溶解度表大全-无机盐-有机物
溶解度与物质的溶解特性

溶解度与物质的溶解特性物质的溶解是指溶质分子或离子在溶剂中逐渐分散和混合的过程,溶解度是指在特定条件下溶质能溶解在溶剂中的最大量。
溶解度和物质的溶解特性之间存在着密切的关系,物质的溶解特性直接影响其溶解度的大小和溶解过程的速率。
本文将从溶解度的概念、影响溶解度的因素以及不同物质的溶解特性等方面进行论述。
一、溶解度的概念溶解度是指在一定温度和压力下,在溶剂中能够溶解的物质的最大量。
通常以溶质在100克溶剂中溶解的质量来表示,单位为克/100克溶剂。
溶解度与冷却和浓缩过程有关,通常在饱和溶液的实验条件下确定。
二、影响溶解度的因素1. 温度:一般来说,溶解度随着温度的升高而增加。
这是因为在高温下,溶质分子或离子的热运动增强,与溶剂分子之间的相互作用减弱,使溶质更容易分散和溶解在溶剂中。
2. 压力:对于固体和液体溶质在液体溶剂中的溶解度,压力的变化对其溶解度影响较小。
但是对于气体溶质在液体溶剂中的溶解度,压力的升高会导致溶解度的增加,这与亨利定律有关。
3. 溶剂的性质:不同溶剂具有不同的溶解能力。
如极性溶剂对极性溶质有较好的溶解能力,而非极性溶剂对非极性溶质有较好的溶解能力。
4. 溶质的性质:溶质的分子或离子的大小、极性、电荷等性质对其溶解度有影响。
例如,小分子具有较大的溶解度,而大分子则溶解度较小;极性分子在极性溶剂中溶解度较高,而非极性分子在非极性溶剂中溶解度较高。
三、不同物质的溶解特性1. 无机盐的溶解特性:无机盐通常以离子的形式溶解在水中。
根据溶解度的大小,可以将无机盐分为可溶性盐和不溶性盐。
可溶性盐在水中能够完全溶解,形成电离的离子,而不溶性盐仅在水中溶解极少量。
2. 有机物的溶解特性:有机物通常是以分子的形式溶解在溶剂中。
有机物的溶解度主要受分子间相互作用力的影响。
极性有机物在极性溶剂中溶解度较高,而非极性有机物则在非极性溶剂中溶解度较高。
3. 气体的溶解特性:气体在液体中的溶解度受溶剂和气体压力的影响。
无机盐在超临界水中的溶解度研究

无机盐在超临界水中的溶解度研究闫正文;廖传华;廖玮;朱跃钊【摘要】采用连续式超临界反应器,考察温度对于无机盐在超临界水中的溶解度影响.结果表明,随着温度的升高,无机盐的溶解度逐渐降低;在相同温度下,氯盐的溶解度随阳离子离子半径及电荷数的增大而减小,即NaCl>KCl>CaCl2,硫酸盐的溶解度也呈现同一规律,即Na2SO4>K2SO4>CaSO4;在相同温度下,阳离子相同,盐的溶解度随阴离子离子半径和电荷数的增大而减小,即NaCl>Na2SO4,KCl>K2SO4,CaCl2>CaSO4;当温度从380 ℃升至400 ℃时,无机盐溶解度下降趋势最快,NaCl、KCl和CaCl2的溶解度区间分别为:213.72 ×10 -6~165.24 ×10 -6, 185.81×10 -6~143.64×10 -6,80.23×10 -6~7.26×10 -6,Na2SO4、K2SO4和CaSO4的溶解度区间分别为:85.72× 10 -6~14.36×10 -6,60.24×10 -6~5.12×10 -6,0.048×10 -6~0.012×10 -6.%The solubility of inorganic salts was researched by using continuous supercritical reactor,the impact of temperature on the solubility was investigated.The results showed that:With the increase of tem-perature,the solubility of inorganic salts was reduced;At the same temperature,with the increase of cation ion radius and the number of charge,the solubility of chlorine salts are decreased:NaCl>KCl>CaCl2, and the solubility of sulfates also show the same law:Na2SO4>K2SO4>CaSO4;At the same temperature, the inorganic salts have the same anion,with the increase of anion ion radius and the number of charge, the solubility of salts arereduced:NaCl>Na2SO4,KCl>K2SO4,CaCl2>CaSO4;When the temperature from 380℃ to 400℃,the decline of the solubility of inorganic salts isfastest,the interval of the solubility of NaCl,KCl and CaCl2:213.72×10 -6~165.24×10 -6,185.81×10 -6~143.64×10 -6,80.23×10 -6~7.26×10 -6,and the interval of the solubility of Na2SO4,K2SO4and CaSO4:85.72×10 -6~14.36× 10 -6,60.24×10 -6~5.12×10 -6,0.048×10 -6~0.012× 10 -6.【期刊名称】《应用化工》【年(卷),期】2018(047)003【总页数】3页(P514-516)【关键词】超临界水氧化;温度;无机盐;溶解度【作者】闫正文;廖传华;廖玮;朱跃钊【作者单位】南京工业大学机械与动力工程学院,江苏南京 210009;南京工业大学机械与动力工程学院,江苏南京 210009;南京工业大学机械与动力工程学院,江苏南京 210009;南京工业大学机械与动力工程学院,江苏南京 210009【正文语种】中文【中图分类】TQ01;TQ110超临界水的物理、化学性质均较常态下的水发生了显著的变化,既不同于液态水,也有别于气态水[1]。
药剂学药物溶解与溶出及释放

6. 温度的影响
• 温度对溶解度影响取决于溶解过程是吸热△Hs>0,还是
放热△Hs<0。当△Hs>0时,溶解度随温度升高而升 高;如果△Hs<0时,溶解度随温度升高而降低。
• 药物溶解过程中,溶解度与温度关系式(3-2)为:
lnS2/S1= △Hs/R(1/T1-1/T2)
式中: S1 、S2—分别在温度T1和T2下的溶解度;
2. 溶剂化作用和水合作用
• 药物离子的水合作用与离子性质有关,阳离子和 水之间的作用力很强,一般单价阳离子结合4个水 分子。
• 水合作用于离子大小、表面积也有关 • 药物的溶剂化会影响药物在溶剂中的溶解度。
3.多晶型的影响
• 晶型不同,导致晶格能不同,药物的熔点、溶解速度、 溶解度等也不同。
• 无定型的溶解度和溶解速度比结晶型的大。
乙醇胺
乌拉坦,尿素 菸酰胺,尿素,乙酰胺,苯甲酸钠,水杨酸 钠 , 磷 酸 酯 , PAS-Na,Vc-Na, 吡 嗪 酰 胺 , 四甲基尿素 ,乌拉坦 菸酰胺,乙酰胺,脲,PEG4000
安络血 氢化可的松
去氧甾醇 葡萄糖酸钙
氯霉素 四环素,土霉素
链霉素 红霉素 新霉素
安定
水杨酸钠,菸酰胺,乙酰胺 苯甲酸钠,邻、对、间羟苯甲酸钠,菸酰胺, 二乙胺 苯甲酸钠,邻、对、间羟苯甲酸钠
酰胺类化合物如乌拉坦尿素烟酰胺乙酰常见的难溶性药物与其应用的助溶剂药物助溶剂碘化钾聚乙烯吡咯烷酮pvp咖啡因苯甲酸钠枸橼酸钠水杨酸钠对氨基苯甲酸钠菸酰胺可可豆碱水杨酸钠苯甲酸钠菸酰胺茶碱二乙胺其他脂肪族胺菸酰胺苯甲酸钠芦丁乙醇胺盐酸奎宁乌拉坦尿素核黄素菸酰胺尿素乙酰胺苯甲酸钠水杨酸钠磷酸酯pasnavna吡嗪酰胺四甲基尿素乌拉坦对羟基苯甲酸甲水杨酸钠菸酰胺乙酰胺氢化可的松苯甲酸钠邻对间羟苯甲酸钠菸酰胺二乙胺苯甲酸钠邻对间羟苯甲酸钠葡萄糖酸钙乳酸钙糖酸钙枸橼酸钠nacl氯霉素nn二甲基甲酰胺nn二甲基乙酰胺琥珀酸钠四环素土霉素水杨酸钠对羟基苯甲酸钠菸酰胺链霉素蛋氨酸甘草酸红霉素乙酰琥珀酸酯v新霉素精氨酸安定水杨酸2加入增溶剂
单水氢氧化锂溶解度 化工手册

单水氢氧化锂溶解度化工手册全文共四篇示例,供读者参考第一篇示例:单水氢氧化锂是一种重要的无机化合物,广泛应用于锂电池、玻璃制造、陶瓷工业等领域。
在化工生产过程中,掌握单水氢氧化锂的溶解度是非常重要的,可以帮助我们合理设计生产工艺,提高生产效率。
本手册将介绍单水氢氧化锂的溶解度相关知识,帮助读者更好地了解该化合物在溶液中的行为特性。
一、单水氢氧化锂的基本性质单水氢氧化锂的化学式为LiOH,是一种白色固体,能溶解于水中,生成碱性溶液。
在常温下,单水氢氧化锂是吸湿性的,能吸收空气中的水分。
在高温下,它还会分解释放氧化锂和水蒸气。
单水氢氧化锂具有较强的腐蚀性,应注意避免与皮肤接触或吸入其蒸气。
单水氢氧化锂在水中的溶解度是指在一定温度下,单位质量的水中能溶解多少量的LiOH。
通常以克/升或摩尔/升来表示。
单水氢氧化锂的溶解度受温度、压力等因素的影响,一般来说,溶解度随温度的升高而增加。
单水氢氧化锂的溶解度与浓度的关系是一个动态平衡过程,随着溶液中LiOH浓度的增加,其溶解度会逐渐减小。
当达到溶解度极限时,称之为饱和状态。
此时溶液中LiOH颗粒和水中LiOH分子的生成速度相等,溶液处于动态平衡状态。
三、单水氢氧化锂的溶解度的影响因素1. 温度:温度是影响单水氢氧化锂溶解度的主要因素之一。
一般情况下,温度升高可以提高溶解度,因为高温有利于分子间的热运动,使得单水氢氧化锂更容易溶解。
2. 压力:压力对单水氢氧化锂的溶解度影响较小,一般情况下可以忽略不计。
3. pH值:单水氢氧化锂是一种碱性物质,因此溶液的pH值会影响其溶解度。
通常情况下,pH值越高,溶解度越大。
4. 其他溶质:当溶液中存在其他溶质时,会影响单水氢氧化锂的溶解度。
有些溶质会与Li+或OH-形成化合物,降低LiOH的溶解度。
1. 重量法:将一定量的LiOH加入一定量的水中,搅拌使其充分溶解,用天平测量其溶解后的总质量,据此计算溶解度。
2. 密度法:测定在不同温度下的LiOH溶液的密度,根据密度和溶液中LiOH的摩尔浓度计算溶解度。
不同温度下无机盐在水中的溶解度
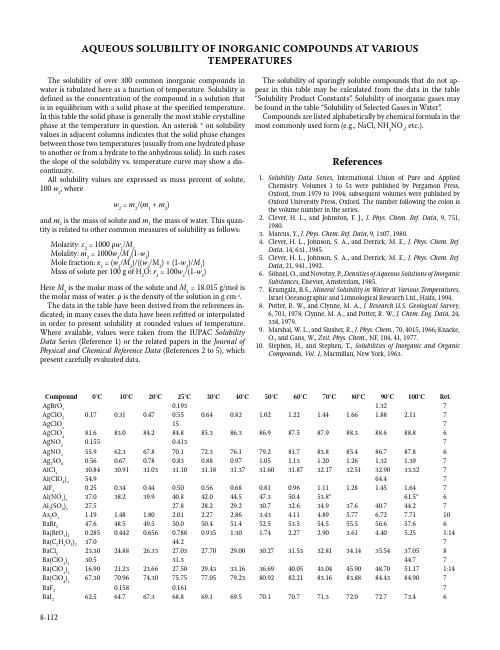
AQUEOUS SOLUBILITY OF INORGANIC COMPOUNDS AT VARIOUSTEMPERATURESThe solubility of over 300 common inorganic compounds in water is tabulated here as a function of temperature. Solubility is defined as the concentration of the compound in a solution that is in equilibrium with a solid phase at the specified temperature. In this table the solid phase is generally the most stable crystalline phase at the temperature in question. An asterisk * on solubility values in adjacent columns indicates that the solid phase changes between those two temperatures (usually from one hydrated phase to another or from a hydrate to the anhydrous solid). In such cases the slope of the solubility vs. temperature curve may show a dis-continuity.All solubility values are expressed as mass percent of solute, 100⋅w2, wherew2 = m2/(m1 + m2)and m2 is the mass of solute and m1 the mass of water. This quan-tity is related to other common measures of solubility as follows: Molarity: c2 = 1000 ρw2/M2Molality: m2 = 1000w2/M2(1-w2)Mole fraction: x2 = (w2/M2)/{(w2/M2) + (1-w2)/M1}Mass of solute per 100 g of H2O: r2 = 100w2/(1-w2)Here M2 is the molar mass of the solute and M1 = 18.015 g/mol is the molar mass of water. ρ is the density of the solution in g cm-3. The data in the table have been derived from the references in-dicated; in many cases the data have been refitted or interpolated in order to present solubility at rounded values of temperature. Where available, values were taken from the IUPAC Solubility Data Series (Reference 1) or the related papers in the Journal of Physical and Chemical Reference Data (References 2 to 5), which present carefully evaluated data.The solubility of sparingly soluble compounds that do not ap-pear in this table may be calculated from the data in the table “Solubility Product Constants”. Solubility of inorganic gases may be found in the table “Solubility of Selected Gases in Water”. Compounds are listed alphabetically by chemical formula in the most commonly used form (e.g., NaCl, NH4NO3, etc.).References1. Solubility Data Series, International Union of Pure and AppliedChemistry. Volumes 1 to 53 were published by Pergamon Press, Oxford, from 1979 to 1994; subsequent volumes were published by Oxford University Press, Oxford. The number following the colon is the volume number in the series.2. Clever, H. L., and Johnston, F. J., J. Phys. Chem. Ref. Data, 9, 751,1980.3. Marcus, Y., J. Phys. Chem. Ref. Data, 9, 1307, 1980.4. Clever, H. L., Johnson, S. A., and Derrick, M. E., J. Phys. Chem. Ref.Data, 14, 631, 1985.5. Clever, H. L., Johnson, S. A., and Derrick, M. E., J. Phys. Chem. Ref.Data, 21, 941, 1992.6. Söhnel, O., and Novotny, P., Densities of Aqueous Solutions of InorganicSubstances, Elsevier, Amsterdam, 1985.7. Krumgalz, B.S., Mineral Solubility in Water at Various Temperatures,Israel Oceanographic and Limnological Research Ltd., Haifa, 1994.8. Potter, R. W., and Clynne, M. A., J. Research U.S. Geological Survey,6, 701, 1978; Clynne, M. A., and Potter, R. W., J. Chem. Eng. Data, 24, 338, 1979.9. Marshal, W. L., and Slusher, R., J. Phys. Chem., 70, 4015, 1966; Knacke,O., and Gans, W., Zeit. Phys. Chem., NF, 104, 41, 1977.10. Stephen, H., and Stephen, T., Solubilities of Inorganic and OrganicCompounds, Vol. 1, Macmillan, New York, 1963.Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. AgBrO30.193 1.327 AgClO20.170.310.470.550.640.82 1.02 1.22 1.44 1.66 1.88 2.117 AgClO3157 AgClO481.683.084.284.885.386.386.987.587.988.388.688.86 AgNO20.1550.4137 AgNO355.962.367.870.172.376.179.281.783.885.486.787.86 Ag2SO40.560.670.780.830.880.97 1.05 1.13 1.20 1.26 1.32 1.397 AlCl330.8430.9131.0331.1031.1831.3731.6031.8732.1732.5132.9033.327 Al(ClO4)354.964.47 AlF30.250.340.440.500.560.680.810.96 1.11 1.28 1.45 1.647 Al(NO3)337.038.239.940.842.044.547.350.453.8*61.5*6 Al2(SO4)327.527.828.229.230.732.634.937.640.744.27 As2O3 1.19 1.48 1.80 2.01 2.27 2.86 3.43 4.11 4.89 5.77 6.727.7110 BaBr247.648.549.550.050.451.452.553.554.555.556.657.66 Ba(BrO3)20.2850.4420.6560.7880.935 1.30 1.74 2.27 2.90 3.61 4.40 5.251:14 Ba(C2H3O2)237.044.27 BaCl223.3024.8826.3327.0327.7029.0030.2731.5332.8134.1435.5437.058 Ba(ClO2)230.531.344.77 Ba(ClO3)216.9021.2323.6627.5029.4333.1636.6940.0543.0445.9048.7051.171:14 Ba(ClO4)267.3070.9674.3075.7577.0579.2380.9282.2183.1683.8884.4384.907 BaF20.1580.1617 BaI262.564.767.368.869.169.570.170.771.372.072.773.468-112Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. Ba(IO3)20.01820.02620.03420.03960.045*0.058*0.0730.0900.1090.1310.1560.1821:14 Ba(NO2)231.136.641.844.346.851.656.260.564.668.572.175.610 Ba(NO3)2 4.7 6.38.29.310.212.414.717.019.321.523.525.56 Ba(OH)2 1.67 4.688.4193352741007 BaS 2.79 4.78 6.978.219.5812.6716.1820.0524.1928.5533.0437.617 Ba(SCN)262.67 BaSO30.00111:26 BeCl240.541.77 Be(ClO4)259.57 BeSO426.6927.5828.6129.2229.9031.5133.3935.5037.7840.2142.7245.287 CaBr2555659616368717310 CaCl236.7039.1942.1344.83*49.12*52.85*56.05*56.7357.4458.2159.0459.948 Ca(ClO3)263.264.265.566.367.269.071.073.275.5*77.4*77.778.01:14 Ca(ClO4)265.37 CaF20.00130.001610 CaI264.666.067.668.369.070.872.474.076.078.079.681.07 Ca(IO3)20.0820.1550.2430.3050.384*0.517*0.5900.6520.811*0.665*0.6681:14 Ca(NO2)238.639.544.548.67 Ca(NO3)250.153.156.759.060.965.477.878.178.278.378.478.56 CaSO30.00590.00540.00490.00410.00350.00300.00260.00230.00200.00191:26 CaSO40.1740.1910.2020.2050.2080.2100.2070.2010.1930.1840.1730.1639 CdBr236.043.049.953.456.460.3*60.3*60.560.760.961.361.66 CdC2O40.00605 CdCl247.250.153.254.656.3*57.3*57.557.858.158.5158.9859.56 Cd(ClO4)258.766.97 CdF2 5.82 4.65 4.18 3.765 CdI244.144.945.846.346.847.949.050.251.552.754.155.46 Cd(IO3)20.0915 Cd(NO3)255.457.159.661.062.866.570.686.186.586.887.187.46 CdSO443.143.143.243.443.644.143.542.541.440.238.536.76 CdSeO442.0440.5939.0238.1837.2935.3533.1530.6527.8424.6921.2417.495 Ce(NO3)357.9959.8061.8963.0564.31*67.0*68.671.1*74.9*79.280.983.11:13 CoCl230.3032.6034.8735.9937.1039.2741.3843.4645.5047.5149.5151.507 Co(ClO4)250.053.07 CoF2 1.47 CoI258.0061.7865.3566.9968.5171.1773.4175.2976.8978.2879.5280.707 Co(NO2)20.0760.497 Co(NO3)245.547.049.450.852.456.060.162.664.967.76 CoSO419.923.026.127.729.232.334.435.935.533.230.627.86 Co(SCN)250.77 CrO362.262.362.662.863.063.564.164.765.566.267.167.96 CsBr55.27 CsBrO3 1.16 1.93 3.01 3.69 4.46 6.328.6011.3214.4517.9621.8325.981:30 CsCl61.8363.4864.9665.6466.2967.5068.6069.6170.5471.4072.2172.961:47 CsClO3 2.40 3.87 5.947.228.6912.1516.3321.1426.4532.1037.8943.421:30 CsClO40.79 1.01 1.51 1.96 2.57 4.28 6.559.2912.4115.8019.3923.077 CsI30.937.243.245.948.653.357.360.763.665.967.769.26 CsIO3 1.08 1.58 2.21 2.59 3.02 3.96 5.06 6.297.709.2010.7912.451:30 CsNO38.4613.018.621.825.132.039.045.751.957.362.166.26 CsOH757 Cs2SO462.663.464.164.564.865.566.166.767.367.868.368.86 CuBr255.87 CuCl240.841.742.643.143.744.846.047.248.549.951.352.76 Cu(ClO4)254.359.37 CuF20.0757 Cu(NO3)245.249.856.359.261.162.063.164.565.967.569.271.06 CuSO412.414.416.718.019.322.225.428.832.436.340.343.56 CuSeO410.616.07 Dy(NO3)358.7959.9961.4962.3563.2965.4368.0471.581:13 Er(NO3)361.5863.1564.8465.7566.6968.7070.9673.6477.751:13 Eu(NO3)355.256.758.559.460.462.564.61:13Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. FeBr254.664.8*7 FeCl233.2*39.4*48.7*7 FeCl342.744.947.947.751.674.876.784.684.384.384.484.76 Fe(ClO4)263.3967.767 FeF3 5.597 Fe(NO3)340.1546.577 Fe(NO3)241.4446.677 FeSO413.517.020.822.824.828.832.835.533.630.427.124.06 Gd(NO3)356.357.759.260.161.062.965.267.971.51:13 HIO373.4574.1074.9875.4876.0377.2078.4679.7881.1382.4883.8285.141:30 H3BO3 2.61 3.57 4.77 5.48 6.278.1010.312.915.919.323.127.36 HgBr20.260.370.520.610.720.96 1.26 1.63 2.08 2.61 3.23 3.954 Hg(CN)2 6.577.839.3310.211.113.115.518.221.224.628.332.36 HgCl2 4.24 5.05 6.17 6.817.629.5312.0215.1819.1624.0629.9036.624 HgI20.00410.00550.00720.01220.01994 Hg(SCN)20.0704 Hg2Cl20.00043 Hg2(ClO4)273.879.8*85.3*7 Hg2SO40.0380.0430.0480.0510.0540.0590.0650.0700.0760.0820.0880.0934 Ho(NO3)363.81:13 KBF40.280.340.450.550.75 1.38 2.09 2.82 3.58 4.34 5.12 5.9010 KBr35.037.339.440.441.443.244.846.247.648.849.850.86 KBrO3 2.97 4.48 6.427.558.7911.5714.7118.1421.7925.5729.4233.281:30 KC2H3O268.4070.2972.0972.9273.7075.0876.2777.3178.2279.0479.8080.557 KCl21.7423.6125.3926.2227.0428.5930.0431.4032.6633.8634.9936.051:47 KClO3 3.03 4.67 6.747.939.2112.0615.2618.7822.6526.8831.5336.651:30 KClO40.70 1.10 1.67 2.04 2.47 3.54 4.94 6.748.9911.7114.9418.676 KF30.9039.847.350.4153.260.07 KHCO318.6221.7324.9226.628.1331.3234.4637.5140.456 KHSO427.129.732.333.635.037.840.543.446.249.0251.8254.66 KH2PO411.7414.9118.2519.9721.7725.2828.9532.7636.7540.9645.4150.121:31 KI56.057.659.059.760.461.662.863.864.865.766.667.46 KIO3 4.53 5.967.578.449.3411.0913.2215.2917.4119.5821.7824.031:30 KIO40.160.220.370.510.70 1.24 1.96 2.83 3.82 4.89 6.027.177 KMnO4 2.74 4.12 5.967.068.2811.1114.4218.166 KNO273.774.675.375.776.076.777.478.078.579.179.680.16 KNO312.017.624.227.731.338.645.752.258.063.067.370.86 KOH48.750.853.254.756.157.958.659.560.661.863.164.66 KSCN63.866.469.170.471.674.176.578.981.183.385.387.36 K2CO351.351.752.352.753.154.054.956.057.258.459.661.06 K2CrO437.138.138.939.439.840.541.341.942.643.243.844.36 K2Cr2O7 4.307.1210.913.115.520.826.331.736.941.545.548.96 K2HAsO448.5*63.6*79.8*7 K2HPO457.059.161.562.764.167.7*72.7*1:31 K2MoO464.766.57 K2SO351.3051.3951.4951.5551.6251.7651.9352.1152.3252.5452.7953.061:26 K2SO47.118.469.9510.711.412.914.215.516.717.718.619.36 K2S2O349.0*62.3*75.7*7 K2S2O522.126.731.133.135.239.042.646.049.152.054.61:26 K2SeO368.4*68.5*68.5*7 K2SeO452.7052.9353.1753.3053.4353.7053.9954.3054.6154.9455.2655.607 K3AsO451.5*55.6*73*7 K3Fe(CN)623.927.631.132.834.337.239.641.743.545.046.147.06 K3PO444.351.47 K4Fe(CN)612.517.322.023.925.629.232.535.538.240.641.443.16 LaCl349.048.548.648.949.350.552.154.056.358.961.76 La(NO3)355.056.958.960.061.163.666.369.9*74.1*1:13 LiBr58.460.162.764.465.967.868.369.069.870.771.772.86 LiBrO361.0362.6264.4465.4466.5168.9071.68*73.24*74.4375.6676.9378.321:30 LiC2H3O223.7626.4929.4231.0232.7236.4840.6545.1549.9354.9160.0465.267 LiCl40.4542.46*45.29*45.8146.2547.3048.4749.7851.2752.9854.98*56.34*1:47Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. LiClO373.275.6*80.8*82.183.485.9*87.1*88.289.691.393.495.71:30 LiClO430.132.635.537.038.641.945.549.253.257.261.371.46 LiF0.1200.1260.1310.1347 LiH2PO455.87 LiI59.460.561.762.363.064.365.867.368.881.381.782.66 LiIO343.81:30 LiNO24145495153566063666810 LiNO334.837.642.750.557.960.162.264.065.767.268.569.76 LiOH10.810.811.011.111.311.712.212.713.414.215.116.16 LiSCN54.57 Li2CO3 1.54 1.43 1.33 1.28 1.24 1.15 1.070.990.920.850.780.727 Li2C2O4 5.877 Li2HPO39.078.407.777.477.18 6.64 6.16 5.71 5.30 4.91 4.53 4.167 Li2SO426.325.925.625.525.325.024.824.524.324.023.823.66 Li3PO40.0271:31 Lu(NO3)371.11:13 MgBr249.349.850.350.650.951.552.152.853.554.255.055.76 Mg(BrO3)243.045.248.049.451.054.357.961.665.369.0*70.9*71.71:14 Mg(C2H3O2)236.1837.5538.9239.617 MgC2O40.0387 MgCl233.9634.8535.5835.9036.2036.7737.3437.9738.7139.6240.7542.158 Mg(ClO3)253.3554.4056.8158.6660.91*65.46*67.3369.2771.0172.4473.481:14 Mg(ClO4)247.848.749.650.150.551.352.16 MgCrO432.06*35.39*7 MgCr2O758.967.07 MgF20.0137 MgI254.756.158.259.460.863.965.065.065.065.065.165.26 Mg(IO3)2 3.19* 6.70*7.928.529.1110.4511.9913.715.617.619.61:14 Mg(NO2)2477 Mg(NO3)238.439.540.841.642.444.145.947.950.052.270.672.06 MgSO30.320.370.460.520.610.87*0.85*0.760.690.640.620.601:26 MgSO418.221.725.126.328.230.933.435.636.935.934.733.36 MgS2O330.734.17 MgSeO431.4*35.7*47*7 MnBr256.0057.7259.3960.1960.9662.4163.7565.0166.1967.3268.4269.507 MnCl238.740.642.543.644.747.049.454.154.755.255.756.16 MnF20.80* 1.01*0.487 Mn(IO3)20.270.347 Mn(NO3)250.561.77 MnSO434.637.338.638.938.937.736.334.632.830.828.826.76 NH4Br37.540.242.743.945.147.349.451.353.054.656.157.47 NH4Cl22.9225.1227.2728.3429.3931.4633.5035.4937.4639.4041.3343.241:47 NH4ClO410.814.117.819.721.725.829.833.637.340.743.846.66 NH4F41.743.244.745.546.347.849.350.952.554.17 NH4HCO310.613.717.619.922.427.934.241.449.358.167.678.07 NH4H2AsO425.229.032.734.536.339.743.146.249.352.255.07 NH4H2PO417.822.026.428.831.236.241.647.253.059.265.772.47 NH4I60.762.163.464.064.665.866.867.868.769.670.471.16 NH4IO3 3.70 4.20 5.647.631:30 NH4NO255.759.064.968.87 NH4NO354.060.165.568.070.374.377.780.883.485.888.290.36 NH4SCN64.481.17 (NH4)2C2O4 2.31 3.11 4.25 4.94 5.737.569.7312.215.118.321.825.77 (NH4)2HPO436.438.240.041.042.044.146.248.550.953.355.958.67 (NH4)2S2O565.567.969.870.571.372.372.973.11:26 (NH4)2S2O837.0040.4543.8445.4947.1150.2553.2856.2359.1362.007 (NH4)2SO332.234.937.739.140.643.747.050.654.558.91:26 (NH4)2SO441.342.142.943.343.844.745.646.647.548.549.550.56 (NH4)2SeO349.051.153.454.756.058.962.065.469.17 (NH4)2SeO454.027 (NH4)3PO415.57Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. NaBr44.445.947.748.649.651.653.754.154.354.554.754.96 NaBrO320.023.2226.6528.2829.8632.8335.5538.0540.3742.521:30 NaCHO230.837.945.748.750.652.053.555.06 NaC2H3O226.528.831.833.535.539.945.158.359.360.561.762.96 NaCl26.2826.3226.4126.4526.5226.6726.8427.0327.2527.5027.7828.051:47 NaClO22.744.47 NaClO297.0*95.3*7 NaClO344.2746.6749.350.151.253.655.557.058.560.563.367.11:30 NaClO461.964.166.267.268.370.472.574.174.775.476.176.76 NaF 3.52 3.72 3.89 3.97 4.05 4.20 4.34 4.46 4.57 4.66 4.75 4.826 NaHCO3 6.487.598.739.329.9111.1312.4013.7015.0216.3717.7319.107 NaHSO422.233.310 NaH2PO436.5441.0746.0048.6851.5457.89*61.7*62.3*65.968.71:31 NaI61.262.463.964.865.767.769.872.074.774.874.975.16 NaIO3 2.43 4.407.78*8.65*9.6011.6713.9916.5219.25*21.1*22.924.71:30 NaIO412.627 NaNO241.943.445.145.946.848.750.752.855.057.259.561.86 NaNO342.244.446.647.748.851.053.255.357.559.661.763.86 NaOH30394650535863677174767910 NaSCN52.957.160.262.763.564.265.065.966.967.969.06 Na2B4O7 1.23 1.71 2.50 3.07 3.82 6.029.714.917.119.923.528.06 Na2CO3 6.4410.817.923.528.732.832.231.731.331.130.930.96 Na2C2O4 2.62 2.95 3.30 3.48 3.65 4.00 4.36 4.71 5.06 5.41 5.75 6.086 Na2CrO422.632.344.646.746.948.951.053.455.355.555.856.16 Na2Cr2O762.163.164.465.266.168.070.172.374.677.079.680.76 Na2HAsO4 5.6*29.3*67*7 Na2HPO4 1.66 4.197.5110.5516.34*35.17*44.64*45.2046.8148.7850.5251.531:31 Na2MoO430.638.839.439.439.840.341.041.742.643.544.545.56 Na2S11.113.215.717.118.622.126.728.130.233.036.441.06 Na2SO312.016.120.923.526.3*27.3*25.924.823.722.822.121.51:26 Na2SO416.1321.9429.22*32.35*31.5530.9030.3930.0229.7929.678 Na2S2O333.136.340.643.345.952.062.365.768.869.470.171.06 Na2S2O538.439.540.040.641.843.044.245.546.848.149.51:26 Na2SeO347.3*45*7 Na2SeO411.736.9*42.1*7 Na2WO441.641.942.342.642.943.644.445.346.247.348.449.56 Na3PO4 4.287.3010.812.614.116.622.928.432.437.640.443.56 Na4P2O7 2.23 3.28 4.81 6.627.0010.1014.3820.0727.3136.0332.3730.676 NdCl349.049.349.750.050.451.252.253.354.555.857.158.56 Nd(NO3)355.7657.4959.3760.3861.4363.6966.2769.471:13 NiCl234.736.138.540.341.742.143.245.046.146.246.446.66 Ni(ClO4)251.152.87 NiF2 2.50 2.527 NiI255.4057.6859.7860.6961.5062.8063.7364.3864.8065.0965.307 Ni(NO3)244.146.048.449.851.354.658.361.063.165.667.969.06 NiSO421.424.427.428.830.3*32.0*34.135.837.739.942.344.86 Ni(SCN)235.487 NiSeO421.626.2*45.6*7 PbBr20.4490.6200.8410.966 1.118 1.46 1.892 PbCl20.660.810.98 1.07 1.17 1.39 1.64 1.93 2.24 2.60 2.99 3.422 Pb(ClO4)281.57 PbF20.06030.06490.06700.06932 PbI20.0410.0520.0670.0760.0860.1120.1440.1870.2430.3152 Pb(IO3)20.00257 Pb(NO3)228.4632.1335.6737.3839.0542.2245.1747.9050.4252.7254.8256.752 PbSO40.00330.00380.00420.00440.00470.00520.00582 PrCl348.048.148.649.049.550.852.354.156.158.36 Pr(NO3)357.5059.2061.1662.2463.40*65.7*67.870.273.41:13 RbBr47.450.152.653.854.957.058.860.662.163.564.865.96 RbBrO30.97 1.55 2.36 2.87 3.45 4.87 6.648.7811.2914.1517.3220.761:30 RbCl43.5845.6547.5348.4249.2750.8652.3453.6754.9256.0857.1658.151:47Compound0°C10°C20°C25°C30°C40°C50°C60°C70°C80°C90°C100°C Ref. RbClO3 2.10 3.38 5.14 6.227.4510.3513.8517.9322.5327.5732.9638.601:30 RbClO41 1.5177 RbF757 RbHCO353.77 RbI55.858.661.162.363.465.467.268.870.371.672.773.86 RbIO3 1.09 1.53 2.07 2.38 2.74 3.52 4.41 5.42 6.527.749.0010.361:30 RbNO316.425.034.639.444.253.160.867.272.276.179.081.26 RbOH63.47 Rb2CrO438.2743.267 Rb2SO427.330.032.533.734.836.938.740.341.843.044.144.96 SbCl385.790.87 SbF379.483.17 Sc(NO3)357.059.361.662.863.966.268.51:13 Sm(NO3)354.8356.3358.0859.0560.0862.3865.05*68.1*70.874.21:13 SmCl348.048.248.448.649.250.06 SnCl246647 SnI20.97 3.877 SrBr246.048.350.651.752.955.257.659.962.364.666.869.06 Sr(BrO3)218.5322.0025.3927.0228.5931.5534.2136.5738.64*40.2*40.841.01:14 SrCl231.9432.9334.4335.3736.4338.9341.9445.44*46.81*47.6948.7049.878 Sr(ClO2)213.013.614.114.314.514.915.315.615.97 Sr(ClO3)263.2963.4263.6463.7763.9364.2964.7065.1665.6566.1866.7467.311:14 Sr(ClO4)270.04*75.35*78.44*7 SrF20.0110.0217 SrI262.562.863.563.964.565.867.369.070.872.774.779.26 Sr(IO3)20.1020.1260.1520.1650.1790.2060.2330.2590.2840.3070.3280.3461:14 Sr(MnO4)2 2.57 Sr(NO2)241.944.358.67 Sr(NO3)228.234.641.044.547.047.447.948.448.949.550.150.76 Sr(OH)20.9 2.27 SrSO30.00151:26 SrSO40.01357 SrS2O38.813.217.720.022.226.87 Tb(NO3)360.661.021:13 Tl2SO4 2.65 3.56 4.61 5.19 5.807.098.469.8911.3312.7714.1815.536 Tm(NO3)367.91:13 UO2(NO3)249.5251.8254.4255.8557.5561.5967.071:55 Y(NO3)355.5756.9358.7559.8661.11*63.3*64.967.972.51:13 Yb(NO3)370.51:13 ZnBr279.380.181.883.084.185.685.886.186.386.686.887.16 ZnC2O40.00100.00190.00265 ZnCl276.679.080.381.481.882.483.083.784.485.286.06 Zn(ClO4)244.29*46.27*48.707 ZnF2 1.535 ZnI281.181.281.381.481.581.782.082.382.683.083.383.76 Zn(IO3)20.580.640.690.770.825 Zn(NO3)247.850.854.454.658.579.180.187.589.96 ZnSO30.17860.17900.17940.18030.18125 ZnSO429.132.035.036.638.241.343.042.141.039.938.837.66 ZnSeO433.0634.9837.3838.7940.345。
高中化学溶解度表

高中化学溶解度表高中化学溶解度表是指在一定温度下,不同物质在水中的溶解度的一张表格。
在化学实验中,了解物质的溶解度对于进行溶液的配制、反应的进行以及物质的纯度检验等都至关重要。
以下是一些常见物质在水中的溶解度数据:1. 无机盐溶解度:- 氯化钠(NaCl):359 g/100 mL- 硝酸银(AgNO3):123 g/100 mL- 硫酸铜(CuSO4):31.6 g/100 mL- 碳酸钙(CaCO3):0.0013 g/100 mL- 硫酸铁(FeSO4):92 g/100 mL2. 有机物溶解度:- 葡萄糖(C6H12O6):91 g/100 mL- 乙醇(C2H5OH):97.2 g/100 mL- 甲苯(C6H5CH3):0.09 g/100 mL- 苯胺(C6H5NH2):4.8 g/100 mL- 氯仿(CHCl3):0.8 g/100 mL需要注意的是,溶解度受温度、压力和溶剂的性质等因素的影响。
通常溶解度是指在标准条件下的数值,即在25摄氏度和标准大气压下的溶解度。
但对于某些物质来说,温度的变化可能会导致它们的溶解度剧烈改变,例如氯化铵(NH4Cl)在0摄氏度时溶解度为37.2 g/100 mL,而在100摄氏度时溶解度却可达到223 g/100 mL。
溶解度表的数据可以通过实验测量得到,也可以通过已有的文献资料或数据库获取。
了解物质的溶解度对于化学实验的设计和操作非常重要。
比如,在配制溶液时,根据所需溶质的溶解度来确定所需的溶质量;在溶液反应中,溶液中物质的溶解度决定了反应的速度和平衡位置;在纯度检验中,溶液中溶解度过高或过低可能意味着溶质的纯度问题。
此外,了解溶解度还有助于理解溶液的饱和与过饱和现象。
当溶质的溶解度达到一定限度时,溶液就处于饱和状态,继续溶解的物质会以固体形式析出。
而在过饱和状态下,溶质的溶解度超过了平衡溶解度,溶液稳定性较差,稍有扰动即可使物质析出。
总之,高中化学溶解度表为学生提供了有关物质的重要信息,有助于他们更好地理解和应用化学知识。
营养学之矿物质
营养学之矿物质人体中几乎含有自然界存在的所有元素。
其中,有20多种是构成人体组织、维持生理功能和生化代谢所必需的。
这些元素在体内按严格的规律和方式,有条不紊地进行一系列互相联系的化学反应。
其中碳、氢、氧、氮构成有机物质,如蛋白质、脂肪和碳水化合物及水分,其余各种元素统称为矿物质或无机盐。
无机盐约占人体重量的5%。
(一)矿物质的分类通常,可依据矿物质在人体内的含量对其进行分类。
若人体的需要量相对较多,含量大于0.01%,一般计量单位在克的水平者,如钙、磷、钠、钾、氯、硫、镁等,统称为常量元素或宏量元素。
若需要量相对较少,含量小于0.01%,一般计量单位仅为毫克或微克的水平者,如铁、碘、铜、锌、硒、锰、钴、铬、钼、氟、镍、硅、矾、锡等,即统称为微量元素或痕量元素。
上述14种微量元素是目前认为是人体所必需的微量元素。
矿物质在体内的含量一般可随年龄增长而增加,但各元素间比例变动不大。
(二)矿物质的特点1.矿物质在体内不能合成,必须由食物和饮水中摄取。
摄入体内的矿物质经机体新陈代谢,每天都有一定量随粪、尿、汗、头发、指甲及皮肤粘膜脱落而排出体外。
因此,矿物质必须不断地从膳食中供给。
2.各种矿物质在体内的分布有其专一性。
如铁主要在红细胞,碘主要在甲状腺,钴主要在红骨髓,锌主要在肌肉,钙、磷主要在骨骼和牙齿,钒主要在脂肪组织等。
3.各种矿物质之间存在协同或拮抗作用。
如膳食中的钙和磷比例不合适,可影响两种元素的吸收;过量的镁可干扰钙的代谢;过量的锌能影响铜的代谢;过量的铜可抑制铁的吸收等。
4.某些微量元素在体内虽需要量很少,但其生理剂量与中毒剂量范围较窄。
若摄入过多易产生毒性作用。
(三)矿物质的生理功能1.参与机体组织的构成无机盐是骨、牙、神经、肌肉、筋腱、腺体、血液的重要组成成分。
在头发、指甲、皮肤以及腺体分泌物中,都含有本身所特有的一种或多种元素。
如钙、磷、镁是骨骼和牙齿的重要成分;磷和硫是蛋白质的成分;铁为血红蛋白的组成成分等。
常用有机溶剂性质(极性、沸点、溶解性等)
常用有机溶剂性质粘度(20℃)/mPa·s; —介电常数名称沸点密度粘度波长极性E T(30) 介电分子量溶解性水100 1 1 268 10.2 63.1 58.8 18二甲亚砜189 2.24 268 7.2 45 48.9 78.14 DMSO能与水、醇、醚、丙酮、乙醛、吡啶、乙酸乙酯等混溶,不溶于乙炔以外的脂肪烃化合物乙二醇197 1.1155 19.9 210 6.9 56.3 26.33 62.07 与水/乙醇/丙酮/醋酸甘油吡啶等混溶,微溶于醚等,不溶于石油烃及油类.能够溶解氯化锌/氯化钠/碳酸钾/氯化钾/碘化钾/氢氧化钾等无机物.甲醇64.9 0.7914 0.6 210 6.6 55.5 32.6 32.04 溶于水、乙醇、乙醚、苯等二甲基甲酰胺152.8 0.92 270 6.4 43.8 36.71 73.10 能和水及大部分有机溶剂互溶,是高沸点的极性(亲水性)非质子性溶剂,能促进SN2反应机构的进行苯胺184 4.4 - 6.3 44.3 6.98乙酸118 1.28 230 6.2 51.9 6.19乙腈81.1 0.37 210 6.2 46 37.5 41.05 相对密度0.79,与水混溶,溶于醇等多数有机溶剂硝基甲烷101 0.67 330 6 46.3 38.6丙酮56.5 0.32 330 5.4 42.2 20.5 58.08 与水、乙醇、氯仿、乙醚及多种油类混溶吡啶115 0.97 305 5.3 40.2 12.3二恶烷; 二氧六环102 1.04 1.54 220 4.8 36 2.21 88.11 与水混溶,可混溶于多数有机溶剂2-丁酮80 0.8054 0.43 330 4.5 72.11 甲基乙基酮能溶于4份水中,但温度升高时溶解度降低,20℃时,水中溶解度26.8%(w),水在2-丁酮中的溶解度11.8%(w)。
溶于乙醇和乙醚,可与油混溶。
溶解度表大全-无机盐-有机物
物质水溶液溶解度表以化学品中特征元素的拼音顺序排列。
所有数据都为1atm下水溶液溶解度的数据,单位为g/100cm31.锕、氨、铵 (2)2.钯、钡、铋、铂、钚 (3)3.氮、镝 (4)4.铒 (4)5.钒 (4)6.钆、钙、锆、镉、铬、汞、钴、硅 (4)7.铪、氦、钬 (7)8.镓、钾、金 (7)9.钪 (8)10.镧、锂、硫、镥、铝 (9)11.镁、锰 (9)12.钠、镍、钕 (10)13.硼、铍、钋、镨 (12)14.氢、铅 (12)15.铷 (13)16.铯、钐、砷、铈、锶 (14)17.铊、碳、铽、锑、铁、铜、钍 (15)18.锡、氙、锌、溴 (17)19.氩、氧、铟、钇、镱、银、铀、铕 (19)20.有机化合物 (22)21.酸碱盐溶解性表 (23)1.锕、氨、铵物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃氢氧化锕Ac(OH)3 0.0022氨NH3 88.5 70 56 44.5 34 36.5 20 15 11 8 7 叠氮化氨NH2N2 16 25.3 37.1苯甲酸氨NH4C7H5O2 20碳酸氢氨NH4CO3 11.9 16.1 21.7 28.4 36.6 59.2 109 170 354 溴化氨NH4Br 60.6 68.1 76.4 83.2 91.2 108 125 135 145 碳酸氨(NH4)2CO3100氯酸氨NH4ClO328.7氯化氨NH4Cl 29.4 33.2 37.2 41.4 45.8 50.4 55.3 60.2 65.6 71.2 77.3 氯铂酸铵(NH4)2PtCl60.289 0.374 0.499 0.637 0.815 1.44 2.16 2.61 3.36 铬酸铵(NH4)2CrO425 29.2 34 39.3 45.3 59 76.1重铬酸铵(NH4)2Cr2O718.2 25.5 35.6 46.5 58.5 86 115 156 砷酸二氢铵NH4H2AsO433.7 48.7 63.8 83 107 122磷酸二氢铵NH4H2PO422.7 39.5 37.4 46.4 56.7 82.5 118 173 氟硅酸铵(NH4)2SiF6 18.6甲酸铵NH4HCO2 102 143 204 311 533磷酸一氢铵(NH4)2HPO4 42.9 62.9 68.9 75.1 81.8 97.2碳酸氢铵NH4HSO4 100酒石酸氢铵NH4HC4H4O6 1.88 2.7碘酸铵NH4IO3 2.6碘化铵NH4I 155 163 172 182 191 209 229 250 硝酸铵NH4NO3 118 150 192 242 297 421 580 740 871 高碘酸铵(NH4)5IO6 2.7草酸铵(NH4)2C2O4 2.2 3.21 4.45 6.09 8.18 14 22.4 27.9 34.7 高氯酸铵NH4ClO4 12 16.4 21.7 37.7 34.6 49.9 68.9高锰酸铵NH4MnO4 0.8磷酸铵(NH4)3PO4 26.1硒酸铵(NH4)2SeO4 96 105 115 126 143 192硫酸铵(NH4)2SO4 70.6 73 75.4 78 81 88 95 103 亚硫酸铵(NH4)2SO3 47.9 54 60.8 68.8 78.4 104 114 150 153 酒石酸铵(NH4)2C4H4O6 45 55 63 70.5 76.5 86.9硫氰酸铵NH4SCN 120 144 170 208 234 346硫代硫酸铵(NH4)2S2O3 2.15钒酸铵NH4VO3 0.48 0.84 1.32 2.422.钯、钡、铋、铂、钚物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃氢氧化钯Pd(OH)2 4.106×10-10氢氧化钯Pd(OH)4 5.247×10-14乙酸钡Ba(C2H3O2)258.8 62 72 75 78.5 75 74 74.8 砷酸钡Ba3(AsO4)2 2.59×10-9叠氮化钡Ba(N3)2 12.5 16.1 17.4溴酸钡Ba(BrO3)2 0.29 0.44 0.65 0.95 1.31 2.27 3.52 0.95 1.31 溴化钡BaBr2 98 101 104 109 114 123 135 149 碳酸钡BaCO3 1.4×10-3氯酸钡Ba(ClO3)220.3 26.9 33.9 41.6 49.7 66.7 84.8 105 氯化钡BaCl231.2 33.5 35.8 38.1 40.8 46.2 52.5 55.8 59.4 氯酸钡Ba(ClO2)243.9 44.6 45.4 47.9 53.8 66.6 80.8 铬酸钡BaCrO4 2.78×10-4氰化钡Ba(CN)280亚铁氰化钡Ba2Fe(CN)69.7×10-3氟化钡BaF20.159 0.16 0.162氟硅酸钡BaSiF6 2.8×10-2甲酸钡Ba(HCO2)226.2 28 31.9 34 38.6 44.2 47.6 51.3磷酸氢钡BaHPO4 1.3×10-2亚磷酸氢钡BaHPO30.687氢氧化钡Ba(OH)2·8H2O 1.67 2.48 3.89 5.59 8.22 20.9 101碘酸钡Ba(IO3)2 3.5×10-2 4.6×10-2 5.7×10-2碘化钡BaI2182 201 223 250 264 291 301 钼酸钡BaMoO46×10-3硝酸钡Ba(NO3)2 4.95 6.67 9.02 11.5 14.1 20.4 27.2 34.4 亚硝酸钡Ba(NO2)250.3 60 72.8 102 151 222 261 325 草酸钡BaC2O4·2H2O 3×10-3氧化钡BaO 3.8高氯酸钡Ba(ClO4)2239 336 416 495 575 653 高锰酸钡Ba(MnO4)2 1.5×10-2焦磷酸钡Ba2P2O79×10-3硒酸钡BaSeO45×10-3硫酸钡BaSO4 2.45×10-4硫化钡BaS 2.88 4.89 7.86 10.4 14.9 22.7 49.9 67.3 60.3 砷酸铋BiAsO4 7.3×10-4氢氧化铋Bi(OH)3 2.87×10-7碘化铋BiI37.76×10-4磷酸铋BiPO4 1.1×10-10硫化铋Bi2S3 1.56×10-20氢氧化铂Pt(OH)2 3.11×10-11物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃溴化铂PtBr4 1.35×10-7氟化钚PuF3 3.14×10-4氟化钚PuF4 3.62×10-4碘酸钚Pu(IO3)48.0×10-23. 氮、镝物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃一氧化氮NO 0.56x10-3一氧化二氮N2O 0.112铬酸镝Dy2(CrO403.10H2O0.6634.铒物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃氢氧化铒Er(OH)3 1.363×10-55.钒物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃无氧化二钒V2O5 1.363×10-56.钆、钙、锆、镉、铬、汞、钴、硅物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃乙酸钆Gd(C2H3O2)?4H2O11.6碳酸氢钆Gd(HCO3)3 5.61溴酸钆Gd(BrO3)3?9H2O50.2 70.1 95.6 126 166氢氧化钆Gd(OH)3 1.882×10-5硫酸钆Gd2(SO4)3 3.98 3.3 2.6 2.32氯化钙CaCl2 59.5 64.7 74.5 100 128 137 147 154 159 乙酸钙Ca(C2H3O2)2?2H2O37.4 36 34.7 33.8 33.2 32.7 33.5 31.1 29.7 砷酸钙Ca3(AsO4)2 3.63×10-3叠氮化钙Ca(N3)245苯甲酸钙Ca(C7H5O2)23H2O 2.32 2.45 2.72 3.02 3.42 4.71 6.87 8.55 8.7 碳酸轻钙Ca(HCO3)216.1 16.6 17.1 17.5 17.9 18.4 溴酸钙Ca(BrO3)2230溴化钙CaBr2125 132 143 213 273 295 312 霰石CaCO3-霰石7.75×10-4方解石CaCO3-方解石 6.17×10-4氯酸钙Ca(ClO3)2209铬酸钙CaCrO4 4.5 2.25 1.83 1.49 0.83磷酸二氢钙Ca(H2PO4)2 1.8氟化钙CaF2 8.6×10-3氟硅酸钙CaSiF60.518甲酸钙Ca(HCO2)216.1 16.6 17.1 17.5 17.9 18.4 磷酸氢钙CaHPO4 4.3×10-3氢氧化钙Ca(OH)20.189 0.182 0.173 0.16 0.141 0.121 8.6×10-27.6×10-2碘酸钙Ca(IO3)29×10-20.24 0.38 0.52 0.65 0.66 0.67碘化钙CaI2 64.6 66 67.6 70.8 74 78 81 钼酸钙CaMoO4 4.1×10-3硝酸钙Ca(NO3)2?4H2O 102 115 129 152 191 358 363 亚硝酸钙Ca(NO2)2?4H2O 63.9 84.5 104 134 151 166 178 草酸钙CaC2O4 6.7×10-4高氯酸钙Ca(ClO4)2188高锰酸钙Ca(MnO4)2388磷酸钙Ca3(PO4)22×10-3硒酸钙CaSeO4?2H2O 9.73 9.77 9.22 8.79 7.14硫酸钙CaSO4?2H2O 0.223 0.244 0.255 0.264 0.265 0.244 0.234 0.205 钨酸钙CaWO4 2.39×10-3氟化锆ZrF4 1.32硫酸锆Zr(SO4)2?4H2O 52.5砷酸镉Cd3(AsO4)27.09×10-6苯甲酸镉Cd(C7H5O2)2 2.81溴酸镉Cd(BrO3)2125溴化镉CdBr256.3 75.4 98.8 129 152 153 156 160 碳酸镉CdCO3 3.93×10-5氯酸镉Cd(ClO3)2299 308 322 348 376 455氯化镉CdCl2100 135 135 135 135 136 140 147 氰化镉Cd(CN)2 2.2×10-2亚铁氰化镉Cd2Fe(CN)68.74×10-5氟化镉CdF2 4甲酸镉Cd(HCO2)28.3 11.1 14.4 18.6 25.3 59.5 80.5 85.2 94.6 氢氧化镉Cd(OH)2 2.70×10-4碘酸镉Cd(IO3)29.7×10-2碘化镉CdI278.7 84.7 87.9 92.1 100 111 125 硝酸镉Cd(NO3)2122 136 150 194 310 713草酸镉CdC2O4.3H2O 6.05×10-3高氯酸镉Cd(ClO4)2180 188 195 203 221 243 272 磷酸镉Cd3(PO4)2 6.24×10-6硒酸镉CdSeO472.5 68.4 64 58.9 55 44.2 32.5 27.2 22 硫酸镉CdSO475.4 76 76.6 78.5 81.8 66.7 63.1 60.8 硫化镉CdS 1.29×10-12钨酸镉CdWO4 4.64×10-2硝酸铬Cr(NO3)3108 124 130 152物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃高氯酸铬Cr(ClO4)3 104 123 130硫酸铬Cr2(SO4)3?18H2O220叠氮化亚汞Hg2(N3)2 2.73×10-2溴化亚汞Hg2Br2 1.35×10-6碳酸亚汞Hg2CO3 4.35×10-7氯化亚汞Hg2Cl2 3.25×10-5铬酸亚汞Hg2CrO4 2.31×10-3氰化亚汞Hg2(CN)2 2.27×10-12高氯酸亚汞Hg2(ClO4)2) 282 325 407 455 499 541 580硫酸亚汞Hg2SO4 4.28×10-2乙酸汞Hg(C2H3O2)225苯甲酸汞Hg(C7H5O2)2?H2O 1.1溴酸汞Hg(BrO3)2?2H2O0.08溴化汞HgBr20.3 0.4 0.56 0.66 0.91 1.68 2.77 4.9 氯酸汞Hg(ClO3)225氯化汞HgCl2 3.63 4.82 6.57 8.34 10.2 16.3 30 61.3 氰化汞Hg(CN)29.3碘酸汞Hg(IO3)2 2.37×10-3碘化汞HgI2 6×10-3草酸汞HgC2O4 1.1×10-2硫化汞HgS 2.94×10-25硫氰酸汞Hg(SCN)2 6.3×10-2溴酸钴Co(BrO3)2?6H2O45.5溴化钴CoBr2 91.9 112 128 163 227 241 257 氯酸钴Co(ClO3)2 135 162 180 195 214 316氯化钴CoCl2 43.5 47.7 52.9 59.7 69.5 93.8 97.6 101 106 氟化钴CoF2 1.36氟硅酸钴CoSiF6?6H2O 118碘酸钴Co(IO3)2?2H2O 1.02 0.9 0.88 0.82 0.73 0.7 碘化钴CoI2 203硝酸钴Co(NO3)2 84 89.6 97.4 111 125 174 204 300亚硝酸钴Co(NO2)2 7.6×10-20.24 0.4 0.61 0.85高氯酸钴Co(ClO4)2 104硫酸钴CoSO4 25.5 30.5 36.1 42 48.8 55 53.8 45.3 38.9 二氧化硅SiO2 1.2×10-27.铪、氦、钬物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃氢氧化铪(III)Hf(OH)3 4.50×10-4氢氧化铪(IV)Hf(OH)4 4.50×10-6氦He 0.6氢氧化钬(III)Ho(OH)3 2.52×10-5硫酸钬(III)Ho2(SO4)3?8H2O 8.18 6.1 4.528.镓、钾、金物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃氢氧化镓Ga(OH)38.62×10-9草酸镓Ga2(C2O4)3?4O20.4硒酸镓Ga2(SeO4)3?16H2O 18.1乙酸钾KC2H3O2216 233 256 283 324 350 381 398 砷酸钾K3AsO419叠氮化钾KN3 41.4 46.2 50.8 55.8 61 106 苯甲酸钾KC7H5O265.8 70.7 76.7 82.1溴酸钾KBrO3 3.09 4.72 6.91 9.64 13.1 22.7 34.1 49.9 溴化钾KBr 53.6 59.5 65.3 70.7 75.4 85.5 94.9 99.2 104 溴铂酸钾K2PtBr6 1.89碳酸钾K2CO3105 109 111 114 117 127 140 148 156 氯酸钾KClO3 3.3 5.2 7.3 10.1 13.9 23.8 37.5 46 56.3 氯化钾KCl 28 31.2 34.2 37.2 40.1 42.6 45.8 48.8 51.3 53.9 56.3 铬酸钾K2CrO456.3 60 63.7 66.7 67.8 70.1 74.5 氰化钾KCN 50重铬酸钾K2Cr2O7 4.7 7 12.3 18.1 26.3 45.6 73砷酸二氢钾KH2AsO4 19物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃磷酸二氢钾KH2PO414.8 18.3 22.6 28 35.5 40.7 50.2 60.5 70.4 78.5 83.5 铁氰化钾K3Fe(CN)630.2 38 46 53 59.3 70 91 亚铁氰化钾K4Fe(CN)614.3 21.1 28.2 35.1 41.4 54.8 66.9 71.5 74.2 氟化钾KF 44.7 53.5 94.9 108 138 142 150甲酸钾KHCO2313 337 361 398 471 580 658碳酸氢钾KHCO322.5 27.4 33.7 39.9 47.5 65.6磷酸二氢钾K2HPO4 150硫酸氢钾KHSO4 36.2 48.6 54.3 61 76.4 96.1 122 氢氧化钾KOH 95.7 103 112 126 134 154 178 碘酸钾KIO3 4.6 6.27 8.08 10.3 12.6 18.3 24.8 32.3 碘化钾KI 128 136 144 153 162 168 176 192 198 206硝酸钾KNO3 13.9 21.9 31.6 45.3 61.3 85.5 106 138 167 203 245 亚硝酸钾KNO2 279 292 306 320 329 348 376 390 410 草酸钾K2C2O425.5 31.9 36.4 39.9 43.8 53.2 63.6 69.2 75.3 高氯酸钾KClO4 0.76 1.06 1.68 2.56 3.73 7.3 13.4 17.7 22.3 高碘酸钾KIO4 0.17 0.28 0.42 0.65 1 2.1 4.4 5.9高锰酸钾KMnO4 2.83 4.31 6.34 9.03 12.6 22.1过二硫酸钾K2S2O8 4.7磷酸钾K3PO4 81.5 92.3 108 133硒酸钾K2SeO4107 109 111 113 115 119 121 122 硫酸钾K2SO4 7.4 9.3 11.1 13 14.8 18.2 21.4 22.9 24.1 四苯硼钾KBC24H20 1.8×10-5硫氰酸钾KSCN 177 198 224 255 289 372 492 571 675 硫代硫酸钾K2S2O396 155 175 205 238 293 312钨酸钾K2WO451.5三氯化金AuCl368三碘化金AuI3 1.30×10-10草酸金(V) Au2(C2O4)50.2589.钪物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃草酸钪Sc2(C2O4)3?6H2O 6×10-3硫酸钪Sc2(SO4)3?5H2O 54.610.镧、锂、硫、镥、铝物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃乙酸镧La(C2H3O2)3?H2O 16.9溴酸镧La(BrO3)398 120 149 200碘酸镧La(IO3)3 4.58×10-2钼酸镧La2(MoO4)3 2.47×10-3硝酸镧La(NO3)3100 136 168 247硒酸镧La2(SeO4)350.5 45 45 45 45 18.5 5.4 2.2硫酸镧La2(SO4)3 3 2.72 2.33 1.9 1.67 1.26 0.91 0.79 0.68 钨酸镧La2(WO4)3?3H2O 6.06乙酸锂LiC2H3O2 31.2 35.1 40.8 50.6 68.6叠氮化锂LiN3 61.3 64.2 67.2 71.2 75.4 86.6 100 苯甲酸锂LiC7H5O238.9 41.6 44.7 53.8溴酸锂LiBrO3154 166 179 198 221 269 308 329 355 溴化锂LiBr 143 147 160 183 211 223 245 266 碳酸锂Li2CO3 1.54 1.43 1.33 1.26 1.17 1.08 1.01 0.85 0.72 氯酸锂LiClO3241 283 372 488 604 777氯化锂LiCl 69.2 74.5 83.5 86.2 89.8 98.4 112 121 128铬酸锂Li2CrO4.2H2O 142重铬酸锂Li2Cr2O7.2H2O 151磷酸二氢锂LiH2PO4126氟化锂LiF 0.16氟硅酸锂Li2SiF6.2H2O 73甲酸锂LiHCO232.3 35.7 39.3 44.1 49.5 64.7 92.7 116 138 亚磷酸氢锂Li2HPO3 4.43 9.97 7.61 7.11 6.03 氢氧化锂LiOH 12.7 12.7 12.8 12.9 13.0 13.3 13.8 15.3 17.5 碘化锂LiI 151 157 165 171 179 202 435 440 481 钼酸锂Li2MoO482.6 79.5 79.5 78 73.9 硝酸锂LiNO353.4 60.8 70.1 138 152 175亚硝酸锂LiNO270.9 82.5 96.8 114 133 177 233 272 324 草酸锂Li2C2O48高氯酸锂LiClO442.7 49 56.1 63.6 72.3 92.3 128 151高锰酸锂LiMnO471.4磷酸锂Li3PO4 3.82×10-2硒化锂Li2Se 57.7亚硒酸锂Li2SeO325 23.3 21.5 19.6 17.9 14.7 11.9 11.1 9.9 硫酸锂Li2SO436.1 35.5 34.8 34.2 33.7 32.6 31.4 30.9酒石酸锂Li2C4H4O642 31.8 27.1 26.6 27.2 29.5硫氰酸锂LiSCN 114 131 153钒酸锂LiVO3 2.5 4.82 6.28 4.38 2.67二氧化硫SO2 9.4氢氧化镥(III)Lu(OH)3 1.16×10-5硫酸镥(III) Lu2(SO4)3?8H2O 57.9氯化铝AlCl343.9 44.9 45.8 46.6 47.3 48.1 48.6 49 氟化铝AlF30.56 0.56 0.67 0.78 0.91 1.1 1.32 1.72 硝酸铝Al(NO3)360 66.7 73.9 81.8 88.7 106 132 153 160 高氯酸铝Al(ClO4)3122 128 133硫酸铝Al2(SO4)3 31.2 33.5 36.4 40.4 45.8 59.2 73 80.8 89 氢氧化铝Al(OH)3 1.0×10-411.镁、锰物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃乙酸镁Mg(C2H3O2)256.7 59.7 53.4 68.6 75.7 118苯甲酸镁Mg(C7H5O2)2?H2O 5溴酸镁Mg(BrO3)2?6H2O 58溴化镁MgBr298 99 101 104 106 112 125 碳酸镁MgCO3 3.9×10-2氯酸镁Mg(ClO3)2 114 123 135 155 178 242 268氯化镁MgCl252.9 53.6 54.6 55.8 57.5 61 66.1 69.5 73.3物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃铬酸镁MgCrO4?7H2O 137氟化镁MgF2 7.32×10-3氟硅酸镁MgSiF626.3 30.8 34.9 44.4甲酸镁Mg(HCO2)214 14.2 14.4 14.9 15.9 17.9 20.5 22.2 22.9 氢氧化镁Mg(OH)29.63×10-4碘酸镁Mg(IO3)27.2 8.6 10 11.7 15.2 15.5 15.6碘化镁MgI2120 140 173 186钼酸镁MgMoO413.7硝酸镁Mg(NO3)262.1 66 69.5 73.6 78.9 78.9 91.6 106草酸镁MgC2O40.104高氯酸镁Mg(ClO4)249.6磷酸镁Mg3(PO4)2 2.59×10-4硒酸镁MgSeO420 30.4 38.3 44.3 48.6 55.8亚硒酸镁MgSeO3 5.45×10-2硫酸镁MgSO422 28.2 33.7 38.9 44.5 54.6 55.8 52.9 50.4 硫代硫酸镁MgS2O350溴化锰MnBr2127 136 147 157 169 197 225 226 228 碳酸锰MnCO3 4.88×10-5氯化锰MnCl263.4 68.1 73.9 80.8 88.5 109 113 114 115 亚铁氰化锰Mn2Fe(CN)6 1.88×10-3氟化锰MnF210.6 0.67 0.44 0.48 氟硅酸锰MnSiF6?6H2O 140氢氧化锰Mn(OH)2 3.22×10-4硝酸锰Mn(NO3)2102 118 139 206草酸锰MnC2O4?2H2O 0.02 0.028 0.033硫酸锰MnSO452.9 59.7 62.9 62.9 60 53.6 45.6 40.9 35.312.钠、镍、钕物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃乙酸钠CH3COONa 36.2 40.8 46.4 54.6 65.6 139 153 161 170 叠氮化钠NaN338.9 39.9 40.8苯甲酸钠NaC7H5O253.0溴酸钠NaBrO324.2 30.3 36.4 42.6 48.8 62.6 75.7 90.8 溴化钠NaBr 80.2 85.2 90.8 98.4 107 118 120 121 121 碳酸钠Na2CO37 12.5 21.5 39.7 49 46 43.9 43.9氯酸钠NaClO379.6 87.6 95.9 105 115 137 167 184 204 氯化钠NaCl 35.7 35.8 35.9 36.1 36.4 37.0 37.1 37.8 38 38.5 39.2 铬酸钠Na2CrO431.7 50.1 84 88 96 115 125 126 氰化钠NaCN 40.8 48.1 58.7 71.2 水解物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃重铬酸钠Na2Cr2O7163 172 183 198 215 269 376 405 415 磷酸二氢钠NaH2PO456.5 69.8 86.9 107 133 172 211 234 氟化钠NaF 3.66 4.06 4.22 4.4 4.68 4.89 5.08 甲酸钠HCOONa 43.9 62.5 81.2 102 108 122 138 147 160 碳酸氢钠NaHCO37 8.1 9.6 11.1 12.7 16氢氧化钠NaOH 98 109 119 129 174碘酸钠NaIO3 2.48 4.59 8.08 10.7 13.3 19.8 26.6 29.5 33 碘化钠NaI 159 167 178 191 205 257 295 302 钼酸钠Na2MoO444.1 64.7 65.3 66.9 68.6 71.8硝酸钠NaNO373 80.8 87.6 94.9 102 122 148 180 亚硝酸钠NaNO271.2 75.1 80.8 87.6 94.9 111 113 160 草酸钠Na2C2O4 2.69 3.05 3.41 3.81 4.18 4.93 5.71 6.5 高氯酸钠NaClO4167 183 201 222 245 288 306 329 高碘酸钠NaIO4 1.83 5.6 10.3 19.9 30.4磷酸钠Na3PO4 4.5 8.2 12.1 16.3 20.2 20.9 60 68.1 77 焦磷酸钠Na4P2O7 2.26硒酸钠Na2SeO413.3 25.2 26.9 77 81.8 78.6 74.8 73 72.7 硫酸钠Na2SO4 4.9 9.1 19.5 40.8 48.8 45.3 43.7 42.7 42.5 硫代硫酸钠Na2S2O371.5 73 77.6 90.8 97.2 溴酸镍Ni(BrO3)2·6H2O 28溴化镍NiBr2113 122 131 138 144 153 154 155 碳酸镍NiCO39.64×10-4氯酸镍Ni(ClO3)2111 120 133 155 181 221 308氯化镍NiCl253.4 56.3 66.8 70.6 73.2 81.2 86.6 87.6 氟化镍NiF2 2.55 2.56 2.56 2.59 碘酸镍Ni(IO3)20.74 0.062 1.43碘化镍NiI2124 135 148 161 174 184 187 188 硝酸镍Ni(NO3)279.2 94.2 105 119 158 187 188高氯酸镍Ni(ClO4)2105 107 110 113 117焦磷酸镍Ni2P2O7 1.017×10-3硫酸镍NiSO4·6H2O 44.4 46.6 49.2 55.6 64.5 70.1 76.7 乙酸钕(III) Nd(C2H3O2)3·H2O 26.2溴酸钕(III) Nd(BrO3)343.9 59.2 75.6 95.2 116氯化钕(III) NdCl396.7 98 99.6 102 105钼酸钕(III) Nd2(MoO4)3 1.9×10-3硝酸钕(III) Nd(NO3)3127 142 145 159 211硒酸钕(III) Nd2(SeO4)345.2 44.6 41.8 39.9 39.9 43.9 7 3.3硫酸钕(III) Nd2(SO4)313 9.7 7.1 5.3 4.1 2.8 2.2 1.213.硼、铍、钋、镨物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃硼酸H3BO3 5.7三氧化二硼B2O3 2.2碳酸铍BeCO30.218氯化铍BeCl242 42钼酸铍BeMoO4 3.02硝酸铍Be(NO3)297 102 108 113 125 178草酸铍BeC2O4·3H2O 63.5高氯酸铍Be(ClO4)2147硒酸铍BeSeO4·4H2O 49硫酸铍BeSO437 37.6 39.1 41.4 45.8 53.1 67.2 82.8 硫化钋(II) PoS 2.38×10-14乙酸镨(III) Pr(C2H3O2)3·H2O 32溴酸镨(III) Pr(BrO3)355.9 73 91.8 114 144氯化镨(III) PrCl3104钼酸镨(III) Pr2(MoO4)3 1.5×10-3硝酸镨(III) Pr(NO3)3112 162 178硫酸镨(III) Pr2(SO4)319.8 15.6 12.6 9.89 2.56 5.04 3.5 1.1 0.9114.氢、铅物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃砷化氢AsH38×10-2氯化氢HCl 81 75 70 65.5 61 57.5 53 50 47 43 40 硫化氢H2S 0.33乙酸铅Pb(C2H3O2)219.8 29.5 44.3 69.8 116叠氮化铅Pb(N3)2 2.49×10-2溴酸铅Pb(BrO3)27.92溴化铅PbBr20.45 0.63 0.86 1.12 1.5 2.29 3.32 3.86 4.55 碳酸铅PbCO37.27×10-5氯酸铅Pb(ClO3)2 3.7×10-2氯化铅PbCl20.67 0.82 1 1.2 1.42 1.94 2.54 2.88 3.2 铬酸铅PbCrO4 1.71×10-5亚铁氰化铅PbFe(CN)6 5.99×10-4氟化铅PbF2 4.63×10-2氟硅酸铅PbSiF6190 222 403 428 463 磷酸氢铅PbHPO4 3.46×10-4亚磷酸氢铅PbHPO3 2.19×10-2氢氧化铅Pb(OH)2 1.62×10-4碘酸铅Pb(IO3)2 2.4×10-3碘化铅PbI20.044 0.056 0.069 0.09 0.124 0.193 0.294 0.42钼酸铅PbMoO4 1.16×10-5硝酸铅Pb(NO3)237.5 46.2 54.3 63.4 72.1 91.6 111 133 草酸铅PbC2O4 6.50×10-4高氯酸铅Pb(ClO4)2·3H2O 440硒酸铅PbSeO40.0131硫酸铅PbSO4 3.84×10-3硫化铅PbS 6.77×10-13酒石酸铅PbC4H4O6 2.5×10-3硫氰酸铅Pb(SCN)20.553硫代硫酸铅PbS2O30.02钨酸铅PbWO4 2.84×10-2氢氧化铅(IV) Pb(OH)47.23×10-1115.铷物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃乙酸铷RbC2H3O286溴酸铷RbBrO3 3.6 5.1溴化铷RbBr 90 99 108 119 132 158氯酸铷RbClO3 2.1 3.1 5.4 8 11.6 22 38 49 63 氯化铷RbCl 77 84 91 98 104 115 127 133 143 铬酸铷Rb2CrO462 67.5 73.6 78.9 85.6 95.7重铬酸铷Rb2Cr2O7 5.9 10 15.2 32.3氟化铷RbF 300氟硅酸铷Rb2SiF60.157甲酸铷RbHCO2443 554 614 694 900碳酸氢铷RbHCO3110氢氧化铷RbOH 180碘酸铷RbIO3 1.96碘化铷RbI 144硝酸铷RbNO319.5 33 52.9 81.2 117 200 310 374 452 高氯酸铷RbClO4 1.09 1.19 1.55 2.2 3.26 6.27 11 15.5 22 高碘酸铷RbIO40.65硒酸铷Rb2SeO4159硫酸铷Rb2SO437.5 42.6 48.1 53.6 58.5 67.5 75.1 78.6 81.816.铯、钐、砷、铈、锶物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃乙酸铯CsC2H3O21010叠氮化铯CsN3307溴酸铯CsBrO30.21 3.66 4.53 5.3溴化铯CsBr 108氯酸铯CsClO3 3.8 6.2 9.5 13.8 26.2 45 58 79 氯化铯CsCl 146 175 187 197 208 230 250 260 271 铬酸铯Cs2CrO471.4氟化铯CsF 322氟硼酸铯CsBF40.818甲酸铯CsHCO2335 381 450 694碘酸铯CsIO3 2.6碘化铯CsI 44.1 58.5 76.5 96 124 150 190 205硝酸铯CsNO39.33 14.9 23 33.9 47.2 83.8 134 163 197 草酸铯Cs2C2O4313高氯酸铯CsClO40.8 1 1.6 2.6 4 7.3 14.4 20.5 30 高锰酸铯CsMnO40.228硒酸铯Cs2SeO4244硫酸铯Cs2SO4167 173 179 184 190 200 210 215 200 乙酸钐Sm(C2H3O2)3·3H2O 15溴酸钐Sm(BrO3)334.2 47.6 62.5 79 98.5氯化钐SmCl392.4 93.4 94.6 96.9硫酸钐Sm2(SO4)3·8H2O 2.7 3.1五氧化二砷As2O565.8三硫化二砷As2S3 4.454×10-4三氧化二砷As2O3 2乙酸铈Ce(C2H3O2)30.35氯化铈CeCl3100氢氧化铈Ce(OH)39.43×10-5碘酸铈Ce(IO3)30.123硝酸铈Ce(NO3)3234磷酸铈CePO47.43×10-11硒酸铈Ce2(SeO4)339.5 37.2 35.2 33.2 32.6 13.7 4.6硫酸铈Ce2(SO4)3·2H2O 21.4 9.84 7.24 5.63 3.87氢氧化铈Ce(OH)4 1.98×10-9乙酸锶Sr(C2H3O2)237 42.9 41.1 39.5 38.3 36.8 36.1 36.2 36.4 溴酸锶Sr(BrO3)2·H2O 30.9溴化锶SrBr285.2 93.4 102 112 123 150 182 223 氯酸锶SrClO3175氯化锶SrCl243.5 47.7 52.9 58.7 65.3 81.8 90.5 101 铬酸锶SrCrO48.5×10-20.09物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃甲酸锶Sr(HCO2)29.1 10.6 12.7 15.2 17.8 25 31.9 32.9 34.4 氢氧化锶Sr(OH)2·8H2O 0.91 1.25 1.77 2.64 3.95 8.42 20.2 44.5 91.2 碘酸锶Sr(IO3)20.19碘化锶SrI2165 178 192 218 270 365 383 钼酸锶SrMoO4 1.11×10-2硝酸锶Sr(NO3)239.5 52.9 69.5 88.7 89.4 93.4 96.9 98.4硒酸锶SrSeO40.656硫酸锶SrSO40.0113 0.0129 0.0132 0.0138 0.0141 0.0131 0.0116 0.0115硫代硫酸锶SrS2O3·5H2O 2.5钨酸锶SrWO4 3.96×10-417.铊、碳、铽、锑、铁、铜、钍物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃叠氮化亚铊TlN30.171 0.236 0.364溴酸亚铊TlBrO30.306溴化亚铊TlBr 0.022 0.032 0.048 0.068 0.097 0.117碳酸亚铊Tl2CO3 5.3氯酸亚铊TlClO3 2 3.92 12.7 36.6 57.3 氰化亚铊TlCN 16.8氟化亚铊TlF 78碳酸氢亚铊TlHCO3500氢氧化亚铊TlOH 25.4 29.6 35 40.4 49.4 73.3 106 126 150 碘酸亚铊TlIO30.0668碘化亚铊TlI 2×10-36×10-30.015 0.035 0.07 0.12 硝酸亚铊TlNO3 3.9 6.22 9.55 14.3 21 46.1 110 200 414 草酸亚铊Tl2C2O4 1.83高氯酸亚铊TlClO4 6 8.04 13.1 19.7 28.3 50.8 81.5磷酸亚铊Tl3PO40.15焦磷酸亚铊Tl4P2O740硒酸亚铊Tl2SeO4 2.17 2.8 8.5 10.8 硫酸亚铊Tl2SO4 2.73 3.7 4.87 6.16 7.53 11 14.6 16.5 18.4 钒酸亚铊TlVO30.87二氧化碳CO20.178一氧化碳CO 2.6×10-3硫酸铽Tb2(SO4)3·8H2O 3.56溴酸铽Tb(BrO3)3·9H2O 66.4 89.7 117 152 198三氟化锑SbF3385 444 562 水解三氯化锑SbCl3602 910 1090 1370 水解溴化亚铁FeBr2101 109 117 124 133 144 168 176 184 碳酸亚铁FeCO3 6.55×10-5氯化亚铁FeCl249.7 59 62.5 66.7 70 78.3 88.7 92.3 94.9 氟硅酸亚铁FeSiF6·6H2O 72.1 74.4 77 84 88 100 氢氧化亚铁Fe(OH)2 5.26×10-5硝酸亚铁Fe(NO3)2·6H2O 113 134草酸亚铁FeC2O4·2H2O 8×10-3高氯酸亚铁Fe(ClO4)2·6H2O 299硫酸亚铁FeSO4·7H2O 28.8 40 48 60 73.3 101 79.9 68.3 57.8砷酸铁FeAsO4 1.47×10-9氯化铁FeCl3·6H2O 74.4 91.8 107氟化铁FeF39.1×10-2氢氧化铁Fe(OH)3 2.10×10-9碘酸铁Fe(IO3)30.36硝酸铁Fe(NO3)3·9H2O 112 138 175物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃高氯酸铁Fe(ClO4)3289 368 422 478 722硫酸铁Fe2(SO4)3·9H2O 440氯化亚铜CuCl 9.9×10-3氰化亚铜CuCN 1.60×10-9氢氧化亚铜CuOH 8.06×10-7碘化亚铜CuI 2.0×10-5硫化亚铜Cu2S 1.36×10-15硫氰酸亚铜CuSCN 8.43×10-7溴化铜CuBr2107 116 126 128 131碳酸铜CuCO3 1.46×10-4氯酸铜Cu(ClO3)2242氯化铜CuCl268.6 70.9 73 77.3 87.6 96.5 104 108 120 铬酸铜CuCrO4 3.41×10-2氟化铜CuF27.5×10-2氟硅酸铜CuSiF673.5 76.5 81.6 84.1 91.2 93.2甲酸铜Cu(HCO2)212.5氢氧化铜Cu(OH)2 1.72×10-6碘酸铜Cu(IO3)2·2H2O 0.109硝酸铜Cu(NO3)283.5 100 125 156 163 182 208 222 247 高氯酸铜Cu(ClO4)2146硒酸铜CuSeO412 14.5 17.5 21 25.2 36.5 53.7亚硒酸铜CuSeO3 2.76×10-3硫酸铜CuSO4·5H2O 23.1 27.5 32 37.8 44.6 61.8 83.8 114 硫化铜CuS 2.4×10-17氟化钍(IV) ThF4·4H2O 0.914碘酸钍(IV) Th(IO3)4 3.69×10-2硝酸钍(IV) Th(NO3)4186 187 191硒酸钍(IV) Th(SeO4)2·9H2O 0.65硫酸钍(IV) Th(SO4)2·9H2O 0.74 0.99 1.38 1.99 318.锡、氙、锌、溴物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃溴化亚锡SnBr285氯化亚锡SnCl284氟化亚锡SnF230碘化亚锡SnI20.99 1.17 1.42 2.11 3.04 3.58 4.2 硫酸亚锡SnSO418.9氙Xe 24乙酸锌Zn(C2H3O2)230溴化锌ZnBr2389 446 528 591 618 645 672 碳酸锌ZnCO3 4.69×10-5氯酸锌Zn(ClO3)2145 152 209 223氯化锌ZnCl2342 353 395 437 452 448 541 614 氰化锌Zn(CN)2 5.8×10-2氟化锌ZnF2 1.6甲酸锌Zn(HCO2)2 3.7 4.3 6.1 7.4 11.8 21.2 28.3 38碘酸锌Zn(IO3)2·2H2O 7.75×10-2碘化锌ZnI2430 432 445 467 490 510 硝酸锌Zn(NO3)298 138 211草酸锌ZnC2O4·2H2O 2.52×10-3高锰酸锌Zn(MnO4)233.3硫酸锌ZnSO441.6 47.2 53.8 61.3 70.5 75.4 71.1 60.5 亚硫酸锌ZnSO3.2H2O 0.16酒石酸锌ZnC4H4O60.022 0.041 0.06 0.104 0.59一氯化溴BrCl 1.519.氩、氧、铟、钇、镱、银、铀、铕物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃氩Ar 4氧O2 1.52×10-2 1.17×10-29.4×10-37.8×10-3 6.2×10-3硫酸镱Yb2(SO4)344.2 37.5 22.2 17.2 10.4 6.4 5.8 4.7 乙酸钇Y(C2H3O2)3·4H2O 9.03溴酸钇Y(BrO3)3·9H2O 168溴化钇YBr363.9 75.1 87.3 101 116 123氯化钇YCl377.3 78.1 78.8 79.6 80.8氟化钇YF3 5.77×10-3硝酸钇Y(NO3)393.1 106 123 143 163 200硫酸钇Y2(SO4)38.05 7.67 7.3 6.78 6.09 4.44 2.89 2.2溴化铟InBr3571氯化铟InCl3210 212氟化铟InF311.2氢氧化铟In(OH)3 3.64×10-8碘酸铟In(IO3)3 6.7×10-2硫化铟In2S3 2.87×10-14乙酸银AgC2H3O20.73 0.89 1.05 1.23 1.43 1.93 2.59叠氮化银AgN37.93×10-4溴酸银AgBrO30.11 0.16 0.23 0.32 0.57 0.94 1.33溴化银AgBr 1.33×10-5碳酸银Ag2CO3 3.50×10-3氯酸银AgClO310.4 15.3 20.9 26.8氯化银AgCl 1.92×10-4 5.2×10-5亚氯酸银AgClO20.248铬酸银Ag2CrO4 2.16×10-3氰化银AgCN 1.47×10-7重铬酸银Ag2Cr2O70.159氟化银AgF 85.9 120 172 190 203硝酸银AgNO3122 167 216 265 311 440 585 652 733 草酸银Ag2C2O4 3.27×10-3氧化银Ag2O 1.3×10-3高氯酸银AgClO4455 484 525 594 635 793 高锰酸银AgMnO40.9硫酸银Ag2SO40.57 0.7 0.8 0.89 0.98 1.15 1.3 1.36 1.41 钒酸银AgVO3 1.46×10-2硫酸铀U(SO4)2.8H2O 11.9 17.9 29.2 55.8醋酸铀酰UO2(C2H3O2)2·2H2O 7.69氯化铀酰UO2Cl2320甲酸铀酰UO2(HCO2)2·H2O 7.2碘酸铀酰UO2(IO3)2·H2O 0.124物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃硝酸铀酰UO2(NO3)298 107 122 141 167 317 388 426 474 草酸铀酰UO2C2O40.45 0.5 0.61 0.8 1.22 1.94 3.16 硫酸铀酰UO2SO4·3H2O 21氢氧化铕Eu(OH)3 1.55×10-5硫酸铕Eu2(SO4)3·8H2O 2.5620.有机化合物物质化学式0℃10℃20℃30℃40℃50℃60℃70℃80℃90℃100℃D-半乳糖C6H12O610.3 68.3 苯胺C6H7N 3.6苯酚C6H5OH 8.3 混溶D-甘露糖C6H12O6248果糖C6H12O6375 538海藻糖C12H22O1168.9麦芽糖C12H22O11108棉籽糖C18H32O16?5H2O 14木糖C5H10O5117尿素CO(NH2)2108 167 251 400 733 D-葡萄糖C6H12O683乳糖C12H22O118蔗糖C12H22O11181.9 190.6 202 216.7 235.6 259.6 288.8 323.7 365.1 414.9 476化学基础数据-物质溶解度表21.酸碱盐溶解性表- 21 -。
氯化钠 氯化镁 氯化钾溶解度曲线
氯化钠、氯化镁和氯化钾是常见的无机盐,它们在水溶液中的溶解度受到温度的影响,因此可以通过实验得到它们的溶解度曲线。
下面,我们将分别介绍氯化钠、氯化镁和氯化钾在水中的溶解度曲线。
一、氯化钠在水中的溶解度曲线1. 实验方法:选取一定质量的氯化钠固体,逐渐加入一定体积的水,通过测定不同温度下溶液中的氯化钠浓度,得到氯化钠在不同温度下的溶解度数据。
2. 实验结果:实验结果表明,氯化钠在水中的溶解度随温度的升高而增加,符合一定的溶解度曲线规律。
3. 溶解度曲线特点:根据实验数据绘制氯化钠在水中的溶解度曲线,可以看出在较低温度下溶解度较低,随着温度的增加溶解度逐渐增大,但在一定温度范围内溶解度的增加速率逐渐减小。
二、氯化镁在水中的溶解度曲线1. 实验方法:与氯化钠相似,选取一定质量的氯化镁固体,逐渐加入一定体积的水,通过测定不同温度下溶液中的氯化镁浓度,得到氯化镁在不同温度下的溶解度数据。
2. 实验结果:实验结果表明,氯化镁在水中的溶解度随温度的升高而增加,但增加的速率相对较大,溶解度的增加呈现较为明显的趋势。
3. 溶解度曲线特点:根据实验数据绘制氯化镁在水中的溶解度曲线,可以观察到在较低温度下溶解度较低,随着温度的增加溶解度迅速增大,增加速率较大,但在高温下溶解度的增加速率开始减小。
三、氯化钾在水中的溶解度曲线1. 实验方法:同样选取一定质量的氯化钾固体,逐渐加入一定体积的水,通过测定不同温度下溶液中的氯化钾浓度,得到氯化钾在不同温度下的溶解度数据。
2. 实验结果:实验结果表明,氯化钾在水中的溶解度随温度的升高而增加,但增加的速率相对较小,溶解度的增加呈现较为平缓的趋势。
3. 溶解度曲线特点:根据实验数据绘制氯化钾在水中的溶解度曲线,可以观察到在较低温度下溶解度较低,随着温度的增加溶解度缓慢增大,增加速率较小,但在高温下溶解度的增加速率更为缓慢。
氯化钠、氯化镁和氯化钾在水中的溶解度受到温度的影响,其溶解度曲线表现出不同的特点。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
物质水溶液溶解度表
以化学品中特征元素的拼音顺序排列。
所有数据都为1atm下水溶液溶解度的数据,单位为g/100cm3
1.锕、氨、铵 (2)
2.钯、钡、铋、铂、钚 (3)
3.氮、镝 (4)
4.铒 (4)
5.钒 (4)
6.钆、钙、锆、镉、铬、汞、钴、硅 (4)
7.铪、氦、钬 (7)
8.镓、钾、金 (7)
9.钪 (8)
10.镧、锂、硫、镥、铝 (9)
11.镁、锰 (9)
12.钠、镍、钕 (10)
13.硼、铍、钋、镨 (12)
14.氢、铅 (12)
15.铷 (13)
16.铯、钐、砷、铈、锶 (14)
17.铊、碳、铽、锑、铁、铜、钍 (15)
18.锡、氙、锌、溴 (17)
19.氩、氧、铟、钇、镱、银、铀、铕 (19)
20.有机化合物 (22)
21.酸碱盐溶解性表 (23)
1.锕、氨、铵
2.钯、钡、铋、铂、钚
3. 氮、镝
4.铒
5.钒
6.钆、钙、锆、镉、铬、汞、钴、硅
7.铪、氦、钬
8.镓、钾、金
9.钪
10.镧、锂、硫、镥、铝
11.镁、锰
12.钠、镍、钕
13.硼、铍、钋、镨
14.氢、铅
15.铷
16.铯、钐、砷、铈、锶
17.铊、碳、铽、锑、铁、铜、钍
18.锡、氙、锌、溴
19.氩、氧、铟、钇、镱、银、铀、铕
20.有机化合物
化学基础数据-物质溶解度表
- 21 - 21.
酸碱盐溶解性表。
