哈佛大学高等有机化学讲义Lecture12
合集下载
高等有机化学-Lecture 11

R H C C OH
+
R C C OH2+
R C C
2. Formation of a Nitrene
R
C O
N
N
N
R
C O
N
+
N2
Step II. Migration
Step III. The migration origin (A) acquires an octet Rearrangement with substitution (combinations with a nucleophile) Rearrangement with elimination (loss of H+)
O2N
COOH PhNH2 Br
O2N
Ph N OH Br PCl5
O2N
O2N NHCOPh H2O
Br
NH2 PhCOOH Br
The shift will keep the stereostructure of chiral center
CH3 C N HO C2H5 C CH2C2H5 H H2SO4 Et2O
-
Ph
PhCH2 CH2COOR
Intramolecular SN2 attack(structure maintaining):
Me COCH3 H Cl
Me Cl H
Me H
KOH
O
HO
-
Me H
O
OH H+
Me COOH H Me
CH2
-
Me
Me
KOH
H
HO
O
CH2
O OH
H
H+
+
R C C OH2+
R C C
2. Formation of a Nitrene
R
C O
N
N
N
R
C O
N
+
N2
Step II. Migration
Step III. The migration origin (A) acquires an octet Rearrangement with substitution (combinations with a nucleophile) Rearrangement with elimination (loss of H+)
O2N
COOH PhNH2 Br
O2N
Ph N OH Br PCl5
O2N
O2N NHCOPh H2O
Br
NH2 PhCOOH Br
The shift will keep the stereostructure of chiral center
CH3 C N HO C2H5 C CH2C2H5 H H2SO4 Et2O
-
Ph
PhCH2 CH2COOR
Intramolecular SN2 attack(structure maintaining):
Me COCH3 H Cl
Me Cl H
Me H
KOH
O
HO
-
Me H
O
OH H+
Me COOH H Me
CH2
-
Me
Me
KOH
H
HO
O
CH2
O OH
H
H+
第四章.哈工大大学化学精品课讲义

(5) 缓冲溶液的缓冲范围
ca pH pK lg c盐
a
弱酸及其强碱盐 缓冲溶液的pH值计算式
缓冲液的pH值与 pK a 及ca/c盐比值有关。 缓冲能力的大小取决于ca与c盐的大小及其比值的大小。 当ca/c盐=1时,(一般缓冲溶液的ca/c盐在0.1与10之间) 缓冲能力最大,因而: 缓冲溶液的缓冲范围为:
溶液中始终存在着解离产生的正、负离子和未解离的 分子之间的平衡,此称为解离平衡。
HAc (aq) H+ (aq) +Ac- (aq)
[ c ( H ) c ] [ c ( Ac ) c ] Ka 解离常数 c( HAc) c
K
对于不同的一元弱酸,既表示其解离程度,又代表其酸性的强弱。
将 (2) 代入 (1) 式, 得:
K
ca
表示一元弱酸浓度、 解离度和解离常数 12 之间的关系。
2. 一元弱酸和一元弱碱的离解平衡
(4) 与 K
b 的关系
K
a
ca
H2O(aq) 对于一元弱碱 HN3·
同样方法可得
c(OH ) c cb Kb
NH4+(aq) + OH-(aq)
K sp G RT ln K 可由实验测得,亦可由 r m sp计算,
20
附录列出了的值。
4.5.2 沉淀-溶解平衡
2. 溶度积规则
在第二章中, 通过比较反应商(J )和标准平衡常数( K )来判 断反应自发进行的方向,这一规律同样适用于难溶电解质的溶 解平衡。显然,J 在这里为离子积, 为溶度积,因此 K sp
化学工作者所考虑的溶液一般是气体、液体或固体等溶 本章主要研究液体溶于液体中形成的液体溶液。液体溶
ca pH pK lg c盐
a
弱酸及其强碱盐 缓冲溶液的pH值计算式
缓冲液的pH值与 pK a 及ca/c盐比值有关。 缓冲能力的大小取决于ca与c盐的大小及其比值的大小。 当ca/c盐=1时,(一般缓冲溶液的ca/c盐在0.1与10之间) 缓冲能力最大,因而: 缓冲溶液的缓冲范围为:
溶液中始终存在着解离产生的正、负离子和未解离的 分子之间的平衡,此称为解离平衡。
HAc (aq) H+ (aq) +Ac- (aq)
[ c ( H ) c ] [ c ( Ac ) c ] Ka 解离常数 c( HAc) c
K
对于不同的一元弱酸,既表示其解离程度,又代表其酸性的强弱。
将 (2) 代入 (1) 式, 得:
K
ca
表示一元弱酸浓度、 解离度和解离常数 12 之间的关系。
2. 一元弱酸和一元弱碱的离解平衡
(4) 与 K
b 的关系
K
a
ca
H2O(aq) 对于一元弱碱 HN3·
同样方法可得
c(OH ) c cb Kb
NH4+(aq) + OH-(aq)
K sp G RT ln K 可由实验测得,亦可由 r m sp计算,
20
附录列出了的值。
4.5.2 沉淀-溶解平衡
2. 溶度积规则
在第二章中, 通过比较反应商(J )和标准平衡常数( K )来判 断反应自发进行的方向,这一规律同样适用于难溶电解质的溶 解平衡。显然,J 在这里为离子积, 为溶度积,因此 K sp
化学工作者所考虑的溶液一般是气体、液体或固体等溶 本章主要研究液体溶于液体中形成的液体溶液。液体溶
哈佛大学高等有机化学讲义Lecture34
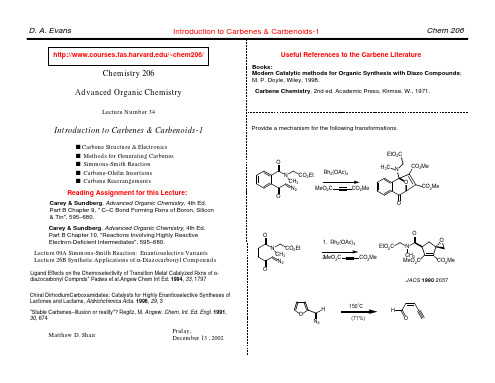
Carey & Sundberg, Advanced Organic Chemistry, 4th Ed. Part B Chapter 9, " C–C Bond Forming Rxns of Boron, Silicon & Tin", 595–680. Carey & Sundberg, Advanced Organic Chemistry, 4th Ed. Part B Chapter 10, "Reactions Involving Highly Reactive Electron-Deficient Intermediates", 595–680. Lecture 09A Simmons-Smith Reaction: Enantioselective Variants Lecture 26B Synthetic Applications of α-Diazocarbonyl Compounds
s the History of the Singlet-Triplet Gap
Year 1932 Method Qual. Thermochem Qual. QM Ab initio Kinetics SCF MINDO Expt An Initio Expt Expt Author Muliken Walsh Gallup Harrison Hase Pople Dewar Lineberger Schaeffer Zare Haydon HCH Angle Grnd State 90-100° 180° 160° 138° –– 132° 134° 138° ––– ––– ––– singlet triplet triplet triplet triplet triplet triplet triplet triplet triplet triplet S–T Splitting kcal/mol –– small 30 >33 8–9 19 8.7 19.5 19.7 8.1 8.5
s the History of the Singlet-Triplet Gap
Year 1932 Method Qual. Thermochem Qual. QM Ab initio Kinetics SCF MINDO Expt An Initio Expt Expt Author Muliken Walsh Gallup Harrison Hase Pople Dewar Lineberger Schaeffer Zare Haydon HCH Angle Grnd State 90-100° 180° 160° 138° –– 132° 134° 138° ––– ––– ––– singlet triplet triplet triplet triplet triplet triplet triplet triplet triplet triplet S–T Splitting kcal/mol –– small 30 >33 8–9 19 8.7 19.5 19.7 8.1 8.5
哈佛大学高等有机化学讲义Lecture33A

33A-06 3/14/96 11:22 PM
Shea, K. J.; Zandi, K. S.; Staab, A. J.; Carr. R. Tetrahedron Lett. 1990, 31, 5885.
CO2Et
R=H 90
R = Me >99
: :
t-Bu t-Bu
Si
OO
10
<1
R
CO2Et
Note: Intermolecular variant gives 1:1 ratio with opposite regiochemistry.
Gillard, J. W.; Fortin, R.; Grimm, E. L.; Maillard, M.; Tjepkema, M.; Bernstein, M. A.; Glasser, R. Tetrahedron Lett. 1991, 32, 1145.
- Function of tether length and steric bulk of alkyl substituents on silicon
❏ Functional Group Protection
- Serves as protecting group before and after reaction ❏ Facile Refunctionalization
- Protodesilylation, Tamao oxidation, allylsilane additions, and transmetallations are possible
Diels-Alder To Form 5-Atom Tether Ring:
OH SiR2Cl
哈佛大学高等有机

k1 kA kB k2
To relate this quantity to !G values, recall that !Go = -RT ln Keq or Keq = e-!G°/RT, k1 = e-!G1/RT, and k2 = e-!G2/RT. Substituting this into the above equation:
K. A. Beaver, D. A. Evans
Leading References:
Conformational Analysis and Reactivity: Curtin-Hammett Principle
Case 1: "Kinetic Quench"
Chem 206
J. I. Seeman, J. Chem. Ed. 1986, 63, 42-48. J. I. Seeman, Chem Rev. 1983, 83, 83-134. See also Eliel, pp. 647-655
H. Yamamoto et.al, Angew. Chem. Int. Ed. 2005, 44, 4389-4391 (pdf)
TIPSO
H Me H
Me H H O
Al(t-BuO)3
R H Me H O H
" "
Me H O R
t-BuOOH
4 sieves toluene, -20C
HO
Olefin Addition Reactions–2
! ! Sharpless Epoxidation continued..... Hydrogenation
Problem 579. The following publication (J. Org. Chem. 1991, 56, 5553) reported the surprisingly selective olefin epoxidation illustrated below. In this reaction, olefin B in 1 was found to be much less reactive than olefin A. Using your knowlege of stereoelectronic effects, provide an explanation for the reduced reactivity of olefin B in diene 1.
To relate this quantity to !G values, recall that !Go = -RT ln Keq or Keq = e-!G°/RT, k1 = e-!G1/RT, and k2 = e-!G2/RT. Substituting this into the above equation:
K. A. Beaver, D. A. Evans
Leading References:
Conformational Analysis and Reactivity: Curtin-Hammett Principle
Case 1: "Kinetic Quench"
Chem 206
J. I. Seeman, J. Chem. Ed. 1986, 63, 42-48. J. I. Seeman, Chem Rev. 1983, 83, 83-134. See also Eliel, pp. 647-655
H. Yamamoto et.al, Angew. Chem. Int. Ed. 2005, 44, 4389-4391 (pdf)
TIPSO
H Me H
Me H H O
Al(t-BuO)3
R H Me H O H
" "
Me H O R
t-BuOOH
4 sieves toluene, -20C
HO
Olefin Addition Reactions–2
! ! Sharpless Epoxidation continued..... Hydrogenation
Problem 579. The following publication (J. Org. Chem. 1991, 56, 5553) reported the surprisingly selective olefin epoxidation illustrated below. In this reaction, olefin B in 1 was found to be much less reactive than olefin A. Using your knowlege of stereoelectronic effects, provide an explanation for the reduced reactivity of olefin B in diene 1.
哈佛大学高等有机化学讲义Lecture15
Paterson, Tet Lett 1991, 32, 7601
favored O R Me disfavored R
H N R
O Li H
‡ Me R
OLi
Me
Me
Me
Me
Me
Me
Me
Me
Me R
OH
O
OH
Me O
O
O
O O
LDA, TMSCl Et3N
Me
LM–NR2
O Me H Li R H N R
OMe OMe
O NEt2
O Et2N Me Me Et 92% (E:Z = 98:2) OH Me
CH3C(NMe2)(OMe)2
O NEt2
H H
s Compare the two variants:
Me S OH
60% Hg(OAc)2, EVE
OEt
CH3C(OEt)3 CH3CH2CO2H (cat) 138oC
O O O O O
+ π2s]
π*
light
π*
new HOMO
C C
bonding
X
[2+2]
O O
[4+2]
O O
bonding
C T
s The related reaction of 2 ethylenes is nonconcerted: [2 + 2] cycloaddition
Y Y Y
批注本地保存成功开通会员云端永久保存去开通
D. A. Evans
Cycloaddition Reactions: Part–1
s Other Reading Material:
favored O R Me disfavored R
H N R
O Li H
‡ Me R
OLi
Me
Me
Me
Me
Me
Me
Me
Me
Me R
OH
O
OH
Me O
O
O
O O
LDA, TMSCl Et3N
Me
LM–NR2
O Me H Li R H N R
OMe OMe
O NEt2
O Et2N Me Me Et 92% (E:Z = 98:2) OH Me
CH3C(NMe2)(OMe)2
O NEt2
H H
s Compare the two variants:
Me S OH
60% Hg(OAc)2, EVE
OEt
CH3C(OEt)3 CH3CH2CO2H (cat) 138oC
O O O O O
+ π2s]
π*
light
π*
new HOMO
C C
bonding
X
[2+2]
O O
[4+2]
O O
bonding
C T
s The related reaction of 2 ethylenes is nonconcerted: [2 + 2] cycloaddition
Y Y Y
批注本地保存成功开通会员云端永久保存去开通
D. A. Evans
Cycloaddition Reactions: Part–1
s Other Reading Material:
高等有机化学--lecture 14

Here are some estimates of the strain based on "homodesmotic reactions" (agrees with experiments for carbon skeletons): theoretical strain energy (kcal/mol) cyclopropane (C3H6) 26.8 25.7 34.9 15.2
E. Kwan
Lecture 14: Small Ring Conformational Analysis
Chem 106
Geminal Hyperconjugations The effect of the D-C-A bond angle is illustrated by the relatively small hyperconjugations in propane vs. cyclopropane: Propane (CC to *CC = 0.52 kcal/mol)
D A D A
vicinal interaction
D
A
D
A
gemin normally talk about geminal interactions? It turns out that their strength depends a lot on the D-C-A angle. In a small ring, the angle is small, the overlap is good, and therefore the interaction is strong.
E. Kwan
Lecture 14: Small Ring Conformational Analysis Small Ring Conformational Analysis
高等有机化学精品讲义:2 (英文精品)

The standard approach is to use computations to predict bond lengths, transition state energies, vibrational frequencies, etc. These calculations correlate well with experimental observations. Therefore, it is reasonable to think that other useful information might be contained in the wavefunctions produced by these calculations. However, the canonical molecular orbitals (CMOs) are relatively delocalized entities that are hard to interpret chemically.
The NBO algorithim converts the wavefunctions/CMOs into other basis sets that are mathematically equivalent (span the same space), but easier to understand:
amide resonance
anomeric effect
hyperconjugation
non-Lewis corrections to Lewis structures
Helpful References
1. Coulson's Valence, 3rd ed. McWeeny, R. Oxford: Oxford University Press, 1979.
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Con
HOMO
disrotatory
Examples
Ground State Conrotatory
Excited State Disrotatory
Con
LUMO
HOMO
Disrotatory
Conrotatory
Activation Energy (kcal/mol) for electrocyclic ring opening
dis-out
favorable H Me R A
Three-Atom Electrocyclizations (4 electrons)
H H A H A Dis?? R C H A Con?? R H A A H
relative rate
1
4
40,000
Ψ3
Ring-fused Cyclopropyl Systems When the cis substiltutents on the cyclopropyl ring are tied together in a ring the following observsations have been made
D. A. Evans
Pericyclic Reactions: Part–2
s Other Reading Material:
Chem 206
/~chem206/
Chemistry 206 Advanced Organic Chemistry
Electrocyclic Processes-1
Chem 206
Controtation and on to the indicated bonding and anti-bonding orbitals of cyclobutene:
LUMO
Excited State (Photochemical Process) disrotatory conrotatory
A H R A H Dis?? R C H A
Does solvolysis proceed via cation 1 followed by rearrangement to 2 (Case 1), or does it proceed directly to 2 (Case 2)?
Ψ3 fast +X– 2 Me H H Case 2 Ψ2 nonbonding X
The Nazarov Reaction
O OH +H+ A A A A f f A –H+ A O R Con?? A A
p
Chem 206
R
p
R Dis?? A A
p
A
A
Denmark, S. E. In Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 5; pp 751. O O +H
Predict the stereochemical outcome of this reaction.
Ph O O O Ph
h
O O
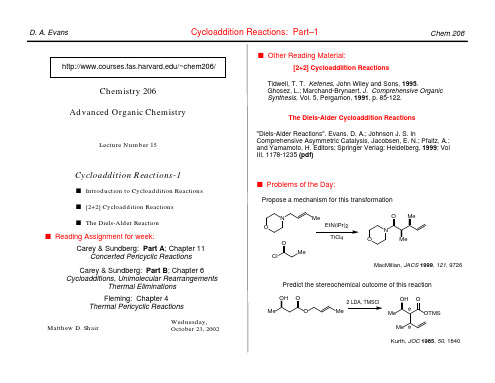
s Reading Assignment for week: Carey & Sundberg: Part A; Chapter 11 Concerted Pericyclic Reactions Fleming: Chapter 4 Thermal Pericyclic Reactions
Evans, Breit
Electrocyclic Processes-2: Torquoselectivity
How do we explain?
R R R
Chem 206
Donor substituents prefer con–out mode Pi acceptor substituents prefer con–in mode
Torquoselectivilty is defined as the predisposition of a given R substituent for a given conrotatory motion Houk et al. Acc. Chem. Res 1996, 29, 471
R
con in
Houk, et. al. Acc. Chem. Res. 1996, 29, 471-477. Houk, et. al. JOC. 1996, 61, 2813-2825. Monday, Columbus Day, October 14, 2002
Ph
heat
h
Huisgen, TL, 1964, 3381.
View the 2 conrotatory modes by looking at the breaking sigma bond from this perspective
H
con
H
out
R R H H
Examples:
Donor substituents prefer con–out mode Pi acceptor substituents prefer con–in mode
HOMO Dis
C
Note that there are two disrotatory modes
R R X Dis R X R R Dis R Sterically favored R Favored for R = ring R R LUMO
X
Me H Me C C H
HOMO Dis
+
H
R
+
+X–
X
TsO Ψ1 cation anion H
TsO H
Me
TsO H
H Me
H Me
relative rate 1
LUMO
4
DePuy, Accts. Chem. Res. 1967, 1, 33
40,000
+
R
H A H A
R Dis
+
H A C H C A
HOMO
X
LUMO
H Me H C Me Me
Ph
O
Suggest a mechanism for the following reaction.
H CO2Me MeO2C CO2Me H
heat
H CO2Me
Matthew D. Shair
H
Bloomfield, TL, 1969, 3719.
Evans, Breit
Electrocyclic Reaction - Selection Rules Ground State (Thermal process) 4n π e(n = 1,2...) 4n+2 π e(n = 0,1,2...) conrotatory
27
Disrotatory
O Ph
Conrotatory
Disrotatory
Ph O
O
O O
Disrotatory
Conrotatory
Ph Ph O O O
R Con R R R R
R
Con
R
Ph O
O
O
Ph
R Sterically favored
Ph Huisgen, TL, 1964, 3381. Ph O
O Cl base O– Cl –Cl– 3-exo-tet disallowed O– O
dis-in
•• Ar N
H CO2Me
MeO2C products
•• (–)
Evans, Breit
Five-Atom Electrocyclizations (4 electrons)
Electrocyclic Processes-3
Activation Energy (kcal/mol) for electrocyclic ring opening
Conrotatory
Disrotatory
42
45
Disrotatory Conrotatory
Conrotatory
H
29
H Criegee, Chem. Ber. 1968, 101, 102.
s Woodward-Hoffmann Theory R. B. Woodward and R. Hoffmann, The Conservation of Orbital Symmetry, Verlag Chemie, Weinheim, 1970. s Frontier Molecular Orbital Theory I. Fleming, Frontier Orbitals and Organic Chemical Reactions, John-Wiley and Sons, New York, 1976. s Dewar-Zimmerman Theory T. H. Lowry and K. S. Richardson, Mechanism and Theory in Organic Chemistry, 3rd Ed., Harper & Row, New York, 1987. s General Reference R. E. Lehr and A. P. Marchand, Orbital Symmetry: A Problem Solving Approach, Academic Press, New York, 1972.
Me H
R
R
H H
Me Me
Evans, Breit
HOMO
disrotatory
Examples
Ground State Conrotatory
Excited State Disrotatory
Con
LUMO
HOMO
Disrotatory
Conrotatory
Activation Energy (kcal/mol) for electrocyclic ring opening
dis-out
favorable H Me R A
Three-Atom Electrocyclizations (4 electrons)
H H A H A Dis?? R C H A Con?? R H A A H
relative rate
1
4
40,000
Ψ3
Ring-fused Cyclopropyl Systems When the cis substiltutents on the cyclopropyl ring are tied together in a ring the following observsations have been made
D. A. Evans
Pericyclic Reactions: Part–2
s Other Reading Material:
Chem 206
/~chem206/
Chemistry 206 Advanced Organic Chemistry
Electrocyclic Processes-1
Chem 206
Controtation and on to the indicated bonding and anti-bonding orbitals of cyclobutene:
LUMO
Excited State (Photochemical Process) disrotatory conrotatory
A H R A H Dis?? R C H A
Does solvolysis proceed via cation 1 followed by rearrangement to 2 (Case 1), or does it proceed directly to 2 (Case 2)?
Ψ3 fast +X– 2 Me H H Case 2 Ψ2 nonbonding X
The Nazarov Reaction
O OH +H+ A A A A f f A –H+ A O R Con?? A A
p
Chem 206
R
p
R Dis?? A A
p
A
A
Denmark, S. E. In Comprehensive Organic Synthesis; Trost, B. M., Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 5; pp 751. O O +H
Predict the stereochemical outcome of this reaction.
Ph O O O Ph
h
O O
s Reading Assignment for week: Carey & Sundberg: Part A; Chapter 11 Concerted Pericyclic Reactions Fleming: Chapter 4 Thermal Pericyclic Reactions
Evans, Breit
Electrocyclic Processes-2: Torquoselectivity
How do we explain?
R R R
Chem 206
Donor substituents prefer con–out mode Pi acceptor substituents prefer con–in mode
Torquoselectivilty is defined as the predisposition of a given R substituent for a given conrotatory motion Houk et al. Acc. Chem. Res 1996, 29, 471
R
con in
Houk, et. al. Acc. Chem. Res. 1996, 29, 471-477. Houk, et. al. JOC. 1996, 61, 2813-2825. Monday, Columbus Day, October 14, 2002
Ph
heat
h
Huisgen, TL, 1964, 3381.
View the 2 conrotatory modes by looking at the breaking sigma bond from this perspective
H
con
H
out
R R H H
Examples:
Donor substituents prefer con–out mode Pi acceptor substituents prefer con–in mode
HOMO Dis
C
Note that there are two disrotatory modes
R R X Dis R X R R Dis R Sterically favored R Favored for R = ring R R LUMO
X
Me H Me C C H
HOMO Dis
+
H
R
+
+X–
X
TsO Ψ1 cation anion H
TsO H
Me
TsO H
H Me
H Me
relative rate 1
LUMO
4
DePuy, Accts. Chem. Res. 1967, 1, 33
40,000
+
R
H A H A
R Dis
+
H A C H C A
HOMO
X
LUMO
H Me H C Me Me
Ph
O
Suggest a mechanism for the following reaction.
H CO2Me MeO2C CO2Me H
heat
H CO2Me
Matthew D. Shair
H
Bloomfield, TL, 1969, 3719.
Evans, Breit
Electrocyclic Reaction - Selection Rules Ground State (Thermal process) 4n π e(n = 1,2...) 4n+2 π e(n = 0,1,2...) conrotatory
27
Disrotatory
O Ph
Conrotatory
Disrotatory
Ph O
O
O O
Disrotatory
Conrotatory
Ph Ph O O O
R Con R R R R
R
Con
R
Ph O
O
O
Ph
R Sterically favored
Ph Huisgen, TL, 1964, 3381. Ph O
O Cl base O– Cl –Cl– 3-exo-tet disallowed O– O
dis-in
•• Ar N
H CO2Me
MeO2C products
•• (–)
Evans, Breit
Five-Atom Electrocyclizations (4 electrons)
Electrocyclic Processes-3
Activation Energy (kcal/mol) for electrocyclic ring opening
Conrotatory
Disrotatory
42
45
Disrotatory Conrotatory
Conrotatory
H
29
H Criegee, Chem. Ber. 1968, 101, 102.
s Woodward-Hoffmann Theory R. B. Woodward and R. Hoffmann, The Conservation of Orbital Symmetry, Verlag Chemie, Weinheim, 1970. s Frontier Molecular Orbital Theory I. Fleming, Frontier Orbitals and Organic Chemical Reactions, John-Wiley and Sons, New York, 1976. s Dewar-Zimmerman Theory T. H. Lowry and K. S. Richardson, Mechanism and Theory in Organic Chemistry, 3rd Ed., Harper & Row, New York, 1987. s General Reference R. E. Lehr and A. P. Marchand, Orbital Symmetry: A Problem Solving Approach, Academic Press, New York, 1972.
Me H
R
R
H H
Me Me
Evans, Breit
