4.第二章.Mineral
土壤学习题与答案

土壤学试题与答案一按章节复习第一章绪论一、填空1.德国化学家李比希创立了(矿质营养)学说和归还学说,为植物营养和施肥奠定了理论基础。
2.土壤形成的五大自然因素是(母质)、(气候)、(生物)、(地形)和时间。
3.发育完全的自然土壤剖面至少有(表土层)、(淀积层)和母质层三个层次。
4.土壤圈处于(岩石圈)、(大气圈)、(生物圈)、(水圈)的中心部位,是它们相互间进行物质,能量交换和转换的枢纽。
5.土壤四大肥力因素是指(水分)、(养分)、(空气)和(热量)。
6.土壤肥力按成因可分为(自然肥力)、(人工肥力);按有效性可分为(有效肥力)、(潜在肥力)二、判断题1.(√)没有生物,土壤就不能形成。
2.(×)土壤三相物质组成,以固相的矿物质最重要。
3.(×)土壤在地球表面是连续分布的。
4.(×)土壤的四大肥力因素中,以养分含量多少最重要。
5.(×)一般说来,砂性土壤的肥力比粘性土壤要高,所以农民比较喜欢砂性土壤。
6.(√)在已开垦的土壤上自然肥力和人工肥力紧密结合在一起,分不出哪是自然肥力,哪是人工能力。
三、名词解释1. 土壤:是具有肥力特性因而能生产植物收获物的地球陆地疏松表层。
2. 土壤肥力:土壤能适时地供给并协调植物生长所需的水、肥、气、热、固着条件和无毒害物质的能力。
3. 土壤剖面:在野外观察和研究土壤时,从地面垂直向下直到母质挖一断面。
四、简答题1. 土壤在农业生产和自然环境中有那些重要作用?(1)土壤是植物生长繁育和生物生产的基地,是农业的基本生产资料。
(2)土壤耕作是农业生产中的重要环节。
(3)土壤是农业生产中各项技术措施的基础。
(4)土壤是农业生态系统的重要组成部分。
2. 土壤是由哪些物质组成的?土壤和土壤肥力的概念是什么?土壤是由固体、液体和气体三相物质组成的疏松多孔体。
3. 简述“矿质营养学说”和“归还学说”。
矿质营养学说:土壤中矿物质是一切绿色植物唯一的养料,厩肥及其它有机肥料对于植物生长所起的作用,并不是其中所含的有机质,而是由于这些有机质在分解时形成的矿物质。
(完整版)营养与食品卫生学重点

营养学:是研究人体营养规律及其改善措施的科学。
营养:是指人体摄取、消化、吸收和利用食物中营养物质以满足机体生理需要的生物学过程。
《皇帝内经素问》:“五谷为养,五果为助,五畜为益,五菜为充,气味合而服之,以补精益气”。
现代营养学分为三个时期:(始于18世纪中叶)。
1.营养学的萌芽与形成期(1785--1945年):1983:提出“蛋白质”;亮氨酸/苏氨酸;1920:“维生素”。
2.营养学的全面发展与成熟期(1945--1985年):公共营养兴起。
3.营养学发展的突破与孕育期(1985年--):植物化学物、分子营养学、新营养学。
第一章营养学基础营养素(nutrient):是指食物中可给人体提供能量、机体构成成分和组织修复以及生理调节功能的化学成分。
营养素六大类:水、脂肪、糖类、蛋白质、矿物质、维生素。
C、H、O、N占人体96%以上;细胞内液ICF(2/3)、外液ECF(1/3);骨密度(BMD);血液5L。
蛋白质(protein)必需氨基酸:指人体不能合成或合成速度不能满足机体需要,必须从食物中直接获得的氨基酸。
8+1:蛋氨酸、赖氨酸、缬氨酸、异亮氨酸、苯丙氨酸、亮氨酸、色氨酸、苏氨酸;组氨酸(婴儿)。
条件必需氨基酸:半胱氨酸←←蛋氨酸、酪氨酸←←苯丙氨酸。
氨基酸模式:蛋白质中各种必需氨基酸的构成比例。
(色氨酸为1)。
完全蛋白质:种类齐全,模式接近,可维持成人健康,也可促进儿童生长发育。
参考蛋白—鸡蛋蛋白质。
限制氨基酸:食物蛋白质中一种或几种必需氨基酸相对含量较低,导致其它氨基酸在体内不能被充分利用而浪费造成其营养价值降低,这些相对含量较低的氨基酸称为限制氨基酸。
蛋白质互补作用:不同食物间相互补充必需氨基酸不足的作用。
蛋白质的功能:1.构成机体组织;2.构成特殊生理活性物质;3.供能:1g食物蛋白质在体内产生16.7kJ能量。
小肠:为蛋白质吸收的主要场所。
氨基酸池:指存在于人体各组织、器官和体液中的游离氨基酸;氨基酸转运子分为两类:钠依赖型、非钠依赖型。
第二章有机化合物概论.ppt

或O
苯(结构式) 苯(骨架式)
3. 立体结构式
CH3 CH3 乳酸
CH3 CH3
HO
CHO
C H
H
COOHCOOH
C
C
H
H OH
O
HOOC HOOC
• 表示分子的空间构型 • 粗实线(锲形实线)表示在纸平面的前方 • 粗虚线(锲形虚线)表示在纸平面的后方 • 实线表示在纸平面上
4. 有机化合物分子结构的模型表示
结构简式
2. 骨架式(碳架式)
戊烷
CH3-CH-CH2-CH2-CH3 CH3
丙三醇
C| H2-C| H-C| H2 OH OH OH
OH OH
OH
HH
•链状和环状化合H 物C常用H 此方法表示
•碳环己碳烷键之间的HH夹CC角12CC0°HH
H
C
H
•取代基不能省略 H H
H
C
HC
CH
HC
CH
C
H
CH3OH
OH
O=
3、羰基(carbonyl): —C—
有机物类别:醛类 CH3CHO (醛基) 酮类 CH3COCH3(酮基)
O=
4、羧基(carboxyl): —C-OH 有机物类别:羧酸类 CH3COOH
5、氨基(amidogen):—NH2
• 有机物类别:伯胺类
—NH2
6、硝基(nitryl):—N • 有机物类别:硝基化合物
定义
有机化合物:是碳氢化合物(烃)及其衍生物
研究碳氢化合物及其衍生物的化学一中—种的化原—合子有物或机分原化子子学
团,被其他原子 或原子团所取代 而衍生的产物
• CO、CO2、碳酸盐等含碳的化合物的 化学性质与无机化合物相似,属于无机 物
植物生理学名词解释

植物生理学名词解释第一章植物的水分生理1.水势:(water potential)水溶液的化学势与纯水的化学势之差,除以水的偏摩尔体积所得商。
2.渗透势:(osmotic potential)亦称溶质势,是由于溶质颗粒的存在,降低了水的自由能,因而其水势低于纯水水势的水势下降值。
3.压力势:(pressure potential)指细胞的原生质体吸水膨胀,对细胞壁产生一种作用力相互作用的结果,与引起富有弹性的细胞壁产生一种限制原生质体膨胀的反作用力。
4.质外体途径:(apoplast pathway)指水分通过细胞壁、细胞间隙等没有细胞质部分的移动,阻力小,移动速度快。
5.共质体途径:(symplast pathway)指水分从一个细胞的细胞质经过胞间连丝,移动到另一个细胞的细胞质,形成一个细胞质的连续体,移动速度较慢。
6.渗透作用:水分从水势高的系统通过半透膜向水势低的系统移动的现象。
7.根压:(root pressure)由于水势梯度引起水分进入中柱后产生的压力。
8.蒸腾作用:(transpiration)指水分以气体状态,通过植物体的表面(主要是叶子),从体内散失到体外的现象。
9.蒸腾速率:(transpiration rate)植物在一定时间内单位叶面积蒸腾的水量。
10.蒸腾比率:(transpiration ratio)光合作用同化每摩尔CO2所需蒸腾散失的水的摩尔数。
11.水分利用率:(water use efficiency)指光合作用同化CO2的速率与同时蒸腾丢失水分的速率的比值。
12.内聚力学说:(cohesion theory)以水分具有较大的内聚力足以抵抗张力,保证由叶至根水柱不断来解释水分上升原因的学说。
13.水分临界期:(critical period of water)植物对水分不足特别敏感的时期。
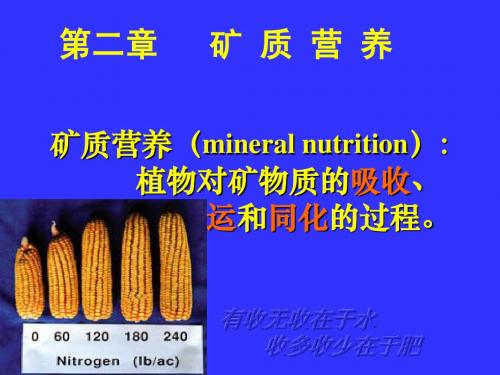
第二章植物的矿质营养1.矿质营养:(mineral nutrition)植物对矿物质的吸收、转运和同化。
第二章 矿质营养(一)2014
Potassium Deficiencies:
Yellow and brown coloration of leaf margins 缺钾:叶缘变黄和棕色
S
吸收形式:SO42- 生理作用: 1、形成含S氨基酸 2、是CoA、VB1、VB7成分 病症: 1、影响蛋白质的合成,叶色黄绿或发红 2、影响叶绿素合成 3、植株矮小 思考- S的缺素症和N的缺素症有何异同?原因?
常见的有钴(Co) 、硒(Se) 、钒(V) 、铝(Al) 、钛(Ti)、锂(Li)、碘(I)等。
钴(Co):豆科植物根瘤菌固氮所必需的。
Si:有利于水稻茎秆发育,在有硅存在时,增强水稻的抗倒和抗病能力。 Na:有利于甜菜叶片中的淀粉转化为蔗糖有利于光合产物向根的运输。 Na还参与保卫细胞和
缺乏症:
缺Ca时的病症主要表现在幼叶和根、茎的生长点。 (本节其它内容自学)
缺钙症状
1)幼叶淡绿色 继而叶尖出现典 型的钩状,随后 坏死。 2)生长点坏死 钙是难移动,不 易被重复利用的 元素,故缺素症 状首先表现在幼 茎幼叶上,如大 白菜缺钙时心叶 呈褐色“干心 病” ,蕃茄 “脐腐病”。
水稻缺Ca,新叶发 黄,生长点坏死
(2)植物缺乏该元素时表现出特有的病症特征(症状),当加入该元素时,
可预防或消除病症,恢复正常,且其功能不能被其它元素所代替。 (3)该元素对植物的营养作用(效果)是直接的(而不是由于土壤的物理、
化学、微生物条件的改善而产生的间接效果)。
◆研究植物营养的方法 ――人工培养法
人工培养法或无土栽培法(soil-less culture)指不用土壤而用人工配制的营养液 进行培养植物的方法。它包括四种:
第二章
矿 质 营 养
第二章 植物的矿质营养

硫不足时,蛋白质含量显著减少,叶色黄绿, 植株矮小。
(7) 铁 ①叶绿素合成所必需;细胞色素和非血红素铁
蛋白的组成成分。 ②Fd的组分。因此,参与光合作用。
缺铁时,由幼叶脉间失绿黄化,但叶脉仍为绿 色;严重时整个新叶变为黄白色。
(8)硼 是细胞壁的成分,与甘露醇、甘露聚糖、 多聚甘露糖醛酸等形成复合物。
一、植物体内的元素
105℃ 植物材料
水分 (10%—95%) 挥发
600 ℃ 干物质
有机物(90%—95%)
(5%—90%)
灰分 (5%—10%)
残留
植物体内的元素包括:
1.矿质元素(mineral element),灰分 元素 (ash element)
2.非矿质元素
1)矿质元素:将植物烘干并充分燃烧后, 余下一些不能挥发的残烬称为灰分,而以 氧化物形式存在于灰分中的元素称为灰分 元素。灰分元素直接或间接来自于土壤矿 质,故亦被称为矿质元素。
研究热点:生物固氮、植物中氨基酸的合成
学习内容
1 植物必需的矿质元素及其生理作 用 2 植物细胞对矿质元素的吸收 3 植物体对矿质元素的吸收 4 矿物质在植物体内运输 5 合理施肥的生理基础
第一节 植物必需的矿质元素
植物对矿物质的吸收、转运和 同化称为矿质营养(mineral nutrition)。
植株缺氮时,植物生长矮小,分枝、分蘖少,叶 片小而薄,株型紧凑,叶片发黄易发生早衰, 且由下部叶片开始逐渐向上。
小麦缺氮
苹果缺氮
(2) 磷
①磷是细胞质(磷脂)和细胞核(核酸)的组成成分。
②磷是核苷酸的组成成分。核苷酸的衍生物(如ATP、 FMN、NAD+、NADP+和CoA等)在新陈代谢中占有 极其重要的地位。
【推荐】mineralPPT资料
• Which side of Mike’s stomach hurts? Ask the patient what is wrong and give him or her some advice.
Student A (Doctor)
Make a list of the food and decide whether what they eat is junk food or not.
Student A
Student A
(Doctor)
(Patient)
Ask the patient My left arm is
what is wrong and broken. It really
give him or her hurts.
some advice.
Student A (Doctor)
Ask the patient what is wrong and give him or her some advice.
Student B
Student B
Student B
(Patient)
(Doctor)
(Patient)
I am coughing all Ask the patient I can’t sleep at
Pre-listening
• Did you often have a stomachache?
• What caused it? Do you still remember?
• How did it disappear later? • What lesson can you learn from
calorie(卡路里),
量元素), fiber(纤维
土壤学习题与答案
土壤学试题与答案一按章节复习第一章绪论一、填空1.德国化学家李比希创立了(矿质营养)学说和归还学说,为植物营养和施肥奠定了理论基础。
2.土壤形成的五大自然因素是(母质)、(气候)、(生物)、(地形)和时间。
3.发育完全的自然土壤剖面至少有(表土层)、(淀积层)和母质层三个层次。
4.土壤圈处于(岩石圈)、(大气圈)、(生物圈)、(水圈)的中心部位,是它们相互间进行物质,能量交换和转换的枢纽。
5.土壤四大肥力因素是指(水分)、(养分)、(空气)和(热量)。
6.土壤肥力按成因可分为(自然肥力)、(人工肥力);按有效性可分为(有效肥力)、(潜在肥力)二、判断题1.(√)没有生物,土壤就不能形成。
2.(×)土壤三相物质组成,以固相的矿物质最重要。
3.(×)土壤在地球表面是连续分布的。
4.(×)土壤的四大肥力因素中,以养分含量多少最重要。
5.(×)一般说来,砂性土壤的肥力比粘性土壤要高,所以农民比较喜欢砂性土壤。
6.(√)在已开垦的土壤上自然肥力和人工肥力紧密结合在一起,分不出哪是自然肥力,哪是人工能力。
三、名词解释1. 土壤:是具有肥力特性因而能生产植物收获物的地球陆地疏松表层。
2. 土壤肥力:土壤能适时地供给并协调植物生长所需的水、肥、气、热、固着条件和无毒害物质的能力。
3. 土壤剖面:在野外观察和研究土壤时,从地面垂直向下直到母质挖一断面。
四、简答题1. 土壤在农业生产和自然环境中有那些重要作用?(1)土壤是植物生长繁育和生物生产的基地,是农业的基本生产资料。
(2)土壤耕作是农业生产中的重要环节。
(3)土壤是农业生产中各项技术措施的基础。
(4)土壤是农业生态系统的重要组成部分。
2. 土壤是由哪些物质组成的?土壤和土壤肥力的概念是什么?土壤是由固体、液体和气体三相物质组成的疏松多孔体。
3. 简述“矿质营养学说”和“归还学说”。
矿质营养学说:土壤中矿物质是一切绿色植物唯一的养料,厩肥及其它有机肥料对于植物生长所起的作用,并不是其中所含的有机质,而是由于这些有机质在分解时形成的矿物质。
《土壤学》作业及复习题09
《土壤学》作业及复习题绪论一、名词解释:–土壤圈–土壤–土壤肥力–土壤生产力–土壤肥力的生态相对性二、填空1.自然界土壤由、、三相物质组成。
2.土壤的本质属性是。
3.四大肥力因子是。
4.土壤是由____、____、___三相组成的,固相约占土壤总体积的___,包括____和____。
5.土地日是每年的。
6.我国耕地面积占土地面积的。
7.土壤生产力的大小由()和()共同决定。
三、问答题1.土壤与土壤圈、土壤肥力与土壤生产力;肥力四因素是什么?★2. 简述土壤的基本物质组成。
★3.简述城市绿地土壤的特征。
★4.怎样提高土壤的生产力?5.土壤的基本特性?6.土壤在植物生长繁育中有哪些特殊作用?通过学习本课程您是如何认识土壤在农业生产中的重要作用的?第一章土壤矿物质(Soil mineral )一、名词解释:–风化作用–成土母质–硅铝铁率–同晶代换–粘土矿物二、填空1.土壤矿物质来自__,其矿物组成按其成因可分为____和____。
2.矿物按成因分为()和()。
3.根据成因将岩石分为()、()和()三大类。
4.岩石风化作用的类型有()、()和()三大类5.近代气候下形成的母质按其搬运方式和堆积特点,可分为母质和母质。
6.层状硅酸盐粘土矿物的基本结构单位是()和()。
7.土壤次生矿物种类主要有_____、______、______。
8.高岭石晶层一面为,另一面为,因而重叠时有_键.9.粘土矿物的基本构造单元是()和()。
10.方土壤的粘土矿物以()为主,北方土壤的粘土矿物主要以()为主。
11.高岭石是类型的黏土矿物,蒙脱石是类型的黏土矿物。
12.三、单项选择1、下列风化过程不属于化学风化的为(): A、溶解 B、水解 C、水化 D、水蚀2、自然界最难风化的矿物种类是(): A、辉石 B、方解石 C、白云母 D、石英3、下列矿物中属于次生矿物的为()。
A.石英 B. 正长石 C. 角闪石 D. 方解石4、影响土壤物理风化的主要因素是()A.温度 B. 结冰 C. 流水 D. 风蚀5、影响土壤化学风化的主要因素是()A.溶解 B. 水化 C. 水解 D. 氧化6、分布最广的成土岩石是()。
智慧树答案租船运输理论与实务知到课后答案章节测试2022年
第一章1. 在租船业务中,Charterer有租家、承租人、租船人等多种称谓。
答案:对2. 航次租船又称为程租。
答案:对3. DHD的全称为Demurrage Half Despatch。
答案:错4. 在国际航运产业链中,对货物运输的需求派生与国际贸易,来自贸易合同的卖方或买方。
答案:对5. 金康标准租船合同范本中的仲裁地点的选择不包括:答案:香港6. 关于租船经纪人及其业务,以下说法正确的是:答案:市场行情、船舶、货物等方面的信息对租船经纪业务至关重要。
7. 关于港口代理人及其业务,以下说法错误的是:答案:港口代理通常由租船经纪人担任。
8. 关于租船合同范本POLCOALVOY,以下说法错误的是:答案:这是一个包运合同范本。
9. 关于各租船合同范本,以下属于定期租船合同的有:答案:BALTIME;NYPE10. 关于租船业务的成本要素,以下说法正确的有:答案:航次成本通常属于变动成本。
;海上运输总成本主要由资本成本、营运成本和航次成本三部分构成。
;航次成本通常包括港口使费、燃油费、运河通行费等。
;资本成本和营运成本在一定时期内通常是固定的,属于固定成本。
第二章1. 国际干散货运输市场基本上是一个完全竞争市场。
答案:对2. 原油运输通常是按“美元/吨”来计收运费的。
答案:错3. 对于一家船东来说,新造船市场、货运市场、船舶买卖市场、拆船市场都和他有关,他有可能在这四个市场都发生交易行为。
答案:对4. 海上货物运输市场分班轮运输市场和租船运输市场两类,其价格形成机制是相似的。
答案:错5. 航次租船业务属于以下哪一个细分市场?答案:Freight Market6. FFA是指:答案:远期运费协议7. VLCC是指:答案:超大型油轮8. 在国际航运市场供需中,影响“需”的因素不包括:答案:船队规模9. 根据市场报告信息“Mineral Hong Kong (175,000 dwt, 14/54.7L 14.5/47.3B, 2006 built) delivery worldwide 1 Nov-31 Dec 2008,redelivery worldwide, 3 years, $52,500 daily. (Glory Wealth)”,以下判断正确的有:答案:是定期租船;$52,500是每天租金水平;54.7和47.8均表示油耗水平10. 根据市场报告信息“ORE / Seven Islands to Rotterdam - Rubena N, 180,000t, $19.50 per tonne, fio 7 days sc, 20-30 May. (TKS)”,以下判断正确的有:答案:5月30日为解约日;是航次租船第三章1. 船舶总载重吨DWT表示船舶可以装载的货物吨数,反映船舶的载重能力。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Chapter ⅡMineralA mineral is a naturally occurring substance formed through geological processes that has a characteristic chemical composition, a highly ordered atomic structure, and specific physical properties. A rock, by comparison, is an aggregate of minerals and need not have a specific chemical composition. Minerals range in composition from pure elements and simple salts to very complex silicates with thousands of known forms. The study of minerals is called mineralogy.PartⅠMineral definitionTo be classified as a true mineral, a substance must be a solid and have a crystalline structure. It must also be a naturally occurring, homogeneous substance with a defined chemical composition. Traditional definitions excluded organically derived material. However, the International Mineralogical Association in 1995 adopted a new definition:a mineral is an element or chemical compound that is normally crystalline and that has been formed as a result of geological processes.The modern classifications include an organic class - in both the new Dana and the Strunz classification schemes.The chemical composition may vary between end members of a mineral system. For example the plagioclase feldspars comprise a continuous series from sodium-rich albite(NaAlSi3O8) to calcium-rich anorthite(CaAl2Si2O8) with four recognized intermediate compositions between. Mineral-like substances that don't strictly meet the definition are sometimes classified as mineraloids. Other natural-occurring substances are nonminerals. Industrial minerals is a market term and refers to commercially valuable mined materials.A crystal structure is the orderly geometric spatial arrangement of atoms in the internal structure of a mineral. There are 14 basic crystal lattice arrangements of atoms in three dimensions, and these are referred to as the 14 "Bravais lattices". Each of these lattices can be classified into one of the six crystal systems, and all crystal structures currently recognized fit in one Bravais lattice and one crystal system. This crystal structure is based on regular internal atomic or ionic arrangement that is often expressed in the geometric form that the crystal takes. Even when the mineral grains are too small to see or are irregularly shaped, the underlying crystal structure is always periodic and can be determined by X-ray diffraction. Chemistry and crystal structure together define a mineral. In fact, two or more minerals may have the same chemical composition, but differ in crystal structure. For example, pyrite and marcasite are both iron sulfide, but their arrangement of atoms differs. Similarly, some minerals have different chemical compositions, but the same crystal structure: for example, halite(made from sodium and chlorine), galena(made from lead and sulfur) and periclase(made from magnesium and oxygen) all share the same cubic crystal structure.Crystal structure greatly influences a mineral's physical properties. For example, though diamond and graphite have the same composition (both are pure carbon), graphite is very soft, while diamond is the hardest of all known minerals. This happens because the carbon atoms in graphite are arranged into sheets which can slideeasily past each other, while the carbon atoms in diamond form a strong, interlocking three-dimensional network.There are currently more than 4,000 known minerals, according to the International Mineralogical Association, which is responsible for the approval of and naming of new mineral species found in nature. Of these, perhaps 100 can be called "common,"50 are "occasional," and the rest are "rare" to "extremely rare."PartⅡ Differences between minerals and rocksA mineral is a naturally occurring solid with a definite chemical composition and a specific crystalline structure. A rock is an aggregate of one or more minerals. (A rock may also include organic remains and mineraloids.) Some rocks are predominantly composed of just one mineral. For example, limestone is a sedimentary rock composed almost entirely of the mineral calcite. Other rocks contain many minerals, and the specific minerals in a rock can vary widely. Some minerals, like quartz, mica or feldspar are common, while others have been found in only one or two locations worldwide. The vast majority of the rocks of the Earth's crust consist of quartz, feldspar, mica, chlorite, kaolin, calcite, epidote, olivine, augite, hornblende, magnetite, hematite, limonite and a few other minerals. Over half of the mineral species known are so rare that they have only been found in a handful of samples, and many are known from only one or two small grains.Commercially valuable minerals and rocks are referred to as industrial minerals. Rocks from which minerals are mined for economic purposes are referred to as ores (the rocks and minerals that remain, after the desired mineral has been separated from the ore, are referred to as tailings).Mineral composition of rocksA main determining factor in the formation of minerals in a rock mass is the chemical composition of the mass, for a certain mineral can be formed only when the necessaryelements are present in the rock. Calcite is most common in limestones, as these consist essentially of calcium carbonate; quartz is common in sandstones and in certain igneous rocks which contain a high percentage of silica.Other factors are of equal importance in determining the natural association or paragenesis of rock-forming minerals, principally the mode of origin of the rock and the stages through which it has passed in attaining its present condition. Two rock masses may have very much the same bulk composition and yet consist of entirely different assemblages of minerals. The tendency is always for those compounds to be formed which are stable under the conditions under which the rock mass originated. A granite arises by the consolidation of a molten magma at high temperatures and great pressures and its component minerals are those stable under such conditions. Exposed to moisture, carbonic acid and other subaerial agents at the ordinary temperatures of the Earth's surface, some of these original minerals, such as quartz and white mica are relatively stable and remain unaffected; others weather or decay and are replaced by new combinations. The feldspar passes into kaolinite, muscovite and quartz, and any mafic minerals such as pyroxenes, amphiboles or biotite have been present they are often altered to chlorite, epidote, rutile and other substances. These changes are accompanied by disintegration, and the rock falls into a loose, incoherent, earthy mass which may be regarded as a sand or soil. The materials thus formed may be washed away and deposited as sandstone or siltstone. The structure of the original rock is now replaced by a new one; the mineralogical constitution is profoundly altered; but the bulk chemical composition may not be very different. The sedimentary rock may again undergo metamorphism. If penetrated by igneous rocks it may be recrystallized or, if subjected to enormous pressures with heat and movement during mountain building, it may be converted into a gneiss not very different in mineralogical composition though radically different in structure to the granite which was its original state.PartⅢPhysical properties of mineralsClassifying minerals can range from simple to very difficult. A mineral can be identified by several physical properties, some of them being sufficient for full identification without equivocation. In other cases, minerals can only be classified by more complex chemical or X-ray diffraction analysis; these methods, however, can be costly and time-consuming.Physical properties commonly used are:∙Crystal structure and habit: See the above discussion of crystal structure. A mineral may show good crystal habit or form, or it may be massive, granular or compact with only microscopically visible crystals.Talc Rough diamond∙Hardness: the physical hardness of a mineral is usually measured according to the Mohs scale. This scale is relative and goes from 1 to 10. Minerals with a given Mohs hardness can scratch the surface of any mineral that has a lower hardness than itself.o Mohs hardness scale: Talc Mg3Si4O10(OH)21.Gypsum CaSO4·2H2O2.Calcite CaCO33.Fluorite CaF24.ApatiteCa5(PO4)3(OH,Cl,F) 5.Orthoclase KAlSi3O8 6.Quartz SiO27.Topaz Al2SiO4(OH,F)2 8.Corundum Al2O3 9.Diamond C (pure carbon)∙Luster indicates the way a mineral's surface interacts with light and can range from dull to glassy (vitreous).o Metallic -high reflectivity like metal: galena and pyriteo Sub-metallic -slightly less than metallic reflectivity: magnetiteo Non-metallic lusters:▪Adamantine - brilliant, the luster of diamond also cerussite and anglesite▪Vitreous -the luster of a broken glass: quartz▪Pearly - iridescent and pearl-like: talc and apophyllite▪Resinous - the luster of resin: sphalerite and sulfur▪Silky - a soft light shown by fibrous materials: gypsum and chrysotile▪Dull/earthy -shown by finely crystallized minerals: the kidney ore variety of hematite∙Color indicates the appearance of the mineral in reflected light or transmitted light for translucent minerals (i.e. what it looks like to the naked eye).o Iridescence - the play of colors due to surface or internal interference. Labradorite exhibits internal iridescence whereas hematite and sphalerite often sho w the surface effect.∙Streak refers to the color of the powder a mineral leaves after rubbing it on an unglazed porcelain streak plate. Note that this is not always the same color as the original mineral.∙Cleavage describes the way a mineral may split apart along various planes. In thin sections, cleavage is visible as thin parallel lines across a mineral.∙Fracture describes how a mineral breaks when broken contrary to its natural cleavage planes.o Chonchoidal fracture is a smooth curved fracture with concentric ridges of the type shown by glass.o Hackley is jagged fracture with sharp edges.o Fibrouso Irregular∙Specific gravity relates the mineral mass to the mass of an equal volume of water, namely the density of the material. While most minerals, including all the commonrock-forming minerals, have a specific gravity of 2.5 - 3.5, a few are noticeably more or less dense, e.g. several sulfide minerals have high specific gravity compared to the common rock-forming minerals.Other properties: fluorescence(response to ultraviolet light), magnetism, radioactivity, tenacity(response to mechanical induced changes of shape or form), piezoelectricity and reactivity to dilute acids.PartⅣ Chemical properties of mineralsMinerals may be classified according to chemical composition. They are here categorized by anion group. The list below is in approximate order of their abundance in the Earth's crust. The list follows the Dana classification system which closely parallels the Strunz classification.Silicate classquartz HaliteThe largest group of minerals by far are the silicates (most rocks are ≥95% silicates), which are composed largely of silicon and oxygen, with the addition of ions such as aluminium, magnesium, iron, and calcium. Some important rock-forming silicates include the feldspars, quartz, olivines, pyroxenes, amphiboles, garnets, and micas.Carbonate classThe carbonate minerals consist of those minerals containing the anion (CO3)2-and include calcite and aragonite (both calcium carbonate), dolomite (magnesium/calciumcarbonate) and siderite(iron carbonate). Carbonates are commonly deposited in marine settings when the shells of dead planktonic life settle and accumulate on the sea floor. Carbonates are also found in evaporitic settings (e.g. the Great Salt Lake, Utah) and also in karst regions, where the dissolution and reprecipitation of carbonates leads to the formation of caves, stalactites and stalagmites. The carbonate class also includes the nitrate and borate minerals.Sulfate classSulfates all contain the sulfate anion, SO42-. Sulfates commonly form in evaporitic settings where highly saline waters slowly evaporate, allowing the formation of both sulfates and halides at the water-sediment interface. Sulfates also occur in hydrothermal vein systems as gangue minerals along with sulfide ore minerals. Another occurrence is as secondary oxidation products of original sulfide minerals. Common sulfates include anhydrite(calcium sulfate), celestine(strontium sulfate), barite(barium sulfate), and gypsum (hydrated calcium sulfate). The sulfate class also includes the chromate, molybdate, selenate, sulfite, tellurate, and tungstate minerals.Halide classThe halides are the group of minerals forming the natural salts and include fluorite (calcium fluoride), halite (sodium chloride), and sylvite (potassium chloride). Halides, like sulfates, are commonly found in evaporitic settings such as playa lakes and landlocked seas such as the Dead Sea and Great Salt Lake. The halide class includes the fluoride, chloride, bromide and iodide minerals.Oxide classOxides are extremely important in mining as they form many of the ores from which valuable metals can be extracted. They also carry the best record of changes in the Earth's magnetic field. They commonly occur as precipitates close to the Earth's surface, oxidation products of other minerals in the near surface weathering zone, andas accessory minerals in igneous rocks of the crust and mantle. Common oxides include hematite (iron oxide), magnetite (iron oxide), chromite (iron chromium oxide), spinel (magnesium aluminium oxide - a common component of the mantle), ilmenite (iron titanium oxide), rutile (titanium dioxide), and ice (hydrogen oxide). The oxide class includes the oxide and the hydroxide minerals.Sulfide classMany sulfide minerals are economically important as metal ores. Common sulfides include pyrite (iron sulfide - commonly known as fools' gold), chalcopyrite(copper iron sulfide), pentlandite(nickel iron sulfide), and galena(lead sulfide). The sulfide class also includes the selenides, the tellurides, the arsenides, the antimonides, the bismuthinides, and the sulfosalts (sulfur and a second anion such as arsenic).Phosphate classThe phosphate mineral group actually includes any mineral with a tetrahedral unit AO4where A can be phosphorus, antimony, arsenic or vanadium. By far the most common phosphate is apatite which is an important biological mineral found in teeth and bones of many animals. The phosphate class includes the phosphate, arsenate, vanadate, and antimonate minerals.Element classThe elemental group includes metals and intermetallic elements (gold, silver, copper), semi-metals and non-metals (antimony, bismuth, graphite, sulfur). This group also includes natural alloys, such as electrum(a natural alloy of gold and silver), phosphides, silicides, nitrides and carbides (which are usually only found naturally in a few rare meteorites).Organic classThe organic mineral class includes biogenic substances in which geological processes have been a part of the genesis or origin of the existing compound. Minerals of the organic class include various oxalates, mellitates, citrates, cyanates, acetates, formates, hydrocarbons and other miscellaneous species. Examples include whewellite, mellite, fichtelite, carpathite, evenkite and abelsonite.Vocabularymineralogy:矿物学,矿物成分crystalline:结晶质的homogeneous:均匀的,均质的,单相的,齐性的,其次的plagioclase:斜长石feldspars:长石,长石英albite:钠长石anorthite:钙长石mineraloids:似矿物,准矿物,胶质矿物geometric:几何的crystal lattice:晶格ionic:离子diffraction:衍射,绕射pyrite:黄铁矿marcasite:白铁矿,黄铁矿halite石盐,岩盐chlorine:氯,氯气galena:方铅矿sulfur:硫,自然硫periclase:方镁石magnesium:镁graphite:石墨,方晶石墨interlocking:嵌合,穿插,岩层交替,岩层咬合calcite:方解石quartz:石英chlorite:亚氯酸盐,氯泥石kaolin:高岭土,高岭石epidote:绿帘石augite:普通辉石hornblende:普通角闪石magnetite:磁铁矿hematite:赤铁矿,赤铁矿粉,赤血石limonite:褐铁矿tailings:尾渣,尾矿paragenesis:共生,共生组合,共生关系carbonic acid:碳酸subaerial:地表的,地面上的,陆上的kaolinite高岭石muscovite:白云母mafic:镁铁质的pyroxenes:辉石类amphiboles:角闪石,闪石类biotite:黑云母chlorite:亚氯酸盐,氯泥石rutile:金刚石metamorphism:变质(作用)gneiss:片麻岩equivocation:推诿、躲闪、说话支吾、含糊其词talc:滑石hardness:硬度Mohs scale:摩氏硬度标(计)Vitreous:玻璃质,玻璃状fluorite:萤石apatite:磷灰石orthoclase:正长石topaz:黄玉corundum:刚玉adamantine:坚硬无比的,金刚石制的,,冷铸钢粒cerussite:白铅矿anglesite:铅矾(硫酸铅矿)apophyllite:鱼眼石resinous:树脂的,沥青的sphalerite:(胶)闪锌矿,隐晶闪锌矿fibrous:纤维的,纤维状的chrysotile纤蛇纹石,温石棉translucent:半透明(的)iridescence:晕彩,晕色Labradorite:拉长石,富拉玄武岩,淡辉长岩streak:条痕,条纹,细脉,矿线,薄层,夹层cleavage(矿物)解理,(岩石)劈理chonchoidal fracturehackley :锯齿状Fibrous 纤维的,纤维状的fluorescence:荧光tenacity:韧性,粘滞性piezoelectricity:压电性,压电现象aragonite:文石siderite:菱铁矿,天蓝石,毒铁矿,普通角闪石,铁陨石类planktonic:浮游的,漂游的karst:喀斯特,岩溶stalactites:钟乳石,石钟乳,熔岩钟乳stalagmites:石笋,熔岩笋nitrate:硝酸盐,硝化,硝酸根,硝酸脂borate:硼酸盐hydrothermal:热液的,水热的anhydrite:硬石膏celestine:天青石barite:重晶石gypsum石膏chromate:铬酸盐molybdate:钼酸盐selenate:硒酸盐类sulfite:亚硫酸盐tellurate:碲酸盐tungstate:钨酸盐halite:石盐,岩盐halides:卤化物sylvite:钾石岩(钾盐)sal ammoniac:playa lakes:干盐湖,雨季糊bromide:溴化物iodide:碘化物,碘(根)chromite:铬铁矿spinel:尖晶石ilmenite:钛铁矿,铌钛矿titanium:钛chalcopyrite:黄铜矿pentlandite:镍黄铁矿selenides:硒化物类tellurides:碲化物arsenides:砷化物antimonides:锑化物bismuthinide:铋化物sulfosalts:硫盐,含硫盐,磺酸盐phosphate:磷酸盐phosphorus:磷antimony:锑,自然锑arsenic:砷,自然砷,砒霜vanadium:钒arsenate:砷酸盐vanadate:钒酸盐antimonate:锑酸盐intermetallic:金属间的bismuth:铋,自然铋electrum:银金矿,银矿,琥珀phosphides:磷化物silicides:硅化物nitrides:氮化物carbides:电石,碳化物,硬质合金片,碳钙石biogenic:生物起源的,生物成因的oxalates:草酸盐mellitates:citrates:柠檬酸盐cyanates:幅度,幅面,版式acetates:醋酸根,醋酸盐formates:安排形式,排列程序,格式,标组hydrocarbons:烃,碳氢化合物miscellaneous:混杂,综合,混合whewellite:水草酸钙石mellite:密蜡石fichtelite:白脂晶石,菲希特尔石carpathite:黄地蜡evenkite:鳞石蜡abelsonite:卟啉镍石。
