毕赤酵母实验操作手册
毕赤酵母手册

毕赤酵母表达实验手册作者:Jnuxz 来源:丁香园时间:2007-9-5大肠杆菌表达系统最突出的优点是工艺简单、产量高、周期短、生产成本低。
然而,许多蛋白质在翻译后,需经过翻译后的修饰加工,如磷酸化、糖基化、酰胺化及蛋白酶水解等过程才能转化成活性形式。
大肠杆菌缺少上述加工机制,不适合用于表达结构复杂的蛋白质。
另外,蛋白质的活性还依赖于形成正确的二硫键并折叠成高级结构,在大肠杆菌中表达的蛋白质往往不能进行正确的折叠,是以包含体状态存在。
包含体的形成虽然简化了产物的纯化,但不利于产物的活性,为了得到有活性的蛋白,就需要进行变性溶解及复性等操作,这一过程比较繁琐,同时增加了成本。
大肠杆菌是用得最多、研究最成熟的基因工程表达系统,当前已商业化的基因工程产品大多是通过大肠杆菌表达的,其主要优点是成本低、产量高、易于操作。
但大肠杆菌是原核生物,不具有真核生物的基因表达调控机制和蛋白质的加工修饰能力,其产物往住形成没有活性的包涵体,需要经过变性、复性等处理,才能应用。
近年来,以酵母作为工程菌表达外源蛋白日益引起重视,原因是与大肠杆菌相比,酵母是低等真核生物,除了具有细胞生长快,易于培养,遗传操作简单等原核生物的特点外,又具有真核生物时表达的蛋白质进行正确加工,修饰,合理的空间折叠等功能,非常有利于真核基因的表达,能有效克服大肠杆菌系统缺乏蛋白翻译后加工、修饰的不足。
因此酵母表达系统受到越来越多的重视和利用。
[1]。
同时与大肠杆菌相比,作为单细胞真核生物的酵母菌具有比较完备的基因表达调控机制和对表达产物的加工修饰能力。
酿酒酵母(Saccharomyces.Cerevisiae)在分子遗传学方面被人们的认识最早,也是最先作为外源基因表达的酵母宿主。
1981年酿酒酵母表达了第一个外源基因----干扰素基因[2],随后又有一系列外源基因在该系统得到表达[3、4、5、6]。
干扰素和胰岛素虽然已经利用酿酒酵母大量生产并被广泛应用,当利用酿酒酵母制备时,实验室的结果很令人鼓舞,但由实验室扩展到工业规模时,其产量迅速下降。
毕赤酵母发酵工艺手册

毕赤酵母发酵工艺手册1. 引言欢迎使用毕赤酵母发酵工艺手册。
本手册旨在介绍毕赤酵母发酵的基本原理、工艺步骤以及相关注意事项。
通过遵循本手册,您可以更好地理解和掌握毕赤酵母的发酵过程,从而在生产中取得更好的效果。
2. 毕赤酵母发酵基本原理- 毕赤酵母是一种常见的酵母菌,其发酵能力强,适用于多种发酵产品的生产。
- 发酵是指通过酵母菌对底物中的糖类进行代谢,产生酒精和二氧化碳的过程。
- 毕赤酵母在发酵过程中需要适宜的温度、pH值和营养物质等条件。
3. 毕赤酵母发酵工艺步骤1. 发酵前准备:- 准备好所需的发酵基质,包括糖类、氮源和维生素等。
- 对基质进行消毒处理,确保无害菌的存在。
2. 接种毕赤酵母:- 选择合适的毕赤酵母培养液进行接种,注意接种量的控制。
- 将毕赤酵母培养液均匀加入发酵基质中。
3. 发酵条件控制:- 控制发酵温度在合适的范围内,一般为25-30摄氏度。
- 监测发酵基质的pH值,保持在适宜的范围内。
- 提供足够的氧气供给,促进酵母的生长和代谢。
4. 发酵过程监测:- 定期对发酵过程中的温度、pH值和酵母数量等进行监测和记录。
- 根据监测结果及时调整发酵条件,确保发酵过程稳定进行。
5. 发酵结束:- 当发酵基质中的糖类被完全代谢,产物达到预期时,发酵过程结束。
- 将发酵产物经过处理和提取,得到最终的产品。
4. 注意事项- 在发酵过程中,应注意卫生和消毒,以防止杂菌的污染。
- 严格控制发酵条件,避免过高或过低的温度、pH值对发酵效果产生不利影响。
- 根据不同的发酵产品,可能需要调整发酵步骤和条件,建议根据具体要求进行调整。
- 在使用本工艺手册时,请参考其他文献和专业意见,确保准确性和可靠性。
以上是关于毕赤酵母发酵工艺手册的简要介绍。
希望本手册能对您在毕赤酵母的发酵工艺中提供帮助和指导。
如有任何问题,请随时与我们联系。
谢谢!。
毕赤酵母发酵手册
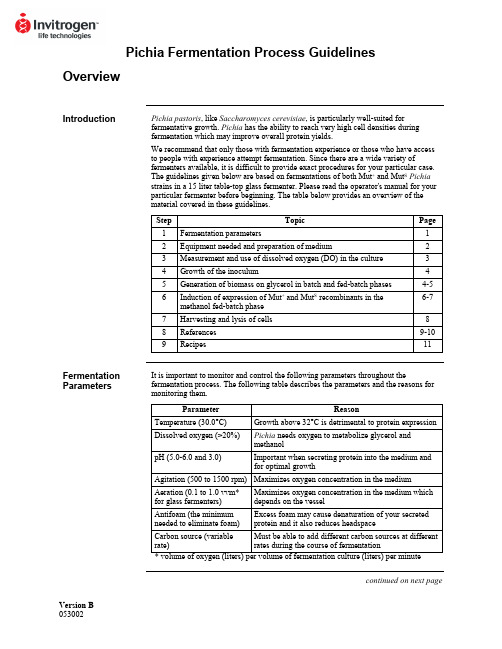
Version B Pichia Fermentation Process GuidelinesOverviewIntroduction Pichia pastoris, like Saccharomyces cerevisiae, is particularly well-suited forfermentative growth. Pichia has the ability to reach very high cell densities duringfermentation which may improve overall protein yields.We recommend that only those with fermentation experience or those who have accessto people with experience attempt fermentation. Since there are a wide variety offermenters available, it is difficult to provide exact procedures for your particular case.The guidelines given below are based on fermentations of both Mut+ and Mut S Pichiastrains in a 15 liter table-top glass fermenter. Please read the operator's manual for yourparticular fermenter before beginning. The table below provides an overview of thematerial covered in these guidelines.Step Topic Page1 Fermentationparameters 12 Equipment needed and preparation of medium 23 Measurement and use of dissolved oxygen (DO) in the culture 34 Growth of the inoculum 45 Generation of biomass on glycerol in batch and fed-batch phases 4-56 Induction of expression of Mut+ and Mut S recombinants in themethanol fed-batch phase6-77 Harvesting and lysis of cells 88 References 9-109 Recipes 11Fermentation Parameters It is important to monitor and control the following parameters throughout thefermentation process. The following table describes the parameters and the reasons for monitoring them.Parameter Reason Temperature (30.0°C) Growth above 32°C is detrimental to protein expression Dissolved oxygen (>20%) Pichia needs oxygen to metabolize glycerol andmethanolpH (5.0-6.0 and 3.0) Important when secreting protein into the medium andfor optimal growthAgitation (500 to 1500 rpm) Maximizes oxygen concentration in the mediumAeration (0.1 to 1.0 vvm*for glass fermenters)Maximizes oxygen concentration in the medium whichdepends on the vesselAntifoam (the minimumneeded to eliminate foam)Excess foam may cause denaturation of your secretedprotein and it also reduces headspaceCarbon source (variablerate)Must be able to add different carbon sources at differentrates during the course of fermentationcontinued on next pageOverview, continuedRecommended Equipment Below is a checklist for equipment recommendations.• A jacketed vessel is needed for cooling the yeast during fermentation, especially during methanol induction. You will need a constant source of cold water (5-10°C). This requirement may mean that you need a refrigeration unit to keep the water cold. • A foam probe is highly recommended as antifoam is required.• A source of O2--either air (stainless steel fermenters at 1-2 vvm) or pure O2(0.1-0.3 vvm for glass fermenters).• Calibrated peristaltic pumps to feed the glycerol and methanol.• Automatic control of pH.Medium Preparation You will need to prepare the appropriate amount of following solutions:• Fermentation Basal Salts (page 11)• PTM1Trace Salts (page 11)• ~75 ml per liter initial fermentation volume of 50% glycerol containing 12 ml PTM1 Trace Salts per liter of glycerol.• ~740 ml per liter initial fermentation volume of 100% methanol containing 12 mlPTM1Trace Salts per liter of methanol.Monitoring the Growth of Pichia pastoris Cell growth is monitored at various time points by using the absorbance at 600 nm (OD600) and the wet cell weight. The metabolic rate of the culture is monitored by observing changes in the concentration of dissolved oxygen in response to carbon availability (see next page).Dissolved Oxygen (DO) MeasurementIntroduction The dissolved oxygen concentration is the relative percent of oxygen in the mediumwhere 100% is O2-saturated medium. Pichia will consume oxygen as it grows, reducing the dissolved oxygen content. However, because oxygen is required for the first step ofmethanol catabolism, it is important to maintain the dissolved oxygen (DO) concentra-tion at a certain level (>20%) to ensure growth of Pichia on methanol. Accuratemeasurement and observation of the dissolved oxygen concentration of a culture willgive you important information about the state and health of the culture. Therefore, it isimportant to accurately calibrate your equipment. Please refer to your operator's manual.Maintaining the Dissolved Oxygen Concentration (DO) 1. Maintaining the dissolved oxygen above 20% may be difficult depending on theoxygen transfer rates (OTR) of the fermenter, especially in small-scale glassvessels. In a glass vessel, oxygen is needed to keep the DO above 20%, usually~0.1-0.3 vvm (liters of O2per liter of fermentation culture per minute). Oxygen consumption varies and depends on the amount of methanol added and the protein being expressed.2. Oxygen can be used at 0.1 to 0.3 vvm to achieve adequate levels. This can beaccomplished by mixing with the air feed and can be done in any glass fermenter.For stainless steel vessels, pressure can be used to increase the OTR. Be sure toread the operator's manual for your particular fermenter.3. If a fermenter cannot supply the necessary levels of oxygen, then the methanol feedshould be scaled back accordingly. Note that decreasing the amount of methanol may reduce the level of protein expression.4. To reach maximum expression levels, the fermentation time can be increased todeliver similar levels of methanol at the lower feed rate. For many recombinantproteins, a direct correlation between amount of methanol consumed and theamount of protein produced has been observed.Use of DO Measurements During growth, the culture consumes oxygen, keeping the DO concentration low. Note that oxygen is consumed whether the culture is grown on glycerol or methanol. The DO concentration can be manipulated to evaluate the metabolic rate of the culture and whether the carbon source is limiting. The metabolic rate indicates how healthy the culture is. Determining whether the carbon source is limiting is important if you wish to fully induce the AOX1 promoter. For example, changes in the DO concentrations (DO spikes) allow you to determine whether all the glycerol is consumed from the culture before adding methanol. Secondly, it ensures that your methanol feed does not exceed the rate of consumption. Excess methanol (> 1-2% v/v) may be toxic.Manipulation of DO If carbon is limiting, shutting off the carbon source should cause the culture to decrease its metabolic rate, and the DO to rise (spike). Terminate the carbon feed and time how long it takes for the DO to rise 10%, after which the carbon feed is turned back on. If the lag time is short (< 1 minute), the carbon source is limiting.Fermenter Preparation and Glycerol Batch PhaseInoculum Seed Flask Preparation Remember not to put too much medium in the baffled flasks. Volume should be 10-30% of the total flask volume.1. Baffled flasks containing a total of 5-10% of the initial fermentation volume ofMGY or BMGY are inoculated with a colony from a MD or MGY plate or from a frozen glycerol stock.2. Flasks are grown at 30°C, 250-300 rpm, 16-24 hours until OD600= 2-6. Toaccurately measure OD600> 1.0, dilute a sample of your culture 10-fold before reading.Glycerol Batch Phase 1. Sterilize the fermenter with the Fermentation Basal Salts medium containing 4%glycerol (see page 11).2. After sterilization and cooling, set temperature to 30°C, agitation and aeration tooperating conditions (usually maximum rpm and 0.1-1.0 vvm air), and adjust the pH of the Fermentation Basal Salts medium to 5.0 with 28% ammonium hydroxide(undiluted ammonium hydroxide). Add aseptically 4.35 ml PTM1trace salts/liter of Fermentation Basal Salts medium.3. Inoculate fermenter with approximately 5-10% initial fermentation volume from theculture generated in the inoculum shake flasks. Note that the DO will be close to 100% before the culture starts to grow. As the culture grows, it will consumeoxygen, causing the DO to decrease. Be sure to keep the DO above 20% by adding oxygen as needed.4. Grow the batch culture until the glycerol is completely consumed (18 to 24 hours).This is indicated by an increase in the DO to 100%. Note that the length of timeneeded to consume all the glycerol will vary with the density of the initial inoculum.5. Sampling is performed at the end of each fermentation stage and at least twice daily.We take 10 ml samples for each time point, then take 1 ml aliquots from this 10 mlsample. Samples are analyzed for cell growth (OD600and wet cell weight), pH, microscopic purity, and protein concentrations or activity. Freeze the cell pellets and supernatants at -80°C for later analysis. Proceed to Glycerol Fed-Batch Phase,page 5.Yield A cellular yield of 90 to 150 g/liter wet cells is expected for this stage. Recombinant protein will not yet be produced due to the absence of methanol.Introduction Once all the glycerol is consumed from the batch growth phase, a glycerol feed isinitiated to increase the cell biomass under limiting conditions. When you are ready toinduce with methanol, you can use DO spikes to make sure the glycerol is limited.Glycerol Fed-Batch Phase 1. Initiate a 50% w/v glycerol feed containing 12 ml PTM1trace salts per liter of glycerol feed. Set the feed rate to 18.15 ml/hr /liter initial fermentation volume.2. Glycerol feeding is carried out for about four hours or longer (see below). A cellularyield of 180 to 220 g/liter wet cells should be achieved at the end of this stage while no appreciable recombinant protein is produced.Note The level of expressed protein depends on the cell mass generated during the glycerolfed-batch phase. The length of this feed can be varied to optimize protein yield. A rangeof 50 to 300 g/liter wet cells is recommended for study. A maximum level of 4%glycerol is recommended in the batch phase due to toxicity problems with higher levelsof glycerol.Important If dissolved oxygen falls below 20%, the glycerol or methanol feed should bestopped and nothing should be done to increase oxygen rates until the dissolvedoxygen spikes. At this point, adjustments can be made to agitation, aeration, pressure oroxygen feeding.Proteases In the literature, it has been reported that if the pH of the fermentation medium islowered to 3.0, neutral proteases are inhibited. If you think neutral proteases aredecreasing your protein yield, change the pH control set point to 3.0 during the glycerolfed-batch phase (above) or at the beginning of the methanol induction (next page) andallow the metabolic activity of the culture to slowly lower the pH to 3.0 over 4 to 5 hours(Brierley, et al., 1994; Siegel, et al., 1990).Alternatively, if your protein is sensitive to low pH, it has been reported that inclusion ofcasamino acids also decreases protease activity (Clare, et al., 1991).Introduction All of the glycerol needs to be consumed before starting the methanol feed to fullyinduce the AOX1 promoter on methanol. However, it has been reported that a "mixedfeed" of glycerol and methanol has been successful to express recombinant proteins(Brierley, et al., 1990; Sreekrishna, et al., 1989). It is important to introduce methanolslowly to adapt the culture to growth on methanol. If methanol is added too fast, it willkill the cells. Once the culture is adapted to methanol, it is very important to use DOspikes to analyze the state of the culture and to take time points over the course ofmethanol induction to optimize protein expression. Growth on methanol also generates alot of heat, so temperature control at this stage is very important.Mut+ Methanol Fed-Batch Phase 1. Terminate glycerol feed and initiate induction by starting a 100% methanol feedcontaining 12 ml PTM1trace salts per liter of methanol. Set the feed rate to 3.6 ml/hr per liter initial fermentation volume.2. During the first 2-3 hours, methanol will accumulate in the fermenter and thedissolved oxygen values will be erratic while the culture adapts to methanol.Eventually the DO reading will stabilize and remain constant.3. If the DO cannot be maintained above 20%, stop the methanol feed, wait for theDO to spike and continue on with the current methanol feed rate. Increaseagitation, aeration, pressure or oxygen feeding to maintain the DO above 20%. 4. When the culture is fully adapted to methanol utilization (2-4 hours), and is limitedon methanol, it will have a steady DO reading and a fast DO spike time (generally under 1 minute). Maintain the lower methanol feed rate under limited conditions for at least 1 hour after adaptation before doubling the feed. The feed rate is then doubled to ~7.3 ml/hr/liter initial fermentation volume.5 After 2 hours at the 7.3 ml/hr/liter feed rate, increase the methanol feed rate to~10.9 ml/hr per liter initial fermentation volume. This feed rate is maintainedthroughout the remainder of the fermentation.6. The entire methanol fed-batch phase lasts approximately 70 hours with a total ofapproximately 740 ml methanol fed per liter of initial volume. However, this may vary for different proteins.Note: The supernatant may appear greenish. This is normal.Yield The cell density can increase during the methanol fed-batch phase to a final level of 350 to 450 g/liter wet cells. Remember that because most of the fermentation is carried out ina fed-batch mode, the final fermentation volume will be approximately double the initialfermentation volume.Fermentation of Mut S Pichia Strains Since Mut S cultures metabolize methanol poorly, their oxygen consumption is very low. Therefore, you cannot use DO spikes to evaluate the culture. In standard fermentations of a Mut S strain, the methanol feed rate is adjusted to maintain an excess of methanol in the medium which does not exceed 0.3% (may be determined by gas chromatography). While analysis by gas chromatography will insure that nontoxic levels of methanol are maintained, we have used the empirical guidelines below to express protein in Mut S strains. A gas chromatograph is useful for analyzing and optimizing growth of Mut S recombinants.continued on next pageMethanol Fed-Batch Phase, continuedMut S Methanol Fed- Batch Phase The first two phases of the glycerol batch and fed-batch fermentations of the Mut S strains are conducted as described for the Mut+ strain fermentations. The methanol induction phases of the Mut+ and Mut S differ in terms of the manner and amount in which the methanol feed is added to the cultures.1. The methanol feed containing 12 ml PTM1trace salts per liter of methanol is initiated at 1 ml/hr/liter initial fermentation volume for the first two hours. It is then increased in 10% increments every 30 minutes to a rate of 3 ml/hr which ismaintained for the duration of the fermentation.2.. The vessel is then harvested after ~100 hours on methanol. This time may be variedto optimize protein expression.Harvesting and Lysis of CellsIntroduction The methods and equipment listed below are by no means complete. The amount of cells or the volume of supernatant will determine what sort of equipment you need.Harvesting Cells and Supernatant For small fermentations (1-10 liters), the culture can be collected into centrifuge bottles (500-1000 ml) and centrifuged to separate the cells from the supernatant.For large fermentations, large membrane filtration units (Millipore) or a Sharples centrifuge can be used to separate cells from the supernatant. The optimal method will depend on whether you need the cells or the supernatant as the source of your protein and what you have available.Supernatants can be loaded directly onto certain purification columns or concentrated using ultrafiltration.Cell Lysis We recommend cell disruption using glass beads as described in Current Protocols inMolecular Biology, page 13.13.4. (Ausubel, et al., 1990) or Guide to ProteinPurification (Deutscher, 1990). This method may be tedious for large amounts of cells.For larger amounts, we have found that a microfluidizer works very well. Frenchpressing the cells does not seem to work as well as the glass beads or the microfluidizer.ReferencesIntroduction Most of the references below refer to papers where fermentation of Pichia wasperformed. Note that some of these are patent papers. You can obtain copies of patentsusing any of the following methods.• Patent Depository Libraries. U. S. patents and international patents granted underthe Patent Cooperation Treaty (PCT) are available on microfilm. These can be copiedand mailed or faxed depending on length. There is a fee for this service. The referencelibrarian at your local library can tell you the location of the nearest Patent DepositoryLibrary.• Interlibrary Loan. If you are not near a Patent Depository Library, you may request acopy of the patent through interlibrary loan. There will be a fee for this service.• U. S. Patent Office. Requests may be made directly to the Patent Office, Arlington,VA. Please call 703-557-4636 for more information on cost and delivery.• Private Library Services. There are private companies who will retrieve and sendyou patents for a fee. Two are listed below:Library Connection: 804-758-3311Rapid Patent Services: 800-336-5010Citations Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A.,Struhl, K., eds (1990) Current Protocols in Molecular Biology. GreenePublishing Associates and Wiley-Interscience, New York.Brierley, R. A., Siegel, R. S., Bussineau, C. M. Craig, W. S., Holtz, G. C., Davis, G. R.,Buckholz, R. G., Thill, G. P., Wondrack, L. M., Digan, M. E., Harpold, M. M.,Lair, S. V., Ellis, S. B., and William, M. E. (1989) Mixed Feed RecombinantYeast Fermentation. International Patent (PCT) Application. Publication No.WO 90/03431.Brierley, R. A., Bussineau, C., Kosson, R., Melton, A., and Siegel, R. S. (1990)Fermentation Development of Recombinant Pichia pastoris Expressing theHeterologous Gene: Bovine Lysozyme. Ann. New York Acad. Sci.589: 350-362.Brierley, R. A., Davis, G. R. and Holtz, G. C. (1994) Production of Insulin-Like GrowthFactor-1 in Methylotrophic Yeast Cells. United States Patent5,324,639.Clare, J. J., Romanos, M. A., Rayment, F. B., Rowedder, J. E., Smith, M. A., Payne, M.M., Sreekrishna, K. and Henwood, C. A. (1991) Production of EpidermalGrowth Factor in Yeast: High-level Secretion Using Pichia pastoris StrainsContaining Multiple Gene Copies. Gene105: 205-212.Cregg, J. M., Tschopp, J. F., Stillman, C., Siegel, R., Akong, M., Craig, W. S.,Buckholz, R. G., Madden, K. R., Kellaris, P. A., Davis, G. R., Smiley, B. L.,Cruze, J., Torregrossa, R., Veliçelebi, G. and Thill, G. P. (1987) High-levelExpression and Efficient Assembly of Hepatitis B Surface Antigen in theMethylotrophic Yeast Pichia pastoris. Bio/Technology5: 479-485.Cregg, J. M., Vedvick, T. S. and Raschke, W. C. (1993) Recent Advances in theExpression of Foreign Genes in Pichia pastoris. Bio/Technology11: 905-910.Deutscher, M. P. (1990) Guide to Protein Purification. In: Methods in Enzymology (J.N. Abelson and M. I. Simon, eds.) Academic Press, San Diego, CA.continued on next pageReferences, continuedCitations, continuedDigan, M. E., Lair, S. V., Brierley, R. A., Siegel, R. S., Williams, M. E., Ellis, S. B., Kellaris, P. A., Provow, S. A., Craig, W. S., Veliçelebi, G., Harpold, M. M. andThill, G. P. (1989) Continuous Production of a Novel Lysozyme via Secretionfrom the Yeast Pichia pastoris. Bio/Technology7: 160-164.Hagenson, M. J., Holden, K. A., Parker, K. A., Wood, P. J., Cruze, J. A., Fuke, M., Hopkins, T. R. and Stroman, D. W. (1989) Expression of Streptokinase inPichia pastoris Yeast. Enzyme Microbiol. Technol.11: 650-656.Laroche, Y., Storme, V., Meutter, J. D., Messens, J. and Lauwereys, M. (1994) High-Level Secretion and Very Efficient Isotopic Labeling of Tick AnticoagulantPeptide (TAP) Expressed in the Methylotrophic Yeast, Pichia pastoris.Bio/Technology12: 1119-1124.Romanos, M. A., Clare, J. J., Beesley, K. M., Rayment, F. B., Ballantine, S. P., Makoff,A. J., Dougan, G., Fairweather, N. F. and Charles, I. G. (1991) RecombinantBordetella pertussis Pertactin p69 from the Yeast Pichia pastoris High LevelProduction and Immunological Properties. Vaccine9: 901-906.Siegel, R. S. and Brierley, R. A. (1989) Methylotrophic Yeast Pichia pastoris Produced in High-cell-density Fermentations With High Cell Yields as Vehicle forRecombinant Protein Production. Biotechnol. Bioeng.34: 403-404.Siegel, R. S., Buckholz, R. G., Thill, G. P., and Wondrack, L. M. (1990) Production of Epidermal Growth Factor in Methylotrophic Yeast Cells. International Patent(PCT) Application. Publication No. WO 90/10697.Sreekrishna, K., Nelles, L., Potenz, R., Cruse, J., Mazzaferro, P., Fish, W., Fuke, M., Holden, K., Phelps, D., Wood, P. and Parker, K. (1989) High LevelExpression, Purification, and Characterization of Recombinant Human TumorNecrosis Factor Synthesized in the Methylotrophic Yeast Pichia pastoris.Biochemistry28(9): 4117-4125.©2002 Invitrogen Corporation. All rights reservedRecipesFermentation Basal Salts Medium For 1 liter, mix together the following ingredients:Phosphoric acid, 85% (26.7 ml)Calcium sulfate 0.93 gPotassium sulfate 18.2 gMagnesium sulfate-7H2O 14.9gPotassium hydroxide 4.13 gGlycerol 40.0g Water to 1 literAdd to fermenter with water to the appropriate volume and sterilize.PTM1 Trace Salts Mix together the following ingredients:Cupric sulfate-5H2O 6.0gSodium iodide 0.08 gManganese sulfate-H2O 3.0gSodium molybdate-2H2O 0.2gBoric Acid 0.02 g Cobalt chloride 0.5 g Zinc chloride 20.0 gFerrous sulfate-7H2O 65.0gBiotin 0.2gSulfuric Acid 5.0 mlWater to a final volume of 1 literFilter sterilize and store at room temperature.Note: There may be a cloudy precipitate upon mixing of these ingredients. Filter-sterilize as above and use.11。
GS115毕赤酵母表达菌使用说明

编号
名称
北京华越洋生物 NRR01030 GS115 毕赤酵母表达菌
基 本 信 息 :
名称:GS115 毕赤酵母表达菌
规格:300ul 甘油菌
储 存 温 度 : -‐80℃
发突变为组氨酸野生型的概率一般低于 10-‐8。GS115 毕赤酵母可以在 YPD
培养基中生长,或者在补充有组氨酸的 minimal media 中生长,但是无法
在单独的 minimal media 中生长。GS115 毕赤酵母在做质粒转化的时候,
可 采 用 电 转 化 的 方 式 将 质 粒 转 入 。
基 因 组 :
His4( 基 因 5 是毕赤酵母菌株,是巴斯德毕赤酵母的一种,属于真核细胞。
一般的针对原核生物的抗生素例如卡那和氨苄对酵母是无效的,因此为了
操作说明:
1,本品包含一份甘油菌,使用本甘油菌时可以不用完全融解,在甘油菌表
面蘸取少量涂板或进行液体培养即可。也可以完全融解后使用,但随着冻融次数
的增加,细菌的活力会逐渐下降。
2,为保证菌种纯正,避免其它细菌污染,尽量先划平板,然后再挑单克隆
菌落进行后续操作。
毕赤酵母适宜的生长温度是 28 至 30 度,温度超过 32 度对蛋白的表
达是有害的,并可能导致细胞的死亡。GS115 毕赤酵母是是组氨酸缺陷型
(His4 基因型),如果表达载体上携带有组氨酸基因,可补偿宿主菌的组
氨酸缺陷,因此可以在不含组氨酸的培养基上筛选转化子。这些受体菌自
养。细菌在 30-‐35℃培养箱中培养 24-‐48h,真菌在 23-‐28℃培养箱中培养 24-‐72h
(必要时,可适当延长培养时间)。
毕赤酵母实验操作手册

毕赤酵母实验操作手册精心整理毕赤酵母表达实验手册大肠杆菌表达系统最突出的优点是工艺简单、产量高、生产成本低。
然而,许多蛋白质在翻译后,需经过翻译后的修饰加工,如磷酸化、糖基化、酰胺化及蛋白酶水解等过程才能转化成活性形式。
大肠杆菌缺少上述加工机制,不适合用于表达结构复杂的蛋白质。
另外,蛋白质的活性还依赖于形成正确的二硫键并折叠成高级核生物表达系统基因表达调控和蛋白修饰功能,避免了产物活性低,包涵体变性、复性等等间题[1]。
与大肠杆菌相比,酵母是单细胞真核生物,具有比较完备的基因表达调控机制和对表达产物的加工修饰能力,人们对酿酒酵母(Saccharomyces.Cerevisiae)分子遗传学方面的认识最早,酿酒酵母也最先作为外源基因表达的酵母宿主.1981年酿酒酵母表达了第一个外源基因一干扰素基因,随后又有一系列外源基因在该系统得到表达。
虽然干扰素和胰岛素已大量生产并在人群中广泛应用,但很大部分表达由实验室扩展到工业规模时,培养基中维特质粒高拷贝数的选择压力消失,质粒变得不稳定,拷贝数下降,而大多数外源基因的高效表达需要高拷贝数的维特,因此引起产量下降。
同时,实验室用培养基复杂而昂贵,采用工业规模能够接受的培养母(一。
⑷毕赤酵母中存在过氧化物酶体,表达的蛋白贮存其中,可免受蛋白酶的降解,而且减少对细胞的毒害作用。
Pichia.pastoris基因表达系统经过近十年发展,已基本成为较完善的外源基因表达系统,具有易于高密度发酵,表达基因稳定整合在宿主基因组中,能使产物有效分泌并适当糖基化,培养方便经济等特点。
利用强效可调控启动子AOX1,已高效表达了HBsAg、TNF、EGF、破伤风毒素C片段、基因工程抗体等多种外源基因,证实该系统为高效、实用、简便,以提高表达量并保持产物生物学活性为突出特征的外源基因表达系统,而且非常适宜子扩大为工业规模[4]。
目前美国FDA已能评价来自该系统的基因工程产品,最近来自该系统的Cephelon制剂已获得FDA批准,所以该系统被认为是安全的.Pichia.pastoris表达系统在生物工程领域将发挥越有中,需带有信号肽序列。
毕赤酵母的摇瓶发酵方法[指南]
![毕赤酵母的摇瓶发酵方法[指南]](https://img.taocdn.com/s3/m/3ffac097d5d8d15abe23482fb4daa58da1111c5e.png)
毕赤酵母的摇瓶发酵方法:一、摇瓶发酵方法:毕赤酵母摇瓶发酵方法分为两个阶段,1、酵母菌株生长阶段;2、脂肪酶诱导表达阶段。
1、酵母生长阶段。
准备试剂:1000ml BMGY培养基,1000ml BMMY培养基,10X的甲醇,摇瓶1L(灭菌),温控摇床,50ml离心管(灭菌)。
紫外分光光度计,石英比色皿。
以下所有操作均在超净台内或者无菌条件下完成。
(1)往灭好菌的IL摇瓶中加入100mlBMGY培养基,然后加入约1ml脂肪酶菌株(培养基:菌液=100:1),用透气膜封口(透气,但是细菌不能透过)。
置于温控摇床上,温度调至300C,转速为250-300rpm/min,使酵母生长,OD600=2.0-6.0,时间约为15-24小时。
(2)将发酵液转入50ml离心管,1500g-3000g离心5min。
去掉上清,用BMMY 培养基将菌体浓度稀释至OD600=1.0,约有500ml左右。
将稀释后的发酵液分别加入到1L的药瓶中,每个摇瓶150ml发酵液(绝不能超过200ml)。
(3)将摇瓶置于温控摇床上,温度调至300C,转速为250-300rpm/min,使酵母表达脂肪酶,每24小时加入一次5%的甲醇,使甲醇的终浓度为0.5%。
连续诱导表达48小时。
(4)将发酵液进行12000rpm/min离心5min,取上清(若上清仍混浊,可反复离心);进行酶活分析和蛋白含量分析。
BMGY培养基的配制(1000ml):20g蛋白胨(peptone),10g酵母提取物(Yeast Extract),加水至700ml;1210C高温灭菌20min。
然后分别在无菌条件下加入10X YNB 100ml,10X 磷酸钾缓冲液(PH6.0)100ml,10X甘油 100ml。
BMMY培养基的配制方法(1000ml):20g蛋白胨(peptone),10g酵母提取物,加水至700ml;1210C高温灭菌20min。
然后分别在无菌条件下加入10X YNB 100ml,10X 磷酸钾缓冲液(PH6.0)100ml,10X甲醇 100ml。
毕赤酵母转化方法

毕赤酵母转化方法
实验概要
本实验介绍了毕赤酵母电转化方法。
该方法无需产生去壁细胞,是产生毕赤酵母重组子的简便方法。
与去壁细胞效率相似。
实验步骤
1. 细胞准备:
1) 在含5mlYPD 的50ml 离心管中,培养毕赤酵母,30 度过夜。
2) 取0.1-0.5ml 过夜培养物,接种含500ml 新鲜培养基的2L 摇瓶,过夜生长至OD600=1.3-1.5。
3) 在4 度,1500g离心5min收集细胞,用500ml预冷的灭菌水悬浮细胞。
4) 如上离心,用250 ml预冷的灭菌水悬浮细胞。
5) 如上离心,用20ml预冷的1M山梨醇悬浮细胞。
6) 如上离心,用1 ml 预冷的1M山梨醇悬浮细胞,至终体积约1.5ml。
注意:可冻存80ul 等量的电感受态细胞,但转化效率会下降很多。
2. 转化:
1) 取80ul上述细胞与5-20ug线性化DNA(溶于5-10ulTE)混合,转入预冷的0.2cm 电转杯中。
2) 在冰上放置5min。
3) 根据所使用装置推荐的酿酒酵母参数进行电击。
4) 立即加入1ml 预冷的1M山梨醇至杯中,将内容物转移至灭菌离心管中。
5) 分成200-600ul等份,涂于MD 或RDB平板上。
6) 在30 度孵育平板至克隆产生,筛选Mut /Muts表型。
毕赤酵母表达(pichia pastoris expression )实验手册(3)

毕赤酵母表达(pichia pastoris expression )实验手册(3)液体YPD培养基可常温保存;琼脂YPD平板在4℃可保存几个月。
加入Ze ocin 100ug / ml,成为YPDZ培养基,可以4℃条件下保存1~2周。
2.4 YPDS + Zeocin 培养基(Yeast Extract Peptone Dextrose Medi um):yeast extract 1%peptone 2%dextrose (glucose) 2%sorbitol 1 M+agar 2%+ Zeocin 100 μg/ml不管是液体 YPDS培养基,还是YPDS + Zeocin 培养基,都必须存放4℃条件下,有效期1~2周。
2.5 MGYMinimal Glycerol Medium (最小甘油培养基)(34%YNB;1%甘油;4*10-5%生物素)。
将800ml灭菌水、100ml的 10* YNB母液、2ml的500*B母液和100ml的10*GY母液混匀即可,4℃保存,保存期为2个月。
2.6 MGYHMinimal Glycerol Medium + Histidine (最小甘油培养基 + 0.004%组氨酸)在1000ml的MGY培养基中加入 10ml的100*H母液混匀,4℃保存,保存期为2个月。
2.7 RDRegeneration Dextrose Medium (葡萄糖再生培养基)(含有:1mol/L的山梨醇;2%葡萄糖;1.34%YNB;4*10-5%生物素;0. 005%氨基酸)1. 将186g的山梨醇定容至700ml,高压灭菌;2. 冷却后于45℃水浴;3. 将100ml的10*D、100ml的10*YNB;2ml的500*B;10ml的100*AA等母液和88ml无菌水混匀,预热至45℃后,与步骤2 的山梨醇溶液混合。
4℃保存。
2.8 RDHRegeneration Dextrose Medium + Histidine (葡萄糖再生培养基 + 0.004%组氨酸)在RD培养基配制的第三步中,在加入10ml的100*H母液,同时无菌水的体积减少至78ml即可,其余配制方法与RD相同。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
毕赤酵母表达实验手册大肠杆菌表达系统最突出的优点是工艺简单、产量高、生产成本低。
然而,许多蛋白质在翻译的修饰加工,如磷酸化、糖基化、酰胺化及蛋白酶水解等过程才能转化成活性形式。
大肠杆菌缺少适合用于表达结构复杂的蛋白质。
另外,蛋白质的活性还依赖于形成正确的二硫键并折叠成高级结表达的蛋白质往往不能进行正确的折叠,是以包含体状态存在。
包含体的形成虽然简化了产物的纯的活性,为了得到有活性的蛋白,就需要进行变性溶解及复性等操作,这一过程比较繁琐,同时增与大肠杆菌相比,酵母是低等真核生物,具有细胞生长快,易于培养,遗传操作简单等原核生物的生物时表达的蛋白质进行正确加工,修饰,合理的空间折叠等功能,非常有利于真核基因的表达,菌系统缺乏蛋白翻泽后加工、修饰的不足。
因此酵母表达系统受到越来越多的重视和利用。
大肠杆菌是用得最多、研究最成熟的基因工程表达系统,当前已商业化的基因工程产品大多是通过其主要优点是成本低、产量高、易于操作。
但大肠杆菌是原核生物,不具有真核生物的基因表达调加工修饰能力,其产物往住形成没有活性的包涵体,需要经过变性、复性等处理,才能应用。
近年程菌表达外源蛋白日益引起重视,主更是因为酵母是单细胞真核生物,不但具有大肠杆菌易操作、化生产的特点,还具有真核生物表达系统基因表达调控和蛋白修饰功能,避免了产物活性低,包涵间题[1]。
与大肠杆菌相比,酵母是单细胞真核生物,具有比较完备的基因表达调控机制和对表达产物的们对酿酒酵母(Saccharomyces.Cerevisiae)分子遗传学方面的认识最早,酿酒酵母也最先作为外宿主.1981年酿酒酵母表达了第一个外源基因一干扰素基因,随后又有一系列外源基因在该系统得素和胰岛素已大量生产并在人群中广泛应用,但很大部分表达由实验室扩展到工业规模时,培养基数的选择压力消失,质粒变得不稳定,拷贝数下降,而大多数外源基因的高效表达需要高拷贝数的量下降。
同时,实验室用培养基复杂而昂贵,采用工业规模能够接受的培养基时,往往导致产量的酵母的局限,人们发展了以甲基营养型酵母(methylotrophic yeast)为代表的第二代酵母表达系甲基营养型酵母包括:Pichia、Candida等.以Pichia.pastoris(毕赤巴斯德酵母)为宿主的外源来发展最为迅速,应用也最为广泛,已利用此系统表达了一系列有重要生物学活性的蛋自质。
毕赤用,原因在于该系统除了具有一般酵母所具有的特点外,还有以下几个优点[1、2、3];⑴具有醇氧化酶AOX1基因启动子,这是目前最强,调控机理最严格的启动子之一。
⑵表达质粒能在基因组的特定位点以单拷贝或多拷贝的形式稳定整合。
(即同源重组)⑶菌株易于进行高密度发酵,外源蛋白表达量高。
⑷毕赤酵母中存在过氧化物酶体,表达的蛋白贮存其中,可免受蛋白酶的降解,而且减少对细胞 Pichia.pastoris基因表达系统经过近十年发展,已基本成为较完善的外源基因表达系统,具表达基因稳定整合在宿主基因组中,能使产物有效分泌并适当糖基化,培养方便经济等特点。
利用AOX1,已高效表达了HBsAg、TNF、EGF、破伤风毒素 C片段、基因工程抗体等多种外源基因,证实用、简便,以提高表达量并保持产物生物学活性为突出特征的外源基因表达系统,而且非常适宜子扩目前美国FDA已能评价来自该系统的基因工程产品,最近来自该系统的Cephelon制剂已获得FDA 认为是安全的. Pichia.pastoris表达系统在生物工程领域将发挥越来越重要的作用,促进更多外高效表达,提供更为广泛的基因工程产品[2、3]。
近年来,Invitrogon公司开发了毕赤酵母表达系统的系列产品,短短几年已经有300多种外源有效表达,被认为是目前最有效的酵母表达系统。
毕赤酵母宿主菌常用的有GS115和KM71两种,都具有HIS4营养缺陷标记。
其中,GS115茵株具有+,即甲醇利用正常型;而KM71菌株的AOX1位点被ARG4基因插入,表型为Muts,即甲醇利用缓适用于一般的酵母转化方法。
Pichia.pastoris酵母菌体内无天然质粒,所以表达载体需与宿主染色体发生同源重组,将外合于染色体中以实现外源基因的表达[5].包括启动子、外源基因克隆位点、终止序列、筛选标记穿梭质粒,先在大肠杆菌复制扩增,然后被导入宿主酵母细胞。
为使产物分泌胞外,表达载体还需毕赤酵母表达系统有多种分泌型表达质粒,有许多蛋白在毕赤酵母得到了高效分泌表达。
胞外白的N末端加上一段信号肽序列,引导重组蛋白进入分泌途径,可使蛋白蛋白质在分泌到胞外之后毕赤酵母对外源蛋白自身的信号序列识别能力差,在本试验中所使用pPICZαA质粒,其信号肽来配因子(α-factor),能很好的达到以上的要求。
并且作为新一代的毕赤酵母分泌表达质粒,它还具有Zeocin抗性标记基因,给我们筛选转化子的工作带来很大的便利[1、2]。
pPICZαA质粒是作为新一代的毕赤酵母分泌表达质粒,它的主要特点简介如下:⑴具有强效可调控启动子AOX1(alcohol oxidase,醇氧化酶);⑵具有Zeocin抗性筛选标记基因,重组转化子可直接用Zeocin进行筛选,即在YPDZ平板上生长都有外源基因的整合,大大简化了重组转化酵母的筛选过程[5]。
在操作过程中,Zeocin也可用pPICZαA的大肠杆菌转化子,不必另外使用Amp,经济而又简便;。
⑶在表达载体A0X1 5’端启动子序列下游,有供外源基因插入的多克隆位点,多克隆位点下游有列;⑷分泌效率强的信号肽α-factor.Invitrogen公司开发的毕赤酵母表达系统的系列产品作为目前被应用为最为广泛的酵母表达有:醇氧化酶可调控的强启动子,能高密度发酵,重组蛋白表达量高。
外源基因整合在酵母基因组同时,高效分泌表达质粒能将外源蛋白表达后,进行翻译后加工处理,将外源蛋白分泌到细胞外,的活性,而且,有利于产物的纯化。
一.毕赤酵母表达常用溶液及缓冲液的配制1.1 各种母液的配制10*YNB (含有硫酸铵、无氨基酸的13.4%酵母基础氮源培养基) 4℃保存。
34g酵母基础氮源+100g硫酸铵,溶于1000ml水中,过滤除菌。
500*B (0.02%生物素 Biotin) 4℃保存保存期为1年。
20mg的生物素溶于100ml水100*H (0.4%Histidine 组氨酸) 4℃保存保存期为1年。
400mg的L-组氨酸溶于100 50℃以促进溶解),过滤除菌。
10*D (20%Dextrose 葡萄糖)保存期为1年。
200g葡萄糖溶于1000ml水中,灭菌10*M (5%Methanol 甲醇)保存期为2个月。
将5ml的甲醇与95ml水混匀,10*GY (10%Glycerol 甘油)保存期为1年以上。
将100ml甘油和900ml水混匀后菌。
100*AA (0.5% of each Amino Acid,各种氨基酸) 4℃保存保存期为1年。
分别酸、L-蛋氨酸、L-赖氨酸、L-亮氨酸和L-异亮氨酸溶于100ml水中,过滤除菌。
1M 磷酸钾溶液(potassium phosphate buffer,pH6.0),将1mol/L的K2HPO4溶液132ml与1m 868ml混匀,其pH为6.0,如需调节pH,则使用磷酸和氢氧化钾调节pH。
1.2 常用溶液及缓冲夜1.2.1 碱裂解法抽提质粒DNA所用溶液:溶液Ⅰ:50mmol / L glucose,100mmol / L EDTA,25mmol / L Tris-HCI (pH 8.0)溶液Ⅱ:0.2mol/L NaOH,1% SDS(临用时配制)溶液Ⅲ:29.44g KAc,11.5ml Acetic acid,加ddH2O至100 ml。
4℃保存。
1.2.2 10% 甘油(Glycerol):将100ml甘油和900ml水混匀后,高压灭菌或过滤除菌。
保存期为1年以上。
1.2.3 Rnase-H2O:1ul Rnase 加入1ml 灭菌 dd H2O。
4℃保存。
1.2.4 TE缓冲液:10mmol / Tris-CI(pH 8.0), lmmol / L EDTA(pH 8.0)1.2.5 STE缓冲液:0.1mol / L, 10mmol / L Tris-HCl (pH 8.0), 1mmol / L EDTA (pH 8.0)1.2.6 SCE缓冲液:1mol / L Sorbitol (山梨醇), 10mmol / L 柠檬酸钠, 10mmol / L EDTA1.2.7 1M potassium phosphate buffer (pH 6.0):132 ml 1M K2HPO4868 ml 1M KH2PO41.2.8 50X TAE 琼脂糖凝胶电泳缓冲液,pH 8.0(1L):242 g Tris57.1 ml Acetic Acid37.2 g EDTA二.毕赤酵母表达的培养基配制[5]2.1 LB(Luria-Bertani)培养基:Trypton l%Yeast Extract 0.5%NaCl l%PH 7.0制作平板时加入 2%琼脂粉。
121℃高压灭菌 20min。
可于室温保存。
用于培养pPICZαA原核宿主入Zeocin 25ug / ml。
2.2 LLB(Low Salt LB)培养基:Trypton l%Yeast Extract 0.5%NaCl 0.5%PH 7.0制作平板时加入 2%琼脂粉。
121℃高压灭菌 20min。
可于室温保存数月。
用于培养pPICZαA原核加入Zeocin 25ug / ml,可以4℃条件下保存1~2周。
2.3 YPD (又称YEPD)Yeast Extract Peptone Dextrose Medium,(Yeast Extract Peptone Dextrose Medium,酵母浸旋葡萄糖培养基)Trypton 2%dextrose (glucose) 2%+agar 2%+Zeocin 100 µg/ml液体YPD培养基可常温保存;琼脂YPD平板在4℃可保存几个月。
加入Zeocin 100ug / ml,成为4℃条件下保存1~2周。
2.4 YPDS + Zeocin 培养基(Yeast Extract Peptone Dextrose Medium):yeast extract 1%peptone 2%dextrose (glucose) 2%sorbitol 1 M+agar 2%+ Zeocin 100 µg/ml不管是液体YPDS培养基,还是YPDS + Zeocin 培养基,都必须存放4℃条件下,有效期1~2周。
2.5 MGYMinimal Glycerol Medium (最小甘油培养基)(34%YNB;1%甘油;4*10-5%生物素)。
