16_Sharpless Asymmetric Epoxidation Reaction哈佛有机化学讲义
有机催化的不对称氧化反应

有机催化的不对称氧化反应Sharpless不对称环氧化反应(Sharpless Epoxidation)
Sharpless不对称环氧化反应,是一种不对称选择的化学反应,可以用来从一级或者二级烯丙醇制备2,3-环氧醇。
该反应大约在1970年代开始得到系统研究,80年代后日臻成熟。
环氧化产物的立体化学是由反应中使用的手性酒石酸酯的非对映体(通常为酒石酸二乙酯或者酒石酸二异丙酯)决定的。
氧化试剂为过氧叔丁醇。
反应中使用一个催化剂可以形成产物的对映体选择性,该催化剂通过四异丙氧基钛和酒石酸二乙酯反应获得。
反应在存在3Å分子筛(3Å MS)的条件下只需5-10 mol%的催化剂量。
Sharpless不对称环氧化反应的成功取决于五大主要原因:
首先,环氧化合物能够简单的转化为二醇、氨基
醇或者醚,所以在天然产物的全合成当中形成手性的环氧化合物是非常重要的步骤。
第二,该反应能够和许多一级或者二级烯丙醇反应。
第三,夏普莱斯环氧化的产物通常具有超过90%的ee值(对映体过量)。
第四,通过夏普莱斯环氧化模型可以预测出产物的手性。
最后,夏普莱斯环氧化的反应试剂都是商业化的且非常廉价易得。
反应机理
用于环氧化的氧化剂是叔丁基氢过氧化物。
该反应由Ti(OiPr)4催化,Ti(OiPr)4通过氧原子结
合氢过氧化物,烯丙基醇基和不对称酒石酸酯配体(假设的过渡态如下所示)。
化学反应官能团转化速查

i 5-7-g f e d c b a e d c b a i h g f ed c ba h g f e d cb a h g f e dc b a 6-4-3-1-2-i h g f ed c b a C=C -C(O)-CH 3CH-CH CH-CX Functional Group InterconversionC=CC C C=CC C RCH 2-SO 2Ph RC CH C C C-NH 2; C-NO 2C-OHC(OR)2; C(SR)2C=C-OR; C=C-SR C C C NC=N-OH, C=N-H C=SC=O C=O C-C(O)Z C=C C=O C-OH C-X C-N C-H C-N C=O C---OH C-OC(O)R C-X C-OCH 2OR C-NH 2C-OR C-H C-OH C=C C-H C(O)OR C-(OR)2C-OH C-ORC-CHO C-CO 2H C-CN C=C C=O C-S C-X C-OH C-H C=C j C(O)XhC Nj kC-HC-Br 8-C-Xi C-OHC-OH C(O)Z dc b a ed c b a f C-NH 2C-Hj CX-CYC CXC=O h g f iC CH RCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXjC O C-NH 2C-CN9-C-CH 3C-Xa e C=O1-adry pyridine: from CaH 2 and distilledtriflatemesylate tosylate S O O O RCH 2CF 3S OO O RCH 2CH 3CH 3CH 3CH 3OH (2). for 3' alcohol:(1). for 1', 2' alcohol:1-i h g f e dc b a C-CHO C-CO 2HC-CN C=C C=O C-SC-NH 2C-X C-OH C-H CH 3CH3CH 3H S RCH 2-HCH 3SOO O RCH 2CH 3S OOCl RCH 2OHpurification textbook~ $ 30 / Kg toluenesulfonyl chloride (s)methanesulfonyy chloride (l)~ $ 30 / Kg jC(O)XPh 2SiHCl / InCl 3PhPhPhPhJOC, 2001, 66, 7741.ii. Ph 2SiHCl / InCl 3i. p -TsCl // LiAlH 4i. ClC(S)OPh // n -Bu 3SnH Cl 2via:a unique Lewis acid catalyst, acceleratedeoxgyenationInCl 3indium trichlorideii. Et 3SiH / Lewis acidJ. Org. Chem. 2000, 65, 6179JOC, 2000, 65, 6179.CHCl 2rt, 3 hr1-bBu 3SnH: (l), easy to remove Ph 3SnH: (s), hard to remove Me 3SnH: too volatile, toxicunstable in acid, form H 2 gas; stable in weak baseNaBH 3CN: stable at pH 5-6hygroscopic, dried self, suggest: buy small amount each time(Grignard reagent)JOC, 1969, 34, 3923.HBrNa / NH 3; Li / NH 3; Na / EtOH Zn; Fe; Sn; Mg(3). metal reduction(2). hydride reduction(1). free radical reductionJACS, 1972, 94, 8905.n -Bu SnH HBrNaBH 4 / InCl 3 / CH 3CNradical reagentn -Bu 3SnH / AlBN JA CS, 2002, 124, 906.i iii NaBH 3CNi LiAlH 4i ii ii NaBH 4ii THL, 1969, 3095.JOC, 1976, 41, 3064.iv LiBHEt 3 (super hydride)mechanism uncertain, probably radicalburn filter paper if dryRaney Nickel: Ni - Al alloy, suspensionJCS Perkin Trans I, 1973, 654.(3). L iAlH 4 / CuCl 2NaBH 4 / NiCl 2NaBHEt 3 / FeCl 2 (or CoCl 2, VCl 3)(2). Li / NH 3(1). Raney Ni1-d1-c RCH 2-HRCH 2NH 2radical mechanismChemistry:R-SH R-S-R R 2SO R 2SO 2R-SS-Rremove: Hg +; Ni(1).(2).Ar-H2H PO Ar-NH 2RCH 2NH 2RCH 2NMe 3R=CH 2R-CH 3(4).X-RCH 2NMe 3OH -p-TsClNaH p-TsCl2(3).Ar-NH 2Ar-Hbasicneutral acidic1-e(2). thioketal:(3). Wolff-Kishner reduction:(5). Tosylhydrazone reduction (Shapiro reaction):(modified Wolff-Kishner reduction):)(6). enol derivatives: SHSH / BF 3, CH 2Cl 2 // RaNiN 2H 4, OH -, heatTsNHNH 2Tf 2O /N// H 2 / PtO 2preparation: HgCl 2 into Znsimilar: Sn / HCl(4). Pd-C / HCO 2NH 4: mild, efficient(7). Et 3SiH / CF 3COOHPhONO 21-fb.p. ~ 230 Chighly toxic, cancer suspected agent?= HMPT: h exa m ethyl p hosphoric t riamide (Me 2N)3P=O 1-g (1). K / Al 2O 3 K / HMPA (2). Na / NH 31-h JOC, 1980, 45, 3227HMPA: h exa m ethyl p hosphor a mide (Me 2N)3P=Oyes for white mouse, uncertain for humanmodified to: N N O1-i(1). RhCl(PPh 3)3 (Wilkinson's cat)(2). Rh(DPPD)2+ Cl -DPPD = Ph 2P-CH 2CH 2-PPh 21-jHSiEt 3 / B(C 6F 5)3activator / hydride sourceRCH 2OROORR OROR RCH 2 OCH 2CH 2OH(3). AlCl 3 / LiAlH 4(2). HCl / NaBH 3(CN)(1). h ν / HSiCl 32-bN NH/ TBDMS-ClTBDPS-ClEt 3N / TMS-Clacid: H 2SO 4H 3PO 4BF 3-Et 2ORC-OCH 2CH=CH2RC-OCPh 3 = RC -OTr RC-O t BuRC-OCH 3RC-OSiR 3RC-OCH 2Ph = RC-OBZl = RC-OBni. Willianson synthesis OK: Si - Cl bond longii. stability of silyl in acid/base: RC-O-TBDPS > RC-O-TBDMS >> RC-O-TBS iii. abbrev.: TBDMS = tert-butyl-dimethylsilyl = TBS =Silyl group:(RO-Tr)Trityl group: (tirphenylmethyl)i. S N 1 reactionii. abbreviation: triphenylmethyl = trityl = -CPh 3 = -Tr iii. advantage: high MW, easy to handle (small amount become large amount)baseBr Willianson synthesis (base, S N 2) not work: elimination side-product with baset -Butyl group:i. abbreviation: benzyl = PhCH 2 = Bzl = Bn ii. deprotecting: H 2 / Pd-CPhCH 2-ClPhCH 2-Br: reactivity goodPhCH 2-I: reactivity better than PhCH 2Br, generated in situ, PhCH 2Br + NaIPhCH 2-X: Benzyl- group:i. Williamson ether synthesis, S N 2 typeii. not a good protecting group, too stable to convert back to alcohol Me group:CH 3-X: CH 3I; CH 3OSO 2R; (CH 3)3O + BF 4-, (CH 3)2SO 4base: NaH, n -BuLi, Ag 2O(4). t -Bu: acid cat /(3). allyl: base /Br (6). silyl: Et 3N / R 3SiCl(5). trityl: py // Ph 3C-Br(2). PhCH 2-: base / PhCH 2-X e d cb a 2-RC=C RC-H RC(O)ORRC-(OR)2RC-OH RC-OR (1). Me: base / CH 3-X2-a (7). acetal / ketal: (see 3e)fRC-CNgenerate H 2, or butane gasJOC, 1988, 53, 2985.trimethyloxonium tetrafluoroborateJCS, 1930, 2166.(8). ArF / CsFROHradical mechanism: SiCl 3t-BuORaNi with C=S2-c2-d (1). hv / HSiCl 3(2). HCl / tBu-OO-t Bu(4). BF 3 / NaBH 42-e2-e.vi. H 2O 2, t -BuOH, MnSO 4 // NaHCO 3, pH 8JA CS, 2001, 123, 2933.HO 22COnew, cheap,, simple, green chemistryconvenient, inexpensive, powerful.JOC, 1980, 45, 4758.JOC, 1982, 47, 2670.OOHOOBr via:Br 2 / ROH2-f ROH / HClEtCNEt C OEt OEtOEtJA CS, 1942, 64, 1825.JOC, 2001, 66, 521.C-OH C-H C-OR C-NH 2C-X 3-a b c d3-a(1). [PhI(OAc)-O]2-Mn(TPP)(2). organic electrochemistry(3). X 2 / hv // OH -3-a.13-a.23-a.3(1) Me 3SiCl // MPCBA//H 3O +(2). O 2, LDA, (EtO)3PJA CS, 1975, 97, 6909.i h g f e C=O C---OH C-OC(O)RC-OCH 2OR C=Cj C O(1). Me: application: deprotecting (2). PhCH 2-(5). trityl:(6). silyl: (3). allyl: (4). t-Bu: RC-OCH 2RC-OSiR 3RC-OCH 3RC-OtBuRC-OCPh 3 = RC-OTr RC-OCH 2CH=CH 2OH - Me i. HOAc: weak acid: good leaving groupii. H 2i. F - : HF, Py-H + F -; n +--SiMe 3-SiBuMe 2-SiBuPh 2if HOBr: OK for TMDMSJOC, 1987, 52, 4973.OCOCF 3+3-b triphenylmethylorganic base: TMG3-c(1). OH -(2). KO 2 / DMSO 3-d not practically useful: R-OH cheaper than R-XJOC, 1975, 40, 1678.(2). Na 2[Fe(CN)5(NO)] / K 2CO 3 / H 2O3-e(1). Symmetry:ketal: use H 3O +acetal: use H 3O +(2). unsymetry:RO-MOM RO-MEM RO-MTM RO-THPi. H 3O +p -TsOH / MeOHi. H 3O +; ii. ZnBr 2 / CH 2Cl 2HgCl 2 / CH 3CN (aq.)actually, acetal exchange (3). Ag 2O / H 2OTHL, 1975, 3183.JOC, 1986, 51, 3913.RO 2C (CH 2)3CHRNH 2RO 2C (CH 2)3OHNa 2[Fe(CN)5(NO)]2323-f(1). base: KHCO 3 (or K 2CO 3, NH 3) / MeOH; NaOH (1 %, or 0.5 N)(3). RED: (2). acid: H 3O +PPh 3 / DEAD / RCO 2H // OH -3-gMitsunobu inversionSynthesis, 1981, 1.JOC, 1987, 52, 4235.common esters:formate = HCO 2R ------------------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH trifluoroacetate = CF 3CO 2R ------------ KHCO 3 (or K 2CO 3, or NH 3) / MeOH acetate = CH 3CO 2R = ROAc --------- KHCO 3 (or K 2CO 3, or NH 3) / MeOH benzoate = PhCO 2R = ROBz -------- NaOH (1 %) / MeOH pivalate = t Bu-CO 2R = ROPv ------ NaOH (0.5 N) / EtOH*HOi LiAlH 4ii. NaAlH 2(OCH 2CH 2OCH 3)CH 3O 2CCO 2CH 3HOOHNaAlH 2(OCH 2CH 2OCH 3)266hydride:electron:Na / NH 3AGIEE, 2002, 41, 3028.JACS, 1972, 94, 7159.LAH ------------ almost all: ald, ketone, acie, ester, acyl X, anhydrideNaBH4 --------------- not for acid, ester (but LiBH4 work for ester)B2H6 --------------- not for ester, acyl X, anhydride;from top:LiAlH4; NaBH4; Na / NH3Al (O i Pr)3 / i PrOH ----------- Meerwein-Pondorf-Verley rxnIrCl4 / i PrOH / P(OMe)3 ------ Henbest rxnLiBH(sec Bu)3 ------------------ H. C. Brownfrom bottom:(2). stereoselective:(1). regioselective:3-h(3). HCHO reagent:Me CHO MeOHHCHOJACS, 1935, 511, 903.CH3CHO C(CH2OH)42Org.Syn, 1925, 4, 53.HCHO / KOHHCHO / Ca(OH)2Synthesis, 1994, 1007.PhNO2OPhNO2H OHBH / SMeJOC, 2001, 66, 7514.JOC, 2003, 68, 2030.OBH3 / THF99.5 % transsolvent: THF, SMe23-iR3B, HOCH2CH2OH // H2O2 // NaOHJOC, 1986, 51, 4925.C O R BRR3BRRRRR3C B OHOCH2CH2OHR3C BOO H2O2OHR3BOOO HO BOR3CH2OR3C OHpractice3-k OOHOHOHOHOOHOH OHOHOHJOC, 1967, 32, 3452.H 2O 2: dangerous,skin whiten, metal decomposeHg (OAc)2: toxic, hard to remove (3). B 2H 6, H 2O 2 / OH -, H 2O(2). Hg(OAc)2, H 2O // NaBH 4(1). H 3O +3-j3-j.13-j.2hydration:(1). KMnO 4 / NaOH (2). OsO 4(3). H 2O 2/HCO 2H (4). Na / EtOHcistran cis +trancis3Me2NNN CH3HCl3hνN CH3HHN CH3HH+N CH3ClNHCHC2R3C NH2R C NR2R C NHRR3C OHR2C OHRC OHR C NH2tertiarysecondaryprimaryCompare nomenclature class:not a very useful reactionC-NC-HC-NC-XC-OHC=OC=C4-abcdefg4-a2NH2Ph I OAcOAc SOONHSOON I Ph Fe(TPP)Cl SOONH2(insertion)TPPNNNNPh2. NaN3N C O1. SO2Cl2CO2CCO OhiC NC(O)XC-C(O)XNH 22RC NO 2RC NH 2iiiiii4-b CF 3CO 3H // Fe / HOAc1. many reducing agents4-b.14-b.21.2.3.4.Fe 3(CO)12 / CH 3OH JOC, 1972, 37, 930.NaBH 4 / Pd-C Na 2S Sn / HCl Vogel's 12.57Vogel's 12.58Vogel's 12.595.H 2 / Pt (S)-CJACS, 1965, 87, 2767.sulfided platium not affect: aromatic rings, ketones, halides, nitriles, amide, eastersJACS, 1933, 55, 4579.2HCHO NMe 2CO 2EtNH 2CO 2EtRC NCC NR C CRC NH 2iC N R N N+-C NR R'ii1. HCHO / HCO 2H 1. RBCl 2 / base1. HC(OEt)3 // NaBH 4;2. R 2CO // NaBH 3CN NH 2N CH 3CH 3HCHO N 3NHBCl 2NH 2COOHN COOHHCH 3NaBH 4b.3 2. HCHO // H 2 / Pd-CN 3NO 2MeO 2CNaBH 422rt NH 2NO 2MeO 2CSynthesis, 1979, 537.mild conditionhigh yieldnot affect:: NO 2, C=C, CN, COOR, COOH2. NaBH 4 / CoCl 2-6H 2Onot good, usually contain polyalkylation products2. Delepine3. NaN 3/ RED4-d4-c 5. Unpolung4. NaN 3 / RED3. Delepine2. Gabriel:1. NH 3N OO K N 2H 42Oi. LAH, NaBH 4ii. H 2 / catiii Zn / HCl; Al (Hg)i. Mg // NH 2Clii. Mg // PhSCH 2-N 3commercial available, tetramer of Me 3N24. CBr 4, PPh 3, NaN 3, DMF // PPh 3 / THFJOC, 2000, 65, 7110.urotropine (乌洛托品)methenamine (六甲烯胺)hexamethylenetetramine (环六亚甲基四胺)内服后遇酸分解出 HCHO,可做尿道消毒剂, 治膀胱炎B 2H 6 / H 2NOSO 3HB 2H 6 / H 2NO CH 3CN / H 3O +B 2H 6 / NH 2Cl C-C-NHCOCH 3C=CC-C-NH 24-freductive amination!Leuckart reactionmost generalvia: hydrazone4. PhNHNH 2 // Al (Hg)2. Me 3SiN 3 // LiAlH 43. NH 3 (excess) // RaNi / H 21. RNH 2 // NaBH 3CN5. NH 4+HCO 2-4-e6. RNH 2 / n -Bu 2SnClH / HMPASynthesis, 2000, 789.5. P 4S 10 // RaNi4. Et 3O + BF 4- // NaBH 43. B 2H 62. NaBH 3(OCOR)1. LiAlH 44-h4-g4-g.a 4-g.b R C NH 2R C NH 2R'formform AlH 3 / THF BrC NBr NH 2JOC, 2000, 65, 8152.AlH 3TH, 1989, 30, 5137.JOC, 1987, 52, 3901.R'Li // NaBH 4R'MgX // NaBH 4R'MgX // Li/NH 3(l)R'2CuLi // NaBH 4TH, 1989, 30, 5139.JOC, 1993, 58, 4313.R C NR C NH 2R'4-iNH 2ONHOCH 3O PhI(OAc)23JOC, 1993, 58, 2478.RCO NH 2RCO NIPh OAcKOHRN C OR NH COOCH 3CH 3OHPhI, OAcPhI(OAc)4-i.2C ONH 2RCH 2PhI(OAc)2 // KOH / CH 3OHC(OR)2C(SR)2h C-NH 2C-NO 2C N C C 5-ag f d c b a 5-C=C-OR C=C-SR C-OH C=N-OH C=N-H C=S C=O C=Ov. via: epoxysilaneRCO CRRCO CH 2R42SOCl H 3O +23OO2-HBrCrO 4OONaBH 3H 3O +3ZnTsNHNH 2MeLiTMSCl MCPBA LAH324CH 2CORRCH 2CORR3SSCH 2CORRPhCHOi. via: α-CO 2Hii. via: α-haloketoneiii. via: aldol processiv. via: thioenol etherRCO CH 2Rdrawback: require simple structure, use many powerful agents: MeLi, LAH, MCPBAe i j C-Br k C-Hii. MCPBAi. hydrolysis5-b5-c C=N-OHC=N-Hi. RaNi ii. TiCl 3iii. KMnO 4 / Al 2O 3H 3O +5-dHg 2+ / H 2O JOC, 1972, 37, 2138.JOC, 1970, 35, 858.HgSO 4 / H 2O / H 2O5-c.15-c.2THL, 2001, 42, 4775.1. DIBAL / H 3O +5-eStenphen reductionmostly for.Syn, 1925, 3, 1874.2. HCl./ SnCl 2 / Et 2O 5-e.1R -CH 2-C N5-e.25-e.3-CH 2-C OHR -CH -C OH R -CH -C O R"R'X / n -BuLiCH 3I R''MgBr H 3O +3.H 3O +5-fH 3O +Hg 2+ / CH3CN (aq)C=C-OR C=C-SROCH 3O+SCH 3O2+O2++O O O SSS SSR SR O O OR OR 5-gHg 2+ / H 3O +H 3O + / solv (aq)H 3O + / solv (aq)Hg 2+ / H 3O +OR OROH OHH 3O + / solv (aq)a very common protecting group, deprotect back to ketoneHC OEt OEt OEtRMgX / H 3O +HC OEt OEt OEtRCHON H+2Cr 2O 7-2N HCl.CrO 3Ag 2O:1. a mild oxidizing agent2. must be freshly prepared: NaOH into AgNO 3 (aq)3. may involve surface change, react with CO 2, lightSwern oxidation: (DMSO, oxalyl chloride, Et 3N)drawback: react at low T Collins reagent: (CrO 3 - 2 Py)1. drawback: use 6 equivalent, a messy reaction 2. must be very dry, fire easily; purify by CaH 23. an old oxidizing material, isolated by Collin.i. PCCii. PDCix. K 2R C OHO aldehyde1st alcohol2nd alcohol1st alcoholR C OHOR C ROR C HO 5-h i. PCC ii. PDCJOC, 1985, 50, 1332.N OCH 3PDC (pyridinium dichormate)(H 2Cr 2O 7 + 2 Py)PCC (pyridinium chlorochromate) (Py-HCl-CrO 3)most widely used use 1 - 1.2 eq.Pfitzner-Moffatt oxidationOO BrDMSOO OOH360 %Synth. Commun., 1986, 16, 1343.JOC, 1977, 42, 1991.Synthesis, 1981, 165.O I OOAcAcOpH 6: weak acid buffer, avoid interfere with ketal groupMcMurray reactioni. Corey approach: subtituted-quinone // H 3O +ii. Watt approacha. PhCHO // MCPBA // H 3O +b. ArPhO // MCPBA // H 3O +c. NBS // KOH // H 3O +PhOPh PhOPhNH 2Ph PhNH 2NC O H // H 3O +O O5-i.15-i.2i. Et 3N // H 3O +Nef reactionii. TiCl 3 / pH 1 or 6iii. SiO 2 / NaOH // H 3O +JACS, 1977, 99, 3861.iv. LDA / MoO 5-Py -v. NaOH // CH 3O OH 3O +vi. KMnO 4 / KOHChem. Rev. 1955, 55, 137.5-k IOOOH O(3 eq.)JACS, 2001, 123, 3183.CH 3CHO2. DDQ / TFA.Synthesis, 1979, 537.JCS, 1932, 1875.Ph-F / DMSO 3.1. SeO 2a select oxidantindrect: change to RC-OH followed by oxidation direct:1. DMAPO / DBU / CH 3CN i. DMSO / AgBF 4RBrDMSO / AgBF 42Bull Soc. Jpn., 1981, 54, 2221.THL, 1974, 917.2. NaIO 4 / DMFO Br84 %oNaIO 4 / DMF a new method 3. DMSO reagents:ii. DMSO / ZnSRCHBrMeRC(O)MeDMSO BrOH OOH JACS, 2003, 68, 2480.ROAgBFTHL, 1975, 4467.C C R CHOHRR C C HC C R R'R C C HR C C ArR C C HC C R PhR C C Hsteric base, prevent Nu attack n -BuLi: not MeLi, or t -BuLi, fire easily RX: R-Br, R-TOS, RCHO, RC(O)Rn -BuLi / R'CHO // Ac 2O // KO t BuClOMeN Liiii.ii. (Ph 3P)2PdCl 2, CuI, Et 2NH / PhIi. n-BuLi / RX6-b6-a b c d e g 6-aC CC CC Csulfonic acid: PhSO 3H; sulfinic acid: PhSO 2H; sulfenic acid: PhSOHiv. CuI, NaI, Na 2CO 3, RC C CH 2ClR C C HCl CH 2C C R'RCCCH 2CCR'Synthesis, 2000, 691.RCH 2-SO 2Ph RC CHh f iRCH(CO 2H)-CH 3-C(O)-CH 3O OOXCRR'=CHXin fact: convert to C=C firstlyii. protect - deprotecti. move to terminal 6-cNH 2NHKuse: KAPAuse: Co (CO)8 // Fe(NO 3)3, EtOHJACS, 1975, 97, 891.6-duse: i. Br 2 / CCl 4 // KO t Bu6-euse: Pb(OAc)4, LiCl // KO t Bu // Br 2/CCl 4 // KO t Fe(NO 3)3: weak oxidizing agentii. Br 2 // KOHJA CS, 1941, 63, 1180.PhPhPh6-fi. NaBH 4, H 3O +, Br 2, KOtBuii. NH 2OH, NaNO 2 / H 2SO 4 // Ac 2O / DMAPiii. LDA, ClPO(OEt)2ON NODMAP:4-N,N-D i m ethyl a minop y ridinemixture ofAc 2O / DMAP:N NC CH 3O6-guse: TsNHNH 2 / EtOH, heatTHL, 1967, 3943.OHO3(l)OCH 3CH CH 2German invention, as acylating agentLDA: Li N(iPr)2, ignored a long time, re-introduced by Michigan State U. became famous, appeared every weekHORLiNH 2 / NH 3 (l)RXuse: LiNH 2 / NH 3 (l) / R-XO Cl6-h6-i.JA CS, 1958, 80, 4599.JA CS, 1955, 77, 3293.Me PhHOSO 2CF 3Me C C Phvia:Me CPhJOC, 1978, 43, 364.ArAr'H Br Ar C C Ar'NaOEtvia:Ar Ar'Br i. NaOEt (when X = Br)ii. BuLi (when X = -OSO 2CF 3)?2minorapplied for reactions: without rearrangement;no regiosiomerCC H OH(CO H) / benzeneOH PhOO Cl ClClClOCl Cl NC NCO iii. Pd-C; or Ni; Pt, Rhii. chloronaili. DDQ use base: DBNi. CH 3I / Ag 2ii. HCHO / HCO 222use: heatuse: heatb 7-i. p-TSOH.H 2O or CSA ii. weak acid: HOAc; HCO 2H; H 2C 2O 4use:C C HIC C H NH 2C C H OC(S)SMe C C H OAc C C H OMs C C H OH a7-i h gCCX C C C CC CHC O C Cf e dc b a 7--C(O)-CH 3CH-CH CH-CX C-OHjCX-CYNaI / Zn (Cu)i. Zn / acetonei. CSCl 2/C COMs OMs C C BrBrCCOH OHc7-CCOH Iii. CSCl 2 / P(OMe)3P NNPhPOCl 3 / py // Snvia:C C IIapplication: i. protect alkene: via Br 2 // ZnCCCCC 36 o C CCCC=C 31 o C CCCC C Cl Cl148 o CC C BrOAcZn / HOAcOAcO AcOAcOOAcOOAc OAcOAcJOC, 1978, 43, 364., 1998, 2113.ii. In / MeOH ii. purify compoundd7-e7-i. WCl 6 / RLi ii. LiPPh 2 / CH 3I product retention product inversionR C HC HCH 2CH 2CH 2OH OClRiii. Na(special structure):7-d.7-d.S R 1R R R 2use: (EtO)3PSynthesis , 1977, 1134.via : betaine, oxaphosphetane (NMR)Onot good for Ph 3P=CH 2function as base:expensivedifficult to prepareOEtCNPPh 3CN3H OPPh 3O CO 2Me+notPh 3P CH EtH C OCO 2Me notPh 3P CH CO 2MeEtH O++++stable ylid gives trans (E)unstable ylid gives cis (Z)water soluble, removed by extraction(comparison: O=PPh 3 highly soluble in organic solvent)use:LiPh SON MeCH 2// Al (Hg)Me 3SiCHR -Li +Ph 3SiCH 2-Li + === Ph 3SiCH 2Br + n-BuLi (exchange)Me 3SiC -H-MgBr === Me 3SiCH 2Cl + Mg (metal reduction)Ph 3SiC -HCH 2Ph === Ph 3SiCH=CH 2 + PhLi (addition to vinylsilane)Me 3SiC -HCO 2Et === Me 3SiCH 2CO 2Et + Li (metalation)Me 3SiCH=PPh 3 === Me 3SiCH 2PPh 3+ X - + KHRO = MeO-, EtO-use: (RO)2PO-CHR'use: Ph 3P-CHR'vi. Sulfoximide (Johnson C.)iii. Silyl Wittig Reaction (Peterson Reaction)ii. Phosphonate Wittig Reaction (Horner-Emmons Modification)i. Wittig Reaction 7-f7-f.Synthesis, 1984, 384.THL, 1981, 2751.JOC, 1968, 33, 780.iv. CH 2(ZnI)2Chem. Lett, 1995, 259.Synlett, 1988, 12, 1369.2CH 2(ZnI)2v. CH 2CHBr 2, Sm, SnI 2 / CrCl 3, THFRO Rvii. Grignard reagent:1. TMSCH MgCluse: TMSCH 2MgClTHL, 1973, 3497.THL, 1988, 4339. 2. NaOAc, AcOHmethylenationOC RR'3H advantages over the Wittig:1. by-products are more easily removed,2. reaction suffers less from steric effects.via:(olefination reaction)1953 discover7-f.2not for Wittig, ylid unstableJOC, 1978, 43, 3253.JACS, 1974, 96, 4706.Chem. Lett, 1973, 1041.TiCl 3-LiAlH 4 / THF TiCl 3 / Mg TiCl 4 / Zn TiCl 4 / K ii. McMurry Couplingi. use: N 2H 4 / H 2S / Pb(OAc)4BASF, 1973, 2147.via:P(OEt)1. H 2S4+1. H 2S4OON SN N N OSN NSON ON NNSN NON NO OSO ON OO OO Og7-form trans alkene:form cis alkene:i. Li / NH 3; or other IA metals ii. Li / EtNH 2iii. LiAlH 4 / THFi. H 2 / Ni 2B (P-2 catalyst)ii. H 2 / Pd-CaCO 3 (Lindlar catalyst)iii. H 2 / Pd-BaSO 4iv. B 2H 6 / HOAc (Diborane)v. N 2H 2vi. HCHO / Pd-C / Et 3Nnot use H 2 / Pt: might convert to alkaneh7-all form trans alkene:i. R 2BH / Br-CN (hydroboration)C CHR HHii. DIBAL / n-BuLi / CH 3I (hydroalumination)iii. Cp 2ZrClH / RX (hydrozirconation)application: protecting groupvia dihalidevia halohydrinvia epoxidevia diene-olefin additionC=C C CX XC CH XC=CC COC=CC=CC=C C=CC=CC CC Cnot for double bond might moveMnO2 / Ph3P CH3 Br- / MTBDNNNCH3MTBD via diradicalJA CS, 1998, 100, 877.Ph Ph7-i7-j8-a 8-a.28-a.38-a.41. HI 3. TsCl / C 62. PI 3JCS, 1905, 87, 1592.CH 3OH CH 3I PI 38-a.12. F 3S-NEt 21.(DAST)(Ishikawa reagent)SN SF O OOO Chem. Rev., 1996, 96, 1737.2FCH SO O OH ONCHCl 2CH 3$ 65 / 500 g $ 80 / 50 gPBr 3PI 3$ 35 / 1000 g PBr 3$ 500 / 125 gJOC, 1993, 58, 3800.8-C-XC-OH C(O)Z d c b a C-NH 2C=O 2. PPh 3 / I 2e C-H8-d8-d.RC O OHR Br Ber.1942, 75, 296.8-d.2ClOCl8-b NaNO 2 / HCl / HBF 4 /Chem Rev., 1956, 56, 219.8-c CF 2Br 2 / ZnFF JCS.PT I, 1993, 335.8-e8-e.1I86 %I 2 / HNO 3JACS, 1917, 39,437.8-e.3I / HNO PhCH 2C(O)CH 3PhCHC(O)CH 3FN SF OOF +Chem. Rev., 1996, 96, 1737.F-TEDA-BF 4, 1994, 149.F 2-N 2 / CFCl 3-CHCl 3JOC, 1988, 53, 2803.90 %adamantane1. regioselective fluorination at the more substituted positions2. electrophilic in natureF -N CFCl 3-CHCl 3ON XR 1R 3OOR 2H Mg(ClO 4)2R 1R 3O OR 2X NBX / Mg(ClO 4)2JOC, 2002, 67, 7429.8-e.2X = Cl, Br, IX = Cl, Br, INBXNBX:i.ii.iii.iv.RRFR = CH 3CO, COCF 3, CCl 3, NO 2 HF / electrolysis1.4-1.6 V already industrilizedNF 3O / TBAH / CH 3CNv.TBHA: TetrabutylammoniumhydroxideTHL, 2003, 44, 2799.9-a9-C-CH 3C-X a (CH 3)3AlMe 3Al98 %Organomet. Chem. Rev., 1996, 4, 47.CH 2Cl 2bridgehead methylation。
有机化学反应概要(修订版)
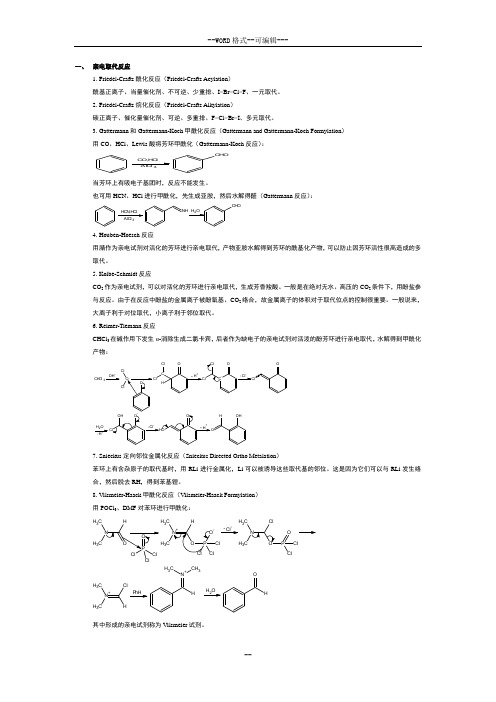
1. Friedel-Crafts 酰化反应(Friedel-Crafts Acylation )酰基正离子、当量催化剂、不可逆、少重排、I>Br>Cl>F 、一元取代。
2. Friedel-Crafts 烷化反应(Friedel-Crafts Alkylation )碳正离子、催化量催化剂、可逆、多重排、F>Cl>Br>I 、多元取代。
3. Gattermann 和Gattermann-Koch 甲酰化反应(Gattermann and Gattermann-Koch Formylation ) 用CO 、HCl 、Lewis 酸将芳环甲酰化(Gattermann-Koch 反应):CO,H Cl AlCl 3C H O当芳环上有吸电子基团时,反应不能发生。
也可用HCN 、HCl进行甲酰化,先生成亚胺,然后水解得醛(Gattermann 反应):HCN,HCl AlCl 3NH OH 2CHO4. Houben-Hoesch 反应用腈作为亲电试剂对活化的芳环进行亲电取代,产物亚胺水解得到芳环的酰基化产物,可以防止因芳环活性很高造成的多取代。
5. Kolbe-Schmidt 反应CO 2作为亲电试剂,可以对活化的芳环进行亲电取代,生成芳香羧酸。
一般是在绝对无水、高压的CO 2条件下,用酚盐参与反应。
由于在反应中酚盐的金属离子被酚氧基、CO 2络合,故金属离子的体积对于取代位点的控制很重要。
一般说来,大离子利于对位取代,小离子利于邻位取代。
6. Reimer-Tiemann 反应CHCl 3在碱作用下发生α-消除生成二氯卡宾,后者作为缺电子的亲电试剂对活泼的酚芳环进行亲电取代,水解得到甲酰化产物:CHCl 3OH-C ClCl O-OC -ClClHH+~C -OClCl Cl--OClO H 2H+-O-Cl OHCl--OO H H+~OHOH7. Snieckus 定向邻位金属化反应(Snieckus Directed Ortho Metalation )苯环上有含杂原子的取代基时,用RLi 进行金属化,Li 可以被诱导这些取代基的邻位。
长春碱

【药物名称】Vinblastine sulfate, 29060-LE, NSC-49842, Velbe, Velsar, Velban 【化学名】Vincaleukoblastine sulfate【CAS登记号】143-67-9, 865-21-4 (free base)【结构式】【分子式】C46-H58-N4-O9.H2-O4-S【分子量】909.061【原研厂家】Janssen (Originator), Lilly (Licensee) 【作用类别】ONCOLYTIC DRUGS, Vinca Alkaloids 【研发状态】Launched【合成情况】〖来源〗J Am Chem Soc〖合成路线〗Preparation of vinblastine, vincristine, and leurosidine, antitumor alkaloids from Catharanthus spp. (Apocynaceae)〖合成方法〗The indole alkaloid catharanthine (I) is treated with m-chloroperbenzoic acid to provide the N-oxide (II). Condensation of (II) with the alkaloid vindoline (III) under Polonovski reaction conditions leads to the bis-indole adduct (IV), which is further reduced to the tetrahydropyridine compound (V). Catalytic hydrogenation of tetrahydropyridine (V) furnishes (VI). This is then oxidized to the N-oxide (VII) (3). 〖作者〗Mangeney, P.; Andriamialisoa, R.Z.; Langlois, N.; Langlois, Y.; Potier, P.〖参考〗Mangeney, P.; Andriamialisoa, R.Z.; Langlois, N.; Langlois, Y.; Potier, P.; Preparation of vinblastine, vincristine, and leurosidine, antitumor alkaloids from Catharanthus spp. (Apocynaceae). J Am Chem Soc 1979, 101, 8, 2243〖出处〗J Am Chem Soc1979,101,(8):2243〖备注〗J Am Chem Soc 〖合成路线〗〖标题〗Preparation of vinblastine, vincristine, and leurosidine, antitumor alkaloids from Catharanthus spp. (Apocynaceae)〖合成方法〗N-Oxide (VII) is subjected to a new Polonovski rearrangement, leading to enamine (VIII). Exposure of (VIII) to thallium triacetate gives rise to the iminium salt (IX), which undergoes further reduction and acetate ester hydrolysis in the presence of NaBH4 to yield the desired compound (3).〖作者〗Mangeney, P.; Andriamialisoa, R.Z.; Langlois, N.; Langlois, Y.; Potier, P.〖参考〗Mangeney, P.; Andriamialisoa, R.Z.; Langlois, N.; Langlois, Y.; Potier, P.; Preparation of vinblastine, vincristine, and leurosidine, antitumor alkaloids from Catharanthus spp. (Apocynaceae). J Am Chem Soc 1979, 101, 8, 2243〖出处〗J Am Chem Soc1979,101,(8):2243〖备注〗〖来源〗Heterocycles 〖合成路线〗〖标题〗A highly efficient and commercially important synthesis of the antitumor Catharantus alkaloids vinblastine and leurosidine from catharanthine and vindoline〖合成方法〗In a similar procedure, coupling of catharanthine-N-oxide (I) with vindoline (II) under Polonovski reaction conditions leads to the iminium salt (III). This is subsequently converted into the desired enamine (IV) employing NADH as the reducing reagent. Aeration of enamine (IV) in the presence of FeCl3, followed by reductive work up leads to the title compound (4).〖作者〗Kutney, J.P.; Choi, L.S.L.; Nakano, J.; Tsukamoto, H.; McHugh, M.; Boulet, C.A. 〖参考〗Kutney, J.P.; Choi, L.S.L.; Nakano, J.; Tsukamoto, H.; McHugh, M.; Boulet, C.A.;A highly efficient and commercially important synthesis of the antitumor Catharantus alkaloids vinblastine and leurosidine from catharanthine and vindoline. Heterocycles 1988䟀:䞠: 篍끈5䁀:〖出处〗Heterocycles1988,27,(8):1845 〖备注〗〖来源〗J Org Chem〖合成路线〗〖标题〗Enantioselective syntheses of vinblastine, leurosidine, vincovaline, and20'-epi-vincovaline〖合成方法〗In an alternative synthesis, which does not utilize the alkaloid catharanthine, Sharpless asymmetric epoxidation of 2-ethyl-2-propenol (I) in the presence of ( )-diethyl tartrate provides (R)-2-ethyl-2,3-epoxypropanol (II). Subsequent addition of allylmagnesium chloride (III) to epoxide (II) leads to diol (IV), which is further protected as the corresponding acetonide (V) with 2,2 dimethoxypropane and p-TsOH. Ozonolysis of the terminal olefin (V) gives rise to aldehyde (VI). Condensation of aldehyde (VI) with the indoloazapine (VII) furnishes the bridged azepine (VIII) as a diastereomeric mixture. Without isolation, this mixture of amines is N-alkylated with benzyl bromide, and the resultant ammonium salts (IX) are subjected to rearrangement in boiling MeOH in the presence of Et3N to provide an equimolecular mixture of the tetracyclic diastereoisomers (X) and (XI) (5).〖作者〗Kuehne, M.E.; Matson, P.A..; Bornmann, W.G.〖参考〗Kuehne, M.E.; Matson, P.A..; Bornmann, W.G.; Enantioselective syntheses of vinblastine, leurosidine, vincovaline, and 20'-epi-vincovaline. J Org Chem 1991, 56, 2, 513〖出处〗J Org Chem1991,56,(2):513〖备注〗〖来源〗J Org Chem〖合成路线〗〖标题〗Enantioselective syntheses of vinblastine, leurosidine, vincovaline, and20'-epi-vincovaline〖合成方法〗Acidic hydrolysis of the mixture of diastereoisomeric acetonides (X) and (XI) leads to the respective diols, which are then separated by flash chromatography. After selective tosylation of the primary hydroxyl group of isomer (XII) withp-toluenesulfonic anhydride, the remaining tertiary hydroxyl is protected by silylation with trimethylsilyl triflate to furnish (XIII). Chlorination of (XIII) withtert-butyl hypochlorite leads to (XIV), which is then coupled to vindoline (XV) in the presence of AgBF4 to afford the bis-indolyl derivative (XVI) (5).〖作者〗Kuehne, M.E.; Matson, P.A..; Bornmann, W.G.〖参考〗Kuehne, M.E.; Matson, P.A..; Bornmann, W.G.; Enantioselective syntheses of vinblastine, leurosidine, vincovaline, and 20'-epi-vincovaline. J Org Chem 1991, 56, 2, 513〖出处〗J Org Chem1991,56,(2):513 〖备注〗〖来源〗J Org Chem〖合成路线〗〖标题〗Enantioselective syntheses of vinblastine, leurosidine, vincovaline, and20'-epi-vincovaline〖合成方法〗Reductive opening of (XVI) with KBH4 in AcOH produces the indoloazonine system (XVII). Intramolecular cyclization of the amino tosylate (XVII) in refluxing MeOH leads to the quaternary salt (XVIII). The N-benzyl group of (XVIII) is then removed by hydrogenolysis over Pd/C to give the silyl ether of vinblastine (XIX). The trimethylsilyl group of (XIX) is finally removed with tetrabutylammonium fluoride to furnish the target compound (5).〖作者〗Kuehne, M.E.; Matson, P.A..; Bornmann, W.G.〖参考〗Kuehne, M.E.; Matson, P.A..; Bornmann, W.G.; Enantioselective syntheses of vinblastine, leurosidine, vincovaline, and 20'-epi-vincovaline. J Org Chem 1991, 56, 2, 513〖出处〗J Org Chem1991,56,(2):513〖备注〗〖来源〗J Org Chem 〖合成路线〗An alternative enantioselective generation of intermediates in the total synthesis of vinblastine: Enantioselection in secodine-type reactions induced byalpha-ferrocenylethyl N-substituents〖合成方法〗In an improved method for the preparation of intermediate (XI), indolodiazepine (I) is condensed with the chiral ferrocenylethyl acetate (II) to produce the N-alkylated compound (III) as an inseparable mixture of diastereoisomers. Subsequent condensation of (III) with aldehyde (IV) and rearrangement in refluxing benzene gives rise to the tetracyclic compound (V) as a single diastereoisomer. Acetolysis of (V) provides amine (VI) along with minor amounts of a partly epimerized compound, which can be removed by chromatography. Then, alkylation of (VI) with benzyl bromide yields the key synthetic intermediate (XI) (6).〖作者〗Kuehne, M.E.; Bandarage, U.K.〖参考〗Kuehne, M.E.; Bandarage, U.K.; An alternative enantioselective generation of intermediates in the total synthesis of vinblastine: Enantioselection insecodine-type reactions induced by alpha-ferrocenylethyl N-substituents. J Org Chem 1996, 61, 3, 1175J Org Chem1996,61,(3):1175 〖备注〗〖来源〗J Am Chem Soc〖合成路线〗Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloid vinblastine and a lower-half analogue: Solvent effect on the stereochemistry of the crucial C-15/C-18' bond〖合成方法〗In a nonoxidative coupling sytrategy, the required tetracyclic precursor (X) has been prepared by two methods. Alkylation of thiolactam (I) with 2 (bromomethyl)-1-butene (II) gives the thioiminium salt (III), which undergoesthio-Claisen rearrangement in the presence of DBU in THF to produce (IV) as a mixture of cis and trans isomers. After conversion of thiolactams (IV) into the corresponding lactams with m-chloroperoxybenzoic acid (mCPBA), the desired isomer (V) is isolated employing preparative HPLC. Double bond dihydroxylation with N-methylmorpholine-N-oxide in the presence of a catalytic amount of OsO4 leads to diol (VI) as a diastereomeric mixture. Protection of diols (VI) with1-methoxycyclohexene (VII) affords the corresponding mixture of cyclohexylidene ketals (VIII). After conversion of (VIII) to the respective thiolactams with Lawesson's reagent in hot toluene, separation of isomers employing preparative HPLC furnishes thiolactam (IX). Then, desulfuration of thiolactam (IX) by means of Raney nickel gives rise to intermediate (X) (7).〖作者〗Magnus, P.; Mendoza, J.S.; Stamford, A.; Ladlow, M.; Willis, P.Magnus, P.; Mendoza, J.S.; Stamford, A.; Ladlow, M.; Willis, P.; Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloid vinblastine and a lower-half analogue: Solvent effect on the stereochemistry of the crucial C-15/C-18'䟀:䞠: 篍끈5䁀:t䛠:䟀: 箭䊘:.篆괈0枘0〖出处〗J Am Chem Soc1992,114,(26):10232〖备注〗〖来源〗J Am Chem Soc〖合成路线〗〖标题〗Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloid vinblastine and a lower-half analogue: Solvent effect on the stereochemistry of the crucial C-15/C-18' bond〖合成方法〗In an alternative, stereospecific synthesis of (X), Sharpless asymmetric epoxidation of 2-ethyl-2-propen-1-ol (XI) in the presence of (-)-diethyl tartrate provides the (R)-epoxide (XII). Ring opening of (XII) with sodium thiophenoxide produces diol (XIII), which is further protected with 1-methoxycyclohexene (VII), yielding ketal (XIV). Sulfide (XIV) is then oxidized with mCPBA to afford sulfoxide (XV) as a mixture of diastereoisomers. Pummerer rearrangement of sulfoxides (XV) in boiling Ac2O, followed by alkaline hydrolysis of the intermediatealpha-acetoxy sulfides furnishes aldehyde (XVI). Then, aldol condensation of aldehyde (XVI) with thiolactam (I) employing stannous triflate andN-ethylpiperidine gives adduct (XVII). Dehydration of alcohol (XVII) to the alpha,beta-unsaturated thiolactam (XVIII) is accomplished by tosylation withp-toluenesulfonic anhydride, followed by tosylate elimination in the presence of DBU. Desulfuration of thiolactam (XVIII) with deactivated Ra-Ni leads to (XIX). Then, hydrogenation of olefin (XIX) produces a mixture of epimers, from which the target isomer (X) can be isolated by column chromatography (7).〖作者〗Magnus, P.; Mendoza, J.S.; Stamford, A.; Ladlow, M.; Willis, P.〖参考〗Magnus, P.; Mendoza, J.S.; Stamford, A.; Ladlow, M.; Willis, P.; Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloid vinblastine and a lower-half analogue: Solvent effect on the stereochemistry of the crucial C-15/C-18'䟀:䞠: 篍끈5䁀:t䛠:䟀: 箭䊘:.篆괈0枘0〖出处〗J Am Chem Soc1992,114,(26):10232〖备注〗〖来源〗J Am Chem Soc〖合成路线〗〖标题〗Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloid vinblastine and a lower-half analogue: Solvent effect on the stereochemistry of the crucial C-15/C-18' bond〖合成方法〗The tetracyclic intermediate (X) is coupled to vindoline (XX) upon treatment with p-nitrobenzyl chloroformate to furnish the bis-indole adduct (XXI) as a mixture of epimers at the carbomethoxy group. Isolation of the desired isomer, followed by acidic ketal hydrolysis leads to diol (XXII). This is then oxidized to thealpha-hydroxy aldehyde (XXIII) with SO3-pyridine in DMSO. Finally, hydrogenolysis of the p-nitrobenzyl carbamate, with concomitant intramolecular reductive amination of the aldehyde group produces the title bis-indole alkaloid 〖作者〗Magnus, P.; Mendoza, J.S.; Stamford, A.; Ladlow, M.; Willis, P.〖参考〗Magnus, P.; Mendoza, J.S.; Stamford, A.; Ladlow, M.; Willis, P.; Nonoxidative coupling methodology for the synthesis of the antitumor bisindole alkaloidvinblastine and a lower-half analogue: Solvent effect on the stereochemistry of the crucial C-15/C-18'䟀:䞠: 篍끈5䁀:t䛠:䟀: 箭䊘:.篆괈0枘0〖出处〗J Am Chem Soc1992,114,(26):10232〖备注〗〖来源〗J Am Chem Soc〖合成路线〗〖标题〗Stereocontrolled total synthesis of (+)-vinblastine〖合成方法〗A stereocontrolled total synthesis of vinblastine has been reported.3-Ethyl-5-phenyl-4-pentenal (I) is transformed into cyanohydrin (II), which is further acetylated with Ac2O in pyridine to furnish the racemic alpha-acetoxy nitrile (III). Enzymatic hydrolysis of cyanohydrin acetate (III) leads to (S)-cyanohydrin (IV) as a mixture of diastereomers at the 4-ethyl group. Ozonolysis of (IV) produces the cyclic hemiacetal (V), which is subsequently dehydrated to the dihydrofuran (VI) employing mesyl chloride and triethylamine. The cyano group of (VI) is then reduced to the primary amine (VII) with LiAlH4 in THF. Acylation of amine (VII) with 2,4-dinitrobenzenesulfonyl chloride leads to the corresponding sulfonamide (VIII) (8).〖作者〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.〖参考〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.; Stereocontrolled total synthesis of (+)-vinblastine. J Am Chem Soc 2002, 124, 10, 2137〖出处〗J Am Chem Soc2002,124,(10):2137〖备注〗〖来源〗J Am Chem Soc 〖合成路线〗〖标题〗Stereocontrolled total synthesis of (+)-vinblastine〖合成方法〗Treatment of 7-mesyloxyquinoline (IX) with thiophosgene, followed by reduction with NaBH4 gives rise to isothiocyanate (X). After protection of the hydroxyl group of (X) as the tetrahydropyranyl ether (XI), nucleophilic addition of the anion of benzyl methyl malonate to the isothiocyanate group affords thioanilide (XII). The radical cyclization of (XII) in the presence of AIBN and Bu3SnH furnishes indole (XII). After protection of indole (XIII) as the N-Boc derivative (XIV), the benzyl ester group is removed by hydrogenolysis, producing the mono-methyl malonate (XV). Decarboxylative Mannich reaction of (XV) with formaldehyde and dimethylamine leads to the indolylacrylate (XVI). The tetrahydropyranyl group is then selectively removed by means of CSA in MeOH to provide tryptophol (XVII) (8, 9).〖作者〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.〖参考〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.; Stereocontrolled total synthesis of (+)-vinblastine. J Am Chem Soc 2002, 124, 10, 2137〖出处〗J Am Chem Soc2002,124,(10):2137〖备注〗〖来源〗Angew Chem. Int Ed〖合成路线〗〖标题〗First de novo synthesis of the bisindole alkaloid vinblastine〖合成方法〗Treatment of 7-mesyloxyquinoline (IX) with thiophosgene, followed by reduction with NaBH4 gives rise to isothiocyanate (X). After protection of the hydroxyl group of (X) as the tetrahydropyranyl ether (XI), nucleophilic addition of the anion of benzyl methyl malonate to the isothiocyanate group affords thioanilide (XII). The radical cyclization of (XII) in the presence of AIBN and Bu3SnH furnishes indole (XII). After protection of indole (XIII) as the N-Boc derivative (XIV), the benzyl ester group is removed by hydrogenolysis, producing the mono-methyl malonate (XV). Decarboxylative Mannich reaction of (XV) with formaldehyde and dimethylamine leads to the indolylacrylate (XVI). The tetrahydropyranyl group is then selectively removed by means of CSA in MeOH to provide tryptophol (XVII) (8, 9).〖作者〗Schneider, C.〖参考〗Schneider, C.; First de novo synthesis of the bisindole alkaloid vinblastine. Angew Chem. Int Ed 2002, 41, 22, 4217〖出处〗Angew Chem. Int Ed2002,41,(22):4217 〖备注〗〖来源〗J Am Chem Soc〖合成路线〗〖标题〗Stereocontrolled total synthesis of (+)-vinblastine〖合成方法〗Mitsunobu coupling between tryptophol (XVII) and sulfonamide (VIII) yields theN-sulfonyl tryptamine derivative (XVIII). Hydration of the cyclic enol ether, with simultaneous Boc group cleavage under acidic conditions affords lactol (XIX). Subsequent deprotection of the dinitrobenzenesulfonyl group of (XIX) with piperidine in MeOH proceeds with rearrangement of the deprotected amino lactol to the cyclic hydroxy enamine (XX). This, upon further heating, undergoes a Diels-Alder type reaction to afford the pentacyclic vindoline ring system (XXI). Following a previously reported synthetic pathway for (XXI), regioselective elimination of the secondary alcohol function, followed by protective group exchange at the phenolic hydroxyl, and oxidation with benzeneseleninic anhydride leads to the C4-alcohol (XXII). Further introduction of the C3-hydroxyl with m chloroperbenzoic acid, and reduction of the resultant imine with NaBH3CN provides diol (XXIII). After reductive methylation of the indoline N of (XXIII) with HCHO/NaBH3CN, acetylation of the secondary hydroxyl group produces ( ) vindoline (XXIII) (8, 9).〖作者〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.〖参考〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.; Stereocontrolled total synthesis of (+)-vinblastine. J Am Chem Soc 2002, 124, 10, 2137〖出处〗J Am Chem Soc2002,124,(10):2137〖备注〗〖来源〗Angew Chem. Int Ed〖合成路线〗〖标题〗First de novo synthesis of the bisindole alkaloid vinblastine〖合成方法〗Mitsunobu coupling between tryptophol (XVII) and sulfonamide (VIII) yields theN-sulfonyl tryptamine derivative (XVIII). Hydration of the cyclic enol ether, with simultaneous Boc group cleavage under acidic conditions affords lactol (XIX). Subsequent deprotection of the dinitrobenzenesulfonyl group of (XIX) with piperidine in MeOH proceeds with rearrangement of the deprotected amino lactol to the cyclic hydroxy enamine (XX). This, upon further heating, undergoes a Diels-Alder type reaction to afford the pentacyclic vindoline ring system (XXI). Following a previously reported synthetic pathway for (XXI), regioselective elimination of the secondary alcohol function, followed by protective group exchange at the phenolic hydroxyl, and oxidation with benzeneseleninic anhydride leads to the C4-alcohol (XXII). Further introduction of the C3-hydroxyl with m chloroperbenzoic acid, and reduction of the resultant imine with NaBH3CN provides diol (XXIII). After reductive methylation of the indoline N of (XXIII) with HCHO/NaBH3CN, acetylation of the secondary hydroxyl group produces ( ) vindoline (XXIII) (8, 9).〖作者〗Schneider, C.〖参考〗Schneider, C.; First de novo synthesis of the bisindole alkaloid vinblastine. Angew Chem. Int Ed 2002, 41, 22, 4217〖出处〗Angew Chem. Int Ed2002,41,(22):4217〖备注〗〖来源〗J Am Chem Soc〖合成路线〗〖标题〗Stereocontrolled total synthesis of (+)-vinblastine〖合成方法〗4-Ethylpent-4-enoic acid (XXIV) is coupled to (R)-4-benzyl-2-oxazolidinone (XXV) via its mixed anhydride with pivaloyl chloride, producing the N-acyl oxazolidinone (XXVI). Diastereoselective cyanoethylation of (XXVI) with acrylonitrile leads to (XXVII). Reduction of imide (XXVII) with NaBH4, followed by protection of the resulting alcohol with t-butyldiphenylsilyl chloride furnishes the silyl ether (XXVIII). The nitrile function of (XXVIII) is then reduced by means of DIBAL to the aldehyde (XXIX), which is further treated with hydroxylamine to yield the corresponding oxime (XXX). Exposure of oxime (XXX) to NaOCl generates an intermediate nitrile oxide, which undergoes intramolecular 1,3-dipolar cycloaddition to the isoxazoline (XXXI). Subsequent reductive cleavage of the isoxazoline (XXXI) N-O bond with Zn/AcOH leads to compound (XXXII). Cyclohexanone (XXXII) is then converted to lactone (XXXIII) under Baeyer-Villiger conditions. Further methanolysis of lactone (XXXIII) gives rise to the open-chain dihydroxyester (XXXIV). Sequential protection of the primary hydroxyl group with triethylchlorosilane and the tertiary hydroxyl with trimethylchlorosilane furnishes the fully silylated triol (XXXV) (8). The lithium enolate of ester (XXXV) is then condensed with isothiocyanate (XXXVI) to produce thioanilide (XXXVII) (8, 9).〖作者〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.〖参考〗Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T.; Stereocontrolled total synthesis of (+)-vinblastine. J Am Chem Soc 2002, 124, 10, 2137〖出处〗J Am Chem Soc2002,124,(10):2137〖备注〗〖来源〗Angew Chem. Int Ed〖合成路线〗。
最全官能团转换

1-a C-OH
(1). for 1', 2' alcohol:
C-H
O CH3 S Cl O
O RCH2 O S O O CH3 tosylate mesylate triflate toluenesulfonyl chloride (s) ~ $ 30 / Kg methanesulfonyy chloride (l) ~ $ 30 / Kg purification textbook dry pyridine: from CaH2 and distilled
Functional Group Interconversion
C-H 1- a b c d e f g h i j C C 6- a b c d e f g h i RC CH RCH2-SO2Ph C C C=C RCH(CO2H)-CH3 -C(O)-CH3
O X O O
C-OR C-OH C-X C-NH2 C-S C=O C=C C-CN C-CO2H C-CHO C(O)X C=C 7- a CH-CX b CH-CH c CX-CY
(4). RCH2NH2
RCH2NMe3 X
-
Ag2O
RCH2NMe3 OH-
R=CH2
R-CH3
1-d
C-S
C-H
radical mechanism
EtO2C HN MeO2C S N O
JOC, 1985, 50, 427.
EtO2C CH2Ph
Raney Nickel: Ni - Al alloy, suspension
iii NaBH3CN
NaBH3CN: stable at pH 5-6 JOC, 1976, 41, 3064. Br
有机人名反应(3)

NH3
O NH4OCH R
NH2
+ CO2
R'
HO R
NH2 R'
-H2O R
NH R'
HCO2H R
NH2 R' O
O H
Lieben Iodoform Reaction
Lieben Iodoform Reaction
O R CH3 1) NaIO 2) NaOH 3) HCl
I2 +
R2 R1
McMurry Reaction
O
TiCl3 + LiAlH4 THF, reflux
12%
Bomse, D. S.; Morton, T. H. Tetrahedron Letters 1975, 781.
McMurry Reaction
O
TiCl3 + LiAlH4 THF, reflux
68%
Meerwein-Ponndorf-Verley Reaction
Meerwein-Ponndorf-Verley Reaction
O + R R' OH Al[OCH(CH3)2]3 R OH + R' O
+ Al[OCH(CH3]3
OH
Al[OCH(CH3)2]3
R R' O
H O
R R' O
Reformatsky Reaction
O + R
1
O Zn OEt Br R
1
OH
O OEt
R
2
R2
Zn
+ ZnBrX
O OEt ZnBr
药物合成反应 第六章 氧化反应

1. Chromium Regent • (1)Jones :CrO3/acetone/H2SO4
对酸敏感化合物不能用此法; 如果起始原料是醛,可氧化成酸;
• (2)Sarret and Collins Regent
• 制备存在危险性; • 产品从吡啶中分离困难;
Example
(3)PCC、PDC
(氧环在位阻小的一侧形成)
PH值有影响:
2.不与羰基共轭的烯键的环氧化
O
CH3 H
CH3 H
+ CH3CO3H
CH3 H
C
C
CH3 H
+ CH3CO2H
烯烃在试剂的作用下,生成环氧化合物的反应称为环氧化反应。
O OH
+
OH R
+
H O
反 应 机 理
R
C O
+
[
R
C O
C O
O
-
O
] -
OH R C O O
• 2. 氧化生成酮、羧酸 • 应用特点
KMnO4、Na2Cr2O7、Cr2O3和稀HNO3作 氧化剂
空气氧化
用硝酸铈铵作氧化剂, 苄位亚甲基氧化成酮
SeO2试剂
(82%)
二 羰基a位活性烃基的氧化
1.形成a-羟酮
(1)反应通式
• (2)影响因素
加BF3可催化酮的烯醇化,KC有利,从而有 利于乙酰化。
• ②铬酰氯为氧化剂
(Chromychlorde)CrO2Cl2
机理:(自由型)
Etard复合体
机理:(离子型)
(Etard复合体)
• (3)影响因素 • ①反应温度
夏普莱斯不对称环氧化反应

夏普莱斯不对称环氧化反应一、简介夏普莱斯不对称环氧化反应(Sharpless Asymmetric Epoxidation,SAE)是一种通过手性催化剂促进的不对称环氧化反应。
该反应由美国化学家K. Barry Sharpless于1980年代初发明,被认为是合成手性分子的重要方法之一。
该反应可以用于合成具有生物活性的天然产物和药物分子。
二、反应机理夏普莱斯不对称环氧化反应的催化剂通常是含有钼或钨等过渡金属离子的配合物。
以钼为例,其配合物通常是Mo(O2CCH3)4或Mo (O2CCH3)6等。
这些配合物可以与氢氧化钠和季铵盐(如TBHP)一起作为反应体系中的催化剂。
在反应中,烯丙醇首先被氧化成α-羟基醛,然后与季铵盐发生亲核加成生成间隔式亚胺中间体。
接着,在催化剂的作用下,亚胺中间体发生环氧化反应生成手性环氧体。
最后,通过水解得到手性1,2-二醇产物。
三、影响因素1. 催化剂的选择:不同的过渡金属催化剂对反应的效果有所不同,Mo (O2CCH3)4和Mo(O2CCH3)6等配合物通常具有较好的催化活性。
2. 反应溶剂:反应中需要使用极性溶剂,如乙醇、二甲基甲酰胺等。
3. 温度:反应通常在0℃至-78℃的低温下进行。
4. 季铵盐用量:过多的季铵盐可能会导致副反应,而过少则会降低反应速率和产率。
四、优点与局限夏普莱斯不对称环氧化反应具有以下优点:1. 可以合成手性环氧体,是制备手性分子的有效方法之一。
2. 该反应操作简单、产率高、对环境友好。
然而,该反应也存在一些局限:1. 该反应只适用于含有α,β-不饱和键的烯丙醇类化合物。
2. 反应体系中需要使用季铵盐等高价催化剂,成本较高。
五、总结夏普莱斯不对称环氧化反应是一种通过手性催化剂促进的不对称环氧化反应,可以用于合成具有生物活性的天然产物和药物分子。
该反应具有操作简单、产率高、对环境友好等优点,但也存在一些局限。
随着化学合成技术的不断发展,夏普莱斯不对称环氧化反应在有机合成领域中仍具有广泛的应用前景。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
R 2 R 1R 3HOO R 3 OH OHOHOHOHOHOH2MyersSharpless Asymmetric Epoxidation ReactionChem 215Reviews:Katsuki, T.; Martin, V. S. Org. React. 1996, 48, 1-300.Johnson, R. A.; Sharpless, K. B. In Catalytic Asymmetric Synthesis , Ojima, I., Ed.; VCH: New York, 1993, pp. 103-158.Johnson, R. A.; Sharpless, K. B. In Comprehensive Organic Synthesis , Trost, B. M.; Fleming, I., Eds., Pergamon Press: New York, 1991, Vol. 7, pp. 389-436.Pfenninger, A. Synthesis 1986, 89-116.Asymmetric Epoxidation of Allylic Alcohols:RSubstitution patterns:• Z -disubstituted olefins are least reactive and selective.OHExamples of Sharpless Epoxidation:3R 2OHR 1Ti(O i-Pr)4,(+)-DETt -BuOOH, 3Å-MSCH 2Cl 2, –20 °CR 2 OH R 1OH• 5-10 mol% catalyst in the presence of 3 or 4 Å-MS. (+)-DET = EtO C CO 2Et• 10-20 mol% excess tartarate vs. Ti(O i Pr)4 required. • (+)- and (–)-DET are readily available and i nexpensive.OH4.7 • (+)- and (–)-DIPT, diisopropyl tartarate, are also available and sometimes lead to higher selectivity. Gao, Y.; Hanson, R. M.; Klunder, J. M.; Ko, S. Y.; Masamune, H.; Sharpless, K. B. J. Am. Chem. Soc. 1987, 109, 5765-5780. Mnemonic for selectivity:L-(+)-DET "O"H 3CBnO OCH 3 OHO OH100 (+)-DET (142)–2014 80 80D-(–)-DET "O"Katsuki, T.; Sharpless, K. B. J. Am. Chem. Soc. 1980, 102, 5974-5976. CH 3CH 3 OCH 35 (+)-DET (7.4) –20 0.75 95 91Application of Mnemonic:CH 3CH 3CH 3PhOHPh120(–)-DET (150)–2059094OOHAE-(–)-DETOHAE-(+)-DETOOHFrom: Gao, Y.; Hanson, R. M.; Klunder, J. M.; Ko, S. Y.; Masamune, H.; Sharpless, K. B. J. Am. Chem. Soc. 1987, 109, 5765-5780 and Johnson, R. A.; Sharpless, K. B. In Catalytic 97%, 86% ee 97%, 86% eeAsymmetric Synthesis , Ojima, I., Ed.; VCH: New York, 1993; pp. 103-158.M. Movassaghiproduct Ti(%) tartarate (%) °C h yield (%) ee (%)OOH 5(+)-DIPT (6.0)26590PhOOH 5(+)-DIPT (7.5)–20389>98PrO OH (+)-DET (5.9)–12118895OOH10C 7H 15(+)-DET (14)–10297486CH 3Ph O OH5 (+)-DIPT (7.5)–35279>98K i n e t i c R e s o l u t i o n :• P r o d u c t s a r e d i a s t e r e o m e r i c . • U s i n g t h e S h a r p l e s s m n e m o n i c , c o n t a c t b e t w e e n t h e C 1 s u b s t i t u e n t (R ) a n d t h e c a t a l y s t p r e d i c t s s l o w r e a c t i n g i s o m e r .C h i r a l S u b s t r a t e :C H 3 OOC H 3O O C H 3OOH 3CH 3C H 3C+ O H O H O H(+)-D E T "O " (+)-D E T "O " O s y n R a t i o (s y n : a n t i )O a n t i R e a g e n t R 2 R 2R 3 R 3RHm -C P B A V O (a c a c )2-T B H P T i (O i P r )4-T B H P T i (O i P r )4-(–)-D I P T -T B H P T i (O i P r )4-(+)-D I P T -T B H P1 : 1.4 1 : 1.8 1 : 2.3 1 : 90 22 : 1R 1 R 1 H O H H O Rs l o wf a s tM A T C H E D M I S M A T C H E D k r e l = k f a s t /k s l o w • P r o d u c t s a r e d i a s t e r e o m e r i c . • S e n s e o f i n d u c t i o n i s d o m i n a t e d b y t h e c a t a l y s t . • T h e C 4 c e n t e r r e i n f o r c e s a n d e r o d e s t h i s i n "M A T C H E D " a n d "M I S M A T C H E D " c a s e s , r e s p e c t i v e l y , a s s h o w n .• W i t h t h e e x c e p t i o n o f Z -d i s u b s t i t u t e d a l l y l i c a l c o h o l s , k r e l > 25. • W h e n k r e l = 25, t h e e e o f u n r e a c t e d a l c o h o l i s e s s e n t i a l l y 100% a t 60% c o n v e r s i o n . • A l l y l i c t e r t i a r y a l c o h o l s a r e n o t s u c c e s s f u l y e p o x i d i z e d u n d e r S h a r p l e s s c o n d i t i o n s . • F a c t o r s m a y c o m b i n e f o r h i g h s e l e c t i v i t y :K o , S . Y .; L e e , A . W . M .; M a s a m u n e , S ; R e e d , L . A ., I I I ; S h a r p l e s s , K . B .; W a l k e r , F . J . T e t r a h e d r o n 1990, 46, 245-264.OO H O H(–)-D I P T40% c o n v e r s i o n H 3CH 3CH o m o a l l y l i c , b i s h o m o a l l y l i c a n d t r i s h o m o a l l y l i c :(±) 70% y i e l d >95% e e• R a t e s o f e p o x i d a t i o n a r e u s u a l l y s l o w e r . • E n a n t i o f a c i a l s e l e c t i v i t y o f t h e c a t a l y s t i s r e v e r s e d f o r a l l t h r e e . • E n a n t i o f a c i a l s e l e c t i v i t y i s g e n e r a l l y l o w e r .• D i s u b s t i t u t e d o l e f i n i s m o r e r e a c t i v e t h a n m o n o s u b s t i t u t e d o l e f i n (k r e l ~100). • k f a s t /k s l o w f o r c h i r a l E -p r o p e n y l c a r b i n o l s i s ~100.T i (O i -P r )4 (1.0 e q u i v ) H 3CO HT B H P , –20 °C 1-4 dH 3C O H E x c e r c i s e : A p p l y t h e S h a r p l e s s m n e m o n i c t o p r e d i c t t h e s t e r e o c h e m i s t r y o f t h i s p r o d u c t .O S h a r p l e s s , K . B .; B e h r e n s , C . H .; K a t s u k i , T .; L e e , A . W . M .; M a r t i n , V . S .; T a k a t a n i , M .; V i t i , S . M .; W a l k e r , F . J .; W o o d a r d , S . S . P u r e A p p l. C h e m . 1983, 55, 589.50%, 41% e e• A l l y l i c 1,2-d i o l s d o n o t f o l l o w t h e S h a r p l e s s m n e m o n i c :R o s s i t e r , B . E .; S h a r p l e s s , K . B . J . O r g . C h e m . 1984, 49, 3707-3711.O H O H O H T i (O i -P r )4 (1.0 e q u i v ) (+)-D E T (1.2 e q u i v )O O H (+)-D I P T OC H 3+H C H 3 T B H P , 0 °C 48 h71% 90% e e 10% 90%e eO O H22%, 29% e eE x c e r c i s e : W h a t i s o m e r w o u l d y o u h a v e p r e d i c t e d u s i n g t h e S h a r p l e s s m n e m o n i c ?H o s o k a w a , T .; K o n o , T .; S h i n o h a r a , T .; M u r a h a s h i , S .-I . J . O r g a n o m e t a l. C h e m . 1989, 370, C 13-C 16. F o r o t h e r e x a m p l e s s e e : J o h n s o n , R . A .; S h a r p l e s s , K . B . I n C a t a l y t i c A s y m m e t r i c S y n t h e s i s , O j i m a , I ., E d .; V C H : N e w Y o r k , 1993, p p . 103-158 a n d K a t s u k i , T .; M a r t i n , V . S . O r g . R e a c t . 1996, 48, 1-300.T a k a n o , S .; I w a b u c h i , Y .; O g a s a w a r a J . A m . C h e m . S o c . 1991, 113, 2786-2787.M . M o v a s s a g h i。
