A LCD Response Time Compensation Feature
Circadian and photic regulation of clock and clock-controlled proteins

Circadian and photic regulation of clockand clock-controlled proteins in the suprachiasmatic nuclei of calorie-restricted miceJorge Mendoza,Paul Pe´vet and Etienne ChalletInstitut de Neurosciences Cellulaires et Inte´gratives,De´partement de Neurobiologie des Rythmes,UMR7168⁄LC2,CNRS et Universite´Louis Pasteur,5rue Blaise Pascal,67084Strasbourg cedex,FranceKeywords:calorie restriction,circadian rhythms,clock proteins,photic resettingAbstractIn mammals,behavioural and physiological rhythms as well as clock gene expression in the central suprachiasmatic clock(SCN)are phase-shifted by a timed calorie restriction(T-CR;animals receiving at midday66%of their daily food intake).The molecular mechanism of SCN depends on feedback loops involving clock genes and their protein products.To understand how T-CR mediates its synchronizing effects,we examined the rhythmic expression of three clock proteins,PERIOD(PER)1,2and CLOCK,and one clock-controlled protein(i.e.vasopressin;AVP)in the SCN of mice either fed ad libitum(AL)or with T-CR.Moreover,we evaluated expression of these proteins in the SCN of AL and T-CR mice following a1-h light pulse.The results indicate that,while PER1and AVP rhythms were phase-advanced in T-CR mice,the PER2rhythm showed an increased amplitude.CLOCK was expressed constitutively in AL mice while in T-CR it was significantly reduced,especially after feeding time.A light pulse produced a delayed increase in PER1and a larger increase in PER2expression in the SCN of T-CR mice than in AL animals.In addition,light exposure triggered an increase in AVP-ir cells in both AL and T-CR mice,and also of CLOCK expression but in T-CR mice only.The circadian changes in clock and clock-controlled proteins and their acute responses to light in the SCN of T-CR mice demonstrate that metabolic cues induced by a calorie restriction modulate the translational regulation of the SCN clock.IntroductionIn mammals,the circadian timing system is hierarchical,with the main clock located in the suprachiasmatic nuclei(SCN)of the hypothalamus and subordinate oscillators in some cerebral structures and peripheral tissues(Hastings et al.,2003).The clockwork in the SCN operates in a self-sustained fashion,involving interacting positive and negative transcriptional and post-translational feedback loops(Reppert& Weaver,2001).In the positive loop,the transcription factors CLOCK and BMAL1drive rhythmic transcription of Period(Per1–3)and Cryptochrome genes(Cry1–2;Lowrey&Takahashi,2004).PERs and CRYs form heterodimers that translocate to the nucleus,where CRYs act as negative regulators by interacting with CLOCK and⁄or BMAL1, forming a negative feedback loop(Kume et al.,1999;Shearman et al., 2000).The nuclear orphan receptor genes Rev-erb a and Ror a are activated by the CLOCK:BMAL1heterodimer to produce proteins that have opposing actions on Bmal1transcription(Preitner et al.,2002; Sato et al.,2004).Transcription of clock-controlled genes such as arginine vasopressin(Avp)is also regulated by CLOCK:BMAL1(Jin et al.,1999).Light is the most potent cue for the entrainment of the SCN(Daan&Aschoff,2001).Retinal ganglion cells project directly to the SCN via the retinohypothalamic tract(Hannibal,2002).Light resetting of the mammalian SCN clock involves acute induction of immediate–early genes(Kornhauser et al.,1990)and Per genes (Albrecht et al.,1997;Shigeyoshi et al.,1997).On the other hand, temporally restricted feeding(i.e.limited duration of daily food access) is a strong synchronizer for rhythmic gene expression in peripheral oscillators(Damiola et al.,2000;Stokkan et al.,2001).Under constant darkness conditions(but not in a light–dark cycle),temporally restricted feeding or a palatable diet in addition to regular food ad libitum are able to entrain the SCN clock(Mistlberger,1994;Holmes& Mistlberger,2000;Castillo et al.,2004;Caldelas et al.,2005;Lamont et al.,2005;Mendoza et al.,2005a).In contrast to temporally restricted feeding paradigms,which do not reduce calorie intake and which allow enough food to be eaten daily,a calorie restriction is known to be responsible for many beneficial physiological changes(slowing of ageing,extended lifespan,delayed onset of major age-related diseases) in various species(Bordone&Guarente,2005).In rats and mice under a light–dark cycle,a timed calorie restriction(i.e.66%of the animal’s daily food intake given each day at the same time),but not temporally restricted feeding,leads to phase advances of behavioural and physiological circadian rhythms,whatever the feeding time over the daily cycle(Challet et al.,1997,1998).Moreover,the temporal organization of the SCN clockwork and its behavioural and clock-gene responses to light are affected by calorie restriction(Mendoza et al., 2005b),suggesting that these effects are related to calorie reduction. Clock mechanisms depend on the regulation not only of clock gene mRNA but also of their protein products.In order to characterize possible SCN translational modifications induced by a timed calorie restriction,we thus investigated the rhythmic expression of clock proteins and their photic responses in the SCN of mice.Correspondence:Dr Jorge Mendoza,as above.E-mail:jmendoza@neurochem.u-strasbg.frReceived1March2007,revised12April2007,accepted10May2007European Journal of Neuroscience,Vol.25,pp.3691–3701,2007doi:10.1111/j.1460-9568.2007.05626.x ªThe Authors(2007).Journal CompilationªFederation of European Neuroscience Societies and Blackwell Publishing LtdMaterials and methodsAnimals and housingAdult male C3H mice(Charles River,Lyon,France)werefirst housed in groups offive with food and water available ad libitum.After 15days of habituation,mice were then housed singly in cages equipped with a running wheel(10cm diameter)in light-proof, ventilated chambers(23±1°C)under a12–12h light–dark cycle (LD;light,200lux;darkness,0lux).Under LD conditions,zeitgeber time(ZT)was defined relative to lights-on(ZT-0)and lights-off (ZT-12).All experiments were performed in accordance with the Principles of Laboratory Animal Care(National Institutes of Health publication86–23,revised1985)and the French national laws. Experimental designIn thefirst experiment,after3weeks of basal conditions,mice were randomly divided into two groups:a group of mice fed ad libitum (AL)with a mean spontaneous food intake of4.6±0.04g and a timed calorie-restricted group(T-CR)which was given66%(i.e.3.0±0.02g)of the daily food intake at ZT-6.After3weeks of calorie restriction,T-CR mice as well as the AL control mice were killed and brains were removed for immunohistochemistry.Sampling was performed at six temporal points with4-h intervals(ZT-0,-4,-8, -12,-16and-20)over a24-h cycle(n¼4per group and temporal point).Because sampling started at ZT-0,animals killed at ZT-0and ZT-4could not be fed at ZT-6on the day of killing in contrast to animals killed at other ZT points.In the second experiment,for the analysis of clock proteins during light resetting we used other groups of mice under the same light(LD 12:12)and feeding conditions(AL vs.T-CR)as in the previous experiment.At the end of3weeks under each feeding condition, animals were exposed to a1-h light pulse(200lux)at ZT-16and were subsequently killed in darkness at two different times(i.e.4or8h after the onset of the light pulse)at ZT-20and ZT-0(n¼6T-CR and n¼4AL for each time point).According to the molecular phase response curve to light in mice under T-CR(Mendoza et al.,2005b), ZT-16is close to the phase when light leads to the most marked change(reduced photic induction)of Per1mRNA levels in the SCN, and the only time point studied when Per2mRNA levels were affected (increased photic induction)by T-CR compared to AL conditions. According to the behavioural phase response curve to light,ZT-16is close to the time when light led to similarly large phase delays in the locomotor activity rhythm in both AL and T-CR mice(Mendoza et al., 2005b).This similar resetting response contrasts with the altered light-induced phase shifts at other nocturnal ZT points in T-CR,when Per2 expression is not triggered by light.The lack of differences between AL and T-CR mice in behavioural phase delays induced by light at ZT-15(Mendoza et al.,2005b)may be specifically due to the overexpression of Per2by light(as a compensation for reduced photic induction of Per1?).Therefore,although not evaluated,we did not necessarily expect alteration in behavioural phase shifts after light exposure at ZT-16between AL and T-CR mice. ImmunohistochemistryAt the selected intervals,animals were killed with an isoflurane overdose and perfused transcardially with50mL of0.9%saline followed by50mL of cold4%paraformaldehyde(PAF)in0.1m phosphate buffer(pH7.4).Brains were removed,postfixed overnight in4%PAF at4°C and transferred to a cryoprotectant buffered sucrose solution(30%)for72h at4°C.Brains were then frozen in isopentane at)60°C and stored at)80°C.Four series of30-l m coronal cryosections through the SCN were prepared on a cryostat at)19°C and collected in0.1m phosphate-buffered saline.Free-floating sections were washed in cold50-m m Tris-buffered saline(TBS; pH7.4;Sigma,St Louis,USA)and incubated in a solution of3%of H2O2(Sigma)in TBS for30min at room temperature.Brain sections were then rinsed in TBS,and incubated for2h in a blocking solution in3%normal horse or goat serum(NHS or NGS)and5%of bovine serum albumin in TBS with0.1%Triton X-100(0.1%TBS-X), followed by an incubation in the primary antibody(in0.1%TBS-X plus NHS or NGS)for48h at4°C.We used a goat polyclonal anti-PER1antibody(1:2000,raised against an epitope mapping the N-terminus of human Per1,SC-7724;Santa Cruz Biotechnology, Santa Cruz,CA,USA),a rabbit polyclonal anti-PER2(1:1000, affinity-purified raised against an epitope mapping the C-terminus of mouse Per2;Alpha Diagnostic International,San Antonio,TX,USA), a goat polyclonal anti-CLOCK(1:1000,raised against an epitope mapping the N-terminus of clock of mouse origin,SC-6927;Santa Cruz Biotechnology),and a rabbit anti-arginine vasopressin(A VP; Truus,1:20000,a gift from Dr Ruud Buijs,Netherlands Institute for Brain Research,Amsterdam,the Netherlands).Following incubation in the primary antibody,sections were rinsed in TBS-X and incubated for2h at4°C with a biotinylated antirabbit IgG made in goat,or antigoat IgG made in horse(Vector Laboratories,Burlingame,CA, USA),diluted1:500with0.4%TBS-X.Following incubation with secondary antibody,sections were rinsed in TBS-X and incubated for 1h at room temperature with an avidin–biotin–peroxidase complex (Vectastain Standard Elite ABC Kit;Vector).Following incubation with the ABC reagents,sections were rinsed twice for10min in TBS, and incubated with0.025%3,3¢-diaminobenzidine(Sigma)with 0.01%H2O2in50-m m Tris buffer.After thisfinal incubation,sections were rinsed with TBS,wet-mounted onto gel-coated slides,dehydra-ted through a series of alcohols,soaked in xylene,and coverslipped. The specificity of antibodies was established in mouse brain by preadsorption control experiments.Antibody binding to antigen was blocked by adding the PER1or CLOCK peptides(PER1,sc-7724-P; CLOCK,sc-6927-P;1mg⁄mL,both diluted1:100;Santa Cruz Biotechnology),the PER2peptide(Mouse PER2blocking peptide, Alpha Diagnostic International;1mg⁄mL,diluted1:100)and the vasopressin peptide(H-1780,1mg⁄mL,diluted1:10;Bachem, Bubendorf,Switzerland)to the primary incubation solution.Addition of each peptide prevented respective immunostaining in the SCN. Cell and protein quantificationSections were viewed using a monitoring CCD video camera with Viewfinder Lite software coupled to a microscope(Leica).For the image analysis we used NIH ImageJ software.Positive cells(cells were considered positive when the signal was>3·the background value)were counted in the whole central SCN(two or three sections per animal),based upon the mouse brain atlas(from bregma )0.46mm;Paxinos&Franklin,2001).To estimate the number of stained nuclei,a standard-sized oval encompassing the whole medial SCN was placed over one SCN,and the number of cells within the oval was obtained.The number of immunoreactive(ir)nuclei was expressed as the mean number of immunopositive cells per section.In addition,because not only A VP-positive cells but also A VPfibres are present in the SCN,and these may affect cell counting,in a second analysis expression of A VP was quantified by measuring staining density.The optical density(OD)of A VP-ir cells in the SCN was3692J.Mendoza et al.ªThe Authors(2007).Journal CompilationªFederation of European Neuroscience Societies and Blackwell Publishing LtdEuropean Journal of Neuroscience,25,3691–3701measured in the same sections as for the cell number analysis.The OD was expressed in arbitrary units corresponding to grey levels.To calculate the OD,the background intensity of staining was subtracted from the intensity of staining in the middle SCN.The background intensity was measured in an area devoid of A VP cell bodies or fibres (lateral hypothalamic area)in the same coronal section as the SCN analysis.Statistical analysisThe data from all experiments were evaluated using one-,two-or three-way anova followed by an LSD post hoc test,corrected for multiple comparisons.A value of P <0.05was considered statistically significant.Statistical analysis was performed using the statistical package Statistica version 4.5(StatSoft,1993).ResultsBody weight was reduced significantly ( 20%)in T-CR mice during the first week of food restriction and remained stable up to the end of the experiment (Fig.1).Repeated-measures anova showed significant differences between groups and time (Group,F 1,47¼45.2,P <0.001;Time,F 5,235¼70.5,P <0.001).Such a body weight loss is fully reversible after 1week of food ad libitum (Challet et al .,1998;Mendoza et al .,2005b).Circadian and clock-controlled protein expression in calorie-restricted micePER1expression was examined through the SCN in animals under AL and T-CR conditions.One-way anova showed significant diurnal expression of PER1expression in both AL and T-CR mice (Table 1).Although PER1was not significantly different between groups (Group effect,F 1,37¼1.38,NS),there was a trend for a reduced expression of PER1in T-CR animals compared to AL animals (Fig.2).Furthermore,there was a significant difference for the factor time and for thegroup ·time interaction (Time effect,F 5,37¼274.1,P <0.01;interaction,F 5,37¼13.8,P <0.01).PER1acrophase that occurred at ZT-12in AL mice appeared to be phase-advanced to ZT-8in T-CR animals in keeping with changes of Per mRNA patterns that we previously reported (Mendoza et al .,2005b).As illustrated in Fig.2,the abundance of PER2-ir nuclei in the SCN revealed a pronounced daily rhythm under both AL and T-CR conditions (Time effect,F 5,37¼196.3,P <0.01;Table 1).The acrophase of the cycle under both feeding conditions occurred after the light–dark transition,slightly later than that of PER1.The daily peak in PER2expression was modulated by T-CR,leading to an increased amplitude compared to AL conditions (Fig.2).This amplitude difference was reflected in a significant difference between groups (Group effect,F 1,37¼18.4,P <0.01)and a significant interaction of group ·time (interaction,F 5,37¼4.2,P <0.01).Expression of CLOCK-ir was high throughout the SCN at all ZT in AL mice (Fig.3).In contrast,CLOCK-ir expression in the SCN of T-CR mice was slightly rhythmic,with decreased levels at ZT-8(Fig.3;Time effect,F 5,37¼2.6,P <0.05;Table 1).The total number of CLOCK-ir nuclei in AL mice was larger than that in T-CR mice (Group effect,F 1,37¼9.3,P <0.01),indicating that the constitutive level of CLOCK in the SCN cells can be changed by feeding conditions.In both AL and T-CR mice,a rhythm of A VP-positive cells was found essentially in the dorsal region of the SCN (Fig.3;Time effect,F 5,37¼4.4,P <0.002;Table 1).The anova does not show a difference between groups (Group effect,F 1,37¼2.1,NS),but there was a significant difference in the group ·time interaction (interac-tion,F 5,37¼5.5,P <0.001),revealing a clear phase advance of the rhythm in T-CR mice (Fig.3).Of note,a small population of A VP-ir cells was detected in the ventrolateral region of the middle SCN (see arrow in Fig.3).In addition,these results were checked with anova on the OD data;this showed a clear difference between groups,time and the interaction group ·time (Group effect,F 1,37¼6.2,P <0.01;Time effect,F 5,37¼9.1,P <0.001;interaction:F 5,37¼12.6,P <0.001),confirming a phase advance of the A VP rhythm by the T-CR (data not shown).Responses of circadian proteins to light in calorie-restricted miceIn both AL and T-CR animals,1h of light exposure at ZT-16led to a marked increase in PER1-ir in the medial SCN (Fig.4;F 1,28¼260.9,P <0.001)The effect of light was significant 4and 8h later (P <0.05).In T-CR mice,light induction of PER1intheTable 1.Statistical analysis of the diurnal expression of clock and clock-controlled proteins in the SCN of mice under ad libitum and T-CRF -valueP -value Ad libitum (F 5,18)PER-1302.60.001*PER-271.50.001*CLOCK 0.40.8A VP 7.00.001*T-CR (F 5,19)PER-192.00.001*PER-2131.50.001*CLOCK 3.80.01*A VP3.30.02**P <0.05,one-way anova .Timed calorie restriction alters the SCN clock 3693ªThe Authors (2007).Journal Compilation ªFederation of European Neuroscience Societies and Blackwell Publishing Ltd European Journal of Neuroscience ,25,3691–3701SCN was lower than in AL mice at ZT-20but higher at ZT-0(Fig.4).In addition,in an analysis for the rostral and caudal SCN regions the difference was significant only at ZT-0(P <0.001;data not shown).Light exposure increased the number of PER2-ir cells in the SCN of both AL and T-CR mice at ZT-20(Fig.5;F 1,28¼96.3,P <0.001).In T-CR mice,the light-increased number of PER2-ir cells was higher than in AL animals at ZT-20(Fig.5;P <0.05).The number of PER2-ir cells 8h after light exposure (i.e.ZT-0)was lower than at ZT-20in the SCN from both AL and T-CR mice (P <0.001),although the values were still higher than those in dark control animals.The same effect was present in rostral and caudal SCN (rostral,F 1,28¼153.2,P <0.001;caudal,F 1,28¼107.5,P <0.001;data not shown).Incidentally,we observed that PER2-ir in response to light was expressed in the SCN regions overlapping those cells expressing A VP (including a small ventrolateral region;see arrow in Fig.5).Fig .2.Rhythmic expression of PER1and PER2in the SCN of AL and T-CR mice.(A)Photomicrographs,and (B)mean (±SEM)number of PER1-and PER2-ir nuclei in the SCN from mice under a 12-h LD cycle and AL (black symbols)or T-CR (white symbols)at different ZTs (ZT-0,lights on;ZT-12,lights off).Black and white top bars indicate the LD cycle.Vertical arrow indicates the mealtime.*P <0.05.Scale bar,200l m.3694J.Mendoza et al.ªThe Authors (2007).Journal Compilation ªFederation of European Neuroscience Societies and Blackwell Publishing LtdEuropean Journal of Neuroscience ,25,3691–3701The CLOCK-ir cell number in the SCN was increased4h after the light pulse(Fig.6;F1,28¼15.8,P<0.001).Moreover,this increase depended on feeding conditions.The number of CLOCK-ir cells in the SCN at ZT-20was significantly increased in T-CR mice(P<0.001). In contrast,the small increase in CLOCK induction at ZT-0in the SCN of AL mice exposed to light was not significant(P¼0.08).In the rostral and caudal SCN no differences following either light treatment or feeding conditions were present(data not shown).A VP-ir cell number in the medial SCN was increased8h after light stimulation(Fig.7;F1,26¼22.2,P<0.001)in both AL and T-CR animals.No difference related to feeding conditions was found (F1,26¼0.9,NS).The A VP-ir increase in response to lightwasevident in the dorsomedial SCN,including the small set of cells in the ventrolateral region which also expressed PER2in response to light stimulation (see arrow in Fig.7).DiscussionIn mice,restricted feeding without calorie restriction may affect the behavioural phase angle of photic entrainment (Holmes &Mistlberger,2000;Sharma et al .,2000),while no clear effect of a timed normocaloric feeding was reported on clock gene expression in the SCN (Mendoza et al .,2005b).This difference on the effects at behavioural and molecular levels of these studies could reside in the feeding paradigms used.In our previous experiment (Mendoza et al .,2005b),100%of the daily food intake of the mice was given each day at the same time (i.e.normocaloric feeding)while in the behavioural study reported by Holmes &Mistlberger (2000)mice were under a temporally restricted feeding with 3h of daily food access.It will be interesting in further studies to investigate clock gene expression in mice exposed to this temporal restriction of food access.By contrast,timed calorie restriction (T-CR)modifies entrainment of the SCN to light,at both behavioural and molecular levels,as reflected by transcriptional alteration of the SCN clockwork (Challet et al .,1998;Mendoza et al .,2005b).Here we confirmed and extended our previous data by showing that T-CR also affects translational regulation of SCN clockwork and its light resetting,thus demonstrating that metabolic cues induced by a calorie restriction interact with synchronization tolight.Fig .4.Light exposure stimulated PER1translation in the mouse SCN.(Top)Representative photomicrographs show coronal sections through the middle SCN of mice kept in an LD cycle,under AL or T-CR conditions,and exposed to a 1-h light pulse at ZT-16.Mice were killed 4or 8h after the beginning of the light pulse or dark control exposure (at ZT-20and ZT-0,respectively);OC,optic chiasm;3V ,third ventricle.(Bottom)Semi-quantitative analysis of PER1-ir cells in the mouse SCN 4and 8h after 1h light exposure at ZT-16(white bars)or after exposure to darkness (black bars)in mice under AL or T-CR conditions.Data are presented as the mean ±SEM.Statistical differences between groups were determined by three-way anova followed by an LSD post hoc test.*P <0.05for differences between light conditions;#P <0.05between feeding conditions.Scale bar,200lm.Fig . 5.Light exposure triggered PER2translation in the mouse SCN.(Top)Representative photomicrographs show coronal sections through the SCN at central level of mice kept in an LD cycle,under AL or T-CR conditions,and exposed to a 1-h light pulse at ZT-16.Mice were killed 4or 8h after the beginning of the light pulse or control dark exposure (at ZT-20and ZT-0,respectively).Arrow shows the small group of cells in the A VP ventrolateral region of SCN expressing PER2.(Bottom)Semi-quantitative analysis of PER2-ir cells in the mouse SCN 4and 8h after 1h light exposure at ZT-16(white bars)or after exposure to darkness (black bars)in mice under AL or T-CR conditions.*P <0.05for differences between light conditions;#P <0.05between feeding conditions.For details see Fig.4.3696J.Mendoza et al.ªThe Authors (2007).Journal Compilation ªFederation of European Neuroscience Societies and Blackwell Publishing LtdEuropean Journal of Neuroscience ,25,3691–3701Altered daily pattern of circadian and clock-controlled proteins during T-CRIn AL mice,rhythmic expression of PER1and PER2has overlapping distribution within the SCN,in accordance with previous findings (Field et al .,2000;LeSauter et al .,2003;Maywood et al .,2003).PER1rhythm was phase-advanced in T-CR mice in comparison with control-fed animals.Moreover,the amplitude of the rhythm was modified by feeding conditions,showing a reduction in the cell number in almost the whole SCN from T-CR mice.Thus,there was a global effect of T-CR on the SCN clockwork without any apparent region-specific response.Because a normocaloric feeding does not change the daily oscillation of Per1expression in the SCN (Mendoza et al .,2005b),this suggests that food intake per se does not affect clock gene expression in the SCN.Therefore,it is unlikely that PER1changes found in the SCN of T-CR mice after feeding time (ZT-8and ZT-12)are due to the fact that mice sampled at these times were fed on the day of killing.If no food at all was provided for the groups of mice sampled from ZT-8to ZT-20,we would not have been able to avoid any possible effect of a longer duration of fasting (ranging from 26to 38h of fasting)compared to the daily fasting conditions (i.e.<24h)under T-CR conditions.The phase and amplitude effects observed at both mRNA and protein levels indicate that the timing and amplitude of PER1oscillation entrained to a LD cycle are changed by T-CR.In T-CR mice,consistent with the lack of shifting effect of T-CR on mRNA Per2expression (Mendoza et al .,2005b),daily expression of PER2does not differ between AL and T-CR.However,the amplitude of PER2oscillation was higher in T-CR mice than in AL mice.The mechanism leading to this PER2up-regulation is not clear.A recent paper raises the possibility that PER1represses light-inducedPER2Fig . 6.Light exposure increased CLOCK translation in the mouse SCN of T-CR mice.(Top)Representative photomicrographs show coronal sections through the SCN of mice kept in an LD cycle,under AL or T-CR conditions,and exposed to a 1-h light pulse at ZT-16.The light pulse induced a small but significant increase in CLOCK-ir in the SCN 4h after exposure in T-CR mice.(Bottom)Semi-quantitative analysis of CLOCK-ir cells in the mouse SCN 4and 8h after a 1-h light exposure at ZT-16(white bars)or after exposure to darkness (black bars)in mice under AL or T-CR conditions.*P <0.05for differences between light conditions.For details see Fig.4.Fig .7.Light exposure increased A VP translation in the mouse SCN of both AL and T-CR mice.(Top)Representative photomicrographs show coronal sections through the medial SCN of mice kept in an LD cycle,under AL or T-CR conditions,and exposed to a 1-h light pulse at ZT-16.Arrow shows the small group of cells in the ventrolateral region of SCN expressing A VP.The light pulse induced a significant increase in A VP-ir in the SCN 8h after exposure in both AL and T-CR mice.(Bottom)Semi-quantitative analysis of A VP-ir cells in the mouse SCN after a 1-h light exposure at ZT-16(white bars)or after exposure to darkness (black bars)in mice under AL or T-CR conditions.*P <0.05for differences between light conditions.For details see Fig.4.Timed calorie restriction alters the SCN clock 3697ªThe Authors (2007).Journal Compilation ªFederation of European Neuroscience Societies and Blackwell Publishing Ltd European Journal of Neuroscience ,25,3691–3701and attenuates phase-resetting by light(Masubuchi et al.,2005).The apparent lower amplitude of PER1expression could then partly be involved in the up-regulation of PER2expression.Moreover,acute photic responses of PER2further support this view,because its levels were increased in T-CR mice compared to AL animals.Nevertheless, even if light exposure leads to increased responses of both PER1and PER2expression,the difference in the entrainment patterns suggests that the two proteins respond to T-CR in different fashions. Furthermore,SCN resetting by T-CR has been reported to be altered in both Per1-and Per2-mutant mice(Feillet et al.,2006).Whereas Per1-mutant mice entrained to a T-CR do not show phase advances of the locomotor activity rhythm after transfer to constant darkness, Per2-mutant mice submitted to T-CR show instead larger phase advances than wild-type mice(Feillet et al.,2006).Taken together, these data and our results suggest that PER1and PER2are implicated in the phase-shifting effects of T-CR.The cellular redox state can modulate binding of CLOCK:BMAL1 (heterodimers)to DNA(Rutter et al.,2002).The circadian PER translation could then be modified by T-CR,because a chronic hypocaloric diet may affect the cellular metabolic state of the SCN clock. CLOCK is a nuclear protein expressed constitutively in the SCN of AL mice,contrasting with the rhythmic expression of PER1and PER2 (Field et al.,2000;Maywood et al.,2003;present study).It has already been proposed that CLOCK participates in resetting by T-CR because heterozygous Clock⁄+mice do not show phase advances of the circadian rhythm of locomotor activity induced by T-CR(Challet et al.,2000).Here we show that CLOCK expression was affected by T-CR,showing a general reduction in the number of immunopositive cells over the daily cycle in the SCN,with a more marked reduction 2h after mealtime.In hamsters,a short photoperiod has been shown to induce a circadian rhythm of Clock mRNA in the SCN with low levels from the beginning to the middle of light phase,suggesting that Clock is implicated in the SCN adaptation to photoperiodic changes(Tournier et al.,2003).Under natural short photoperiods,there is a reduction in food availability that is associated with many physiological changes (e.g.decreased body temperature and metabolic state,and weight loss;Malan,1978;Pe´vet,1987;Goldman,2001).Moreover,torpor produces changes in SCN clock genes in Siberian hamsters(Herwig et al.,2006)and its occurrence can be increased by food restriction (Paul et al.,2004).In mice,which are not considered a photoperiodic species,changes in body temperature(Feillet et al.,2006)and body weight as well as modifications of CLOCK expression in the SCN under T-CR conditions(present study)may reflect a short photo-period-like hypometabolic condition in this species.This hypothesis is supported by the fact that the nocturnal peak in pineal melatonin in T-CR mice is not only phase-advanced but is also longer(i.e.short photoperiod-like)than that in AL mice,in spite of similar LD (12–12h)conditions(Mendoza et al.,2005b).In nocturnal rodents fed ad libitum,A VP mRNA and protein oscillations in the SCN have been reported to peak at the middle and the end of the day,respectively(Dardente et al.,2004;Van der Zee et al.,2005).In the present study,we found that daily levels of A VP show a phase advance in T-CR mice compared to AL mice,in full accordance with previous data on A VP mRNA(Mendoza et al., 2005b).Moreover,we noted a reduction in the daily amplitude of A VP immunoreactivity during T-CR.Consistent with thisfinding,a daytime decrease in A VP expression has already been reported in rats under calorie restriction at both mRNA and protein levels(Andrade et al., 2004).In addition,the A VP release pattern from SCN is reduced in food-restricted rats(Kalsbeek et al.,2000).Taken together,these results show that food restriction,coupled or not with calorie restriction,decreases SCN A VP synthesis and release,indicating damped oscillations of this circadian output.Altered light induction of circadian proteins during T-CRLight resetting is associated with an up-regulation of Per mRNA in the SCN during the night(Albrecht et al.,1997;Shigeyoshi et al.,1997; Dardente et al.,2002;Yan&Silver,2002;Hamada et al.,2004).In mice fed ad libitum,a phase-delaying light pulse has been shown to increase PER1and PER2levels within4–9h(Field et al.,2000; Harmar et al.,2002;Yan&Silver,2004),with the number of positive cells similar to the present data(e.g.Harmar et al.,2002).In response to1-h light exposure,expression of PER1and PER2 changed depending on the feeding condition and on the time after light pulse.In T-CR animals,light-induced PER1expression was delayed, but it still occurred in the ventral SCN(i.e.in retinorecipient, vasoactive intestinal polypeptide-and gastrin-releasing peptide-con-taining cells;Dardente et al.,2002;Yan&Okamura,2002;Yan& Silver,2002;Karatsoreos et al.,2004),as observed in AL mice.This regional response is in accordance with previous studies in AL animals (von Gall et al.,2003;Yan&Silver,2004).Moreover,in T-CR mice we found that PER1expression was increased8h after light exposure whereas it was lower4h earlier.We have previously reported a reduced induction of Per1transcription in the SCN of T-CR mice1h after light exposure(Mendoza et al.,2005b).The present time course at the translational level supports the interpretation that light induction of Per1mRNA response could actually be delayed instead of being simply reduced.Moreover,because PER1oscillation in the SCN clock is advanced at least by1h in T-CR mice(present study),photic stimulation may have occurred at a slightly different circadian time. This suggests that the different PER1induction by light between AL and T-CR mice might be due to a different SCN phase at the moment of stimulation.This point,however,does not explain the delay of PER1 induction in T-CR mice that we attribute to the effects of calorie restriction.Moreover,according to the behavioural phase response curve to light,both AL and T-CR mice at ZT-16were still in the phase-delaying zone of light resetting(Mendoza et al.,2005b).For PER2expression at ZT-20,the responses to light were higher in the SCN of T-CR mice than AL animals.This specific light response profile of PER2is consistent with our data on mRNA(Mendoza et al.,2005b). Similar to previous results obtained in mice exposed to light at circadian time16(Yan&Silver,2004),PER2expression was preferentially triggered in the dorsal part(i.e.A VP cells).We also noticed light-induced PER2expression in the small ventrolateral region expressing A VP. Light exposure has been already shown to increase Clock mRNA levels in the rat SCN,suggesting that Clock is somehow involved in photic signal transduction of the circadian clock(Abe et al.,1999). However,we could not confirm this result at the translational level because no significant light-induced CLOCK expression was clearly detected in AL mice.By contrast,there was a light-induced increase in CLOCK cells in the SCN of T-CR mice,indicating that T-CR may change the molecular mechanisms underlying photic synchronization. Heterozygous Clock⁄+mice have high-amplitude phase resetting responses to6-h light pulses,an effect mediated by either reduced pacemaker amplitude or altered light inputs to the SCN clock (Vitaterna et al.,2006).Interestingly,Clock–⁄–mice do not show expected phase delays after light in the early night,but they show much larger late-night,light-induced phase advances than wild-type mice,suggesting that CLOCK may indeed have a role in the light input pathway or in SCN light sensitivity(DeBruyne et al.,2006). After a light pulse,the number of A VP-ir cells was enhanced in the medial SCN,and this increase was more marked(and significant)8h3698J.Mendoza et al.ªThe Authors(2007).Journal CompilationªFederation of European Neuroscience Societies and Blackwell Publishing LtdEuropean Journal of Neuroscience,25,3691–3701。
LCD Monitor Reliability Issues-PPT文档资料

Copyright © 2004 reliability solutions all rights reserved
LCD Monitor Reliability Testing
ITO CORROSION
ITO LINES
Copyright © 2004 reliability solutions all rights reserved
• Will result in accelerated ITO corrosion and loss of signal leading to dead line on screen which cannot be repaired
• Accelerated testing in humid conditions very effective in accelerating failure mechanism, LCD supplier must be able to show Env test data to prove LCD panel robustness
• Poor bonding evident if ‘Tab Peel Test’ is performed on sample LCD panels
– Poor control of production environment can result in moisture / chemicals trapped between Tab chip carrier and Glass panel
Copyright © 2004 reliability solutions all rights reserved
LCD Monitor Reliability Testing
数显连续测厚规 使用说明书

数显连续测厚规中使用本仪器时,请遵守说明书上记载的规格、功能和使用注意事项,超出使用范围会影响本仪器的安全性能。
出口管制条例本产品为「外汇及外贸法」的限制对象产品。
欲拿到外国时,请事先与本公司进行协商。
请勿拆解电池,也不要使电池短路,或擅自给电池充电或加热。
否则可能引起电池发热、破裂及电池漏液进入人的眼中。
万一误吞了电池,要马上请医生检查。
在商品或包装上印有的此标记图形是指在EU(欧洲)诸国废弃此产品时不可与一般家庭垃圾同样丢弃。
为了减少WEEE(废电气电子设备)埋入土壤的数量,减少对环境污染的影响,请协助努力做到商品再利用和再生。
产业垃圾分别处理的EU(欧洲)诸国的电气 电子设备的废弃时的注意事项注意概述关于处理方法的详细内容,请向附近的经销商或代理商咨询。
Digital Thickness Gauge with Roller InsertsGBSafety PrecautionsTo ensure operator safety, use this instrument in conformance with the directions and specifications given in this User’s M anual.Notes on Export RegulationsYou shall agree to commit no act which would, direct or indirect, violate any law or regulation of Japan or your country, or any other international treaty, relating to the export or re-export of any commodities.If a battery is swallowed, immediately consult a doctor.This symbol on the product or on its packaging indicates that this product shall not be treated as household waste. To reduce the e-nvironmental impact of WEEE (Waste Electrical and Electronic E-quipment) and minimize the volume of WEEE entering landfills, please reuse and recycle. For further information, please contact your local dealer or distributors.Disposal of Old Electrical & Electronic Equipment (Applicable In the European Union and other European countries with sep-arate collection systems) CAUTIONInstructionDo not disassemble, short-circuit, charge, or heat the battery.herwise the battery content may leak to come into contact the eye, or cause battery heating or explosion.Ot with UsageAttention1.mm/in:it is a Metric and inch conversion key, press this key for mm/in system conversion.2.ON/OFF:power on/off key.3.SET/ZERO:setting and zero setting.Zero setting key. It can set zero in any position within the measuring range. Press for a short time, show zero or preset value.Press for a long time, the LCD shows "SET ",you can preset value by pressing "pre-" and "pre+".4.pre-、pre+:press the button for a short time, you can set the value yourself. Or press the button for a long time, you can set the value one by one.5.TOL:tolerance function.Press this button for one time, you can set the maximum tolerance and minimum toleran- ce. Set the value one by one. The LCD will show "ok "if the value is qualified.6.If the measuring rod have dust, that will influence the movement of measuring rod, so be- f ore using, please use a clean and without oil cloth to clean the measuring rod.数显连续测厚规是将数显表安装在表架上,其滚轮测头测量面相对于表架上滚轮测头测量面的之间的距离(厚度),由数显表读数的一种测量工具。
显示器件制造专业进阶考核试卷

3.视角
4.色准度
5. Cell
6.有源矩阵
7.阴影技术/IPS技术/VA技术
8.响应时间
9.环氧树脂/硅胶
10. QA
四、判断题
1. ×
2. ×
3. √
4. ×
5. √
6. √
7. ×
8. ×
9. √
10. ×
五、主观题(参考)
1. LCD依靠背光源和液晶层的扭转控制光线通过,LED则是通过发光二极管直接发光。LCD优点是成本较低,亮度较高;缺点是视角较小,功耗较高。LED优点是视角广,功耗低;缺点是成本较高,亮度均匀性较差。
2. AMOLED采用有源矩阵驱动,每个像素都有独立的控制电路,适用于大尺寸、高分辨率显示;PMOLED使用被动矩阵驱动,适用于小尺寸、低分辨率显示。
3. Array工艺形成TFT矩阵,CF工艺生产彩色滤光片,Cell工艺将液晶材料注入两块基板之间形成液晶盒。Array决定显示器的驱动性能,CF影响色彩表现,Cell是液晶显示的核心部分。
A. Retina Display
B. Super AMOLED
C. IGZO
D. UHD
( )
14.以下哪个因素会影响显示器件的功耗?
A.亮度
B.显示面积
C.驱动方式
D.所有上述因素
( )
15.下列哪个环节属于LED显示器件的制造流程?
A. Array
B. CF
C. Chip
D. SMT
( )
16.以下哪种技术主要用于降低OLED显示器件的功耗?
A.像素关闭技术
B.亮度调节技术
C.驱动电路优化
D.所有上述技术
DELL服务器面板指示灯错误代码
Yes
Any
E1222
VCACHE #PwrGd
High
VCACHE # voltage regulator has failed.
AC Cycle or SEL clear
Yes
Any
E1223
VRM #PwrGd
High
VRM # voltage regulator has failed.
Failing device is reseated/replaced/repaired.
Yes
Any
W1228
ROMBห้องสมุดไป่ตู้att< 24 hr
Low
This is a predictive failure warning message telling the user that the PERC5I RAID battery has less then 24 hours of charge left init. Wee provide this message as a warning message to the customer.
System Phase When Event Can Occur?
E1210
CMOSBatt
Low
CMOS battery is missing or the voltage is outside of the allowable range.
Failing device is reseated/replaced/repaired.
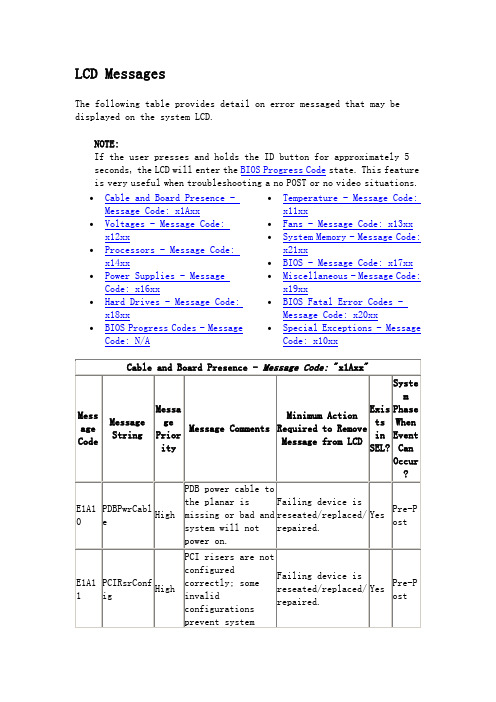
LCD Messages
The following table provides detail on error messaged that may be displayed on the system LCD.
SAE Audio LP26 双入六出音频处理器说明书

The LP26 loudspeaker processor from SAE Audio is a 2-in 6-out processing platform offering an advanced functionality and increased flexibility within the affordable processors segment. Featuring a fully configurable signal routing and output specific functions like crossover filtering, 10 band parametric equalizer, signal delay and level limiter, the LP26 is much more than a simple crossover system. Together with the included Windows user interface software for unit configuration and real-time monitoring, the LP26 is the most usefuldevice to improve the sounding performance of any small or mid sized application.Features Applications 2 balanced XLR inputs and 6 matched impedance XLR outputs.96kHz sampling rate with 24bit depth.64 bit digital signal processing.98dB dynamic range.Nominal input level at 0dBu.Maximum input level at 23dBu.Nominal output level at 0dBu.Maximum output level at 12dBu.Operational frequency range from 20Hz to 20kHz.Processing latency below 1ms.16 character LCD display.100 presets memory on hardware unit.220v / 110v AC mains selector.Adjustable input gain from -48dB to +12dBFully configurable signal routing, any output may be fed fromany input.Crossover filtering on each output channel with Butterworth (6,12, 28 and 24db/Octave), Bessel (6, 12, 28 and 24db/Octave)and Linkwitz-Riley (12 and 24db/Octave) filter types.10 band fully parametric (frequency, Q and gain) equalizer oneach output channel, with multiple equalization filter behaviorsincluding High and Low Shelves, Band pass, High and Low pass,Bell and Notch filters.5ms delay (aprox. 1.7m) available on each output channel.Limiter available on each output channel for added safety of thespeakers.180° phase inverter and mute button on each output.Full configuration and monitoring from Windows PC computerusing onboard USB interface and included software.FunctionalityPortable or installed small and mid sized sound reinforcement applications.Signal filtering and optimization on multi-way speaker systems.LP26Loudspeaker ProcessorLP26 rear panelNo. 39 WenJiao Road East, HeShun, NanHai, FoShan, GD, China.TEL: +86 - 757 - 8512 - 9007FAX: +86 - 757 - 8568 - 8191LP2620Hz - 20kHz< 0.05% 5ms> 98dBOutput gain Input gain 3kg525 x 195 x 110 (1U )483 x 127 x 45 (1U )ModelInputs Outputs 2 balanced inputs6 unbalanced outputsInput impedance > 10k ohms.From +12dB to -50dB, 0.25dB incrementFrom +12dB to -50dB , 0.25dB incrementOther ParametersFrequency response (-0.3dB)THD+N Max delay Dynamic range Crossover / EQ operational frequency band 20Hz - 20kHzMinimun load impedance Maximum output level 600 ohms+9dBu (600 ohms load)Password protection Yes ConnectorsSignal inputsSignal outputsComputer linkMains powerXLR Female socket XLR Male socket 3 cores ICE socket USB Dimension / WeightDevice presets50Language optionsEnglish, Germany, Spainish, French, Dutch Limiter release timeLimiter thresholdSampling rate Phase inversion LCD display Adjustable from 10 to 100 Adjustable from -48dB to 9dB 96kHz, 24 bit depthYes2 x 16 characterWeight Product dimensions (mm)Packing dimensions (mm)Mains power Plugable US standard 6.5A power wire. AC 115v / 230v / 50~60Hz. ±10%Dimension (mm)SpecificationsSAE reserves the right to make any changes to the product specifications without prior notice. Final specifications to be found in the user manual. Equalizer Q Equalizer gain 10-band EQ type Crossover filteringAdjustable from 0.2 to 25Adjustable from -12dB to +12dBBell, Notch, All Pass, Band Pass, Low/High Pass, Low/High ShelfBessel, Butterworth (6, 12, 24 and 48dB/Octave), Linkwitz -Riley (12 and 24dB /Octave )。
4)SUPER_IPS WHITE PAPER
White PaperThe Importance of S-IPS Technologyfor LCD TelevisionsIf you attended the Consumer Electronics Show earlier this year, youexperienced the high level of excitement surrounding liquid crystal display (LCD) televisions and you sensed the expectation that LCD TVs will bethe next “big thing” in consumer electronics—such a big thing that it will fuel the consumer electronics and display industries for years to come. There are, however, still a few worms in this apple—three worms, to be precise. LCD-TV is a premium product that has sold for a premium price, but its performance has not always supported its premium price and product identity. The performance problems have been:1. Limited Viewing Angle:As a viewer moves to the side – so that he or she is not looking at the screen straight on – the brightness and contrast of the imagedecrease, making viewing less pleasurable and sometimesimpossible. In extreme cases, the viewer may experience gray-scale inversion, which makes portions of the image look like a photographic negative.2. Color Change with Viewing Angle:As a viewer moves to the side, colors—especially skin tones andother mid-range colors—change, often get “washed out”, thus,seriously compromising viewer enjoyment.3. Smearing or Blurring of Moving Images:Still images are sharp and clear, but become progressively smearedas they begin to move quickly. At its worst, this can cause the on-screen image of a football, for instance, to disappear or appearrepeatedly, when the ball is thrown.Super In-Plane Switching (S-IPS) is a LCD technology that solves these problems. It is solving them now, in LCD TVs currently being sold by several well-known TV manufacturers.What S-IPS DoesThe kinds of LCDs that are used in TV sets work by changing the polarization of light waves as the light passes through the liquid-crystal (LC) material, and then blocking more or less of the light depending on the change in polarization. Traditional LC materials – called “twisted nematic”materials – change the polarization of the light by tilting their molecules out of the plane of the display (Fig. 1b). This mechanism is fine for laptop computers (notebook PCs) that are normally viewed head on, but light that passes through the display at angles that aren’t small is changed in a different way from light that goes through the display at a right angle. This is what gives rise to brightness, contrast and color distortions.S-IPS solves these problems by using a switching mode that keeps all of the molecules in the plane of the display at all times (Fig. 1a), and does it in such a way that light passing straight through the display at an angle is not treated very differently than light passing straight through the display. As aresult, brightness, contrast and color rendition are much more constant, regardless of where in the room a viewer is sitting, or what angle you view the screen from. This enables large and wide LCD monitors and TVs. (Fig.2).12 Milliseconds Gray to GrayS-IPS dramatically improves viewing angle and off-axis color fidelity, but does not by itself cure the smearing of moving images.LCD designers have worked hard for years to bring the total response time of an LCD down to below 16.67 milliseconds, which would theoretically allow the LCD to faithfully follow all standard video signals (the time for 1 frame of 60 frames/second film). They first measured the switching time from black to white to black because that was the easiest thing to measure, but it is not representative of the video content most people watch.With some more research, it became obvious that switching times from one gray level to another are generally more useful in predicting real front of screen image quality and performance with real video content much slower than switching times from black to white. To minimize smearing, it is necessary to get all of these gray-to-gray switching times down below 17 milliseconds. Through a combination of optimizing the LC material and designing a new electric driving system based on a proprietary “Over Driving Circuit” (ODC), LG.Philips LCD’s engineers have succeeded in reducing gray-to-gray switching times down to about 12 milliseconds.What It MeansA well-designed LCD TV set incorporates an S-IPS LCD panel with ODC displays images, as well as a wide-viewing angle, no color shift, and a 12 milliseconds gray-to-gray response time for no-smear motion video. This is finally a premium TV that’s truly premium.Of course, there are technologies that are attempting to compete with S-IPS LCD TV. There are two other “wide-viewing angle” LCD technologies. The first, sometimes called “wide-view twisted nematic” (WVTN LCD), is amore-or-less traditional twisted nematic (TN) panel with its viewing angle increased by using extra compensation films. This works reasonably wellfor notebook PCs and small-screen TVs, but the films are expensive and there are still problems with brightness, gray-level-inversion, contrast and color distortions at larger viewing angles.The other large-screen LCD technology is multi-domain vertical alignment (MVA) LCD. Invented about five years ago VA (Fig. 1c), like IPS, improves on the performance of traditional LCDs by changing the internal configuration of the LCD cell and uses a different LC mode. But the MVA configuration still switches by changing the tilt angle of the LC molecules, and it still requires expensive compensation films. It does a good job of maintaining brightness and contrast at large viewing angles, but color distortions can be severe (Fig. 3). When it comes to offering a premium, wide-screen viewing experience, neither of these technologies can matchS-IPS.Stepping back one step, the wide-screen technology that competes most directly with larger LCD TVs in general is the plasma display panel (PDP). PDPs have good color rendition and fast response time, but they still have some motion artifacts and they have a problem with real-world image contrast as well as “burn-in” and lifetime issues. The large contrast ratios –sometimes well over 1000 – that manufacturers claim for PDPs in normal usage are measured in a dark room. In a moderately lit living room, the contrast ratio can drop to a not-very-impressive 50 or less. LCDs are brighter than PDPs, weigh between one half to two thirds as much, use much less energy and produce much less heat, have a higher resolution and have between two to four times the lifetime of PDPs . (LCD technology also enables more pixels closer together more easily than a PDP, enabling true “high definition” HDTVs.) LCD TVs are the wide-screen, flat-panel television technology that will deliver on the promises the consumer electronics industry has been making for years, just as it delivered the best consumermonitor to the PC “IT” industry today.。
针对高清液晶电视的响应时间补偿算法设计与实现_陈善军
本科毕业设计论文题 目 针对高清液晶电视的响应时间补偿算法设计与实现专业名称 计算机科学与技术学生姓名 陈善军指导教师 魏廷存毕业时间 2010.7摘要随着液晶电视向大屏幕,高分辨率全色显示方向的发展,人们对视频画面显示质量的要求日益提高。
而液晶显示器的响应时间过慢会导致图像质量的降低,液晶显示中“残像”、“拖尾”的现象,即是因为响应时间过长而导致。
降低响应时间在液晶显示器的显示中有重要意义。
如何降低液晶显示器的响应时间是液晶显示器应用到大屏幕电视中所需研究的重要课题。
目前已经有很多这方面的研究,从不同的出发点提出了许多改进技术。
本文采用过驱动处理技术,对数据帧进行过驱动处理,以降低液晶显示器的响应时间,来提高图像画质。
所谓灰阶响应时间是指液晶单元针对输入信号从一个灰度值过渡到另一个灰度值所需要的时间,即液晶单元从一种分子排列状态转变成另外一种分子排列状态所需要的时间,响应时间愈小愈好,它反应了液晶显示器各像素点对输入信号反应的速度,其原理是在液晶分子内施加电压,使液晶分子扭转与恢复.。
常说的25ms、16ms就是指的这个反应时间,反应时间越短则使用者在看动态画面时越不会有尾影拖曳的感觉。
一般将灰阶响应时间分为两个部分:上升时间(Rise time)和下降时间(Fall time),一般上升时间和下降时间是不同的。
关键词:响应时间,过驱动,液晶显示器ABSTRACTWith the development of high-definition LCD TV to the big screen, high resolution full color display, the image quality requirements of LCD display is increased. But the slow response time of LCD will result in lower image quality, the “image sticking”, “tail” phenomenon of liquid crystal display is result from the long response time. Reduce the response time on the LCD display is important. How to reduce the response time of LCD is an important research subject in applied the LCD to large screen TV. There has been a lot of this research in this area and from different starting points put forward a lot of improvement techniques. In this paper, we use the over-driven processing to process the data fames to reduce the response time and improve the image quality.Gray to gray response time is the time the LCD unit required for the input signal transition from one gray level to another gray value, that is, the time the liquid crystal cell required for change its arrangement of molecule from one state to another state, which reflects the response rate of the input singal of the LCD pixel, the response time is the smaller the better. The principle is applying voltage to liquid crystal molecules, make the liquid crystal molecules twist or recovery. Often said of 25ms, 16ms are the response times, the shorter response time, there will be no tail drag feeling when the user looking at the dynamic screen. Generally, we divide the black and white response time into two parts: the rise time and fall time, generally, rise time and fall time are different.Key words: response time, over-drive, LCD目录第一章绪论 (6)1.1应用背景 (6)1.2研究现状 (6)1.3主要工作 (7)1.4论文组织 (7)第二章算法实现原理 (9)2.1液晶显示器概述 (9)2.2液晶显示器的显示原理 (9)2.3过驱动算法的实现原理 (10)第三章针对高清液晶电视的过驱动算法 (15)3.1算法流程图 (15)3.2图像数据色彩空间转换 (16)3.3双线性插值方法 (17)3.4详细处理过程 (18)3.5代码实现 (19)3.6PSNR的计算 (19)3.7测试效果 (19)3.8算法复杂度分析 (22)3.9误差分析 (22)第四章针对高清液晶电视过驱动处理的改进算法 (24)4.1三角插值的基本原理: (24)4.2算法流程图 (25)4.3详细处理过程 (26)4.4代码实现 (27)4.5两种插值的比较: (27)4.6测试效果 (27)4.7算法复杂度分析 (30)4.8误差分析 (30)第五章未来的工作 (31)5.1处理后图像帧的动态显示 (31)5.2与硬件的结合 (31)5.3针对硬件存储空间的优化 (31)5.4更多的尝试 (31)第六章全文总结 (32)参考文献33致谢34毕业设计小结 (35)附录36附录1:双线性插值过驱动算法主要代码实现 (36)附录2:三角插值过驱动算法主要代码实现 (37)附录3:BMP文件读写函数 (38)附录4:BMP图像读取与写出 (42)附录5:查找表 (44)第一章绪论1.1 应用背景在现在的显示器市场上,液晶显示器(LCD, Liquid Crystal Display)凭借其体积小,功耗低等特点已经逐渐取代传统的CRT显示器,占领了大部分显示器市场,由下图可以看出,LCD显示器与CRT显示器相比在市场上所占比例越来越大。
认证机构的审核发现和纠正措施表的指导性文件
申请者提交给认证机构的审核发现和纠正措施表的指导性文件OverviewThe Corrective Action Submission Form is the 认证机构form that records any non compliances raised during a IATF 16949 audit activity. One form is produced for every minor or major non compliance raised during the audit. A single non compliance may incorporate more than one requirement of either the technical specification i16949elf or an organizations’ defined processes.概述纠正措施提交表是认证机构用于记录在16949 审核活动中发现的任何不符合项。
审核中发现的每一个轻微不符合或者重大不符合对应一张表格。
一个单独的不符合项可能包含技术规范本身或者被组织定义的过程中不止一个的要求The FindingSection 1 of the form will be completed by the auditor and will define the process failure which led to the non compliance, the clause of the technical specification or organizations’ processes to which it did not comply and finally a summary of the objective evidence gathered which suppor16949 the raising of the finding.发现表格的第一部分由审核员填写,并定义导致不符合的过程失效,列出没有遵循的技术规范或者组织过程的条款,提供所收集到的支持该发现提出的客观证据的汇总The ResponseOnce the finding has been raised an organization must take immediate action to contain the issue. For example if an un-calibrated gage was seen on the shop floor the gage would be immediately removed to prevent continued use. If unidentified par16949 were observed on the shop floor they would immediately be quarantined while their status was determined.回应一旦提出发现,客户必须采取包含该问题的临时措施。
SG-Ultra Max数位体积电子体积矩说明书
SG-Ultra Max Digital Hydrometer/Density Meter | p: +1-414-962-3377 | toll free: +1-877-805-3377 | fax: +1-414-962-3660Model # SG-ULTRA MAXSG-Ultra Max KitProduct Overview The SG-Ultra Max measures the density and density-related values of yoursample within seconds. Results appear on the large LCD screen with back-light and are ready for storage, printout or export to a PC. The lightweightand robust design enable on-site operation in a wide range of environments.Pump spills do not enter the instrument. The SG-Ultra Max digital hydrom-eter features leakproof housing for operation in industrial and field appli -cations including battery testing for utilities and telecoms, food & beveragetesting, quality control, biotechnology and many more. Supported measuringunits include: density, density at reference temperature, specific gravity (sg),Alcohol, API, °Baumé, °Brix and five programmable custom functions toensure your measurement requirements.Advantages • 99.999% Accurate, tests specific gravity and temperature simultaneously• User-Friendly Compact, lightweight, and allows for one-hand measure-ment. Large LCD display screen with backlight.• Robust Rugged, comes with protective carrying case. Sustains toughfield environments with leakproof housing• Wireless Communication Infared data interface for data exchange with aPC and data export to a printer (data transfer accessories included).• Stores up to 1,024 total measurement results including timestamp andsample ID.• Automatic temperature compensation•Reliable CE Compliant and One Year Warranty*Sample must not freeze in the measuring cell.Kit Includes:• Kit Includes:• SG-Ultra Max Body• 180 mm Sample Tube• 2 mL Plastic Syringes• Luer Adapter 1/4” UNF (for syringefilling)• IrDA Adapter• Interface for Exporting Data to PC•Carrying Case• User ManualSG-Ultra Max Body。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
The transition time between any two gray levels in a nematic LCD depends on several factors which can be divided into two classes, factors related to the forcing torque, i.e. the torque attempting to move the molecules, and factors related to the resistance to movement such as flow dynamics, viscosity etc. The forces related to the resistance to movement are intrinsic to the LC material and its environment such as pre-tilt, cell thickness, temperature, director orientation distribution, etc.
Displays Group, National Semiconductor, Santa Clara, California, USA
Abstract
The electro-optic response times of the principal LCD modes are not fast enough for video applications. Applying a one-frame acceleration voltage, in lieu of the correct video command, can compensate the slow response time. This paper discusses the operation and design choices of a timing controller with an embedded LCD response time compensator (RTC).
48.3 / R. I. McCartney
48.3: A Liquid Crystal Display Response Time Compensation Feature Integrated into an LCD Panel Timing Controller
Richard I. McCartney
there is a partial frame of both the old and the new frame visible on the screen with a progressively moving tear boundary through it. This scan-and-hold aspect of the LCD is nearly ideal for presentation of static images such as computer-generated spreadsheets and word documents where flicker is minimal compared with CRTs, but it is undesirable from the standpoint of video applications. Uniform gray-to-gray response times coupled with mechanisms to modulate the backlight are among the expected solutions to meet the impulse requirements for video applications.
The AMLCD pixel’s luminance, however, is virtually constant over the entire frame. For an equivalently bright LCD, the LCD pixel would be the luminance of the average luminance shown in Figure 1. Since LCDs are progressively scanned, at every instant
The forcing torque lies in the balance of two torques, the exciting torque that is induced by an electric field and a restoring torque induced by the spring constants working to establish long-range nematic order2. The response time required to tation to another is dependent on both factors which can be deduced from knowledge of the present state of the crystalline deformation and whether the movement is due to an increase or a decrease in the torque from the electric field.
The frame impulse requirement originates in the difference between how AMLCDs operate and the way that the human visual system senses motion from changing still frames. Each still frame represents a sample of the image frozen at an instant in time. When these still frames are flashed momentarily on the retina at rates faster than the visual system can track, the frames fuse into continuous motion. Film projectors at theaters approximate this impulse rendering. CRTs and Plasma displays approximate this rendering as well since the luminance from a phosphor pixel decays away rapidly. Figure 1 shows measured data of a CRT pixel’s luminance profile.
Targeted LCD-TV screen sizes range from 15 inches through greater than 50 inches. Acknowledging some overlap, these sizes are substantially larger than desktop monitor applications. Generation 5, 6 and 7 factories are being brought on-line to support these new sizes.
1. Background and History
Active-matrix LCD technology which enabled the Notebook PC and the thin, flat computer Monitor through a progression of technological innovations now ready to take on television. Television imposes several new challenges on the current AMLCD art including: a broader color envelope, very large sizes, image frame impulsing and faster, more uniform response time between gray levels. The broader color envelope originates in video standards. AMLCD NBPCs and monitor applications have actually taken liberties to reduce the color envelope as a method to improve transmissivity and thereby reduce backlight power and gain other benefits. A number of methods are being developed to improve the color envelope while still maintaining high transmissivity.
Until recently, it wasn’t widely appreciated that in general, the response times of the commercially important LCD modes, in particular TN, IPS and MVA, are inadequate to show high quality video1. This is due to several factors including that computer applications typically have very low video content and what little video content there is tends to be poor quality (e.g. internet video streams, file based video clips and game graphics). Furthermore, the LCD industry specifications report only the off-to-on response times of the panel. This is the fastest response mode of the liquid crystal. Response times of 15 to 25 milliseconds is representative and would be adequate if all gray-to-gray transitions were at this rate. However, the gray-to-gray response times can be many times longer, i.e. hundreds of milliseconds. These gray-to-gray transitions account for the poor quality of motion video when it is due the LCD response time.
