枸橼酸铁FDA说明书
FDA认证颜料
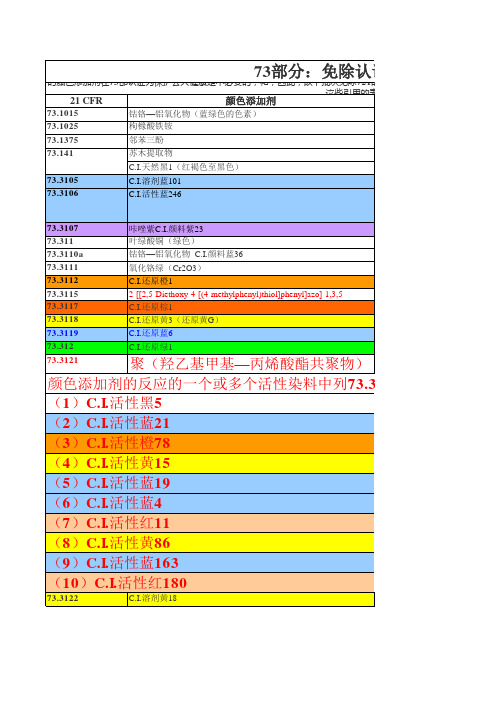
73.1015 73.1025 73.1375 73.141 73.3105 73.3106 钴铬—铝氧化物(蓝绿色的色素) 枸橼酸铁铵 邻苯三酚 苏木提取物 C.I.天然黑1(红褐色至黑色) C.I.溶剂蓝101 C.I.活性蓝246
的颜色添加剂在73部认证为保护公共健康是不必要的;和,因此,以下批次免除721的认证要求(C)FD&C法案的。 这些引用的表,代表颜色指数”。 21 CFR 颜色添加剂
17095-24-8 73049-92-0 4424-06-0 12226-47-0 2580-78-1 13324-20-4 12226-08-3 61951-86-8 72847-56-4 72828-03-6
隐型眼镜
6407-78-9
隐型眼镜 128-70-1
隐型眼镜 隐型眼镜 隐型眼镜
6-Ethoxy-2-(6-ethoxy-3oxobenzo[b]thien-2(3H)ylidene)benzo[b]thiophen-3 (2H)one. 1328-53-6 1309-37-1;1317-60-8;1332-37-2 13463-67-7
73.3107 73.311 73.3110a 73.3111 73.3112 73.3115 73.3117 73.3118 73.3119 73.312 73.3121
咔唑紫C.I.颜料紫23 叶绿酸铜(绿色) 钴铬—铝氧化物 C.I.颜料蓝36 氧化铬绿(Cr2O3) C.I.还原橙1 2-[[2,5-Diethoxy-4-[(4-methylphenyl)thiol]phenyl]azo]-1,3,5C.I.还原棕1 C.I.还原黄3(还原黄G) C.I.还原蓝6 C.I.还原绿1
柠檬酸铁

用途:用作食品铁质强化剂、营养增补剂。用于饼干、钙质奶粉等;用作放射性医药品,
用于检查铁代谢异常及造血功能;饲料添加剂。
性质:
潜在危险:
(1)危险性类别: (2)侵入途径: (3)健康危害: (4)环境危害: (5)燃爆危险:
意外预防: 食入:
(2)消防措施 危险特性: 有害燃烧产物: 灭火方法:
品名:(中文) 柠檬酸铁
(英文)Ferric citrate
别名:(中文) 枸橼酸铁
(英文)
CAS No.:6043-74-9
柠檬酸铁
化学式: 分子量:244.94
理化特性
(1)成分/组成信息: (2)外观与性状:红褐色透明小薄片结晶,或结晶性粉末。 (3)熔点(℃): (4)沸点(℃): (5)相对密度(水=1): (6)溶解性:不溶于乙醇,在冷水中慢慢溶解,在热水中很易溶解
(3)溅散及泄漏:
管理操作及储藏:
操作注意事项: 储存注意事项:
废品处理及销毁:
(1)废弃物性质: (2)废弃处置方法: (3)废弃注意事项:
说明书-柠檬酸铁片 美国 Keryx Biopharmaceuticals, Inc.

12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility14 CLINICAL STUDIES14.1 Long-term, Randomized, Controlled, Safety and Efficacy Trial14.2 Fixed-Dose Trial16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied16.2 Storage and Handling17 PATIENT COUNSELING INFORMATION17.1 Dosing Recommendations17.2 Adverse Reactions*Sections or subsections omitted from the full prescribing information are not listed.FULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGEAuryxia (ferric citrate) is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis.2 DOSAGE AND ADMINISTRATION2.1 Dosing and Dose AdjustmentThe recommended starting dose is 2 tablets orally 3 times per day with meals. Serum phosphorus levels should be monitored and the dose of Auryxia titrated in decrements or increments of 1 to 2 tablets per day as needed to maintain serum phosphorus at target levels, up to a maximum dose of 12 tablets daily. Dose can be titrated at 1-week or longer intervals.In a clinical trial conducted in the United States, patients required an average of 8 to 9 tablets a day to control serum phosphorus levels.3 DOSAGE FORMS AND STRENGTHSTablet: Auryxia 210 mg ferric iron, equivalent to 1 g ferric citrate, film-coated, peach-colored, and oval-shaped tablet embossed with “KX52”.4 CONTRAINDICATIONSAuryxia is contraindicated in patients with iron overload syndromes (e.g., hemochromatosis) [see Warnings and Precautions (5.1)].5 WARNINGS AND PRECAUTIONS5.1 Iron OverloadIron absorption from Auryxia may lead to excessive elevations in iron stores. Increases in serum ferritin and transferrin saturation (TSAT) levels were observed in clinical trials. In a 56-week safety and efficacy trial in which concomitant use of Auryxia and IV iron was permitted, 55 (19%) of patients treated with Auryxia had a ferritin level >1500 ng/mL as compared with 13 (9%) of patients treated with active control.Assess iron parameters (e.g., serum ferritin and TSAT) prior to initiating Auryxia and monitor iron parameters while on therapy [see Contraindications (4), Overdosage (10) and Clinical Pharmacology (12.2)]. Patients receiving IV iron may require a reduction in dose or discontinuation of IV iron therapy.5.2 Accidental Overdose of IronAccidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison control center immediately.5.3 Patients with Gastrointestinal Bleeding or InflammationPatients with inflammatory bowel disease or active, symptomatic gastrointestinal bleeding were excluded from clinical trials. Safety has not been established in these populations.6 ADVERSE REACTIONS6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to adverse reaction rates in the clinical trials of another drug and may not reflect the rates observed in practice.Adverse reactions to a drug are most readily ascertained by comparison with placebo, but there is little placebo-controlled experience with Auryxia, so this section describes adverse events with Auryxia, some of which may be disease-related, rather than treatment-related.A total of 289 patients were treated with Auryxia and 149 patients were treated with active control (sevelamer carbonate and/or calcium acetate) during the 52-week, randomized, open-label, active control phase of a trial in patients on dialysis. A total of 322 patients were treated with Auryxia for up to 28 days in three short-term trials. Across these trials, 557 unique patients were treated with Auryxia; dosage regimens in these trials ranged from 210 mg to 2,520 mg of ferric iron per day, equivalent to 1 to 12 tablets of Auryxia. In these trials, adverse events reported for Auryxia were similar to those reported for the active control group.Adverse events reported in more than 5% of patients treated with Auryxia in these trials included diarrhea (21%), nausea (11%), constipation (8%), vomiting (7%), and cough (6%).During the 52-week, active-control period, 60 patients (21%) on Auryxia discontinued study drug because of an adverse event, as compared to 21 patients (14%) in the active control arm. Patients who were previously intolerant to any of the active control treatments (calcium acetate and sevelamer carbonate) were not eligible to enroll in the study. Gastrointestinal adverse reactions were the most common reason for discontinuing Auryxia (14%).Auryxia is associated with discolored feces (dark stools) related to the iron content, but this staining is not clinically relevant and does not affect laboratory tests for occult bleeding, which detect heme rather than non-heme iron in the stool.7 DRUG INTERACTIONSTable 1: Oral drugs that can be administered concomitantly withAuryxiaAmlodipineAspirinAtorvastatinCalcitriolClopidogrelDigoxinDiltiazemDoxercalciferolEnalaprilFluvastatinGlimepirideLevofloxacinLosartanMetoprololPravastatinPropranololSitagliptinWarfarinOral drugs that have to be separated from Auryxia and mealsDosing RecommendationsDoxycycline Take at least 1 hour before AuryxiaCiprofloxacin Take at least 2 hours before or after AuryxiaOral medications not listed in Table 1There are no empirical data on avoiding drug interactions between Auryxia and most concomitant oral drugs. For oral medications where a reduction in the bioavailability of that medication would have aclinically significant effect on its safety or efficacy, consider separation of the timing of the administration of the two drugs. The duration of separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate release or an extended release product. Consider monitoring clinical responses or blood levels of concomitant medications that have a narrow therapeutic range.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category B: There are no adequate and well-controlled studies in pregnant women. It is not known whether Auryxia can cause fetal harm when administered to a pregnant woman. Animal reproduction studies have not been conducted.The effect of Auryxia on the absorption of vitamins and other nutrients has not been studied in pregnant women. Requirements for vitamins and other nutrients are increased in pregnancy. An overdose of iron in pregnant women may carry a risk for spontaneous abortion, gestational diabetes and fetal malformation [see Nonclinical Toxicology (13.1)].8.2 Labor and DeliveryThe effects of Auryxia on labor and delivery are unknown.8.3 Nursing MothersData from rat studies have shown the transfer of iron into milk by divalent metal transporter-1 (DMT-1) and ferroportin-1 (FPN-1). Hence, there is a possibility of infant exposure when Auryxia is administered to a nursing woman.8.4 Pediatric UseThe safety and efficacy of Auryxia have not been established in pediatric patients.8.5 Geriatric UseClinical studies of Auryxia included 106 subjects aged 65 years and older (33 subjects aged 75 years and older). Overall, the clinical study experience has not identified any obvious differences in responses between the elderly and younger patients in the tolerability or efficacy of Auryxia.10 OVERDOSAGENo data are available regarding overdose of Auryxia in patients. In patients with chronic kidney disease on dialysis, the maximum dose studied was 2,520 mg ferric iron (12 tablets of Auryxia) per day. Iron absorption from Auryxia may lead to excessive elevations in iron stores, especially when concomitant IV iron is used [see Warnings and Precautions (5.1)].In clinical trials, one case of elevated iron in the liver as confirmed by biopsy was reported in a patient administered IV iron and Auryxia.Because accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age, this product must be kept out of the reach of children. In case of accidental overdose, a doctor or poison control center should be contacted immediately [see Warnings and Precautions (5.2)].11 DESCRIPTIONAuryxia (ferric citrate) is known chemically as iron (+3), x (1, 2, 3-propanetricarboxylic acid, 2 y (H O)hydroxy-),2Auryxia 210 mg ferric iron tablets, equivalent to 1g ferric citrate, are film-coated, peach-colored, and oval-shaped tablets embossed with “KX52”. The inactive ingredients are pregelatinized starch and calcium stearate. In addition, the film-coating contains the following inactive ingredients; hypromellose, titanium dioxide, triacetin, and FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake, FD&C Red#40/Allura Red AC Aluminum Lake, and FD&C Blue #2/Indigo Carmine Aluminum Lake.12 CLINICAL PHARMACOLOGY12.1 Mechanism of ActionFerric iron binds dietary phosphate in the GI tract and precipitates as ferric phosphate. This compound is insoluble and is excreted in the stool. By binding phosphate in the GI tract and decreasing absorption, ferric citrate lowers the phosphate concentration in the serum.12.2 PharmacodynamicsIn addition to effects on serum phosphorus levels, Auryxia has been shown to increase serum iron parameters, including ferritin, iron and TSAT. In dialysis patients treated with Auryxia in a 52-week study in which IV iron could also be administered, mean (SD) ferritin levels rose from 593 (293) ng/mL to 895 (482) ng/mL, mean (SD) TSAT levels rose from 31% (11) to 39% (17) and mean (SD) iron levels rose from 73 (29) mcg/dL to 88 (42) mcg/dL. In contrast, in patients treated with active control, these parameters remained relatively constant [see Contraindications (4) and Warnings and Precautions (5.1)].12.3 PharmacokineticsAbsorption and DistributionFormal pharmacokinetic studies have not been performed with Auryxia. Examination of serum iron parameters has shown that there is systemic absorption of iron from Auryxia [see Contraindications (4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].Drug Interaction StudiesIn vitroOf the drugs screened for an interaction with ferric citrate in vitro, only doxycycline showed the potential for interaction with at least 70% decrease in its concentration. This interaction can be avoided by spacing the administration of doxycycline and ferric citrate [see Drug Interactions (7)].In vivoSix drug interaction studies (N=26-60/study) were conducted to establish the effects of Auryxia (administered as 3 x 2 g/day with meals) on the disposition of concomitantly orally administered clopidogrel, ciprofloxacin, digoxin, diltiazem, glimepiride and losartan in healthy subjects. With the exception of ciprofloxacin, Auryxia did not alter the systemic exposure of the tested drugs, as measured by the area under the curve (AUC) and Cmax of the tested drugs when either co-administered with Auryxia or given 2 hours later. Auryxia decreased the relative bioavailability of concomitantly administered ciprofloxacin by approximately 45%. However, there was no interaction when Auryxia and ciprofloxacin were taken 2 hours apart. Consequently, ciprofloxacin should be taken at least 2 hours before or after Auryxia is dosed [see Drug Interactions (7)].13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, Impairment of FertilityData from carcinogenesis studies have shown that ferric citrate is not carcinogenic in mice and rats when administered intramuscularly or subcutaneously. Ferric citrate was neither mutagenic in the bacterial reverse mutation assay (Ames test) nor clastogenic in the chromosomal aberration test in Chinese hamster fibroblasts.The potential for ferric citrate to impair reproductive performance or to cause fetal malformation has not been evaluated. Skeletal and encephalic malformation was observed in neonatal mice when ferric gluconate was administered intraperitoneally to gravid dams on gestation days 7-9. However, oral administration of other ferric or ferrous compounds to gravid CD1-mice and Wistar-rats caused no fetal malformation.14 CLINICAL STUDIESThe ability of Auryxia to lower serum phosphorus in patients with CKD on dialysis was demonstrated in randomized clinical trials: one 56-week, safety and efficacy trial, consisting of a 52-week active-controlled phase and a 4-week, placebo-controlled, randomized withdrawal period, and one 4-week open-label trial of different fixed doses of Auryxia. Both trials excluded subjects who had an absolute requirement for aluminum containing drugs with meals.14.1 Long-term, Randomized, Controlled, Safety and Efficacy TrialAfter the 2-week washout period during which phosphate binders were held, patients with a mean serum phosphorus of 7.5 mg/dL during washout were randomized 2:1 to Auryxia (N=292) or active control (calcium acetate and/or sevelamer carbonate; N=149). The majority (>96%) of subjects were on hemodialysis. The starting dose of Auryxia was 6 tablets/day, divided with meals. The starting dose ofactive control was the patient’s dose prior to the washout period. The dose of phosphate binder was increased or decreased as needed to maintain serum phosphorus levels between 3.5 and 5.5 mg/dL, to a maximum of 12 tablets/day.As shown in the figure below, serum phosphorus levels declined following initiation of therapy. The phosphorus lowering effect was maintained over 52 weeks of treatment.Figure 1: Serum Phosphorus Control over 52 WeeksFollowing completion of the 52-week active-controlled phase, Auryxia-treated patients were eligible to enter a 4-week placebo-controlled randomized withdrawal phase, in which patients were re-randomized in a 1:1 ratio to receive Auryxia (N=96) or placebo (N=96). During the placebo-controlled period, the serum phosphorus concentration rose by 2.2 mg/dL on placebo relative to patients who remained on Auryxia.Table 2: Effect of Auryxia on serum phosphorus during randomized withdrawalThe LS mean treatment difference and p-value for the change in mean were created via an ANCOVA model with treatment as thefixed effect and Week-52 baseline (phosphorus) as the covariate. Between-treatment differences were calculated as the LS mean (KRX 0502) – LS mean (placebo or active control).Note: Analyses using ANCOVA with last observation carried forward. ANCOVA=analysis of covariance; CI=confidence interval.Primary Endpoint (Week 56)AuryxiaPlaceboTreatment Difference (95% CI)p-valueSerum phosphorus (mg/dL) Mean baseline (Week 52)5.12 5.44 Mean change from baseline (Week 56)-0.241.79−2.18 (−2.59, −1.77)<0.000114.2 Fixed-Dose TrialFollowing a 1- to 2-week washout from all phosphate-binding agents, 154 patients withhyperphosphatemia (mean serum phosphorus of 7.5 mg/dL) and CKD on dialysis were randomized in a 1:1:1 ratio to 1, 6, or 8 tablets/day of Auryxia for 4 weeks. Auryxia was administered with meals;subjects receiving 1 tablet/day were instructed to take it with their largest meal of the day, and subjects on 6 or 8 tablets/day took divided doses in any distribution with meals. Dose-dependent decreases in serum phosphorus were observed by Day 7 and remained relatively stable for the duration of treatment.The demonstrated reductions from baseline to Week 4 in mean serum phosphorus were significantly greater with 6 and 8 tablets/day than with 1 tablet/day (p<0.0001). Mean reduction in serum phosphorus at Week 4 was 0.1 mg/dL with 1 tablet/day, 1.9 mg/dL with 6 tablets/day, and 2.1 mg/dL with 8tablets/day.16 HOW SUPPLIED/STORAGE AND HANDLING 16.1 How Supplieda aTablets: Auryxia 210 mg ferric iron tablets equivalent to 1 g of ferric citrate are supplied as 200 tablets in 400-cc high-density polyethylene bottles. The 210 mg ferric iron tablets are film-coated, peach-colored, and oval-shaped tablets embossed with “KX52.”1 Bottle of 200-count 210 mg ferric iron tablets (NDC 59922-631-01)16.2 Storage and HandlingStorage: Store at 20 to 25°C (68 to 77°F): excursions permitted to 15° to 30°C (59°F to 86°F) [See USP controlled room temperature]. Protect from moisture.17 PATIENT COUNSELING INFORMATION17.1 Dosing RecommendationsInform patients to take Auryxia as directed with meals and adhere to their prescribed diets. Instruct patients on concomitant medications that should be dosed apart from Auryxia.17.2 Adverse ReactionsAdvise patients that Auryxia may cause discolored (dark) stools, but this staining of the stool is considered normal with oral medications containing iron.Auryxia may cause diarrhea, nausea, constipation and vomiting. Advise patients to report severe or persistent gastrointestinal symptoms to their physician.Manufactured for and Distributed by:Keryx Biopharmaceuticals, Inc.One Marina Park Drive, 12th Floor Boston, MA 02210, USAIssued 1/2016 Rev 3.2PRINCIPAL DISPLAY PANEL - NDC: 59922-631-01 - 210 mg 200-ct BottlePRINCIPAL DISPLAY PANEL - NDC: 59922-631-91 - 210 mg 200-ct Professional Samples BottlePRINCIPAL DISPLAY PANEL - NDC: 59922-631-92 - 210 mg 50-ct Professional Samples BottleAURYXIAferric citrate tablet, coatedProduct InformationProduct T ype HUMAN PRESCRIPTION DRUG Ite m Code (Source)NDC:59922-631 Route of Administration ORALActive Ingredient/Active MoietyStrengthIngredient Name Basis ofStrengthTETRAFERRIC TRICITRATE DECAHYDRATE (UNII: Q91187K011) (FERRIC CATION -FERRIC CATION210 mg UNII:91O4LML611)Product CharacteristicsColor PINK Score no sco reShape OVAL Siz e19mmFlavor Imprint Code KX52ContainsPackaging#Item Code Package Description Marketing Start Date Marketing End Date 1NDC:59922-631-01200 in 1 BOTTLE; Type 0: No t a Co mbinatio n Pro duct2NDC:59922-631-91200 in 1 BOTTLE; Type 0: No t a Co mbinatio n Pro duct3NDC:59922-631-9250 in 1 BOTTLE; Type 0: No t a Co mbinatio n Pro ductMarketing InformationMarke ting Cate gory Application Numbe r or Monograph Citation Marke ting Start Date Marke ting End Date NDA NDA20587409/17/2014Labeler - Keryx Biopharmaceuticals, Inc. (073504263)Keryx Biopharmaceuticals, Inc.Revised: 1/2016。
铁剂临床应用主要事项

铁是细菌、微生物生长繁殖不可缺少的物质之一 给予过多的铁剂,微生物生长,繁殖加快,毒性增强 铁缺乏,将限制微生物的生长 大多数微生物通过释放铁螯合伍与宿主的结合蛋白竞争
而获得铁。也有一些细菌通过细胞膜上的转铁蛋白受体 从转铁蛋白中获得铁。
静脉铁剂的安全性小结
• 所有静脉铁剂均能引起急性不良反应 • 所有静脉铁剂都有细胞毒性 • 所有静脉铁剂都可能发生氧化应激反应 • 静脉铁剂的安全性(肾小管上皮细胞损伤)
40
便秘
30
20
10
0
安慰剂
180
200
225
300
4ቤተ መጻሕፍቲ ባይዱ0
每天mg Fe++ 服两周
常用口服铁剂特点
• 硫酸亚铁缓释片(维铁控释片)
组方:硫酸亚铁525mg + Vit.C 500 g、Vit.B6、 Vit.B2 烟酸、泛酸钙、 腺苷辅酶,维生素B12。仿造意大利“Iberet-500”
– 优点:缓慢释放铁离子,减少对胃、肠的刺激作用。 – 缺点:
• 仿造 Iberet-500 药片太大,难于吞服 • 不能咬开服,小儿不能服用 • 胃肠道反应19.20%,吸收不完全28%有黑便 • 在空肠中吸收逃避了最佳吸收部位 • 速释多种维生素是否必要
35
常用口服铁剂特点
• 琥珀酸亚铁
– 优点:与琥珀酸形成亚铁盐,促进吸收 – 缺点:
• 为糖衣片,影响铁剂的吸收 • 稳定性不如硫酸亚铁 • 肠腔中仍有铁离子,有消化道副反应 • 给足治疗剂量0.2tid,日治疗费用较高
•*: NKF-K/DOQI CLINICAL PRACTICE GUIDELINES AND CLINICAL PRACTICE RECOMMENDATIONS FOR ANEMIA IN CHRONIC KIDNEY DISEASE
细菌内毒素检测法用于枸橼酸焦磷酸铁溶液检测的可行性研究

细菌内毒素检测法用于枸橼酸焦磷酸铁溶液检测的可行性研究洪颖,盖雅婷,翁鹭娜,林丽花,张真,李玲玲(厦门市食品药品质量检验研究院,厦门361000)摘要 目的:建立枸橼酸焦磷酸铁溶液的细菌内毒素检测方法。
方法:按《中国药典》2015年版四部通则1143细菌内毒素检查法进行试验和结果判断,采用凝胶法,用不同厂家的鲎试剂对枸橼酸焦磷酸铁溶液3批样品进行干扰试验和细菌内毒素检查。
结果:根据生产单位药品标准和临床实际应用情况,确定枸橼酸焦磷酸铁溶液的细菌内毒素限值为2EU·mL-1。
其最大不干扰浓度为0 17mg·mL-1,可用灵敏度为0 06EU·mL-1及以上的鲎试剂检测其中的细菌内毒素。
结论:枸橼酸焦磷酸铁溶液可进行细菌内毒素检测。
关键词:枸橼酸焦磷酸铁;细菌内毒素检查;干扰试验中图分类号:R921 2 文献标识码:A 文章编号:1009-3656(2021)01-0015-04doi:10 19778/j chp 2021 01 003InvestigationondeterminationofthebacterialendotoxininferricpyrophosphatecitratesolutionHONGYing,GAIYating,WENGLuna,LINLihua,ZHANGZhen,LILingling(XiamenInstituteforFoodandDrugQualityControl,361000,China)Abstract Objective:Toestablishamethodfordeterminationofbacterialendotoxininferricpyrophosphatecitratesolution Methods:TheinterferencetestandbacterialendotoxintestofthreebatchsamplesofferricpyrophosphatecitratesolutionwereperformedwiththeTALsfromdifferentmanufacturersaccordingtothegelmethodofbacterialendotoxintestintheChinesePharmacopeia2015VolumeⅣ Results:Thelimitofbacterialendotoxininferricpyrophosphatecitratesolutionwassetas2EU·mL-1basedonthecriteriainhouseandclinicalpractice Themaximumnon interferenceconcentrationwas0 17mg·mL-1,andthebacterialendotoxincouldbetestedwiththe0 06EU·mL-1orhighersensitivity Conclusion:TheTALcanbeusedinbacterialendotoxintestforferricpyrophosphatecitratesolution.Keywords:ferricpyrophosphatecitratesolution;bacterialendotoxintest;interferencetest 枸橼酸焦磷酸铁溶液为铁替代剂,适用于依赖透析的成人慢性肾脏疾病(HDD CKD)患者维持血红蛋白。
柠檬酸铁简述-20170803

柠檬酸铁简述上市情况:柠檬酸铁(Ferric citrate)最早由台湾宝龄富锦公司研发,后转让给美国Keryx 公司,之后,该公司将此药再授权给日本Tobacco公司。
该药最早于2014年1月在日本获批,商品名为Riona,规格为250mg(以无水枸橼酸铁计),用于改善慢性肾病患者的高磷血症,由日本Tobacco公司负责上市销售。
2014年9月获美国食品和药物管理局批准,商品名为Auryxia,规格为210mg(按Fe计),用于透析的慢性肾病患者控制血磷水平。
2015年9月在欧盟获批,商品名为Fexeric,规格为210mg(按Fe计),用于控制慢性肾病成人患者的血磷水平。
Keryx获得该品的全球许可(部分亚太国家除外)。
台湾、中国等其他地区由宝龄富锦公司负责上市销售。
专利情况:全球专利保护至2024年。
作用机理:柠檬酸铁(Ferric citrate)是一种铁基磷结合剂,在肠道结合磷,可增加磷排泄,降低磷吸收,还可预防异位钙化、继发性甲状旁腺功能亢进和骨异常的进展。
用于慢性肾病患者的高磷血症,控制慢性肾病(CKD)透析患者的血清磷水平。
国内申报情况:国内申报名称为枸橼酸铁片(胶囊),共有13家企业申报,目前仅江苏奥赛康的获得生产批件,获得制剂和原料药临床批件的有7家:协和药业(胶囊、片剂)、江苏柯菲平(片剂)、泰州朗润(片剂)、辰欣药业(片剂)、山东诚创(片剂)、阳光诺合(片剂)、江苏恒瑞(片剂)。
其中江苏恒瑞在枸橼酸铁及片研发项目上已投入研发费用约198万元人民币。
宝龄富锦生技申报枸橼酸铁胶囊进口新药,处于在审评阶段。
江苏奥赛康在2016年11月获批生产,但未查到已上市的产品中标信息。
优缺点:柠檬酸铁具有:容易使用,每餐只需要很少的药片,可增加铁储存的能力,降低贫血药物的使用等优势,包括赛诺菲的碳酸司维拉姆(司维拉姆碳酸盐)及Fresenius的无机磷(醋酸钙)。
不过因为可能引起铁过载,而被FDA要求标签警告这款药物可引起“铁过载”,因此,医生在患者接受治疗期间必须监控患者的铁水平。
枸橼酸检验操作规程

枸橼酸检验操作规程1. 目的建立枸橼酸检验标准操作规程,使枸橼酸检验操作规范化。
2. 范围适用于枸橼酸的质量检验。
3. 术语或定义3.1 GMP:药品生产质量管理规范(Good Manufacturing Practice)的英文简称。
3.2 SMP:标准管理程序(Standard Management Procedure),用于指导工作的管理类文件。
3.3 SOP:标准操作程序(Standard Operating Procedure),用于指导如何完成一项工作的文件。
4. 职责质量控制部对本规程的实施负责。
5. 程序5.1 检验依据5.1.1 《中国药典》2020年版四部(702页)。
5.1.2 枸橼酸质量标准(质量标准编号:)。
5.1.3 《中国药典》2020年版四部。
1.【性状】本品无色的半透明结晶、白色颗粒或白色结晶性粉末;无臭,味极酸;在干燥空气中微有风化性;水溶液显酸性反应。
本品在水中极易溶解,在乙醇中易溶,在乙醚中略溶。
2.【鉴别】2.1鉴别⑴2.1.1仪器与用具红外分光光度计、压片机、玛瑙研钵2.1.2操作方法取本品,在105℃干燥2小时,取干燥后的供试品约1mg,置入玛瑙研钵研细,再取溴化钾粉(约200mg),在玛瑙研钵中充分研磨混匀,移置于直径13mm的压模中,使铺布均匀,加压至20MPa,约60秒取出。
将供试片置于仪器的样品光路中,进行光谱扫描,本品的红外光吸收图谱应与对照的图谱(光谱集263图)一致。
2.2鉴别⑵---枸橼酸盐鉴别反应2.2.1试药稀硫酸、高锰酸钾试液、硫酸汞试液、溴试液、吡啶、醋酐2.2.2操作方法①取供试品溶液2ml(约相当于枸橼酸10mg),加稀硫酸数滴,加热至沸,加高锰酸钾试液数滴,振摇,紫色即消失;溶液分成两份,一份中加硫酸汞试液1滴,另一份中逐滴加入溴试液,均生成白色沉淀②取供试品约5mg,加吡啶-醋酐(3∶1)约5ml,振摇,即生成黄色到红色或紫红色的溶液。
说明书4-3

山梨醇铁注射液说明书山梨醇铁注射液说明书【药品名称】-通用名:山梨醇铁注射液曾用名:商品名:英文名:Iron sorbitex Injection汉语拼音:Shɑnlichuntie Zhusheye本品系山梨醇枸橼酸铁的灭菌胶体溶液,并用糊精和过量的山梨醇使其稳定。
【性状】本品为深棕色胶体溶液。
【药理毒理】药理作用:山梨醇铁属于抗贫血药,1ml含铁量50mg。
铁为人体必须元素,是构成血红蛋白、肌红蛋白、铁蛋白、细胞色素和某些组织酶的组分之一。
急性失血、慢性失血、铁需要相对增加,以及胃肠道铁吸收障碍时,都可因铁的消耗与摄取不平衡而发生缺铁性贫血。
对缺铁患者补充铁剂后,除血红蛋白合成加速外,与组织缺铁和含铁酶活性降低症状有关如:生长迟缓、行为异常、体力不足、黏膜组织变化及皮肤、指甲病变也都能逐渐得以纠正。
毒理作用:尚无有关动物实验资料。
【药代动力学】山梨醇铁是三价铁,仅供肌肉注射,注射后吸收迅速,2小时后血药浓度达到最高峰,24小时内从尿中排出给药量的20%~30%。
不可静脉注射。
如注射量超过血液的铁结合力,血浆中游离铁对机体有毒性作用,因此该药不能与口服铁盐同时应用。
【适应症】一般不做首选铁剂。
主要用于预防和治疗各种不宜口服铁剂者,如溃疡性结肠炎;或口服治疗无效的缺铁性贫血;或者是需要迅速纠正贫血状况者。
【用法用量】深部肌内注射。
1.成人:一次1~2ml,隔1~3日1次;儿童:体重大于6kg,一次1ml,一日1次,体重小于6kg,一次0.5ml,一日1次,贫血纠正后应继续使用一段时间以补充储存铁。
【不良反应】注射后有金属味及注射局部疼痛;少数患者可有发热、心动过速及关节痛等过敏反应;有报道,个别病人因肌肉注射本品出现过敏性休克和/或心脏毒性而死亡。
【禁忌】血色病或含铁血黄素沉着症,溶血性贫血,已知对铁过敏者及肝肾功能损害者禁用。
【注意事项】(1)需深部肌肉注射,进针及出针速度要快,以免药液渗出至皮下。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use Auryxia® safely and effectively. See full prescribing information for Auryxia.Auryxia (ferric citrate) tablet containing 210 mg of ferric iron equivalent to 1 g ferric citrate for oral useInitial U.S. Approval: 2014--------------------INDICATIONS AND USAGE----------------------Auryxia™ is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis (1)-----------------DOSAGE AND ADMINISTRATION---------------x Starting dose is 2 tablets orally 3 times per day with meals(2)x Adjust dose by 1 to 2 tablets as needed to maintain serum phosphorus at target levels, up to a maximum of 12 tabletsdaily. Dose can be titrated at 1-week or longer intervals. (2) ----------------DOSAGE FORMS AND STRENGTHS-------------x Tablets: 210 mg ferric iron, equivalent to 1 g ferric citrate (3) ----------------------CONTRAINDICATIONS------------------------x Iron overload syndromes (e.g., hemochromatosis) (4)-----------------WARNINGS AND PRECAUTIONS----------------x Iron overload: Monitor ferritin and TSAT. Patients may require a reduction in dose or discontinuation of IV iron.(5.1) x Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age.Keep this product out of reach of children. In case ofaccidental overdose, call a doctor or poison control centerimmediately. (5.2)x Patients with gastrointestinal bleeding or inflammation: Safety has not been established. (5.3)------------------------ADVERSE REACTIONS-----------------------x In clinical trials, likely adverse reactions occurring with Auryxia included diarrhea, discolored feces, constipation,nausea, and vomiting (6.1)To report SUSPECTED ADVERSE REACTIONS, contact Keryx Biopharmaceuticals at 1-844-445-3799 or FDA at 1-800FDA-1088 or /medwatch------------------------DRUG INTERACTIONS-----------------------x When clinically significant drug interactions are expected, consider separation of the timing of administration. Consider monitoring clinical responses or blood levels of theconcomitant medication (7)See 17 for PATIENT COUNSELING INFORMATIONRevised: 11/2014FULL PRESCRIBING INFORMATION: CONTENTS*1 INDICATIONS AND USAGE2 DOSAGE AND ADMINISTRATION2.1 Dosing and Dose Adjustment3 DOSAGE FORMS AND STRENGTHS4 CONTRAINDICATIONS5 WARNINGS AND PRECAUTIONS5.1 Iron Overload5.2 Accidental Overdose of Iron5.3 Patients with Gastrointestinal Bleeding or Inflammation6 ADVERSE REACTIONS6.1 Clinical Trials Experience7 DRUG INTERACTIONS8 USE IN SPECIFIC POPULATIONS8.1 Pregnancy8.2 Labor and Delivery8.3 Nursing Mothers8.4 Pediatric Use8.5 Geriatric Use 10 OVERDOSAGE11 DESCRIPTION12 CLINICAL PHARMACOLOGY12.1 Mechanism of Action12.2 Pharmacodynamics12.3 Pharmacokinetics13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment ofFertility14 CLINICAL STUDIES14.1 Long-term, Randomized, Controlled, Safety andEfficacy Trial14.2 Fixed-Dose Trial16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How Supplied16.2 Storage and Handling17 PATIENT COUNSELING INFORMATION17.1 Dosing Recommendations17.2 Adverse Reactions* Sections or subsections omitted from the full prescribing information are not listed.FULL PRESCRIBING INFORMATION1 INDICATIONS AND USAGEAuryxia® (ferric citrate) is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis.2 DOSAGE AND ADMINISTRATION2.1 Dosing and Dose AdjustmentThe recommended starting dose is 2 tablets orally 3 times per day with meals. Serum phosphorus levels should be monitored and the dose of Auryxia titrated in decrements or increments of 1 to 2 tablets per day as needed to maintain serum phosphorus at target levels, up to a maximum dose of 12 tablets daily. Dose can be titrated at 1-week or longer intervals.In a clinical trial conducted in the United States, patients required an average of 8 to 9 tablets a day to control serum phosphorus levels.3 DOSAGE FORMS AND STRENGTHSTablet: Auryxia 210 mg ferric iron, equivalent to 1 g ferric citrate, film-coated, peach-colored, and oval-shaped tablet embossed with “KX52”.4 CONTRAINDICATIONSAuryxia is contraindicated in patients with iron overload syndromes (e.g., hemochromatosis) [see Warnings and Precautions (5.1)].5 WARNINGS AND PRECAUTIONS5.1 Iron OverloadIron absorption from Auryxia may lead to excessive elevations in iron stores. Increases in serum ferritin and transferrin saturation (TSAT) levels were observed in clinical trials. In a 56-week safety and efficacy trial in which concomitant use of Auryxia and IV iron was permitted, 55 (19%) of patients treated with Auryxia had a ferritin level >1500 ng/mL as compared with 13 (9%) of patients treated with active control.Assess iron parameters (e.g., serum ferritin and TSAT) prior to initiating Auryxia and monitor iron parameters while on therapy [see Contraindications (4), Overdosage (10) and Clinical Pharmacology (12.2)]. Patients receiving IV iron may require a reduction in dose or discontinuation of IV iron therapy.5.2 Accidental Overdose of IronAccidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or poison control center immediately.5.3 Patients with Gastrointestinal Bleeding or Inflammation Patients with inflammatory bowel disease or active, symptomatic gastrointestinal bleeding were excluded from clinical trials. Safety has not been established in these populations.6 ADVERSE REACTIONS6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to adverse reaction rates in the clinical trials of another drug and may not reflect the rates observed in practice.Adverse reactions to a drug are most readily ascertained by comparison with placebo, but there is little placebo-controlled experience with Auryxia, so this section describes adverse events with Auryxia, some of which may be disease-related, rather than treatment-related.A total of 289 patients were treated with Auryxia and 149 patients were treated with active control (sevelamer carbonate and/or calcium acetate) during the 52-week, randomized, open-label, active control phase of a trial in patients on dialysis. A total of 322 patients were treated with Auryxia for up to 28 days in three short-term trials. Across these trials, 557 unique patients were treated with Auryxia; dosage regimens in these trials ranged from 210 mg to 2,520 mg of ferric iron per day, equivalent to 1 to 12 tablets of Auryxia. In these trials, adverse events reported for Auryxia were similar to those reported for the active control group.Adverse events reported in more than 5% of patients treated with Auryxia in these trials included diarrhea (21%), nausea (11%), constipation (8%), vomiting (7%), and cough (6%).During the 52-week, active-control period, 60 patients (21%) on Auryxia discontinued study drug because of an adverse event, as compared to 21 patients (14%) in the active control arm. Patients who were previously intolerant to any of the active control treatments (calcium acetate and sevelamer carbonate) were not eligible to enroll in the study. Gastrointestinal adverse reactions were the most common reason for discontinuing Auryxia (14%).Auryxia is associated with discolored feces (dark stools) related to the iron content, but this staining is not clinically relevant and does not affect laboratory tests for occult bleeding, which detect heme rather than non-heme iron in the stool.7 DRUG INTERACTIONSTable 1: Oral drugs that can be administered concomitantly withAuryxiaAmlodipineAspirinAtorvastatinCalcitriolClopidogrelDigoxinDoxercalciferolEnalaprilFluvastatinLevofloxacinMetoprololPravastatinPropranololSitagliptinWarfarinOral drugs that have to be separated from Auryxia and mealsDosing RecommendationsDoxycycline Take at least 1 hour before AuryxiaOral medications not listed in Table 1There are no empirical data on avoiding drug interactions between Auryxia and most concomitant oral drugs. For oral medications where a reduction in the bioavailability of that medication would have a clinically significant effect on its safety or efficacy, consider separation of the timing of the administration of the two drugs. The duration of separation depends upon the absorption characteristics of the medication concomitantly administered, such as the time to reach peak systemic levels and whether the drug is an immediate release or an extended release product. Consider monitoring clinical responses or blood levels of concomitant medications that have a narrow therapeutic range.8 USE IN SPECIFIC POPULATIONS8.1 PregnancyPregnancy Category B: There are no adequate and well-controlled studies in pregnant women. It is not known whether Auryxia can cause fetal harm when administered to a pregnant woman. Animal reproduction studies have not been conducted.The effect of Auryxia on the absorption of vitamins and other nutrients has not been studied in pregnant women. Requirements for vitamins and other nutrients are increased in pregnancy. An overdose of iron in pregnant women may carry a risk for spontaneous abortion, gestational diabetes and fetal malformation [see Nonclinical Toxicology (13.1)].8.2 Labor and DeliveryThe effects of Auryxia on labor and delivery are unknown.8.3 Nursing MothersData from rat studies have shown the transfer of iron into milk by divalent metal transporter-1 (DMT-1) and ferroportin-1 (FPN-1). Hence, there is a possibility of infant exposure when Auryxia is administered to a nursing woman.8.4 Pediatric UseThe safety and efficacy of Auryxia have not been established in pediatric patients.8.5 Geriatric UseClinical studies of Auryxia included 106 subjects aged 65 years and older (33 subjects aged75 years and older). Overall, the clinical study experience has not identified any obvious differences in responses between the elderly and younger patients in the tolerability or efficacy of Auryxia.10 OVERDOSAGENo data are available regarding overdose of Auryxia in patients. In patients with chronic kidney disease on dialysis, the maximum dose studied was 2,520 mg ferric iron (12 tablets of Auryxia) per day. Iron absorption from Auryxia may lead to excessive elevations in iron stores, especially when concomitant IV iron is used [see Warnings and Precautions (5.1)].In clinical trials, one case of elevated iron in the liver as confirmed by biopsy was reported in a patient administered IV iron and Auryxia.Because accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6 years of age, this product must be kept out of the reach of children. In case of accidental overdose, a doctor or poison control center should be contacted immediately [see Warnings and Precautions (5.2)].2F e +3y H 2Oxx=0.70 – 0.87, y = 1.9 – 3.3 11 DESCRIPTIONAuryxia (ferric citrate) is known chemically as iron (+3), x (1, 2, 3-propanetricarboxylic acid, 2-hydroxy-), y (H 2O)Auryxia 210 mg ferric iron tablets, equivalent to 1g ferric citrate, are film-coated, peach-colored, and oval-shaped tablets embossed with “KX52”. The inactive ingredients are pregelatinized starch and calcium stearate. In addition, the film-coating contains the following inactive ingredients; hypromellose, titanium dioxide, triacetin, and FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake, FD&C Red #40/Allura Red AC Aluminum Lake, and FD&C Blue #2/Indigo Carmine Aluminum Lake.12 CLINICAL PHARMACOLOGY 12.1Mechanism of ActionFerric iron binds dietary phosphate in the GI tract and precipitates as ferric phosphate. Thiscompound is insoluble and is excreted in the stool. By binding phosphate in the GI tract anddecreasing absorption, ferric citrate lowers the phosphate concentration in the serum.12.2 PharmacodynamicsIn addition to effects on serum phosphorus levels, Auryxia has been shown to increase serum iron parameters, including ferritin, iron and TSAT. In dialysis patients treated with Auryxia in a52-week study in which IV iron could also be administered, mean (SD) ferritin levels rose from593 (293) ng/mL to 895 (482) ng/mL, mean (SD) TSAT levels rose from 31% (11) to 39% (17) and mean (SD) iron levels rose from 73 (29) mcg/dL to 88 (42) mcg/dL. In contrast, in patients treated with active control, these parameters remained relatively constant [see Contraindications (4) and Warnings and Precautions (5.1)].12.3 PharmacokineticsAbsorption and DistributionFormal pharmacokinetic studies have not been performed with Auryxia. Examination of serum iron parameters has shown that there is systemic absorption of iron from Auryxia [seeContraindications (4), Warnings and Precautions (5.1) and Clinical Pharmacology (12.2)].Drug Interaction StudiesIn vitroOf the drugs screened for an interaction with ferric citrate in vitro, only doxycycline showed the potential for interaction with at least 70% decrease in its concentration. This interaction can be avoided by spacing the administration of doxycycline and ferric citrate [see Drug Interactions (7)].13 NONCLINICAL TOXICOLOGY13.1 Carcinogenesis, Mutagenesis, and Impairment of Fertility Data from carcinogenesis studies have shown that ferric citrate is not carcinogenic in mice and rats when administered intramuscularly or subcutaneously. Ferric citrate was neither mutagenic in the bacterial reverse mutation assay (Ames test) nor clastogenic in the chromosomal aberration test in Chinese hamster fibroblasts.14 CLINICAL STUDIESThe ability of Auryxia to lower serum phosphorus in patients with CKD on dialysis was demonstrated in randomized clinical trials: one 56-week, safety and efficacy trial, consisting of a 52-week active-controlled phase and a 4-week, placebo-controlled, randomized withdrawal period, and one 4-week open-label trial of different fixed doses of Auryxia. Both trials excluded subjects who had an absolute requirement for aluminum containing drugs with meals.14.1 Long-term, Randomized, Controlled, Safety and EfficacyTrialAfter the 2-week washout period during which phosphate binders were held, patients with a mean serum phosphorus of 7.5 mg/dL during washout were randomized 2:1 to Auryxia (N=292) or active control (calcium acetate and/or sevelamer carbonate; N=149). The majority (>96%) of subjects were on hemodialysis. The starting dose of Auryxia was 6 tablets/day, divided with meals. The starting dose of active control was the patient’s dose prior to the washout period. The dose of phosphate binder was increased or decreased as needed to maintain serum phosphorus levels between 3.5 and 5.5 mg/dL, to a maximum of 12 tablets/day.As shown in the figure below, serum phosphorus levels declined following initiation of therapy. The phosphorus lowering effect was maintained over 52 weeks of treatment.Figure 1: Serum Phosphorus Control over 52 WeeksFollowing completion of the 52-week active-controlled phase, Auryxia-treated patients were eligible to enter a 4-week placebo-controlled randomized withdrawal phase, in which patients were re-randomized in a 1:1 ratio to receive Auryxia (N=96) or placebo (N=96). During the placebo-controlled period, the serum phosphorus concentration rose by 2.2 mg/dL on placebo relative to patients who remained on Auryxia. Table 2:Effect of Auryxia on serum phosphorus during randomized withdrawalPrimary Endpoint (Week 56) Auryxia Placebo Treatment Difference(95% CI)p-valueSerum phosphorus (mg/dL)Mean baseline (Week 52)Mean change from baseline (Week 56)5.12 í5.44 1.79í í í<0.0001aaThe LS mean treatment difference and p-value for the change in mean were created via an ANCOVA model with treatment as the fixed effect and Week-52 baseline (phosphorus) as the covariate. Between-treatment differences were calculated as the LS mean (KRX-0502) – LS mean (placebo or active control). Note: Analyses using ANCOVA with last observation carried forward. ANCOVA=analysis of covariance; CI=confidence interval.14.2 Fixed-Dose TrialFollowing a 1-to 2-week washout from all phosphate-binding agents, 154 patients with hyperphosphatemia (mean serum phosphorus of 7.5 mg/dL) and CKD on dialysis were randomized in a 1:1:1 ratio to 1, 6, or 8 tablets/day of Auryxia for 4 weeks. Auryxia was administered with meals; subjects receiving 1 tablet/day were instructed to take it with theirlargest meal of the day, and subjects on 6 or 8 tablets/day took divided doses in any distribution with meals. Dose-dependent decreases in serum phosphorus were observed by Day 7 and remained relatively stable for the duration of treatment. The demonstrated reductions from baseline to Week 4 in mean serum phosphorus were significantly greater with 6 and 8 tablets/day than with 1 tablet/day (p<0.0001). Mean reduction in serum phosphorus at Week 4 was 0.1mg/dL with 1 tablet/day, 1.9 mg/dL with 6 tablets/day, and 2.1 mg/dL with 8 tablets/day.16 HOW SUPPLIED/STORAGE AND HANDLING16.1 How SuppliedTablets: Auryxia 210 mg ferric iron tablets equivalent to 1 g of ferric citrate are supplied as 200 tablets in 400-cc high-density polyethylene bottles. The 210 mg ferric iron tablets are film-coated, peach-colored, and oval-shaped tablets embossed with “KX52.”1 Bottle of 200-count 210 mg ferric iron tablets (NDC 59922-631-01)16.2 Storage and HandlingStorage: Store at 20 to 25°C (68 to 77°F): excursions permitted to 15° to 30°C (59°F to 86°F) [See USP controlled room temperature]. Protect from moisture.17 PATIENT COUNSELING INFORMATION17.1 Dosing RecommendationsInform patients to take Auryxia as directed with meals and adhere to their prescribed diets. Instruct patients on concomitant medications that should be dosed apart from Auryxia.17.2 Adverse ReactionsAdvise patients that Auryxia may cause discolored (dark) stools, but this staining of the stool is considered normal with oral medications containing iron.Auryxia may cause diarrhea, nausea, constipation and vomiting. Advise patients to report severe or persistent gastrointestinal symptoms to their physician.Manufactured for and Distributed by:Keryx Biopharmaceuticals, Inc.750 Lexington Avenue, 20th FloorNew York, NY 10022US Patents Nos 5,753,706; 6,903,235; 7,767,851; 8,093,423; 8,299,298; 8,338,642; 8,609,896; 8,754,257 and pending, comparable and/or related patents.Issued 11/2014 Rev 1。
