美国普通化学笔记5
普通化学笔记

普通化学1、化学反应的半衰期A Akc dtdc u =-= 注意积分计算。
2、关于化学反应的吉布斯自由能的计算 (1)、计算公式:G=H-TS (2)、关于H 的计算:所有稳定单质在标准状态下的焓变为0。
系统吸热焓变0>∆H ;放热反应0<∆H (3)、熵:对同一物质,气态时的熵大于液态,液态大于固态。
同一物质,在同一物态时候,温度升高,熵增加。
分子越复杂,熵越大;混合物或溶液的熵大于纯物质。
(4)、关于0<∆G ;则反应往正方向只发进行,0>∆G ,反应向逆方向自发进行。
0=∆G ,表示反应达到平衡。
(5)、fy w sc w RT G G m r m r ln+∆=∆Θ(注意:生成物用标准状态,压强100KPA )。
3、关于化学平衡(1)、压力对化学平衡的影响:用平衡常数计算。
(2)、温度:注意吸热及放热。
4、关于缓冲溶液的计算:-++=Ac H HAcB 0 A B-Ba Ba A+Ba-++=Ac K KAcA A A [A+Ba][Ba]/[B-Ba]=Ka5、关于氧化还原反应和电化学 (1)、失去电子的反应:氧化反应;充当电池的负极。
(2)、得到电子的反应:还原反应;充当电池的正极。
记住:还原剂被氧化,氧化剂被还原。
(3)、原电池负极写在左边,(-)Zn|Zn 2+(CZn 2+)|| Cu 2+(Ccu 2+)|Cu(+)。
(4)、标准电极:氢电极为0V 。
(5)、能特斯方程:氧化态(1)+ne=还原态(2))21lg(0.059-1/21/2-+=nE E 应用能特斯方程应注意的情况:若电极中有气体,则用相对压力解决。
若有难容固体及液体,则不用写进方程。
关于有氢离子的反应,写在氧化态一边,就作为氧化态处理,写在还原态一边就作为还原态处理。
关于用电动势的大小表示可以判断氧化剂的强弱:电动势越高,高价氧化性越强,低价还原性越强。
电池电动势:E=E+-E-;若算出来为小于0 ,则正负极倒过来。
高中化学选修五笔记(按章节)详解

3、常见异类异构
具有相同C原子数的异类异构有:
a、烯烃与环烷烃(CnH2n)
b、炔烃、二烯烃和环烯烃(CnH2n-2)
c、苯的同系物、二炔烃和四烯烃等(CnH2n-6)
d、饱和一元醇和醚、烯醇和烯醚等(CnH2n+2O)
e、饱和一元醛和酮、烯醛和烯酮等(CnH2nO)
原子
同种物质
组成、结构、性质都相同
分子式、结构式的形式及状态可能不同
无机物或有机物
第三节有机化合物的命名
一、链状有机物的命名
1、烷烃的命名
1)烷基的认识
烃分子失去一个氢原子所剩余的原子团叫做烃基。烷烃失去一个氢原子剩余的原子团就叫烷基,用“—R”表示。
2)烃基的同分异构
碳数较多的烷烃,失去不同位置的氢原子所形成的烃基有所不同,呈现同分异构现象
结构式
乙烯
具有化学式所能表示的意义,能反映物质的结构;能完整地表示出有机物分子中每个原子的成键情况的式子,但不表示空间结构
结构简式乙醇CH源自CH2OH结构式的简便写法,着重突出官能团
键线式
乙酸
表示有机化合物分子的结构,只要求表示出碳碳键以及与碳原子相连的基团,图式中的每个拐点和终点均表示一个碳原子
书写结构简式时要注意:
两者的关系:“官能团”属于“基”,但“基”不一定是“官能团”。
b、基与根
类别
基
根
实例
羟基
氢氧根
区别
电子式
电性
电中性
带一个单位负电荷
存在
有机化合物
无机化合物
电子数
9
10
6)常见有机物的主要类别、官能团和代表物质*
20200209高二化学(选修5内容1.1有机化合物的分类与结构特点)课堂笔记

第一章认识有机化合物YOUJIWUDEDINGYI1.自古以来,人类在生产生活中就利用了各类微生物。
如面团的发酵,酒的酿造,臭豆腐的制作等等。
但当时并没有“有机化合物”的概念。
2.19世纪初,瑞典化学家贝采里乌斯提出“有机化合物”的概念,其内容是“来自于动植物体的化合物”,并认为有机化合物得自天成,不能够通过人工合成。
3.1828年,德国化学家维勒通过加热异氰酸铵的方式得到了尿素,打破了有机物与无机物之间的界限。
NH4OCN→CO(NH2)2YOUJIWUDEDINGYI如今,我们对于有机化合物的定义内容是:绝大部分含碳的化合物,但是不包括碳的氧化物、硫化物(CS);2碳酸、氢氰酸(HCN)、氰酸(HOCN)、硫氰酸(HSCN)及这几种酸对应的盐;碳化硅(SiC)等主要在无机化学中研究的含碳物质。
所有有机物都含有碳元素,大部分有机物都含有氢。
按照组成元素可以将有机物分为烃和烃的衍生物两大类:只含有碳和氢两种元素的有机化合物称为烃,含有碳和氢之外元素的有机化合物称为烃的衍生物。
有机物的通性YOUJIWUDETONGXING1.除少数有机物外,大部分有机物都可以燃烧2.熔点较低,一般不超过400℃3.热稳定性较差,高温易分解4.极性一般较弱,大多不溶于水5.反应速率往往较慢,大多数反应需要一定的条件(如催化剂)6.反应历程比较复杂,副反应较多,因此有机反应通常用“→”表示链状化合物,如CH 2==CH —CH==CH 2、CH 3CH 2CH 2OH 、CH 3CH 2CHO 、CH 3CH 2CH 3环状化合物化合物,如化合物,如1.按碳的骨架分类芳香脂环一、按碳的骨架分类有机化合物2.相关概念辨析(1)不含的碳环化合物,都是脂环化合物。
(2)含一个或多个的化合物,都是芳香化合物。
(3)环状化合物还包括杂环化合物,即构成环的原子除碳原子外,还有其他原子,如氧原子(如呋喃)、氮原子、硫原子等。
(4)链状烃通常又称脂肪烃。
高中化学选修5精编笔记
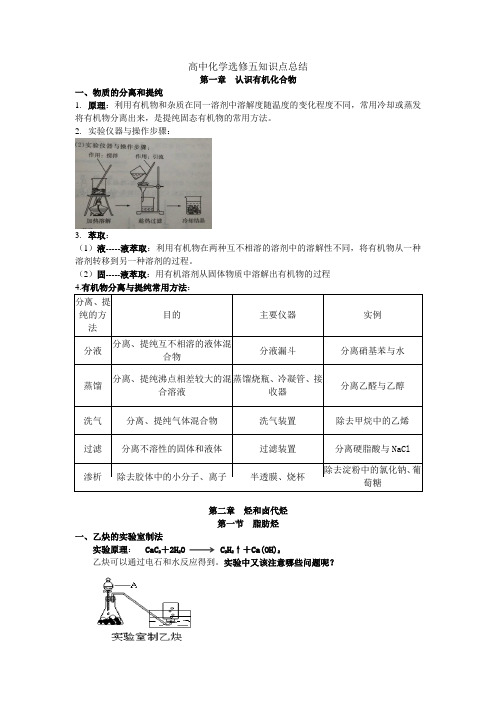
高中化学选修五知识点总结第一章认识有机化合物一、物质的分离和提纯1.原理:利用有机物和杂质在同一溶剂中溶解度随温度的变化程度不同,常用冷却或蒸发将有机物分离出来,是提纯固态有机物的常用方法。
2.实验仪器与操作步骤:3.萃取:(1)液-----液萃取:利用有机物在两种互不相溶的溶剂中的溶解性不同,将有机物从一种溶剂转移到另一种溶剂的过程。
(2)固-----液萃取:用有机溶剂从固体物质中溶解出有机物的过程4.有机物分离与提纯常用方法:分离、提纯的方法目的主要仪器实例分液分离、提纯互不相溶的液体混合物分液漏斗分离硝基苯与水蒸馏分离、提纯沸点相差较大的混合溶液蒸馏烧瓶、冷凝管、接收器分离乙醛与乙醇洗气分离、提纯气体混合物洗气装置除去甲烷中的乙烯过滤分离不溶性的固体和液体过滤装置分离硬脂酸与NaCl渗析除去胶体中的小分子、离子半透膜、烧杯除去淀粉中的氯化钠、葡萄糖第二章烃和卤代烃第一节脂肪烃一、乙炔的实验室制法实验原理:CaC2+2H2O C2H2↑+Ca(OH)2乙炔可以通过电石和水反应得到。
实验中又该注意哪些问题呢?注意:①实验装置在使用前要先检验气密性,只有气密性合格才能使用;②盛电石的试剂瓶要及时密封,严防电石吸水而失效;③取电石要用镊子夹取,切忌用手拿电石;④作为反应容器的烧瓶在使用前要进行干燥处理;⑤向烧瓶里加入电石时,要使电石沿烧瓶内壁慢慢滑下,严防让电石打破烧瓶;⑥电石与水反应很剧烈,向烧瓶里加水时要使水逐滴慢慢地滴下,当乙炔气流达到所需要求时,要及时关闭分液漏斗活塞,停止加水;电石是固体,水是液体,且二者很易发生反应生成C2H2气体。
很显然C2H2的生成符合固、液,且不加热制气体型的特点,那是不是说就可以用启普发生器或简易的启普发生器来制取乙炔呢?⑦实验室中不可用启普发生器或具有启普发生器原理的实验装置.......................作制备乙炔气体的实验装置。
主要原因是:a.反应剧烈,难以控制。
普通化学大一笔记(一)

普通化学大一笔记(一)原子结构•原子的组成•元素周期表•原子核•原子轨道化学键•电子亲和力•电离能•共价键•离子键•金属键•范德华力分子结构•分子的组成•配位化合物•水合物•离子半径和化学键化学反应•化学平衡•反应热•反应速率•化学动力学•电解质•酸碱反应物态转化•相变热•气液平衡•溶解度•溶液的浓度•气体的性质化学物质的实验室制备•常见实验室试剂的制备方法•实验室中常用的仪器•化学实验安全注意事项•实验室垃圾的处理方法化学物质的应用•化学反应在生活和工业中的应用•燃料的选择与应用•氧化还原反应在物质中的应用•化学物质对环境和生物的影响以上是普通化学大一的笔记,希望对学习化学的同学有所帮助。
原子结构原子的组成原子由质子、中子和电子组成。
质子和中子位于原子核中心,电子在核外绕核运动。
元素周期表元素周期表是由元素按照原子序数和化学性质排列的表格。
原子核原子核由质子和中子组成,质子质量为1,带正电荷;中子质量为1,不带电荷。
原子核的半径非常小,约为10^-14m。
原子轨道原子轨道是电子在原子核周围的运动轨道。
常见的有s、p、d、f 四种类型。
化学键电子亲和力电子亲和力是电子与原子结合所释放的能量。
电离能电离能是将原子或离子中的一个电子移出所需的最小能量。
共价键共价键是两个原子共用一对电子的化学键。
离子键离子键是由正负离子间的相互作用形成的化学键。
金属键金属键是由金属中的金属离子和自由电子相互作用形成的键。
范德华力范德华力是分子间的一种作用力,主要由氢键、分子间作用力等形成。
分子结构分子的组成分子由两个或更多原子通过化学键连接而成。
配位化合物配位化合物是由中心金属离子和周围配位体形成的离子或分子。
水合物水合物是由水分子与离子或分子形成的化合物。
离子半径和化学键离子半径的大小决定了离子之间化学键的强度和类型。
化学平衡化学平衡是化学反应达到动态平衡状态的一种特殊情况。
反应热反应热是一种热力学量,表示化学反应过程中所吸收或放出的热量。
普通化学讲义_笔记 (3)

专题一化学,就是研究物质化学运动和变化规律的科学,亦即研究那些具有一定质量、占有一定空间的实物的组成、结构、性质和变化规律,以及伴随这些变化过程的能量关系的科学。
物质有4种不同的物理聚集状态,即气态、液态、固态和等离子态。
气体的基本特征是其具有无限的可膨胀性、无限的掺合性和外界条件(温度、压力)对其体积影响的敏感性。
理想气体状态方程对含有物质的量为n的理想气体,在密闭的容器中其体积(V )、压力(p )和热力学温度(T )之间服从以下关系式: PV = nRT此式称为理想气体状态方程。
式中R 叫做摩尔气体常数,其值等于1 mol 任何理想气体的pV/T 值,其数值可根据阿伏加德罗定律来求得。
R =8.314J •mol -1·K -1 。
在使用理想气体状态方程时,要注意各物理量的量纲与R 数值及其单位的一致,即R =8.314J •mol -1·K-1时,式中n 、p 、V 、T 等物理量只能用它们的基本单位mol 、Pa 、m 3和K 。
理想气体状态方程可表示为另外一些形式,如:RT M m pV = 或RTM p =ρ 二、混合气体分压定律(道尔顿分压定律、阿马格分容定律)在恒温下,把混合气体分离成各个单独组分,并使其与混合气体具有相同的压力,此时该组分气体所占有的体积称为该组分的分体积。
ni n V i V i x )()()(== ∑=)()(i V i V 气—液平衡在临界温度以下,气体转化为液体,但分子的热运动并未停止,处于液体表面的少数分子能克服分子间力,重新飞逸出液面变成气体,此过程称为液体的蒸发(或气化)。
如果把液体放置于密闭的容器中,蒸气分子则不致逃走,已形成的蒸气分子又可能重新撞到液面上而凝聚为液态。
蒸发与凝聚两个过程同时进行,但开始时前者居优势,所以气相中分子逐渐增多,随后分子返回液相的机会增大,到了一定程度,单位时间内分子的出入数目相等,此时两个过程达到平衡: 气体凝聚蒸发液体,此时,液体的蒸发和气体的凝聚似乎已经停止,但实际上这两个过程仍在不断进行,只是它们的速度相等而已,因此,这是一种动态平衡。
高中化学选修五笔记(按章节)详解

选修五部分第一章认识有机化合物第一节有机化合物的分类一、有机物和无机物的区分有机物的含义1、旧义:含碳元素的化合物碳的氧化物、碳酸以及碳酸盐金属碳化物、氰化物除外2、新义:以碳原子为主要骨架的化合物二、有机物的分类1、按碳原子骨架区分1)链状化合物:分子中碳原子连接成链例如:丁烷CH3-CH2-CH2-CH3、乙醇CH3-CH2-OH、乙酸CH3-COOH等2)环状化合物:分子中碳原子连接成环a,分子中不含有苯环b、芳香化合物:如苯、苯甲酸分子中只含有一个苯环2、按官能团分类1)官能团:决定化合物特殊性质的原子或原子团2)烃:只含有碳、氢元素的有机化合物,如:烷烃、烯烃、炔烃、芳香烃3)烃的衍生物:烃分子里的氢原子被其他原子或原子团所取代而形成的一系列新的化合物a、卤代烃:烃分子中的氢原子被卤族原子取代而形成的化合物b、烃的含氧衍生物:烃分子中的氢原子被含氧原子的官能团所取代而形成的化合物4)常见的官能团*5)官能团和根(离子)、基的区别*a、基与官能团基:有机物分子里含有的原子或原子团。
官能团:决定化合物特殊性质的原子或原子团。
两者的关系:“官能团”属于“基”, 但“基”不一定是“官能团”。
b、基与根电中性带一个单位负电荷有机化合物无机化合物9 106)常见有机物的主要类别、官能团和代表物质*第二节有机化合物的结构特点一、有机化合物中碳原子的成键特点1、碳原子有4个价电子,能与其他原子形成4个共价键,碳碳之间的结合方式有单键、双键或三键;多个碳原子之间可以相互形成长短不一的碳链和碳环,碳链和碳环也可以相互结合,所以有机物结构复杂,数量庞大。
2、单键——甲烷的分子结构CH4分子中1个碳原子与4个氢原子形成4个共价键,构成以碳原子为中心、4个氢原子位于四个顶点的正四面体结构甲烷的电子式 甲烷的结构式 甲烷分子结构示意图在甲烷分子中,4个碳氢键是等同的,碳原子的4个价键之间的夹角(键角)彼此相等,都是109°28′。
第6章_美国化学文摘

分子式索引
分子式索引是以全卷报道过的化学物 质的分子式作为索引标题来查找文献 的一种索引。
丏利索引
CA从94卷开始用Patent Index代替以前的专利号索 引(Numerical Patent Index)和专利对照索引 (Patent concordance index)。目前CA共收录32 个国家和2个国际专利组织的专利文献。专利索引以 这些国家或组织的代号字顺编排,其下按专利号顺序 编排,通过专利号等,引出专利文献文摘号或同族专 利情况。
包括的主题内容有: ①化学物质大类,如Amines; ②分类定义不明确的化学物质,如Plastics; ③物化概念和现象,如Absorption; ④各类化学反应,如Oxidation; ⑤化工过程及设备,如Distillation; ⑥生物化学; ⑦动植物的俗名和学名; ⑧矿物岩石; ⑨动物名、植物名、细菌名、器官名、疾病名、治疗方法、手术名、 生物化学的酶类、氨基酸类、激素类等。
本节内容在教 材
P75
第6章
美国《化 学 文 摘》
Chemical Abstract
主讲人:孙鹏
/
6.1《化学文摘》概况
教材P75
CA由美国化学文摘服务社 (CAS) 编辑 出版。
创刊于1907年。
Chemical Abstracts Services
是一部文摘型检索工具之一。
30 31 31
73 74 旧80 新80
1、文摘 (1)分类
CA自1911年设计了30个类目,后来几经变 动,目前共设80个类目,划分成五个部,分别是生物化 学部、有机化学部、大分子化学部、应用化学和化学工程 部以及物理化学、无机化学和分析化学部。 CA每周出版一期,1997年以前,单号期出前三 部30个类目,双号期出第四、第五部包含的31~80类。 而从1997年Vol.126NO.1起至今,每期都出80个类 的文摘,不存在单号期与双号期的差别。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ChemistryChapter 8∙In 1864, English chemists john newlands noticed that when the known elements were arranged in order of atomic mass, every eighth element hadsimilar properties. Newlands referred to this peculiar relationship as thelaw of octaves. Howe ver, this “law” turned out to be inadequate forelements beyond calcium, and newland’s work was not accepted by thescientific community.∙Representative elements are the elements in groups 1A through 7A, all of which have incompletely filled s or p subshells of the highest principalquantum number. With the exception of helium, the noble gases (thegroup 8A elements) all have a completely filled p subshell. The transitionmetals are the elements in groups 1B and 3B through 8B, which haveincompletely filled d subshells or readily produce cations withincompletely filled d subshells (these metals are sometimes referred to asthe d-blok transition elements). The group 2B elements are Zn, Cd, andHg, which are neither representative elements nor transition metals. Thelanthanides and actinides are sometimes called f-block transition elementsbecause they have incompletely filled f subshells∙The outer electrons of an atom, which are those involved in chemical bonding are often called the valence electrons. Having the same number ofvalence electrons accounts for similarities in chemical behavior among theelements within each of these groups.∙Ions, or atoms and ions, that have the same number of electrons and hence the same ground-state electron configuration are said to be isoelectronic.∙Atomic radius of a metal is one-half the distance between the two-nuclei in two adjacent atoms. For elements that exist as diatomic molecules, theatomic radius is one-half the distance between the nuclei of the two atomsin a particular molecule.∙When looking at a periodic table:o The elements are increasing as in atomic radius as you go fromright to left, and from up to down. ****∙Ionic radius is the radius of a cation or an anion. Ionic radius affects the physical and chemical properties of an ionic compound.∙When a neutral atom is converted to an ion, we expect a change in size. If the atoms forms an anion, its size increases, because the nuclear chargeremains the same but the repulsion resulting from the additional electronenlarges the domain of the electron cloud. On the other hand, a cation issmaller than the neutral atom, because removing one or more electronsreduces electron-electron repulsion but the nuclear charge remains thesame, so the electron cloud shrinks.∙Focusing on isoelectronic cations, we see that the radii of tripostive ions (that is, ions that bear three positive charges) are smaller than those ofdipositive ions (that is, ions that bear two positive charges) which in turnare smaller than unipositve ions (that is, ions that bear one positive charge).∙Ionization energy – is the minimum energy required to remove an electron from a gaseous atom in its ground state. The magnitude of ionizationenergy is a measure of the effort required to force an atom to give up anelectron, or of how “tightly” the electron is held in the atom., the higherthe ionization energy the more difficult it is to remove the electron.∙For a many-electron atom, the amount of energy required to remove the first electron, from the atom in its ground state:o Energy + X(g) -> X+(g) + e-o Is called the first ionization energy (I1). In this equation Xrepresent a gaseous atom of any element and e- is an electron.Unlike an atom in the condensed liquid and solid phases, an atomis the gaseous phase is virtually uninfluenced by its neighbors.o Energy + X+(g) -> X2+(g) + e- Second ionizationo Energy + X2+(g) -> X3+(g) + e- Third Ionization∙When a electron is removed from a neutral atom, the repulsion among the remains electrons decreases. Because the nuclear charge remains constant,more energy is needed to remove another electron from the positivelycharged ions. Thus for the same element ionization energies alwaysincrease in this order:o I1<I2<I3<….∙Another property that greatly influences the chemical behavior of atoms is their ability to accept one or more electrons. This ability is called electronaffinity, which is the negative of the energy change that occurs when anelectron is accepted by an atom of an element in the gaseous stateo X(g) + e- -> X-(g) deltaH = -XXXkJ▪If delta h has a positive value (ie. 390 kj/mol) means thatthe process is exothermic▪If delta h has a negative value, that means that the processis endothermic∙Another trend in chemical behavior of the representative elements is the diagonal relationship. Diagonal relationship refers to similarities that existbetween pairs of elements in different groups and period of the periodictable. Specifically the first three members of the second period (Li, Be andB) exhibit many similarities to the elements located diagonally belowthem in the periodic table.If you would like to further understand this chapter, I suggested reading the summary. Or if you would like to learn more about the individual group elements, then I suggest reading the last few pages of this chapter.Chapter 9∙Lewis dot symbol – consists of the symbol of an element and one dot for each valence electron in an atom of the element.∙Covalent bond – a bond in which two electrons are shared by two atoms.Covalent compounds are compounds that contain only covalent bonds.∙Lone pairs – pairs of valence electrons that are not involved in covalent bond formation (ie. F2)∙Lewis structures is a representation of covalent bonding in which shared electron pairs are shown either as lines or as pairs of dots between two atoms, and lone pairs are shown as pairs of dots on individual atoms. Only valence electrons are shown in a Lewis structure.∙Octet rule – an atom other than hydrogen tends to from bonds until it is surrounded by eight valence electrons. In other words, a covalent b ond forms when there are not enough electrons for each individual atom tohave a complete octet. By sharing electrons in a covalent bond, theindividual atoms can complete their octets. The requirement for hydrogen is that it attains the electron configuration of helium, or a total of twoelectrons.o The octet rule works mainly for elements in the second period of the periodic table.∙Atoms can form different types of covalent bonds. In a single bond – two atoms are held together by one electron pair. Many compounds are held together by multiple bonds, that is, bonds formed when two atoms shre two or more pairs of electrons. If two atoms share two pairs of electrons, the covalent bond is called a double bond.∙ A triple bond arises when two atoms share three pairs of electrons, (N2) ∙Bond length – is defined as the distance between the nuclei of two covalently bonded atoms in a molecule.∙The bond HF is called a polar covalent bond, or simply a polar bond, because the electrons spend more time in the vicinity of one atom than the other. The HF bond and other polar bonds can be though of as beingintermediate between a (nonpolar) covalent bond, in which the sharing of electrons is exactly equal, and an ionic bond, in which the transfer of the electron(s) is nearly complete.∙ A property that helps us distinguish a nonpolar covalent bond from a polar covalent bond is electronegativity, the ability of an atom to attract toward itself the electrons in a chemical bond. Elements with highelectronegativity have a greater tendency to attract electrons than doelements with low electronegativity.o Electronegativity is related to electron affinity and ionization energy.o Electronegativity is a relative concept, mea ign tha t an element’s ectronegativity can be measured only in relation theelectronegativity of other elements.o Linus Pauling devised a method for calculating relativeelectronegativities of most elements.∙There is no sharp distinction between a polar bond and an ionic bond, but the following rule is helpful in distinguishing between them. An ionicbond forms when the electronegativity difference between the twobonding atoms is 2.0 more. This rule applies to most but not all ioniccompounds. Sometimes chemists use the quantity percent ionic characterto describe the nature of a bond. A purely ionic bond would have 100percent ionic character, although no such bond is known, whereas anonpolar or purely covalent bond has 0 percent ionic character.∙Electronegativity and electron affinity are related but different concepts.Both indicate the tendency of an atom to attract electrons. However,electron affinity refers to an isolated atom’s attraction for an additionalelectron, whereas electronegativity signifies the ability of an atom in achemical bond (with another atom) to attract the shared electron.Furthermore, the electron affinity is an experimentally measurablequantity, whereas electronegativity is an estimated number that cannot be measured.∙An atom’s formal charge is the electrical charge difference between the valence electrons in an isolated atom and the number of electrons assigned to an atom in a lewis structure.∙To assign the number of electrons on an atom in a lewis structure, we proceed as:o All the ato m’s nonbonding electrons are assigned to the atomo We break the bond(s) between the atom and other atom(s) and assign half of the bonding electrons to the atom∙When you write formal charges, these rules are helpful:o For molecules, the sum of the formal charges must add up to zero because they are electrically neutral species.o For cations, the sum of the formal charges must equal the positive chargeo For anions, the sum of the formal charges must equal the negative charge∙Keep in mind, that formal charges do not represent actual charge separation within the molecule.∙Resonance structure – one of two or more lewis structures for a single molecule that cannot be represented accurately by only one lewis structure.The double-headed arrow indicates that the structures shown areresonance structures.∙The term resonance itself means the use of two or more lewis structures to represent a particular molecule.∙Exceptions to the octet rule:o The incomplete octet:▪In some compounds the number of electrons surround thecentral atom in a stable molecule is fewer than eight.▪Elements in group 3A, particularly boron and aluminum,also tend to form compounds in which they are surroundedby fewer than eight electrons.∙ A resonance structure with a double bond betweenB and F can be drawn that satisfies the octet rule forB.▪The B-N bond is different from the covalent bondsdiscussed so far in the sense that both electrons arecontributed by the N atom. A covalent bond in which oneof the atoms donated both electrons is called a coordinatecovalent bond. Although the properties of a coordinatecovalent bond do not differ from those of a normal covalentbond (because all electrons are alike no matter what theirsource), the distinction is useful for keeping tack of valenceelectrons and assigning formal charges)o Odd-Electron Molecules▪Some molecules contain an odd number of electrons.Among them are nitric oxide (NO) and nitrogen dioxide(NO2)▪Because we need an even number of electrons for completepairing (to reach eight) the octet rule clearly cannot besatisfied for all the atoms in any molecule that has an oddnumber of electronso The expanded octet:▪In a number of compounds there are more than eightvalence electrons around an atom. These expanded octetsare needed only for atoms of elements in and beyond thethird period of the periodic table.∙ A measure of the stability of a molecule is its bond energy, which is the enthalpy change required to break a particular bond in 1 mole of gaseousmolecules. (bond energies in solids and liquids are affected byneighboring molecules.)∙In many cases, it is possible to predict the approximate enthalpy ofreaction by using the average bond energies. Because energy is alwaysrequired to break chemical bonds and chemical bond formation is alwaysaccompanied by a release of energy, we can estimate the enthalpy of areaction by counting the total number of bonds broken and formed in thereaction and recording all the corresponding energy changes. The enthalpyof reaction in the gas phase is given by:o deltaH o = sigma(BE(reactants)) – sigma(BE(products))o where be stands for average bond energy and sigma is thesummation signTo further understand Bond energies, and Lewis dot structures and resonance I suggest taking a deeper look into the textbook.。
