thymosin-alpha1 and interferon-alpha on Th1 and Th2 cytokine synthesis in chronic hepatitis B
盐酸氨溴索对高血压脑出血患者术后的肺保护作用
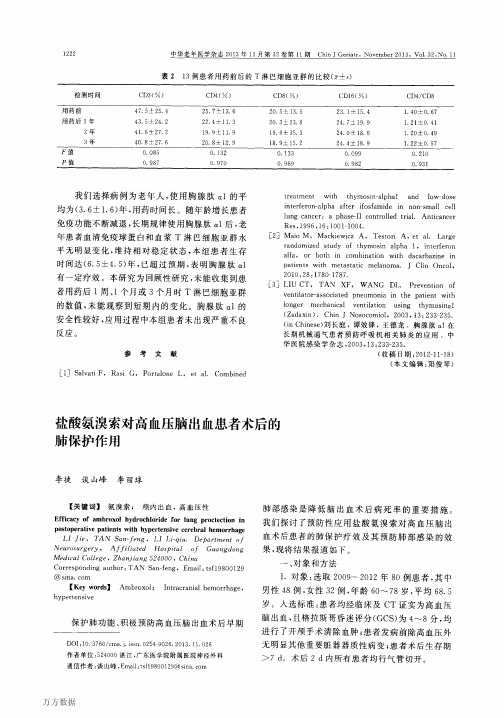
r1]Niederman MS,MandelI
Guidelines for the
management
community—acquired assessment of
pneumonia.Diagnosis,
severity,antimicrobial therapy,and
d内试验组的肺部感染发生率为45.0%(18/
review:
recruiting
Care,
[53
Beeh KM,
Beier
J,
Esperester
A,
et
a1.
Antiinflammatory properties of ambrox01.Eur J Med Res,2008。13:557-562.
(收稿日期:2013-07—23) (本文编辑:石婧)
男性48例,女性32例,年龄60~78岁,平均68.5 岁。入选标准:患者均经临床及CT证实为高血压 脑出血,且格拉斯哥昏迷评分(GCS)为4~8分,均 进行了开颅手术清除血肿;患者发病前除高血压外
保护肺功能、积极预防高血压脑出血术后早期
DOI:10.3760/cma.j.issn.0254 9026.201 3.11.026 作者单位:524000湛江,广东医学院附属医院神经外科
[1]Salvati
F,Rasi
G,Portalone
盐酸氨溴索对高血压脑出血患者术后的
肺保护作用
李捷谈山峰李丽球
【关键词】氨溴索;
postoperative patients with Ll
颅内出血,高血压性
肺部感染是降低脑出血术后病死率的重要措施。 我们探讨了预防性应用盐酸氨溴索对高血压脑出 血术后患者的肺保护疗效及其预防肺部感染的效 果,现将结果报道如下。 一、对象和方法 1.对象:选取2009--2012年80例患者,其中
HIF-1α_纳米抗体的制备及其抑制黑素瘤生长的作用

山西农业科学 2023,51(12):1435-1441Journal of Shanxi Agricultural Sciences HIF-1α纳米抗体的制备及其抑制黑素瘤生长的作用李佳敏1,贾琼1,秦蓉芬1,迟志端1,王富明2,范瑞文1(1.山西农业大学动物医学学院,山西太谷 030801;2.晋中市庄子乡综合便民服务中心,山西晋中 030600)摘要:缺氧诱导因子1α(Hypoxia inducible factor 1α,HIF-1α)参与低氧微环境相关疾病的发生等过程,具有控制肿瘤生长和发展的功能。
黑色素瘤是一种发生于人和动物恶性程度较高的肿瘤。
为探明HIF-1α纳米抗体对黑色素瘤的影响,研究利用前期保存的羊驼源黑色素瘤细胞噬菌体文库筛选HIF-1α纳米抗体,经原核表达与纯化后,通过Western Blot和免疫组织化学验证HIF-1α纳米抗体与抗原的结合性;分别通过CCK-8法、划痕试验以及Western Blot法检测其对B16黑素瘤细胞的增殖和迁移能力及其相关分子表达的影响。
结果表明,经表达和纯化获得的HIF-1α纳米抗体分子质量约为16 ku,没有跨膜结构,具有亲水性。
通过Western Blot和免疫组织化学验证了其具有良好的抗原结合性。
在增殖试验和划痕试验中,与对照组相比,HIF-1α纳米抗体抑制了B16细胞的增殖和迁移,下调了靶基因VEGF的表达,并使细胞增殖和迁移相关蛋白Ras、ERK、RAC和RAF的表达量下调。
预测HIF-1α纳米抗体进入B16细胞内,与抗原结合,通过下游靶基因VEGF下调RAs、ERK、RAC、RAF的表达,从而对细胞增殖和迁移起抑制作用,可作为黑色素瘤治疗的新靶点。
关键词:HIF-1α;纳米抗体;B16细胞;Western Blot法;CCK-8法;细胞增殖;细胞迁移中图分类号:R739.5 文献标识码:A 文章编号:1002‒2481(2023)12‒1435‒07Effect on Preparation of HIF-1α Nano-Antibody and ItsInhibition of Melanoma GrowthLI Jiamin1,JIA Qiong1,QIN Rongfen1,CHI Zhiduan1,WANG Fuming2,FAN Ruiwen1(1.College of Veterinary Medicine,Shanxi Agricultural University,Taigu 030801,China;2.Jinzhong City Zhuangzi Integrated Convenient Service Center,Jinzhong 030600,China)Abstract:The hypoxia inducible factor 1α(HIF-1α) is involved in the occurrence of diseases related to hypoxia microenvironment and has the function of controlling tumor growth and development. As we known, melanoma is a highly malignant tumor occurring in animals and humans. To explore the effect of HIF-1α nano-antibody on melanoma, in this study, the phage library of alpaca-drived melanoma cells previously preserved in our laboratory was used to screen HIF-1α nano-antibodies. After prokaryotic expression and purification, the binding of HIF-1α nano-antibody and its antigen was verified by Western blot and immunohistochemistry. The effects of HIF-1α nano-antibody on the proliferation and migration of B16 melanoma cells and the expression of related molecules were detected by CCK-8, wound healing test, and Western blot methods. The results showed that HIF-1α nano-antibody obtained by expression and purification was hydrophilic protein without transmembrane structure and had a molecular weight of about 16 ku. Western blot and immunohistochemistry results showed that it had good antigenic binding. In the proliferation and wound healing experiments, HIF-1α nano-antibody inhibited the proliferation and migration of B16 cells, down-regulated the expression of target gene VEGF and the proliferation and migration related proteins Ras, ERK, RAC, and RAF, comparing with the control group. In Conclusion, it was predicted that HIF-1α nano-antibody entered B16 cells and combined with antigens and down-regulated the expression of RAs, ERK, RAC, RAF through the downstream target gene VEGF, which inhibited cell proliferation and migration, and could be used as a new target for melanoma treatment.Key words:HIF-1α; nano-antibody; B16 cells; Western Blot method; CCK-8 method; cell proliferation; cell migration氧是生命活动中所必需的物质,且在其中起重要作用[1]。
干扰素α-2b联合α1胸腺肽治疗慢性乙型肝炎疗效分析

干扰素α-2b联合α1胸腺肽治疗慢性乙型肝炎疗效分析雷南凤;刘添皇【期刊名称】《中国医药科学》【年(卷),期】2015(000)022【摘要】目的:探究干扰素α-2b联合α1胸腺肽治疗慢性乙型肝炎的临床疗效。
方法将160例慢性乙型肝炎患者随机分为两组,即对照组经干扰素α-2b治疗,观察组在此基础上经α1胸腺肽治疗。
再比较两组疗效。
结果观察组治疗6个月ALT、AST复常率分别为45.0%、50.0%,对照组分别为18.8%、17.5%,差异有统计学意义(P<0.05);观察组治疗6、9、12个月HBsAg转阴率分别为21.3%、3.2%、32.5%,对照组分别为5.0%、12.5%、15.0%,差异有统计学意义(P<0.05);观察组治疗6、9、12个月HBV DNA转阴率分别为58.8%、62.5%、62.5%,对照组分别为31.3%、36.3%、36.3%,差异有统计学意义(P <0.05);两组治疗6、12个月QOL各领域评分均显著优于治疗前(P<0.05);观察组治疗6个月生理、社会关系评分分别为(63.1±10.2)分、(60.4±12.7)分显著高于对照组,差异具有统计学意义(P<0.05);观察组治疗12个月生理、心理及社会关系评分分别为(65.4±11.1)分、(64.6±13.5)分、(62.4±12.6)分显著高于对照组(P<0.05)。
两组不良反应发生率比较,差异无统计学意义(P>0.05)。
结论慢性乙型肝炎患者经干扰素α-2b联合α1胸腺肽治疗,可有效清除HBV,提高患者免疫力,控制发生不良反应,疗效确切,值得临床推广应用。
%Objective To explore clinical curative effect of interferon alpha-2b combined with thymosin alpha 1 in treatment of chronic hepatitisB.Methods160 patients with chronic hepatitis B were randomly allocated tothe control group and the observation group. The control group was treated by interferon alpha-2b while the observation group was received thymosin alpha 1 at the basis of the treatment in the control group. Curative effects of two groups were compared.ResultsAST and ALT normalization rate of 6 months after treatment of the observation group were 45% and 50% respectively and those of the control group respectively were 18.8% and 17.5% respectively (P<0.05). Negative rates of HBsAg of 6, 9 and 12 months after treatment of the observation group were 21.3%, 3.2% and 32.5% respectively while those of the control group were 5.0%, 12.5% and 15.0% respectively (P<0.05). In addition, negative rates of HBV DNA of 6, 9 and 12 months after treatment of the observation group were 58.8%, 62.5% and 62.5% respectively while those of the control group were 31.3%, 36.3% and 36.3% respectively (P<0.05). QOL scores of 6 and 12 months after treatment of two groups were both significantly better than those before treatment (P<0.05). The scores of physiological and social relations of the observation group were (63.1±10.2) score and (60.4±12.7) score respectively, significantly higher than those of the control group, and the differences had statistical significance (P<0.05). The scores of physical, psychological and social relationship of the observation group were (65.4±11.1)score, (64.6±13.5)score and (62.4±12.6)score respectively, significantly higher than those of the control group (P<0.05). There was no statistical significance when incidences of adverse reactions in two groups were compared (P>0.05).Conclusion Interferon alpha-2b combined with thymosin alpha 1 in treatment of patients with chronichepatitis B can effectively remove HBV, improve patients' immunity and control the occurrence of adverse reactions. It has an accurate curative effect, worthy of clinical promotion and application.【总页数】4页(P46-48,68)【作者】雷南凤;刘添皇【作者单位】广东省梅州市人民医院药剂科,广东梅州514031;广东省梅州市人民医院肝病科,广东梅州514031【正文语种】中文【中图分类】R512.62【相关文献】1.干扰素α-2b联合胸腺肽α1治疗慢性乙型肝炎临床疗效分析 [J], 张经良;李涛2.α-2b干扰素联合胸腺肽治疗HBeAg阳性慢性乙型肝炎的疗效 [J], 肖清华;陈华卿;程进明;陈德永;焦煜全3.α-2b干扰素联合胸腺肽治疗HBeAg阳性慢性乙型肝炎的临床疗效分析 [J], 李静宇4.胸腺肽联合重组人干扰素α-2b治疗慢性乙型肝炎疗效及对外周血T淋巴细胞亚群水平的影响 [J], 马兴梅5.α-2b型干扰素联合胸腺肽治疗慢性乙型肝炎疗效分析 [J], 黄新造;徐军峡;程丹;徐松波因版权原因,仅展示原文概要,查看原文内容请购买。
四逆汤对脓毒症(心肾阳衰证)患者C5a和C3a的影响

脓毒症因其复杂、难治且病死率高,目前广受重症领域关注。
仅美国,每年就有75万人被诊断为严重脓毒症,其中有21.5万人死于上述疾病[1]。
目前有报道的严重脓毒症的死亡率已高达28%~35.5%。
即使严格执行了Surviving Sepsis Campaign(SSC)指南的集束化治疗,死亡率仍在30%左右[1]。
免疫调控紊乱是脓毒症发生的关键机制之一,作为机体固有免疫重要组成部分的补体系统也在其中发挥了重要作用[2-3]。
免疫治疗随之成为脓毒症治疗的研究热点,也已发现拮抗补体有望在脓毒症的治疗中发挥作用[4-5],但目前仅停留于动物实验。
而已进入临床研究的重组IFN-γ(rIFN-γ)、粒细胞集落刺激因子和粒细胞/单核细胞集落刺激因子(G-CSF/GM-CSF)和胸腺肽α1(Tα1),虽被证实对机体免疫状态的存在一定的改善作用,但在死亡率降低方面仍没有令人满意的结果[6-8]。
四逆汤主治阳虚阴寒,甚或亡阳虚脱等危急重症,被广泛应用于脓毒症,尤其是脓毒性休克的治疗[9]。
而已报道的研究也揭示了其可调节机体免疫功能,存在一定的抗四逆汤对脓毒症(心肾阳衰证)患者C5a和C3a 的影响∗陈腾飞肖斌△许钦王施玮陈波(福建中医药大学附属第二人民医院,福建福州350003)中图分类号:R631文献标志码:B文章编号:1004-745X(2020)12-2196-03doi:10.3969/j.issn.1004-745X.2020.12.037【摘要】目的观察四逆汤对脓毒症(心肾阳衰证)患者C5a和C3a的影响。
方法患者60例随机分为对照组与四逆汤组,每组30例。
对照组参照指南给予常规治疗,四逆汤组在对照组的基础上加用四逆汤治疗,每日1剂,早晚温服或鼻饲,连续7d或直至心肾阳衰证消失。
分别于纳入研究的第1天、第3天及第7天以ELISA检测血清中C3a和C5a水平,比较急性生理与慢性健康评分量表Ⅱ(APACHEⅡ)评分、序贯器官衰竭评分表(SOFA)评分和28d死亡率在组间的差异性。
靶向沉默人类真核翻译延长因子1A2可抑制胰腺癌细胞BxPC_3体外迁移和侵袭

DOI: 10.3781/j.issn.1000-7431.2012.08.Copyright 2012 by TUMOR靶向沉默人类真核翻译延长因子1A2可抑制胰腺癌细胞BxPC-3体外迁移和侵袭许 朝,胡端敏,诸 琦上海交通大学医学院附属瑞金医院消化内科,上海 200025[摘要] 目的:探讨靶向沉默人类真核翻译延长因子1A2(homo sapiens eukaryotic translation elongation factor 1 alpha 2,EEF1A2)对胰腺癌BxPC-3细胞体外迁移和侵袭的影响及其可能的分子机制。
方法:应用RT-PCR 和蛋白质印迹法检测4种胰腺癌细胞株中EEF1A2的mRNA 和蛋白表达水平。
将EEF1A2-小干扰RNA (small interference RNA ,siRNA )和阴性对照siRNA 转染到BxPC-3细胞中,随后采用RT-PCR 和蛋白质印迹法分别检测EEF1A2的mRNA 及蛋白表达,应用体外划痕和Transwell 侵袭实验分别检测BxPC-3细胞的体外迁移和侵袭能力,蛋白质印迹法检测转染前后细胞中p-Akt 和总Akt 蛋白的表达变化。
结果:4种胰腺癌细胞株中除SW1990细胞呈EEF1A2低表达外,BxPC-3、Patu8988和Panc-1细胞均呈EEF1A2高表达。
EEF1A2-siRNA 转染BxPC-3细胞48 h 后,EEF1A2 mRNA 及蛋白表达水平较阴性对照siRNA 转染的细胞和空白对照细胞明显降低(P <0.01)。
EEF1A2-siRNA 转染组BxPC-3细胞的体外迁移和侵袭能力明显低于阴性转染对照组和空白对照组(P <0.05)。
EEF1A2-siRNA 转染组细胞中Akt 的磷酸化水平明显低于对照组细胞(P <0.05),而总Akt 蛋白表达水平未显著改变(P >0.05)。
结论:siRNA 干扰下调EEF 1A 2基因表达可明显抑制胰腺癌BxPC-3细胞的体外迁移和侵袭,其机制可能与EEF1A2调控Akt 信号通路有关。
人胸腺肽α1Thymosinα1酶联免疫分析

人胸腺肽α1(Thymosin α1)酶联免疫分析(ELISA)试剂盒使用说明书本试剂仅供研究使用目的:本试剂盒用于测定人血清,血浆及相关液体样本中人胸腺肽α1(Thymosinα1)含量。
实验原理:本试剂盒应用双抗体夹心法测定标本中人胸腺肽α1(Thymosin α1)水平。
用纯化的人胸腺肽α1(Thymosin α1)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入胸腺肽α1(Thymosin α1),再与HRP 标记的胸腺肽α1(Thymosin α1)抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB 显色。
TMB 在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的胸腺肽α1(Thymosin α1)呈正相关。
用酶标仪在450nm 波长下测定吸光度(OD 值),通过标准曲线计算样品中人胸腺肽α1(Thymosin α1)浓度。
样本处理及要求:1.血清:室温血液自然凝固10-20 分钟,离心20 分钟左右(2000-3000 转/分)。
仔细收集上清,保存过程中如出现沉淀,应再次离心。
2.血浆:应根据标本的要求选择EDTA、者柠檬酸钠或肝素作为抗凝剂,混合10-20 分钟后,离心20 分钟左右(2000-3000 转/分)。
仔细收集上清,保存过程中如有沉淀形成,应该再次离心。
3.尿液:用无菌管收集,离心20 分钟左右(2000-3000 转/分)。
仔细收集上清,保存过程中如有沉淀形成,应再次离心。
胸腹水、脑脊液参照实行。
4.细胞培养上清:检测分泌性的成份时,用无菌管收集。
离心20 分钟左右(2000-3000 转/分)。
仔细收集上清。
检测细胞内的成份时,用PBS(PH7.2-7.4)稀释细胞悬液,细胞浓度达到100 万/ml 左右。
通过反复冻融,以使细胞破坏并放出细胞内成份。
离心20 分钟左右(2000-3000 转/分)。
仔细收集上清。
离子交换法分离固相合成胸腺素α1

步离子交换方法制备得到高纯度 T l a 的方法 , 并就其中的关键影响因素展开讨论,以满足
大 规模合 成 T l 备 的要 求 。 a制
2 实验部分
21 仪 器 .
,
 ̄ T x lrr 10 蛋 白 纯 化 系 统 , 瑞 典 安 法 码 西 亚 仪 器 有 限 公 司 ;A I2 0 K A epoe 0 P 0 0 SMS / ,美 国生物 应用 系 统 公司 ;Bek nCol r高效液 相 色谱 仪 ,美 国 B cma cma ut e ek n
脱 方法以及树脂种 类对纯化 效果的影响, 立了一套基于 S ucT Q阴离子 交换树脂 的纯化方案 。 建 ore 1 M 5
利用上述方法纯化 Tt纯度可 达 9. %,收率 6, c l 53 5 0 %,经 E I 分析 ,产 品分子 量与 Td吻合 。 4 S. MS e 该技 术可满足规模化 、低成本纯化 固相合成胸腺素 a 的需要 。 1 关键 词:胸 腺素 a ;离子交换 ;S U C Q l O R M1 ;纯化 5
研究表 明, e 主要的生理功能是诱导 T细胞的分化 、 Tt l 增殖和成熟, 可作为免疫增强剂
用于 免疫 缺 陷和免 疫抑 制 方面 的疾 病 [4 目前 ,临床 上主 要用 于慢 性 肝 炎病毒 以及 恶性 2t - o
肿瘤 的治 疗 。除此 之外 ,Tt还 能 辅助 治疗 以防止肺 癌复 发 ,增加 老年 人 使用 的流感 疫苗 e l 的效 用 。进 一 步 的研 究 还 发现 ,联合 T l F 0和 ZdV ie 药将 可 能成 为一 条治疗 a ,IN. 【 io Udn 用 A DS的新 途径 。 I J 目前 ,国 内外 Tt c l的生 产方 法主 要有 3种 ,即组 织提 取法 、基 因工 程 法和化 学 合成方 法 ,其 中 ,固相化 学合 成法 制 备 Tt c l已成 为主 流技 术之 一 J q 。但 由于 在 F c固相 方法 mo 合成 Tt c l过程 当 中,每 一步 的连 效率 不可 能达 到 10 0 %,在合 成后 产 品 当中必 然 出现许 多 与 目标产 物 结构 上很 相近 的物 质 ,结构差 异很 小 ,物 理化学 性质 也 很相 近 ,这 样 便增加 了
乙肝药品列表英文

HBF Drug Watch Compounds in Development for Chronic Hepatitis B
Updated July 2009
FAMILY/DRUG NAME
MECHANISM
COMPANY
STATUS
Interferons- Mimic naturally-occurring, infection-fighting immune substance produced in the body
Intron A
(Interferon
alfa-2b
Immunomodulator
Schering-Plough
Madison, NJ
FDA Approved
1991
Pegasys
(Peginterferon alfa-2a)
Immunomodulator
Roche
Switzerland
FDA Approved 2005
FDA Approved
1999
Hene
Canada
FDA Approved 2006
GlaxoSmithKline Philadelphia, PA
FDA Approved
1998
Hepsera
(Adefovir Dipivoxil)
Inhibits viral DNA polymerase
Gilead
Foster City, CA
FDA Approved
2002
Baraclude
Post-Exposure and/or Post-Liver Transplant Treatment
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
In vitro effect of thymosin-alpha1and interferon-alpha on Th1 and Th2cytokine synthesis in patients with eAg-negative chronic hepatitis BE.Loggi,1A.Gramenzi,1M.Margotti,1C.Cursaro,1S.Galli,2G.Vitale,1E.Grandini,1A.Scuteri,1R.Vukotic,1P.Andreone1and M.Bernardi11Department of Internal Medicine,Cardioangiology and Hepatology and2Microbiology Section,Department of Clinical and Experimental Medicine,University of Bologna,Bologna,ItalyReceived April2007;accepted for publication October2007SUMMARY.Thymosin alpha-1(T a1)has been shown to be effective in chronic hepatitis B treatment.This study inves-tigated the effect of T a1and interferon-alpha(IFN a)on cytokine production by peripheral blood mononuclear cells (PBMCs)of12patients with eAg-negative chronic hepatitis B(HBV).We evaluated the effect of incubation with T a1, IFN a or both on the synthesis of T-helper1(Th1)cytokines [interleukin-2(IL-2),IFN c]and Th2cytokines(IL-4,IL-10) and of antiviral protein2¢,5¢-oligoadenylate synthetase (2¢,5¢-OAS)in patients and in a group of10healthy controls. Concerning Th1profile,controls showed lower IL-2syn-thesis than HBV patients.In HBV setting,IFN a/T a1combi-nation was able to increase IL-2production significantly, when compared with baseline condition.About the Th2-cytokines,controls showed statistically lower synthesis of IL-4and higher production of IL-10,than HBV patients.In these latter,IFN a increased the synthesis of IL-10compared with baseline.Interestingly,both T a1alone and the IFN a/ T a1combination reversed this effect.Finally,compared with baseline,the synthesis of2¢,5¢-OAS was significantly higher in the presence of T a1and IFN a alone,and in the presence of IFN a/T a1association,while no differences were found be-tween controls and HBV patients.In conclusion,in PBMCs from eAg-negative HBV patients,T a1alone was able to in-crease the antiviral protein synthesis,while in association with IFN a,it stimulated the IL-2synthesis and inhibited the IFN-induced IL-10production.These results need further investigations,but reinforce the idea of an immunothera-peutic approach for chronic hepatitis B.Keywords:hepatitis B,immune response,thymosin alpha-1, T-helper1,T-helper2.INTRODUCTIONHepatitis B virus(HBV)is one of major causes of chronic liver disease worldwide[1].Chronic hepatitis B can range from a persistent asymptomatic carrier state to a more or less progressive chronic infection,potentially evolving to cir-rhosis,hepatocellular carcinoma and liver failure[2].HBV is not a cytopathic virus and liver injury is mainly mediated by the host immune response against virus-infected liver cells and by the production of cytokines.However,the pathoge-netic mechanisms responsible for both viral persistence and variable course of infection have only partly been clarified.It has been shown that a vigorous,polyclonal and multi-specific cytotoxic and T-helper(Th)cell response to HBV is readily detectable in the peripheral blood of patients with self-limited hepatitis B,but is weak,antigenically restricted or undetectable in patients with chronic infection[3].Thus, this T-cell response is believed to play a pivotal role in the outcome of HBV.Disturbed functions of CD4Th lymphocytes in the course of chronic hepatitis B have been well documented[4]. Human CD4T cells are known to be functionally heteroge-neous,containing two distinct Th subsets:Th1-producing interferon-gamma(IFN c)and interleukin(IL)-2,and Th2-releasing IL-4,IL-5and IL-10[5,6].In immunopathogenesis of chronic hepatitis B,it has been suggested that a prefer-ential shift towards a Th1-polarized phenotype could be associated with a spontaneous viral clearance or with a successful control of infection[7],while an early commit-ment towards a Th2synthesis plays a significant role in disease progression[8].On the other hand,some authors also showed that the Th1cytokine profile correlates withAbbreviations:IFN,interferon;IL,interleukin;2¢,5¢-OAS,2¢,5¢-oli-goadenylate synthetase;HBV,hepatitis B virus;PBMCs,peripheralblood mononuclear cells.Correspondence:Pietro Andreone MD,Servizio di SemeioticaMedica,Department of Internal Medicine,Cardioangiology andHepatology,University of Bologna,Via Massarenti,9,Bologna,Italy.E-mail:andreone@med.unibo.itJournal of Viral Hepatitis,2008,15,442–448doi:10.1111/j.1365-2893.2007.00960.xhepatic inflammatory activity and liver damage progression [9].Thymosin alpha-1(T a1)is a synthetic polypeptide of thymic origin that stimulates the maturation of thymocytes, the differentiation into active T cells and restores T-cell function by T-cell-mediated antibody production[10–13]. T a1concentration has been found to be low in patients with chronic HBV[14],and clinical trials with T a1indicates that it could be at least as effective as IFN a[15,16].The aim of the present study was to evaluate theÔin vitroÕeffect of IFN a,T a1,and of their association on the produc-tion of Th1(IL-2,IFN c)and Th2cytokines(IL-4,IL-10),as well as the synthesis of an antiviral protein,the2¢,5¢-oli-goadenylate synthetase(2¢,5¢-OAS),by peripheral blood mononuclear cells(PBMCs)of patients with eAg-negative chronic HBV infection.PATIENTS AND METHODSPatientsTwelve patients[male/female:9/3;median age(years): 44.5,range:19–62]with HBeAg-negative chronic hepa-titis B were enrolled in this study.All patients were hepatitis B surface antigen-positive and hepatitis B e antigen-negative for at least1year and had detectable serum HBV-DNA within the last3months before starting the study.Chronic hepatitis B was confirmed by histo-logical evaluation in all cases.No patient had decompen-sated liver disease,detectable antibodies against hepatitis C,D or human immunodeficiency viruses;none had evi-dence of hepatocellular carcinoma or had received any anti-viral drug.Ten healthy subjects[male/female:6/4;median age (years):38,range:28–52],seronegative for any HBV sero-logical markers,without a clinical history of hepatitis and without symptoms or signs of liver disease were studied as controls.Their demographic characteristics were not signif-icantly different from those of patients.All subjects gave written informed consent to the study that conformed to the Helsinki declaration.MethodsVenous blood samples were collected into heparinized tubes and immediately processed.PBMCs were obtained by centrifugation of blood over Ficoll solution(Sigma-Aldrich,Milan Italy)and washed three times in RPMI1640medium containing10%heat inactivated fetal calf serum(Gibco Invitrogen,Milan,Italy), 2m M L-glutamine,50U/mL penicillin,50l g/mL strepto-mycin and10m m HEPES(Gibco Invitrogen).The cells were resuspended at the concentration of1·106PBMC/mL and were then subjected to four different experimental condi-tions,all in the presence of Concanvalin A(ConA)at 10mg/mL(Sigma);as previously reported[17,18],ConA was used as a polyclonal stimulus for triggering cytokine production.The incubation conditions were as follows:1culture medium alone(baseline condition);2culture medium containing500U/mL of IFN a(Wellfer-on,Glaxo Wellcome;Verona,Italy);3culture medium containing10l g/mL of T a1(SciClone Pharmaceuticals,San Mateo,CA,USA);4culture medium containing IFN a and T a1together.Peripheral blood mononuclear cells of healthy controls were cultured only in the presence of culture medium alone without addition of IFN a and/or T a1.The cells were cultured for24h at37°C in atmosphere containing5%CO2,and then harvested for cytokine analysis:after centrifugation at 500·g,the supernatants were collected and stored at–80°C for subsequent test.The cytokine production was assessed by enzyme-linked immunoadsorbent assay(ELISA)according to manufac-turerÕs instructions(R&D Systems,Minneapolis,MN,USA), using the supernatants harvested from cell cultures.Optical density was determined by plate photometer at450nm,and corrected by subtraction of readings at540nm.The values of patients were obtained by interpolation with the standard curve.Both the patients and the standard samples were as-sessed in duplicate.The activity of2¢,5¢-OAS was determined in the super-natants of the cell cultures incubated in the absence of ConA,using a radioimmunological assay(Eiken Chemical, Tokyo,Japan).The sensitivity of the method was 10–810pmol/dL.Serum HBV markers were tested by Chemioluminescent Microparticles Immunoassay(CMIA; ArchitectÒAbbott Laboratories,Aprilia,Italy).Serum HBV-DNA was measured quantitatively by real-time PCR assay(AffigeneÒHBV trender;Alfa Wassermann,Bologna, Italy).The limit of detection for this test was50IU/mL. Nucleotide polymorphisms at nucleotide(nt)1762and nt1764in the basal core promoter region and at codon28in the precore region were identified by line probe assay Inno-Lipa HBV PreCore(Innogenetics Srl,Pomezia,Italy).Viral genotype was assessed by TrugeneÒHBV Genotyping Kit (Bayer Diagnostic,Tarrytown,NY,USA).Statistical analysisThe data are expressed as median(range).The statistical analysis was performed by non-parametric test;in partic-ular,comparison between the different experimental conditions was analysed by WilcoxonÕs test for paired data and correlations between variables were evaluated by SpearmanÕs correlation coefficient.A value of P<0.05was considered statistically significant.Data processing was carried out with the SPSS statistical package for Windows.T a1and IFN a and cytokine synthesis in HBV443RESULTSThe main biochemical and virological characteristics of pa-tients are listed in Table 1.Th1-cytokinesAt baseline,the concentration of IL-2of HBV-infected pa-tients was significantly higher when compared with healthy controls [435(75–1828)vs 86(25–139)pg/mL,P =0.000](Fig.1a).Concerning the HBV patients,com-pared with the baseline condition,the addition of IFN a or T a 1to the culture medium did not significantly modify the IL-2production (Fig.1b).Interestingly,the association IFN a /T a 1induced a significant increase in IL-2when com-pared both with baseline [1081(239–2930)vs 435(75–1828)pg/mL,P =0.02]and with T a 1alone [1081(239–2930)vs 652.5(100–2195)pg/mL,P =0.01].The IFN c concentrations (Fig.2a)did not show any sig-nificant differences between patients and controls at the baseline conditions [2877(1567–6738)vs 2957(170–8650)pg/mL,P =0.6].In HBV patients,the IFN c level in the different stimulate conditions was not significantly dif-ferent with respect to baseline.However,the association IFN a /T a 1was able to increase IFN c synthesis significantly compared with T a 1alone [3320(1165–9130)vs 3000(315–7780)pg/mL,P =0.03](Fig.2b).No correlation was found between HBV-DNA serum levels and ALT levels and Th1-cytokines both in baseline and in stimulated cultures.Th2-cytokinesAt baseline,compared with controls,the IL-4levels were significantly higher in patients [0(0–83)vs 41(0–207)pg/mL,P =0.01](Fig.3a),while the IL-10levels were signifi-cantly lower [1051(295–3150)vs 2666(439–4118)pg/mL,P =0.003](Fig.4a).As far as HBV patients are con-cerned,IL-4production (Fig.3b)in the presence of IFN a washigher when compared with all the other experimental conditions,even if the increase reached statistical signifi-cance only when compared with the association IFN a /T a 1[67(0–238)vs 15(0–132)pg/mL;P =0.03].Regarding IL-10production (Fig.4b),the addition of IFN a alone resulted in a significant increase in comparison with all the other experimental conditions:baseline [1705(570–4530)vsTable 1Biochemical and virological characteristics of patientsCase no.Sex Age GPT (U/L)HBV-DNA (IU/mL)Nucleotide at 1762/1764Nucleotide at 1896Genotype 1M 47941831823T/AA D 2F 5417787320A/A &A/G G D 3M 56323375A/G G/A D 4M 242562259125T/A A D 5F 49100207525T/A A D 6M 477839310A/G A D 7F 6225100T/A A D 8M 3934390A/G A C 9M 261401907690A/G A D 10M 1922283260T/A G D 11M 42580361350T/A G D 12M36409960A/GAD444 E.Loggi et al.1051.5(295–3150)pg/mL,P=0.003],T a1alone[1705(570–4530)vs656.5(280–2060)pg/mL,P=0.003],and IFN a/T a1together[1705(570–4530)vs982.5(458–23205)pg/mL,P=0.003].Finally,the IL-10concentra-tion in the presence of T a1alone was found significantly lower than baseline condition[656.5(280–2060)vs1051.5 (295–3150)pg/mL,P=0.02].No correlation was found between HBV-DNA serum levels and alanine aminotransferase(ALT)levels and Th2-cyto-kines both in baseline and in stimulated cultures.2¢,5¢-OASAt baseline,no difference was found between2¢,5¢-OAS levels of patients and controls[51[0–14930)vs40(21–139)pmol/mL,P=0.7](Fig.5a).In HBV patients(Fig.5b), the2¢,5¢-OAS level at baseline was significantly lower com-pared with all the other experimental conditions.In fact,the addition of either IFN a[118(0–25690)vs51(0–14930) pmol/mL,P=0.03]or T a1[168(0–22620)vs51(0–14930)pmol/mL,P=0.03]induced a significant increase in2¢,5¢-OAS production,when compared with baseline condition.The IFN a/T a1association produced a further in-crease in2¢,5¢-OAS concentration,but it reached statistical significance only when compared with baseline[255(44–29200)vs51(0–14930)pmol/mL,P=0.01].No corre-lation was found between HBV-DNA serum levels and ALT levels and2¢,5¢-OAS level both in baseline and in stimulated cultures.DISCUSSIONAs HBV is a non-cytopathic virus,the immune response against virus-infected liver cells and the production of inflammatory cytokines are thought to be responsible for both liver disease and viral clearance[19].It has been shown that individuals with self-limited HBV infection mount a vigorous polyclonal Th and cytotoxic(CTL)re-sponse to multiple viral epitopes;these responses can be persistently found following viral clearance[20–23].On the contrary,these responses are usually weak or absent in patients who fail to clear the virus and become chronically infected[3,4,24].Furthermore,a consistent lymphocyte infiltrate with Th-1profile has been found in the liver of patients chronically infected by HBV,suggesting a patho-genetic role in liver injury[25].Although these data are still controversial,there is general agreement about the concept that hyporesponsiveness of HBV-specific T cells is an important determinant of virus persistence in chronic HBV infection.Thus,therapeutic strategies should aim to correct this deficiency[26].T a1and IFN a and cytokine synthesis in HBV445Thymosin-a1is an immunoregulatory agent capable of triggering maturational events in lymphocytes,to augment T-cell function,to promote reconstitution of immune defects, to amplify the expression of class I HLA and to increase natural killer cell activity[11–13,27].In PBMCs of patients with chronic hepatitis C,T a1has been shown to enhance the Th1immune response[17].Data of trials with T a1in chronic hepatitis B indicate that it may be at least as effective as IFN a[15,16].Interestingly,T a1 is better tolerated than IFN a and seems to induce a gradual and sustained increase in virological response overtime after discontinuation of treatment[15,28,29].We performed this study to evaluate the effect of T a1,alone or in association with IFN a on cytokine and antiviral protein2¢,5¢-OAS synthesis from PBMCs of patients with chronic hepatitis B.Despite the limited number of patients,this study dem-onstrated that PBMCs of patients with chronic HBV infection produce more IL-2than those of healthy controls.On the contrary,IFN c production was not different between patients and controls.Thisfinding could simply reflect the absence of stimulation of the immune system in healthy subjects,rather than a Th1polarization in HBV patients,as previously reported[30].A predominant shift of the immune response towards a Th1profile has been suggested to be crucial for the eradication of HBV,while a prevalent Th2response seems to favour viral persistence[21,31].Therefore,it is possible to speculate that the use of immunomodulatory agent capable of reinforcing the Th1response could be advantageous to control HBV infection.Our in vitro data show that T a1alone,as well as IFN a alone are not able to modify either IL-2or IFN c production compared with base-line condition.However,in association,they are able to in-duce a significant increase in IL-2not only compared to baseline but also compared to T a1alone.As we have not studied the local cytokine production in HBV-infected liver samples,this IL-2increased production needs further investigations.IL-2is a pleiotropic cytokine with several antiviral and immunomodulatory proprieties that have been demonstrated to downregulate hepatocellular HBV gene expression by an indirect,noncytopathic process[32].Thus, we can hypothesize that the association between IFN a and T a1could have a role in controlling HBV chronic infection. As far as the Th2cytokines are concerned,at baseline,the behaviours in patients and controls were completely opposed and more difficult to explain.In particular,the higher value of IL-4concentration in patients is not unexpected as it446 E.Loggi et al.reflects the Th2polarization in chronic infection,as previ-ously described[9,33].On the other hand,ourfinding of lower levels of IL-10in HBV patients is in contrast to pre-vious data[30].These discrepancies could be partially be-cause of different experimental design and different study population.IL-10is a potent immunosuppressor that has been recently shown to play a key role in T-cell exhaustion and viral persistence in animal models of viral infection[34]. In HBV patients,Hyodo et al.[30]found a large number of IL-10-secreting cells after stimulation of PBMCs with HBcAg, while no specific stimulation was performed in our study. Furthermore,it should be pointed out that our study sample included only patients with precore mutant infection (HBeAg-negative,HBV-DNA-positive).It is well known that HBeAg is responsible for immunotolerance and T cell anergy [35].We can speculate that in our patients the precore mutation could have some influence in IL-10synthesis,even though further studies are needed in this setting.Our results show that IL-4production by PBMCs of HBV patients was not modified by the different experimental condition compared to baseline.Nevertheless,IFN a alone significantly increased IL-10synthesis,while T a1induced a significant decrease with respect to baseline.Incubation of T a1in combination with IFN a was able to reverse the IFN-induced increase of IL-10.Finally,we confirmed that T a1amplifies the synthesis of IFN-induced antiviral protein 2,5-OAS[17].In summary,T a1alone and IFN a alone were found to reduce and increase respectively only the synthesis of IL-10 by PBMCs of patients with HBeAg-negative chronic HBV infection.Furthermore,both drugs alone or in association significantly increased the antiviral protein2,5-OAS pro-duction.On the other hand,the association T a1plus IFN a induced a significant increase in IL-2and blocked the IL-10 increase induced by IFN a.If this profile could be effectively advantageous in the treatment of chronic hepatitis B,more information is needed about intrahepatic environment.It is well known that nucleoside/nucleotide analogues,widely used for treatment of chronic HBV,are able to achieve the suppression of viral replication,but at the same time,they are inadequate to induce recovery of the immune system [36].In this view,compounds able to modulate the host immune response,should be further investigated to boost or increase HBV-immune response.ACKNOWLEDGEMENTSThis study was in part supported by Associazione per la Ricerca sulle Malattie Epatiche(ARME).REFERENCES1Lok AS,Mc Mahon BJ.Practice Guidelines Committee, American Association for the Study of Liver Diseases,2001.Chronic hepatitis B.Hepatology2001;34:1225–1241.2Villeneuve JP.The natural history of chronic hepatitis B virus infection.J Clin Virol2005;34(Suppl.1):S139–142. 3Bertoletti A,Gehring AJ.The immune response during hepatitis B virus infection.J Gen Virol2006;87:1439–1449.4Chisari FV,Ferrari C.Hepatitis B virus immunopathogene-sis.Annu Rev Immunol1995;13:29–60.5Mosmann TR,Sad S.The expanding universe of T-cell subsets:Th1,Th2and more.Immunol Today1996;17: 138–146.6OÕGarra A.Cytokines induce the development of function-ally heterogeneous T helper cell subsets.Immunity1998;8: 275–283.7Ferrari C,Missale G,Boni C,Urbani S.Immunopathogenesis of hepatitis B.J Hepatol2003;39(Suppl.1):S36–42.8Akpolat N,Yahsi S,Godekmerdan A,Demirbag K,Yalniz M.Relationship between serum cytokine levels and histopath-ological changes of liver in patients with hepatitis B.World J Gastroenterol2005;11:3260–3263.9Jiang R,Feng X,Guo Y et al.T helper cells in patients with chronic hepatitis B virus infection.Chin Med J(Engl)2002;115:422–424.10Li CL,Zhang T,Saibara T et al.Thymosin alpha1acceler-ates restoration of T cell-mediated neutralizing antibody response in immunocompromised hosts.Int Immunophar-macol2002;2:39–46.11Chien RN,Liaw YF.Thymalfasin for the treatment of chronic hepatitis B.Expert Rev Anti Infect Ther2004;2:9–16.12Knutsen AP,Freeman JJ,Mueller KR,Roodman ST,Bou-hasin JD.Thymosin-alpha1stimulates maturation of CD34+stem cells into CD3+4+cells in an in vitro thymic epithelia organ coculture model.Int J Immunophar-macol1999;21:15–26.13Giuliani C,Napolitano G,Mastino A et al.Thymosin-alpha1 regulates MHC class I expression in FRTL-5cells at tran-scriptional level.Eur J Immunol2000;30:778–786.14Sherman KE,Jones CC,Goldstein AL,Naylor PH.Low thy-mosin alpha-1concentrations in patients chronically in-fected with the hepatitis B virus.Viral Immunol1991;4: 195–199.15Andreone P,Cursaro C,Gramenzi A et al.A randomized controlled trial of thymosin-alpha1versus interferon alfa treatment in patients with hepatitis B e antigen antibody and hepatitis B virus DNA positive chronic hepatitis B.Hepatology1996;24:774–777.16Chien RN,Liaw YF,Chen TC,Yeh CT,Sheen IS.Efficacy of thymosin alpha1in patients with chronic hepatitis B:a ran-domized,controlled trial.Hepatology1998;27:383–387. 17Andreone P,Cursaro C,Gramenzi A et al.In vitro effect of thymosin-alpha1and interferon-alpha on Th1and Th2 cytokine synthesis in patients with chronic hepatitis C.J Viral Hepat2001;8:194–201.18Andreone P,Gramenzi A,Loggi E et al.In vitro effect of indomethacin and interferon-alpha on Th1and Th2cyto-kine synthesis in patients with chronic hepatitis C.Cytokine 2004;26:95–101.19Thomas HC,Montano L,Goodall A et al.Immunological mechanism in chronic hepatitis B virus infection.Hepatology 1982;2:116S–121S.T a1and IFN a and cytokine synthesis in HBV44720Penna A,Artini M,Cavalli A et al.Long-lasting memory T cell responses following self-limited acute hepatitis B.J Clin Invest1996;98:1185–1194.21Penna A,Del Prete G,Cavalli A et al.Predominant T-helper 1cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B.Hepatology1997;25: 1022–1027.22Rehermann B,Pasquinelli C,Mosier SM,Chisari FV.Hepa-titis B virus(HBV)sequence variation of cytotoxic T lym-phocyte epitopes is not common in patients with chronic HBV infection.J Clin Invest1995;96:1527–1534.23Rehermann B,Ferrari C,Pasquinelli C,Chisari FV.The hepatitis B virus persists for decades after patientsÕrecovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response.Nat Med1996;2:1104–1108.24Webster GJ,Reignat S,Brown D et al.Longitudinal analysis of CD8+T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B:implications for immunotherapy.J Virol2004;78:5707–5719.25Barnaba V,Franco A,Paroli M et al.Selective expansion of cytotoxic T lymphocytes with a CD4+CD56+surface phenotype and a T helper type1profile of cytokine secretion in the liver of patients chronically infected with Hepatitis B virus.J Immunol1994;152:3074–3087.26Bertoletti A,Naoumov NV.Translation of immunological knowledge into better treatments of chronic hepatitis B.J Hepatol2003;39:115–124.27Serrate SA,Schulof RS,Leondaridis L,Goldstein AL,Sztein MB.Modulation of human natural killer cell cytotoxic activity,lymphokine production,and interleukin2receptor expression by thymic hormones.J Immunol1987;139: 2338–2343.28Chan HL,Tang JL,Tam W,Sung JJ.The efficacy of thymosin in the treatment of chronic hepatitis B virus infection:a meta-analysis.Aliment Pharmacol Ther2001;15:1899–1905.29Lau e of immunomodulatory therapy(other than interferon)for the treatment of chronic hepatitis B virus infection.J Gastroenterol Hepatol2000;15(Suppl.):E46–E52.30Hyodo N,Tajimi M,Ugajin T,Nakamura I,Imawari M.Frequencies of interferon-gamma and interleukin-10 secreting cells in peripheral blood mononuclear cells and liver infiltrating lymphocytes in chronic hepatitis B virus infection.Hepatol Res2003;27:109–116.31Bertoletti A,DÕElios MM,Boni C et al.Different cytokine profiles of intraphepatic T cells in chronic hepatitis B and hepatitis C virus infections.Gastroenterology1997;12:193–199.32Guilhot S,Guidotti LG,Chisari FV.Interleukin-2downre-gulates hepatitis B virus gene expression in transgenic mice by a posttranscriptional mechanism.J Virol1993;67: 7444–7449.33Kobayashi K,Ishii M,Igarashi T et al.Profiles of cytokines produced by CD4-positive T lymphocytes stimulated by anti-CD3antibody in patients with chronic hepatitis C.J Gas-troenterol1998;33:500–507.34Blackburn SD,Wherry EJ.IL-10,T cell exhaustion and viral persistence.Trends Microbiol2007;15:143–146.35Milich DR,Chen MK,Hughes JL,Jones JE.The secreted precore antigen can modulate the immune response to the nucleocapsid:a mechanism for persistence.J Immunol1998;160:2013–2021.36Boni C,Penna A,Bertoletti A et al.Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B.J Hepatol2003;39:595–6.448 E.Loggi et al.。
