聚偏氟乙烯粘结剂溶液干燥条件对锂电池用石墨负极附着力的影响_英文_
锂电池中pvdf粒径-概述说明以及解释

锂电池中pvdf粒径-概述说明以及解释1.引言1.1 概述锂电池作为一种重要的能源存储装置,近年来得到了广泛的应用和研究。
作为锂电池中一个关键的组成部分,PVDF(聚偏氟乙烯)在锂电池中起着至关重要的作用。
PVDF是一种高分子化合物,具有良好的热稳定性、机械性能和化学稳定性,因此被广泛用于锂电池的正负极材料中。
在锂电池中,PVDF作为一种粘结剂,能够将电极材料牢固地粘结在一起,同时还能提供良好的电子导电性和离子传输性。
PVDF具有优异的电化学稳定性,能够有效抑制锂电池中的电解液损耗,在很大程度上提高了锂电池的循环寿命和安全性能。
PVDF的粒径对锂电池的性能有着重要的影响。
当PVDF粒径适中时,能够提供较大的比表面积,增加与电极材料的接触面积,从而促进电池中离子的传输和反应速率。
此外,适度的PVDF粒径还能有效改善电池的力学强度和柔韧性,提高电池的机械稳定性。
然而,PVDF粒径过大或过小都会对锂电池的性能造成不利影响。
当PVDF粒径过大时,其与电极材料的接触面积减小,导致电池的充放电效率降低,电池内阻增大。
而当PVDF粒径过小时,其在电极材料中的分散性变差,容易导致电极材料的电导率下降,影响电池的整体性能。
因此,研究PVDF粒径对锂电池性能的影响,并对其进行优化,对于提高锂电池的能量密度、循环寿命和安全性能具有重要意义。
本文将重点探讨PVDF粒径在锂电池中的作用及其优化方法,并展望未来在这一领域的研究方向。
1.2 文章结构文章结构的部分当前缺少明确的内容。
文章结构是用来组织和引导读者理解文章内容的重要组成部分。
在本篇文章中,可以考虑以下内容来填充文章结构部分:文章结构:本篇文章将按照以下结构组织内容以深入研究PVDF粒径在锂电池中的重要性和影响因素:第一部分,引言。
在引言部分,将对整篇文章的目的和研究背景进行概括性介绍,以便读者对该主题有一个整体的了解。
第二部分,正文。
正文将分为三个小节。
首先,我们将介绍锂电池的背景和应用,包括其在电动汽车、移动设备和储能系统中的重要性。
pvdf粘结剂固化条件

PVDF粘结剂固化条件引言PVDF粘结剂是一种常用的粘结剂,广泛应用于各个领域。
为了确保粘结效果和强度,正确的固化条件是至关重要的。
本文将深入探讨PVDF粘结剂的固化条件,包括温度、时间、压力等因素的影响。
PVDF粘结剂的特性PVDF(聚偏氟乙烯)粘结剂是一种高性能的粘结剂,具有以下特性: 1. 耐高温性能优异 2. 耐腐蚀性强 3. 电绝缘性好 4. 抗紫外线性能好固化温度的影响固化温度是影响PVDF粘结剂固化效果的重要因素之一。
适当的固化温度可以提高粘结强度和耐热性。
以下是不同温度下的固化效果对比:低温固化(<100℃)•优点:–节省能源–缩短固化时间•缺点:–粘结强度较低–耐热性较差中温固化(100-150℃)•优点:–粘结强度适中–耐热性良好•缺点:–固化时间较长高温固化(>150℃)•优点:–粘结强度高–耐热性优异•缺点:–能耗较高–可能导致材料变形综上所述,中温固化是一种较为理想的选择,可以在保证粘结强度和耐热性的同时,尽量减少固化时间。
固化时间的影响固化时间是另一个关键因素,它直接影响到PVDF粘结剂的固化效果和强度。
短时间固化•优点:–节省时间•缺点:–粘结强度较低–固化不完全适当时间固化•优点:–较高的粘结强度–固化较完全•缺点:–固化时间较长长时间固化•优点:–最高的粘结强度–完全固化•缺点:–时间成本较高因此,根据实际需要,在保证固化效果的前提下,选择适当的固化时间是必要的。
固化压力的影响固化压力也是影响PVDF粘结剂固化效果的重要因素之一。
适当的固化压力可以提高粘结强度和粘接面的均匀性。
低压固化•优点:–节省能源•缺点:–粘结强度较低–粘接面不均匀高压固化•优点:–粘结强度高–粘接面均匀•缺点:–能耗较高综上所述,高压固化可以获得更好的固化效果,但需要考虑能耗和设备成本等因素。
固化条件的优化为了获得最佳的固化效果,需要进行固化条件的优化。
以下是一些建议:1.结合实际应用需求,选择适当的固化温度、时间和压力。
锂离子电池粘结剂选择难题,终于有人能讲明白了

锂离子电池粘结剂选择难题,终于有人能讲明白了粘结剂是锂离子电池极片的重要组成材料之一,是将电极片中活性物质和导电剂粘附在电极集流体上的高分子化合物,具有增强活性材料、导电剂和集流体间接触性以及稳定极片结构的作用,是锂离子电池材料中技术含量较高的附加材料。
研究表明,虽然粘结剂在电极片中用量较少,但粘结剂性能的优劣直接影响电池的容量、寿命及安全性。
1.正极binder---PVDF•聚偏氟乙烯PVDF(Poly-vinylidene fluoride)主要是指偏氟乙烯均聚物、偏氟乙烯与其他化合物的共聚物。
•PVDF是结晶性聚合物,结晶度一般为50%左右,熔融温度在140-180 ℃之间。
•由于C-F键长短,键能高(486kJ/mol) ,故PVDF具有良好的抗氧化性、耐化学腐蚀性、耐高温性,特别是在碳酸酯类溶剂( EC、DEC、DMC 等)中稳定性好。
1.1 PVDF主要种类•均聚类PVDF,是VF2的均聚物,如HSV900, 5130等;•共聚物类PVDF,主要使用的是VF2(偏二氟乙烯)/HFP(六氟丙烯)的共聚物,如2801,LBG等。
1.2 PVDF合成方法通常由偏氟乙烯通过悬浮聚合或乳液聚合而成,反应方程式如下所示:CH2=CF2→(CH2CF2)n1.3 分子量对PVDF的影响•不同聚合度的VDF均聚物,其熔点温度差异不大;但PVDF分子量的大小会影响其在溶剂中的溶解难易程度。
•在一定分子量范围内,分子量的提高有助于粘结力和内聚力的提高;l改性对PVDF结晶度/溶胀度影响•掺杂的-HFP量越多,其结晶度越低,导致熔点相应降低;•结晶度降低,聚合物溶胀程度增大(甚至溶解)。
1.4 PVDF面临的问题与挑战过高分子量(>150W)对粘结力的提升效果不明显,但会造成更难溶解2. 负极binder---SBRSBR(丁苯橡胶乳液)由丁二烯及苯乙烯两种单体经自由基乳液聚合而成。
常用的锂离子电池SBR粘结剂除上述两种单体外,通常都引入了新的功能单体,用以提高其离子电导率或粘附力。
PVDF性能及对锂电池性能的影响

PVDF性能及对锂电池性能的影响聚偏氯乙烯(PVDF)是一种特殊的高性能聚合物材料,具有优异的耐热性、耐化学腐蚀性、机械强度、绝缘性质和耐候性等特点。
PVDF在锂电池领域的应用非常广泛,主要有电解质、隔膜和电极材料等方面。
首先,PVDF作为电解质材料,因其具有良好的耐化学腐蚀性和离子导电性能成为锂电池中重要的组成部分。
PVDF作为锂盐的添加剂,可以提高电解液的导电性能和离子传输效率,从而提高锂电池的循环稳定性和能量密度。
另外,PVDF作为电解质材料还具有较高的电化学稳定性和较低的导电阻抗,可以有效减少电池内部的能量损失,提高电池的充放电效率和容量。
其次,PVDF作为锂电池隔膜的材料,具有较高的机械强度、热稳定性和耐化学腐蚀性,可以保证锂电池的安全性和循环寿命。
PVDF隔膜材料具有优良的微孔结构和较低的电阻率,可以有效防止正负极之间的直接接触和短路,同时保证锂离子和电子的传输。
此外,PVDF隔膜材料还具有良好的润湿性和可渗透性,可以增加锂离子在电解液和电极之间的传输速率,提高锂电池的功率密度和循环性能。
再次,PVDF作为锂电池电极材料,主要用于制备锂离子电池的正极材料。
PVDF具有较高的热稳定性和化学稳定性,可以耐受正极材料在高温下的反应。
此外,PVDF还具有良好的可溶性和可处理性,可以方便地与其他材料进行混合和复合,以提高正极材料的电化学性能。
PVDF还具有一定的导电性,可以提高锂离子在电极活动材料中的传输速率,增加锂电池的充放电速率和倍率性能。
总的来说,PVDF作为一种高性能聚合物材料,对锂电池的性能具有重要的影响。
PVDF作为电解质材料可以提高锂电池的导电性能和循环稳定性;作为隔膜材料可以保证锂电池的安全性和循环寿命;作为电极材料可以提高锂电池的充放电速率和倍率性能。
随着锂电池技术的不断发展,PVDF材料在锂电池领域的应用前景将更广阔。
不同干燥条件下聚偏氟乙烯的结晶与电池性能
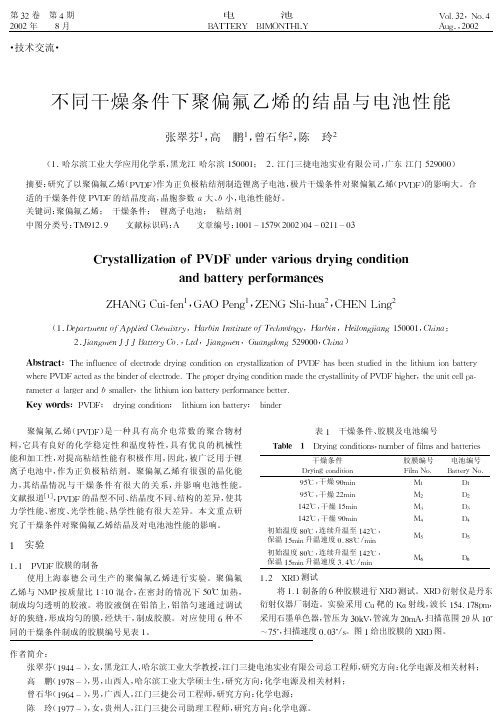
分类 5&’(()*)%’+),-
晶胞特点
结晶度/=
67 68
!大 "小 !>
69 6!
!中 "大 7>
6: 6;
!小 <中 ;>
结果可见,07、08 的循环性能好于其余7种,08 后期最好。 0:、0; 最差。07、08 放电电压比 0: 高8>2/ 多。新电池 07、08 的内阻最小,0: 最大;循环9>>次后,07 最小,08 次之,二者相 差不大,0:、0; 很大。
[:] QL 6’-OXT-(何曼君),5QLA Y$)O(#,T(陈维寿),0ZARH)O[)’(董 西侠)B高分子物理(修订版)[6]BU#’-N#’)(上海):1TV’-\-)]$3()O +4.3$(((复旦大学出版社),9EEF:7!9W7!7B
收稿日期::>>:W9>W9:
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!"
晶系 空间群 晶胞参数 晶胞中的分子数
单斜 #;9DO$9!/, %P5=L4J,&PL=45J,’P5=49J,#PL3=3Q (P9
表5 胶膜!!3面衍射间距实测值与计算值
!"#$% ( $&C-+/,1.))&H<!!3)+,11R+<&/1N+/N,+H,EH+-1N
I>$%晶体
实测间距/J
;:年精心打造的品牌 荣获“国家期刊奖”的杂志
欢迎刊登广告 彩色黑白随你选!
《电池》广告具有长久的影响力! 《电池》的广告为您扬名!
ptfe干法负极副反应

ptfe干法负极副反应
PTFE(聚四氟乙烯)是一种具有优异化学稳定性和耐热性的高分子材料,常用于制备电池的负极材料。
在电池中,PTFE通常用作负极的添加剂,以改善电池的性能。
PTFE干法负极副反应是指在制备电池负极时,PTFE与其他材料之间可能发生的化学反应或物理变化。
从化学反应的角度来看,PTFE在制备电池负极时可能会与其他材料发生副反应。
例如,PTFE在与导电剂或活性材料接触时,可能发生氧化还原反应,导致电池性能的变化。
这些副反应可能会影响电池的循环寿命、充放电性能等方面。
从物理变化的角度来看,PTFE在制备电池负极时也可能发生一些物理变化。
例如,PTFE的添加可能会改变电极的结构或孔隙度,从而影响电极的扩散性能和电荷传输性能。
此外,PTFE的添加还可能影响电极的机械性能和热稳定性。
PTFE的存在可能改变电极的柔韧性和热传导性能,从而影响电池的安全性能和稳定性。
总的来说,PTFE干法负极副反应是制备电池负极过程中需要考
虑的重要因素。
了解PTFE与其他材料之间的相互作用对于优化电池
性能和提高电池循环寿命具有重要意义。
需要在制备过程中进行充
分的研究和实验,以确保PTFE的使用不会对电池性能产生负面影响。
]锂离子电池用PVDF粘结剂调研资料
![]锂离子电池用PVDF粘结剂调研资料](https://img.taocdn.com/s3/m/92c9994630b765ce0508763231126edb6e1a7646.png)
]锂离子电池用PVDF粘结剂调研资料PVDF是一种聚偏氟乙烯材料,被广泛应用于锂离子电池中的粘结剂。
在锂离子电池制造过程中,PVDF粘结剂主要用于固定电池内部的正负极活性材料与电解质膜,同时也能提供电池的导电性和机械稳定性。
以下是与PVDF粘结剂相关的调研资料:1.PVDF粘结剂的特点:-耐高温性能:PVDF具有良好的热稳定性,可以在高温条件下长时间使用,不易发生分解或变形。
-耐化学腐蚀性:PVDF具有良好的耐酸碱性和耐盐水性,可以有效防止电池内部发生化学反应导致电池寿命的缩短。
-优良的粘接性和耐磨性:PVDF粘结剂能够有效地固定电池内部的活性材料和电解质膜,同时具有良好的耐磨性,能够保持电池的机械稳定性。
-优异的导电性能:PVDF具有较高的电导率,可以提供电池内部的良好导电性。
2.PVDF粘结剂在锂离子电池中的应用:-电池正负极活性材料的粘结:PVDF粘结剂能够将正负极活性材料牢固地粘结到电池电极上,确保电极的稳定性和高效率的锂离子传输。
-电解质膜的粘结:PVDF粘结剂能够将电解质膜牢固地固定在电池内部,保证电解质的稳定性和良好的离子传输性能。
-导电剂的粘结:PVDF粘结剂可以用于固定电池中的导电剂,保证电池内部的良好导电性。
-电池封装材料的粘结:PVDF粘结剂还可以用于电池封装材料的固定,确保电池的整体结构的稳定性。
3.PVDF粘结剂的制备方法:PVDF粘结剂可以通过溶液共混、熔融共混等方法制备。
其中,溶液共混是较为常用的一种制备方法,通常使用溶剂将PVDF溶解后与其他材料共混,然后通过溶剂的挥发或加热使PVDF重新沉淀出来,形成PVDF粘结剂。
4.PVDF粘结剂的市场应用和发展趋势:PVDF粘结剂在锂离子电池制造业中得到了广泛应用,因为它具有良好的耐温性、耐化学性和粘接性能,能够满足电池制造过程的要求。
随着锂离子电池市场的快速发展,对PVDF粘结剂的需求也在不断增加。
未来,PVDF粘结剂的研发重点将放在提高其导电性、机械强度和耐老化性能等方面,以满足高能量密度和高安全性的锂离子电池的需求。
锂电池用聚偏氟乙烯粘结剂论文

量 分 数 分 别 为 &’( )"*+""* , 六 氟 丙 烯 ( ,(-)
."*+/"*,0(1 2"*+."*的 / 元共聚的氟橡胶为粘
!""# 年第 $% 卷第 ! 期
!" !!!!!!" !"
化工生产与技术
&’()*+,- ./012+3*04 ,41 5(+’40-067
・ ・ $
氟 化 工
!!!!!!"
锂电池用聚偏氟乙烯粘结剂
张成德 马 圭
(国家氟材料工程技术研究中心, 浙江 衢州 %!8""8 )
摘要 锂电池用粘结剂是制造锂电池的重要材料之一, 可直接影响其性能。介绍了锂电池
收稿日期: !""9E$$E!9
题, 但随着时间的迁移, 在使用过程中, 也有由于电 极的内部应力使电极合剂层从集电体上部分或全部 剥离, 负荷特性变差, 引起容量劣化。 由于 <=> 均聚物存在上述问题, 所以一般采用
<=> 均聚物改性、 <=> 与第 ! 单体及第 % 单体共聚 和 <=> 均聚物与另一种共聚物共混等方法。 不管采
非水系电池 >?.:$$$@ED$@8.9;$@@@B"CB!$;
・ ・ %
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
第38卷第6期Vol.38,No.62008年6月JOU RNAL OF UNIVE RSITY OF SCIENCE AND TECHNOLOGY OF CHINAJun.2008A rt icle ID:0253 2778(2008)06 0623 05Received:2008 01 18;Revised :2008 05 05Foundation item:Supported by Nation al Natu ral Science Foundation of Chin a (50372064).Biography:SH UI J iang lan,male,born in 1979,PhD.Res earch field:Lithium batteries.E mail:shu ijl@ Corresponding author:CH EN Chun hua,Ph D/Prof.E mail:cchchen @Effect of drying condition of poly (vinylidene fluoride)binder solution on film adhesion of graphiteelectrode for lithium batteriesSH U I Jiang lan,DIN G Chu x iong,Y U Yan,CH EN Chun hua(L abor atory of A d v anced F unctional M aterials and Dev ic es,Dep artment of M ater ials Sc ienc e and Eng ine ering ,Univ ersity of S cience and T ec hnology of China ,H e f ei 230026,China)Abstract:T he dr ying conditions of a poly (v inylidene fluoride)(PVDF)solution hav e a great influence on its crystalinity and binding streng th during the preparatio n o f electro de laminates for lithium ion batteries.A peeling experiment w as desig ned to evaluate the m echanical strength o f the PVDF adhering to g raphite.X r ay diffractio n and g alvanostatic cell cycling w ere used to evaluate the influence of PVDF binder treated under different thermal co nditions.T he results indicate that for the PVDF binder that is dried and crystallized at a temperature slig htly hig her than its m elting temperature,it has hig her cr ystallinity and adhesion strength to the g raphite par ticles,w hether it is so aked in electrolyte or not.This binder related mechanical pr operty o f the electr ode can improve the cy cling performance o f the g raphite electro de altho ug h the capacity m ay under go a gr adual rising process during the first 150or so cy cles due to the partial blocking of the electrical conduction path by PV DF initially.Key words:poly (viny lidene fluoride);gr aphite;cry stallinity;adhesion;lithium batteries CLC number:O646 Document code:A聚偏氟乙烯粘结剂溶液干燥条件对锂电池用石墨负极附着力的影响水江澜,丁楚雄,余 彦,陈春华(中国科学技术大学材料科学与工程系先进功能材料与器件实验室,安徽合肥230026)摘要:聚偏氟乙烯(PVDF)溶液的干燥条件对其结晶性、粘结强度有很大影响,对制备锂离子电池的电极材料亦有影响.为此,设计了一种薄膜剥皮试验来评估PVDF 附着于石墨的机械强度;以XRD 和电池循环来测量不同热处理条件下PVDF 粘结剂的影响.结果表明,在高于PVDF 熔点的温度处理的样品,PVDF 具有更高的结晶性和附着力,从而改善了石墨电极的循环性能,还观察到前150次循环过程中的容量逐步上升现象.PVDF 的逐渐溶胀被认为是出现该现象的原因.关键词:聚偏氟乙烯;石墨;结晶性;附着力;锂电池0 IntroductionGraphite is a widely used ano de material in the lithium ion battery.In order to improv e its capacity r etention ability,various attem pts have been made to r ealize surface m odification of the g raphite particles w hich w ill influence the form ation of SEI(so lid electr olyte interphase) film.Such a treatment usually reduces the initial capacity loss(ICL)and impr oves the cycling per for mance of the battery[1~3].In addition,the m icrostr ucture o f the po rous electrode can also be eng ineered dur ing the slur ry preparation by means of apply ing pr essure to the com posite electrode to tailor the poro sity of the electr ode.Pr oper porosity is necessary for the electr ode to have g ood capacity retention ability[4~7].Som e studies m entioned that the dr ying temperature of the composite electrode film has a g reat im pact on the adhesio n streng th of the w hole electrode to the current co llector,and that crystallinity of the PVDF binder plays an important r ole in this streng th.A detailed investigatio n on the effect of elevated dr ying temperature o n the adhesion streng th of the PVDF binding g raphite particles and the corresponding electro chem ical character ization has not been r eported in literature.In this paper,w e investigated this binding str ength betw een the binder PVDF and the g raphite particles,and com pared the cycling per for mance of the co mposite electro de m anufactured w ith different dry ing conditions.It is understo od that the crystallinity of PVDF sho uld differ greatly w hen it is o btained by dry ing its solution at a tem perature either far below or higher than its melting point(about170 ).M or eov er, the cry stallinity of PVDF binder has a gr eat influence o n the adhesion str ength as mentioned above.Therefor e,we selected70 (our usually used dry ing temperature)and200 as the dr ying temperatures to perform a comparative study.We have found that the crystallinity and adhesion strength of PVDF binder to the g raphite in the sample dried at200 are much hig her than that dried at70 ,and this kind o f adhesio n streng th has a crucial influence on the cy cling performance of the graphite electrode.H igher adhesion streng th results in better capacity retention ability.1 ExperimentalThe gr aphite electrodes w ere manufactured by mix ing a graphite pow der(about1 m particle size)(90w t%)w ith10w t%of PVDF binder into a NMP dispersed slurry,follow ed by spreading the slurry using the doctor blade m ethod on a sheet o f copper foil.T he natur ally dried laminate w as further dried in an oven at70 fo r5h or200 fo r20minutes.T he dried electrode films w er e taken out fro m the oven directly to the ro om tem perature about10 .Cycling test w as carried out on coin type cells(2032)w ith the config uration o f Li/1M LiPF6in ethylene car bonate and diethy l carbonate(EC DEC,11v/v)/ graphite film,w hich w ere assem bled in an arg on filled g love box(M BRAUN LABMAST ER130) w here bo th mo isture and oxy gen levels w ere less than1ppm.A Celgard2400microporous polypropylene membrane was used as the separator.To evaluate adhesio n str ength of PVDF binder to the gr aphite par ticles,a peeling exper im ent w as desig ned to simulate the adhesio n betw een g raphite particles and binder PVDF.A12w t%PVDF so lution w as prepar ed by dissolv ing PVDF pow der into NMP(1 m ethyl 2 py rrolido ne,C5H9NO).It w as coated onto the sur face o f tw o pieces o f graphite bars(8m m wide and50mm lo ng)and then dr ied in an oven in air at70 for5h(LT film)and200 for20m inutes(H T film), respectiv ely,in order to simulate the drying conditions of the graphite electrode laminates.We peeled these tw o PVDF film s fro m the g raphite bars at roughly the same peeling strength and velocity w ith a sm all clip and spring balance. Sim ilar ly,tw o PVDF coated g raphite bars w er e made and soaked in the electro lyte solution for18624中国科学技术大学学报第38卷hours at ambient temperature.T he PVDF adhesion o n these soaked bars w ere also measur ed by the peeling method.The thermal stability of the PVDF w as measured by a thermogravimetric analyzer (TGA50,Shimadzu)in the temperatur e range betw een 20and 700 w ith a heating rate of 5 /min.T he cry stal str ucture w as analyzed w ith X r ay diffr actio n (Cu K radiation,Philips X Pert PRO SU PER).Graphite electrode lam inates w er e prepared by casting onto a copper foil a slurr y consisting of g raphite pow der (90w t%)and po ly (v iny lidene fluoride )(PVDF )(10w t%)dispersed in 1 m ethyl 2 pyrr olidinone (NM P ).The lam inatesw ere then dr ied at either 70 o r 200 for 2hours and calendar ed to o btain po rosity betw een 60%~70%.Gr aphite/Li coin cells (2032size)w ere made w ith 1M LiPF 6in ethylene carbonate (EC):diethyl carbo nate (DEC)(1 1by w eig ht)as the electrolyte.Cycling tests on these cells w ere per for med under a constant current density of 0 20mA /cm 2in the vo ltag e rang e betw een 0and 3V.2 Results and discussionT he melting po int o f PVDF is around 170 .Accor ding to the thermo gravimetric analysis (T GA )o f the PVDF pow der used in this study (Fig.1),PVDF do es not deco mpose until 450 .Fig.2show s the X ray diffraction patterns o f the PVDF co atings after drying at 200 (H T film)and 70(LT film ).T he diffraction peakobserv ed at 2 !20.8∀is assigned to the unr esolved (110)and (200)diffractions of ! phase of PVDF,and phase o f PVDF show s diffraction peaks at 2 !18.5∀and 26∀,and are assigned to (020)/(110)and (120)diffractions,respectively [8].Co mparing the XRD patterns of the H T film and the LT film,the peaks of the LT film are broader than tho se of the H T film,w hich indicates that crystallinity of the film dried at 200 is higher than that dried at 70 .In addition,some ! phase has transferred to phase w hen the dryingcondition chang es from 70 /5h to 200 /20min.Different from som e resear ches to evaluate the adhesion strength o f co mposite film to substrates,w e solely w ant to know the adhesion streng th betw een the binder and g raphite because w e believ e that the interaction of the binder to the activ e materials is ver y im po rtant for the electrode stability and electro chem ical perform ance.Thus,a peeling ex periment w as desig ned to ev aluate this streng th and rectang ular g raphite bar s w er e used as substr ates to simulate the true adhesio n of the binder to the graphite particles.Tab.1sho ws the peeling strength for the H T film and the LT film before and after being soaked in the electr olyte.It can be seen that the peeling of the H T film needs fo ur times more for ce than the peeling of the LT film.Besides,w hen w e peel the binder films from the g raphite bar so me graphite particles ar e removed off the bar so that the peeled PVDF film ex hibits a fuscous co lor (Fig.3).The H T film625第6期E ffect of drying cond ition of poly (vi nylid en e flu orid e)bind er solu tion on film adhesion of graphite electrode for lithi um batteri espresents m ore fusco us than the LT film.Because of the higher adhesion strength of the H T film,m ore g raphite particles can be r em oved off from the gr aphite bar.After being soaked in the po lar or ganic electro lyte for 18hours at ambient temperature of about 20 ,the adhesion str ength of both the H T film and the LT film all decreased a little,but the sequence is still H T >LT.The decrease in the adhesion strength after soaking in the electroly te may be due to the sw elling of the amo rphous portion of PVDF w hen the electr olyte solvent is absor bed by the film.As repor ted by Yoo and Despotopoulou et.al [9,10],the crystallinity is an important factor in determ ining the adhesion strength:the higher crystallinity the higher adhesion str ength betw een com posite film and cur rent collector.H ence abov e r esults,combined w ith XRD results,clearly indicate that PVDF m ay crystallize at a tem perature slightly hig her than its m elting point.Co nsequently ,thus r esulted higher crystallinity leads to much higher adhesion strength of PVDF binder to the surface of graphite particles.Tab.1 Peel strength of dried PVDF film from graphite barspeel strength /(N #mm -1)70 /5hpeel stren gth /(N #mm -1)200 /20minbefore electrolyte soakin g 0.110.49after soaking in electrolytefor 18hours0.080.45Th e PVDF film s w ere dried at 70 /4h (a)and 200 /20min (b),respectivelyFig.3 Photo of the peeled PVDF film from graphite barsFig.4sho w s the capacity retention ability oftw o Li/graphite cells w ith the graphite electrodesmentioned above.T he cell w ith electro de dr ied at 70 /5h show s some capacity loss during the first 100cy cles fr om 365to 330m Ah/g,and the capacity fading accelerates after 125cycles.On the other hand,the cell w ith electro de dried at 200 /20min show s very g ood capacity retention ability.The capacity no t only keeps stable but also increases somew hat from 336to 367m Ah/g during the first 165cy cles,about 0 19mAh/g per cy cle.Though the capacity increases initially ,it finally decreases after r eaching the peak value (367mAh/g),that is still below the theo retical capacity o f graphite (372mAh/g ),dur ing a pr olongedcy cling.Li/com posite graphite (drying condition:70 /4h (a)and 200 /20min (b)).Th e pas sing current w as 0.2m A/cm 2Fig.4 Cycling performance of cellsThe lithium insertion extraction in a g raphite electr ode involv es several pro cesses including ∃periodic volume changing in the g raphite flakes,w ith the amo unt of activ e m aterial not decreasing [11],%surface film fo rmation o n the graphite par ticles [1~3,11],and &the sw elling o f po lymer binder PVDF [9,10].It should be mentio ned here that coulo mbic efficiencies o f these tw o kinds of cells are alm ost the same,abo ut 80%.Thus,the reaso n for the cycling performance change in Fig.4sho uld not be only due to the for mation o f SEI,w hich has been intensively investigated [1~4],although some gro ups repo rted that the br eaking o f SEI film can cause the capacity bining the abo ve facto rs ∃,%and &,w e speculate that the contact among gr aphite particles may be deteriorated w ith the incom pact electrode626中国科学技术大学学报第38卷upo n pr olonged cy cling.The loose particle contact can lead to a poor electrical conduction among the par ticles,and to the form ation of surface films w hich are m ore resistiv e.This may certainly cause the capacity loss of the battery.H ig her adhesion str ength betw een the PVDF binder and graphite can alleviate the po ssible detachm ent betw een the PVDF binder and the sur face of the graphite par ticles during the periodic vo lum e chang es o f the g raphite particles.On the other hand,some g raphite particles in the H T electrode may not be utilized fully initially because so me particles are covered closely by the PVDF binder w ith higher crystallinity so that the electronic conduction path is blocked and only a fraction(<100%)o f the g raphite surface is accessible to the electro lyte.H ence the w hole capacity can not be utilized fully at first,but the capacity can rise during prolo ng ed cycling due to the g radual binder swelling by the electroly te solution,as observed in the case of H T electrode(Fig.4).3 ConclusionWe have co mpared lam inated graphite electrodes and pure PVDF films m anufactured by different dry ing pro cesses,i.e.heating at either 70 /5h o r200 /20min.The cell w ith the 200 dried electr ode show s much better capacity retention ability,and the capacity ev en underg oes a little rise fo r the first165cy cles.PV DF dr ied at temperature slightly higher than its m elting po int w ill cause higher crystallinity w hich co nsequently enhances its adhesion strength as a binder w ith the g raphite particles and the stability of the electro de str ucture.Better adhesion o f the electrode can alleviate the issue of the detachment of PVDF binder to the g raphite and prevent loose contact betw een g raphite particles,and thus impr ove the capacity retention ability.A partial sw elling o f the binder can block the contact between the electroly te and the active m aterials,leading to an increase in capacity upon prolonged cycling.The internal interaction betw een the binder and the graphite particles has a crucial effect on the capacity retention ability of the composite g raphite electr ode.References[1]Zhang S S,Xu K,Jow T R.Effect of Li2CO3 co atingon the perfo rmance of natural g raphite in L i ion batter y [J].Electro chem Commun,2003,5:979 982.[2]Zhang S S,Xu K,Jo w T R.Enhanced per for mance ofnatura l g raphite in Li ion battery by o xalatobor ate coat [J].J P ow er Sour ces,2004,129:275 279.[3]Ko maba S,Itabashi T,K aplan B,et al.Enhancementof L i io n batt ery perfor mance of gr aphite anode by so dium ion as an electro ly te addit ive[J].Electro chem Co mmun,2003,5:962 966.[4]Gnanar aj J S,Cohen Y S,L evi M D,et al.T he effectof pressur e o n the elect roanalytical r esponse o f g ra phite ano des and L iCoO2cat ho des for L i io n batter ies[J].J Electro anal Chem,2001,516:89 102.[5]M anev V,N aidenov I,P ur esheva B,et al.Effect ofelectro de po rosity on the perfo rmance of nat ur al Br azilian g r aphite electr odes[J].J P ower Sources, 1995,57:133 136.[6]Buqa H,Goer s D,H o lzapfel M,et al.H ig h ratecapability of g raphite negat ive electro des for lithium io n batter ies[J].J Electro chem Soc,2005,152:A474 A481.[7]Shim J,Str iebel K A.Effect of electro de densit y o ncy cle perfo rmance and irr ev ersible capacity loss fo r natura l gr aphite anode in lithium io n batter ies[J].J Pow er So ur ces,2003,119 121:934 937.[8]Cho y K L,Bai W.P reparation o f o riented poly(viny lidene f luor ide)thin films by a cost effect ive electro st at ic spr ay assisted vapour depo sitio n based met ho d[J].T hin So lid F ilms,2000,372:6 9.[9]Yo o M,Fr anka C W,M o ri S,et al.Effect of poly(v iny lidene fluo ride)binder cry st allinity and g ra phite st ruct ur e o n the mechanical streng th o f the composite ano de in a lithium ion batter y[J].Po lymer,2003,44: 4197 4204.[10]D espoto po ulou M,Burchill M T.Coat ings fo relectro chemical applications[J].P ro gr ess in Or ganic Co atings,2002,45:119 126.[11]K o lty pin M,Cohen Y S,M ar ko vsky B,et al.T hestudy o f lithium insertion deinsertion pr ocesses into co mpo site g raphite electro des by in sit u atomic for ce micro sco py(A FM)[J].Electro chem Commun,2002, 4,17 23.627第6期E ffect of drying cond ition of poly(vi nylid en e flu orid e)bind er solu tion on film adhesion of graphite electrode for lithi um batteri es。
