聚苯并咪唑PBI的制备及性能研究
化学纤维概论-PBI

由于高温拉伸时残存在PBI初生纤维中的溶剂 容易气化而导致“爆米花状”纤维等,所以拉伸 前必须对纤维进行水洗和干燥,除净残存的溶剂 和水。 PBI初生纤维的拉伸可分两级进行,如一级拉 伸比约1.5~3.5倍,二级拉伸比约1.05~1.5倍,二 级拉伸的温度要高于一级拉伸。为防止发生氧化 降解等,拉伸需在400~500℃高温氮气环境下进 行。
制备方法——PBI的合成
工业上可采用熔融本体缩聚工艺制备PBI,即在惰性气体保护下 按照一定比例是单体TAB和DPIP熔融本体缩聚反应合成PBI:
上述的缩聚反应分两个阶段进行。第一阶段为 预缩聚,反应温度270~300℃,反应时间1~2h, 除去副产物苯酚和水得到泡沫状预聚体,将其冷 却和粉碎后再在真空下雨375~400℃固相聚合 2~3h,制成黄棕色粒状PBI树脂。
聚苯并咪唑纤维
聚苯并咪唑纤维发展
聚苯并咪唑纤维于20世纪60年代初由美国空军 材料实验室研制成功。1983年由美国塞拉尼斯公 司正式投产,年生产能力为460t,因生产成本高, 发展缓慢。它是一种典型的杂环高分子耐热纤 维,大分子主链上含有苯并咪唑撑,它主要作 为宇航密封舱耐热防火材料﹐由于核纤维吸湿 率高达15%﹐因此自1983年后﹐又开发了穿著舒 适的高温防护服等民用产品。
制作宇宙飞船中的绳 索、耐烧蚀热屏蔽材料、 减速用阻力降落伞及宇航 员的加压安全服等。曾经 用它制作阿波罗号和空间 试验室宇航员的航天服和 内衣。
应 用
在一般工业中可作石棉代用品,包括耐高温手 套、高温防护服、传送带等,使用温度常为 250~300℃,能在500℃下短时间使用。
可作气体、液体的 耐腐蚀滤材和烟道气 滤袋,可在150~ 200℃范围内使用, 在酸的露点温度以下 不受腐蚀。
(完整word版)苯并咪唑研究进展

苯并咪唑合成研究进展摘要:苯并咪唑类化合物具有广泛的生物活性,如抗癌、抗真菌、消炎、治疗低血糖和生理紊乱等,在药物化学中具有非常重要的意义;并可用于模拟天然超氧化物歧化酶(SOD)的活性部位研究生物活性,以及环氧树脂新型固化剂、催化剂和某些金属的表面处理剂, 还可作为有机合成反应的中间体等.绿色合成苯并咪唑化合物显得尤为重要。
本文主要讲述了苯并咪唑的合成方法,以及在离子鉴定、航空航天等方面的应用介绍。
关键词:苯并咪唑配合物合成应用1合成苯并咪唑类化合物1。
1以邻苯二胺和羧酸(及其衍生物)为原料的合成继1872年Hoebrecker首次合成第一个苯并咪唑类化合物 2,5—二甲基苯并咪唑(1)后,Ladenburg用乙酸和 4-甲基邻苯二胺加热回流,也同样得到化合物1 .从此, 邻苯二胺衍生物和有机酸的关环反应就成为苯并咪唑类化合物制备最通用的方法,但通常需要很强的酸性条件[常采用 HCl、多聚磷酸(PPA)、混酸体系、对甲苯磺酸等作为催化剂]和很高的反应温度[1].1986 年 Gedye 等[2]首次报道了微波作为有机反应的热源,具有速度快、产率高、污染少、在无溶剂条件下, 利用微波间歇加热合成苯并咪唑衍生物。
安全性高等优点。
例如, 路军等[3]只需反应 8 min, 产率一般可达 64%~88%。
Zhang[4]成功报道了以邻苯二胺和原酸酯为原料合成苯并咪唑类化合物。
.他们用路易斯酸为催化剂,在乙醇溶剂中室温搅拌进行反应,合成条为催化剂时, 反应2h, 产率为95%。
用相同的原料,他们[5]还研究件比较温和。
当以ZrCl4了用磺酸作为催化剂,在甲醇体系中室温下合成苯并咪唑类化合物,产率达到 96%, 反应时间也缩短为1h。
1。
2液相合成考虑到载体合成的某些缺点, 研究者们对同样以卤代硝基苯为原料的传统液相合成法也比较重视。
例如,Raju 等[6]报道了在室温下用邻氟取代硝基苯合成含硫和含氧的取代苯并咪唑。
基于聚苯并咪唑功能化离子液体的交联型离子交换膜的制备与性能研究

基于聚苯并咪唑/功能化离子液体的交联型离子交换膜的制备与性能研究燃料电池的核心部件是能够传输离子的离子交换膜,其中质子交换膜(PEMs)是能够传递质子的离子交换膜,它的性能好坏直接决定着电池的性能和使用寿命。
聚苯并咪唑(PBI)具有较好的热稳定性和机械强度,而且其气体渗透性和甲醇渗透率低。
然而,由于PBI中的质子解离度很低,质子传导率仅为10<sup>-7</sup><sup>1</sup>0<sup>-6</sup> S/cm,因而不能直接用于燃料电池。
研究人员通常将其掺杂磷酸以获得较高的质子传导率。
但是,由于磷酸并未固定在主链上且易溶于水,随着反应进行,磷酸容易流失,从而造成电池性能的显著下降。
这是无机酸掺杂聚合物电解质膜所面临的主要问题。
研究表明,聚合离子液体能够有效的提高膜的质子传导率,同时兼具离子液体的特性而不易渗出。
本论文先合成聚阳离子型离子液体(PIL),将其引入以聚苯并咪唑为主链的聚合物体系中,加入交联剂KH560,在酸性条件下水解形成共价交联网络,从而制备了PBI/PIL交联复合型高温质子交换膜。
相较于纯膜,复合膜具有优异的机械性能和质子传导率,特别是cPBI-BF<sub>4</sub>-40膜在170℃无水条件下质子传导率最高可达0.117S/cm,拉伸强度在 6 MPa以上,有希望应用于高温质子交换膜燃料电池。
将PBI作为阴离子交换膜的聚合物主体,同时引入共价交联网络,以此解决力学性能和离子传导率之间的矛盾。
我们首先合成高分子量羟基聚苯并咪唑(PBIOH)和含有可交联功能基团的离子液体(AESP),将二者按比例有序掺杂,得到一系列PBIOH-AESP复合膜。
相较于纯膜,交联膜具有更好的力学性能、热稳定性和化学稳定性。
例如,拉伸强度在碱掺杂之后仍在20 MPa以上,热分解温度在300℃以上,PBIOH-AESP 20膜在90℃具有最高的离子传导率,为0.077 S/cm,相对于纯膜,提高幅度高达148%,即使在3.5 M KOH中浸泡216小时后,仍然具有0.043 S/cm,基本满足燃料电池的需求。
燃料电池用高分子量聚苯并咪唑膜的制备与性能研究
燃料电池用高分子量聚苯并咪唑膜的制备与性能研究杨景帅1,刘佩佩1,李庆峰2,*,Cleemann rs 2,Steenberg Thomas 2,Hjuler A.Hans 2,Jensen J.Oluf 2,Bjerrum J.Niels 2,何荣桓1,*(1.东北大学理学院,辽宁,沈阳,110819,E-mail:herh@ ;2.Department of Energy Conversion and Storage,Technical University of Denmark,Lyngby,Denmark,DK-2800Kgs.E-mail:qfli@dtu.dk )以磷酸(PA )掺杂聚苯并咪唑(PBI )膜为电解质的质子交换膜燃料电池,运行工作温度高达120-200o C ,近年来备受研究者的青睐。
与以全氟磺酸膜为电解质工作温度在80o C 左右的质子交换膜燃料电池相比具有以下技术上的优势,包括:较高的CO 耐受能力,简化的水管理系统,更有效的热利用,一体化的燃料处理系统和电池系统等。
磷酸由于其独特的自解离能力,在高温非水条件下依靠氢键网络能够传递质子[1-2]。
PBI/PA 膜的电导率与膜的磷酸掺杂水平(每摩尔聚合物重复单元结合的磷酸的物质的量)密切相关,而电导率的高低将直接影响燃料电池性能的优劣。
人们尝试了不同方法来提高膜的酸掺杂水平,进而提高膜的电导率。
然而,PBI 膜酸掺杂水平的提高,会显著降低膜的机械强度,进而影响电池的使用寿命[3]。
因此,制备兼具高导电能力和高机械强度的膜材料是推动高温质子交换膜燃料电池进一步发展和商业化的关键问题之一[4]。
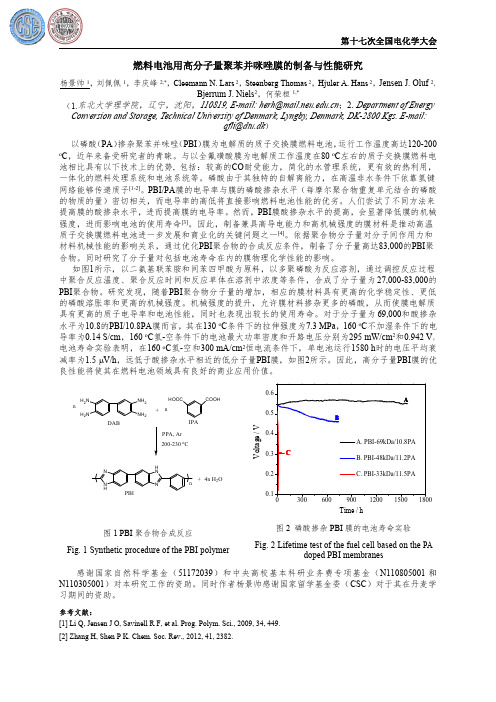
依据聚合物分子量对分子间作用力和材料机械性能的影响关系,通过优化PBI 聚合物的合成反应条件,制备了分子量高达83,000的PBI 聚合物。
同时研究了分子量对包括电池寿命在内的膜物理化学性能的影响。
如图1所示,以二氨基联苯胺和间苯四甲酸为原料,以多聚磷酸为反应溶剂,通过调控反应过程中聚合反应温度、聚合反应时间和反应单体在溶剂中浓度等条件,合成了分子量为27,000-83,000的PBI 聚合物。
多亲水位点侧链修饰聚苯并咪唑膜的制备及其在液流电池中的应用

多亲水位点侧链修饰聚苯并咪唑膜的制备及其在液流电池中的应用一、背景介绍随着能源需求的不断增长,研究新型高效的储能材料成为了人们关注的焦点。
液流电池作为一种新型储能技术,具有高效、可持续等优点,因此备受研究者的青睐。
而聚苯并咪唑(PBI)膜则是液流电池中常用的分离膜材料之一,其优异的化学稳定性和离子传输性能使其在液流电池中具有广泛应用前景。
然而,PBI膜在实际应用中存在着一些问题,如其亲水性差、离子传输速率低等。
因此,在PBI膜表面引入多亲水位点侧链修饰可以有效提高其亲水性和离子传输速率,从而提高液流电池的性能。
二、制备方法1. 合成多亲水位点侧链修饰聚苯并咪唑(PBI-L)单体;2. 将合成得到的PBI-L单体溶解于N-甲基吡咯烷酮(NMP)中,并加入碳酸锂作为反应助剂;3. 将溶液倒入模具中,在120℃下进行热压成膜,得到PBI-L膜;4. 将PBI-L膜放入去离子水中洗涤,去除余胶和碳酸锂等杂质;5. 将洗涤后的PBI-L膜放入80%的乙酸中进行酯化反应,引入羟基官能团;6. 将酯化后的PBI-L膜放入氢氧化钠溶液中进行碱水解反应,得到多亲水位点侧链修饰聚苯并咪唑(PBI-L-OH)膜。
三、性能测试1. 接触角测试:将水滴滴在不同表面上,测量其与表面之间的接触角。
结果显示,PBI-L-OH膜的接触角为72°,而未经修饰的PBI膜接触角为90°以上,说明引入多亲水位点侧链修饰可以显著提高PBI膜的亲水性。
2. 离子传输测试:使用循环伏安法测量电池在不同电压下的电流密度和电势差。
结果显示,在相同条件下,使用PBI-L-OH作为分离膜的液流电池的电流密度和电势差均比使用未经修饰的PBI膜的液流电池高。
四、应用前景多亲水位点侧链修饰聚苯并咪唑膜作为一种新型分离膜材料,在液流电池中具有广泛应用前景。
其亲水性的提高可以显著减少离子传输过程中的阻力,从而提高液流电池的能量密度和功率密度。
聚苯并咪唑树脂的合成与性能研究

II
聚..............................................1
1.1 聚苯并咪唑树脂的概述.....................................................................................................1 1.2 聚苯并咪唑的发展概况.....................................................................................................1 1.3 合成聚苯并咪唑的主要单体与工艺.................................................................................3
学校代码 10530 分 类 号 O633.5
学 号 200706020975 密级
硕士学位论文
聚苯并咪唑树脂的合成与性能研究
学位申请人 指导教师 学院名称 学科专业 研究方向
张海 林原斌 教授
化学学院 有机化学 精细有机合成
二零一零年 五 月 二十 日
Study on the Preparation and Performance of Polybenzimidazole resin
作者签名:
日期: 年 月 日
学位论文版权使用授权书
本学位论文作者完全了解学校有关保留、使用学位论文的规定,同意 学校保留并向国家有关部门或机构送交论文的复印件和电子版,允许论文 被查阅和借阅。本人授权湘潭大学可以将本学位论文的全部或部分内容编 入有关数据库进行检索,可以采用影印、缩印或扫描等复制手段保存和汇 编本学位论文。
聚苯并咪唑的合成及应用研究进展_马涛
Vol 136No 18・36・化 工 新 型 材 料N EW CH EMICAL MA TERIAL S 第36卷第8期2008年8月作者简介:马涛(1978-),男,兰州大学高分子化学与物理专业在读博士,师承李彦锋教授,从事于耐高温高分子材料的研究。
联系人:李彦锋。
聚苯并咪唑的合成及应用研究进展马 涛 李彦锋3 赵 鑫 邵 瑜 宫琛亮 杨逢春(兰州大学化学化工学院,兰州大学生物化工及环境技术研究所,兰州730000)摘 要 介绍了国内外有关聚苯并咪唑高分子材料的研究状况。
论述了聚苯并咪唑的发展,二元酸和四胺单体的合成方法、聚合工艺、种类及国内外应用状况,并对聚苯并咪唑的发展方向和研究热点进行了分析。
关键词 聚苯并咪唑,单体合成,聚合,应用Progress on synthesis and application of polybenzimidazolesMa Tao Li Yanfeng Zhao Xin Shao Yu G ong Chenliang Yang Fengchun (College of Chemist ry and Chemical Engineering ,Instit ute of Biochemical Engineering &Environmental Technology ,Lanzhou U niversity ,Lanzhou 730000)Abstract The progress of polybenzimidazoles was reviewed.The character of polybenzimizoles on phylogeny ,monomer ,polymerization technology ,and applications were detailedly described ,meanwhile ,the developments of poly 2benzimizoles were obviously presented.K ey w ords polybenzimidazole ,monomer synthesis ,polymerization ,application 随着航天技术的发展,特别是航天器飞行速度和有效载荷与结构质量比的提高,耐高温先进复合材料正在成为最主要的航天结构新材料。
聚苯并咪唑的合成分析研究()
CQWU/JL/JWB/ZY084-02学年论文<课程论文、课程设计)题目:聚苯并咪唑的合成研究姓名:施燕学号:200904183010专业年级:10级化学师范成绩:指导教师<职称):孟江平2018 年12月14日聚苯并咪唑的合成研究施燕<重庆文理学院材料与化工学院化学师范1班,重庆永川402160)摘要介绍了国内外有关聚苯并咪唑高分子材料的研究状况。
论述了聚苯并咪唑的发展,二元酸和四胺单体的合成方法、聚合工艺、种类及国内外应用状况,并对聚苯并咪唑的发展方向和研究热点进行了分析。
关键词聚苯并咪唑, 单体合成, 聚合, 应用中图分类号X703.1文献标识码B文章编号Progress on synthesis and application ofpolybenzimizolesSHIYan(Depatment of Chemistry and Environmeal Science, ChongqingUniversity of Arts and Sciences, Yongchuan, Chongqing 402160, China>Abstract The progress of polybenzimidazoles was reviewed. The character of polybenzimizoles on phylogeny,monomer,polymerization technology, and applications were detailedly described, meanwhile, the developments of polybenzimizoles were obviously presented.Keywords poly benzimidazole, monomer synthesis, polymerization, application聚苯并咪唑<PBI)是一种主链上具有咪唑杂环结构的线性并具有碱性的聚合物,具有优异的机械性能、耐化学腐蚀性、耐高温、抗燃性等特性,而且能够与强酸形成一种酸掺杂体系传导质子。
聚苯并咪唑的合成_性能及在燃料电池膜材料中的应用_浦鸿汀
[ 22 ]
[ 15 ]
[ 23 ][ 23 ]源自[2][ 23 ][ 24 ] [2]
[ 24 ]
[ 16 ]
参考文献 [ 17 ] [7] [7] [7] [ 18 ] [ 18 ]
[ 19 ]
[ 20 ]
[ 16 ]
·12 ·
R1 为
;
R2 为以下结构
—CH2 —
CH3
—( CH2 —) 2 C
反应温度与时间
400 ℃,1h
第一步 :270 ℃,115h ;第二步 :360 ℃,110h
副产物
水
苯酚和水
是否发泡
不发泡
由反应过程决定
催化剂
使用催化剂
可选择是否使用催化剂
成本
中等
高
112 溶液聚合法 溶液聚合法是先将四胺或四胺盐酸盐加到多聚磷酸 ( PPA) 中 ,氮气保护 ,加热搅拌使之溶解 ,然后加
亲核取代法是通过先合成含有苯并咪唑环的有亲核取代位的中间体 ,然后在碱性条件下和醇反应得 到 PBI。亲核取代法的优点是反应单体较易制备 ,扩大了可得到的 PBI 种类 。缺点为相对直接缩聚法来 说 ,亲核反应法对反应过程中生成的小分子物质的去除要求更为严格 。Harris 等[14] 用可以自聚合的单体 来合成聚苯并咪唑 ,先合成出一种特定的单体 ,其中含有卤素原子 、羟基和苯并咪唑单元 ,然后将这种单 体自聚合 ,羟基和卤素亲核取代反应得到聚苯并咪唑 。
高迁移率聚合物半导体材料最新进展
高迁移率聚合物半导体材料最新进展高迁移率聚合物半导体材料是一类具有高电子迁移率的有机材料,用于制备有机场效应晶体管(OFETs)等电子器件。
与传统的无机半导体材料相比,高迁移率聚合物半导体材料具有易于加工、柔性、低成本等优点,因此在柔性电子学、可穿戴设备、印刷电子等领域具有广阔的应用前景。
近年来,高迁移率聚合物半导体材料取得了一系列重要进展。
研究人员成功合成了一系列具有高迁移率的聚合物材料。
聚苯并咪唑(PBI)是一种具有高能带电子迁移率的聚合物材料,具有优异的电荷传输性能,可以应用于高性能晶体管器件的制备。
还开发出了一种新型的聚杂环并噻吩(PTTT)材料,具有极高的电子迁移率,使其成为制备高性能OFETs 的理想材料之一。
研究人员通过制备高质量的薄膜结构来提高聚合物材料的电子迁移率。
利用溶液加热处理的方法可以显著提高聚合物材料的晶体质量和晶体度,从而提高电子迁移率。
研究人员还开发了一系列表面修饰技术,如通过介质工程等手段来改善薄膜的晶体结构,从而进一步提高聚合物材料的电子迁移率。
研究人员还通过设计合成新型的聚合物材料来提高其电子迁移率。
引入不对称结构、改变侧链结构等方法可以有效地改善聚合物材料的电子输运性能。
利用共轭聚合物与非共轭聚合物的共混来改变材料的分子排列方式,也可以提高聚合物材料的电子迁移率。
研究人员还通过界面优化等手段来提高聚合物材料的电子迁移率。
利用不同的界面材料来调控聚合物材料与电极之间的能级匹配和界面结合,可以提高电子注入和传输效率,进而提高电子迁移率。
利用界面工程来抑制杂质和缺陷的影响,也可以提高聚合物材料的电子迁移率。
高迁移率聚合物半导体材料的最新进展使其在有机电子器件领域具有了更广泛的应用前景。
通过合成新型的聚合物材料、优化薄膜结构、改善材料分子排列方式以及界面优化等手段,可以进一步提高聚合物材料的电子迁移率,实现更高性能的有机电子器件。
随着新材料合成方法和器件加工技术的不断发展,相信高迁移率聚合物半导体材料将在未来得到更广泛的应用。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Beyond polyimide:Crosslinked polybenzimidazole membranes fororganic solvent nanofiltration(OSN)in harsh environmentsIrina B.Valtcheva,Santosh C.Kumbharkar,Jeong F.Kim,Yogesh Bhole,Andrew G.Livingston nDepartment of Chemical Engineering,Imperial College,Exhibition Road,South Kensington Campus,London SW72AZ,UKa r t i c l e i n f oArticle history:Received3November2013Accepted27December2013Available online18January2014Keywords:Organic solvent nanofiltration(OSN)Polybenzimidazole(PBI)CrosslinkingExtreme pHAcid/basefiltrationa b s t r a c tIn this work,we report a new class of organic solvent nanofiltration(OSN)membranes fabricated frompolybenzimidazole(PBI)which exhibit superior chemical stability compared to other well-known polymericmembranes such as polyimide.Integrally skinned asymmetric PBI membranes were prepared and crosslinkedusing either an aliphatic or an aromatic bifunctional crosslinker.Three batches of membranes with the samecomposition were prepared and crosslinked with each crosslinker.The membrane performance showedexcellent reproducibility in organic solvents and water in terms offlux and retention profile.Also,bothmembranes showed good tolerance towards extreme pH conditions.To critically assess their chemical stabilitythe membranes were tested in realistic chemical process conditions that employ different types of acids andbases,e.g.concentrated dichloroacetic acid in acetonitrile,piperidine in N,N-dimethylformamide(DMF)andmonoethanolamine in water.The membranes modified with aliphatic crosslinker could not retain theirproperties when DMF was used as the organic solvent.This was found to be due to dissolution of PBI in DMFrather than degradation due to pH exposure.On the other hand,it was shown that the membrane modifiedwith the aromatic crosslinker exhibits superior stability and higher permeances in comparison to themembrane crosslinked with the aliphatic crosslinker.The results obtained show that membranes fabricatedfrom crosslinked PBI were stable and have the potential for applications in chemically harsh conditions foundin processes ranging from pharmaceutical to petrochemical industries.&2014Elsevier B.V.All rights reserved.1.IntroductionSeparations of solutes in organic solvents are widely employed inpharmaceutical and chemical industries.Typical techniques used forfractional separation are distillation,adsorption andflash chromato-graphy.These processes usually involve the utilisation of fresh solventsand energy.In particular,flash chromatography is relatively cumber-some to scale-up due to its aspect ratio limitation[1].These operationsresult in increased cost of goods,and raise environmental concerns.Asa result,cost effective alternatives are desired in order to minimise theplant footprint,waste production and energy anicsolvent nanofiltration(OSN)is a membrane-based separation processwhich can provide the necessary molecular discrimination[2],and hasalready shown great potential in terms of savings[1,3]and applic-ability[4–6]to industrial processes.Nanofiltration(NF)is already astate-of-the-art process for water purification and treatment[7,8].However,the available NF and reverse osmosis(RO)membranes are inmost cases not stable in harsh and corrosive environments typicallyrequired for OSN[2].The solvent and pH resistance of the membraneis one of the main challenges for implementing OSN in relevantprocesses.Inorganic materials are ideal for such conditions since theydo not easily dissolve or deform in organic solvents.On the otherhand,they are difficult to fabricate,handle and are more expensive ascompared to organic membranes[2,9].For this reason the majority ofreported OSN membranes are made out of polymeric materials.The most exhaustively studied polymer for application in OSN ispolyimide(PI)[2,10,11].The usual solvents in which PI dissolvesare polar aprotic ones,such as N,N-dimethylformamide(DMF)anddimethyl sulfoxide(DMSO)and these are typically used in membranefabrication.For a PI membrane to be stable in applications using thesesolvents,it needs to be chemically crosslinked with diamines[10,11].PI is stable to some extent in weak acids and low concentrations ofbases but is not recommended for use in inorganic acids and degradeswhen exposed to high concentrations of organic and inorganic bases[12].Apart from solvent stability,tailored membrane performance is adesirable feature,e.g.varying the molecular weight cut-off(MWCO)1of PI membranes[13].A few polymeric membranes have been reported in literaturewhich can withstand either acids or bases in aqueous feed streams[14–16].However,these membranes are based on sulfonatedpolyether ether ketone(SPEEK)or polysulfone(PSf)which willnot withstand many organic solvents.Hence,there is a need forContents lists available at ScienceDirectjournal homepage:/locate/memsciJournal of Membrane Science0376-7388/$-see front matter&2014Elsevier B.V.All rights reserved./10.1016/j.memsci.2013.12.069n Corresponding author.Tel.:þ442075945582;fax:þ442075945639.E-mail address:a.livingston@(A.G.Livingston).1Molecular weight cut-off(MWCO)is defined as the lowest molecular weightof a solute rejected at90%by the membrane.Journal of Membrane Science457(2014)62–72alternative membrane materials which can withstand both organic solvents and acidic/basic conditions.Surface crosslinked chitosan/polyacrylonitrile composite NF membranes have been shown to maintain their stability under basic pH and in several important organic solvents [17].However,the acid/base resistance was only demonstrated in aqueous media.Polybenzimidazole (PBI,Fig.1)has been studied extensively for reverse osmosis [18–21].More recently PBI has gained much atten-tion for applications in gas separation [22],aqueous NF [23],forward osmosis [24]and fuel cells [25,26]due to its outstanding properties (thermal,mechanical and chemical stability in corrosive environ-ments)[27].In addition,PBI has the advantage of possessing excellent stability towards acids and bases [12].PBI dissolves in polar aprotic solvents,such as N ,N -dimethylaceta-mide (DMAc),N -methylpyrrolidone (NMP)and DMSO,from which dope solutions can be prepared and cast.Uncrosslinked PBI hollow fibre membranes were shown to be effective in the removal of chro-mate from alkaline wastewater [28].PBI flat sheet membranes cross-linked with α,α0-dichloro-p-xylene were reported for the concentra-tion and separation of cephalexin from aqueous solutions over a pH range from 2to 10[23].Similar to polyimide membranes,unmodi fied PBI membranes cannot be used for polar aprotic solvents.Several chemical modi fication procedures have been reported in literature including treatment of PBI membranes with α,α0-dichloro-p-xylene [23],divinyl sulphone [29]and 1,4-dibromobutane [30].In this study,we report on the fabrication and performance of crosslinked PBI NF membranes for applications in organic solvents containing acids or bases.The conditions of two pharmaceutical and one chemical process have been chosen to demonstrate the solvent and acid/base resistance of the prepared membranes.Also,the batch-to-batch reproducibility of crosslinked PBI membranes has been evaluated.The membranes were characterised with FTIR,contact angle measurement and SEM.2.Experimental 2.1.MaterialsCelazole s S26polybenzimidazole (PBI,MW ¼27,000g mol À1)solution was purchased from PBI Performance Products Inc.(USA).The solution contains 26wt%polymer solids and 1.5wt%lithium chloride (stabiliser)dissolved in DMAc.Non-woven polypropylenefabric Novatexx 2471was from Freudenberg Filtration Technolo-gies (Germany).All solvents such as DMAc,propan-2-ol (IPA),acetonitrile (MeCN),and DMF were HPLC grade and were used as received from VWR (UK).The chemicals for crosslinking were α,α0-dibromo-p-xylene (DBX)and 1,4-dibromobutane (DBB)from VWR (UK)and Sigma Aldrich (UK),respectively.Polyethylene glycol (PEG)of three different molecular weights was purchased from VWR (UK)and Sigma Aldrich (UK).Dichloroacetic acid (DCA)and piperidine (PIP)were both from Sigma Aldrich (UK);monoetha-nolamine (MEA)was from VWR (UK).De-ionised water was produced by passing tap water through an RO filtration unit.2.2.Fabrication of integrally skinned (IS)asymmetric PBI membranes and chemical crosslinkingCelazole s S26was diluted with DMAc to 17wt%polymer con-centration and stirred continuously at 2170.51C until a homo-geneous dope solution was obtained.The polymer solution was then left overnight to remove air bubbles.Membranes were cast on polypropylene non-woven using a bench top laboratory casting machine with an adjustable knife set at 250m m (Elcometer,UK).Following this,the membranes were immersed in a water precipita-tion bath at 22711C for 24h,and subsequently washed with IPA to remove residual solvent and water.The viscosity of the dope solutions was measured using a rotary viscometer Model 2020from Cannon Instrument Company (USA)with a spindle size 16suitable for high viscosities.All viscosities were recorded at 10rpm spindle speed and at 211C.Membranes were crosslinked by immersion in a solution contain-ing 3wt%DBX or 10wt%DBB in acetonitrile (Fig.1).The reaction was carried out at 801C for 24h under constant stirring and re flux.After crosslinking,the membranes were first immersed in IPA to remove residual reagents and later on impregnated with PEG 400by immer-sion in a PEG 400/IPA (1:1)solution for 4h to preserve the pore structure and allow dry storage.After obtaining the final membrane,the thickness was recorded using a digital micrometre purchased from Mitutoyo (UK).The stated membrane thicknesses include the impregnated polymer film and the polypropylene non-woven and are the mean between six measured points in different parts of the membrane sheet.2.3.Selection of model solutionsTypically,a range of polystyrene (PS)[31]or polyethylene glycol (PEG)oligomers [32]is used to determine the rejection performance of OSN membranes.The main advantage of both methods is that the rejection of a range of homologous solutes can be analysed simulta-neously,and an MWCO curve can be obtained for each membrane.Also,both types of oligomers are soluble in a wide variety of organic solvents.However,PS solutes are more expensive and not soluble in water due to their hydrophobic character.Since the application of crosslinked PBI membranes is intended for but not limited to iterative synthesis of therapeutic drugs [5,33,34],which closely resemble PEG compounds,PEGs in different MW were chosen as the marker solutes in the current study.Three solvent systems,tested in this work,were chosen based on three commercial processes which employ acidic or basic conditions in the production.The first one mirrors one of the reaction condi-tions used in oligonucleotide synthesis,which is 3wt%DCA in MeCN [35].The second system replicates one of the reaction conditions used in peptide synthesis –20wt%piperidine in DMF [36].The third filtration solution –20wt%MEA in DI water –is representative of liquids used in carbon capture and storage (CCS)[37].Fig.1.(a)Chemical crosslinking mechanism of 2,2-(m-phenylene)-5,5-bibenzimida-zole (PBI)with a)α,α0-dibromo-p-xylene (DBX)and (b)1,4-dibromobutane (DBB).I.B.Valtcheva et al./Journal of Membrane Science 457(2014)62–72632.4.Membrane characterisation2.4.1.Fourier transform-infrared spectroscopy (FT-IR)Infrared spectra were recorded on a Perkin Elmer-Spectrum 100.The samples were fixed on a zinc/selenium diamond plate with the separating layer facing the beam.Prior to FT-IR measure-ments the membrane samples were immersed in water followed by IPA to remove traces of residual chemicals and PEG 400.2.4.2.Soak test for stabilityThe stability of crosslinked PBI membranes was compared with the stability of commercially available polyimide membranes (Table 2).Small pieces of DuraMem 200[38](a crosslinked polyimide membrane from Evonik-MET)and lab prepared DBX and DBB crosslinked PBI membranes were cut and washed with DI water and IPA to remove any traces of conditioning chemicals.The pieces were then dried in vacuum and their weight was measured and recorded.Five soak solutions were prepared:20wt%DCA/acetonitrile,20wt%PIP/DMF,20wt%MEA/water,20wt%NaOH/water and 20wt%HCl/water.Two pieces from two batches of membranes with the same composition were soaked in each solution to evaluate reproducibility.Along with these tests,one of each five solutions was left blank.This test was carried out for two months at 201C,after which the membrane pieces were withdrawn from the solutions,washed with water and vacuum dried.Then the weight of the sample was recorded and compared to the initial value.2.4.3.Contact angleThe contact angle of the membranes was obtained with an Easy-Drop Instrument (Kruess,Germany).Water drops of constant size were deposited on the membrane surface using amicropipette.The digital image from the camera was then analysed using a built-in drop shape analysis tool.The reported contact angle for each membrane is an average of 10drop measurements.2.5.Membrane performance and analysisThe filtration experiments were all carried out in cross flow cells connected in series (Fig.2),each holding membrane discs with an effective area of 14cm 2.Pressure and temperature were kept constant at 10bar and 301C,respectively,throughout the experiment.The pump flow rate was set at 40L h À1for all experiments.The permeance B is de fined as the volumetric flow rate of solution per unit membrane area per unit pressure drop and can be calculated using the given equation B ¼V ΔðL m À2h À1bar À1Þð1Þwhere V is the collected permeate volume,A is the effective membrane area,t is the time and Δp is the applied trans-membrane pressure.Feed and permeate samples were taken at different time intervals for rejection R i determination.Rejection was calculated using Eq.(2),where C p,i and C f,i represent the concentration of solute i in the permeate and the feed,respectively.R i ¼1ÀC p ;if ;i100ð%Þð2ÞA solute mixture containing polyethylene glycols (PEG)of threedifferent molecular weights (400,2000and 8000g mol À1)was used to determine the rejection properties of the membranes.The PEGs were dissolved in MeCN,DMF and DI water at a concentration of 1g L À1for each MW (referred to as standard solution from here on).Collected samples containing PEGs,DCA and MEA were analysed using an Agilent HPLC coupled to an evaporative light scattering detector (ELSD,Varian-385).The ELSD evaporator was set at 401C and the nebuliser at 551C.Nitrogen gas was supplied to the detector at a flow rate of 1.5SLM (standard L m À1).A reverse phase column (C18-300,250mm Â4.6mm,ACE Hichrom)was used and the mobile phases were methanol and DI water buffered with 0.1M ammonium acetate.The HPLC pump flowrate was set at 1ml min À1and the column temperature was set at 301C.The concentration of PIP was analysed using an Agilent GC with an HP-5column (5%phenyl methyl siloxane;capillary:30m Â0.530mm Â1.50m m)coupled to a flame ionisation detector (FID).The temperature ramp was from 401C (hold for 1min)until 2001C with an increase of 101C min À1.Table 1Summary of crosslinked PBI membranes prepared from eight different dope solutions of the same composition.Four membranes were crosslinked with DBX and the other four with DBB.Membranes with entry nos.1–6were used for reproducibility evaluation.Entries 7and 8were used to demonstrate the chemical resistance of crosslinked PBI membranes.Entry no.Membrane codeComposition Viscosity (cP)at 211CCrosslinking Thickness(μm)1M1.1–DBX 17wt%PB Iin DMAc 77203wt%DBX 262.5752M1.2–DBX 80903wt%DBX 242.7743M1.3–DBX 68503wt%DBX 251.7744M2.1–DBB 17wt%PBI in DMAc 810010wt%DBB 228.0745M2.2–DBB 750010wt%DBB 230.7746M2.3–DBB 710010wt%DBB 215.2787M1–DBX 17wt%PBI in DMAc 75003wt%DBX 250.8758M2–DBB769010wt%DBB241.377Table 2Percentage weight loss of membrane samples which were inserted in five different acidic/basic solutions.Membrane CodeWeight loss (%)a 20wt%DCA/MeCN20wt%PIP/DMF 20wt%MEA/water (pH ¼12)20wt%HCl/water (pH ¼0)20wt%NaOH/water (pH ¼14)DuraMem 200170397222728173157M1–DBX 070170271170070M2–DBB070372270470170aThe error indicates the standard deviation in %from the average weight loss value of two membrane samples.I.B.Valtcheva et al./Journal of Membrane Science 457(2014)62–72642.6.Viscosity and molar volume of solvent solutionsThe kinematic viscosity of the solvents and solvent mixtures used was determined using a BS/U/M2miniature viscometer at 301C.Three consecutive measurements were taken (with coef fi-cient of variation less than 1%)and the mean was used to calculate the kinematic viscosity.By multiplying with the corresponding density,the dynamic viscosity for each liquid was obtained.The molar volume V m for pure solvents was taken from literature [39]and the molar volume V m ,mix of acidic and basic solutions was calculated according to the equation V m ;mix ¼∑i x i M iρmixðmol cm À3Þð3Þwhere x i and M i are the mole fraction and molecular weight of component i ,respectively,and ρmix is the measured density of the mixture at 301C.2.7.Scanning electron microscopy (SEM)Scanning electron micrographs of membrane surface and cross-section were recorded using a JEOL 5610LV.The samples were first washed with IPA in order to remove PEG 400.The surface samples were prepared by cutting small squares and pasting them onto SEM sample holders covered with carbon tape.For the preparation of cross-section samples,small pieces of membrane were snapped under liquid nitrogen and pasted vertically onto SEM stubs.Finally,the samples were sputtered with gold under argon atmosphere (Emitech K550coater).The SEM was operated at an acceleration voltage of 10kV.2.8.Experimental sequenceEight membranes were cast from eight different dope solutions by diluting the commercial PBI solution to 17wt%of polymer solids.Four membranes were crosslinked with DBX and the other four with DBB.The procedure is described in detail in Section 2.2,and Table 1summarises the composition and physical properties of the eight membranes.Two membrane discs were cut out of each of the eight batches to eliminate errors resulting from defective membrane coupons.First,the reproducibility of crosslinked PBI membranes was determined in different solvents –acetonitrile,DMF and DI water using entries 1–6listed in Table 1.The pure solvent permeance was established for each of the membranes after washing out PEG 400preservative.The steady state value was taken as such after three consecutive hourly measurements were within 5%of each other.Then the standard PEG/solvent mixture was filtered for 24h and rejection was taken at that point.Secondly,the filtrations of acidic and basic solvent mixtures were performed as solvent swap experiments (Fig.3).The evalua-tion of the membrane stability was done by first establishing the membrane performance with solutions containing only the PEG marker solutes for 24h.Then,without removing the membranes from the test cells they were exposed for 24h to the acidic or basic conditions.Next,by filtering pure respective solvents the rig was cleaned from the previous mixtures.The last step involved filtra-tion of the initial solvent containing the PEG markers.The chemical stability of the membranes was evaluated by comparing membrane performance before and after acid/base exposure.The composition and physical properties of the two membranes M1–DBX and M2–DBB can be found in Table 1entry nos.7and 8.DueFig.2.(a)Schematic representation of the cross flow filtration system with pressure gauge P ,flow meter F and temperature control T .The solid lines represent the feed/retentate flow and the dashed lines –the permeate flow;(b)top and cross-section view of a cross flow cell with (1)feed inlet,(2)retentate outlet,(3)permeate outlet,(4)o-rings,(5)membrane disc,and (6)sintereddisc.Fig.3.Filtration sequences followed for evaluating the chemical stability of crosslinked PBI membranes:Sequence 1was used for one set of M1–DBX and M2–DBB membranes and sequence 2was used for a new set of the membranes.I.B.Valtcheva et al./Journal of Membrane Science 457(2014)62–7265to failure of M2–DBB after completing the first filtration sequence (Fig.3–Sequence 1),fresh membrane discs were taken to evaluate their performance upon exposure to MEA (Fig.3–Sequence 2).All results are reported as the mean between the tested samples and the error values represent the one standard deviation from the mean.3.Results and discussion 3.1.Membrane characterisation3.1.1.FT-IRPrior to filtration tests,small membrane samples were used to con firm that the crosslinking was successful.Fig.4shows the IR spectra of uncrosslinked,DBX and DBB crosslinked membranes.The characteristic peaks of PBI were con firmed with spectra of PBI published in literature [40].The peak at 3415cm À1can be attributed to non-hydrogen bonded N –H stretching and the peak at 3145cm À1to hydrogen bonded N –H stretching.Unfortunately,these signals are not useful because O –H stretching is registered as a broad peak in the same area,overlapping the N –H stretches.The peaks in the finger-print area (at 1612,1590,1443,1286,801and 705cm À1)are all attributed to benzene and imidazole rings and their conjugation.Two distinct peaks at 2920cm À1and 2850cm À1were observed in the spectra of the crosslinked membranes which are attributed to C –H (the terminal C of the crosslinker)and C –N (the link between the crosslinker and the polymer backbone)stretches,con firming the crosslinking between the imidazole rings.3.1.2.Soak test for stabilityTo assess the stability of crosslinked PBI membranes,the mem-branes were soaked in different chemical solutions and the observed stability was compared to that of commercially available PI mem-branes (DuraMem 200).Table 2summarises the percentage weight loss of the three membranes after withdrawing them from the corresponding solutions.Polyimide membrane degraded in MEA,PIP,HCl and NaOH.The DCA soak test showed localised swelling of polyimide membrane surface but did not result in membrane disintegration.The two types of crosslinked PBI membranes were found to be stable in all five test solutions based on visible observation and weight loss measurement,except for M2–DBB exposed to HCl/water and PIP/DMF.The PIP/DMF solution turned a slight yellow colour due to dissolution of PBI in DMF.As the five blank solutions remained clear for the tested period,it can be concluded that any visible observation was due to the degradationof the membrane pieces.From Table 2it can be concluded that DBX crosslinked PBI membranes,compared to DuraMem 200and DBB crosslinked membranes,showed superior chemical stability in the pH range from 0to 14.3.1.3.Contact angle measurementsThe contact angles of crosslinked PBI membranes were measured and compared with uncrosslinked PBI samples.It can be seen in Table 3that the contact angles of crosslinked PBI membranes decreased compared to uncrosslinked PBI,suggesting that the mem-branes became more hydrophilic after the crosslinking treatment.The lowest contact angle was measured for DBX treated PBI membranes,decreasing by more than 40%from the value of untreated PBI.This is in agreement with the observed higher permeances of M1–DBX in all tested solvents compared to M2–DBB (described in Section 3.2).Such behaviour has previously been reported by Soroko et al.[41]for TiO 2doped PI membranes.The higher TiO 2content resulted in more hydrophilic surfaces and an increase in ethanol flux.Bhanushali et al.[42]tested various polar and non-polar solvents with commer-cial hydrophilic and hydrophobic membranes.They concluded that hydrophilic membranes provide higher fluxes with polar as opposed to non-polar solvents.3.2.Membrane performance3.2.1.Reproducibility of crosslinked PBI membranesCrosslinked PBI membranes (entry nos.1–6in Table 1)were tested for their reproducibility in the three solvents of interest –acetonitrile,DMF and DI water.Six membrane discs from each batch were tested in each solvent and a new set of discs was used for each solvent.First,the pure solvent permeance of the membranes was measured after 24h of operation and the data is summarised in Table 4.Comparing the pure solvent permeances shown in Table 4,a correlation is evident between solvent viscosity,molar volume,surface tension (Table 5)and the respective permeance.Consistent with the trend reported in literature [43,44],solvent transport through cross-linked PBI membranes increased with decreasing viscosity,molar volume and surface tension (Table 5).Hence,the highest permeance,observed for acetonitrile,can be attributed to the lowest viscosity.On the other hand the lowest permeance was obtained for DMF due to its higher viscosity and higher molar volume.Water has the highestTable 3Contact angles for unmodi fied and modi fied PBI membranes.Membrane Contact angle (deg )a Uncrosslinked PBI 60.572.3M1–DBX 35.375.4M2–DBB55.072.3aThe error indicates the standard deviation between 10drop measurements on the same membrane.Table 4Pure solvent permeances for DBX and DBB crosslinked membranes measured after establishing steady state.MembranePure solvent permeance (L m À2h À1bar À1)a AcetonitrileDMF DI water DBX crosslinked PBI 37777711277DBB crosslinked PBI1777170472aThe error given in the table is the standard deviation from the average calculated value of the total of three membrane batches (entry nos.1–6in Table 1).Fig.4.FT-IR spectra for uncrosslinked,DBB and DBX crosslinked PBI membrane samples.I.B.Valtcheva et al./Journal of Membrane Science 457(2014)62–7266viscosity and surface tension,which should result in the lowest permeance.However,due to its small molar volume,water had an intermediate flux,slightly higher than DMF (Table 4).After obtaining the pure solvent permeance,the pure solvent was replaced by a solution containing the PEG markers.The flux was monitored over time and PEG rejection was measured after 24h.The results are shown in Fig.5.In all filtrations DBB membranes showed a lower permeance than DBX membranes.This was likely due to the more hydrophilic surface of M1–DBX (Table 3)which enhanced the permeance of polar solvents.In Fig.5(a),it is interesting to note that filtration of PEG/acetonitrile through DBX crosslinked membranes led to around 50%decrease in permeance (from 21to 11L m À2h À1bar À1)whereas filtration of PEG/DMF and PEG/DI water resulted in no signi ficant permeance loss (Fig.5(a)).On the other hand,for the membranes crosslinked with DBB no impact of the solvent/solutesystem was observed (Fig.5(b)).It is speculated that such trend comes from the initial swelling of DBX membranes by MeCN,slowly compensated by pressure compaction.Furthermore,Fig.5(c)and (d)show the rejection of PEG markers in the three different solvents.It can be noted that DBX crosslinked membranes had the same rejection pro file in all tested solvents,while DBB crosslinked ones appeared to have a higher rejection of PEGs when water is the solvent and lower in the case of MeCN and DMF.Overall,the error bars in Fig.5indicate that crosslinked PBI membranes have a consistent performance from batch to batch and it can be concluded that all demonstrated effects are reproducible.3.2.2.Filtration under acidic/basic conditionsTo assess the chemical stability membranes M1–DBX and M2–DBB (entry nos.7and 8in Table 1)were used.The experimental solvent sequences,shown in Fig.3,were used to investigate the resistance in acidic and basic environments.The permeance results collected from the filtrations are shown in Fig.6.Similar to the solvent permeance data in Fig.5(a),M1–DBX membranes had higher permeances than M2–DBB membranes (Fig.5(b))in all tested solvents.The solvent/solute permeances followed the same trend as seen in Fig.5and increased in the order:PEG/DMF o PEG/DI water o PEG/acetonitrile.A steady state flux was reached after 24h filtration of PEG/solvent solutions in each case.After washing the rig with fresh solvents to ensure removal of PEGs,the corresponding acidic or basic solution was loaded and circulated for 24h.The permeances for acidic and basic solutions are shown in Fig.6in the middle panel of each graph.In all three cases,an instant dropTable 5Solvents and solvent mixtures used in the study and their characteristics.Solvent mixtureViscosity a (cP)Molar volume b (cm 3mol À1)Surface tension b (mN m À1)Pure Acetonitrile0.3452.728.033wt%DCA/acetonitrile 0.3652.8–Pure DMF0.7877.433.9020wt%PIP/DMF 0.8281.4–DI water0.8318.171.6720wt%MEA/DI water1.2721.2–aMeasured viscosity at 301C.bValues for pure solvents taken from [39]at 301C and for mixtures calculated using Eq.(3)at 301C.Fig.5.Average solvent permeance pro files over time of (a)PBI membranes crosslinked with DBX and (b)PBI membranes crosslinked with DBB;average rejection of PEGs after 24h for (c)PBI membranes crosslinked with DBX and (d)PBI membranes crosslinked with DBB.The error bars represent one standard deviation from the average value of the measurement,where each point summarises the average value from tests on M1.1–DBX,M1.2–DBX,M1.3–DBX panels (a)and (c)and M2.1–DBB,M2.2–DBB,M2.3–DBB panels (b)and (d).I.B.Valtcheva et al./Journal of Membrane Science 457(2014)62–7267。
