Vesicle-mediated protein transport pathways to the vacuole in Schizosaccharomyces pombe
植物戊糖磷酸途径及其两个关键酶的研究进展

植物学通报 2004, 21 (2): 139 ̄145Chinese Bulletin of Botany植物戊糖磷酸途径及其两个关键酶的研究进展①黄 骥 王建飞 张红生②(南京农业大学作物遗传与种质创新国家重点实验室南京210095)摘要戊糖磷酸途径是植物体中糖代谢的重要途径,主要生理功能是产生供还原性生物合成需要的NADPH,可供核酸代谢的磷酸戊糖以及一些中间产物可参与氨基酸合成和脂肪酸合成等。
葡萄糖-6-磷酸脱氢酶和6-磷酸葡萄糖酸脱氢酶是戊糖磷酸途径的两个关键酶,广泛的分布于高等植物的胞质和质体中。
本文综述了植物戊糖磷酸途径及其两个关键酶的分子生物学的研究进展,讨论了该途径在植物生长发育和环境胁迫应答中的作用。
关键词戊糖磷酸途径,葡萄糖-6-磷酸脱氢酶,6-磷酸葡萄糖酸脱氢酶Advances on Plant Pentose Phosphate Pathwayand Its Key EnzymesHUANG Ji WANG Jian-Fei ZHANG Hong-Sheng②(National Key Laboratory of Crop Genetics & Germplasm Enhancement,Nanjing Agricultural University, Nanjing210095)Abstract The pentose phosphate pathway in plant is a very important metabolic pathway which supplies the major sources of reduced nicotinamide adenine dinucleotide phosphate (NADPH) and the ribulose 5-phosphate, ribose 5-phosphate and erythrose 5-phosphate involved in synthesis of nucleotides, aromatic amino acids and fatty acids in non-photosynthetic tissues. This paper mainly reviews the advances of research on plant pentose phosphate pathway and its key enzymes: glu-cose-6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase. Its possible functions involved in plant development or responding to environmental stresses are discussed.Key words Pentose phosphate pathway, Glucose-6-phosphate dehydrogenase, 6-phosphogluc-onate dehydrogenase戊糖磷酸途径(pentose phosphate pathway, PPP)是植物体中糖代谢的重要途径,其主要生理功能是产生供还原性生物合成需要的NADPH以及可供核酸代谢的磷酸戊糖,一些中间产物则可参与氨基酸合成和脂肪酸合成等。
肠道转运蛋白SR-B1_调节Caco-2_细胞脂质转运的研究

医学食疗与健康 2023年2月下第21卷第6期·实验研究·*基金项目:国家自然科学基金青年科学基金项目(编号:31701599)作者简介:郑梦熳(1995.09—),女,硕士研究生,研究方向:公共卫生。
△通信作者:刘雨薇(1988.02—),女,博士研究生,副教授,研究方向:食品与营养。
E-mail:***************.cn肠道转运蛋白SR-B1调节Caco-2细胞脂质转运的研究郑梦熳 郭毅 李文韵 吴岷 徐铭婧 邵嫚嫚 刘雨薇△(复旦大学公共卫生学院,上海 200032)【摘要】目的:研究肠道转运蛋白SR-B1在Caco-2细胞脂质转运中的作用。
方法:使用慢病毒载体构建SR -B 1敲低Caco-2细胞(Caco-2 KD)与对照Caco-2 NC 细胞,使用UHPLC-HRMS/MS 测定Caco-2 KD 细胞与Caco-2 NC 细胞在玉米油胶束孵育前后的脂质组,观察SR -B 1在Caco-2细胞摄取玉米油胶束脂质中的作用。
结果:Caco-2 NC 与Caco-2 KD 细胞中TG、DG、LPC、LPE、Cer 与So 的含量在胶束孵育后显著增加(P <0.05),但与Caco-2 NC 细胞相比,Caco-2 KD 细胞中TG 与DG 的细胞内增量分别减少了(32.07±0.75)%、(65.21±7.50%),差异显著(P <0.001)。
结论:SR-B1在Caco-2细胞摄取玉米油胶束TG 与DG 的过程中起重要促进作用。
【关键词】脂质组;SR-B1;Caco-2细胞;甘油三酯;甘油二酯【中图分类号】R541.4 R363 【文献标识码】A 【文章编号】2096-5249(2023)06-0060-04食品营养与健康受到广泛关注[1-2],其中肠细胞的脂质吸收与转运蛋白调节是营养学研究的重点,特定受体在不同脂质吸收过程中的作用机制仍须探索。
生物化学:第十章 三羧酸循环

The Citric Acid Cycle Oxidizes Two-Carbon Units
The cycle starts with the condensation of oxaloacetate (C4) and acetyl CoA (C2) to give citrate (C6), which is isomerized to isocitrate (C6). Oxidative decarboxylation of this intermediate gives –ketoglutarate (C5). The second molecule of carbon dioxide comes off in the next reaction, in which -ketoglutarate is oxidatively decarboxylated to succinyl CoA (C4). The thioester bond of succinyl CoA is cleaved by inorthophosphate to yield succinate, and a high phosphoryl transfer potential compound in the form of GTP is concomitantly generated. Succinate is oxidized to fumarate (C4), which is then hydrated to form malate (C4). Finally, malate is oxidized to regenerate oxaloacetate (C4). Thus, two carbon atoms from acetyl CoA enter the cycle, and two carbon atoms leave the cycle as CO2 in the successive decarboxylations catalyzed by isocitrate dehydrogenase and ketoglutarate dehydrogenase. In the four oxidation–reduction reactions in the cycle, three pairs of electrons are transferred to NAD and one pair to FAD. These reduced electron carriers are subsequently oxidized by the electrontransport chain to generate approximately 9 molecules of ATP. In addition, 1 molecule of a compound having a high phosphoryl transfer potential is directly formed in the citric acid cycle. Hence, a total of 10 molecules of compounds having high phosphoryl transfer potential are generated for each two-carbon fragment that is completely oxidized to H2O and CO 2. 44 浙江大学医学院徐立红
细胞生物学-物质的跨膜运输(翟中和第四版)-含注释!!!
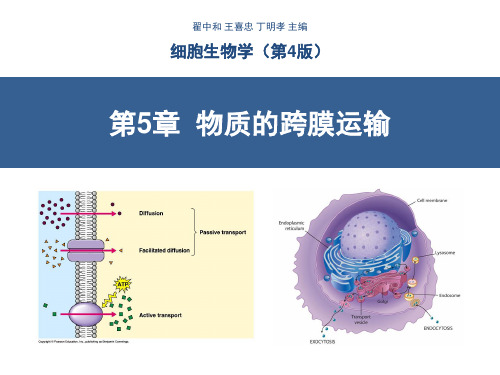
动物、植物细胞主动运输比较
三、ABC 超家族
• ABC 超家族也是一 类ATP 驱动泵 • 广泛分布于从细菌 到人类各种生物中, 是最大的一类转运 蛋白 • 通过ATP 分子的结 合与水解完成小分 子物质的跨膜转运
(一)ABC转运蛋白的结构与工作模式
• 4 个“核心”结构域
– 2 个跨膜结构域,分别含6 个跨
H+/K+ ATPase Control of acid secretion in the stomach
二、V 型质子泵和 F 型质子泵
• V 型质子泵广泛存在 于动物细胞的胞内体 膜、溶酶体膜,破骨 细胞和某些肾小管细 胞的质膜,以及植物、 酵母及其他真菌细胞 的液泡膜上 (V 为 vesicle) • 转运 H+ 过程中不形成 磷酸化的中间体
导兴奋)
B. 配体门通道(胞外配体)
(突触后膜接收乙酰胆碱的
受体)
C. 配体门通道(胞内配体)
D. 应力激活通道(内耳的 听毛细胞)
含羞草“害羞”的机制
• 估计细胞膜上与物质转运有关的蛋白占核基因编码蛋白的 15~30%,细 胞用在物质转运方面的能量达细胞总消耗能量的2/3。
• 两类主要转运蛋白:
P型泵的主要特点:都是跨膜蛋白,并且是由一条多肽完成 所有与运输有关的功能,包括ATP的水解、磷酸化和离子 的跨膜运输。
Na+-K+ATP酶的分子结构:
α β 两种亚基组成的二聚体。
α 亚基具有ATP酶的活性;
β 亚基是具有组织特异性的糖蛋白。
(一)Na+-K+ 泵(Na+-K+ ATPase)
Figure 11-14 Molecular Biology of the Cell (© Garland Science 2008)
囊泡运输

Rabs 作用 : 通过效应因子 , 调节 SNAREs 复合体的形成 ,
促进和调节运输小泡的停泊和融合。
第三节 胞吞作用(endocytosis)
胞吞作用是病毒及生物大分子颗粒等进入细 胞的一种方式 • 过程中消耗一定的能量 • 分为 吞噬作用(phagocytosis) 胞饮作用(pinocytosis) 受体介导的胞吞作用
hand-over-hand I left-right
II left-left or right-right
马达蛋白在复杂网络的运动
微丝与微管 和中间纤维 共同组成细 胞骨架
MTOC 微管组织中心
囊泡运输的定向融合机制
“定向和融合的关键环节”
配体-受体
融合复合物
囊泡与靶膜的融合是一个涉及多种蛋白的识别 与锚泊结合、装配与去装配的复杂调控过程, 具有高度的特异性
细胞囊泡运输
一、2013年诺贝尔生理学、医学奖
• 2013年诺贝尔生理学或医学奖被授予三位 科学家,来自耶鲁大学、出生于美国的詹 姆斯•罗斯曼(James E. Rothman);加州大学 伯克利分校的兰迪•谢克曼(Randy W. Schekman);来自斯坦福大学、二十世纪80 年代移居美国的德国人托马斯•祖德霍夫 (Thomas C. Sudhof)
高尔基复合体→溶酶体
参与的分子:网格蛋白、衔接蛋白、发动蛋白
A. 网格蛋白有被小泡形态特征电镜图;B. 网格蛋白有被小泡结构特征示意图
网格蛋白:
构成网架结构,形成囊泡外被。
成纤维细胞的网格蛋白衣被电子显微镜照片
衔接蛋白:在网格蛋白结构外框与囊膜间隙中填 充、覆盖。
具有识别特异的跨膜 蛋白受体,并将其连 接到网格蛋白上的作 用。衔接蛋白一端与 网格蛋白重链结合, 催化网格蛋白的聚合; 另一端又与被转运的 “货物”分子衔接。
医学细胞生物学-5 细胞内膜系统与囊泡运输

第五章细胞内膜系统与囊泡运输溶酶体23第一节内质网——内质网(endoplasmic reticulum)是一类由大小、形态各异的膜性囊泡所构成的细胞器。
约占细胞总膜面积的50%,占细胞总体积的10%。
是封闭的网络系统。
4一、内质网的形态结构●内质网是由平均膜厚度为5~6nm的膜性小管、小泡和扁囊彼此相连所构成的三维管网结构系统。
●在不同组织细胞中,或同一种细胞的不同发育阶段及不同生理功能状态下,内质网的形态结构、数量分布和发达程度有很大差别。
56内质网的基本类型分为粗面内质网(rough endoplasmic reticulum RER)和滑面内质网(smooth endoplasmic reticulum,SER)。
●RER呈扁平囊状,排列整齐,有核糖体附着。
●SER呈分支管状或小泡状,无核糖体附着。
78●合成脂类●解毒作用●糖原代谢●贮积钙离子●蛋白质的合成●蛋白质的修饰加工●新生多肽的折叠、组装与运输●膜的生成●物质的运输9特化类型●髓样小体:存在于视网膜的色素上皮细胞中,光面内质网发生特化,其管囊连接成了网状,形成紧密平行排列的髓样结构。
●肌质网:肌纤维是高度特化的细胞,其内质网也发生特化,为纵行小管状结构,沿肌原纤维有规律地分布,其主要功能是储积Ca2+。
●环孔片层:平行排列成堆,形态结构类似核膜,常见于生殖细胞或病理分化的细胞等快速增殖细胞的细胞质中。
被认为是糙面内质网的局部特化。
10二、内质网的化学组成(一)脂类和蛋白质是内质网的主要化学组成成分●脂类和蛋白质比例大约1:2。
●脂类主要成分为磷脂,磷脂酰胆碱含量较高,鞘磷脂含量较少,没有或很少含胆固醇。
(二)内质网含有以葡萄糖-6-磷酸酶为主要标志性酶的诸多酶系内质网约有30多种膜结合蛋白,另有30多种位于内质网腔。
11(三)网质蛋白是内质网网腔中普遍存在的一类蛋白质●网质蛋白的共同特点是在其多肽链的羧基端(C端)均含有一个驻留信号(KDEL或HDEL 四氨基酸序列)。
V-ATPase对肿瘤细胞糖酵解的影响
2021年1月第11卷第2期·综 述·V-ATPase对肿瘤细胞糖酵解的影响闫 娜 王金花▲内蒙古医科大学附属人民医院病理科,内蒙古呼和浩特 010010[摘要]V-ATPase是一类特殊的膜转运蛋白,通过驱动质子转运以及参与信号转导调节细胞各种生理过程。
V-ATPase在细胞中的主要功能及作用已有大量研究,而关于其激活或抑制对于细胞糖酵解的影响,以及在肿瘤细胞所起作用的相关材料比较零散。
通过整理近年来关于V-ATPase以及肿瘤糖酵解等的相关文献发现,V-ATPase在肿瘤细胞发生、发展、转移等过程中起到了至关重要的作用。
因此,总结V-ATPase的结构功能,以及其对细胞糖酵解的影响甚至在癌细胞中起的作用,有利于提高对于V-ATPase的进一步认识,并便于探索其在肿瘤治疗新方案,尤其靶向治疗研究中的作用。
[关键词] Vacuolar ATPase;糖酵解;肿瘤;代谢[中图分类号] R730.2 [文献标识码] A [文章编号] 2095-0616(2021)02-0047-04 Impacts of V-ATPase on glycolysis of tumor cellsYAN Na WANG JinhuaDepartment of Pathology, the Affiliated People's Hospital of Inner Mongolia Medical University, Inner Mongolia, Hohhot 010010, China[Abstract] V-ATPase is a sort of special membrane transporter, which regulates various physiological processes ofcells by driving proton transport and participating in signal transduction. The key functions and effects of V-ATPase incells have been researched extensively, while the related materials about its activation or inhibition on cell glycolysisand its effects in tumor cells are scattered. By sorting out the related literatures about V-ATPase and tumor glycolysisin recent years, it is discovered that V-ATPase plays a vital effect in the occurrence, development and metastasis of tumor cells. Therefore, summarizing the structure and function of V-ATPase, its impacts on cell glycolysis and evenits effects in cancer cells will help to improve the further understanding of V-ATPase and investigate its effects in new tumor treatment programs, especially in targeted therapy research.[Key words] Vacuolar ATPase; Glycolysis; Tumors; Metabolism运输ATPase存在于真核生物和部分微生物体内,能够水解ATP,释放能量用于跨膜运输蛋白质等,参与人体生命活动。
细胞生物学-第8章-蛋白质分选与膜泡运输
二、COPII包被膜泡的装配与运输
• 负责从内质网高尔基体的物质运输; • COPII包被蛋白由5种蛋白亚基组成:Sar1、 Sec12、
Sec23/Sec24、 Sec13/Sec31、Sec16 • 包被蛋白的装配是受控的; • COPII包被膜泡具有对转运物质的选择性并使之浓缩。
COPII蛋白能识别并结合跨膜内质网蛋白胞质面一端的信 号序列(Asp-X-Glu) 内质网腔面的受体能与ER腔中的可溶性蛋白(如分泌蛋白 )结合。
6.组成型分泌
7.调节型分泌
8.分选到溶酶体
9.胞吞途径
蛋白质的分泌与胞吞途径概观
膜泡运输
• 在供体膜上如何形成膜泡? • 如何运输膜? • 膜泡如何锚定靶膜并与之融合?
在供体膜上如何形成膜泡?
电子显微镜下观察,发现膜泡表面有一层蛋白质
包被蛋白的功能:
1、形成膜泡:使供体膜弯曲 2、选择膜泡成分:被运输的货物 3、靶膜融合:包含与靶膜融合相关蛋白
• 蛋白质分选的运输方式: 1.门控运输;2.跨膜运输;3.膜泡运输;4.细胞 质基质中的蛋白质运输。
• 蛋白质分选的信号机制: 信号肽;信号识别颗粒;信号识别颗粒的受体 ;ER膜上的易位子等。
• 3种类型的有被小泡: 1. 网格蛋白/接头蛋白小泡 ;2. COPI小泡;3. COPII小泡
anchor sequence, STA)
• 内在信号锚定序列(internal signal anchor sequence, SA)
膜整合蛋白 一次跨膜
膜整合蛋白 两次跨膜
多肽跨膜次数的确定
内质网膜整合蛋白的拓扑学类型
膜整合蛋白 方向性
蛋白分选机制
1、蛋白质自身信号序列 2、靶细胞器上包含特定的信号识别装置
诺如病毒参考文献
Vaccine21(2003)376–385A modified cholera holotoxin CT-E29H enhances systemicand mucosal immune responses to recombinant Norwalkvirus–virus like particle vaccineSangeeta B.Periwal a,Kristin R.Kourie a,Nandini Ramachandaran b,Susan J.Blakeney b, Sylvia DeBruin a,Duzhang Zhu c,Timothy J.Zamb b,Larry Smith a,Steve Udem b, John H.Eldridge a,c,Khushroo E.Shroff a,∗,Patricia A.Reilly ba Department of Viral Vaccine Immunology,Wyeth–Ayerst Research,Pearl River,NY10965,USAb Department of Virology,Wyeth–Ayerst Research,Pearl River,NY10965,USAc Department of Bacterial Vaccine Immunology,Wyeth–Lederle Vaccines,211Bailey Road,West Henrietta,NY14586,USAAbstractIn this study,we evaluated the potential of a genetically modified cholera toxin,CT-E29H as an adjuvant for recombinant Norwalk virus like particle(NV-VLP)vaccine.This detoxified mutant,containing E to H substitution at amino acid29of the CT-A1subunit,was adminis-tered with a recombinant Norwalk virus like particle vaccine to Balb/c mice by mucosal routes to monitor the induction of mucosal,humoral and cellular responses.We observed that a low dose of NV-VLP(5g)with the adjuvant delivered by the intranasal route(IN)was more ef-fective than the highest dose(200g)delivered by oral route at inducing both cellular and NV-VLP specific IgG and IgA responses.Higher counts of antigen specific IgA secreting cells were observed in the Peyer’s Patches(PP)following delivery of the vaccine with CT-E29H as compared to delivery of vaccine by mucosal routes without CT-E29H.Furthermore,there was an increase in antigen specific cells producing IL-4from animals that received the vaccine with the adjuvant.Delivery of the vaccine by the oral route results in antigen specific CD4+and CD8+T cells in PP and spleen.Addition of CT-E29H results in an increase of antigen specific CD4+cell population in PP and both CD4+and CD8+populations in the spleen.These cellular and cytokine responses suggest that combining the vaccine with CT-E29H results in a stronger Th2type response.Collectively,these results indicate that immune responses to NV-VLP vaccine are qualitatively and quantitatively improved when the vaccine is delivered along with CT-E29H,and thus merits its further consideration as a mucosal adjuvant.©2002Elsevier Science Ltd.All rights reserved.Keywords:Norwalk virus like particle;Cholera toxin mutant;Mucosal1.IntroductionGenerally,non-replicating vaccines are less immunogenic than live vaccines at mucosal surfaces.Therefore,there is a great need for substances that can act as adjuvants for the local immune response to orally or intranasally(IN)admin-istered non-replicating immunogens.Cholera toxin(CT), the enterotoxin produced by Vibrio cholerae,has proved to be an exceptionally potent mucosal adjuvant.It has been shown in numerous studies to significantly enhance intesti-nal IgA responses[1–3]and to function as a strong adjuvant for systemic immune responses[4,5].The toxicity associated with CT prevents its use as an adjuvant in vaccine formulations.Several groups have now modified this toxin in order to reduce its toxicity,while ∗Corresponding author.Tel.:+1-845-602-5455;fax:+1-845-602-3628. E-mail address:shroffk@(K.E.Shroff).preserving its adjuvant capacity[6,7].One such detoxified version of CT,designated as CT-E29H,has been shown in previous studies to have markedly reduced toxicity as determined by Y-1adrenal and mouse gut weight assays [8],while maintaining the adjuvant effect of the parenteral cholera toxin[9,10].CT-E29H differs from native CT by a single amino acid substitution(E to H)at amino acid29in the CT-A1subunit of the native CT.Its binding capacity to GM1-gangloside is equivalent to that of CT[10,11].In this study,we tested the potential of CT-E29H as a mucosal adjuvant for Norwalk virus like particle(NV-VLP) vaccine.Norwalk is one in a group of non-cultivable human caliciviruses that have been recognized as the causative agent for sporadic and epidemic outbreaks of acute gastroenteritis [12].The study of this virus has been hampered by the inabil-ity to culture this virus in vitro and also due to the lack of a suitable animal model.However,when NV capsid protein is0264-410X/02/$–see front matter©2002Elsevier Science Ltd.All rights reserved. PII:S0264-410X(02)00618-7S.B.Periwal et al./Vaccine 21(2003)376–385377expressed in Sf9cells infected with recombinant baculovirus it spontaneously forms virus like particles (VLPs)[13].Al-though,these VLPs are artificial products,they have proven to be morphologically and antigenically similar to the native virion [13].More recently,Guerrero et al.,have shown that NV-VLPs administered by the intranasal route in mice can induce systemic and mucosal immune responses [14].The results presented here show that administration of CT-E29H along with NV-VLP results in enhanced systemic and mucosal cellular as well as humoral immune bining 5g of CT-E29H to a low dose of NV-VLP (5g)significantly enhanced immune responses compared to the same low dose of NV-VLP administered without the adjuvant.2.Materials and methods 2.1.AnimalsSix-to-eight-weeks-old female Balb/c mice purchased from Taconic Farms (Germantown,NY)were used through-out these studies.All mice were housed and maintained in a facility approved by the American Association for Accreditation of Laboratory Animal Care.2.2.Vaccine and adjuvantNV-VLPs were prepared by infection of Sf9cells with recombinant baculovirus expressing the NV capsid protein as previously described [13].Briefly,500ml cultures of Sf9cells in Insect Xpress medium (BioWhittaker,Walkersville,MD)were grown in 2l spinner flasks prior to infection at an MOI =0.5and incubation at 28◦C for 6days.Culture supernatants were clarified by centrifugation at 3500×g for 30min at 4◦C.NV-VLPs were concentrated by centrifu-gation through a sucrose at 96,000×g for 3h at 4◦C.NV-VLP were resuspended in water and banded in a cesium chloride (36%w/v)isopycnic gradient by centrifugation at 92,000×g for 16h at 4◦C.The fraction containing NV-VLP was isolated,dialyzed against PBS and sterile filtered.CT-E29H was prepared as described previously [10].2.3.Immunization of miceGroups of five mice were used in all experiments.For in-tranasal delivery,mice were immunized with 5g NV-VLP with or without 5g CT-E29H.A total of 20l was in-stilled in the nares of mice tranquilized with ketamine.For oral delivery,mice were pre-treated with 100l of a solution consisting of eight parts of Hank’s balanced salt solution and two parts 7.5%sodium bicarbonate in order to neutral-ize stomach acidity.Immediately following pre-treatment,mice were gavaged with 100l of NV-VLP or NV-VLPplus CT-E29H.All mice were boosted two times at a 3-week interval and euthanized 2weeks following last boost.2.4.Collection of samplesFecal samples were collected 2weeks following the fi-nal boost.Blood was collected in Microtainer ®tubes (Bec-ton Dickinson,NJ)from the retro-orbital plexus during the experimental period and by cardiac puncture at the termi-nation of the experiment.Serum was collected and stored at −20◦C.Fresh fecal matter (approximately 100l)was collected from the small intestine of each animal in sepa-rate tubes containing 0.1ml of sterile PBS,0.02%sodium azide,1%BSA,1mM PMSF and 5mM EDTA.The tubes were vortexed for approximately 30s;the particulates were allowed to settle for 15min,then vortexed again and cen-trifuged at 1000×g for 10min.Supernatants were collected and an additional 200l of PBS was added prior to storing the samples at −20◦C.2.5.Proliferation assayLymphoproliferation was carried out as previously de-scribed [15].Briefly,2×105spleen cells were cultured in the presence or absence of 1g/ml of NV-VLP in com-plete RPMI media with 10%FCS.After 4days incuba-tion at 37◦C in the presence of 5%CO 2,cultures were pulsed overnight with 1Ci of [3H]thymidine per well for 12h.Incorporation was measured on a beta counter.The responses are reported as the stimulation index (SI),which is defined as the counts obtained in the presence of anti-gen,divided by the counts obtained in the absence of anti-gen.Cells from spleen and PP were enriched for CD4+or CD8+T cells by depletion of the alternate subset using the MiniMACS magnetic separation system (Miltenyi Biotec,Auburn,CA)before plating them out at 2×105cells/ml for a proliferation assay.The purity of the enriched pop-ulations was determined to be 85–90%by flow cytometry (not shown).2.6.ELISAThis assay was performed on sera and fecal extracts.Briefly,96well,flat bottom plates (Immulon)were coated overnight at 4◦C with purified NV-VLP at a concentration of 1g/ml.The plates were washed with PBS +0.1%Tween-80(PBST)and were blocked with 4%BSA for 1h at room tem-perature.A total of 50l of five-fold dilution of serum start-ing at 1:25,or 50l of fecal extracts were added to the plates.After 1-h incubation,the plates were washed with PBST five times,and a 1:20,000dilution of biotinylated anti-mouse IgG (Jackson Immunoresearch Lab,West Grove,PA)or a 1:5000dilution of biotinylated anti-mouse IgA (Southern Biotechnology Associates,Birmingham,AL)was added and the plates incubated for 1h.The plates were washed with PBST before adding 50l of Avidin plus Enzyme conjugate378S.B.Periwal et al./Vaccine21(2003)376–385(ABC Kit,Vector,CA)to each well and incubated for30min at room temperature.The plates were washed with PBST before adding the substrate3,3 -,5,5 -tetramethylbenzidine (TMB).The color was allowed to develop for10min and then the reaction was stopped by addition of100l of2.5N H2SO4.The plates were read at450nm on an Optimax (Molecular Devices)ELISA reader.The serum titers were reported as GMT.2.7.IgA ELISPOT assayThis assay was performed as previously described[16]. Briefly,96well nitrocellulose Immulon II plates(Millipore, Bedford,MA)were coated with0.1g per well of NV-VLP overnight at4◦C.The plates were washed under aseptic con-ditions with PBS and blocked with complete RPMI contain-ing10%FCS for1h at room temperature.Cells from the Peyer’s patches(PP)were serially diluted three-fold start-ing at1×105cells/well,and plated in triplicates.The plates were incubated overnight at37◦C in5%CO2.Following incubation,the plates were washed with PBS and incubated for2h at room temperature with a1:100dilution of goat anti-mouse IgA-alkaline phosphatase(Southern Biotechnol-ogy Associates,Birmingham,AL).Plates were washedfive times with PBS–0.05%Tween80before being developed using an alkaline phosphatase substrate(BioRad,Hercules, CA).The reaction was stopped by washing with distilled water.NV-specific IgA secreting cells were quantified by counting spots using a ZEISS Elispot counter.2.8.Interferon-gamma and IL-4ELISPOT assayBriefly,96-well Immulon II plates(Millipore,Bedford, MA)were coated with1g per well of purified rat anti-mouse interleukin-4(IL-4)monoclonal antibody(BVD4-1D11;Pharmingen,San Diego,CA),or purified rat anti-mouse gamma interferon(IFN-gamma)monoclonal antibody(R4-6A2;Pharmingen,San Diego,CA)per well overnight at4◦C.Plates were washed,blocked with com-plete RPMI medium,and seeded in triplicates with100l of2×105cells/well(in three-fold dilution)of spleno-cyctes that were stimulated with antigen for3days in culture at conditions described in the proliferation assay section.Plates were incubated overnight at37◦C in5% CO2.One hundred microliters(1:250)of biotinylated rat anti-mouse IFN-gamma(XMG1.2;Pharmingen)or bi-otinylated rat anti-mouse IL-4(BVD6-2462:Pharmingen) antibody was added and incubated for2h at room tempera-ture.Strepavidin–alkaline phosphatase conjugate(Mabtech, Nacka,Sweden)was added,and the plates were incubated at room temperature for2h.Plates were developed using an alkaline phosphatase substrate(BioRad).The reaction was stopped by washing with distilled water.Interferon-gamma and IL-4secreting cells were quantified by counting spots using a ZEISS Elispot counter.2.9.Gut and respiratory associated lymphoid tissue (GALT,RALT)organ cultureIntestinal fragment and PP cultures were performed as previously described[17].Briefly,PP were dissected from the gut and3–4cm long segments of small intestine were cut open longitudinally and washed3–5times in sterile Hank’s buffer.The trachea was dissected,cut longitudinally and di-vided into two pieces.Lungs were dissected and cut into four equal parts.The pieces of trachea and lungs were washed in Hank’s buffer and cultured along with intact PP and small intestine fragments.The cultures were set-up in sterile24 wellflat bottom plates(Costar,Cambridge,MA)in2ml of Kennet’s H-Y media(JRH Biosciences,Lenexa,KS)con-taining10%FBS(Sigma,St.Louis,MO)1%glutamine and 0.25g/ml Fungizone(Gibco,Grand Island,NY)for7days under90%O2/10%CO2at37◦C in a modular incubator chamber(Billups-Rothenberg,Del Mar,CA).Culture super-natants were collected and frozen at−20◦C until assayed by ELISA.2.10.Data and statistical analysesGeometric mean titers(GMTs)of IgG in sera were de-termined for every group of mice.All non-responders were included in the computation of GMT and standard devia-tions were calculated on the log-transformed titers.GMTs were compared using Student’s t-test.Mucosal antibody responses in fecal extracts are expressed ing/ml using mouse IgA and IgG as the standards.3.Results3.1.Intranasal route of deliveryA low dose of NV-VLPs(5g per dose)was adminis-tered by IN route in the absence or presence of CT-E29H (5g).After IN immunization of NV-VLP with CT-E29H, the splenic T cells responded strongly to recall antigen in vitro as shown in Fig.1A.The antigen stimulated prolifera-tive response of cells from mice immunized with NV-VLP and CT-E29H yielded a SI of7,which was significantly (P<0.05)higher than that of na¨ıve cells or cells from mice immunized with NV-VLP in the absence of adjuvant.Fol-lowing IN administration of NV-VLP without CT-E29H, all mice exhibited a NV-VLP specific IgG response,with a GMT of6234.Co-administration of NV-VLP with CT-E29H significantly enhanced(P<0.05)the serum IgG GMT to greater than50,000as shown in Fig.1B. Supernatants from in vitro organ cultures were assayed to detect NV-VLP specific IgA antibodies.Animals were sac-rificed2weeks after the second boost;lung,trachea,small intestine and Peyer’s patches(PP)were collected and pooled from multiple mice in the same group.Tissues were cultured for7days in a modular incubator chamber under10%CO2S.B.Periwal et al./Vaccine 21(2003)376–385379and 90%O 2.NV-VLP specific IgA antibody levels in the culture supernatants were determined by ELISA as shown in Fig.1C .Animals immunized with NV-VLP and CT-E29H had high levels of NV-VLP specific IgA antibodies in the su-pernatants of organ culture from mucosal sites local tovac-Fig.1.(A)Lymphoproliferation assay following IN delivery of NV-VLPs.Splenocyctes were cultured in complete RPMI for 4days in the absence or presence of 1g/ml of NV-VLP.Cultures were pulsed with [3H]-thymidine overnight and its incorporation measured on a beta counter.Proliferation is represented as stimulation index (SI).The()represents significant (P <0.05)increase in SI;(B)ELISA assay for total anti Norwalk IgG antibody in sera of animals that received NV-VLP vaccine by IN route.Titers are expressed as geometric mean titer (GMT).The()represents significant increase (P <0.05)in serum titers.Preimmunization sera had titers of less than 100;(C)ELISA assay for detection of NV-specific IgA in supernatants from fragment cultures of lung,trachea,PP and small intestine (SI),from mice that received the NV-VLP vaccine by IN route.Each data point represents an average optical density value at 450nm ±S.D.;(D)ELISPOT assay for detection of NV-specific IgA antibody spot forming cells in PP from mice that received the NV-VLP vaccine by IN route.cine administration such as lung,trachea,and also at distal mucosal sites,such as small intestine and PP.The levels of antibody detected in the supernatants directly reflect the ex-tent of infiltration of NV-VLP specific IgA secreting plasma cells in the mucosal tissues at the time of sample collection.380S.B.Periwal et al./Vaccine 21(2003)376–385Fig.1.(Continued ).Two weeks after the last boost,PP cells were collected and pooled for each group.Single-cell suspensions were prepared and NV-VLP specific IgA-secreting cells were enumerated by an ELISPOT assay.Fig.1D shows that mice immunized with NV-VLP with CT-E29H had a significantly higher number of NV-VLP specific IgA spot forming cells in their PP as compared to the other groups (P <0.006).This result provides further proof that IN vaccination with CT-E29H formulated NV-VLP results in the dissemina-tion of cells destined to produce IgA to distal mucosal sites.3.2.Oral route of deliveryPrevious studies demonstrated that oral immunization of mice with NV-VLP requires a high dose of antigen (200g)to consistently elicit measurable immune re-sponses [18].In order to determine the effect of the ad-juvant,CT-E29H,on the immunogenicity of NV-VLP,mice were immunized at days 0,21and 42with a con-stant dose of NV-VLP (200g)in the absence or pres-ence of various doses of CT-E29H in increasing dosage (1–50g).S.B.Periwal et al./Vaccine21(2003)376–385381Fig.2.(A)Lymphoproliferation assay following oral delivery of NV-VLP vaccine along with different doses(1–50g per dose)of CT-E29H.Splenocyctes were cultured in presence of1g/ml of NV-VLP for96h.Cultures were pulsed with[3H]-thymidine overnight and its incorporation measured on a beta counter.Proliferation is represented as stimulation index(SI);(B)ELISPOT assay for detection of interferon-gamma and IL-4spot forming cells in spleens from mice that received the NV-VLP vaccine along with different doses(1–50g per dose)of CT-E29H by the oral route;(C)ELISPOT assay for detection of NV-specific IgA antibody spot forming cells in PP from mice that received the NV-VLP vaccine with or without different doses of CT-E29H(1–50g per dose)by oral route.382S.B.Periwal et al./Vaccine 21(2003)376–385Fig.2.(Continued ).Immunization of mice with NV-VLP and 10g of CT-E29H,following pretreatment with sodium bicarbonate,gave the strongest stimulation index (SI =5)in splenic proliferation assay as shown in Fig.2A .NV-VLP vaccine administered in the absence of adjuvant resulted in a fee-ble cellular response (SI =1.6).Mice immunized with NV-VLP plus 10g of CT-E29H also had the highest IL-4and IFN-gamma spot producing cells as shown in Fig.2B .There was marked increase in IL-4spot forming cells in all groups that received CT-E29H compared to groups without adjuvant.In contrast,mice immunized with NV-VLP in the absence of adjuvant had a cytokine response biased towards IFN-gamma.3.2.1.Effect of sodium bicarbonate pretreatment prior to oral delivery of vaccineIn these studies,delivering the CT-E29H formulated vac-cine following pretreatment with sodium bicarbonate was found to result in higher antigen specific responses.This was evident in the cellular immune response as measured in a proliferation assay (Fig.2A ),higher numbers of Norwalk-specific IgA secreting cells in PP (Fig.2C ),and higher lev-els of IgA in fecal extracts (Fig.3).3.2.2.Mucosal antibody responses at local siteA Norwalk-specific fecal IgA response was induced in 100%(5/5)of mice by the oral administration of 200g ofNV-VLP in the absence of the adjuvant (Fig.3).The mag-nitude of the response was significantly higher (P <0.05)in the group that received 200g of VLP orally in the pres-ence of 10g of CT-E29H than in the group that received NV-VLP alone.No specific IgA was detected in mice that received a low dose of NV-VLP (50g)orally even when 10g of CT-E29H was co-administered (data not shown).Serum from all animals immunized with NV-VLP alone by the oral route had low levels of Norwalk-specific antibod-ies (GMT =4947±S .D .).However,the group immunized with NV-VLP combined with 10g CT-E29H,had signifi-cantly higher serum IgG titers (GMT =39,206;P <0.05)as shown in Fig.4.3.3.Characterization of T lymphocyctes in PP and spleen following oral delivery of the NV-VLPs combined with CT-E29HWe determined the predominant antigen reactive T cell subsets (CD4+or CD8+)in PP and spleen following oral delivery of NV-VLP,and determined if CT-E29H affected the prevalence of either subset within these tissues.PP and spleen cells were isolated from mice immunized with NV-VLP alone,or in formulation with CT-E29H (10g).Isolated cells were enriched for either CD4+or CD8+T cells as described in methods.Enriched populations were examined by flow cytometry and determined to be overS.B.Periwal et al./Vaccine 21(2003)376–385383Fig.3.ELISA assay for detection of NV-specific IgA responses in fecal wash from mice that received the NV-VLP vaccine with or without different doses of CT-E29H (1–50g per dose)by oral route.Error bars represent standard deviation.The()represent the significant increase in IgA titers (P <0.003)when the mice are immunized with NV-VLP along with the adjuvant.90%pure (data not shown).Table 1shows that the pre-dominant T cell subset in PP that proliferates upon expo-sure to antigen is CD4+.CD8+T cells in the PP did not proliferate in response to in vitro re-stimulation.In con-trast,both the CD4+and CD8+T cell subsets in spleen responded to in vitro stimulation.The highest levels of T cell proliferation were obtained from mice that had re-ceived NV-VLP vaccine along with CT-E29H.This is the first time that NV-VLP specific CD4+T cells have been detected in the PP following immunization with NV andCT-E29H.Fig. 4.ELISA assay for total anti Norwalk IgG antibody in sera of animals that received NV-VLP vaccine with or without different doses of CT-E29H (1–50g per dose)by oral route.This graph has combined data from two separate studies.One study which is indicated with (᭹)had serum samples collected on week 7and the other study at week 6.Titers are reported as GMT.Table 1Lymphproliferation assay of enriched CD4+and CD8+subsets of T cells from PP and spleens of mice that received NV-VLP vaccine with or without different doses of CT-E29H (1–50g per dose)by oral route GroupsCD4+CD8+PPSpleen PP Spleen NV-VLP1.22.50.7 4.8NV-VLP +CT-E29H3.1 3.6 1.9 6.8Na¨ıve1111Splenocyctes were cultured in complete RPMI for 4days in the ab-sence or presence of 1g/ml of NV-VLP.Cultures were pulsed with [3H]-thymidine overnight and its incorporation measured on a beta counter.Proliferation is represented as stimulation index (SI).4.DiscussionMucosal surfaces of the gastrointestinal,genitourinary and respiratory tracts are the primary sites of entry for most infectious agents,yet most current vaccines are delivered parenterally and induce little or no specific mucosal im-munity.The development of mucosal vaccines for human use would considerably benefit from the identification of safe and effective mucosal adjuvants.Mucosal adjuvants such as the heat labile enterotoxin (LT)of E.coli and CT are highly effective in animal models but are generally considered toxic for use in humans [19–21].A previously described genetically modified version of CT,known as CT-E29H,has reduced toxicity while retaining its ability to work as an adjuvant [10].We have studied the effectiveness of this adjuvant for a vaccine against Norwalk virus,based on VLP,which previously was shown to be immunogenic when mucosally administered [22].384S.B.Periwal et al./Vaccine21(2003)376–385In our studies,the Norwalk VLP based vaccine combined with CT-E29H and administered by the IN route generates higher levels of systemic,cellular and mucosal humoral responses than does immunization via the oral route.Three immunizations with NV-VLP given by IN in the presence of adjuvant gave local and distal mucosal humoral responses (Fig.1C).The induction of a distal mucosal response is demonstrated by the detection of antigen specific IgA form-ing cells in the PP(Fig.1D).Further,evidence of distal mucosal immunity was obtained through the presence of Norwalk specific IgA in the supernatants of PP and small intestine organ cultures from animals that were given the vaccine with the adjuvant intranasally(Fig.1C).These distal immune responses in the PP and SI were generated in mice that received5g of vaccine intranasally. Considering that we were previously unable to detect any responses to50g of NV-VLP delivered orally,we believe that the responses observed in the PP and SI following in-tranasal delivery did not result from passage of the vac-cine into the gastrointestinal tract during intranasal delivery. Thus,generation of mucosal immunity at the distal site is attributed to the ability of lymphoid cells to migrate specif-ically to various mucosal compartments that form the com-mon mucosal immune system[23].Oral immunization,especially in the case of non-replicating antigens,presents several challenges that must be overcome to achieve efficacious vaccination.Most im-portantly,the immunogen must maintain its native structure and antigenicity as it makes its way past the high acidic environment of the stomach,and it must also be stable to proteolytic enzyme digestion in the gut.An earlier report [18]suggests that NV-VLPs are stable at the acidic pH of the stomach;it is possible that some degradation of the VLPs may occur as these particles traverse the gastroin-testinal tract.Also,NV-VLPs have been shown to be safe and immunogenic when administered by the oral route in humans[22].In mice,a low dose of50g of NV-VLP given along with a pretreatment of sodium bicarbonate did not give an immune response(data not shown)but,a dose of200g of NV-VLP given with a pretreatment with sodium bicarbonate and adjuvant induces a cellular and mucosal humoral response.This response is much better than the one obtained when the vaccine is given without a pretreatment of the sodium bicarbonate at the same dose. Oral delivery of NV-VLP combined with CT-E29H gen-erated a strong local humoral response as demonstrated by presence of antigen-specific IgA antibodies in fecal extracts and IgA secretory cells in PP.We compared the effect of NV-VLP vaccine formulated with four different doses of CT-E29H(1,5,10and50g per dose)on mucosal,cellular and humoral responses.The10g dose of CT-E29H gen-erated a significantly higher response in proliferation assay and yielded higher antigen-specific IgA secreting cells in PP as compared to the other doses of CT-E29H in the for-mulation.Although,the sera antibody titer for animals that received the vaccine formulated with50g of CT-E29H ap-pears to be higher(Fig.4),than the sera titer from animals that received the vaccine formulation at10g the difference was not statistically significant.The lower dose(10g)of CT mutant generated higher cellular(Fig.2A and B)and humoral responses(Figs.2C and3)as compared to dose of 50g of CT mutant.This was an intriguing observation as in previous studies we have found a linear relationship be-tween the dose of the CT mutant and the systemic immune responses when5,100,200g doses of CT-E29H were ad-ministered by the intramuscular route.Oral route of delivery also showed elevated levels of IL-4producing cells.This ob-servation,combined with high titers of IgG1subclass of an-tibody(data not shown)detected in the serum suggests that CT-E29H directs a predominantly Th2like response.This is in line with enhanced mucosal Th2type response reported by Yamamoto et al.[24]for another non-toxic mutant of CT. However,unlike the diverse mucosal responses generated by the nasal route of delivery,there was no evidence of an anti-NV response in the respiratory tract(data not shown) by the oral route of delivery suggesting that the intranasal route might be the preferred route for vaccine delivery.Thus, our results provide evidence for CT-E29H being a potent mucosal adjuvant when combined with NV-VLP vaccine. References[1]Lange S,Holmgren J.Protective antitoxic cholera immunity in mice;influence of route and number of immunizations and mode of action of protective antibodies.Acta Pathol Microbiol Immunol Scand C 1978;86(C):145–52.[2]Pierce NF.The role of antigen form and function in primary andsecondary intestinal immune responses to cholera toxin and toxoid in rats.J Exp Med1978;148(1):195–206.[3]Lycke N,Lindholm L,Holmgren J.IgA isotype restriction in themucosal but not in the extramucosal immune response after oral immunization with cholera toxin or cholera toxin B subunit.Int Arch Allergy Appl Immunol1983;72(2):119–27.[4]Kateley JR,Kasarov L,Friedman H.Modulation of in vivo antibodyresponses to cholera toxin.J Immunol1975;114(1Pt1):81–4. [5]Holmgren J,Lindholm L.Cholera toxin,ganglioside receptors andthe immune response.Immunol Commun1976;5(9):737–56.[6]Pizza M,Giuliani MM,Fontana MR,Monaci E,Douce G,DouganG,et al.Mucosal vaccines:non toxic derivatives of LT and CT as mucosal adjuvants.Vaccine2001;19(17–19):2534–41.[7]Douce G,Fontana M,Pizza M,Rappuoli R,Dougan G.Intranasalimmunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin.Infect Immun1997;65(7):2821–8.[8]Sack DA,Sack RB.Test for enterotoxigenic Escherichia coli usingY-1adrenal cells in miniculture.Infect Immun1975;11(2):334–6.[9]Siadat-Pajouh M,Cai L.Protective efficacy of rotavirus2/6-virus-likeparticles combined with CT-E29H,a detoxified cholera toxin adjuvant.Viral Immunol2001;14(1):31–47.[10]Tebbey PW,Scheuer CA,Peek JA,Zhu D,LaPierre NA,Green BA,et al.Effective mucosal immunization against respiratory syncytial virus using purified F protein and a genetically detoxified cholera holotoxin,CT-E29H.Vaccine2000;18(24):2723–34.[11]Jobling MG,Holmes RK.Analysis and structure and function of theB subunit of cholera toxin by the use to site-directed mutagenesis.Mol Microbiol1991;5(7):1755–67.[12]Kapikian AZ,Estes MK,Chanock RM.Norwalk group of viruses.In:Fields BN,Knipe DM,Howley PM,editors.Fields virology.Philadelphia:Raven Press;1996,p.783–810.。
植物SNARE 蛋白的结构与功能
植物学通报 2005, 22 (6): 715 ̄722 Chinese Bulletin of Botany专题介绍植物 SNARE 蛋白的结构与功能①鲍永美 王州飞 张红生 ②(南京农业大学作物遗传与种质创新国家重点实验室 南京 210095)摘要在真核生物细胞囊泡运输过程中的膜融合主要是由SNARE蛋白介导的, SNARE蛋白的结构高度保守。
研究发现, 植物中的SNARE蛋白促进植物细胞板形成, 能与离子通道蛋白相互作用, 有利于 植物的正常生长发育, 能提高植物的抗病性及参与植物的向重力性作用。
应用基因组学和蛋白质组学 技术结合细胞学水平上的分析方法有助于深入揭示植物SNARE蛋白家族成员的功能, 明确SNARE蛋 白在信号转导途径中的作用, 阐明动植物免疫系统的区别和联系。
关键词 SNARE蛋白, 结构, 功能Structure and Function of SNAREs in PlantBAO Yong-Mei WANG Zhou-Fei ZHANG Hong-Sheng②(State Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University, Nanjing 210095)AbstractThe membrane fusion in vesicle trafficking in the cells of eukaryotic organisms ismediated by soluble-N-ethyl-maleimide-sensitive fusion protein attachment protein receptor (SNARE) proteins, which are highly conserved in their structures from various species, including yeast, animals and plants. Increasing research has demonstrated many tissue- or subcellularspecific components of SNAREs involved in the formation of the cell plate, interacting with ion channel proteins, and gravity sensing in plants. SNARE proteins might play important roles in plant growth, development and response to abiotic and biotic stresses. The application of genomics and proteomics approaches, as well cytological methods, will accelerate our understanding of diverse functions of the plant SNARE family and their specific location in the signal transduction pathway, and the differentiation and relation between animal and plant immunity systems. Key words SNARE protein, Structure, Function 植物细胞内可溶性物质的运输主要是通 过细胞内的囊泡(vesicles)在不同细胞器膜结 构间穿梭运输而实现(Sanderfoot et al., 1999), 囊泡充当了整个系统的运输工具。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
CELL STRUCTURE AND FUNCTION 28: 399–417 (2003) REVIEW© 2003 by Japan Society for Cell Biology
Vesicle-mediated Protein Transport Pathways to the Vacuole in Schizosaccharomyces pombe
Kaoru Takegawa*, Tomoko Iwaki, Yasuko Fujita, Tomotake Morita, Akira Hosomi, andNaotaka Tanaka
Department of Life Sciences, Faculty of Agriculture, Kagawa University, Miki-cho, Kagawa 761-0795, Japan
ABSTRACT.The vacuole of Saccharomyces cerevisiae plays essential roles not only for osmoregulation and ionhomeostasis but also down-regulation (degradation) of cell surface proteins and protein and organellar turnover.Genetic selections and genome-wide screens in S. cerevisiae have resulted in the identification of a large number ofgenes required for delivery of proteins to the vacuole. Although the complete genome sequence of the fission yeastSchizosaccharomyces pombe has been reported, there have been few reports on the proteins required for vacuolarprotein transport and vacuolar biogenesis in S. pombe. Recent progress in the S. pombe genome project of hasrevealed that most of the genes required for vacuolar biogenesis and protein transport are conserved between S.pombe and S. cerevisiae. This suggests that the basic machinery of vesicle-mediated protein delivery to the vacuoleis conserved between the two yeasts. Identification and characterization of the fission yeast counterparts of thebudding yeast Vps and Vps-related proteins have facilitated our understanding of protein transport pathways tothe vacuole in S. pombe. This review focuses on the recent advances in vesicle-mediated protein transport to thevacuole in S. pombe.
Key words:Vacuolar protein sorting/Schizosaccharomyces pombe/sorting signal/glycosylation
IntroductionEukaryotic cells are characterized by a number of differ-ent membrane-bound compartments, which serve specialroles in the cell. In the yeast Saccharomyces cerevisiae, thevacuole is the most prominent organelle and is morphologi-cally distinguished by its large volume of about one-quartercomparing the total cell volume. The vacuole of S.cerevisiae is an acidic organelle and its internal pH is main-tained at 6.2 by a vacuolar-type protein-pumping ATPase.The vacuole also serves as an important storage reservoirfor amino acids, small ions and polyphosphates, and isessential for osmoregulation and ion homeostasis (Jones etal., 1997). Resident vacuolar proteins pass through the earlystages of the secretory pathway and are sorted in the late
Golgi, away from proteins destined for the cell surface(Klionsky et al., 1990). This routing of proteins to the vacu-ole is one of the major diversions in protein flow throughthe secretory pathway and serves as an excellent paradigmfor protein sorting processes.In an effort to identify the trans-acting cellular machineryresponsible for this localization process, genetic selectionsin S. cerevisiae were used to isolate mutants that missortand secrete the vacuolar hydrolase carboxypeptidase Y(CPY) (Bankaitis et al., 1986; Rothman and Stevens, 1986).Many of the resulting vacuolar protein-sorting (vps)mutants were also isolated with screens for vacuolar pepti-dase deficiencies (pep; Jones, 1977) and vacuolar morpho-logical defects (vam; Wada et al., 1992). Together, thesemutants define more than 40 complementation groups andhave been categorized into six classes (A–F) with respect totheir vacuolar protein sorting, morphology, and acidificationdefects (Banta et al., 1988; Raymond et al., 1992). Recently,genome-wide screens for mutants defective in vacuolar pro-tein sorting and vacuole morphology have been reported(Avano et al., 2002; Seeley et al., 2002; Bonangelino etal., 2002). Bonangelino et al. identified 362 mutant strains(7.8% of the total) that secreted various amounts of CPY to
*To whom correspondence should be addressed: Kaoru Takegawa,Department of Life Sciences, Faculty of Agriculture, Kagawa University,Miki-cho, Kagawa 761-0795, Japan.Tel: +81–87–891–3116, Fax: +81–87–891–3021E-mail: takegawa@ag.kagawa-u.ac.jpAbbreviations: VPS, vacuolar protein sorting; MVB, multivesicularbody; CPY, carboxypeptidase Y; ER, endoplasmic reticulum; GFP, greenfluorescent protein; PtdIns, phosphatidylinositol; SNARE, soluble NSFattachment protein receptor.
