[Nd(C_7H_5O_2)_2(C_4H_6SO_2N)]·2H_2O的合成及体外抗肿瘤活性的初步研究
配位化合物(习题参考答案)
由(2)、(3)计算结果看出,AgCl 能溶于稀 NH3·H2O,而 AgBr 须用浓 NH3·H2O 溶解。 12.解: (1)[HgCl4]2− + 4 I−
K =
ψ
K fψ ([HgI 4 ] 2 − ) K fψ ([HgCl 4 ] )
= 5.78 ×10 14
K ψ 很大,故反应向右进行。
y = 0.49
可见 KCN 可溶解较多的 AgI。 10.解:设 1.0 L 1.0 mol·L−1 氨水可溶解 x mol AgBr,并设溶解达平衡时 c([Ag(NH3)2]+) = x mol·L−1(严格讲应略小于 x mol·L−1)c(Br− ) = x mol·L−1 AgBr(s) + 2NH3·H2O [Ag(NH3)2]+ + Br− + 2H2O 平衡浓度/(mol·L−1) 6.0 − 2 x x x
(2)[Cu(CN)2]− + 2NH3·H2O [Cu(NH3)2]+ + 2CN− + 2H2O
−
Kψ =
K fψ ([Cu(NH 3 ) 2 ] + ) K fψ ([Cu(CN) 2 ] )
= 7.24×10−14
(3)[Fe(NCS)2]+ + 6F−
[FeF6]3− + 2SCN−
+
Kψ =
PDF 文件使用 "pdfFactory" 试用版本创建
[CoF6]3-
[Ru(CN)6]4-
[Co(NCS)4]2―
6.解:已知:[MnBr4]2―μ=5.9 B.M,[Mn(CN)6]3―μ=2.8 B.M。 由: µ= n(n+2) 式求得:
无机及分析化学答案全(南京大学)(第四版)-第八章

无机及分析化学答案全(南京大学)(第四版)-第八章第8章习题答案1.命名下列配合物:(1)K2[Ni(CN)4] (2)(NH4)2[FeCl5(H2O)](3)[Ir(ONO)(NH3)5]Cl2 (4)Na2[Cr(CO)5]解:(1)四氰根合镍(Ⅱ)酸钾(2)五氯?一水合铁(III)酸铵(3)二氯化亚硝酸根?五氨合铱(III)(4)五羰基合铬(-Ⅱ)酸钠(参考P172)2.写出下列配合物(配离子)的化学式?(1)硫酸四氨合铜(Ⅱ) (2)四硫氰?二氨合铬(III)酸铵(3)二羟基?四水合铝(III)离子(4)二苯合铬(0)解:(1)[Cu(NH3)4]SO4 (2)(NH4)[Cr(NH3)2(SCN)4](3)[Al(H2O)4(OH-)2]+ (4)[Cr(C6H6)2]6.试用价键理论说明下列配离子的键型(内轨型或外轨型)、几何构型和磁性大小。
(1)[Co(NH3)6]2+ (2)[Co(CN)6]3-解:(1)Co最外层价电子排布为:27Co:3d74s2Co2+的最外层价电子排布为:27Co2+:3d74s0[ ][ ][ ][ ][ ] [ ] [ ][ ][ ] [ ][ ][ ][ ][ ]3d7 4S0 4P0 4d0 [ ][ ][ ][ ][ ] [ ][ ][ ][ ][ ][ ] [ ][ ][ ]SP3d2杂化、成键,形成[Co(NH3)6]2+因为:形成[Co(NH3)6]2+时用的是Co2+最外层4S、4P、4d 空轨道以SP3d2杂化、成键,而且中心离子Co2+形成配离子的前后单电子数没变,所以:该[Co(NH3)6]2+配合离子是外轨型,SP3d2杂化,几何构型为正八面体。
因为:以SP3d2杂化、成键形成[Co(NH3)6]2+后,具有3个单电子,所以:[Co(NH3)6]2+的磁矩为:,因为具有单电子分子是顺磁性分子,无单电子分子是抗磁性分子,所以形成[Co(NH3)6]2+后,具有3个单电子,[Co(NH3)6]2+是顺磁性分子。
分子生物学课后习题答案

第一章第一章 绪论绪论o DNA 重组技术和基因工程技术。
DNA 重组技术又称基因工程技术,目的是将不同DNA 片段(基因或基因的一部分)按照人们的设计定向连接起来,在特定的受体细胞中与载体同时复制并得到表达,产生影响受体细胞的新的遗传性状。
细胞的新的遗传性状。
DNA 重组技术是核酸化学、蛋白质化学、酶工程及微生物学、遗传学、细胞学长期深入研究的结晶,究的结晶,而限制性内切酶而限制性内切酶DNA 连接酶及其他工具酶的发现与应用则是这一技术得以建立的关键。
的关键。
DNA 重组技术有着广泛的应用前景。
重组技术有着广泛的应用前景。
首先,首先,DNA 重组技术可以用于大量生产某些在正常细胞代谢中产量很低的多肽,如激素、抗生素、酶类及抗体,提高产量,降低成本。
其次,DNA 重组技术可以用于定向改造某些生物的基因结构,使他们所具有的特殊经济价值或功能成百上千倍的提高。
能成百上千倍的提高。
o 请简述现代分子生物学的研究内容。
1、DNA 重组技术(基因工程)2、基因表达调控(核酸生物学)3、生物大分子结构功能(结构分子生物学)4、基因组、功能基因组与生物信息学研究、基因组、功能基因组与生物信息学研究第二章第二章 遗传的物质基础及基因与基因组结构遗传的物质基础及基因与基因组结构o 核小体、DNA 的半保留复制、转座子。
核小体是染色质的基本结构单位。
是由H2A 、H2B 、H3、H4各两分子生成八聚体和由大约200bp 的DNA 构成的。
核小体的形成是染色体中DNA 压缩的第一步。
压缩的第一步。
DNA 在复制过程中,每条链分别作为模板合成新链,产生互补的两条链。
这样新形成的两个DNA 分子与原来DNA 分子的碱基顺序完全一样。
因此,每个子代分子的一条链来自亲代DNA ,另一条链则是新合成的,这种复制方式被称为DNA 的半保留复制。
转座子是存在染色体DNA 上的可自主复制和移位的基本单位。
转座子分为两大类:插入序列和复合型转座子。
CH7WOANS

Chapter 7. Nucleic Acid1. Definition(1). Cyclic nucleotides(2). Chargaff’s Rule(3). Double Helix(4). B- form DNA and Z-form DNA(5). 5’-Cap of mRNA(6). Denaturation and renaturation(7). Tm2. Mono-choice questions(1) The compound that consists of ribose linked by an N-glycosidic bond to N-9 of adenine is:A. a deoxyribonucleoside.B. a purine nucleotide.C. a pyrimidine nucleotide.D.adenosine monophosphate.E.adenosine.(2) A major component of RNA but NOT of DNA is:A. adenine.B.cytosine.C.guanine.D.thymine.E.uracil.(3) The difference between a ribonucleotide and a deoxyribonucleotide is:A. a deoxyribonucleotide has an —H instead of an —OH at C-2.B. a deoxyribonucleotide has α configuration; ribonucleotide has the β configuration atC-1.C. a ribonucleotide has an extra —OH at C-4.D. a ribonucleotide has more structural flexibility than deoxyribonucleotide.E. a ribonucleotide is a pyranose, deoxyribonucleotide is a furanose.(4) The phosphodiester bonds that link adjacent nucleotides in both RNA and DNA:A.always link A with T and G with C.B.are susceptible to alkaline hydrolysis.C.are uncharged at neutral pH.D.form between the planar rings of adjacent bases.E.join the 3' hydroxyl of one nucleotide to the 5' hydroxyl of the next.(5) The DNA oligonucleotide abbreviated pATCGAC:A.has 7 phosphate groups.B.has a hydroxyl at its 3' end.C.has a phosphate on its 3' end.D.has an A at its 3' end.E.violates Chargaff's rules.(6) The experiment of Avery in which nonvirulent bacteria were made virulent by transformation was significant because it showed that:A.bacteria can undergo transformation.B.genes are composed of DNA only.C.mice are more susceptible to pneumonia than are humans.D.pneumonia can be cured by transformation.E.virulence is determined genetically.(7) Chargaff's rules state that in typical DNA:A. A = G.B. A =C.C. A = U.D. A + T = G + C.E. A + G = T + C.(8) Based on Chargaff's rules, which of the following are possible base compositions for double-stranded DNA?%A %G %C%T %UA. 5 45 45 5 0B. 20 20 20 20 20C. 35 15 35 15 0D.All of the above.E.None of the above.(9) In the Watson-Crick model of DNA structure:A.both strands run in the same direction, 3' 5'; they are parallel.B.phosphate groups project toward the middle of the helix, where they are protectedfrom interaction with water.C.T can form three hydrogen bonds with either G or C in the opposite strand.D.the distance between the sugar backbone of the two strands is just large enough toaccommodate either two purines or two pyrimidines.E.the distance between two adjacent bases in one strand is about 3.4 Å.(10) Which of the following is NOT true of all naturally occurring DNA?A.Deoxyribose units are connected by 3',5'-phosphodiester bonds.B.The amount of A always equals the amount of T.C.The ratio A+T/G+C is constant for all natural DNAs.D.The two complementary strands are antiparallel.E.Two hydrogen bonds form between A and T.(11) In the Watson-Crick model of DNA structure (now called B-form DNA):A. a purine in one strand always hydrogen bonds with a purine in the other strand.B.A–T pairs share three hydrogen bonds.C.G–C pairs share two hydrogen bonds.D.the 5' ends of both strands are at one end of the helix.E.the bases occupy the interior of the helix.(12) The double helix of DNA in the B-form is stabilized by:A.covalent bonds between the 3' end of one strand and the 5' end of the other.B.hydrogen bonding between the phosphate groups of two side-by-side strands.C.hydrogen bonds between the riboses of each strand.D.nonspecific base-stacking interaction between two adjacent bases in the same strand.E.ribose interactions with the planar base pairs.(13) B-form DNA in vivo is a ________-handed helix, _____ Å in diameter, with a rise of ____ Å per base pair.A.left; 20; 3.9B.right; 18; 3.4C.right; 18; 3.6D.right; 20; 3.4E.right; 23; 2.6(14) In double-stranded DNA:A.only a right-handed helix is possible.B.sequences rich in A–T base pairs are denatured less readily than those rich in G–Cpairs.C.the sequence of bases has no effect on the overall structure.D.the two strands are parallel.E.the two strands have complementary sequences.(15) Which of the following is a palindromic sequence?A.AGGTCCTCCAGGTTCCGCAAGGC.GAATCCCTTAGGD.GGATCCCCTAGGE.GTA TCCCATAGG(16) Which of the following are possible base compositions for single-stranded RNA?%A %G %C%T%UA. 5 45 45 0 5B. 25 25 25 0 25C. 35 10 30 0 25D.All of the above.E.None of the above.(17) Double-stranded regions of RNA:A.are less stable than double-stranded regions of DNA.B.can be observed in the laboratory, but probably have no biological relevance.C.can form between two self-complementary regions of the same single strand of RNA.D.do not occur.E.have the two strands arranged in parallel (unlike those of DNA, which areantiparallel).(18) When double-stranded DNA is heated at neutral pH, which change does not occur?A.The absorption of ultraviolet (260 nm) light increases.B.The covalent N-glycosidic bond between the base and the pentose breaks.C.The helical structure unwinds.D.The hydrogen bonds between A and T break.E.The viscosity of the solution decreases.(19) Which of the following deoxyoligonucleotides will hybridize with a DNA containing the sequence 5'AGACTGGTC3' ?A.5'CTCA TTGAG3'B.5'GACCAGTCT3'C.5'GAGTCAACT3'D.5'TCTGACCAG3'E.5'TCTGGA TCT3'(20) The ribonucleotide polymer 5'GTGATCAAGC3' could only form a double-stranded structure with:A.5'CACTAGTTCG3'.B.5'CACUAGUUCG3'.C.5'CACUTTCGCCC3'.D.5'GCTTGA TCAC3'.E.5'GCCTAGTTUG3'.(21) In the laboratory, several factors are known to cause alteration of the chemical structure of DNA. The factor(s) likely to be important in a living cell is (are):A.heat.B.low pH.C.oxygen.D.UV light.E.both C and D.(22) In living cells, nucleotides and their derivatives can serve as:A.carriers of metabolic energy.B.enzyme cofactors.C.intracellular signals.D.precursors for nucleic acid synthesis.E.all of the above.(23) ATP is NOT a nucleoside because it ________.A. has phosphate groupsB. has three phosphates instead of just oneC. lacks the deoxyribosyl groupD. is not connect to a carbohydrate group(24) According to Chargaff’s observations of nucleotide composition of DNA samplesA.% of (G + C) + % of (A + T) = 100%.B.A = T.C.G = C.D.%A + %G + %C + %T = 100%.E.All of the above(25) The rise and pitch of B-DNA are 0.33 nm and 3.40 nm, respectively. About how many helical urns are there in a fragment 1 mm in length?A. 3030B. 294C. 330D. 0.0034E. Cannot calculate from the information given.(26) Regions of DNA that are most easily unwound haveA. about half G and half C.B. alternating A and G.C. greater G:C content.D. greater A:T content.(27) Which is NOT true of the different conformations of DNA?A. Z-DNA is a left-handed spiral.B. A-DNA and B-DNA are right-handed spirals.C. A-DNA and Z-DNA segments are limited to short regions of DNA.D. Both A-DNA and B-DNA are dehydrated.(28) In addition to knowing the chemical structures of the nucleotides, Watson and Crick used________ of Franklin and Wilkins and the chemical equivalencies of Chargaff in order to propose their model of DNA structure.A. sequence informationB. UV spectraC. % (G + C) and % (A + T)D. X-ray diffraction data(29) In proteins, amino acids are linked by peptide bonds; in polynucleotides, nucleotides are linked byA. phosphoanhydride bonds.B. 3’-5’phosphodiester bonds.C. 5’-3’phosphodiester bonds.D. B and CE. All of the above(30) It is easier to melt DNA richer in AT than GC becauseA. it is more heat sensitive.B. there is one less hydrogen bond.C. the helix pitch is longer in AT rich regions.D. All of the above(31) As B-DNA is gradually heated, the absorbance at 260 nmA. increases.B. decreases.C. stays the same.D. is half way between that of poly (AT and poly (GC).(32) Which of the following is mismatched?A. rRNA: 80% of cellular RNAB. tRNA: carry amino acids during protein synthesisC. mRNA: stable RNA carrying the coded information from DNAD. small RNA: catalytic with or without proteins(33) Which type of RNA is the most abundant in living cells (by percent)?A. ribosomalB. messengerC. smallD. transfer(34) Which is NOT a difference between RNA and DNA?A. The sugar ring of RNA is more oxidized than that in DNA.B. RNA contains uracil; DNA usually does not.C. RNA cannot form helices.D. RNA is single-stranded; DNA is double-stranded.(35) How does a nucleotide differ from a nucleoside?A. Nucleosides are found in DNA, whereas nucleotides are found in RNA.B. Purines are only found in nucleotides.C. Nucleosides contain only deoxyribose sugars.D. A nucleotide is a nucleoside with a phosphate ester linked to the sugar .E. None of the above.(36) The feature(s) of DNA deduced by Watson and Crick includedA. two antiparallel polynucleotide chains coiled in a helix around a common axis.B. the pyrimidine and purine bases lie on the inside of the helix.C. the bases are nearly perpendicular to the axis.D. All of the above.E. None of the above.(37) The chemical forces that contribute to the stability of the DNA due to the base stacking present in the DNA helix areA. hydrogen bonds.B. van der Waals.C. disulfide bonds.D. B and C.E. None of the above.3. Short answer questions(1). A viral DNA is analyzed and found to have the following base composition, in mole percent: A = 32, G = 16, T = 40, C = 12.A. What can you immediately conclude about this DNA?B. What kind of secondary structure do you think it would have?(2). Give the following sequence for one strand of a double-strand oligonucleotide:5’ ACCGTAAGGCTTTAG 3’A. Write the sequence for the complementary DNA strand.B. Write the sequence of the RNA complementary to the strand shown above.(3). A stretch of double-stranded DNA contains 1000 bp, and its base composition is 58%(G+C). How many thymine residues are in this region of DNA?(4). Do the two complementary strands of a segment of DNA have the same base composition?Does (A+G) equal (C+T)?(5). In samples of DNA isolated from two unidentified species of bacteria, X and Y, adenine makes up 32% and 17%, respectively, of the total bases. What relative proportions of adenine, guanine, thymine, and cytosine would you expect to find in the two DNA samples? What assumptions have you made? One of these species was isolated from a hot spring (64℃). Suggest which species is the thermophilic bacterium. What is the basis for your answer?(6). Calculate the weight in grams of a double-helical DNA molecule stretching from the earth to the moon (~320,000 km). The DNA double helix weighs about 1 X 1018 g per 1,000 nucleotide pairs; each base pair extends 3.4 Å. For an interesting comparison, your body contains about 0.5 g of DNA!(7). Compare hydrogen bonding in the αhelix of proteins and in the double helix of DNA. Include the answer the role of hydrogen bonding in stabilizing these two structures.(8). Describe qualitatively how the t m for a double-stranded DNA depends upon its nucleotide composition.(9). Write the structure of cAMP and cGMP molecules.。
生物化学习题及答案(王镜岩编著版)

生物化学习题及答案(王镜岩编著版)第九章核酸的生物合成(一)名词解释1.半保留复制:双链DNA的复制方式,其中亲代链分离,每一子代DNA分子由一条亲代链和一条新合成的链组成。
2.不对称转录:转录通常只在DNA的任一条链上进行,这称为不对称转录。
3.逆转录:Temin和Baltimore各自发现在RNA肿瘤病毒中含有RNA指导的DNA聚合酶,才证明发生逆向转录,即以RNA为模板合成DNA。
4.冈崎片段:一组短的DNA片段,是在DNA复制的起始阶段产生的,随后又被连接酶连接形成较长的片段。
在大肠杆菌生长期间,将细胞短时间地暴露在氚标记的胸腺嘧啶中,就可证明冈崎片段的存在。
冈崎片段的发现为DNA复制的科恩伯格机理提供了依据。
5.复制叉:复制DNA分子的Y形区域。
在此区域发生链的分离及新链的合成。
6.领头链:DNA的双股链是反向平行的,一条链是5/→3/方向,另一条是3/→5/方向,上述的起点处合成的领头链,沿着亲代DNA 单链的3/→5/方向(亦即新合成的DNA沿5/→3/方向)不断延长。
所以领头链是连续的。
7.随后链:已知的DNA聚合酶不能催化DNA 链朝3/→5/方向延长,在两条亲代链起点的3/ 端一侧的DNA链复制是不连续的,而分为多个片段,每段是朝5/→3/方向进行,所以随后链是不连续的。
8.有意义链:即华森链,华森——克里格型DNA中,在体内被转录的那股DNA链。
简写为W strand。
9.光复活:将受紫外线照射而引起损伤的细菌用可见光照射,大部分损伤细胞可以恢复,这种可见光引起的修复过程就是光复活作用。
10.重组修复:这个过程是先进行复制,再进行修复,复制时,子代DNA链损伤的对应部位出现缺口,这可通过分子重组从完整的母链上,将一段相应的多核苷酸片段移至子链的缺口处,然后再合成一段多核昔酸键来填补母链的缺口,这个过程称为重组修复。
11.内含子:真核生物的mRNA前体中,除了贮存遗传序列外,还存在非编码序列,称为内含子。
1现代基础化学(第二版) 课后答案(第三版也可用) 化学工业出版社

《现代基础化学》答案化学工业出版社第一章,习题答案,P44-461. E = -B/n2 (B=2.18⨯ 10-18 J∆E = -B/52 + B/22 = 4.58 ⨯ 10-19 Jhv = ∆E, λν = cλ = c ⨯h ÷∆E = 434 nm3.①②不存在;③④存在4.Ni 3s2 3p6 3d8 4s2的四个量子数是:3s2: n= 3, l=0, m=0, m s = +1/2; n= 3 , l=0, m=0, m s = -1/23p6: n= 3, l=1, m=-1, m s = +1/2; n= 3, 1=1, m=-1, m s = -1/2; n= 3, 1=1, m=0, m s = +1/2 n= 3, 1=1, m=0, m s = -1/2; n= 3, 1=1, m=1, m s = +1/2; n= 3, 1=1, m=1, m s = -1/2 3d8: n= 3, 1=2, m=-2, m s = +1/2; n= 3, 1=2, m=-2, m s = -1/2; n= 3, 1=2, m=-1, m s = +1/2 n= 3, 1=2, m=-1, m s = -1/2; n= 3, 1=2, m=0, m s = +1/2; n= 3, 1=2, m=0, m s = -1/2n= 3, 1=2, m=1, m s = +1/2; n= 3, 1=2, m=2, m s = +1/24s2: n= 4, 1=0, m=0, m s = +1/2; n= 4, 1=0, m=0, m s = -1/25.①3d ②4f ③2s ④3d ⑤1s ⑥3p; 结果:⑤< ③< ⑥< ①= ④< ②6.①n=4;②l=1;③m=0;④m s=1/2或-1/27.8.①n>3;3d②l = 1;2p③m= -1 /0/+1; 3p④ m s = +1/2 或-1/2, 4s9.48Cd 4s24p64d105s2.10.①有三个19K;24Cr;29Cu②均第四周期, K: s区,,IA族;Cr: d区VIB族;Cu: ds区IB族11.(1) Ar: 1s22s22p63s23p6 (2)Fe: 1s22s22p63s23p64s23d6(3) I: 1s22s22p63s23p63d104s24p64d105s25p5 ;(4) Ag: 1s22s22p63s23p63d104s24p64d105s112.①(1)Ca: s区, 第四周期,IIA族;(2) Cl: p区,第三周期,VIIA;(3) Ti: d区,第四周期,IVB;(4) Hg: ds区, 第六周期, IIB族②Ca: +2,Cl: +7,Ti: +4,Hg: +2③(1)<(3)<(4)<(2)13.铁原子3d64s2;26Fe14.48;[Kr]4d105s2, IIB族;48Cd15.甲:3s23p5,VIIA,非金属,电负性高;乙:3d24s2,IVB,金属,电负性低。
大学化学(第二版)部分习题参考答案
2.303×8.314×10-3×1500
∴ Kө = 102.588 = 3.87×102
【P33: 第5题】解题思路
解: 查附录4可知:
CuO(s) +CO(g)=Cu(s) +CO2(g)
ΔfHmө(298.15K)/(kJ·mol-1) -157.3 -110.5 0 -393.5
Smө(298.15K)/(J·mol-1·K-1) 42.6 197.7 33.2 213.7
=
(1000/100)1 ·(1000/100)3
大学化学(第二=版)1部0分0习题参考
(PNH3/Pө)2
(1000/100)2
ΔrGm(T) = ΔrGmө(T) + 2.303RTlgQ = -21.63 + 2.303×8.314×10-3×573.15×lg(100) = 0.318(kJ·mol-1)
NH3·H2O与NH4Cl 组成弱碱-弱碱盐缓冲体系:
pOH = pKbӨ-lgc(cN(HNH3·H4+2)O)= -lg(KbӨ)-lg
0.033 0.033
=4.75
pH =14 –pOH =14 -4.75 =9.25
【P59:第4题】 在烧杯中盛放20.0cm3 0.100mol·dm-3氨
=-500.08 kJ·mol-1
大学化学(第二版)部分习题参考
△rSmө(298.15K) =∑viSmө(生成物) -∑viSmө(反应物) =[ 1×106.7] –[1×39.75 + 1×248.22 + 1/2×205.14]
= -283.84J·mol-1·K-1
ΔrGmө(T)≈ΔrHmө(298.15K)-T·ΔrSmө(298.15K)<0时自发进行。
教材习题解答
第一章 习题一、填空题1.稀溶液的依数性包括 蒸气压下降 , 沸点升高 , 凝固点降低 , 渗透现象 。
2.引起溶胶聚沉的诸多因素中,最重要的是 电解质的聚沉作用 。
3.在15℃和97 kPa 压力下,15 g 氮气所占有的体积为 13 升。
4.在20℃和97 kPa 压力下,0.842 g 某气体的体积是0.400 L ,这气体的摩尔质量是 52.89 g •mol -1 。
5.试比较下列溶液的沸点:0.1 mol •L -1蔗糖水溶液 < 0.1 mol •L -1NaCl 水溶液 < 0.1 mol •L -1Na 2SO 4水溶液。
6.试比较下列溶液的凝固点:0.1 mol •L -1蔗糖水溶液 = 0.1 mol •L -1甲醇水溶液 = 0.1 mol •L -1苯甲醇水溶液。
7.试比较下列溶液的渗透压:0.1 mol •L -1蔗糖水溶液 < 0.1 mol •L -1NaCl 水溶液 < 0.1 mol •L -1Na 2SO 4水溶液。
二、选择题1.下列溶液性质中哪一种不是依数性? ( D ) A. 凝固点 B. 沸点 C. 渗透压 D. 颜色2.在容易聚沉的溶胶中加入适量的大分子物质溶液,以使溶胶的稳定性大大增加,这叫做什么作用? ( B )A. 敏化作用B. 保护作用C. 加聚作用D. 聚沉作用3.等体积:0.1 mol •L -1KI 和:0.1 mol •L -1AgNO 3溶液混合制成的AgI 溶胶,下列电解质中,聚沉能力最强的是 ( C )A. Na 2SO 4B. MgSO 4C. FeCl 3D. K 3[Fe(CN)6]4.溶胶的基本特征之一是 ( D ) A. 热力学上和动力学上皆稳定的系统 B. 热力学上和动力学上皆不稳定的系统 C. 热力学上稳定而动力学上不稳定的系统 D. 热力学上不稳定和动力学上稳定的系统5.25℃时,0.01mol•kg -1的糖水的渗透压为∏1,而0.01mol•kg -1的尿素水溶液的渗透压为∏2,则 ( C )A. ∏1<∏2B. ∏1>∏2C. ∏1=∏2D. 无法确定6.当AgNO 3的稀溶液与KI 的稀溶液作用时,若 AgNO 3过量时,此溶胶( B ) A. 不带电 B. 带正电 C. 带负电 D. 无法确定7.加入下列哪一种溶液,能使As 2S 3胶体溶液凝聚最快 ( A ) A. Al 2(SO 4)2 B. CaCl 2 C. Na 3PO 4 D. MgCl 28.当不挥发性溶质溶于溶剂形成稀溶液后,则 ( A ) A. 溶剂蒸气压降低 B. 溶液的蒸气压升高C. 溶液的蒸气压不变D. 溶液的蒸气压可能升高也可能降低 三、是非题1.真实气体在低温高压下可以近似地看作理想气体。
第06章 配位反应 习题解答
解:C
第06章(02407)已知E (Hg2+/Hg)=0.857V,K ([Hg(CN)4]2-)=2.51041;
则E ([Hg(CN)4]2-/Hg)=()。
(A)0.37V;(B)0.37V;(C)1.59V;(D)1.59V。
(A)取代反应为:[MX4]2-+4Y- [MY4]2-+4X-;
(B)由于K ([MX4]2-)<K ([MY4]2-),所以该反应的K >1。
(C)当Y-的量足够时,反应必然向右进行。
(D)配离子的这种取代反应,实际应用中并不多见。
解:D
第06章(02400)已知E (Fe3+/Fe2+)=0.771V;[Fe(CN)6]3-和[Fe(CN)6]4-的K 分别为1.01042和
(A)K =K ·K ;(B)K =K /K ;
(C)K =K /K ;(D)K =1/(K ·K )。
解:A
第06章(02399)某金属离子M2+可以生成两种不同的配离子[MX4]2-和[MY4]2-,K ([MX4]2-)<K ([MY4]2-)。若在[MX4]2-溶液中加入含有Y-的试剂,可能发生某种取代反应。下列有关叙述中,错误的是()。
解:错
第06章(02371)在[Ni(NH3)6]2+溶液中加入乙二胺(en),将会有[Ni(en)3]2+生成。()
解:对
第06章(02372)在FeCl3溶液中先加入少量KCNS(s),再加入适量的NaF溶液,最终溶液呈血红色。()
解:错
第06章(02373)已知K ([HgCl4]2-)=1.171015,K ([HgI4]2-)=1.4810-30。
普通化学:第十章配位化合物课后答案
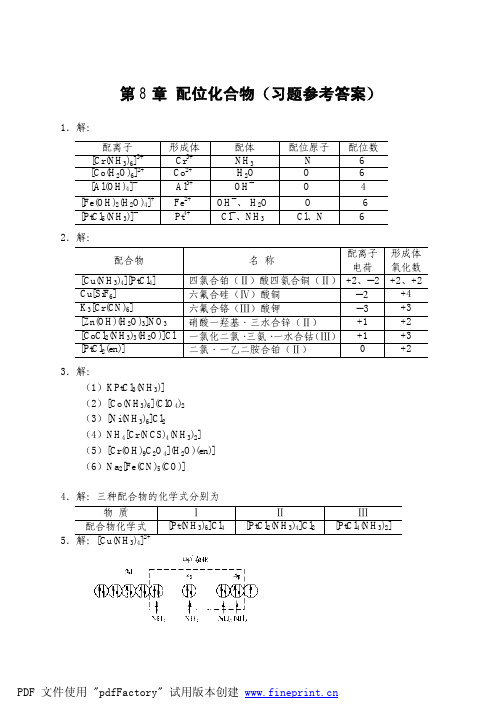
第十章配位化合物习题答案1.指出下列配离子的形成体、配体、配位原子及中心离子的配位数。
[Cr(NH3)6]3+Cr3+NH3N 6[Co(H2O)6]2+Co2+H2O O 6[Al(OH)4]-Al3+OH- O 4[Fe(OH)2(H2O)4]+Fe3+OH-H2O O 6[PtCl5(NH3)]-Pt4+Cl-NH3O, Cl 62.写出下列配合物的化学式:(1) 三氯·一氨合铂(II)酸钾K[PtCl3(NH3)](2) 高氯酸六氨合钴(II) [Co(NH3)6](ClO4)2(3) 二氯化六氨合镍(II) [Ni(NH3)6]Cl2(4) 四异硫氰酸根·二氨合铬(III)酸铵(NH4) [Cr(NCS)4(NH3)2](5) 一羟基·一草酸根·一水·一乙二胺合铬(III) [Cr(OH)(C2O4)(H2O)(en)](6) 五氰·一羰基合铁(II)酸钠Na3 [Fe(CN)5(CO)]3.命名下列配合物,并指出配离子的电荷数和形成体的氧化数。
配合物名称配离子电荷形成体的氧化数[Cu(NH3)4][PtCl4] 四氯合铂(II)酸四氨合铜(II) +2,-2 +2,+2 Cu[SiF6] 六氟合硅(IV)酸铜-2 +4K3[Cr(CN)6] 六氰合铬(III)酸钾-3 +3 [Zn(OH)(H2O)3]NO3硝酸一羟基·三水合锌(II) +1 +2 [CoCl2(NH3)3H2O)]Cl 一氯化二氯·三氨·一水合钴(III) +1 +34.有下列三种铂的配合物,用实验方法确定它们的结构,其结果如下:物质I II III化学组成PtCl4·6NH3PtCl4·4NH3PtCl4·2NH3溶液的导电性导电导电不导电可被AgNO3沉淀的Cl-数 4 2 不发生根据上述结果,写出上列三种配合物的化学式。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
文 章编号 10.8 32 1)605.5 324(010.9 00 0
[ (7 5 22 4 6ON) 2 2 NdCH 0 )( H S 2 ] H O的合成及体外抗肿 C . 瘤活性 的初步研究
黄熠 , 伟军 唐 ,王岐 本 ,程庆
(. 1 湘南学院化学与生命科学 系,湖 南郴0 4 3 0 ; . , 2 0 0 2湘南学院基础 医学部.湖南郴此本文 以硫代 脯氨 酸和苯 甲酸为 配体合 成 了一种新 的稀 土配合 物 NdC H O )(4 S 2 ・H O 为 (7 5 22 H6ON) 2 ,并对 该 C 2 配合物进行 了体外抗肿瘤活性实验, 结果表明其对人宫颈癌 H l细胞有较好的抑制作用. e a
1 实验
11 仪器 和试剂 .
收稿 B期 :2 1.93 0 1 .0 0 作者简介 : ̄ ( 6。 男, 1 5) 9 , 湖南郴州人, 副教授 , 研究方 向: 稀土配合物合 成与应 用
基金项 目:湖南省教育厅资助课题(6 74 0C 8) 郴州市科技 局资助项 1 [ 117 ・  ̄ 2 0 号) (0 6
第6 期
黄熠 等: d 752 (46 2) HO的 [ ( 10)cHS N1 2 合成及体外 N Ct 2 O . 2 抗肿瘤活性的初步 研究
91 5
测定, 稀土的含量用E T 滴定法测定I , DA J 结晶水的含量用差减法求得, GD A曲线佐证. 引 用T . T 结果表 明,所合
成的化合物的化学组成为I (7 5 2 (4 6O N ] H O Nd H O) C H S 2 ) 2 2 . C 2 ・
中 图分 类 号 : 9 4 R 7 . R l: 9 91
d i 1.99 .s.0 32 8 . 1 .1 0 o: 036 /i n10 —4 32 11. js 0 2
文献 标 识 码 :A
肿瘤是危害人类健康 的重大因素, 国恶性肿瘤的发病率及死亡率均呈上升趋势, 我 肿瘤的预 防和治疗任务 十分艰巨. 尽管 目 前抗肿瘤药物数不胜数, 但大多数存在着毒副作用大、 价格昂贵等缺点,因此开发更加高效低 毒廉 价 的抗肿 瘤药物 一直 是 国内外 的研究热 点 . 甲酸及 其钠 盐是 目前 国家允许 使 用并订有 国家标 准 的食 品防 苯 腐剂之一, 常用于食品、药剂和 日用品的防腐, 但对肝功能衰弱者不适宜l 且有研究显示, q ; 苯甲酸具有叠加毒 性作用 . J大量研究证实稀土配合物的生物活性较原配体会有不同程度的提高,而毒性作用降低【 稀土元素具 j j . 有抗炎杀菌和抗肿瘤活性 J利用其协 同作用有助于寻找更加高效、 , 低毒的抗菌、 消炎、 防腐和抗肿瘤新药 J . 硫代脯氨酸是一种抗癌药物¨ ¨, J对头颈部鳞状细胞癌疗效显著, 但对卵巢癌、 乳腺癌、 肾癌和甲状腺癌疗效一
第3 7卷第 6期
西南民族 大学学报 ・ 自然科 学版
J u n l f o t we t i e s y f r t n l isNa u a ce c d t n o r a uh s v ri o i a i e ・ t r l in e E i o o S Un t Na o t S i
摘 要:以硫代脯氨 酸和苯 甲酸为配体合成 了一种新 的稀土配合 物,用红外光谱 、热重差热分析 、元素分析和化 学分析 确定它的化学式为 NdC H5 ) C H6 2 ・H O. ( 7 02 2( 4 NO S) 2 2 体外试验表明对 H l e a细胞 的增殖有较好 的抑制作 用.
关键词:稀土配合物 ; 抗肿瘤活性; 硫代脯氨酸:苯甲酸
1 Nd C Hs ) C H6 S・H2 的合成 . 2 ( 7 O2 ( 4 NO2) 0 2 2
取 1m o d l H O配 成 3m 溶 液 ;取 2 m lCH C O 0 m l Cy 2 N 6 0l 0 m o 6 5O H与 2m o的N O 0 m l a H混 合溶 解 ,1 m l 0m o CHS 2 4 7ON与1 m o的N O 混合溶解. 0 m l aH 将上述两配体溶液混合加到三 E瓶中, 0 水浴中加热, l 在6  ̄ C 边搅拌, 边 滴加N C3 H O d l6 2 溶液. ・ 反应6 小时, 静置 1小时, 2 过滤. 先用无水 乙醇洗涤, 再用蒸馏水洗涤至用A N 3 g O 溶液检 查无C一 将得到的固态化合物, l , 于真空干燥器 中干燥至恒重. 化合物中的碳、 氢、 氮和硫元素含量用元素分析仪
F I一5 型傅立叶变换红外光谱仪; ai E 型元素分析仪; C -1 TR 60 V r Ll o I I H T 型微机热分析天平; 462 C 2 2 0— 型 O 孵 箱; J 7 Y 一 5型超 净工作 台; NM一62型酶 联免疫检 测仪 ;6孔细 胞培养板 . 8 D 90 9 CH C O , R 6 sO H A 试剂, 含量大于9 . 湖南湘中化学试剂厂; d l6 2 , R 9 %, 5 N Cy HO A 试剂, 含量大于9%, 9 天津市 北联精细化学品开发有限公司; 4 7O N B 试剂, CH S 2 , R 含量大于9 . 武汉远成科技有限公司; 9 %, 0 无水 乙醇, R A 试 剂, 上海南翔试剂有限公司; a H A 试剂, NO ,R 广东汕头陇西化工厂; ME 上海沪宇生物科技有限公司; D M, 胎牛 血 清,天津灏 洋 生物制 品科技有 限公 司; T( MT 噻唑蓝 ) ,上 海恒远 生物科 技有 限公 司;二 甲亚砜DMS O,上海诚 临化 工物 质有 限公 司分装 ( 国生产 );人宫颈癌 ( ea 法 H l细胞株 ),生研 ( 上海 )生化试剂 有 限公 司.
