朗道混合生化校准品
生化类体外诊断试剂开瓶稳定性研究

生化类体外诊断试剂开瓶稳定性研究摘要】分析生化类体外诊断试剂的开瓶稳定性。
方法:以朗道质控品为检测样本,利用全自动生化分析仪对总蛋白试剂盒、丙氨酸氨基转移酶检验试剂盒和肌酐检测试剂盒进行检测,分析开瓶时间对检测结果的影响。
结果:在开瓶时间不断增加的情况下,总蛋白(TP)、肌酐(Cre)与谷丙转氨酶(ALT)的误差指标的相对偏差均呈现出上升趋势。
结论:不同类型的生化类体外诊断试剂的开瓶稳定性存在差异。
生化类体外诊断试剂开瓶以后需要尽快使用,在每次检测实施前都需要重新校准。
【关键词】生化类体外诊断试剂;总蛋白;肌酐;谷丙转氨酶随着临床检验技术的不断发展,生化试剂在临床检验领域的作用日渐突出。
医疗设备产业的发展进步,让生化试剂的产品类型呈现出了多样化的特点[1]。
受生产规模、技术水平及产品质量等因素的影响,来自不同厂家的生化类体外试剂的产品质量存在一定的差异[2]。
在临床检验领域,生化试剂中的部分组分在受热、受潮及受光以后出现的分解、失活现象也会给临床诊断结果的准确性带来不利的影响。
受业务量的影响,一些医院在试剂开瓶以后,往往需要利用很长的时间消耗试剂,试剂开瓶后放置时间对临床诊断结果的影响是一些研究者所关注的内容。
本次研究旨在分析生化类体外诊断试剂的开瓶稳定性。
1.资料及方法1.1一般资料本次研究以朗道质控品为检测样本,利用全自动生化分析仪开展开瓶稳定性实验,对总蛋白试剂盒、丙氨酸氨基转移酶检验试剂盒和肌酐检测试剂盒进行检测。
应用于本次研究的朗道质控品中包含有TP、Cre与ALT等物质,参考值分别为45.0g/L、138U/L与366μmol/L。
总蛋白试剂盒的组成成分以硫酸铜、铝氧化钠、酒石酸钾钠与碘化钾为主。
丙氨酸氨基转移酶检测试剂盒中包含有Tris-HCl、L-丙氨酸、α-酮戊二酸、NADH、乙二醇和L-乳酸脱氢酶。
肌酐检测试剂盒的组成成分以苦味酸及氢氧化钠为主。
应用于本次研究的实验设备为DS-800全自动生化分析仪。
朗道质控和校准明细
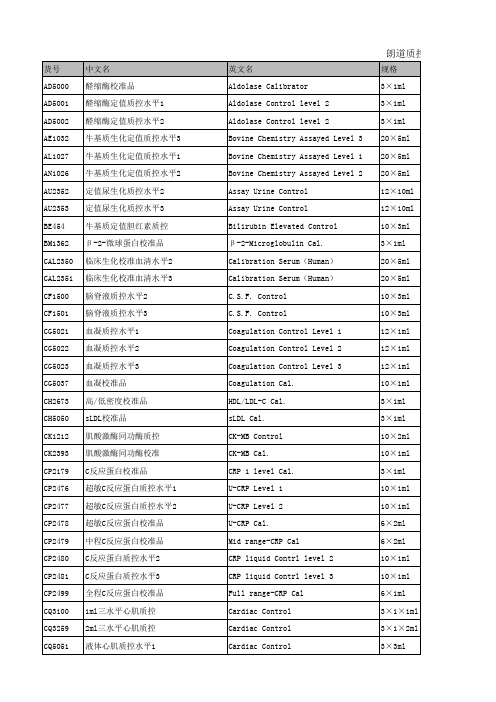
GSP Cal.
3×1ml
GSP Contrl.
3×1ml
GSP Contrl.
3×1ml
Glutamine Calibrator
10×5ml
Glutamine Control Level 1
5×5ml
Glutamine Control Level 2
5×5ml
GM1378 谷氨酰胺定值质控水平3 GY1369 甘油定值质控水平1 HA3444 糖化血红蛋白校准品 HA5072 糖化血红蛋白质控品 HA5083 血红蛋白F&A2 质控品 HD1667 药物监测质控水平1 HD1668 药物监测质控水平2 HD1669 药物监测质控水平3 HE1532 人定值多项生化质控血清水平3 HE5068 人定值生化质控血清水平3 HN1530 人定值多项生化质控血清水平2 HN5067 人定值生化质控血清水平2 HP1359 结合珠蛋白校准品 IA2633 三水平免疫多项质控 IA2638 免疫多项质控水平1 IA2639 免疫多项质控水平2 IA2640 免疫多项质控水平3 IA3109 多项免疫质控水平1 IA3110 多项免疫质控水平2 IA3111 多项免疫质控水平3 IA3112 多项免疫三水平质控 IAS3113 免疫特殊质控I 水平1 IAS3114 免疫特殊质控I 水平2 IAS3115 免疫特殊质控I 水平3 IAS3117 免疫特殊质控II 水平1 IAS3118 免疫特殊质控II 水平2 IAS3119 免疫特殊质控II 水平3 IE2492 免疫球蛋白E校准品 IT2691 特定蛋白校准(11项) IT2692 特定蛋白校准(6项) IT3861 免疫球蛋白复合校准品 IT3899 高敏IgG校准品 IT3982 高敏IgG质控品水平1 IT3983 高敏IgG质控品水平2
生化复合校准品产品技术要求雷诺华

医疗器械产品技术要求编号:
生化复合校准品
2性能指标
2.1外观
2.1.1包装外观
试剂盒的包装应整洁;图案与文字应清晰无误;标签应粘贴端正,无污迹;封盖应紧密,无漏
液。
2.1.2试剂外观
产品校准品LEVEL 1 应为粉色透明液体,LEVEL 2 为蓝色透明液体,LEVEL 3 为无色透明液体、无沉淀和明显悬浮物、絮状物。
2.2试剂装量
试剂每瓶装量应不低于标示量。
2.3均匀性
2.3.1瓶内均匀性
瓶内均匀性应符合表1 的要求。
表 1 瓶内均匀性
2.3.2瓶间均匀性
瓶间均匀性应符合表2 的要求。
表 2 瓶间均匀性
2.4溯源性
2.4.1赋值程序
提供赋值程序文件至少一个批次的赋值记录。
2.4.2校准品的互换性
制造商需提供校准品互换性的技术文件。
2.4.3赋值的准确性
偏倚满足表3 的要求。
表 3 准确度。
生化室作业指导书3.29

目录修订页编写者:陈晓玲审核者:王沛批准者:王沛血清总胆红素(T-BIL)测定1 检验目的指导本室工作人员规范操作本检测项目,确保检测结果的准确。
2 实验原理在酸性溶液中,样本中的总胆红素被钒酸盐氧化生成胆绿素的同时,在450nm波长处可引起吸光度的下降。
其在450nm波长处引起吸光度的变化值与样本中总胆红素的浓度成正比。
通过在450nm波长处测定吸光度的变化值即可测得样本中总胆红素的浓度。
3 标本3.1 病人准备:无特殊要求。
最好用禁食的标本以减少乳糜血的干扰。
3.2 类型:血清3.3 标本存放:15~25℃保存可稳定2天;2~8℃保存可稳定7天;-20℃保存可稳定3个月,如冰冻保存,不可反复冻融。
3.4 标本运输:常温条件下避光保存运输。
3.5 标本拒收标准:标本溶血、细菌污染、脂血、非避光保存运输的标本。
4 实验材料4.1 试剂:上海复星长征医学科学有限公司总胆红素试剂盒(沪食药监械(准)字2014第2400166号 YZB/沪 1546-40-2014)4.1.1 试剂组成试剂1(R1):柠檬酸缓冲液100mmol/L 表面活性剂 1.0%试剂2(R2):磷酸盐缓冲液200mmol/L 偏钒酸钠 3.8mmol/L4.1.2 试剂准备:试剂为即用式。
4.1.3 试剂稳定性与贮存编写者:陈晓玲审核者:王沛批准者:王沛4.1.3.2有效期:在2~8℃避光、密封的储存条件下,试剂盒自检定合格之日起有效期为18个月。
4.1.4 变质指示:当试剂有浊度时,表明有细菌污染,不能继续使用。
4.1.5 注意事项:此试剂为体外诊断用,不要入口,毒性还末确定;避免和眼睛,皮肤或衣服接触,如果接触到,立即用大量的水冲洗受损害的部位15分钟,接触到眼睛或吞服,立即寻找医疗保护。
4.2 校准品:使用上海复星长征医学科学有限公司提供的TBIL校准品对自动分析仪进行校准。
4.3 质控品:使用正常值、病理值复合控制品。
朗道质控和校准明细
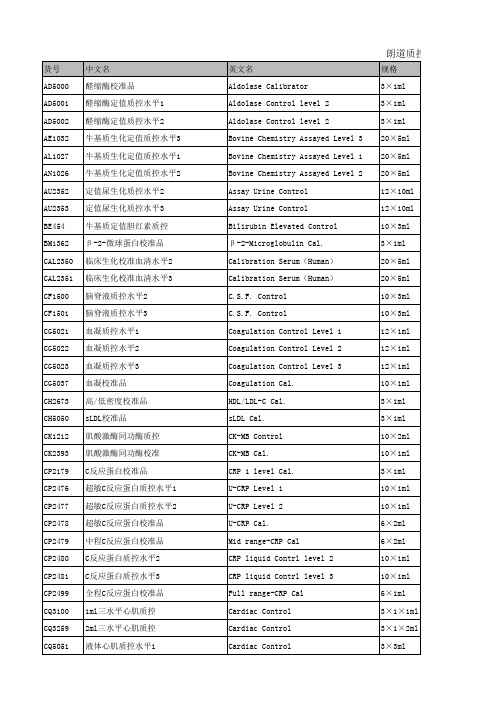
IT3984 LE2661 LE2662 LE2663 LE2668 LE2669 LE2670 LE5013 LE5014 LE5015 LE5016 LE5017 LE5018 LIA3105 LIA3106 LIA3107 LIA3108 LO2306 LP3023 LP3404 LP3406 LP5047 LUE5049 LUE5071 LUE5095 LUL5069 LUL5093 LUN5048 LUN5070 LUN5094 MA1361 MA1567 MC1379 MC1380
高敏IgG质控品水平3 3ml脂类多项质控水平1 3ml脂类多项质控水平2 3ml脂类多项质控水平3 1ml脂类多项质控水平1 1ml脂类多项质控水平2 1ml脂类多项质控水平3 sLDL质控水平1 sLDL质控水平2 sLDL质控水平3 载脂蛋白质控水平1 载脂蛋白质控水平2 载脂蛋白质控水平3 液体免疫质控水平1 液体免疫质控水平2 液体免疫质控水平3 三水平液体免疫质控 抗“O ”校准品 载脂蛋白A1&B校准品 脂蛋白(a)校准品 脂蛋白(a)质控品 载脂蛋白校准品2 92项液体生化质控水平3 103项液体生化质控水平3 103项液体生化质控水平3 103项液体生化质控水平1 103项液体生化质控水平1 92项液体生化质控水平2 103项液体生化质控水平2 103项液体生化质控水平2 尿微量白蛋白质控水平1&2 尿微量白蛋白校准品 多种分析物定值质控水平1 多种分析物定值质控水平2
液体心肌质控水平2 液体心肌质控水平3 高敏肌钙蛋白T质控品 胱抑素C校准品 胱抑素C正常值质控 胱抑素C高值质控 大麻素校准品 迷幻药校准品 EDDP校准品 乙醇校准/质控品 多药物校准 多药物质控水平1 多药物质控水平2 EDDP质控水平1 EDDP质控水平2 迷幻药质控水平1 迷幻药质控水平2 大麻素质控水平1 大麻素质控水平2 苯并二氮校准品 苯并二氮质控水平1 苯并二氮质控水平2 氨/乙醇定值质控水平1 氨/乙醇定值质控水平2 氨/乙醇定值质控水平3 H-FABP校准品 H-FABP质控水平1 H-FABP质控水平2 糖化血清蛋白校准品 糖化血清蛋白质控水平1 糖化血清蛋白质控水平3 谷氨酰胺定值校准品 谷氨酰胺定值质控水平1 谷氨酰胺定值质控水平2
朗道2530-746UN校准血清靶值

CALIBRATION SERUM LEVEL 2 (CAL 2)20 x 5mlCAT. NO. CAL 2350 SIZE:2014-01LOT NO. 746UN EXP:INTENDED USEFor use as a Calibrator in clinical chemistry assays. RANDOX Calibration Sera are based on lyophilised human serum. The concentrations and activities are suitable for calibration of clinical chemistry assays on a wide range of automatic analysers. Constituent concentrations are available at 2 levels.SAFETY PRECAUTIONS AND WARNINGSHuman source material from which this product has been derived has been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), and Hepatitis C Virus (HCV) antibody and found to be NON-REACTIVE. FDA approved methods have been used to conduct these tests. However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samples should be handled as though capable of transmitting infectious diseases and disposed of accordingly.For IN VITRO diagnostic use only.STORAGE AND STABILITYUnreconstituted serum is stable up to the expiry date shown on the side of each individual bottle. Once reconstituted, the components of the Calibration Sera are stable for 8 hours at +25°C, 7 days at +2°C to +8°C and 1 month at -20°C when frozen once (see limitations).PREPARATION FOR USESerum must only be reconstituted using the following procedure:1. Open the vial carefully, avoiding any loss of material.2. Reconstitute by pipetting exactly 5ml of distilled water at +20°C to +25°C, into the vial.3. Replace the rubber stopper and leave to stand for 30 minutes out of bright light before use.4. Swirl gently several times during the reconstitution period to ensure that the contents are completely dissolved.5. Prior to use, mix the contents by inverting the vial. Do not shake the vial as the formation of foam should be avoided. Ensure that no lyophilised material remains unreconstituted.6. The serum is then ready for use with either a manual test or with an automated instrument.MATERIALS PROVIDEDCalibration Serum - Level 2Cat No. CAL2350 20 x 5mlMATERIALS REQUIRED BUT NOT PROVIDEDCalibrated Pipette, double deionised water.LIMITATIONSAfter reconstitution, Bicarbonate is stable for 8 hours in the closed bottle and 1 hour in the open bottle.For Total and Prostatic Acid Phosphatase, the material should be stabilised by adding 1 drop (25H l - 30H l) of 0.7M Acetic acid solution to 1ml of the serum exactly 30 minutes after reconstitution. After stabilisation, Total & Prostatic Acid Phosphatase are stable for 2 hours at +25°C, 2 days at +2°C to +8°C and 1 month when frozen once at -20°C. Alkaline Phosphatase levels in the reconstituted serum will rise over the stability period. It is recommended that the reconstituted serum be allowed to stand for 1 hour at +25°C before measurement.Bilirubin in the serum is light sensitive and it is recommended that the serum be stored in the dark. Stored in the dark it is stable for 1 day at +2°C to +8°C. Do not store at +15°C to +25°C. Do not freeze.Bacterial contamination of the reconstituted serum will cause reductions in the stability of many components. Different lot numbers of this calibrator should not be interchanged as the values assigned to the calibrators vary from lot to lot.LOT NO. 746UNVALUE ASSIGNMENTEach batch of serum is distributed to approximately 3000 laboratories worldwide and values are assigned by a consensusof results obtained by these laboratories. The Calibration values for each instrument have been determined in at least 10 independent laboratories. Values are verified against a master lot of calibrator which is traceable to reference methods or reference materials. In some cases values may be assigned at Randox Laboratories in comparison to a master lot ofcalibrator which is traceable to reference methods or reference materials.If an instrument specific value is not available, refer to the Mean of all Instruments section. If necessary contact Randox Laboratories - Technical Support, Northern Ireland, tel: (028) 9442 2413 or email Technical.Support@NOTES ® All trademarks recognised.(1) Values established by reference laboratories officially recognised by the Federal Chamber of Physicians inGermany.(2) DGKC : German Society for Clinical Chemistry(3) IFCC : International Federation of Clinical Chemistry(4) SCE : Scandinavian Committee on Enzymes14 Nov ’11 nePage 1 of 3Page 2 of 3Page 3 of 3Page 1 of 2Page 2 of 2Page 1 of 1Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 1Page 1 of 1Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 4Page 2 of 4Page 3 of 4Page 4 of 4Page 1 of 2Page 2 of 2Page 1 of 2Page 2 of 2Page 1 of 7Page 2 of 7Page 3 of 7Page 4 of 7Page 5 of 7Page 6 of 7Page 7 of 7Page 1 of 1Page 1 of 1Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 2Page 2 of 2Page 1 of 3Page 2 of 3Page 3 of 3Page 1 of 1Page 1 of 1Page 1 of 2Page 2 of 2Page 1 of 2Page 2 of 2。
03小试验证实验记录
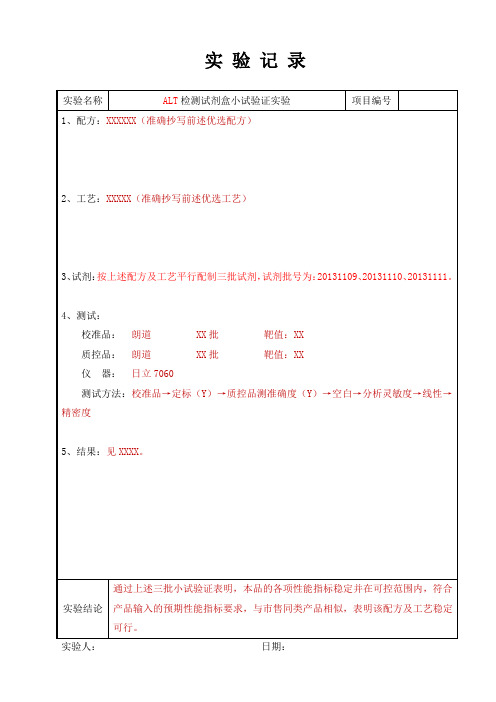
实验记录
实验名称ALT检测试剂盒小试验证实验项目编号
1、配方:XXXXXX(准确抄写前述优选配方)
2、工艺:XXXXX(准确抄写前述优选工艺)
3、试剂:按上述配方及工艺平行配制三批试剂,试剂批号为:20131109、20131110、20131111。
4、测试:
校准品:朗道 XX批靶值:XX
质控品:朗道 XX批靶值:XX
仪器:日立7060
测试方法:校准品→定标(Y)→质控品测准确度(Y)→空白→分析灵敏度→线性→精密度
5、结果:见XXXX。
实验结论通过上述三批小试验证表明,本品的各项性能指标稳定并在可控范围内,符合产品输入的预期性能指标要求,与市售同类产品相似,表明该配方及工艺稳定可行。
实验人:日期:。
朗道多项生化定值质控

0843 LIQUID ASSAYED CHEMISTRY CONTROL PREMIUM PLUS - LEVEL 2 (LIQ CHEM ASY PREMIUM PLUS 2) Cat. No. LAN4214Lot No.833UNSize: 12 x 5 ml Expiry: 2015-03INTENDED USEThis product is intended for in vitro diagnostic use, in the quality control of diagnostic assays. The Liquid Assayed Chemistry Control Premium Plus is for the control of accuracy.DEVICE DESCRIPTIONThe Liquid Assayed Chemistry Control Premium Plus is supplied at 3 levels, level 1, 2 and 3. Target values and ranges are supplied for the analytes listed in the values section at all three levels.SAFETY PRECAUTIONS AND WARNINGSFor in vitro diagnostic use only. Do not pipette by mouth. Exercise the normal precautions required for handling laboratory reagents.Human source material from which this product has been derived has been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), and Hepatitis C Virus (HCV) antibody and found to be NON-REACTIVE. FDA approved methods have been used to conduct these tests.However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samples should be handled as though capable of transmitting infectious diseases and disposed of accordingly.Health and Safety Data Sheets are available on request.STORAGE AND STABILITYOPENED: Store refrigerated (+2°C to + 8°C). Thawed serum is stable for 7 days at +2°C to +8°C, with the following exceptions: Troponin T is stable for 3 days at +2°C to +8°C. Only the required amount of product should beremoved. After use, any residual product should NOT BE RETURNED to the original vial.UNOPENED: Store frozen at -20°C to -70°C. Stable to expiration date printed on individual vials (see Limitations). LIMITATIONSFor Total Acid Phosphatase, the material should be stabilised by adding 1 drop (25 µl - 30 µl) of 0.7M Acetic acid solution to 1 ml of the serum after thawing. After stabilisation, Total Acid Phosphatase is stable for 7 days at +2°C to +8°C.Bilirubin in the serum is light sensitive and it is recommended that the serum is stored in the dark.ALT, Total Acid Phosphatase, Alkaline Phosphatase, Total and Direct Bilirubin values may gradually decrease during the products shelf life.Bacterial contamination of the thawed serum will cause reductions in the stability of many components.The control should not be used as a calibration material.PREPARATION1. Allow the frozen control to thaw at room temperature (+15°C to +25°C) until completely thawed. Swirl the contents to ensurehomogeneity.2. Refer to the Control section of the individual analyser application.3. Refrigerate any unused material. Prior to reuse, mix contents thoroughly.MATERIALS PROVIDEDLiquid Assayed Chemistry Control Premium Plus - Level 2 12 x 5 mlMATERIALS REQUIRED BUT NOT PROVIDEDNoneASSIGNED VALUESEach lot of serum is submitted to a number of external laboratories. Values are assigned from a consensus of results obtained by these laboratories and internal testing conducted at Randox Laboratories Ltd. With each batch, a control range is provided for individual parameters and each parameter method.If an instrument specific value is not available, refer to the Mean of all Instruments section. If necessary, contact Randox Laboratories – Customer Technical Services, Northern Ireland, Tel: +44 (0) 28 9445 1070 or email Technical.Services@Rev. 20 Nov ’13 nePAGE 1 OF 2Page 2 of 2420/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 3 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 4 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 5 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 6 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 7 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 8 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel:+44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 9 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 10 of 24 20/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 11 of 24 20/11/2013___________________________________________________________________________________________________Page 12 of 24 20/11/2013___________________________________________________________________________________________________Page 13 of 24 20/11/2013___________________________________________________________________________________________________Page 14 of 24 20/11/2013___________________________________________________________________________________________________Page 15 of 24 20/11/2013___________________________________________________________________________________________________Page 16 of 24 20/11/2013___________________________________________________________________________________________________Page 17 of 24 20/11/2013___________________________________________________________________________________________________Page 18 of 24 20/11/2013___________________________________________________________________________________________________Page 19 of 24 20/11/2013___________________________________________________________________________________________________Page 20 of 24 20/11/2013___________________________________________________________________________________________________Page 21 of 24 20/11/2013___________________________________________________________________________________________________Page 22 of 24 20/11/2013___________________________________________________________________________________________________Page 23 of 2420/11/2013___________________________________________________________________________________________________Page 24 of 2420/11/2013___________________________________________________________________________________________________。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
朗道混合生化校准品
1 控制品用途
RANDOX 校准品用于临床检验分析系统的校准。
2 控制品说明
RANDOX提供两种水平的校准。
分为二水平和三水平,均为冻干粉。
3 控制品储存
不论是否打开控制品,都必须储存在2~8℃。
4 控制品稳定性
4.1 未开瓶:在 2~8℃避光、密封的储存条件下可稳定至失效期。
4.2 开瓶:该校准复溶后(未污染、原瓶内、加盖条件下),在 15℃~25℃环境下至少可以稳定 8 小时,在 2℃~8℃环境下至少可以稳定 7 天,在-18℃~-24℃环境下(仅冻融一次)至少可以稳定 28 天(参阅“产品的局限性”说明)。
5 控制品注意事项
仅用于体外诊断用途。
不要用嘴吸取移液器。
采取处理实验室试剂要求的常规预防措施。
可索要健康和安全数据表。
该质控品采用人基质,对所有捐献者的血清均进行了艾滋 HIV(HIV1、HIV2)抗体、乙型肝炎表面抗原(HbsAg)和丙型肝炎病毒(HCV)抗体的测试,发现均呈阴性。
测试所采用的方法均通过美国 FDA 认证。
然而,目前没有一种方法能够完全确保该血清无传染物质,因此质控品与所有的病人样品均应当按照可能传播疾病的样品小心处理。
6 控制品准备
本控制品为干粉。
使用蒸馏水后复溶后使用。
7 控制品使用方法:
7.1 小心打开瓶盖,避免固体内容物的任何损失。
7.2在 20℃~25℃的室温下,用精密量具准确量取瓶签所标毫升数的蒸馏水复溶 1 瓶血清。
7.3盖上橡皮塞,拧紧瓶盖。
7.4轻轻旋转小瓶,确保内容物完全溶解。
7.5将小瓶倒置,确保所有的冻干物完全溶解。
7.6为避免产生气泡,勿摇晃小瓶,使用前避光放置 30 分钟。
取出的血清不应放回原始瓶中,避免交叉污染。
8 定值和范围
每批生产的校准品将提交给若干第三方实验室,由这些实验室得到可靠的结果后完成赋值。
