维莫非尼片说明书
M-莫达非尼胶囊 说明书

核准日期:警告:莫达非尼胶囊说明书请仔细阅读说明书并在医师指导下使用已有使用莫达非尼后出现严重的、威胁生命的皮疹的报导,包括史蒂文斯- 约翰逊(Stevens - Johnson)综合征、中毒性表皮坏死松解症和伴有嗜酸性粒细胞增多和全身症状的药物皮疹。
这些严重皮疹的发生率的底值估计约为每百万人群中有1-2 例。
没有已知的因素可以预测莫达非尼所致的严重皮疹的发生率和程度。
几乎所有使用莫达非尼后出现皮疹的病例均发生在治疗开始后的1-5 周内。
然而,偶有在长时间治疗之后发生的病例(如3 个月)。
因此治疗的持续时间并不能作为一个预测首次发生皮疹风险的指标。
通常情况下在皮疹发生之后应立即停药,除非能够确定皮疹的发生与莫达非尼无关。
即使停用莫达非尼也不一定能够阻止皮疹进展到威胁生命或导致永久致残或毁容的程度。
莫达非尼禁用于治疗任何儿科适应症。
【药品名称】通用名:莫达非尼胶囊英文名:Modafinil Capsule汉语拼音:Modafeini Jiaonang【成份】化学名称:2- [(二苯甲基)亚磺酰基]乙酰胺。
化学结构式为:分子式:C H NO S15 15 2分子量:273.35【性状】本品内容物为白色或类白色粉末。
[适应症]本品适用于阻塞性睡眠呼吸暂停引起嗜睡的成年患者的促醒。
莫达非尼用于辅助治疗阻塞性睡眠呼吸暂停。
如果病人选择连续气道正压通气(continuous positive airway pressure,CPAP)治疗,在使用本品前应给 CPAP 足够的治疗时间发挥最大效应。
如果莫达非尼用作 CPAP 的辅助治疗,则必须定期评估 CPAP 的依从性。
任何情况下谨慎诊断和治疗根本的睡眠障碍是致关重要的。
处方医生应意识到有些患者可能有不止一种的睡眠障碍导致其嗜睡。
长期使用莫达非尼的效应在安慰剂对照试验中尚未得到系统地评估。
选择长期使用该处方药治疗阻塞性睡眠呼吸暂停的医生需对用药个人定期进行长期使用本品的再评价。
安今益-mims

制造商
拜耳医药保健 (Bayer)
成份
每片含雌二醇 Estradiol 1mg, 屈螺酮 Drospirenone 2mg
适应症
用于绝经超过1年的女性所出现的雌激素缺乏症状的激素替代治疗。
用量
尚未使用雌激素或复方激素制剂的妇女可在任何时候开始使用 ;使用HRT的妇女应在完成当前治疗周期后开始使用。每日1片,连服28天,即1盒药服完后立即开始服用下1盒。如果发生1片药漏服的情况,应尽快服用。如果漏服时间>24小时,不需补服。
制造商:
拜耳医药保健 (Bayer)
不良反应
主要为乳房疼痛、出血和点滴出血,不规则出血常常随着连续治疗而缓解。其他常见不良反应包括抑郁、情绪不稳、神经质、头痛、腹痛、恶心、腹胀、良性乳房肿瘤、乳房胀大、子宫肌瘤增大、子宫颈良性肿瘤、月经紊乱、生殖道分泌物。参见产品说明书。
药物相互作用
慎与具有肝酶诱导活性的药物(如乙内酰脲类、巴比妥类、去氧苯巴比妥、卡马西平和利福平),某些抗生素(如青霉素和四环素),西咪替丁、酮康唑等CYP 3A4抑制剂合用。用药期间快速饮酒可使循的血压下降现象。参见产品说明书。
注意事项
本药不能作为避孕药使用。治疗期间如果发现下列情况,应立即停药 :偏头痛或首次出现频繁的异常严重的头痛,或有其他可能是脑血管闭塞的前驱症状时 ;于妊娠期或既往使用性激素时首发的胆汁淤积性黄疸或胆汁淤积性瘙痒再次发作 ;发生血栓性疾病,或可疑血栓形成。当出现胆囊疾病、痴呆,或其风险因素出现或恶化时,应考虑是否有必要停止治疗。
服药与进食
服药不受进食影响 (最好在每日同一时间服药。).
禁忌
肿瘤特药服务

肿瘤特药服务服务内容当用户在等待期后经二级或二级以上公立医院专科医生初次诊断罹患保险合同所定义的恶性肿瘤时,联合全国逾200+家的院边及DTP药房,提供院外药店取药和送药到家的服务。
药品目录本服务提供的特药覆盖治疗期内国内已上市的抗癌靶向药物及免疫治疗药物,并持续更新。
序号商品名通用名平均月治疗费用1易瑞沙吉非替尼片1641元2伊瑞可吉非替尼片1494元3特罗凯盐酸厄洛替尼片18800元4凯美纳盐酸埃克替尼片5765元5吉泰瑞马来酸阿法替尼片6000元6泰瑞沙甲磺酸奥希替尼片15300元7多泽润达可替尼16980元8爱必妥西妥昔单抗注射液25900-28490元9泰欣生尼妥珠单抗注射液13600元10赛可瑞克唑替尼胶囊15600元11安圣莎盐酸阿来替尼胶囊15632元12赞可达塞瑞替尼胶囊29700元13万珂注射用硼替佐米11280-22560元/3周14昕泰注射用硼替佐米8560-17120元/3周15千平注射用硼替佐米8400-16800元/3周16齐普乐注射用硼替佐米8360-16720元/3周17益久注射用硼替佐米8300-16700元/3周18恩莱瑞枸橼酸伊沙佐米胶囊14799元19赫赛汀注射用曲妥珠单抗前三周11000元,后续每三周5500元(三周方案较常用)20帕捷特帕妥珠单抗注射液前三周共9900元,后续治疗每三周4950元21泰立沙甲苯磺酸拉帕替尼片10500元22艾瑞妮马来酸吡咯替尼片11351元23安维汀贝伐珠单抗注射液19340元/3周24福可维盐酸安罗替尼胶囊6818元/3周25爱优特呋喹替尼胶囊7938元/月26艾坦甲磺酸阿帕替尼片12249元/月27拜万戈瑞戈非尼片16464元/月28多吉美甲苯磺酸索拉非尼片11400元29索坦苹果酸舒尼替尼胶囊11573-13950元30维全特培唑帕尼片23200元31乐卫玛甲磺酸仑伐替尼胶囊50400元32英立达阿昔替尼片12420元33格列卫甲磺酸伊马替尼片32000元34昕维甲磺酸伊马替尼片3800元35诺利宁甲磺酸伊马替尼片3120元36格尼可甲磺酸伊马替尼胶囊3560元37依尼舒达沙替尼片7038元38达希纳尼洛替尼胶囊36100元39施达赛达沙替尼片14041元40可瑞达帕博利珠单抗注射液35836元/月41欧狄沃纳武利尤单抗注射液37040元/月42拓益特瑞普利单抗注射液14400元/月43达伯舒信迪利单抗注射液5686元/3周44艾瑞卡注射用卡瑞利珠单抗39600元/月45飞尼妥依维莫司片18080元46佐博伏维莫非尼片32880元47利普卓奥拉帕利片20280元48美罗华利妥昔单抗注射液16062元/3周49汉利康利妥昔单抗注射液13048元/月50亿珂伊布替尼胶囊22680元51爱谱沙西达本胺片18480元52瑞复美来那度胺胶囊21644元53立生来那度胺胶囊3960元54安显来那度胺胶囊5380元55齐普怡来那度胺胶囊3980元/月56恩度重组人血管内皮抑制素注射液8820元/3周57爱博新哌柏西利胶囊29800元58泽珂阿比特龙片17390.40元服务项目病案管理:医生团队将从第一次接触患者起通过线上一对一为患者提供病程管理服务,包含治疗方案记录及跟进、用药及不良反应的问询回访、安抚家属处方审核:由专业的药师提供肿瘤特药的处方协助审核服务,从医药专业方向判定用药合理性,并给予专业判断意见特药直赔:为客户提供药品直赔服务,无须用户垫付药费送药上门:对于特药处方审核通过的,提供送药上门便利服务服务安排时间病案管理用药全程处方审核资料齐全后1个工作日特药直赔确认客户信息后,1个工作日协调药房备药(如当地药房缺货需2-3天)送药上门处方审核通过后,1个工作日服务有效期保单生效起一年内有效。
维莫非尼片说明书

核准日期:2017年3月10日修改日期:2017年5月26日2017年11月29日2018年7月24日2020年2月13日2021年2月26日2022年1月17日2022年2月22日维莫非尼片说明书请仔细阅读说明书并在医师指导下使用【药品名称】通用名:维莫非尼片商品名:佐博伏®,英文商品名Zelboraf®英文名:Vemurafenib film-coated tablets汉语拼音:Weimofeini Pian【成份】本品主要活性成分为维莫非尼,以维莫非尼和琥珀酸醋酸羟丙甲纤维素固体分散体存在。
化学名称:丙烷-1-磺酸{3-[5-(4-氯苯基)-1H- 吡咯并[2,3-b]吡啶-3-羰基]-2,4-二氟代苯基}- 酰胺。
化学结构式:分子式:C23H18ClF2N3O3S分子量:489.93【性状】两面凸起、粉白色至橙白色的薄膜衣片。
【适应症】佐博伏®适用于治疗经CFDA批准的检测方法确定的BRAF V600突变阳性的不可切除或转移性黑色素瘤。
【规格】240 mg【用法用量】患者必须经由CFDA批准的检测方法确定的证明肿瘤为BRAF V600突变阳性,才可使用佐博伏®治疗。
佐博伏®不能用于BRAF野生型黑色素瘤患者。
首剂药物应在上午服用,第二剂应在此后约12小时,即晚上服用。
每次服药均可随餐或空腹服用。
用一杯水送服药物,服药时整片吞下佐博伏®片剂。
不应咀嚼或碾碎佐博伏®片剂。
标准剂量佐博伏®的推荐剂量为960 mg(四片240 mg片剂),每日两次。
治疗持续时间建议佐博伏®治疗应持续至疾病进展或发生不可接受的毒性反应(参见表1和表2)。
漏服如果漏服一剂计划的药物,可在下一剂服药4小时以前补服漏服的药物,以维持每日两次的给药方案。
不应同时服用两剂药物。
呕吐如果佐博伏®服药后发生呕吐,患者不应追加剂量,而应按常规剂量继续治疗。
纳入门诊特殊管理的药品
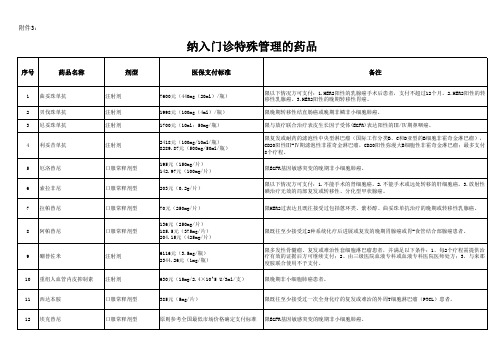
纳入门诊特殊管理的药品
序号 药品名称 剂型 医保支付标准 备注
限以下情况方可支付:1.HER2阳性的乳腺癌手术后患者,支付不超过12个月。2.HER2阳性的转 移性乳腺癌。3.HER2阳性的晚期转移性胃癌。 限晚期转移性结直肠癌或晚期非鳞非小细胞肺癌。 限与放疗联合治疗表皮生长因子受体(EGFR)表达阳性的Ⅲ/Ⅳ期鼻咽癌。 限复发或耐药的滤泡性中央型淋巴瘤(国际工作分类B、C和D亚型的B细胞非霍奇金淋巴瘤), CD20阳性Ⅲ-Ⅳ期滤泡性非霍奇金淋巴瘤,CD20阳性弥漫大B细胞性非霍奇金淋巴瘤;最多支付 8个疗程。 限EGFR基因敏感突变的晚期非小细胞肺癌。 限以下情况方可支付:1.不能手术的肾细胞癌。2.不能手术或远处转移的肝细胞癌。3.放射性 碘治疗无效的局部复发或转移性、分化型甲状腺癌。 限HER2过表达且既往接受过包括蒽环类、紫杉醇、曲妥珠单抗治疗的晚期或转移性乳腺癌。
9
硼替佐米
注射剂
限多发性骨髓瘤、复发或难治性套细胞淋巴瘤患者,并满足以下条件:1、每2个疗程需提供治 疗有效的证据后方可继续支付;2、由三级医院血液专科或血液专科医院医师处方;3、与来那 度胺联合使用不予支付。 限晚期非小细胞肺癌患者。
10
重组人血管内皮抑制素
注射剂
630元(15mg/2.4×10^5 U/3ml/支)
30
塞瑞替尼
口服常释剂型
198元(150mg/粒) 448元(50mg/粒); 359.4元(37.5mg/粒); 263.5元(25mg/粒); 155元(12.5mg/粒) 112元(240mg/片) 189元(140mg/粒) 4933元(4mg/粒); 3957.9元(3mg/粒); 3229.4元(2.3mg/粒) 2980元(5ml:3750IU/支); 1477.7元(2ml:1500IU/支) 7911元(30mg/瓶); 5800元(20mg/瓶)
新型抗肿瘤药物临床应用指导原则(2018年版)

关于印发新型抗肿瘤药物临床应用指导原则(2018年版)的通知国卫办医函〔2018〕821号各省、自治区、直辖市及新疆生产建设团卫生计生委:为规范新型抗肿瘤药物临床应用,我委组织原国家卫生计生委合理用药专家委员会牵头制定了《新型抗肿瘤药物临床应用指导原则(2018年版)》(可在我委官方网站“医政医管”栏目下载)。
现印发你们,请认真组织学习,贯彻执行。
该指导原则将定期修订更新,指导临床合理应用抗肿瘤药物。
附件:新型抗肿瘤药物临床应用指导原则(2018年版)2018年9月14日(信息公开形式:主动公开)新型抗肿瘤药物临床应用指导原则(2018年版)目录第一部分新型抗肿瘤药物临床应用指导原则抗肿瘤药物临床应用的基本原则一、病理组织学确诊后方可使用 (01)二、基因检测后方可使用 (02)三、严格遵循适应证用药 (04)四、体现患者治疗价值 (04)五、特殊情况下的药物合理使用……………………………………………………………六、重视药物相关性不良反应………………………………………………………………抗肿瘤药物临床应用管理05 05一、医疗机构建立抗肿瘤药物临床应用管理体系 (06)二、抗肿瘤药物临床应用实行分级管理 (09)三、细胞或组织病理学诊断 (10)四、培训、评估和督查 (10)第二部分各系统肿瘤的药物临床应用指导原则呼吸系统肿瘤用药一、吉非替尼 (12)二、厄洛替尼 (14)三、埃克替尼 (15)四、马来酸阿法替尼 (16)五、奥希替尼 (17)六、克唑替尼 (18)七、贝伐珠单抗 (20)八、重组人血管内皮抑制素 (21)九、盐酸安罗替尼 (21)十、塞瑞替尼…………………………………………………………………………………十一、纳武利尤单抗…………………………………………………………………………22 24消化系统肿瘤用药一、瑞戈非尼 (26)二、甲苯磺酸索拉非尼 (28)三、曲妥珠单抗 (29)四、甲磺酸阿帕替尼 (29)五、苹果酸舒尼替尼 (30)六、甲磺酸伊马替尼 (31)七、依维莫司 (32)八、贝伐珠单抗 (33)九、西妥昔单抗 (35)血液肿瘤用药一、甲磺酸伊马替尼 (37)二、达沙替尼 (39)三、尼洛替尼 (39)四、利妥昔单抗 (40)五、西达本胺 (42)六、伊布替尼 (43)七、硼替佐米 (45)八、来那度胺 (46)九、沙利度胺 (48)十、芦可替尼 (49)泌尿系统肿瘤用药一、依维莫司 (50)二、甲苯磺酸索拉非尼 (51)三、苹果酸舒尼替尼 (52)四、阿昔替尼 (52)五、培唑帕尼 (53)乳腺癌用药一、曲妥珠单抗 (54)二、甲苯磺酸拉帕替尼 (56)皮肤及软组织肿瘤用药一、甲磺酸伊马替尼 (58)二、维莫非尼 (59)三、依维莫司 (60)头颈部肿瘤用药一、尼妥珠单抗 (62)二、甲苯磺酸索拉非尼 (63)第一部分新型抗肿瘤药物临床应用指导原则为规范新型抗肿瘤药物临床应用,提高肿瘤合理用药水平,保障医疗质量和医疗安全,维护肿瘤患者健康权益,特制定新型抗肿瘤药物临床应用指导原则。
莫达菲尼
详细说明特价供应产品莫达非尼片【通用名称】莫达非尼片【中文名称】莫达非尼【英文名称】Modafinil【英文别名】2-[(Diphenylmethyl)sulfinyl]-acetamide【汉语拼音】Modafeini【适应症状】抑郁症患者.特发性嗜睡或发作性睡眠症.【用法用量】口服.每日睡前1.5小时服50~100mg,每4~5天增加50毫克,直至最适剂量(每日200~400毫克)【性状】本品为白色或类白色的结晶性粉末;无臭、几乎无味.本中种在甲醇中略溶,在乙醇或乙酸乙酯中微溶,在水中几乎不溶.【注意事项】严重肝损害的患者剂量减半,肾功能不全和老年患者服用剂量要酌减,左室肥大、有缺血性心电图改变、胸痛、心律失常或有临床表现的二尖瓣脱垂的患者及近期发生心肌梗塞、不稳定型心绞痛或有精神病史者禁用或慎用.【包装规格】胶囊剂(片剂):每粒(片)含本品20mg,100mg,200mg,瓶装100粒(片)【剂型】片剂(胶囊剂)【价格】20mg×100粒(片):¥180/瓶100mg×100粒(片):¥860/瓶200mg×100粒(片):¥1100/瓶20mg×1000粒(片):¥1800/瓶莫达非尼片药品名称:通用名称:莫达非尼片英文名称:Modafinil适应症:抑郁症患者。
特发性嗜睡或发作性睡眠症用法用量:口服。
每日睡前1.5小时服50~100mg,每4~5天增加50毫克,直至最适剂量(每日200~4 00毫克)注意事项:严重肝损害的患者剂量减半,肾功能不全和老年患者服用剂量要酌减,左室肥大、有缺血性心电图改变、胸痛、心律失常或有临床表现的二尖瓣脱垂的患者及近期发生心肌梗塞、不稳定型心绞痛或有精神病史者禁用或慎用不良反应:本品具有较大的安全性,对血压和心率无影响,无活动增多、耐受性或反弹性思睡等不良反应,也无潜在的成瘾性。
主要不良反应有恶心、神经过敏和焦虑,加量过快服药可出现轻至中度头痛。
依维莫司说明书,印度飞尼妥(依维莫司片)使用说明书
依维莫司说明书,印度飞尼妥(依维莫司片)使用说明书依维莫司片商品名:飞尼妥,AFINITOR汉语拼音称:YiWeiMiSiPian英文名称:Everolimus Tablets有效成份:依维莫司性状:飞尼妥(依维莫司)为白色或类白色片剂。
研发厂家:瑞士诺华制药版本:诺华依维莫司、印度依维莫司对接机构:印丽康医疗【依维莫司飞尼妥适应症】依维莫司主要适用于治疗以下患者:1、既往接受舒尼替尼或索拉非尼治疗失败的晚期肾细胞癌成人患者。
2、不可切除的、局部晚期或转移性的、分化良好的(中度分化或高度分化)进展期胰腺神经内分泌瘤成人患者。
3、需要治疗干预但不适于手术切除的结节性硬化症(TSC)相关的室管膜下巨细胞星形细胞瘤(SEGA)成人和儿童患者。
【印度依维莫司微信:ybk577】【依维莫司飞尼妥用法用量】依维莫司片(飞尼妥)推荐剂量为每次口服10mg,每天一次,与食物同服或不同服皆可。
在每天同一时间服用。
用一杯水整片吞服片剂,不应咀嚼或压碎。
对于无法吞咽片剂的患者,用药前将依维莫司片剂放入一杯水中(约30ml)轻轻搅拌至完全溶解(大约需要7分钟)后立即服用。
用相同容量的水清洗水杯并将清洗液全部服用,以确保服用了完整剂量。
依维莫司需要吃多久才能停药?只要患者服用依维莫司存在临床获益就应持续治疗,或使用至出现不能耐受的毒性反应时。
【依维莫司副作用】服用依维莫司最常见不良反应(发生率≥30%)是咽炎、感染、无力、疲乏、咳嗽和腹泻。
【注意事项】1、非-感染性肺炎:建议监测临床症状,如果出现非-感染性肺炎,减低飞尼妥(依维莫司)剂量或停用飞尼妥(依维莫司)直至症状缓解。
2、感染:飞尼妥会增加感染风险。
建议监测体征和症状,及时治疗。
3、妊娠中使用:当给予妊娠妇女飞尼妥(依维莫司)时可能危害胎儿。
应告知妇女飞尼妥(依维莫司)对胎儿的潜在危害。
【禁忌】禁用于对飞尼妥及其它雷帕霉素衍生物或任何辅料过敏的患者。
诺华_依维莫司片英文说明书_2015.05.11
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ZORTRESS® (everolimus) safely and effectively. See full prescribing information for ZORTRESS.ZORTRESS (everolimus) tablets for oral useInitial U.S. Approval: 2010WARNING: MALIGNANCIES AND SERIOUS INFECTIONS,KIDNEY GRAFT THROMBOSIS; NEPHROTOXICITY; AND MORTALITY IN HEART TRANSPLANTATIONSee Full Prescribing Information for Complete Boxed Warning.• Only physicians experienced in immunosuppressive therapy and management of transplant patients should use Zortress. (5.1)• Increased susceptibility to infection and the possible development of malignancies may result from immunosuppression. (5.2, 5.3)• Increased incidence of kidney graft thrombosis. (5.4)• Reduced doses of cyclosporine are required for use in combination with Zortress in order to reduce nephrotoxicity. (2.4, 2.5, 5.6, 12.7,12.8)• Increased mortality in a heart transplant clinical trial. Use in heart transplantation is not recommended. (5.7)---------------------------RECENT MAJOR CHANGES--------------------------Dosage and Administration (2) 1/2015 Warnings and Precautions, Management ofImmunosuppression (5.1) 1/2015 Warnings and Precautions, Interstitial Lung Disease/Non-Infectious Pneumonitis (5.10) 11/2015---------------------------INDICATIONS AND USAGE---------------------------• Zortress is indicated for the prophylaxis of organ rejection in adult patients: • Kidney transplant: at low-moderate immunologic risk. Use in combination with basiliximab, cyclosporine (reduced doses) and corticosteroids. (1.1) • Liver transplant: Administer no earlier than 30 days post-transplant. Use in combination with tacrolimus (reduced doses) and corticosteroids. (1.2, 5.5) Limitations of Use (1.3)Safety and efficacy has not been established in the following:• Kidney transplant patients at high immunologic risk.• Recipients of transplanted organs other than kidney or liver• Pediatric patients (<18 years)-------------------------DOSAGE AND ADMINISTRATION--------------------• Kidney transplantation: starting oral dose of 0.75 mg twice daily as soon as possible after transplantation. (2.1)• Liver transplantation: starting oral dose of 1.0 mg twice daily starting 30 days after transplantation. (2.2)• Monitor everolimus concentrations: Adjust maintenance dose to achieve trough concentrations within the 3-8 ng/mL target range (using LC/MS/MS assay method (2.1, 2.2, 2.3)• Administer consistently with or without food at the same time as cyclosporine or tacrolimus. (2.6, 12.3)• Mild hepatic impairment: Reduce initial daily dose by one-third (2.7) • Moderate or Severe hepatic impairment: Reduce initial daily dose by one-half. (2.7, 12.6)-------------------------DOSAGE FORMS AND STRENGTHS------------------Zortress is available as 0.25 mg, 0.5 mg, and 0.75 mg tablets. (3)----------------------------------CONTRAINDICATIONS--------------------------• Hypersensitivity to everolimus, sirolimus, or to components of the drug product. (4)----------------------------WARNINGS AND PRECAUTIONS------------------• Angioedema (increased risk with concomitant ACE inhibitors): Monitor for symptoms and treat promptly. (5.8)• Delayed Wound Healing/Fluid Accumulation: Monitor symptoms; treat promptly to minimize complications. (5.9)• Interstitial Lung Disease/Non-Infectious Pneumonitis: Monitor for symptoms or radiologic changes; manage by dose reduction or discontinuation until symptoms resolve; consider use of corticosteroids. (5.10)• Hyperlipidemia (elevations of serum cholesterol and triglycerides): Monitor and consider anti-lipid therapy. (5.11)• Proteinuria (increased risk with higher trough concentrations): Monitor urine protein. (5.12)• Polyoma Virus Infections (activation of latent viral infections; BK-virus associated nephropathy): Consider reducing immunosuppression. (5.13)• TMA/TTP/HUS (concomitant use with cyclosporine may increase risk): Monitor for hematological changes or symptoms. (5.15)• New Onset Diabetes After Transplantation: Monitor serum glucose. (5.16) • Male Infertility: Azospermia or oligospermia may occur. (5.17, 13.1)• Immunizations: Avoid live vaccines. (5.18)----------------------------------ADVERSE REACTIONS--------------------------Most common adverse reactions were as follows:Kidney transplantation (incidence ≥20%): peripheral edema, constipation, hypertension, nausea, anemia, UTI, and hyperlipidemia. (6.1);Liver transplantation (incidence>10%): diarrhea, headache, peripheral edema, hypertension, nausea, pyrexia, abdominal pain, and leukopenia (6.1)To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA1088 or /medwatch.-----------------------------------DRUG INTERACTIONS-------------------------Strong-moderate CYP3A4 inhibitors (e.g., cyclosporine, ketoconazole, erythromycin, verapamil) and CYP3A4 inducers (e.g., rifampin) may affect everolimus concentrations. (7.1) Consider Zortress dose adjustment (5.14)---------------------------USE IN SPECIFIC POPULATIONS-------------------• Pregnancy: Based on animal data may cause fetal harm. (8.1)• Nursing Mothers: Discontinue drug or nursing. (8.3)See 17 for PATIENT COUNSELING INFORMATION and Medication GuideRevised: 11/2015FULL PRESCRIBING INFORMATION: CONTENTS* WARNING: MALIGNANCIES AND SERIOUS INFECTIONS, KIDNEY GRAFT THROMBOSIS; NEPHROTOXICITY; AND MORTALITY IN HEART TRANSPLANTATION1 INDICATIONS AND USAGE1.1 Prophylaxis of Organ Rejection in Kidney Transplantation1.2 Prophylaxis of Organ Rejection in Liver Transplantation1.3 Limitations of Use2 DOSAGE AND ADMINISTRATION2.1 Dosage in Adult Kidney Transplant Patients2.2 Dosage in Adult Liver Transplant Patients2.3 Therapeutic Drug Monitoring -Everolimus2.4 Therapeutic Drug Monitoring-Cyclosporine in KidneyTransplant Patients2.5 Therapeutic Drug Monitoring-Tacrolimus in Liver TransplantPatients2.6 Administration2.7 Hepatic Impairment3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS4.1 Hypersensitivity Reactions5 WARNINGS AND PRECAUTIONS5.1 Management of Immunosuppression5.2 Lymphomas and Other Malignancies5.3 Serious Infections5.4 Kidney Graft Thrombosis5.5 Hepatic Artery Thrombosis5.6 Zortress and Calcineurin Inhibitor-Induced Nephrotoxicity5.7 Heart Transplantation5.8 Angioedema5.9 Wound Healing and Fluid Accumulation5.10 Interstitial Lung Disease/Non-Infectious Pneumonitis5.11 Hyperlipidemia5.12 Proteinuria5.13 Polyoma Virus Infections5.14 Interaction with Strong Inhibitors and Inducers of CYP3A45.15 Thrombotic Microangiopathy/Thrombotic ThrombocytopenicPurpura/Hemolytic Uremic Syndrome (TMA/TTP/HUS)5.16 New Onset Diabetes After Transplant5.17 Male Infertility5.18 Immunizations5.19 Interaction with Grapefruit Juice5.20 Patients with Hereditary Disorders/Other6 ADVERSE REACTIONS6.1 Serious and Otherwise Important Adverse Reactions6.2 Clinical Studies Experience6.3 Postmarketing Experience7 DRUG INTERACTIONS7.1 Interactions with Strong Inhibitors or Inducers of CYP3A4 and Pglycoprotein7.2 Cyclosporine (CYP3A4/P-gp Inhibitor and CYP3A4 Substrate)7.3 Ketoconazole and Other Strong CYP3A4 Inhibitors7.4 Erythromycin (Moderate CYP3A4 Inhibitor)7.5 Verapamil (CYP3A4 and P-gp Substrate)7.6 Atorvastatin (CYP3A4 substrate) and Pravastatin (P-gp substrate)7.7 Simvastatin and Lovastatin7.8 Rifampin (Strong CYP3A4/P-gp Inducers)7.9 Midazolam (CYP3A4/5 substrate)7.10 Other Possible Interactions7.11 Octreotide7.12 Tacrolimus8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use8.6 Hepatic Impairment8.7 Renal Impairment10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.3 Pharmacokinetics12.5 Drug-Drug Interactions12.6 Specific Populations12.7 Everolimus Whole Blood Concentrations Observed in Kidneyand in Liver Transplant Patients12.8 Cyclosporine Concentrations Observed in Kidney TransplantPatients12.9 Tacrolimus Concentrations in Liver Transplant13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIES14.1 Prevention of Organ Rejection after Renal Transplantation14.2 Prevention of Organ Rejection after Liver Transplantation16 HOW SUPPLIED/STORAGE AND HANDLING17 PATIENT COUNSELING INFORMATION* Sections or subsections omitted from the full prescribing information are not listedFULL PRESCRIBING INFORMATIONWARNING: MALIGNANCIES AND SERIOUS INFECTIONS, KIDNEY GRAFT THROMBOSIS;NEPHROTOXICITY; AND MORTALITY IN HEART TRANSPLANTATIONMalignancies and Serious Infections• Only physicians experienced in immunosuppressive therapy and management of transplant patients should prescribe Zortress. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for maintenance therapy should have completeinformation requisite for the follow-up of the patient. [See Warnings and Precautions (5.1)]• Increased susceptibility to infection and the possible development of malignancies such as lymphoma and skin cancer may result from immunosuppression. [See Warnings and Precautions (5.2 and 5.3)]Kidney Graft Thrombosis• An increased risk of kidney arterial and venous thrombosis, resulting in graft loss, was reported, mostly within the first 30 days post-transplantation. [See Warnings and Precautions (5.4)]Nephrotoxicity• Increased nephrotoxicity can occur with use of standard doses of cyclosporine in combination with Zortress.Therefore reduced doses of cyclosporine should be used in combination with Zortress in order to reduce renaldysfunction. It is important to monitor the cyclosporine and everolimus whole blood trough concentrations. [See Dosage and Administration (2.4 and 2.5) and Warnings and Precautions (5.6) and Clinical Pharmacology (12.7 and12.8)]Mortality in Heart Transplantation• Increased mortality, often associated with serious infections, within the first three months post-transplantation was observed in a clinical trial of de novo heart transplant patients receiving immunosuppressive regimens with or without induction therapy. Use in heart transplantation is not recommended. [See Warnings and Precautions (5.7)]1 INDICATIONS AND USAGE1.1 Prophylaxis of Organ Rejection in Kidney TransplantationZortress is indicated for the prophylaxis of organ rejection in adult patients at low-moderate immunologic risk receiving a kidney transplant. [See Clinical Studies (14.1)] Zortress is to be administered in combination with basiliximab induction and concurrently with reduced doses of cyclosporine and with corticosteroids. Therapeutic drug monitoring of everolimus and cyclosporine is recommended for all patients receiving these products. [See Dosage and Administration (2.2 and 2.3)] 1.2 Prophylaxis of Organ Rejection in Liver TransplantationZortress is indicated for the prophylaxis of allograft rejection in adult patients receiving a liver transplant. Zortress is to be administered no earlier than 30 days post-transplant concurrently in combination with reduced doses of tacrolimus and with corticosteroids [See Warnings and Precautions (5.5) and Clinical Studies (14.2)]. Therapeutic drug monitoring of everolimus and tacrolimus is recommended for all patients receiving these products. [See Dosage and Administration (2.3 and 2.5)]1.3 Limitations of UseThe safety and efficacy of Zortress has not been established in the following populations:Kidney transplant patients at high immunologic riskRecipients of transplanted organs other than kidney and liver [See Warnings and Precautions (5.7)]Pediatric patients (<18 years).2 DOSAGE AND ADMINISTRATIONPatients receiving Zortress may require dose adjustments based on everolimus blood concentrations achieved, tolerability, individual response, change in concomitant medications and the clinical situation. Optimally, dose adjustments of Zortress should be based on trough concentrations obtained 4 or 5 days after a previous dosing change. Dose adjustment is required if the trough concentration is below 3 ng/mL. The total daily dose of Zortress should be doubled using the available tablet strengths (0.25 mg, 0.5 mg or 0.75 mg). Dose adjustment is also required if the trough concentration is >8ng/mL on 2 consecutive measures; the dose of Zortress® should be decreased by 0.25 mg b.i.d. [See Therapeutic Drug Monitoring (2.3) and Clinical Pharmacology (12.3)]2.1 Dosage in Adult Kidney Transplant PatientsAn initial Zortress dose of 0.75 mg orally twice daily (1.5 mg per day) is recommended for adult kidney transplant patients in combination with reduced dose cyclosporine, administered as soon as possible after transplantation. [See Therapeutic Drug Monitoring (2.3, 2.4), Clinical Studies (14.1)]Oral prednisone should be initiated once oral medication is tolerated. Steroid doses may be further tapered on an individualized basis depending on the clinical status of patient and function of graft.2.2 Dosage in Adult Liver Transplant PatientsStart Zortress at least 30 days post-transplant. An initial dose of 1.0 mg orally twice daily (2.0 mg per day) is recommended for adult liver transplant patients in combination with reduced dose tacrolimus. [See Therapeutic Drug Monitoring (2.3 and 2.5), Clinical Studies (14.2)]Steroid doses may be further tapered on an individualized basis depending on the clinical status of patient and function of graft.2.3 Therapeutic Drug Monitoring -EverolimusRoutine everolimus whole blood therapeutic drug concentration monitoring is recommended for all patients. The recommended everolimus therapeutic range is 3 to 8 ng/mL. [See Clinical Pharmacology (12.7)] Careful attention should be made to clinical signs and symptoms, tissue biopsies, and laboratory parameters. It is important to monitor everolimus blood concentrations, in patients with hepatic impairment, during concomitant administration of CYP3A4 inducers or inhibitors, when switching cyclosporine formulations and/or when cyclosporine dosing is reduced according to recommended target concentrations. [See Clinical Pharmacology (12.7, 12.8)]There is an interaction of cyclosporine on everolimus, and consequently, everolimus concentrations may decrease if cyclosporine exposure is reduced. There is little to no pharmacokinetic interaction of tacrolimus on everolimus, and thus, everolimus concentrations do not decrease if the tacrolimus exposure is reduced. [See Drug Interactions (7.2)]The everolimus recommended therapeutic range of 3 to 8 ng/mL is based on an LC/MS/MS assay method. Currently in clinical practice, everolimus whole blood trough concentrations may be measured by chromatographic or immunoassay methodologies. Because the measured everolimus whole blood trough concentrations depend on the assay used, individual patient sample concentration values from different assays may not be interchangeable. Consideration of assay results must be made with knowledge of the specific assay used. Therefore, communication should be maintained with the laboratory performing the assay.2.4 Therapeutic Drug Monitoring-Cyclosporine in Kidney Transplant PatientsBoth cyclosporine doses and the target range for whole blood trough concentrations should be reduced, when given in a regimen with Zortress, in order to minimize the risk of nephrotoxicity. [See Warnings and Precautions (5.6), Drug Interactions (7.2), Clinical Pharmacology (12.8)]The recommended cyclosporine therapeutic ranges when administered with Zortress are 100 to 200 ng/mL through Month 1 post-transplant, 75 to 150 ng/mL at Months 2 and 3 post-transplant, 50 to 100 ng/mL at Month 4 post-transplant, and 25 to 50 ng/mL from Month 6 through Month 12 post-transplant. The median trough concentrations observed in the clinical trial ranged between 161 to 185 ng/mL through Month 1 post-transplant and between 111 to 140 ng/mL at Months 2 and 3 post-transplant. The median trough concentration was 99 ng/mL at Month 4 post-transplant and ranged between 46 to 75 ng/mL from Months 6 through Month 12 post-transplant. [See Clinical Pharmacology (12.8) and Clinical Studies (14.1)] Cyclosporine, USP Modified is to be administered as oral capsules twice daily unless cyclosporine oral solution or intravenous administration of cyclosporine cannot be avoided. Cyclosporine, USP Modified should be initiated as soon as possible -and no later than 48 hours -after reperfusion of the graft and dose adjusted to target concentrations from Day 5 onwards.If impairment of renal function is progressive the treatment regimen should be adjusted. In renal transplant patients, the cyclosporine dose should be based on cyclosporine whole blood trough concentrations. [See Clinical Pharmacology (12.8)]In renal transplantation, there are limited data regarding dosing Zortress with reduced cyclosporine trough concentrations of 25 to 50 ng/mL after 12 months. Zortress has not been evaluated in clinical trials with other formulations ofcyclosporine. Prior to dose reduction of cyclosporine it should be ascertained that steady-state everolimus whole blood trough concentration is at least 3 ng/mL. There is an interaction of cyclosporine on everolimus, and consequently, everolimus concentrations may decrease if cyclosporine exposure is reduced. [See Drug Interactions (7.2)]2.5 Therapeutic Drug Monitoring-Tacrolimus in Liver Transplant PatientsBoth tacrolimus doses and the target range for whole blood trough concentrations should be reduced, when given in a regimen with Zortress, in order to minimize the potential risk of nephrotoxicity. [See Warnings and Precautions (5.6), Clinical Pharmacology (12.9)]The recommended tacrolimus therapeutic range when administered with Zortress are whole blood trough (C-0h) concentrations of 3 to 5 ng/mL by three weeks after the first dose of Zortress (approximately Month 2) and through Month 12 post transplant.The median tacrolimus trough concentrations observed in the clinical trial ranged between 8.6 to 9.5 ng/mL at Weeks 2 and 4 post-transplant (prior to initiation of everolimus). The median tacrolimus trough concentrations ranged between 7 to 8.1 ng/mL at Weeks 5 and 6 post-transplant, between 5.2 to 5.6 ng/mL at Months 2 and 3 post-transplant, and between 4.3 to 4.9 ng/mL between Months 4 and 12 post-transplant. [See Clinical Pharmacology (12.9), Clinical Studies (14.2)] Tacrolimus is to be administered as oral capsules twice daily unless intravenous administration of tacrolimus cannot be avoided.In liver transplant patients, the tacrolimus dose should be based on tacrolimus whole blood trough concentrations. [See Clinical Pharmacology (12.9)]In liver transplantation, there are limited data regarding dosing Zortress with reduced tacrolimus trough concentrations of 3 to 5 ng/mL after 12 months. Prior to dose reduction of tacrolimus it should be ascertained that the steady-state everolimus whole blood trough concentration is at least 3 ng/mL. Unlike the interaction between cyclosporine and everolimus, tacrolimus does not affect everolimus trough concentrations, and consequently, everolimus concentrations do not decrease if the tacrolimus exposure is reduced.2.6 AdministrationZortress tablets should be swallowed whole with a glass of water and not crushed before use.Administer Zortress consistently approximately 12 hours apart with or without food to minimize variability in absorption and at the same time as cyclosporine or tacrolimus. [See Clinical Pharmacology (12.3)]2.7 Hepatic ImpairmentWhole blood trough concentrations of everolimus should be closely monitored in patients with impaired hepatic function. For patients with mild hepatic impairment (Child-Pugh Class A), the initial daily dose should be reduced by approximately one-third of the normally recommended daily dose. For patients with moderate or severe hepatic impairment (Child-Pugh B or C), the initial daily dose should be reduced to approximately one-half of the normally recommended daily dose. Further dose adjustment and/or dose titration should be made if a patient’s whole blood trough concentration of everolimus, as measured by an LC/MS/MS assay, is not within the target trough concentration range of 3 to 8 ng/mL. [See Clinical Pharmacology (12.6)]3 DOSAGE FORMS AND STRENGTHSZortress is available as 0.25 mg, 0.5 mg, and 0.75 mg tablets.Table 1. Description of Zortress (everolimus) TabletsDosage Strength 0.25 mg 0.5 mg 0.75 mg Appearance White to yellowish, marbled, round, flat tablets with bevelled edgeImprint “C” on one side and “NVR”on the other “CH” on one side and“NVR” on the other“CL” on one side and“NVR” on the other4 CONTRAINDICATIONS4.1 Hypersensitivity ReactionsZortress is contraindicated in patients with known hypersensitivity to everolimus, sirolimus, or to components of the drug product.5 WARNINGS AND PRECAUTIONS5.1 Management of ImmunosuppressionOnly physicians experienced in management of systemic immunosuppressant therapy in transplantation should prescribe Zortress. Patients receiving the drug should be managed in facilities equipped and staffed with adequate laboratory and supportive medical resources. The physician responsible for the maintenance therapy should have complete information requisite for the follow-up of the patient. In limited data with the complete elimination of CNI (calcineurin inhibition), there was an increased risk of acute rejection.5.2 Lymphomas and Other MalignanciesPatients receiving immunosuppressants, including Zortress, are at increased risk of developing lymphomas and other malignancies, particularly of the skin. The risk appears to be related to the intensity and duration of immunosuppression rather than to the use of any specific agent.As usual for patients with increased risk for skin cancer, exposure to sunlight and ultraviolet light should be limited by wearing protective clothing and using a sunscreen with a high protection factor.5.3 Serious InfectionsPatients receiving immunosuppressants, including Zortress, are at increased risk of developing bacterial, viral, fungal, and protozoal infections, including opportunistic infections. [See Warnings and Precautions (5.13), Adverse Reactions (6.1, 6.2)] These infections may lead to serious, including fatal, outcomes. Because of the danger of over immunosuppression, which can cause increased susceptibility to infection, combination immunosuppressant therapy should be used with caution.Antimicrobial prophylaxis for Pneumocystis jiroveci (carinii) pneumonia and prophylaxis for cytomegalovirus (CMV) is recommended in transplant recipients.5.4 Kidney Graft ThrombosisAn increased risk of kidney arterial and venous thrombosis, resulting in graft loss, has been reported, usually within the first 30 days post-transplantation. [See Boxed Warning]5.5 Hepatic Artery ThrombosisMammalian target of rapamycin (mTOR) inhibitors are associated with an increase in hepatic artery thrombosis (HAT). Reported cases mostly have occurred within the first 30 days post-transplant and most also lead to graft loss or death. Therefore, Zortress should not be administered earlier than 30 days after liver transplant.5.6 Zortress and Calcineurin Inhibitor-Induced NephrotoxicityIn kidney transplant recipients, Zortress with standard dose cyclosporine increases the risk of nephrotoxicity resulting in a lower glomerular filtration rate. Reduced doses of cyclosporine are required for use in combination with Zortress in order to reduce renal dysfunction. [See Boxed Warning, Indications and Usage (1.1), Clinical Pharmacology (12.8)]In liver transplant recipients, Zortress has not been studied with standard dose tacrolimus. Reduced doses of tacrolimus should be used in combination with Zortress in order to minimize the potential risk of nephrotoxicity. [See Indications and Usage (1.2), Clinical Pharmacology (12.9)]Renal function should be monitored during the administration of Zortress. Consider switching to other immunosuppressive therapies if renal function does not improve after dose adjustments or if the dysfunction is thought to be drug related. Caution should be exercised when using other drugs which are known to impair renal function.5.7 Heart TransplantationIn a clinical trial of de novo heart transplant patients, Zortress in an immunosuppressive regimen with or without induction therapy, resulted in an increased mortality often associated with serious infections within the first three months post-transplantation compared to the control regimen. Use of Zortress in heart transplantation is not recommended.5.8 AngioedemaZortress has been associated with the development of angioedema. The concomitant use of Zortress with other drugs known to cause angioedema, such as angiotensin converting enzyme (ACE) inhibitors may increase the risk of developing angioedema.5.9 Wound Healing and Fluid AccumulationZortress increases the risk of delayed wound healing and increases the occurrence of wound-related complications like wound dehiscence, wound infection, incisional hernia, lymphocele and seroma. These wound-related complications may require more surgical intervention. Generalized fluid accumulation, including peripheral edema (e.g., lymphoedema) and other types of localized fluid collection, such as pericardial and pleural effusions and ascites have also been reported.5.10 Interstitial Lung Disease/Non-Infectious PneumonitisA diagnosis of interstitial lung disease (ILD) should be considered in patients presenting with symptoms consistent with infectious pneumonia but not responding to antibiotic therapy and in whom infectious, neoplastic and other non-drug causes have been ruled-out through appropriate investigations. Cases of ILD, implying lung intraparenchymal inflammation (pneumonitis) and/or fibrosis of non-infectious etiology, some reported with pulmonary hypertension (including pulmonary arterial hypertension [PAH]) as a secondary event, have occurred in patients receiving rapamycins and their derivatives, including Zortress. Most cases generally resolve on drug interruption with or without glucocorticoid therapy. However, fatal cases have also occurred.5.11 HyperlipidemiaIncreased serum cholesterol and triglycerides, requiring the need for anti-lipid therapy, have been reported to occur following initiation of Zortress and the risk of hyperlipidemia is increased with higher everolimus whole blood trough concentrations. [See Adverse Reactions (6.2)] Use of anti-lipid therapy may not normalize lipid levels in patients receiving Zortress.Any patient who is administered Zortress should be monitored for hyperlipidemia. If detected, interventions, such as diet, exercise, and lipid-lowering agents should be initiated as outlined by the National Cholesterol Education Program guidelines. The risk/benefit should be considered in patients with established hyperlipidemia before initiating an immunosuppressive regimen containing Zortress. Similarly, the risk/benefit of continued Zortress therapy should be reevaluated in patients with severe refractory hyperlipidemia. Zortress has not been studied in patients with baseline cholesterol levels >350 mg/dL.Due to an interaction with cyclosporine, clinical trials of Zortress and cyclosporine in kidney transplant patients strongly discouraged patients from receiving the HMG-CoA reductase inhibitors simvastatin and lovastatin. During Zortress therapy with cyclosporine, patients administered an HMG-CoA reductase inhibitor and/or fibrate should be monitored for the possible development of rhabdomyolysis and other adverse effects, as described in the respective labeling for these agents. [See Drug Interactions (7.7)]5.12 ProteinuriaThe use of Zortress in transplant patients has been associated with increased proteinuria. The risk of proteinuria increased with higher everolimus whole blood trough concentrations. Patients receiving Zortress should be monitored for proteinuria. [See Adverse Reactions (6.2)]5.13 Polyoma Virus InfectionsPatients receiving immunosuppressants, including Zortress, are at increased risk for opportunistic infections; including polyoma virus infections. Polyoma virus infections in transplant patients may have serious, and sometimes fatal, outcomes. These include polyoma virus-associated nephropathy (PVAN), mostly due to BK virus infection, and JC virus associated progressive multiple leukoencephalopathy (PML). PVAN has been observed in patients receiving immunosuppressants, including Zortress. PVAN is associated with serious outcomes; including deteriorating renal function and kidney graft loss. [See Adverse Reactions (6.2)] Patient monitoring may help detect patients at risk for PVAN. Reductions in immunosuppression should be considered for patients who develop evidence of PVAN or PML. Physicians should also consider the risk that reduced immunosuppression represents to the functioning allograft.5.14 Interaction with Strong Inhibitors and Inducers of CYP3A4Co-administration of Zortress with strong CYP3A4-inhibitors (e.g., ketoconazole, itraconazole, voriconazole, clarithromycin, telithromycin, ritonavir, boceprevir, telaprevir) and strong CYP3A4 inducers (e.g., rifampin, rifabutin) is not recommended without close monitoring of everolimus whole blood trough concentrations. [See Drug Interactions (7)]。
维罗非尼 (Votrient)治疗的疾病及其副作用
维罗非尼 (Votrient)治疗的疾病及其副作用维罗非尼(Votrient)治疗的疾病及其副作用维罗非尼是一种被广泛应用于治疗多种肿瘤的口服抗癌药物。
它的主要成分是帕西替尼(Pazopanib),可抑制血管生成并减缓肿瘤的生长。
本文将介绍维罗非尼治疗的疾病范围以及可能产生的副作用。
维罗非尼可以用于多种类型的癌症治疗,包括肾细胞癌、软组织肉瘤和甲状腺癌等。
肾细胞癌是肾脏起源的一种常见癌症,维罗非尼通过靶向血管内皮生长因子受体(Vascular Endothelial Growth Factor Receptor,VEGFR)来抑制肿瘤的生长和扩散,从而治疗肾细胞癌。
维罗非尼还可以用于软组织肉瘤的治疗。
软组织肉瘤是一种恶性肿瘤,常见于人体各个部位的软组织,如肌肉、脂肪和血管等。
维罗非尼可通过阻断成纤维细胞生长因子受体(Fibroblast Growth Factor Receptor,FGFR)、血管内皮生长因子受体(VEGFR)和血小板源性生长因子受体(Platelet-Derived Growth Factor Receptor,PDGFR)来干扰肿瘤的生长和扩散。
此外,维罗非尼还适用于治疗甲状腺癌。
甲状腺癌是一种常见的内分泌系统肿瘤,维罗非尼通过抑制血管内皮生长因子受体(VEGFR)和甲状腺肿瘤相关基因活化突变(RTK)来阻止癌细胞的生长和扩散。
虽然维罗非尼在肿瘤治疗中发挥着重要作用,但它也会带来一些副作用。
常见的维罗非尼副作用包括乏力、食欲不振、恶心、腹泻和高血压等。
乏力是患者最常遇到的副作用之一,可能导致日常生活能力受限。
此外,维罗非尼还可能引发一系列胃肠道反应,包括食欲不振、恶心和腹泻等。
这些副作用可能会影响患者的生活质量,因此在接受治疗过程中,需密切关注患者的症状并及时处理。
另外,维罗非尼还可能引发高血压。
由于抑制血管内皮生长因子受体(VEGFR)的活性,维罗非尼可能导致血管收缩,进而引发高血压。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
用一杯水送服药物,服药时整片吞下维莫非尼片剂。不应咀嚼或碾碎维莫非尼片剂。 治疗持续时间
第 3 页/共 27 页
ms,且QTc相对于治疗前的变化 值≤ 60 ms
特殊人群剂量说明 老年人:对于年龄≥ 65 岁的患者,无特殊剂量调整需求。 儿童:维莫非尼对 18 岁以下患者的安全性和有效性尚未确立。维莫非尼未批准用于 18
岁以下的患者(参见【药代动力学】)。 肾功能受损:对于轻度或中度肾功能受损的患者,无需进行起始剂量调整(参见【药代
第 2 次出现(不可耐受)2 级或 3 级 AE, 暂时中断治疗,直至不良事件恢复至 0-1 级。恢复用药时给药剂量为
或治疗中断后未缓解
480mg,每日两次(如果之前剂量已经降低到 480mg,每日两次,永
久性停药)
第 3 次出现(不可耐受)2 级或 3 级 AE, 永久性停药 或治疗中断后未缓解
4级
建议维莫非尼治疗应持续至疾病进展或发生不可接受的毒性反应(参见表 1 和表 2)。 漏服
如果漏服一剂药物,可在下一剂服药 4 小时以前补服漏服的药物,以维持每日两次的给 药方案。不应同时服用两剂药物。 呕吐
如果维莫非尼服药后发生呕吐,患者不应追加剂量,而应按常规剂量继续治疗。
剂量调整 (参见【注意事项】和【不良反应】)
治疗期间第2次发生QTc> 500 ms,且QTc相对于治疗前的变化 值≤ 60 ms
治疗期间第3次发生QTc > 500
推荐剂量调整方案 不建议开始服用该药 永久性停药
暂时中断治疗,直至QTc降至500 ms以下 参见【注意事项】中的监测指标 恢复用药时给药剂量为720mg,每日两次 (如果之前已经 降低过剂量,恢复给药剂量为480mg,每日两次) 暂时中断治疗,直至QTc下降至500 ms以下 参见【注意事项】中的监测指标 恢复用药时给药剂量为480mg,每日两次(如果之前剂量 已经降低到480mg,每日两次,永久性停药) 永久性停药
对于伴有症状的不良事件或 QTc 间期延长的处理,可能需要按照表 1 或表 2 降低剂量、 暂时中断用药或停止维莫非尼治疗。对于出现皮肤鳞状细胞癌(cuSCC)不良事件,不建议 调整剂量或中断用药。不建议采用低于 480 mg 每日两次的剂量。
第 2 页/共 27 页
表1 基于不良事件的剂量调整方案
动力学】)。对于重度肾功能受损的患者,由于数据不足,无法确定是否需要进行剂量调整。 肝功能受损:对于轻度或中度肝功能受损的患者,无需进行起始剂量调整(参见【药代
动力学】)。对于重度肝功能受损的患者中,由于数据不足,无法确定是否需要进行剂量调 整。
【不良反应】 临床试验中的不良反应
安全性特征总结 维莫非尼的临床开发研究项目作为一个整体项目进行分析时,预估共 6300 例患者接受 过维莫非尼治疗。 患有不可切除或转移性黑色素瘤的患者: 药物不良反应(ADR)来自 2 项临床试验,一项为在初治的 BRAF V600 突变阳性的不 可切除或转移性黑色素瘤患者(N = 675)中进行的 III 期临床研究(NO25026),另一项为 在至少一次既往系统性治疗失败的 BRAF V600 突变阳性的转移性黑色素瘤患者(N = 132) 中进行的 II 期临床研究(NP22657)。 在 III 期开放性研究(NO25026)中,被随机分配至维莫非尼组的患者接受每日 2 次口 服,起始剂量为 960 mg;被随机分配至阳性对照组的患者接受 1000 mg/m2 的达卡巴嗪治疗, 采用静脉途径给药,每 3 周一次。维莫非尼中位治疗时间 6.6 个月,与此相比,达卡巴嗪的 中位治疗时间 0.8 个月。II 期临床研究(NP22657)是一项开放性、非对照、单组研究。在 这项研究中,患者接受维莫非尼 960 mg 每日两次的治疗,中位治疗时间 5.7 个月。 最常见的任意级别 ADR(任一研究≥ 30%)为关节痛、疲乏、皮疹、光敏反应、脱发、
毒性级别(CTC-AE)a
建议的剂量调整方案
1 级或 2 级(可耐受)
维持维莫非尼 960 mg,每日两次
2 级(不可耐受)或 3 级
第 1 次出现(不可耐受)2 级或 3 级 AE 暂时中断治疗,直至不良事件恢复至 0-1 级。恢复用药时给药剂量为 720mg,每日两次 (如果之前已经降低过剂量,恢复给药剂量为 480mg,每日两次)
一项在既往接受过治疗的转移性黑色素瘤患者中开展的非对照、开放 II 期临床研究中 观察到暴露-依赖的 QT 延长。对于 QTc 延长的管理可能需要特定的监测手段(参见【注意 事项】)。
表2 基于QT间期延长的剂量调整方案
QTc值 基线时QTc > 500 ms QTc延长同时满足QTc > 500 ms 和相对于治疗前的变化值 > 60 ms 治疗期间第1次发生QTc > 500 ms,且QTc相对于治疗前的变化 值≤ 60 ms
【性状】
第 1 页/共 27 页
两面凸起、粉白色至橙白色的薄膜衣片。
【适应症】 维莫非尼适用于治疗经 CFDA 批准的检测方法确定的 BRAF V600 突变阳性的不可切
除或转移性黑色素瘤。
【规格】
240 mg
【用法用量】 患者必须经由CFDA批准的检测方法确定的证明肿瘤为BRAF V600 突变阳性,才可使用
核准日期:2017 年 3 月 10 日 修改日期:2017 年 5 月 26 日
2017 年 11 月 29 日
维莫非尼片说明书
请仔细阅读说明书并在医师指导下使用 【药品名称】 通 用 名:维莫非尼片 商 品 名:佐博伏 ®, 英文商品名 Zelboraf ®
英 文 名:Vemurafenib film-coated tablets
第 1 次出现任何 4 级 AE
永久性停药或暂时中断治疗,直至不良事件恢复至 0-1 级。恢复用药 时给药剂量为 480mg,每日两次(如果之前剂量已经降低到 480mg, 每日两次,永久性停药)
第 2 次出现任何 4 级 AE
永久性停药
a 临床不良事件的强度根据不良事件常见术语标准(CTC-AE)4.0版进行评级
汉语拼音:Weimofeini Pian
【成份】 本品主要活性成分为维莫非尼,以维莫非尼和琥珀酸醋酸羟丙甲纤维素固体分散体存在。 化学名称:丙烷-1-磺酸 {3-[5-(4-氯苯基)-1H- 吡咯并[2,3-b]吡啶-3-羰基]-2,4-二氟代苯
基}- 酰胺。 化学结构式:
分 子 式:C23H18ClF2N3O3S 分 子 量:489.93
