice were further examined with a non-pathogenic E.coli (DH5a strain)that expressed a chicken ovalbumin (OVA)protein fragment (amino acids 139–386).Oral gavage with OVA-DH5a E.coli revealed profoundly decreased luminal OVA-specific IgA,but not IgG1,consistent with impaired bacteria-specific IgA antibody responses (Figure 5B).Figure 3.Treg-Cell-Specific MyD88Deficiency Aggravates the Severity of Experimental Colitis(A)Survival curves of the following mouse groups treated with 5%DSS for 5days then followed thereafter:Myd88fl/fl,Foxp3EGFPcre Myd88D /D ,and Foxp3EGFPcre Myd88D /D mice that were given cell-sorted CD4+EGFP +splenic Treg cells from either Foxp3EGFPcre (MyD88-sufficient)or Foxp3EGFPcre Myd88D /D (MyD88-deficient)mice.(B)Body weight loss after treatment of mouse groups as in (A)with 3.5%DSS for 5days.(C)Colon length and histological scores of the respective mouse groups in (B)at day 9after DSS treatment.(D–I)Flow cytometric analysis (D and G)and frequencies of total and IL-10+CD4+Foxp3+Treg cells (D–F)and IFN-g -and IL-17-secreting CD4+Foxp3–Tconv cells (G–I)isolated from the cLP of the respective mouse groups in (B)and (C)at day 9after DSS treatment.(J–M)Groups of Foxp3EGFPcre and Foxp3EGFPcre Myd88D /D mice were subjected to DSS-induced colitis and treated with either isotype control or neutralizing anti-IL-17A mAbs at days 0,2,and 4of DSS treatment.(J)Colon histology (hematoxylin and eosin staining;403magnification;scale bar represents 100m m).(K and L)Body weight loss (K)and colon length and clinical score (L)of the respective mouse groups.(M)Frequencies and absolute numbers of IL-17-and IFN-g -secreting cLP CD4+Foxp3–Tconv cells of the respective mouse groups.Results are representative of three independent experiments.n =9–10mice/group for (A);**p <0.01by log rank test.n =4–7mice/group for (B)–(M);*p <0.05;**p <0.01;***p <0.001by one-way ANOVA.Error bars represent SEM.See also Figure S2.294Immunity 43,289–303,August 18,2015ª2015Elsevier Inc.To determine whether impaired gut anti-microbial IgA re-sponses were associated with altered small intestinal microbiota communities,we performed direct highly parallel pyrosequenc-ing of 16S ribosomal DNA (rDNA)amplicons derived from illeal tissues of Foxp3EGFPcre Myd88D /D and Myd88fl/flmice (see Sup-plemental Experimental Procedures section for description of the sequencing methods and bioinformatic analysis).Analysis of microbiota diversity via the Shanon measure revealed no dif-ferences between Myd88fl/fland Foxp3EGFPcre Myd88D /D mice (data not shown ).However,Unifrac analysis showed significant differences in the microbial community structure between Myd88fl/fland Foxp3EGFPcre Myd88D /D mice (Figure 5C).Statisti-cal testing at the level of bacterial phyla revealed increased rela-tive abundance of Firmicutes and decreases in Bacteroidetes and Tenericutes phyla in Foxp3EGFPcre Myd88D /D as compared to Myd88fl/flmice (Figure 5D).Analysis of taxa that discriminated between the two groups of mice identified 6taxa at the genus level,encompassing 16operational taxonomic units (OTUs)(Tables S1and S2).The most abundant of the latter,OTU0006,was detected in Foxp3EGFPcre Myd88D /D but not Myd88fl/flsam-ples (Figure 5E and Table S1).It was identified by phylogenetic placement and sequence alignment as SFB,consistent with the role of IgA in immunity to SFB (Figure S5;Gaboriau-Routhiauet al.,2009;Ivanov et al.,2009;Le´cuyer et al.,2014;Suzuki etal.,Figure 4.Treg-Cell-Specific MyD88Deficiency Impairs PP Tfr and Tfh Cell Differentiation and Intestinal IgA Production(A–D)Flow cytometric analysis,frequencies,and absolute numbers of Tfh cells (CD4+CXCR5+PD-1hi )(A),CXCR5+IL-21+cells within the Tfh cell populations (B),Tfr cells (CD4+Foxp3+CXCR5+PD-1hi )(C),and IgA +B220+B cells (D)in PP of Foxp3EGFPcre Myd88D /D and Myd88fl/flmice.(E)Serum and intestinal lavage fluid concentrations of IgA,IgG,IgG1,and IgG2a in Foxp3EGFPcre Myd88D /D and Myd88fl/flmice.(F–H)Flow cytometric analysis,and frequencies and absolute numbers of Tfh (F),Tfr (G),and IgA +B220+(H)cells isolated from the PP of Tcra –/–mice 2weeks after the transfer of CD4+EGFP +Treg cells isolated from either Foxp3EGFPcre or Foxp3EGFPcre Myd88D /D mice.(I)Luminal concentration of IgA and IgG1in the mouse groups from (H).Results are representative of three independent experiments.n =3–5mice/group;*p <0.05;**p <0.01by Student’s unpaired two-tailed t test.Error bars represent SEM.See also Figures S3and S4.Immunity 43,289–303,August 18,2015ª2015Elsevier Inc.2952004).Magnetic cell sorting of IgA-bound fecal bacteria followed by real-time PCR analysis revealed that SFB sharply segregated into the IgA +fraction in Myd88fl/flmice,in agreement with recent studies (Palm et al.,2014).In contrast,it was increased in both the IgA +and IgA –fractions in Foxp3EGFPcre Myd88D /D mice (Figure 5F).These studies suggest that the SFB dysbiosis in Foxp3EGFPcre Myd88D /D mice was related to the defective anti-bacterial IgA response.Dysbiosis in Foxp3EGFPcre Myd88D /D mice was associated with increased bacterial translocation into deep tissues.Oral gavage of OVA-DH5a E.coli resulted in its increased recovery in the fecal pellets and livers of Foxp3EGFPcre Myd88D /D mice as compared to Myd88fl/flcontrols (Figure 5G).More broadly,the livers and lungs of Foxp3EGFPcre Myd88D /D had increased bacterial loads as compared to those of Myd88fl/flmice,with increased recovery of Lactobacilli and Pasteurella species in both organs (Figure 5H).Figure 5.Treg-Cell-Specific MyD88Deficiency Promotes Dysbiosis and Bacterial Translocation to Deep Tissues(A)Flow cytometric analysis and frequencies of IgA-and IgG1-coated bacteria in the fecal pellets of Rag1–/–,Foxp3EGFPcre Myd88D /D ,and Myd88fl/flmice.(B)OVA-specific luminal IgA and IgG1concentrations in mice (A)gavaged with OVA-DH5a bacteria.(C)Weighted Unifrac plot of ileal mucosa-associated microbial communities,visualized with principal coordinate analysis (PCoA),in Foxp3EGFPcre Myd88D /D versus Myd88fl/flmice (p value =0.001by AMOVA).(D)Relative abundance of different microbial phyla in ileal mucosa-associated microbiota in Foxp3EGFPcre Myd88D /D and Myd88fl/flmice (*false discovery rate <0.05).(E)Relative abundance of SFB taxa in illeal mucosa-associated microbiota in Foxp3EGFPcre Myd88D /D and Myd88fl/flmice.(F)Real-time PCR analysis of SFB 16S rRNA in IgA +and IgA –fecal bacterial fractions of Myd88fl/fland Foxp3EGFPcre Myd88D /D mice.(G)Stool (left)and liver (right)OVA-DH5a bacterial load [expressed as log colony forming units (lg CFU)/g]in Foxp3EGFPcre Myd88D /D and Myd88fl/flmice treated with the OVA-DH5a bacteria by oral gavage.(H)Loads of total bacteria,Lactobacilli ,and Pasteurella (all expressed as CFU/g tissue)in the lung and liver of Myd88fl/fland Foxp3EGFPcre Myd88D /D mice.Results are representative of three independent experiments;n =5mice/group.*p <0.05;**p <0.01by Student’s unpaired two-tailed t test.Error bars represent SEM.See also Figure S5and Tables S1and S2.296Immunity 43,289–303,August 18,2015ª2015Elsevier Inc.。
内质网应激通过AKT-TSC-mTOR信号通路调控自噬

f.acto璐,which could also be partly restored by 4一PBA.CoIlSidering the recent repon that
s眦y constitutiVe actiVation of mTOR triggers ER s仃ess,our
may help propose a
内质网应激导致II也l寡聚化及自身磷酸化,并激活其RNA内切酶活性。II迮1 可切割X盒结合蛋白l(XBPl)的mRNA,使其成为成熟的mRNA。XBPl的成熟 IIl】[ⅢA编码碱性含有亮氨酸锌指结构的转录因子,它能增强分子伴侣蛋白BiP等的 转录活性。分子伴侣蛋白表达的上调可促进内质网功能恢复,帮助新生蛋白的正确 折叠包装。PERK属于真核细胞蛋白质翻译起始复合体蛋白激酶家族成员,是位于 内质网的I型膜蛋白。其N端可感受内质网应激的信号,而C端有丝/苏氨酸蛋白 激酶功能域。PERK活化后能够特异性地磷酸化真核翻译起始因子2a(eIF2a)第 5l位丝氨酸。eIF2a被磷酸化后则失去起始蛋白质翻译的活性,进而使得胞内蛋白 合成的整体水平被下调。ATF6是II型膜蛋白,其C端位于内质网腔内。活化的ATF6 的N端切割段可转移到细胞核内促进转录因子XBPl等基因的转录。
finally result in the restoration of the homeostasis.However,ER s仃ess that can not be
rescued results in autophagy锄d ceU deatll,while the precise mechaIlism w硒largely
ummown.Here we demonStrated that ER stress—induced cell death w2Ls mediated by
checkpoint vsx基本配置

3. Once VSX is installed the “show app vap-group” will show the status as Not Running as VSX has not been configured yet, so it is normal. bell2# show application vap-group VAP_Group = vsx Application = VSX vsx_1 vsx_2 Operational State = NOT RUNNING Operational State = NOT RUNNING Version = NGX Admin State = ENABLED
VS (VirtSys)
Management 192.168.72.180-181
VR (EVR)
interface fastethernet 1/1 logical management circuit management interface fastethernet 1/2 logical cust circuit cust interface fastethernet 1/3 logical evr circuit evr 2. Install VSX, during installation you will be prompted for management interface name and IPs, provide those. Welcome to the Check Point VPN-1 VSX NGX Configuration Program for the X Series platforms. ========================================================================= This program will allow you to install VPN-1 VSX NGX Enforcement Module on X Series platforms Checking available options. Please wait... Configuring VAP Group "vsx" with VAPs: 1 2
Akt信号转导通路总结

<The actions of Akt in the cell are numerous and diverse, but all result in anti-apoptosis, or pro-cell proliferation effects. These physiological roles of Akt include involvement in metabolism, protein synthesis, apoptosis pathways, transcription factor regulation and the cell cycle. Akt exerts its effects in the cell by phosphorylating a variety of downstream substrates. The downstream targets of Akt include BAD (BCL2 Antagonist of Cell Death), Caspase9, FKHR (Forkhead Transcriptional Factor), GLUTs (Glucose Transporters), eNOS (Nitric Oxide Synthase), PFK2(6-Phosphofructo-2-Kinase), PFK1(6-Phosphofructo-Kinase), mTOR (Mammalian Target of Rapamycin), IKK (I-KappaB Kinase), NF-KappaB (NuclearFactor-KappaB), GSK3 (Glycogen Synthase Kinase-3), WNK1(WNK Lysine deficient Protein Kinase-1), PRAS40 (Proline-Rich Akt Substrate 40 kDa), p47Phox, YAP (Yes-Associated Protein-1), Htt (Huntingtin), Ataxin, AR (Androgen Receptor), ASK1 (Apoptosis Signal-Regulating Kinase-1), MDM2 (Mouse Double Minute-2), CREB (cAMP Response Element-Binding Protein),p21CIP1 (Cyclin Dependent Kinase Inhibitor-p21), p27KIP1 (Cyclin Dependent Kinase Inhibitor-p27) , Chk1 (Cell Cycle Checkpoint Kinase-1), XIAP(X-Linked Inhibitor of Apoptosis Protein), Raf1 (v-Raf1 Murine Leukemia Viral Oncogene Homolog-1), PDE3B (Phosphodiesterase 3B cGMP-Inhibited), TSC(Tuberous Sclerosis Gene) and GABA(A)R (Gamma-Aminobutyric Acid Receptor-A) (Ref.4).Akt inhibits apoptosis by phosphorylating the BAD component of the BAD/BclXL (Bcl2 Related Protein Long Isoform) complex. Phosphorylated BAD binds to 14-3-3 causing dissociation of the BAD/BclXL complex and allowing cell survival. Akt activates IKK, which ultimately leads to NF-KappaB activation and cell survival. Other direct targets of Akt are members of the FKHRL1 (Forkhead-Related Family of Mammalian Transcription Factor-1). In the presence of survival factors, Akt1 phosphorylates FKHRL1, leading to the association of FKHRL1 with 14-3-3 proteins and its retention in the cytoplasm. Survival factor withdrawal leads to FKHRL1 dephosphorylation, nuclear translocation, and target gene activation. Within the nucleus, FKHRL1 most likely triggers apoptosis by inducing the expression of genes that are critical for cell death, such as the TNFSF6 (Tumor Necrosis Factor Ligand Superfamily Member-6) gene. Another notable substrate of Akt is the death protease Caspase9. Phosphorylation of Caspase9 decreases apoptosis by directly inhibiting the protease activity. Akt also activates TERT (Telomere Reverse Transcriptase), which is responsible for telomere maintenance and DNA stability. Akt has been linked to angiogenesis, through the activation of eNOS, which influences long-term blood vessel growth. Akt can regulate several levels of Glucose metabolism. It enhances Glucose-uptake in Insulin-responsive tissues by inducing the expression of GLUT1 and GLUT3 andthe translocation of GLUT4 to the plasma membrane; the GLUTs transport glucose into the cell. Akt also activates Glycogen synthesis by phosphorylating and inactivating GSK3, which leads to the activation of Glycogen Synthase and CyclinD1. Akt phosphorylates PDE3B on Ser273. This activates PDE3B and results in regulation of intracellular levels of cyclic nucleotides in response to Insulin. Akt induces glycolysis through the phosphorylation and activation PFK2, which in turn activates PFK1. These enzymes convert Fructose-6-Phosphate into Fructose-1, 6-Bisphosphate, a key step in Glucose metabolism. Akt may also be involved in activation of the nutrient-dependent Thr/Ser kinase, mTOR. Activation of mTOR results in the phosphorylation of ribosomal protein S6 kinase, p70S6K. Akt also phosphorylates the two tumor suppressor genes TSC1 and TSC2, which are negative regulators of the mTOR-S6K pathway. Phosphorylation of TSC1 and TSC2 results in suppression of their inhibitory activity and may also target the proteins for degradation. Activation of mTOR also results in phosphorylation and inactivation of eIF4EBP (Eukaryotic Initiation Factor-4E Binding Protein), an inhibitor of the translation initiation factor eIF4E. Nonphosphorylated PHASI binds to eIF4E (Eukaryotic Initiation Factor-4E) and inhibits protein synthesis. Akt also phosphorylates GAB2 (GRB2-Associated Binding Protein-2) on Ser159. Phosphorylation of Ser159 on Gab2 by Akt/PKB appears to negatively regulate GAB2 tyrosine phosphorylation by the ErbBreceptor tyrosine kinases, although the underlying mechanism has not been solved (Ref.5 & 6).The transcription factor CREB is directly phosphorylated at Ser133 by Akt. This causes an increased affinity of CREB for its co-activator protein, CRB (Crumbs). The heterodimer, now an active transcription factor, promotes transcription of genes that contain CREs (cAMP responsive elements) in their promoter, such as the anti-apoptotic genes Bcl2 and Mcl1. Akt also phosphorylates AR at two serine residues, Ser210 and Ser270, which causes a decrease in AR activity on the p21 promoter. In addition to causing cell cycle progression, this also results in apoptosis inhibition in certain cell types, through other actions of AR. YAP is another transcription factor that is phosphorylated by Akt, and is of importance because it does not contain an Akt consensus sequence. Akt phosphorylates Ser127 on YAP, which causes association with 14-3-3 proteins, nuclear export and cytoplasmic localization. Akt has also been shown to phosphorylate p21 directly, on Thr145. p21 is a member of the Cip/Kip family of CDK inhibitors that arrest the cell cycle and therefore limit cell proliferation. p21 can also promote cell cycle progression, via mediating the assembly and activity of cyclin D1-CDK4/6 complexes. P27 is another cyclin-dependent kinase inhibitor, of the Kip family. P27 inhibits CDK2 and CDK4/6 complexes, which is located in the nuclear localization signal. NLS targets protein to nucleus via nuclear import machinery, and phosphorylation in this region of p27 results innuclear exclusion. 14-3-3 proteins bind phosphorylated p27 and cause active export from nucleus. Without p27 in the nucleus, the cyclin-CDK complexes form and promote cell cycle progression. Akt also phosphorylates MDM2. MDM2 is phosphorylated at many sites, only two of which have been identified. Ser166 is phosphorylated by Akt. Akt phosphorylation of MDM2 allows its entry into the nucleus where it targets p53 for degradation (Ref.7, 8, 9 & 10). PRAS40 is a 40 kDa substrate of AKT. Activated AKT phosphorylates PRAS40 on threonine 246, enabling PRAS40 to bind to 14-3-3. AKT and PRAS40 are components of the PI3K pathway. This pathway plays a role in glucose uptake, cell growth, and apoptosis inhibition. The precise function of PRAS40 is not yet known; however, it has been hypothesized that PRAS40 interacts with SH3 and WW domain containing proteins, and may change the function of these proteins. Akt phosphorylates, both in vitro and in vivo, the GABA(A)R, the principal receptor mediating fast inhibitory synaptic transmission in the mammalian brain. Akt-mediated phosphorylation increases the number of GABA(A)Rs on the plasma membrane surface, thereby increasing thereceptor-mediated synaptic transmission in neurons. XIAP is a physiological substrate of Akt. Akt interacts with and phosphorylates XIAP at serine 87. Phosphorylation of XIAP by Akt inhibits both its autoubiquitination and cisplatin-induced ubiquitination. These effects reduce XIAP degradation and the increased levels of XIAP are associated with decreasedcisplatin-stimulated Caspase3 activity and programmed cell death. Htt isalso a substrate of Akt and phosphorylation of Htt by Akt is crucial to mediate the neuroprotective effects of IGF1 (Insulin-Like Growth Factor-I). WNK1 is a physiologically relevant target of Insulin signaling through PI3K and Akt and functions as a negative regulator of Insulin-stimulated mitogenesis (Ref.11, 12 & 13). Akt also phosphorylates Ataxin1 and modulate neurodegeration.14-3-3 protein mediates the neurotoxicity of Ataxin1 by binding to and stabilizing Ataxin1, thereby slowing its normal degradation. Akt also decreases ASK1 kinase activity by phosphorylating a consensus Akt site at serine 83 of ASK1. Akt also interacts with the JIP1 (JNK Interacting Protein-1) scaffold and inhibits the ability of JIP1 to form active JNK signaling complexes. The binding of Akt to JIP1 is isoform specific; Akt1 but not Akt2 interacts with JIP1. Thus, Akt can inhibit one or more steps within the JNK signaling pathway, depending on the complement of components that form the functional JNK signaling module. Akt mediates PI3K-dependent p47Phox phosphorylation, which contributes to respiratory burst activity in human neutrophils. AKT impair Chk1 through phosphorylation, ubiquitination, and reduced nuclear localization to promote genomic instability in tumor cells. Akt and its upstream regulators are deregulated in a wide range of solid tumors and hematologic malignancies, hence the Akt pathway is considered a key determinant of biologic aggressiveness of these tumors, and a major potential target for novel anti-cancer therapies (Ref.14 & 15).?。
AZD7762 860352-01-8 GlpBio

Peptides, Inhibitors, AgonistsProduct Data SheetProduct Name: AZD7762Cat. No.:GC10546Chemical Name: 3-(carbamoylamino)-5-(3-fluorophenyl)-N-[(3S)-piperidin-3-yl]thiophene-2-carboxamideCHEMICAL PROPERTIESCas No.: 860352-01-8Molecular Formula: C17H19FN4O2SMolecular Weight: 362.42Storage: PowderSolubility: >18.1mg/mL in DMSOChemical Structure:BackgroundAZD7762 is a novel ATP competitive inhibitor of checkpoint kinases. Chk family checkpoint kinases include Chk1 and Chk2. They are activated in response to DNA damage and phosphorylate CDC25A, CDC25C protein phosphatases, which delay cell cycle progression. Therefore, Chk activation initiates cell cycle checkpoint, causes cell cycle arrest, and allows DNA repair.AZD7762 is a potent selective inhibitor of Chk1. It binds to the ATP binding pocket and compete ATP binding in a reversible manner. AZD7762 inhibits Chk1 phosphorylation of CDC25C peptide with an IC50 of 5 nM. The Ki is 3.6 nM. It is equally potent against Chk2 but less potent against CAM, Yes, Fyn, Lyn, Hck and Lck. [1]AZD7762 prevents cell cycle arrest and DNA repair in DNA damaged tumor cells, causing tumor cell apoptosis. Hence, it potentiates the antitumor activity of DNA damaging agents and can be used as a chemosensitizing agent. [2]Half life of AZD7762 is 1-2 hours in mice [3]References:[1]Polanski, R. ,Hodgkinson, C.L.,Fusi, A., et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin.Cancer.Res 20(4), (2014).[2]https://clinicaltrials. gov/ct2/show/NCT01791595 term=AZD+3965&rank=1Research Update1. Effect of Hydrofluoric Acid Concentration and Etching Time on Bond Strength to Lithium Disilicate Glass Ceramic. Oper Dent. 2017 Nov/Dec;42(6):606-615. doi: 10.2341/16-215-L. Epub 2017 Jul 14. PMID:28708007AbstractThe aim of this study was to evaluate the influence of different concentrations of hydrofluoric acid (HF) associated with varied etching times on the microshear bond strength (μSBS) of a resin cement to a lithium disilicate glass ceramic. Two hundred seventy-five ceramic blocks (IPS e.max Press [EMX], Iv oclar Vivadent), measuring 8 mm × 3 mm thickness, were randomly distributed into five groups according to the HF concentrations (n=50): 1%, 2.5%, 5%, 7.5%, and 10%.2. Does acid etching morphologically and chemically affect lithium disilicate glass ceramic surfaces? J Appl Biomater Funct Mater. 2017 Jan 26;15(1):e93-e100. doi: 10.5301/jabfm.5000303. PMID:27647389AbstractBACKGROUND: This study evaluated the surface morphology, chemical composition and adhesiveness of lithium disilicate glass ceramic after acid etching with hydrofluoric acid or phosphoric acid.METHODS: Lithium disilicate glass ceramic specimens polished by 600-grit silicon carbide paper were subjected to one or a combination of these surface treatments: airborne particle abrasion with 50-μm alumina (AA), etching with 5% hydrofluoric acid (HF) or 36% phosphoric acid (Phos), and application of silane coupling agent (Si).3. Fatigue failure load of feldspathic ceramic crowns after hydrofluoric acid etching at different concentrations. J Prosthet Dent. 2018 Feb;119(2):278-285. doi: 10.1016/j.prosdent.2017.03.021. Epub 2017 May 26. PMID:28552291AbstractSTATEMENT OF PROBLEM: Hydrofluoric acid etching modifies the cementation surface of ceramic restorations, which is the same surface where failure is initiated. Information regarding the influence of hydrofluoric acid etching on the cyclic loads to failure of ceramic crowns is lacking.PURPOSE: The purpose of this in vitro study was to evaluate the influence of different hydrofluoric acid concentrations on the fatigue failure loads of feldspathic ceramic crowns.。
急性淋巴细胞白血病的分子诊断和治疗研究
急性淋巴细胞白血病的分子诊断和治疗研究Acute Lymphoblastic Leukemia Research from Molecular Diagnosis to Treatment OptionsAbstract:Acute lymphoblastic leukemia (ALL) is the most common hematological malignancy in children and remains a significant cause of morbidity and mortality in adults. The molecular diagnosis of ALL provides a precise understanding of the heterogeneous nature of the disease and helps to identify high-risk patients who require intensive treatment. Several molecular abnormalities have been identified, including mutations in leukemia-associated genes, chromosomal translocations, and gene fusions. These molecular alterations provide opportunities for the development of targeted therapies that can improve patients' outcomes. This review provides an overview of molecular diagnostic techniques and emerging targeted therapies that have shown promising results in the treatment of ALL.Keywords: Acute lymphoblastic leukemia, Molecular diagnosis, Targeted therapy, Chromosomal translocations, Gene fusions IntroductionAcute lymphoblastic leukemia (ALL) is a hematological malignancy characterized by the proliferation of immature lymphoid cells in the bone marrow, leading to the infiltration of other organs. ALL is the most common cancer in children, accounting for approximately 30% of all childhood malignancies worldwide. In adults, ALL is a rare disease accounting for only 20% of all leukemias. However, it remains a significant cause ofmorbidity and mortality, particularly in patients with relapsed or refractory disease. The prognosis of ALL is influenced by several factors, including age, initial response to therapy, and the presence of specific genetic abnormalities. The development of molecular diagnostic techniques has led to a better understanding of the molecular heterogeneity of ALL and has paved the way for the development of targeted therapies.Molecular diagnosis of ALLThe diagnosis of ALL is based on the presence of blast cells in the bone marrow or peripheral blood, along with clinical manifestations. Molecular diagnosis techniques have been developed to provide a better understanding of the molecular basis of the disease and to identify high-risk patients who require aggressive treatment. These techniques include cytogenetic analysis, fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), and next-generation sequencing (NGS). Cytogenetic analysis is a classical technique that involves the visualization of chromosomal abnormalities using banding techniques. Chromosomal translocations involving the immunoglobulin and T-cell receptor genes are common in ALL and are associated with specific subtypes of the disease. For example, the t(9;22) Philadelphia chromosome is present in approximately 25% of adult ALL cases and is associated with a poor prognosis. FISH is a more sensitive technique that allows the detection of chromosomal translocations, gene fusions, and copy number variations using fluorescent probes. PCR is a highly sensitive technique that amplifies specific DNA sequences and is widely used to detect fusion genes and minimal residual disease(MRD) in ALL patients. NGS is a next-generation sequencing technique that allows the simultaneous detection of multiple mutations and copy number variations in a single assay, providing a comprehensive genomic analysis of the disease.Targeted therapies in ALLThe identification of specific molecular abnormalities in ALL has led to the development of targeted therapies that can improve patients' outcomes. The success of targeted therapies in ALL depends on the identification of patient subgroups that are likely to benefit from these therapies, as well as the development of drugs that can target the specific molecular abnormalities.Chromosomal translocations involving the B-cell receptor (BCR) and the T-cell receptor (TCR) genes are common in ALL and result in the formation of fusion proteins that drive leukemogenesis. Several drugs have been developed to target these fusion proteins, including imatinib and dasatinib, which target the BCR-Abl fusion protein, and ponatinib, which targets the T315I mutation in the BCR-Abl fusion protein.Gene fusions involving the mixed-lineage leukemia (MLL) gene are common in infant ALL and are associated with a poor prognosis. Several drugs have been developed to target MLL fusion proteins, including DOT1L inhibitors and CDK9 inhibitors, which target the epigenetic regulation of gene expression. ConclusionMolecular diagnosis techniques have provided a better understanding of the heterogeneous nature of ALL and haveidentified specific molecular abnormalities that can be targeted by drugs. The use of targeted therapies in ALL is still in its early stages, and more research is needed to identify patient subgroups that are likely to benefit from these therapies, as well as the development of new drugs that can target specific molecular abnormalities. The success of targeted therapies in ALL depends on a better understanding of the disease's biology and the development of personalized treatment strategies.Targeted therapies have revolutionized the treatment of several cancers, including ALL. These therapies provide a more precise approach to cancer treatment by targeting specific molecular abnormalities that drive tumor growth and spread, while sparing normal tissue. Successful targeted therapies in ALL have led to improved outcomes for many patients, particularly those with high-risk disease.Imatinib and dasatinib are two drugs that target the BCR-Abl fusion protein associated with the t(9;22) translocation. These drugs have improved outcomes in patients with Philadelphia-positive ALL, leading to increased survival rates and reduced chemotherapy toxicity. Ponatinib is a third-generation BCR-Abl inhibitor that has shown promise in treating patients with the T315I mutation, which confers resistance to imatinib and dasatinib. Gene fusions involving the MLL gene are common in infant ALL and are associated with a poor prognosis. These fusions result in the recruitment of histone-modifying enzymes that alter gene expression and drive leukemogenesis. Drugs targeting epigenetic regulators, such as DOT1L and CDK9 inhibitors, represent a promising approach to treating MLL-rearranged ALL.Other targeted therapies under investigation in ALL include immune checkpoint inhibitors, chimeric antigen receptor (CAR) T-cell therapy, and antibody-drug conjugates. Immune checkpoint inhibitors, such as nivolumab and pembrolizumab, release the brakes on T-cell function, allowing them to attack cancer cells more effectively. CAR T-cell therapy involves extracting a patient's own T-cells and genetically modifying them to target specific cancer antigens before infusing them back into the patient. Antibody-drug conjugates combine a targeted antibody that binds to a cancer cell surface antigen with a toxic payload, resulting in targeted cell death. Blinatumomab, a bispecific T-cell engager antibody, targets the CD19 antigen on leukemia cells and redirects T-cells to attack them.Despite the success of targeted therapies in ALL, there are still challenges to overcome. Many targeted therapies require specific molecular abnormalities to be present in order to be effective, which limits their use to certain patient subgroups. Additionally, the development of resistance to targeted therapies can lead to treatment failure and disease progression. Therefore, ongoing research efforts are needed to identify new targets and develop strategies to overcome resistance.In conclusion, targeted therapies have shown promising results in the treatment of ALL by targeting specific molecular abnormalities that drive leukemogenesis. The development of personalized treatment strategies based on the molecular profile of the disease holds great potential for improving outcomes and reducing treatment-related toxicity. Ongoing research efforts are needed tooptimize and expand the use of targeted therapies in the management of ALL.Targeted therapies have revolutionized cancer treatment and become an essential component of the standard of care for several cancers, including ALL. These therapies provide a more precise approach to cancer treatment by targeting specific molecular abnormalities that drive tumor growth and spread, while sparing normal tissues. In ALL, targeted therapies have dramatically improved outcomes for high-risk patients, and ongoing research is identifying new molecular targets and developing strategies to overcome resistance.One of the most successful targeted therapies in ALL is tyrosine kinase inhibitors (TKIs) that target the BCR-Abl fusion protein associated with the t(9;22) translocation. Imatinib was the first TKI approved for the treatment of Philadelphia-positive ALL, leading to improved outcomes in these patients. However, imatinib resistance can develop, and dasatinib and ponatinib are second and third-generation TKIs with increased potency and activity against imatinib-resistant mutations.The development of resistance to targeted therapies is also a significant challenge, limiting their long-term effectiveness. One strategy to overcome resistance is to use combination therapies that target multiple pathways simultaneously. For example, the combination of a TKI with a CDK9 inhibitor has shown preclinical efficacy in MLL-rearranged ALL. Another approach is to develop new drugs that target molecular mechanisms leading to resistance, such as the Bruton's tyrosine kinase inhibitor acalabrutinib, which blocks the activation of the PI3K/AKT signaling pathway and has shown efficacy against TKI-resistant ALL cells in preclinicalmodels.Immune checkpoint inhibitors have shown promising results in several cancers, including melanoma and lung cancer, by restoring T-cell function and enhancing anti-tumor immunity. In ALL, early-phase clinical trials are ongoing to assess the safety and efficacy of immune checkpoint inhibitors, and preliminary results suggest potential benefits in relapsed/refractory disease.CAR T-cell therapy is a rapidly evolving immunotherapy that uses genetically engineered T-cells to target cancer cells, offering a personalized approach to cancer treatment. In ALL, CAR T-cell therapy targeting the CD19 antigen has shown high response rates, leading to FDA approval of tisagenlecleucel and axicabtagene ciloleucel for relapsed/refractory Philadelphia-negative ALL. Ongoing research is optimizing the manufacturing process and developing novel CAR constructs to improve efficacy and reduce toxicity.In conclusion, targeted therapies have revolutionized the treatment of ALL by improving outcomes and reducing toxicity. However, challenges remain, including the development of resistance, the need for specific molecular abnormalities, and toxicities associated with some targeted therapies. Ongoing research is needed to optimize and expand the use of targeted therapies by identifying new targets and developing strategies to overcome resistance, improve efficacy, and reduce toxicity. Ultimately, the goal is to develop personalized treatment strategies that maximize the benefits of targeted therapies for all patients with ALL.。
细胞衰 老与肿 瘤发 生
生命科学Chinese Bulletin of Life Sciences第20卷 第3期2008年6月Vol. 20, No.3Jun., 2008细胞衰老与肿瘤发生胡 兵*,安红梅,沈克平(上海中医药大学附属龙华医院,上海200032)摘 要:细胞衰老(cell senescence)是指细胞在信号转导作用下不可逆地脱离细胞周期并丧失增殖能力后进入的一种相对稳定的状态。
细胞衰老有增殖衰老与早熟衰老两种形式:增殖衰老由端粒缩短激发的信号转导激发,与TP53/CDKN1a (p21WAF-1/Cip1)/pRB/E2F 信号通路密切相关;早熟衰老由细胞内在或外在急慢性应激信号引发,与TP53/CDKN1a (p21WAF-1/Cip1)/pRB/E2F 或CDKN2a(p16ink4A ) /pRB/E2F 信号通路相关。
目前研究已经证实早熟衰老是细胞在癌变过程中的天然屏障,是继D NA 修复、细胞凋亡后的第三大细胞内在抗癌机制,在机体防止肿瘤形成中起重要作用。
关键词:细胞衰老;肿瘤发生;生殖衰老;早熟衰老中图分类号:Q255;R730.231 文献标识码:ACellular senescence and carcinogenesisHU Bing*, AN Hong-mei, SHEN Ke-ping(Longhua Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China)Abstract: Cellular senescence, irreversible cell cycle arrest and proliferation ability lost, reflects safeguard machinery that limits the proliferative capacity of the cell exposed to endogenous or exogenous stress signals.Two forms of cell senescence have been found in present, which is replicative senescence and premature senescence. Replicative senescence is initiated by the shortening of telomeres, and related to TP53/CDKN1a (p21WAF-1/Cip1)/pRB/E2F signal pathway. Premature senescence is initiated by endogenous or exogenous acute and chronic stress signals, and related to TP53/CDKN1a (p21WAF-1/Cip1)/pRB/E2F or CDKN2a(p16ink4A ) /pRB/E2F pathway. Premature cellular senescence may act in response to oncogenic activation as a natural barrier to interrupt tumorigenesis at a premalignant level. Cellular senescence is a third pathway of reaction of cells to DNA damage in addition to DNA repair/recovery and cell apoptosis from the damage.Key words: cell senescence; carcinogenesis; replicative senescence; premature senescence文章编号 :1004-0374(2008)03-0447-03收稿日期:2007-12-21;修回日期:2008-01-23基金项目:上海市自然科学基金(07Z R 14109)*通讯作者:E-mail: beearhu@20世纪60年代,Hayflick 在成纤维细胞的体外培养中发现,正常二倍体细胞在体外条件下增殖分裂50到70代即进入一种衰老的状态,无法进一步传代培养,但仍然存活,这种现象被称为“Hayflick 极限”(Hayflick ,1961),学术界称这种现象为细胞衰老(cell senescence)。
细胞增殖异常与疾病
Regulation of the cell cycle Regulation of the cell cycle by extracellular signals
by intracellular signals
1 、细胞周期自身调控机制
Key spot:Cyclin、 CDKs、 CKI、Checkpoint of cell cycle
• G2期:Cdc25(P80)
一个蛋白激酶结合在损 伤的DNA上,激活Chk1 (checkpoint kinase),
Chk1使Cdc25失活,使 其不能活化cdc2,导致 细胞周期中断。
CDC25为一种磷酸酶
Cdc2基因的产物为CDK1 , Cdc2为启动有丝分裂所必 须
MPF:mitosis promoting factor
特异性抑制 CDK4-CyclinD1、CDK6-CyclinD1复合物
▲Cip/Kip: (cyclin inhibition protein/kinase inhibition protein) p21Cipl、P27Kip1和P57Kip1 抑制 多数CDK活性
【抑制途径】
▲
CKIs通过直接结合CDK-cyclin复
2. 细胞周期监控机制受损
(Impairment of checkpoint system)
主要原因:G1/S、G2/M检查点异常 失察结果:探测DNA损伤功能降低
(如发现不了DNA损伤,会导致基因缺失、易位、染色体重排等)
■
G1/S交界处失察
进入S期
▲P53
DNA损伤
G1/S检查 细胞凋亡
▲P53突变或丢失
端粒及端粒酶的研究进展
综述文章编号:1009-0002(2007)04-0667-03端粒及端粒酶的研究进展杨平勋,黄君健军事医学科学院生物工程研究所,北京100850[摘要]端粒是真核细胞染色体末端的特有结构,是由端粒结合蛋白和一段重复序列的端粒DNA组成的一个高度精密的复合体,在维持染色体末端稳定性,避免染色体被核酸酶降解等方面起着重要的作用。
端粒的长度、结构及组织形式受多种端粒结合因子的调控。
由于端粒的重要性,在哺乳动物细胞里,端粒的长度或端粒结构变化与癌症发生及细胞衰老有密切的关系。
由于末端复制问题的存在,随着细胞分裂次数的增加,端粒不断缩短,细胞不可避免的走向衰老或凋亡。
由于在细胞分裂过程中端粒长度的不断缩短与细胞分裂代数增加具有相关性,即端粒长度反应了细胞的分裂次数,因此有人将端粒形象的比喻为生物时钟。
在90%的癌细胞中,端粒酶被重新激活,以此来维持端粒的长度,使细胞走向永生化。
简要综述了端粒、端粒酶及端粒酶结合蛋白的最新研究进展。
[关键词]端粒;端粒酶;端粒结合蛋白[中图分类号]Q26[文献标识码]AAdvancesofTelomereandTelomeraseYANGPing-xun,HUANGJun-jianBeijingInstituteofBiotechnology,Beijing100850,China[Abstract]Telomeresarespecializednucleoproteincomplexesattheendsoflinearchromosomes,consistingofmulti-kilo-baselongarraysofdouble-strandedTTAGGGrepeats,asingle-strandedoverhangofthe3'G-strand,andassociatedpro-teins.Itprotectstheendsoflinearchromosomes.Telomerelength,constructionandorganizationareregulatedbylotsoftelomerebindingproteins.Becauseofthesignificanceoftelomeres,thechangesoftelomeres'lengthorconstructurecancausecellstoentersenescenceorapoptosis.Telomeraseisimportantformaintainingtelomeres,andmostofcancercellshavetelomeraseactivity,so,itcanbeatargetforcurecancer.Thelatestdevelopmentofthetelomere,telomeraseanditsbindingproteinsfunctionwerediscussed.[Keywords]telomereUtelomeraseUtelomere-bindingprotein端粒的发现应归功于20世纪30年代2位遗传学家芭芭拉・麦克林托克和赫尔曼・穆勒的观察结果。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ArabidopsisWEE1KinaseControlsCellCycleArrestinResponsetoActivationoftheDNAIntegrityCheckpointWOA
KristofDeSchutter,1Je´roˆmeJoube`s,1,2ToonCools,AurineVerkest,FlorenceCorellou,3ElenaBabiychuk,ElsVanDerSchueren,TomBeeckman,Sergeı¨Kushnir,DirkInze´,andLievenDeVeylder4
DepartmentofPlantSystemsBiology,FlandersInteruniversityInstituteforBiotechnology,GhentUniversity,B-9052Gent,Belgium
UpontheincidenceofDNAstress,theataxiatelangiectasia–mutated(ATM)andRad3-related(ATR)signalingkinasesactivateatransientcellcyclearrestthatallowscellstorepairDNAbeforeproceedingintomitosis.AlthoughtheATM-ATRpathwayishighlyconservedoverspecies,themechanismsbywhichplantcellsstoptheircellcycleinresponsetothelossofgenomeintegrityareunclear.WedemonstratethatthecellcycleregulatoryWEE1kinasegeneofArabidopsisthalianaistranscriptionallyactivateduponthecessationofDNAreplicationorDNAdamageinanATR-orATM-dependentmanner,respectively.InaccordancewitharoleforWEE1inDNAstresssignaling,WEE1-deficientplantsshowednoobviouscelldivisionorendoreduplicationphenotypewhengrownundernonstressconditionsbutwerehypersensitivetoagentsthatimpairDNAreplication.InducedWEE1expressioninhibitedplantgrowthbyarrestingdividingcellsintheG2-phaseofthecellcycle.WeconcludethattheplantWEE1geneisnotrate-limitingforcycleprogressionundernormalgrowthconditionsbutisacriticaltargetoftheATR-ATMsignalingcascadesthatinhibitthecellcycleuponactivationoftheDNAintegritycheckpoints,couplingmitosistoDNArepairincellsthatsufferDNAdamage.
INTRODUCTIONGenomeintegrityofcellsisthreatenedbyDNAdamagethatistheconsequenceofenvironmentalstressesandendogenouscauses.Tocopewiththesestressconditions,cellshavedevel-opedasetofsurveillancemechanismstomonitorthestatusandstructureofDNAduringcellcycleprogression.InSchizosac-charomycespombe(fissionyeast)andmammals,DNAdamageactivatestheataxiatelangiectasia–mutated(ATM)andRad3-related(ATR)signalingcascadesthatsimultaneouslyturnonDNArepaircomplexesandarrestcelldivision;thismechanismallowscellstorepairdamagedDNAbeforeproceedingintomitosis(ZhouandElledge,2000;Abraham,2001;BartekandLukas,2001;KurzandLees-Miller,2004).ATMrespondsspe-cificallytodouble-strandedbreaks,whereasATRprimarilysensesreplicationstresscausedbyapersistentblockofrepli-cationforkprogression.TheATMandATRkinasestransducethe
DNAstresssignaltothecheckpointkinasesCHK1andCHK2,which,inturn,arrestthecellcyclebydirectlymodulatingtheactivityoftheeffectorsthatcontrolcellcycleprogression(ChenandSanchez,2004;Sancaretal.,2004),thecyclin-dependentkinase(CDK)complexes.CDKcomplexesconsistofacatalytickinasesubunitandaregulatorycyclin.ThesequentialactivationofdifferentCDK/cyclincomplexesdrivesthecellcyclethroughthephosphoryl-ationofmanydifferenttargetsubstrates.CDK/cyclinactivityishighlyregulatedatmultiplelevels.Controlmechanismsincludetheregulatedsynthesisanddestructionofthecyclinsubunits(Peters,1998;Murray,2004),whicharethoughttotargettheCDKstothesubstrates(OhiandGould,1999),andtheassoci-ationofCDKswithinhibitoryproteinsanddockingfactors(Lees,1995).Moreover,CDKactivityispositivelyregulatedbyphos-phorylationofaconservedresidue(Thr-161orequivalent)withintheTloopandnegativelyregulatedthroughphosphorylationofTyr-15andThr-14byWEE1familykinases(BerryandGould,1996).PhosphorylationofTyr-15andThr-14residuesoftheCDKsubunitinhibitsATPbindingandblockssubstraterecognition.Infissionyeastandmammals,rapidactivationoftheCDK/cyclinactivityattheG2-Mboundaryismediatedbyadual-specificityphosphataseCDC25.MaintenanceoftheinhibitionofCDKactivitybyTyr-15phosphorylationistheultimatetargetofDNAdamagecheckpointsignaling.ByactivationofCHK1andCHK2,CDC25isphosphorylatedandtargetedforubiquitin-dependentdestructionorassociationwitha14-3-3protein,resultinginnuclearexportandexclusionofCDC25fromthenuclearpoolofCDK/cyclincomplexes(Boutrosetal.,2006).BothWEE1andthefunctionallyrelatedkinaseMIK1havebeenimplicatedastargetsoftheDNAdamageandreplicationcheck-pointsaswell.InXenopuslaevis(Africanfrog)eggextracts,
1Theseauthorscontributedequallytothiswork.
2Currentaddress:LaboratoiredeBiogene`seMembranaire,Centre
NationaldelaRechercheScientifique,Unite´MixtedeRecherche5200,Universite´VictorSe´galenBordeaux2,146,rueLe´oSaignat,F-33076BordeauxCedex,France.3Currentaddress:LaboratoireArago,CentreNationaldelaRecherche
ScientifiqueParisVI,Unite´MixtedeRecherche7628,BP44,F-66651BanyulssurMerCedex,France.4Towhomcorrespondenceshouldbeaddressed.E-maillieven.deveylder@
psb.ugent.be;fax32-9-3313809.TheauthorresponsiblefordistributionofmaterialsintegraltothefindingspresentedinthisarticleinaccordancewiththepolicydescribedintheInstructionsforAuthors(www.plantcell.org)is:LievenDeVeylder(lieven.deveylder@psb.ugent.be).WOnlineversioncontainsWeb-onlydata.OAOpenAccessarticlescanbeviewedonlinewithoutasubscription.www.plantcell.org/cgi/doi/10.1105/tpc.106.045047
