有机硅化学概论
有机硅资料
有机硅是新材料。
有机硅不但是七大战略性新兴产业之一新材料产业的重要组成部分,而且是其它产业不可或缺的配套材料,在发展战略性新兴产业中具有举足轻重的作用。
中国有机硅工业经过50多年的发展,已形成生产企业众多、产品种类齐全、与其他产业关联度高,发展潜力和市场前景巨大的高新技术产业。
有机硅具有独特性能。
由于硅在化学元素中独有的兼具有机和无机的性质,其Si-O-Si独特的结构决定了有机硅产品的独特性能是在诸多领域是难以被替代的,有机硅化合物是指分子结构中含有Si-C键、且至少有一个有机基是直接与硅原子相连的化合物;以重复的Si-X 键为主链的线型或环状聚合物叫有机硅聚合物;通常所说的有机硅即指有机硅聚合物。
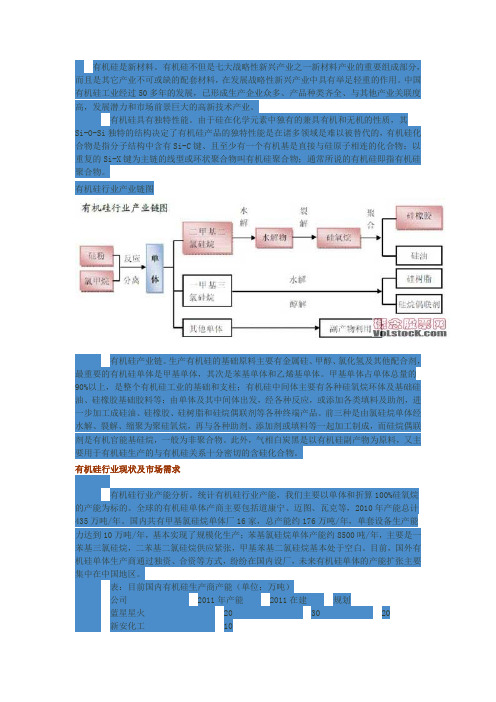
有机硅行业产业链图有机硅产业链。
生产有机硅的基础原料主要有金属硅、甲醇、氯化氢及其他配合剂,最重要的有机硅单体是甲基单体,其次是苯基单体和乙烯基单体。
甲基单体占单体总量的90%以上,是整个有机硅工业的基础和支柱;有机硅中间体主要有各种硅氧烷环体及基础硅油、硅橡胶基础胶料等;由单体及其中间体出发,经各种反应,或添加各类填料及助剂,进一步加工成硅油、硅橡胶、硅树脂和硅烷偶联剂等各种终端产品。
前三种是由氯硅烷单体经水解、裂解、缩聚为聚硅氧烷,再与各种助剂、添加剂或填料等一起加工制成,而硅烷偶联剂是有机官能基硅烷,一般为非聚合物。
此外,气相白炭黑是以有机硅副产物为原料,又主要用于有机硅生产的与有机硅关系十分密切的含硅化合物。
有机硅行业现状及市场需求有机硅行业产能分析。
统计有机硅行业产能,我们主要以单体和折算100%硅氧烷的产能为标的。
全球的有机硅单体产商主要包括道康宁、迈图、瓦克等,2010年产能总计435万吨/年。
国内共有甲基氯硅烷单体厂16家,总产能约176万吨/年,单套设备生产能力达到10万吨/年,基本实现了规模化生产;苯基氯硅烷单体产能约8500吨/年,主要是一苯基三氯硅烷,二苯基二氯硅烷供应紧张,甲基苯基二氯硅烷基本处于空白。
学习课件(有机硅)

05
有机硅的未来发展与挑战
有机硅的发展趋势
01
02
03
环保化
随着环保意识的提高,有 机硅行业将更加注重环保 生产,减少对环境的污染。
高性能化
有机硅材料不断向高性能 化发展,提高其耐温、耐 腐蚀、抗氧化等性能。
多元化
有机硅产品种类不断增多, 应用领域不断拓展,以满 足不同行业的需求。
有机硅面临的挑战与问题
有机硅在汽车制造领域的应用
总结词
提高汽车性能
详细描述
总结词
有机硅在汽车制造中主要用于 生产高性能的密封件、减震件 和涂层。这些产品可以提高汽 车的舒适性、稳定性和耐久性 ,并增强汽车的外观效果。
轻量化材料
详细描述
有机硅材料相对较轻,可以替 代部分金属材料,降低汽车的 整体重量。轻量化设计是汽车 节能减排的重要手段之一,有 利于提高汽车的燃油经济性和 排放性能。
学习课件(有机硅)
• 有机硅简介 • 有机硅的种类与合成 • 有机硅材料的性能与改性 • 有机硅在各领域的应用 • 有机硅的未来发展与挑战
01
有机硅简介
有机硅的定义
有机硅
是指含有硅元素的有机化合物, 也称为硅基有机化合物。
定义解释
有机硅由碳和硅两种元素组成, 其分子结构中碳-硅键的键能高, 使其具有独特的物理和化学性质 。
19世纪
有机硅化合物的研究开始起步。
20世纪40年代
出现商业化的有机硅产品,如 硅橡胶和硅树脂。
21世纪
有机硅材料在各领域的应用更 加广泛,成为现代工业和科技 发展的重要支撑材料之一。
02
有机硅的种类与合成
有机硅单体的合成
01
02
03
有机硅主要分类及概述

有机硅主要分类及概述
有机硅,也被称为胶状硅,是一类采用有机硅树脂或预硅化物作为基料,采用特殊的热固工艺,制成的宽范围的高分子材料。
因其材料具有优
异的机械性能和抗化学腐蚀性能及耐温性能,使其在航空航天、汽车制造、机械制造、能源开发、节能环保等行业中有着广泛的应用。
有机硅材料可
以分为热固性和半热固性两大类。
热固性有机硅,又称热固性有机硅树脂,是由硅氧环或硅氧环类化合物,经过聚合反应,形成芳环结构,制成透明至微薄黄色材料,具有良好
的热稳定性和耐化学腐蚀性,耐热性特别好,承受温度可达260℃。
热固
性硅树脂的主要性能,如粘附力、抗衡硬度、线绕线损失、厚度膨胀、氧
化稳定性、机械性能等,均要求很高。
热固性有机硅的应用,广泛地用于
生产电子工业用电缆和布线,绝缘层和绝缘层,电容器,电阻器和避雷器
等电气元器件,紧固件,汽车零部件等。
半热固性有机硅,也称聚氨酯硅树脂,主要由三氯甲烷、氧化硅及聚
氨酯为原料经过聚合反应制成,具有良好的机械强度、抗老化性能和耐油性,可弯曲,抗拉伸,热收缩性能优异,电绝缘性能也比热固性硅树脂更好。
有机硅:概述二

在有机硅核心分子结构中加入不同分子及化合物能够增强或改变有机硅性质,使其具有令人惊奇的万能用途。
由此产生的材料适应性极强,可以产生 2000 多种不同的形态,如固体、液体、油品、油 脂、半粘软膏、抗泡剂、浸渍剂、脱模剂、硅橡胶等等。
高性能、高保护、多用途,引领生活方式有机硅正改变着我们的现在,描绘着我们的未来源于自然有机硅是一类聚合体的总称,其中包括硅氧烷及硅烷——均为地壳中含量位居第二的天然元素硅的 化合物变体。
以下是部分使用有机硅的重点行业:• 汽车• 航空航天• 建筑• 化妆品• 家用电器• 电子• 食品• 保健• 机械工程• 纸张• 橡胶• 表面涂料• 织物与皮革企业家、消费者及艺术家们都希望通过出色的材料帮助提供创新、经济的解决方案,以满足各行各业的尖端要求。
稳定。
有机硅是非常稳定的化合物,具有出色的抗风雨及老化的特性,并且能够承受极限的温度及气候的变化。
有机硅不会与化学物质发生反应,可以抵抗盐、风、水及辐射等气候的影响,并且具有出色的绝缘性能。
洁净。
有机硅可以与化学物质及材料共用,或是在化学物质及材料上使用,并且通常不会产生有害的副产品或气味。
有机硅可以防水,并且不会促进细菌及真菌的滋生。
使用有机硅制造的产品易于清洁。
在建材方面,有机硅可以防止潮湿及霉菌的损害。
采用有机硅制造的医疗器械易于消毒。
耐用。
有机硅有极强的抗磨损及抗撕裂的性能。
与许多合成材料不同,有机硅在长时间暴露于恶劣环境之后仍可保持其基本的化学及物理性质。
有机硅不但自身非常可靠,还可以延长许多材料的使用寿命。
适应性强、万能用途。
有机硅使用非常方便。
相对而言,其在许多行业中易于生产、易于使用。
有机硅:概述二构建生活的先进化学品有机硅是现代生活的基本构成成分,具有极为宽广的化学及物理特性,从而成为从航空到纺织各个行业用作增强性能的首选材料。
A V O 8 5 1 6有机硅可以用于先进的生产及加工过程,并且常常推动可以简化生产流程的新型技术的产生。
有机硅培训

3)具有抗静电性; 4)适于染烫发质用香波和护发产品, 有锁色效果;
5)形成一层滑爽而牢固的膜,深层 修复受损的头发。
氨端聚二甲基硅氧烷
3 甲基-氨基-苯基(改性)硅油
——氨基(改性)硅油 (氨基硅油和二甲基硅油疏水性的对比)
氨基硅油比二甲基硅油更有效地改善头发表面的疏水性能
1
结构:
二甲基硅油
——结构与主要特点
聚二甲基硅氧烷及其衍生物是由这4种结构组合
主要特点:
聚二甲基硅氧烷的基本结构
2
环状聚硅氧烷
——化学式
2
环状聚硅氧烷
——挥发速率曲线
D4和D5改何去何从? “化学品注册,评估,授权和限制 (REACH)法规”(EC No 1907/2006):2015年开始了一项限制环 五聚二甲基硅氧烷(D5)和环四聚二甲 基硅氧烷(D4)的项目,旨在限制这两 种成分在洗去型化妆品中的添加量为 0.1%。
6
有机硅微米球形粉末
——硅石
1、滚珠效果和提高铺展性能 提供优异的润滑性能、特别在化妆品的应用上,这种特性能提高产品的铺展性,它能给予 产品丝滑的触感,并在彩妆上使用有防结块的功能; 2、吸收皮脂和汗水 在应用中能吸收皮脂和汗水,这让使用者获得更长时间的清爽感觉。最适合用于控油产品 使用; 3、软焦效果/哑光 小粒径的硅石能使光线在面部散射和部分反射,具有淡化皮肤皱纹的效果。硅石的光扩散 性能起到面部粉层的视觉隐形效果,为化妆品提供自然装饰的效果。 4、持久的遮盖效果 在使用中具有独特的光学功能,它吸油后不会变的完全透明,能为皮肤提供更持久的遮盖 效果。 5、添加功能性成分 硅粉可以根据顾客需求而成为功能性粉末、由于硅粉的吸收性能、SILNOS能添加其他的 功能性物质。 6、容易添加 能在化妆品配方中容易的分散和混合。根据客户的使用目的,硅粉具有极佳的化学稳定性 和热稳定性,这些因素减少了产品使用的局限性,因此它在各个领域中使用方便、应用广 泛。
有机硅化合物化学第一章

有机硅材料具有独特的结构
• 聚硅氧烷Si原子上充足的甲基将高能量的 聚硅氧烷主链屏蔽起来。 • C--H无极性,使分子间相互作用力十分微 弱。 • Si--O键长较长,Si--O--Si键键角大。 • Si--O键是具有50%离子键特征的共价键。
有机硅产品具有如下性能
• • • • 耐温特性 耐候性 生理惰性 低表面张力和低表面能
碳和硅化学键的比较
• 原子的大小不同 • 原子的电负性不同 • 价层的空轨道不同
硅原子的化学键特征
硅是第三周期元素,它具有碳不具有的3d轨 道,它核外有5各空的d轨道,在一定条件下, 硅的空3d轨道可以参与成键,这是硅化合物 和碳化合物最大的不同点。 • 硅原子的3d轨道参与成键有两种方式,一 是组成spd杂化轨道,二是利用它的3d轨道, 接受外界提供未成键电子对。
第二阶段:成长期
• 这一时期的杰出代表是英国化学家,诺丁 汉大学教授F.S.Kipping,他在有机硅化学 领域做了四十余年工作,他的突出贡献是 在1904年将格氏反应成功的应用于制备有 机硅化合物。该合成方法称为日后有机硅 化合物合成的基本方法。至今仍是主要的 有机硅化合物合成方法之一。 • 1904年,W.Dilthey得到了六苯基环三硅氧 烷—第一个环状聚硅氧烷化合物。
有机硅化合物的主要化学键
1)Si--O键 Si--O键是具有重要意义的化学 键,因为它是形成有机硅聚合物的骨架,主 链,是最基本的键型 2)Si--C键 Si--C键是构成有机硅化合物的特 征键,也是有机聚硅氧烷侧基德健型。它赋 予硅化合物的有机性能。 3)Si--X键 Si--X键具有很强的极性,是一种 活性很强的化学键。 4)Si--N键 Si--N键 5)Si--Si键
从1863年至今的100多年,有机硅化合物化学经历 了初创期、成长期、发展期、繁荣及发展期
工业硅基础知识系列之:有机硅

工业硅基础知识系列之:有机硅有机硅化合物是指含有硅碳键的化合物,且至少有一个有机基团通过硅碳键结合到硅原子上。
如甲基硅烷CH3SiH3、二甲基二氯硅烷(C2H5)2SiCl2等都是有机硅化合物,而SiC、Si3N4等则属于无机硅化合物。
自然界中至今未发现有机硅化合物的存在,只有在动物羽毛和禾本科植物中发现有硅酸酯类化合物,但这类物质并不含有硅碳键(Si-C),而只是含有硅-氧碳键(Si—O—C)。
有机硅高聚物的种类繁多,包括聚硅氧烷、聚硅烷、聚碳硅烷、聚氮硅烷等。
有机聚硅氧烷是其中最重要的一类,其结构可表示如下:其中,R为有机基团(如甲基、苯基等);n为硅原子上连接的有机基团数(n =1、2、3), m为聚合度。
一般认为的有机硅材料主要就是指以含聚硅氧烷主链的低聚或高聚物。
有机聚硅氧烷之所以有广泛的用途,主要由于它们具有其他高分子材料所无法比拟的独特性能:如耐高温、耐低温、防潮、绝缘、耐腐蚀、耐老化及生理惰性等。
有机硅高分子产品品种非常多样,有液体(硅油)、弹性体(硅橡胶)、树脂、乳液等,它们在宇航、航空、电气、电子、轻工、机械、化工、建筑、农业、医学、日常生活等方面均已得到广泛的应用。
有机硅材料在它的组成中既有无机硅氧烷链,又含有有机基团,是一种典型的半无机高分子。
而正是这种结构特点使它成为一种很特殊的高分子材料,并具有其它材料所不能同时具备的耐高温、阻燃、电气绝缘、耐辐射和生理惰性等一系列优良性能。
特别值得一提的是,有机硅工业的发展史不同于通用合成材料。
通用合成材料是以原料制造工艺、大型生产技术及产品的加工为中心发展的;而有机硅则是以产品开发为中心而发展的。
在近几十年来,有机硅单体的生产工艺变化不大,而有机硅技术重点主要在于产品应用上,如有机基团的引入、聚合物结构和交联技术等方面。
有机硅材料可以根据需要,设计出各种不同分子结构以满足各行各业不同场合下的使用要求。
在设计多用途产品时,可以采取下列途径。
有机硅化合物的基本性质

氯硅烷类与相应旳氯甲烷旳偶极矩比较
硅化物 H3SiCl SiH2Cl2 SiHCl3
偶极矩(D) 1.28 1.17 0.85
碳化物 CH3Cl CH2Cl2 CHCl3
偶极矩(D) 1.87 1.56 1.00
是因为氯原子旳p电子进入硅旳3d空轨道,形成dπ-pπ
配键, 构造为
+
H2Si
Cl
, 从而使偶极矩数据下降。
0.63
1.05
p-Me3SiC6H4COOH
0.54
1.11
四、硅成键旳类型和特征
(一)Si-O键
它是形成有机硅高聚物最基本、最主要旳键。 1、特点: (1) Si-O键旳键能很高。热稳定性好 (2) 键长、键角很大。分子柔顺。分子间作用力小,表面张力小. 2、Si-O-Si键旳制备措施: (1)水解法。经过卤硅烷水解来制备。 (2)非水解法。经过缩合反应、开环聚合反应来制备。
2 Me3Si-旳共轭效应
Me3Si-基取代芳香族化合物旳苯环上旳氢原子后,在溶剂 作用下,苯环上旳一部分π电子进入硅旳3d轨道,形成了 dπ-pπ配键,一般称此为-T效应。如图所示。
酸 PhCOOH
两种羧酸旳电离常数
水中(在 60%(wt)乙 25℃)旳电 醇-水中(25℃
离常数
)旳电离常数
Ka×104 Ka×106
键角 O-Si-O
— 115±5°
109° — —
C-Si-C 111±4°
— 106° — —
偶极矩
偶极矩是指分子中正电中心和负电中心间旳距离(偶极长) 与正电荷或负电荷旳电量旳乘积,其单位为D( 德拜)。表 达分子极性旳大小。
硅旳电负性比碳小,按常规推测多种氯硅烷旳偶极矩应 该比相应旳氯甲烷大,但实际成果却相反。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1. Introduction to Silicone ChemistryA. Colas, Dow Corning Europe SA, Seneffe (Belgium)By analogy with ketones, the name “silicone” was given in 1901 by Kipping to describe new compounds of the brut formula R2SiO. These were rapidly identified as being polymeric and actually corresponding to polydialkylsiloxanes. Among them, the most common are polydimethylsiloxanes (PDMS), trimethylsilyloxy terminated with the structure:The methyl groups along the chain can be substituted by many other groups (e.g., phenyl, vinyl or trifluoropropyl). The simultaneous presence of “organic” groups attached to an “inorganic” backbone gives silicones a combination of unique properties and allows their use in fields as different as aerospace (low and high temperature performance), electronics (electrical insulation), health care (excellent biocompatibility) or in the building industries (resistance to weathering).NomenclatureThe main chain unit in PDMS, - (SiMe2O) -, is often shortened to the letter D because, as the silicon atom is connected with two oxygen atoms, this unit is capable of expanding within the polymer in two directions. In a similar way, M, T and Q units can be defined corresponding to [1]:The above polymer (1) can also be described as MD n M. This allows simplifying the description of various structures like (Me3SiO)4Si or tetrakis(trimethylsilyloxy)silane, which becomes M4Q. Superscripts are sometimes used to indicate groups other than methyl (e.g.,D H for HMeSiO2/2).The synthesis of siloxanes has been described elsewhere [1, 2, 3]. In summary, PDMS is obtained from the hydrolysis of dimethyldichlorosilane Me2SiCl2, which leads to a mixture of linear and cyclic oligomers:Higher molecular weight PDMS is obtained after polymerisation, for example, of the above cyclics in the presence of an end-blocker such as hexamethyldisiloxane and catalysed by a strong acid or strong base according to:Using other chlorosilanes, different end-blockers and/or different cyclics leads to many structures including polymers with various functional groups grafted on the polymer chain and/or at the polymer ends (e.g., vinyl, hydrogeno, phenyl, amino alkyl). These can be formulated into solvent-based, emulsion or solventless products.Reactive polymers can be cross-linked into elastomers using:• a peroxide-initiated reaction; in particular, if the silicone polymer carries some vinyl groups• a condensation reaction; for example, between a hydroxy end-blocked PDMS and an alkoxysilane, in presence of tin salt or titanium alkoxide as catalyst •an addition reaction; for example, between a vinyl-functional PDMS and an hydrogenomethyl dimethyl siloxane oligomer, in presence of a organic platinumcomplexSuch polymer, cross-linker and catalyst are formulated with various additives as one-part, ready-to-use products or two-part products to be mixed prior to use and to cure at room temperature or only at elevated temperatures.Physicochemical PropertiesThe position of silicon, just under carbon in the periodic table, led to a belief in the existence of analogue compounds where silicon would replace carbon. Most of these analogue compounds do not exist, or if they do, they behave very differently. There are few similarities between Si-X bonds in silicones and C-X bonds [1-3]:Element (X) Bond length (Å) Ionic character (%)Si - X C - X Si - X C - X Si 2.34 1.88 -- 12C 1.88 1.54 12 --H 1.47 1.07 2 4O 1.63 1.42 50 22Between any given element and silicon, bond lengths are longer than for carbon with this element. The lower silicon electronegativity (1.8) vs. carbon (2.5) leads to a verypolarised Si-O bond, highly ionic and with a large bond energy, 452 kJ/mole (108 kcal/mol). The Si-C bond has a bond energy of ±318 kJ/mole (76 kcal/mol), slightly lower than a C-C bond, while the Si-Si bond is weak, 193 kJ/mole (46.4 kcal/mole). These values partially explain the stability of silicones; the Si-O bond is highly resistant to homolytic scission. On the other hand, heterolytic scissions are easy, as demonstrated by the re-equilibration reactions occurring during polymerisations catalysed by acids or bases. Silicon atoms do not form stable double or triple bonds of the type sp2 or sp with other elements, yet the proximity of the d orbitals allows d -p retro-coordination. Because of this retro-coordination, trialkylsilanols are more acid than the corresponding alcohols. Yet, the involvement of retro-coordination is challenged [4].Another example of the difference between analogues is the tetravalent diphenyldisilanol, (C6H5)2Si(OH)2, which is stable, while its carbon equivalent, a gem-diol, dehydrates. The Si-H bond is weakly polarised, but here in the direction of a hydride, and is more reactive than the C-H bond. Overall, there are few similarities between a silicone polymer and a hydrocarbon polymer.Silicones display the unusual combination of an inorganic chain similar to silicates and often associated with high surface energy but with side methyl groups that are, on the contrary, very organic and often associated with low surface energy [4]. The Si-O bond are strongly polarised and without protection should lead to strong intermolecular interactions. However, the methyl groups, only weakly interacting with each other, shield the main chain.This is made easier by the high flexibility of the siloxane chain; rotation barriers are low, and the siloxane chain can adopt many conformations. Rotation energy around a CH2-CH2 bond in polyethylene is 13.8 kJ/mol but only 3.3 kJ/mol around a Me2Si-O bond, corresponding to a nearly free rotation. The siloxane chain adopts a configuration that can be idealised by saying that the chain exposes a maximum number of methyl groups to the outside, while in hydrocarbon polymers, the relative backbone rigidity does not allow “selective” exposure of the most organic or hydrophobic methyl groups. Chain-to-chain interactions are low, and the distance between adjacent chains is also higher in silicones. Despite a very polar chain, silicones can be compared to paraffin, with a low critical surface tension of wetting [4]. Yet because of their low intermolecular forces, PDMS materials remain liquid in a much wider range of molecular weights and viscosities than hydrocarbons. The surface activity of silicones is displayed in many circumstances [4]:•Polydimethylsiloxanes have a low surface tension (20.4 mN/m) and are capable of wetting most surfaces. With the methyl groups pointing to the outside, this gives very hydrophobic films and a surface with good release properties, particularly if the film is cured after application. Silicone surface tension is also in the most promising range considered for biocompatible elastomers (20 to 30 mN/m).•Silicones have a critical surface tension of wetting (24 mN/m), which is higher than their own surface tension. This means that silicones are capable of wettingthemselves, a property that promotes good film formation and good surface covering.•Silicone organic copolymers can be prepared with surfactant properties, with the silicone as the hydrophobic part (e.g., in silicone polyether copolymers).The low intermolecular interactions in silicones have other consequences [4]: •Glass transition temperatures are very low (e.g., 146 K for a polydimethylsiloxane compared to 200 K for polyisobutylene, the analogue hydrocarbon); cross-linkedPDMS will be elastomeric at RT in the absence of any plasticizers.•The presence of a high free volume compared to hydrocarbons explains the high solubility and high diffusion coefficient of gas into silicones. Silicones have a highpermeability to oxygen, nitrogen and water vapour, even if in this case liquid water is not capable of wetting a silicone surface. As expected, silicone compressibility is also high.•In silicone, the activation energy to the viscous movement is very low, and viscosity is less dependent on temperature compared to hydrocarbon polymers. Moreover,chain entanglements are involved at higher temperature and contribute to limit theviscosity reduction [4].The presence of groups other than methyl along the chain allows modification of some of the above properties:• A small percentage of phenyl groups along the chain perturbs sufficiently to reduce crystallisation and allows the polymer to remain flexible at very low temperatures.The phenyl groups also increase the refractive index.•Trifluoropropyl groups along the chain change the solubility parameter of the polymer from 7.5 to 9.5 (cal/cm3)1/2. These copolymers are used to prepareelastomers with little swelling in alkane or aromatic solvents.Considering the above, many polymeric “architectures” can be prepared of different physical forms (volatile, liquid, viscoelastic, solid) with different functionalities, inert or capable of interacting or reacting with many other compounds. Formulation into convenient products leads to even more products. This explains the wide range of industries where silicones are used.References1 Hardman, B. Encycl. Polym. Sci. Eng. 1989, 15, 204.2 Rochow, E. G. Silicon and Silicones, Springer-Verlag: Berlin, Heidelberg, New York, 1987.3 Noll, W. Chemistry and Technology of Silicones; Academic Press: New York, 1968.4 Owen, M. J. Chemtech. 198111, 288. This article has been updated and republished in Chimie Nouvelle2004, 85, 27.This article has been published in the chapter “Silicones in Industrial Applications” in Inorganic Polymers, an advanced research book by Nova Science Publishers (); edited by Roger De Jaeger (Lab. de Spechtrochimie Infrarouge et Raman, Univ. des Sciences and Tecn. de Lille, France) and Mario Gleria (Inst. di Scienze e Tecn. Molecolari, Univ. di Padoa, Italy). Reproduced here with the permission of the publisher.。
