生物类似药ch COriginal and Biosimilar Epoetin Productsonsistency of 质量分析Quality and Batch-to-Bat
天津大学制药工程专业 考研《制药工艺学》中英文对译

天津⼤学《制药⼯艺学》中英⽂对译pharmaceutical technology制药⼯工艺学pharmaceutical pipeline制药链pharmacopoeia药典。
Roswell Park Memorial Instirute RPMI good manufacturing practices for drugs GMP制药⾏行行业medicines,drugs药品traditional Chinese medicines中药natural medicines天然药物chemical drugs化学药物biologics,biologic products⽣生物制品generics,generic drugs仿制药物me-too-drug仿制药biosimilars⽣生物类似药biotechnology⽣生物技术.Food and druge administration FDA biotechnology pharmaceutical,biopharmaceutical⽣生物制药nucleotide核苷酸nucleoside核⽢甘enzyme酶enzyme inhibitor酶抑制剂immunomodulator免疫调节剂penicillin⻘青霉素antibody engineering抗体⼯工程inducer诱导剂precursor前体prodrug前药transformation遗传转化.conversion⽣生物转化fermentation发酵.strain breeding菌种选育separation and purification分离纯化和提纯.cell growth phase/fermentationproduct synthesis phase/product secretion phase.Murashige&Skoog MScell autolysis phase/fermentation anaphase.generic通⽤用药物metabolism代谢.substrate培养基质primary/secondary metabolism初级/次级代谢.specific growth rate⽐比⽣生⻓长速率lag/log/decline/stationary/death phase延滞期/对数⽣生⻓长期/减数期/稳定期/死亡期coupling model⽣生⻓长与⽣生产偶联型.PEG聚⼄乙⼆二醇semi-coupling model⽣生⻓长与⽣生产半偶联型.starter culture培养物non-coupling model⽣生⻓长与⽣生产⾮非偶联型.storage保存protoplast fusion原⽣生质体融合.DMSO⼆二甲基亚砜master stock/cell bank MSB/MCB主菌种库.glycerol⽢甘油working stock/cell bank WSB/WCB⼯工作菌种库.Streptomyces链霉菌quality control QC质量量控制.cholramphenicol氯霉素China Center for Type Culture Collection CCTCC中国典型培养物保藏中⼼心China General Microbiological Culture Collection Center CGMCC中国普通微⽣生物保藏管理理中⼼心China Center of Industrial Culture Collection CICC中国⼯工业微⽣生物菌种保藏管理理中⼼心National Center for Medical Culture Collection(Bacteria)CMCC中国医学微⽣生物菌种保藏中⼼心America Type Culture Collection ATCCEuropean Collection of Cell Culture ECACCInstiture for Fermentation,Osaka IFONational Collection of Type Culture mNCTCmedium培养基.carbon source碳源nitrogen source氮源.mineral salt⽆无机盐macroelement⼤大量量元素.trace element microelement微量量元素growth factor⽣生⻓长因⼦子.precursor前体accelerant促进剂.fed medium补料料培养基agar琼脂粉.contaminated microbe杂菌contamination污染.phage噬菌体disinfection消毒.sterilization灭菌pathogen病原微⽣生物.filtration sterilization过滤灭菌filter过滤介质VVM空⽓气流量量(单位时间单位体积内通⼊入的标准状况下的空⽓气体积)primary culture原代培养.passage culture传代培养solid surface culture固体表⾯面培养.liquid submerged culture液体深层培养immobilized culture固定化培养.high cell density culture⾼高密度培养intermittent opration间歇式操作.discontinuous operation不不连续培养semi-continuous operation半连续培养.batch operation分批式操作fed batch operation补料料-分批式(流加)操作.chemostat恒化器器MPa罐压.dissolved oxygen DO溶解氧cell concentration菌体浓度.fermentation heat发酵热production heat产⽣生热.loss heat散失热biological heat⽣生物热.agitation heat搅拌热evaporation heat蒸发热.sensible heat显热radiant heat辐射热.oxygen supply供氧oxygen consumption耗氧.dissolved oxygen coefficient溶解氧系数oxygen transfer rate OTR氧传递速率.oxygen uptake rate OUR摄氧速率ventilation通⽓气.respiratory intensity呼吸强度oxygen saturation concentration氧饱和浓度.respiratory quotient RQ呼吸熵critical oxygen concentration临界氧浓度.fill补料料withdraw放料料.foam泡沫defoaming agent消沫剂.surfactant表⾯面活性剂dispersant分散剂.emulsifier乳化剂inertcarrier惰性载体.antibiotic抗⽣生素carbenicillin羧苄⻘青霉素/⻘青霉素G6-aminopenicillanic acid6-APA6-氨基⻘青霉烷酸.cephalosporin C CPC头孢菌素C erythromycin红霉素.amino acid氨基酸hybridomn杂交瘤.vitamin维⽣生素recombinant DNA technology重组DNA技术.recombinant DNA products rDNA制品plasmid质粒.replicon复制⼦子promoter启动⼦子.terminator终⽌止⼦子multiple cloning site MCS多克隆隆位点.transferability转移性incompatibility不不相容性.cloning vector克隆隆载体expression vector表达载体.shuttle vector穿梭载体intergration vector整合载体.inclusion body包涵体yeast酵⺟母.genetic engineering strain基因⼯工程菌yeast intergration plasmid YIP酵⺟母整合载体yeast episomal plasmid YEP酵⺟母附加载体yeast centromere plasmid YCP酵⺟母着丝粒载体centromere sequence CEN着丝粒序列列autonomously replicating sequences ARS⾃自主复制序列列yeast replicating plasmid YRP酵⺟母复制质粒polymerase chain reaction PCR聚合酶链式反应reverse transcription PCR RT-PCR反转录PCRcomplementary DNA cDNAavian myeloblastosis virus AMV禽源成髓细胞瘤病毒moloney murine leukemia virus MMLV⿏鼠源败⾎血病毒莫勒勒尼株diethyl pyrocarbonate DEPC焦碳酸⼆二⼄乙酯.denaturation变性annelling退⽕火.extension链延伸restriction endonuclease限制性核酸内切酶.ligase连接酶recombinant重组⼦子.interferon IFN⼲干扰素recombinant human interferon rhIFNtricarboxylic acid cycle TCA循环三羧酸循环pentose phosphate pathway PPP磷酸戊糖途径.glycosylation糖基化apoptosis凋亡.diploid cell⼆二倍体primary cell原代细胞.passage cell传代细胞immortal cell永久细胞系.Chinese hamster ovary CHO中国仓⿏鼠卵卵巢DHFR⼆二氢叶酸还原酶.methotrexate MTX甲氨蝶呤baby hamster kidney BHK幼仓⿏鼠肾脏dicistron双顺反⼦子long terminal repeat sequences LTRS逆转录病毒的⻓长末端重复序列列cytomegalovirus CMV⼈人巨噬病毒.ubiquitin泛素蛋⽩白bovine growth hormone.BGH⽜牛⽣生⻓长素.toppoisomerase拓拓扑异构酶internal ribosome entry site IRES核糖体进⼊入位点.serum⾎血清minimum essential medium MEM basal medium Eagle’s BME Dulbecco’s modified Eagle’s medium DMEMGlasgow’s modified Eagle’s medium GMEMJoklik’s Park Memorial Eagle’s medium JMEMRoswell Park Memorial Institute RPMIserum-free medium SEM⽆无⾎血清培养基.buffer solution缓冲液balance saline solution BSS平衡盐溶液monolayer anchorage-dependent culture单层贴壁培养.suspension culture悬浮培养microcarrier微载体.microencapsulation method微囊法phosphonate buffer solution PBS磷酸盐缓冲液.scale-down缩⼩小erythropoietin EPO红细胞⽣生成素.luria bertani LB recombinant human erythropoietin rhEPO重组⼈人红细胞⽣生成素synthon合成⼦子.synthetic equivalent合成等价物protocol solvent质⼦子性溶剂.micronization微晶化catalyst催化剂.phase transfer catalyst相转移催化剂TEBAC三⼄乙基苄基氯化铵Mokosza催化剂TO/CMAC三⾟辛基甲基氯化铵Starks催化剂.Brandstrom催化剂四丁基硫酸氢铵chirality⼿手性.enantiomers对应异构体configuration构型.chiral drug⼿手性药物enantiomeric excesses对映体过量量e.e.%.restrosynthesis追溯求源法resolution拆分.omeprazole奥美拉唑paclitaxel,Taxol紫杉醇.cephalosporin C CPC头孢菌素7-aminocephalosporanic acid7-ACA7-氨基头孢烷酸.cefalexin头孢氨苄tetrahydrofuran THF四氢呋喃.quality by design QbD质量量源于设计process analysis technology PAT过程分析技术quality target product profile QTPP⽬目标产品质量量概况critical material attribute CMA关键物料料属性critical process parameter CPP关键⼯工艺参数normal operation range NOR正常操作区间proven acceptable range PAR可接受的区间critical quality attribute CQA关键质量量属性bioreactor⽣生物反应器器key process parameter KPP重要⼯工艺参数.fermenter发酵罐complete stirred tank reactor CSTR全混流反应器器.yield得率piston fluid reactor PFR平推流反应器器.titer效价stirred tank reactor STR搅拌罐.scale-up放⼤大fixed bed reactor固定化床反应器器.draft tube导流筒packed bed reactor PBR填充床反应器器.bubble column⿎鼓泡塔fluidized bed reactor FBR流化床反应器器.air-lift reactor⽓气升式反应器器disk and turbine impeller涡流式搅拌桨.process validation⼯工艺验证marine style impeller推进式搅拌桨.process design⼯工艺设计process mass intensity PMI过程质量量强度.process qualification⼯工艺确认reaction mass efficiency RME反应质量量效率standard operation procedure SOP标准操作规程.continued process verification⼯工艺核实biochemical oxygen demand BOD⽣生化需氧量量.total nitrogen TN总氮chemical oxygen demand COD化学需氧量量.suspended subatance SS悬浮物mixed liquor suspended solids MLSS混合液悬浮固体total organic carbon TOC总有机碳.sludge volume SV污泥泥沉降⽐比sludge volume index SVI污泥泥指数piping&instrument diagram PID⼯工艺控制流程图。
常规诊疗条件下比较依那西普生物类似药(益赛普)与阿达木单抗、英夫利西单抗治疗RA的临床疗效

常规诊疗条件下比较依那西普生物类似药(益赛普)与阿达木单抗、英夫利西单抗治疗RA的临床疗效PresentID: SAT0360原文TANAR- A ETANERCEPT BIOSIMILAR IS AS EFFECTIVE AS ADALIMUMAB AND INFLIXIMAB IN ACOHORT OF REAL-LIFE OF PATIENTS WITH RHEUMATOID ARTHRITISP. Santos-Moreno1,*, G.Saavedra-Martinez2, L. Villarreal3, D. Gomez1, J. Bello-Gualtero1, V. Giraldo4,P. Martinez4, A. Sanchez4, M. Sanchez4, E. Uribe4, M. Boon41Rheumatology, 2Epidemiology,3Psychology, 4Internal medicine, Biomab, Center For Rheumatoid Arthritis,Bogota, Bogota, ColombiaBackground: Clinical response in patients with rheumatoid arthritis (RA) using biologics is well-known. However, there is no direct comparison between biologics in cohorts of patients with RA inreal-life settings, which could have implications in treatment decisions andhealth economics.Objectives: The aim of this study was to describe a direct comparison in effectiveness between two classical antiTNF biologics (Adalimumab, Infliximab) and one Etanercept biosimilar in patients with long-standing RA in a cohort of real-life.Methods: A descriptive cross-sectionalstudy was performed. Were included 158 patients with at least 6 visits torheumatologist in last 24 months in a specialized in RA center. Clinical follow-up was designed by the authors according to DAS28 as follows: every 3-5weeks (DAS28 > 5.1), every 7-9 weeks (DAS28 ≥ 3.1 and ≤ 5.1), and every11-13 weeks (DAS28 < 3.1). Therapy had to be adjusted with DAS28 > 3.2 unless patient′s conditions don’t permit it; we considered this follow-up type as implementation of a T2T strategy. We divided patients in two groups:remission-low disease activity (Rem/LDA) patients and moderate-severe diseaseactivity (MDA/ SDA) patients and the aim of the study was to look at what percentage of patients who were MDA/SDA disease activity reached a low disease activity or remission. 158 patients with RA and using Adalimumab, Infliximab and Etanerceptbiosimilar (Etanar? CP Guojian Pharmaceutical Co Ltd, China) were involved. The Etanercept biosimilar was approved for using in Colombiasince 2007. Descriptive epidemiology was done, the medians were analyzed usingt-Student assuming normality for DAS28 distribution and disease activity was analyzed using Pearson′s statistics.Results: 158 patients were included inthis study, 125 (79.1%) women and 33 (20.9%) men. Average age was 59 +/- 10 y/owith disease duration of 11 years (0.5-47). 158 patients with diagnosis of RA using Adalimumab, Etanercept and Infliximab were involved: Adalimumab 61 (38.6%), Etanercept 25 mg 62 (39.2%), and Infliximab35 (22.2%). At 24 months was observed an increase in percentage of patients in remission and a decrease in percentage of patients in MDA/SDA disease activity statistically significant. for Adalimumab at beginning DAS28-3.6 and 24months later 2.6; for Etanercept biosimilar at beginning DAS28-3.6 and 24months later 2.6 and for Infliximab at beginnngDAS28-3.6 and 24 months later 2.6.There were not statistically significant differences between analyzed biologics. On the other hand, there were fewer adverse events with Etanercept-biosimilar than Adalimumab and Infliximab; it was statistically significant.Conclusions: This study shows that the Etanercept biosimilar is as effective as 2 othertraditional anti-TNF biological for disease activity control in patients with rheumatoid arthritis in a real-life setting with fewer adverse events, which could have implications in treatment decisions and health economics. On the other hand the study proves effectiveness of implementation of a T2T strategyin patients with RA.译文背景:生物制剂治疗RA的临床疗效众所周知。
便宜又好用的肿瘤生物仿制药,你了解有多少?

便宜又好用的肿瘤生物仿制药,你了解有多少?生物仿制药不是假药,而是实实在在的真药和好药。
文丨维生素C来源丨医学界肿瘤频道提到生物仿制药,大家可能嗤之以鼻。
在我国,一提“仿制”两个字,人们往往联想到山寨、盗版、非法等字眼。
而国际上的生物仿制药(biosimilar)却是指对原研专利生物药在其专利失去的市场独占权法律保护后,进行的合法仿制。
从成分上来讲,仿制药与品牌药相差无几,是货真价实的真药。
我们先来了解一下概念:原研药是指原创性的新药,在全球最先提出申请,并获得专利保护的药品,一般有20年的保护期,在保护期内,其他企业不得仿制。
要经过对成千上万种化合物进行层层筛选、严格的临床前和临床试验,才得以获准上市。
一般情况下,原研药需要科研人员花费十几年左右的研发时间和数十亿美元的研发经费。
新药刚上市的时候,都伴随着专利保护和品牌,因此新药又叫“专利药”或者“品牌药”。
关于生物仿制药是指是与已批准的生物原研药相似的一种生物药(包括疫苗、血液及血液成分、体细胞、基因治疗、组织和重组治疗性蛋白等)。
它与原研药具有相同的活性成分,在剂量、剂型、给药途径、安全性和有效性、质量、治疗作用以及适应证上没有显著差异的一种仿制品。
具有降低医疗支出、提高药品可及性、提升医疗服务水平等重要经济和社会效益的作用。
生物仿制药的批准基于它与已经获批的生物制品有高度相似的数据,并且在安全性、纯度和效力方面没有临床意义上的差异。
所以,单从药效上来说,仿制药肯定不是假药,而是实实在在的真药和好药。
美国食品药品监督管理局(FDA)批生物仿制药主要看以下几点是否与原研药相同。
包括:作用机理、给药途径、剂型、剂量规格、生产设备。
为促进我国生物制药产业的健康、有序发展,国家药监局及时组织药品审评中心等技术部门,在借鉴世界卫生组织和国内外相关指导原则及国际生物类似药成功研发案例的基础上,结合我国生物药研发的实际情况和具体国情,在2015年2月制订发布了《生物类似药研发与评价技术指导原则(试行)》。
阿达木生物类似药临床研究设计要点考虑(征求意见稿)

阿达木单抗注射液生物类似药临床试验设计考虑要点(征求意见稿)一、概述阿达木单抗(Adalimumab)系在中国仓鼠卵巢细胞中表达的重组全人源化肿瘤坏死因子α(Tumor Necrosis Factor, TNFα)单克隆抗体注射液,由美国雅培公司研发上市,商品名为:修美乐(Humira®)。
阿达木单抗在美国和欧盟已获批多个适应症[1,2],2010年首次获准进口中国。
目前在中国批准用于:①对改善病情抗风湿药(DMARDs),包括甲氨蝶呤疗效不佳的成年中重度活动性类风湿关节炎患者(RA);②常规治疗效果不佳的成年重度活动性强直性脊柱炎患者(AS);和③需要进行系统治疗或光疗,并且对其它系统治疗(包括环孢素、甲氨蝶呤或光化学疗法)不敏感,或具有禁忌症,或不能耐受的成年中重度慢性斑块状银屑病患者(PsO)[3](见表1)。
阿达木单抗注射液原研产品美国专利已于2016年到期,欧洲专利2018年即将到期[4],国内外制药企业已纷纷加入其生物类似药的研发。
Amgen研发的ABP501(Amgevita®)、BI研发的BI695501(Cyltezo®)和Samsung Bioepis研发的SB5(Imraldi®)均已作为阿达木单抗的生物类似药在欧盟获批上市[5-7],其中的ABP501和BI695501在美国也已按生物类似药获准上市[8,9]。
本文在原国家食品药品监督管理总局已发布的《生物类似药研发与评价技术指导原则(试行)》[10](以下简称“指导原则”)基础上,结合阿达木单抗的特点,重点探讨当前普遍关注的临床研究策略和临床试验设计问题,以期为国内阿达木单抗生物类似药的临床研发提供参考。
二、阿达木单抗生物类似药临床研究策略根据《指导原则》,生物类似药研发总体思路是通过系统的比对试验证明候选药与原研药的相似性为基础,支持其安全性、有效性和质量可控。
依据逐步递进的原则,分阶段进行药学、非临床、临床比对研究。
注射用曲妥珠单抗生物类似药临床研究设计及审评考虑要点(

注射用曲妥珠单抗生物类似药临床研究设计及审评考虑要点(征求意见稿)一、前言曲妥珠单抗(Trastuzumab)是由瑞士罗氏公司研发的一种重组DNA衍生的人源化单克隆抗体,含人IgG1亚型框架,互补决定区源自鼠抗p185 HER2 抗体,能够特异性地作用于人表皮生长因子受体-2(HER2)的细胞外部位第IV亚区,竞争性阻断人体表皮生长因子与HER2的结合,从而抑制肿瘤细胞的生长。
罗氏公司的注射用曲妥珠单抗(Herceptin®,赫赛汀®)最早于1998年9月25日获得美国FDA批准上市,2002年进口中国,目前获批的适应症为:单药用于治疗HER2阳性转移性乳腺癌;联合紫杉醇或者多西他赛用于HER2阳性转移性乳腺癌;HER2阳性的可手术乳腺癌患者的辅助治疗;HER2阳性转移性胃癌。
在欧盟,还获批了早期乳腺癌新辅助治疗的适应症。
赫赛汀®在美国、欧盟及中国获准上市的适应症见表1。
曲妥珠单抗在欧盟的专利已于2014年7月到期,美国专利也将于2019年6月到期,其生物类似药的研发成为热点,目前印度(Hertraz, Mylan)、韩国(Herzuma, Celltrion)和俄罗斯(HERtiCAD, Biocad)各有一个生物类似药上市。
本文在CFDA已发布的《生物类似药研发与评价技术指导原则(试行)》(后简写为《指导原则》)基础上,结合该品种的特点,对曲妥珠单抗生物类似药的临床研究策略和方案设计要点进行探讨,以期为曲妥珠生物类似药的研发相关人员提供参考。
二、曲妥珠单抗生物类似药临床研究策略根据《指导原则》,生物类似药研发总体思路是以比对试验证明其与参照药的相似性为基础,支持其安全、有效和质量可控。
采用逐步递进的顺序,分阶段开展药学、非临床、临床比对试验。
根据前期比对试验结果设计后续比对试验研究的内容。
根据前期药学和药理毒理比对试验结果,曲妥珠单抗生物类似药的临床研发可能会存在以下两种情况:1、药学和药理毒理试验证明候选药与参照药相似,按照生物类似药的路径开展药代动力学比对试验和临床安全有效性比对试验。
托珠单抗注射液生物类似药临床试验指导原则(征求意见稿)

1托珠单抗注射液生物类似药临床试验指导原则2(征求意见稿)34一、前言5托珠单抗注射液(Tocilizumab)由罗氏公司研发,采用哺乳动物6细胞(CHO)表达的抗人白介素6受体单克隆抗体制剂,商品名为:7雅美罗®/Actemra®。
通过阻断白介素6与可溶性及膜结合的白介素6 8受体结合,抑制白介素6的信号转导,从而减少病理性炎症反应。
托9珠单抗自2009年2月起陆续在欧盟、美国、日本等多个国家和地区10获准上市,获批的适应症包括:成人类风湿关节炎(RA),多关节型11幼年特发性关节炎(pJIA)、全身型幼年特发性关节炎(sJIA)、巨细12胞动脉炎(GCA)和细胞因子释放综合征(CRS)等。
目前,托珠单13抗在我国获批的适应症包括RA和sJIA[1]。
14托珠单抗注射液原研产品序列专利已到期[2],国内外众多制药企15业纷纷加入其生物类似药的研发过程中。
为了更好地推动生物类似药16的开发,在原国家食品药品监督管理总局已发布的《生物类似药研发17与评价技术指导原则(试行)》[3]基础上,我们结合该品种的特点及18研发企业相关问题的沟通交流情况,讨论形成了托珠单抗生物类似药19临床试验研究设计要点,以期为业界提供参考。
20二、托珠单抗生物类似药临床研究总体要求21原则上,药代动力学比对试验需要进行1项健康受试者单次给药22药代动力学生物等效性研究,验证候选药与原研药PK特征的相似性。
1临床比对研究建议选择原研进口获批RA适应症人群,与原研药进行21项“头对头”比较的临床等效性研究以支持其按生物类似药注册上3市。
4三、临床研究设计考虑要点5生物类似药临床比对研究设计应当以证明候选药与原研药的相6似性为目的,进行科学合理的研究设计。
7(一)健康受试者药代动力学比对研究8试验设计:参照一般生物等效性研究的设计,结合托珠单抗生物9类似药半衰期较长(稳态浓度下,每四周给药一次,4mg/kg时为11 10天,8 mg/kg时为13天),具有免疫原性等特点,建议采用随机、双11盲、平行对照、单次给药的试验设计。
新型非格司亭生物仿制药 Nivestim(TM) 获准在欧洲用于预防因化疗导致的发热性嗜中性白血球减少症.docx

新型非格司亭生物仿制药Nivestim(TM) 获准在欧洲用于预防因化疗导致的发热性嗜中性白血球减少症- Hospira 的非格司亭Nivestim(TM) 已经获得欧盟委员会(EC) 的批准,用于预防发热性嗜中性白血球减少症(FN) 和化疗后嗜中性白血球减少症(CIN) 引起的存活期的缩短- Nivestim 是一种新型非格司亭,该药集给药便利性、便于储藏性和安全性与一体- 嗜中性白血球减少症是由癌症化疗引起的最严重的血液中毒症,可导致化疗剂量相对常规疗程的减少和/或推迟(1)英格兰LEAMINGTON SPA 2010年6月10日电/美通社亚洲/ --Hospira 今天宣布,欧盟委员会已经批准将Nivestim(TM)(非格司亭)用于预防发热性嗜中性白血球减少症,这种病症是由癌症化疗引起的最严重的血液中毒症(1)。
目前Nivestim 已在欧盟各国获得营销授权。
Nivestim 预计将可降低嗜中性白血球减少症的治疗成本。
德国Freiburg University Medical Center(弗赖堡大学医学中心)内科医学副教授Cornelius Waller 博士表示:“Nivestim 的获批为医疗卫生专业人员和患者带来了切实的好处。
因癌症化疗导致的嗜中性白血球减少症可导致患者无法完成全部的化学疗程。
Nivestim 为医疗卫生专业人员提供了一种具有成本效益且易于使用的选择,帮助患者坚持到底。
”Nivestim 是Hospira 的第二种生物仿制药。
该公司的促红细胞生成素生物仿制药Retacrit(TM) 目前在17个欧洲国家销售。
Hospira 是首个在欧洲销售生物仿制药的美国公司。
该公司的生物仿制药产品线还包括非格司亭的长效版聚乙二醇非格司亭,是该行业最大的产品线之一。
Hospira 首席商务官Ron Squarer 表示:“作为Hospira 扩大生物仿制药产品组合持久承诺的一部分,我们很自豪地宣布,Nivestim 已经获得欧盟委员会的批准。
国家食品药品安全专业技术人员培训考试-美国药事法规
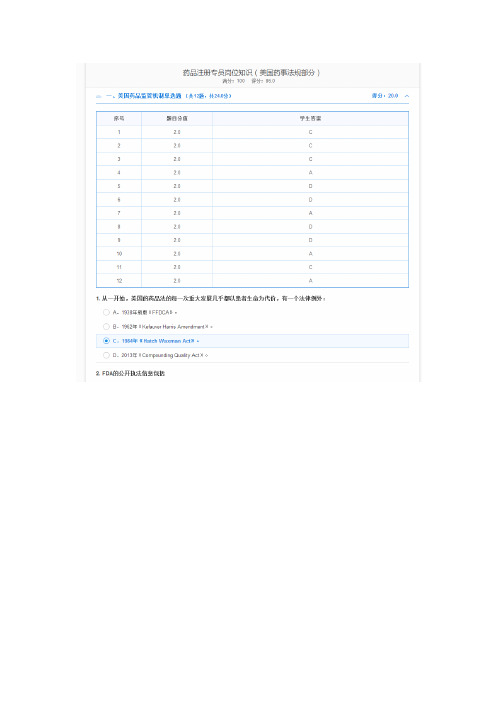
药品注册专员岗位知识(美国药事法规部分)满分:100得分:84.0一、美国药品监管机制单选题(共12题,共24.0分)1. 从一开始,美国的药品法的每一次重大发展几乎都以患者生命为代价,有一个法律例外:A.1938年新版《FFDCA》。
B.1962年《Kefauver Harris Amendment》。
C.1984年《Hatch Waxman Act》。
D.2013年《Compounding Quality Act》。
2. FDA的公开执法信息包括A.现场核查,产品召回,不批准上市。
B.警告信,业内除名,罚款入狱。
C.药品安全警告,违法广告警告信,定期执法报告。
D.短缺药品名录,警告信,消费者预警报告。
3. 美国药品法发展的里程碑节点对药品上市提出的最低要求是A.1906年以后要求上市药品必须符合质量可控、安全、有效的标准。
B.1938年以后要求上市药品必须符合质量可控、安全、有效的标准。
C.1962年以后要求上市药品必须符合质量可控、安全、有效的标准。
D.2004年以后要求上市药品必须符合质量可控、安全、有效的标准。
4. 药品作为特殊民用消费品的原因是A.药品与每个人的生老病死息息相关。
B.药品上市必须符合质量标准。
C.药品是非天然产品,必须被批准才能上市。
D.符合安全、有效、质量可靠标准的药品就能被批准上市。
5. 505(b)2指的是A.仿制药,以ANDA申报。
B.原创药,以NDA申报。
C.原创药的简约版,以NDA申报,但是要和仿制药一起排队。
D.既不是仿制药也不是原创药,以505(b)2申报。
6. 特殊试验设计方案(SPA)是A.在任何原创新药开发的任何时期都可以申请。
B.在任何新药开发的特殊重要阶段都可以申请,需要等FDA的批准。
C.需要等FDA的批准才能实施,太麻烦花时间,所以最好不要申请。
D.如果基于人道主义的原则,需要实施不以人为受试者的三期有效性试验,应当申请。
7. 21 CFR Part 11是对电子记录和电子签字的法规,规定了A.电子数据无论出处必须可靠。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Research ArticleQuality and Batch-to-Batch Consistency of Original and Biosimilar Epoetin ProductsLiem Andhyk Halim 1,Vera Brinks 1,Wim Jiskoot 2,Stefan Romeijn 2,Rob Haselberg 3,Chris Burns 4,Meenu Wadhwa 4,Huub Schellekens 1,*1Department of Pharmaceutics,Utrecht Institute for Pharmaceutical Sciences (UIPS),Utrecht University,3584CG Utrecht,the Netherlands 2Division of Drug Delivery Technology,Leiden Academic Centre for Drug Research,Leiden University,2300RA Leiden,the Netherlands 3Division of BioAnalytical Chemistry,AIMMS research group BioMolecular Analysis,VU University Amsterdam,1081HV Amsterdam,the Netherlands 4Biotherapeutics Group,National Institute for Biological Standards and Control,Hertfordshire,EN63QG,UKa r t i c l e i n f oArticle history:Available online xxxKeywords:recombinant human erythropoietin biosimilarimmunogenicityphysicochemical properties protein characterizationa b s t r a c tComprehensive physicochemical characterization and biological assays are essential parts in assessing quality attributes of biologicals.Here,we compared the quality of different marketed recombinant hu-man erythropoietin (epoetin)products:originators,Eprex and NeoRecormon as well as 2biosimilars,Retacrit and Binocrit.In addition,assessment of batch-to-batch variability was included by collecting 2or more batches of each mon assays which included sodium dodecyl sulfate e polyacrylamide gel electrophoresis,high-performance size-exclusion chromatography,asymmetrical flow field e flow fractionation,capillary zone electrophoresis,and potency testing were used.Of the tested products and among batches of single products,variations in epoetin content,isoform pro files,and potency were found.Ultimately,this study demonstrated the high quality of epoetin products with some degree of variation among products and batches,con firming the “similar but not identical ”paradigm of biologicals.©2016American Pharmacists Association ®.Published by Elsevier Inc.All rights reserved.IntroductionSince the 1980s,the advent of recombinant DNA technology has enabled the development of many innovative recombinant human therapeutic proteins.1,2These products have enabled the treatment of a variety of diseases and have become the fastest growing class of therapeutics.Recombinant human erythropoietin (epoetin)was one of the first authorized recombinant proteins on the market.It is mainly used for the treatment of anemia in patients with chronic kidney disease and cancer.3,4Severe side effects,such as thromboembolic processes and antibody-associated pure red cell aplasia (PRCA)are rare.PRCA may occur if epoetin-induced antibodies are able to neutralize the native endogenous erythropoietin.5,6Epoetin shares its factors for immunogenicity with nearly all therapeutic proteins.The exact mechanisms underlying immunogenicity are still not fullyunderstood.Multiple factors including product-related factors (formulation,contaminants,glycosylation and impurities),storage and handling,route of administration,and patient characteristics play a role in this.7,8Since 2006,the loss of patent and data protection has allowed the introduction of generic versions of therapeutic proteins such as somatropin,filgrastim,and epoetin.However,the generic regula-tory route used for small molecules cannot be used for proteins.Owing to their inherent variability,complexity,and heterogeneity,it is impossible to establish that 2protein products are identical.9,10Individual protein products themselves also demonstrate micro-heterogeneity and batch-to-batch variability so cannot be identical to themselves.Therefore,regulatory frameworks have been established throughout the world requiring an extensive compar-ison in quality,ef ficacy,and safety to show similarity between the original product and the intended copy.11,12If the criteria are met,the duplicate product can be marketed as a biosimilar.As we had access to 4marketed epoetin products,2originators,Eprex and NeoRecormon,and 2biosimilars,Retacrit and Binocrit,we performed quality assessment for these products.Eprex (epoetin alfa)and NeoRecormon (epoetin beta)have been reported to differ in their isoform compositions and biological properties onThis article contains supplementary material available from the authors by request or via the Internet at /10.1016/j.xphs.2015.10.019.*Correspondence to:Huub Schellekens (Telephone:þ31(0)302537306;Fax:þ31(0)302537839).E-mail address:h.schellekens@uu.nl (H.Schellekens).Contents lists available at ScienceDirectJournal of Pharmaceutical Sciencesjournal ho mep age:www.jp harmsci.org/10.1016/j.xphs.2015.10.0190022-3549/©2016American Pharmacists Association ®.Published by Elsevier Inc.All rights reserved.Journal of Pharmaceutical Sciences xxx (2016)1e 9account of the use of different CHO cells strain.13Meanwhile,the quality assessment of Retacrit and Binocrit to their reference product,Eprex,has been shown elsewhere to have slight variation in their quality attributes.14,15Besides quality,batch consistency is also considered important for biologicals.Although a few studies have looked into batch-to-batch variability of an individual epoetin brand,13,16,17there has been no published study on batch-to-batch consistency of multiple epoetin brands marketed in Europe.As we also had the possibility to collect multiple batches from these4epoetin prod-ucts,this comparability study is feasible as a follow-up to a study we published earlier.14Materials and MethodsEpoetin ProductsAll epoetin products(see Table1for an overview)were either obtained from local pharmacies in the Netherlands or provided by Hospira and Sandoz.They were received in the original prefilled syringes and stored as stated on the product specification.As an internal reference standard,epoetin-biological reference prepara-tion(BRP)batch3(EDQM,Strasbourg,France)was included in every experiment to validate the method as recommended in the European Pharmacopeia(Ph.Eur.)monograph on Erythropoietin concentrated solution.18It contains equals parts of epoetin alfa and beta.19Before every test,visual inspection was performed for the potential presence of visible particles.All products remained clear and colorless.In all cases,products were tested within their shelf lives.Sodium Dodecyl Sulfate e Polyacrylamide Gel Electrophoresis The epoetin products were loaded on5%polyacrylamide gel (stacking section)and separated on15%polyacrylamide gel (running section)under nonreducing conditions as previously described by Brinks et al.14Unless indicated otherwise,all materials were obtained from Bio-Rad Laboratories B.V.(Veenendaal,the Netherlands).In short,loading solutions of all epoetin products included24m L of undiluted products and6m L of5Âsample buffer (containing Tris-HCl pH6.8,glycerol,sodium dodecyl sulfate and bromophenol blue).Two micrograms of epoetin-BRP batch3were included on each gel.Before loading,all samples were incubated either at95 C,70 C, or room temperature(±25 C)for10min to facilitate protein unfolding.PageRuler™Prestained Protein Ladder,10-180kDa (Life Technologies,Bleiswijk,the Netherlands)was used as a reference for molecular weight in all cases.Separation was per-formed on Mini-PROTEAN®II Electrophoresis Cell with the following running conditions:30min at70V,followed by60min at 150V.Protein bands were visualized by silver staining method as described by Brinks et al.14High-Performance Size-Exclusion ChromatographyDuring the course of this study,the collection of multiple batches of each epoetin product was rather difficult.Hence, epoetin products were obtained at different time points.Reta-crit and NeoRecormon were obtained back in2010.Subse-quently,Binocrit and Eprex were obtained in early and late 2014,respectively.As there was an urge to analyze unexpired products,high-performance size-exclusion chromatography (HP-SEC)wasfirst performed on a Waters2695Separations Module connected to a Waters2487Dual l Absorbance Detector(Waters Corporation,Milford,MA)for thefirst2 products.The machine was then no longer available,and we had to switch to an Agilent1200HPLC system(Agilent Technologies,Palo Alto,CA)combined with a Wyatt Eclipse (Wyatt Technology Europe GmbH,Dernbach,Germany)to analyze the later products.On both machines,a Tricorn™high-performance Superdex 20010/300GL column(GE Healthcare,Little Chalfont,Buck-inghamshire,United Kingdom)was installed.Auto sampler (Agilent)temperature was set at4 C,and each time,100m L of undiluted product were injected.The eluent was14.4g/L Na2HPO4.2H2O(Sigma-Aldrich,Zwijndrecht,the Netherlands), 0.2g/L KH2PO4,and23.4g/L NaCl(Merck,Darmstadt,Germany)at pH7.4andfiltered through a0.2-m mfilter(Sartorius Stedim, G€o ttingen,Germany).Separation took place at aflow rate of0.5mL/min for60min at 30 C.Absorbance was recorded at280nm and analyzed using either Empower2software version6.20.00.00or Astra software version5.3.4.20.A DAWN®HELEOS™18-angle laser light scattering (MALLS)was part of the Agilent system,therefore allowing estimation of the average molecular weight of eluting compounds. Alternatively,proteins with different molecular weights,(1)lyso-zyme,(2)trypsin,(3)ovalbumin,(4)albumin,and(5)holo-transferrin,were used on the Waters system as calibration standards for molecular weight estimation.All proteins were purchased from Sigma-Aldrich.Subsequently,the protein content was determined from the UV signal at280nm using Beer-Lambert law.For all epoetins, a molar extinction coefficient of22,600MÀ1cmÀ1was used.20Table1List of All Epoetin ProductsBrand Name(INN)Lot Number Declared Potency ExcipientsEprex(epoetin alfa)DDS5L00DGS4W00DHS5T00DIS3M004000IU/0.4mL Sodium dihydrogen phosphate dihydrate,disodium phosphate dihydrate,sodium chloride, glycine,polysorbate80Binocrit(epoetin alfa)45011273041234121110,000IU/1.0mL8000IU/0.8mLSodium dihydrogen phosphate dihydrate,disodium phosphate dihydrate,sodium chloride,glycine,polysorbate80Retacrit(epoetin zeta)8K058L88M072C99F081G99M108N910,000IU/1.0mL Disodium phosphate dihydrate,sodium dihydrogen phosphate dihydrate,sodium chloride, calcium chloride dihydrate,polysorbate20,glycine,leucine,isoleucine,threonine,glutamic acid,phenylalanineNeoRecormon(epoetin beta)H0002H01H0003H0130,000IU/0.6mL Urea,sodium chloride,polysorbate20,sodium dihydrogen phosphate dihydrate,disodium phosphate dodecahydrate,calcium chloride dihydrate,glycine,l-leucine,l-isoleucine,l-threonine,l-glutamic acid,l-phenylalanineL.A.Halim et al./Journal of Pharmaceutical Sciences xxx(2016)1e92One-IU epoetin was set to8.4and8.3ng epoetin protein for epoetin alfa21,22/zeta23and beta,24respectively.Asymmetrical Flow Field e Flow FractionationAsymmetricalflowfield eflow fractionation was performed on an Agilent1200HPLC system(Agilent Technologies)with degasser, cooled auto sampler,and a UV(280nm)and afluorescence de-tector.It was combined with a Wyatt Eclipse(Wyatt Technology Europe GmbH,Dernbach,Germany)and a DAWN®HELEOS™18-angle laser light scattering(MALLS)detector(Wyatt Technology Europe GmbH).Fifty microliters of each undiluted formulation were injected through a350-m m thick,medium wide-spaced in a small channel with a Nadir5-kDa cutoff regenerated cellulose membrane(Wyatt Technology Europe GmbH).The same mobile phase buffer was used as in HP-SEC but wasfiltered through a0.1-m m cellulose nitrate Whatman™filter(GE Healthcare Life Sciences, Pittsburgh,KS).The detectorflow and the focusflow were set to1 and 1.5mL/min,respectively.The Eclipse elution settings are summarized in Table2.Calculation of the molecular weight from the MALLS and UV signals was performed by the Astra software version 5.3.4.20.Protein content was determined as described before.Enzyme-Linked Immunosorbent AssayEpoetin was identified with the Quantikine IVD Human Epoetin ELISA(R&D Systems Europe,Abingdon,Oxon,United Kingdom) according to the manufacturer's instructions.After fractionation by HP-SEC as described by Hermeling et al.,2520m L of each fraction was added to a well containing100m L of assay diluent buffer and 80m L of specimen diluent buffer,provided by the kit Chromogen, was left to react for20min before it was stopped by the addition of acid.The plate was immediately read on an Infinite®M1000PRO microplate reader(Tecan,Giessen,the Netherlands)at450and 600nm(reference wavelength).In each plate,both recombinant human epoetin provided in the kit and Eprex were used as the standards.Capillary Zone ElectrophoresisThe isoform distribution of different epoetin products was assessed by capillary zone electrophoresis(CZE)according to the Ph.Eur.monograph on Erythropoietin concentrated solution.18 Binocrit was analyzed on a7100CE System equipped with a photodiode array detector and ChemStation software from Agilent Technologies(Wilmington,DE);the remaining epoetin products were analyzed on a ProteomeLab™PA800or a PA800Plus Phar-maceutical Analysis System coupled to UV detector and operated with32Karat software from Beckman Coulter(Brea,CA).Epoetin internal reference standard and products were pretreated by direct loading to either Nanosep®(Pall Corporation,Ann Arbor,MI)or Amicon®Ultra(Sigma-Aldrich)centrifugal devices,both with a molecular weight cutoff value of10kDa as described in the Ph.Eur.monograph.Retentates were aliquoted and stored atÀ80 C until just before separation.An uncoated50m m inner diameter fused-silica capillary with an effective length of100cm(Polymicro Technologies,Phoenix,AZ) was used for separation.The CZE buffer consisted of0.01-M tricine (Acros Organics,Geel,Belgium),0.01-M sodium chloride(Merck), 0.01-M sodium acetate(Merck),7-M urea(Amresco,Solon,OH)and 25-mM putrescine(Sigma-Aldrich),pH5.55adjusted with50%(v/v) glacial acetic acid at30 C andfiltered through Minisart®0.45-m mfilter(Sartorius Stedim).The preconditioning of the capil-lary and between-run rinsing was performed,adapting either Ph. Eur.monograph or Zhang et al.26In both methods,epoetin internal reference standard and products were injected hydrodynamically at 0.7psi for40s with a separation voltage of143V/cm.The UV detectorset at214nm was operated at2Hz.The isoform distribution was assessed from3or more independent runs of every batch.In Vitro BioassayAn in vitro bioassay was performed using the erythropoietin-dependent subline UT-7/EPO derived from a human eryth-roleukemia.27Cells were maintained in Iscove's modified Dulbec-co's medium containing10%heat-inactivated fetal calf serum supplemented with L-glutamine(2mM),penicillin(50U/mL), streptomycin(0.05mg/mL),and0.2IU/mL epoetin.Cells were subcultured every2-3days and split1:5when they had reached a cell density of2-5Â105cells/mL.Two-fold dilutions of the epoetin samples ranging from0.1 IU/mL to0.00078IU/mL were incubated with UT-7/EPO cells at a density of0.5Â104cells/well.The plates were incubated at37 C, 5%CO2for48h,and3H-thymidine(thymidine[methyl-3H]1mCi [37MBq]/mL,PerkinElmer,Beaconsfield,United Kingdom) 0.5m Ci/well,diluted in assay medium,added for the last4h of the incubation period.The cells were harvested onto glassfiberfilter mats using a micro96harvester(Molecular Devices,Wokingham, United Kingdom)and the radioactivity incorporated into DNA estimated by scintillation counting using a2450MicroBeta2scin-tillation counter(PerkinElmer,Waltham,MA).Bioactivity estimates of the different preparations were derived relative to the epoetin standard(Third WHO International Standard[third WHO IS]forTable2AF4Elution Program SettingsStep Start(min)Duration(min)Crossflow(mL/min)Elution02 1.8Focus21 1.5Focusþinjection32 1.5Elution510 1.8Elution15100Figure1.SDS-PAGE of all epoetin products under nonreducing condition.Roman number represents the different batches of each products.S is epoetin-BRP,and RT stands for room temperature.Different intensities are most likely due to gel-to-gel variation.L.A.Halim et al./Journal of Pharmaceutical Sciences xxx(2016)1e93erythropoietin,recombinant,for bioassay,11/170available from NIBSC,United Kingdom).In Vivo Potency TestOn account of ethical considerations in the use of animals,only selected epoetin products and batches were assessed for potency in normocythemic mice by measuring the stimulation of reticulocyte production according to the Ph.Eur.monograph for erythropoietin-concentrated solution.18BALB/c female mice received the third WHO IS for erythropoietin and the epoetin products,diluted into a high,middle,and low dose,subcutaneously.Each dilution group consisted of6animals weighing between16and23g.Mice were kept for5days,and blood was withdrawn from the orbital sinus before culling by cervical dislocation.At the end of the assay, reticulocyte concentration as a percentage of total erythrocyte concentration was determined.Potency estimates for the epoetin products were calculated relative to the epoetin standard,byfitting a parallel-line model comparing assay response to log concentra-tion using CombiStats version5.0(1999-2013EDQM/Council of Europe).Assay validity was assessed by analysis of variance with nonlinearity and nonparallelism considered significant at the1% level(p<0.01).Duplicate potency estimates from independent bioassays were combined using CombiStats version5.0.ResultsSodium Dodecyl Sulfate e Polyacrylamide Gel Electrophoresis Possible proteinaceous impurities in the products were checked with sodium dodecyl sulfate e polyacrylamide gel electrophoresis under nonreducing conditions.In all tested products,a single broad band of epoetin was apparent on silver staining(Fig.1),corre-sponding in position and intensity to the single band of the epoetin-BRP batch3.Neither higher molecular weight species nor fragments were found in any batch.In addition,different sample preparations,that is,incubation at either95 C,70 C,or room temperature(25 C),did not induce aggregation and/or degrada-tion.Faint bands identified in the Retacrit and NeoRecormon samples at43kDa and higher were likely due to overloading of prestained protein marker.High-Performance Size-Exclusion ChromatographyHP-SEC was used to characterize soluble aggregates and to quantify epoetin.As demonstrated in Figure2,the main epoetin peak of Eprex and Binocrit(panels a and b)separated on an Agilent HPLC system was detected at29.9and30.1min,respectively.The monomer identities of both epoetin alfa products showed compa-rable average molecular weight close to the theoretical value of 30.4kDa,as estimated by MALLS(Supplementary Fig.1,filled bars).28The additional peaks before the main epoetin peak are probably related to the use of polysorbate80(PS80)as a stabilizer. Thisfinding has also been reported by Hermeling et al.25and will be discussed further below.On a Waters system,the main epoetin peak eluted at29.3and 28.2min for Retacrit and NeoRecormon,respectively(Figs.2b and 2c).Although Retacrit eluted about1min later,its average molec-ular weight was found to be similar to that of NeoRecormon,as estimated by the calibration standards of protein with known molecular weight(Supplementary Fig.1,empty bars).Here,3 possible explanations are suggested.First,the method variability of using proteins with known molecular weight to estimate the molecular weight of epoetin might be a factor.Hence,difference of 1min in elution time cannot be precisely measured in terms of molecular weight.It may also explain the larger estimated average molecular weight of epoetin monomer(57.1kDa)than the theo-retical value(30.4kDa).Second,the difference in elution timebutFigure2.HP-SEC chromatograms of(a)Eprex,(b)Binocrit,(c)Retacrit,and(d)NeoRecormon.The inset is a zoom of the chromatograms of the area between15and30min. The different colors represent the batches of single product.L.A.Halim et al./Journal of Pharmaceutical Sciences xxx(2016)1e94similar estimated molecular weight suggests possible different hydrophilic interactions between the column materials and the 2types of epoetin.Third,differences in glycosylation pattern may lead to differences in the amount of bound water and hence the hydrodynamic volume of the proteins.To study whether the high e molecular weight (HMW)peak was due to PS80or epoetin oligomers or both,(1)0.3mg/mL of PS80,(2)Eprex,and (3)Eprex spiked with 0.3mg/mL of PS80were applied onto the column.As shown in Figure 3a ,the retention times of PS80(peak 1)and Eprex HMW (peak 2)partly overlap.When spiking Eprex with 0.3mg/mL of PS80,the shoulder of Eprex HMW increased signi ficantly and the HMW peak itself shifted to a slightly longer retention time (compare peak 2and peak 3).Conversely,the PS80peak was slightly shifted in the presence of epoetin (compare peak 1and peak 3).These results indicate that epoetin affects the elution behavior of PS80,vice versa ,which compromises an accu-rate assessment of aggregate content.In an attempt to overcome this,we added an equal concentra-tion of PS80to the mobile phase buffer as present in Eprex (0.3mg/mL),with the intention to avoid any PS80signals.25However,although the concept worked for placebo formulation and for the Eprex formulation,the peak became smaller,and for the latter sample,a negative peak appeared (Fig.3b ),again indicating that epoetin and PS80mutually in fluence each other's elution behavior.These data show that it is impossible to accurately determine the amount of epoetin within the HMW species peak.Therefore,as adapted from Hermeling et al.,25fractions (250m L)of Eprex (DDSL500)were collected from 10min until 35min,and theepoetin content therein was assessed by enzyme-linked immuno-sorbent assay.As shown in Figure 4,there was no detectable epoetin dimer or oligomer in the region where HMW species eluted (15-25min).This implies that the different elution behavior of PS80alone,PS80in Eprex formulation,and Eprex spiked with PS80was not solely in fluenced by epoetin.Instead,buffer components or denatured protein could also affect the in fluence behavior of PS80.29Furthermore,the detected HMW species might as well consist of denatured protein which was not detected by the antibodies.The determination of epoetin monomer content in all batches is summarized in Table 3.As expected,a higher EPO monomer con-tent was found in NeoRecormon than that in the other epoetin products,in line with the higher potency as declared on the label.The epoetin monomer content of Binocrit and Retacrit was ~3%and ~14%,respectively,less than that of Eprex.Content discrepancies between batches of 1brand were also apparent.In Eprex,batch DDSL500contained 2%more monomeric epoetin than in DGS4W00.Batch-to-batch variation was found to be the highest in NeoRecormon (~12%).Asymmetrical Flow Field e Flow FractionationAsymmetrical flow field e flow fractionation (AF4)was used as an orthogonal method to HP-SEC for the separationandFigure 3.Zoomed HP-SEC chromatograms of (i)PS80,(ii)Eprex,and (iii)Eprex spiked with 0.3mg/mL PS80in (a)absence or (b)presence of 0.3mg/mL PS80in the mobilephase.Figure 4.Chromatograms of Eprex batch DIS3M00(solid line)and 0.3mg/mL of PS80(dashed line)on Superdex 200column (left y -axis)and results from epoetin speci fic enzyme-linked immunosorbent assay (dotted line)on Eprex column fractions (right y -axis).Table 3Comparison of Content of the 4Epoetin Products Tested Brand NameLotNumber Declared Content (IU/mL)Content UV280(IU/mL)HP-SEC AF4EprexDDS5L0010,0009963±29747±38DGS4W009770±359480±189DHS5T009875±1209614±76DIS3M009825±1009587±38Mean 9858±809607±105Retacrit 8K058L810,0008014±639275±6368M072C98496±5210,059±7009F081G98480±819468±3699M108N98808±4410,450±587Mean 8450±629812±586Binocrit 73041210,0009553±711,008±74245011219400±N.D.10,185±5913412119803±3410,817±265Mean10,767±2711,940±636NeoRecormon H0002H0150,00047,784±35852,158±139H0003H0153,841±9258,582±569Mean50,813±26155,370±414N.D.,not determined.L.A.Halim et al./Journal of Pharmaceutical Sciences xxx (2016)1e 95Figure 5.AF4elugrams of the 4epoetin products (a)Eprex,(b)Binocrit,(c)Retacrit,and (d)NeoRecormon.The inset is a zoom into the elugrams in the area between 10and 25min.The different colors represent the batches of singleproduct.Figure 6.Representative CE-UV analysis of the 4epoetin products.Each product is represented by 1repetition of 1batch.L.A.Halim et al./Journal of Pharmaceutical Sciences xxx (2016)1e 96quanti fication of various sizes of protein monomer and aggregates.As shown in Figure 5,2or more distinct peaks were detected in all epoetin products.The elution time of epoetin monomer slightly differed between products.Monomers of Eprex and Binocrit,which are epoetin alfa,were detected at between 8.6and 8.8min.Monomers of Retacrit (epoetin zeta)and NeoRecormon (epoetin beta)were eluted slightly later at ~8.9-9.2min.As in HP-SEC,differences in elution time between epoetin products were not re flected in the average molecular weight as estimated by MALLS (Supplementary Fig.1).It shows that epoetin possibly interacts with the cellulose membrane.30The peak eluting at ~11min in both epoetin alfa products (Figs.5a and 5b )is likely related to PS80.As shown in Supplementary Figure 2,the peak of PS80alone also has the same elution time.For Retacrit and NeoRecormon (Figs.5c and 5d ),which contain PS20instead of PS80,this particular peak was absent.Peaks eluting earlier than epoetin monomer (<8min)are likely due to the excipients (listed in Table 1).The peaks eluting when the cross flow was stopped (>15min)may be due to larger impurities.However,because small peaks with similar retention times were also observed when injecting placebo for-mulations (results not shown),it is also possible that they may result,at least in part,from contamination of the AF4channel and tubings.In line with the results obtained with HP-SEC,the highest epoetin monomer content was found in NeoRecormon.In addition,the highest content differences between batches ~13%were found in NeoRecormon.The variation between Eprex batches (~3%)was lower than that of other tested products.In contrast,Eprex con-tained the least epoetin monomer content among all epoetin products.These results clearly indicate variation in content deter-mination between HP-SEC and AF4most likely due to different adsorption which hinders the full mass recovery of the injected protein.30Capillary Zone ElectrophoresisMultiple isoforms of epoetin were detected on separation by CZE by an adapted Ph.Eur.method.Owing to the use of different instrumentation,some migration time differences were observed.To correct for this,the time scale of the electropherograms was converted to effective mobility.This conversion enabled good inter-and intraproduct comparison.In all products,the effective mobility of isoforms was observed between 0.001and 0.003cm 2V À1min À1(Fig.6)indicating reproducible migration behavior.Eprex and Binocrit (epoetin alfa)consisted of 6isoforms,whereas Retacrit (epoetin zeta)contained an additional isoform.Neo-Recormon,which is an epoetin beta,contained 8isoforms,similarFigure 7.Relative isoform distribution of (a)different batches of Eprex (n ¼3)and (b)different epoetin products,namely Eprex (n ¼12),Binocrit (n ¼8),Retacrit (n ¼13),and NeoRecormon (n ¼10).The area inside black box represents the acceptance criteria based on Ph.Eur.monograph on Erythropoietin concentrated solution.Error bar indicates standard deviation.Table 4Weighted Mean Potencies (IU/mL)of 4Epoetin Products With Upper and Lower 95%Con fidence Limits ProductBatchDeclared Potency (IU/mL)Epoetin Content as IU/mL (95%Fiducial Limits)In Vitro /In VivoIn Vitro Potency (IU/mL)In Vivo Potency (IU/mL)EprexDDS5L0010,00012,500(12,100-13,000)N.D.N.D.DGS4W00N.D.N.D.N.D.DHS5T00N.D.N.D.N.D.DIS3M0013,100(12,700-13,500)N.D.N.D.Retacrit 8K058L810,0008610(7880-9410)9920(8234-11,951)0.878M072C99190(8300-10,200)N.D.N.D.9F081G99440(9000-9900)N.D.N.D.9M108N99040(8680-9400)11,886(9834-14,365)0.76Binocrit 73041210,00015,300(14,300-16,400)9395(8190-10,777) 1.63450112115,200(14,300-16,400)8015(6828-9408) 1.9034121115,700(14,900-16,600)8544(7573-9640)1.84NeoRecormon H0002H0150,00054,000(52,500-55,500)49,483(42,123-58,128) 1.09H0003H0157,500(55,500-59,500)50,965(43,401-59,847)1.13N.D.,not determined.L.A.Halim et al./Journal of Pharmaceutical Sciences xxx (2016)1e 97。
