Chapter 2-Quantum mechanics
朗道十卷英文名

朗道十卷英文名朗道十卷是世界著名物理学教材。
朗道十卷英文名如下所示。
一、《朗道理论物理学教程第一卷:力学》Course of Theoretical Physics Volume one: Mechanics二、《朗道理论物理学教程第二卷:场论》Course of Theoretical Physics Volume two: The Theory of Fields三、《朗道理论物理学教程第三卷:量子力学(非相对论理论) 》Course of Theoretical Physics Volume three: Quantum Mechanics (Non-relativistic Theory)四、《朗道理论物理学教程第四卷:量子电动力学》Course of Theoretical Physics Volume four: Quantum Electrodynamics 五、《朗道理论物理学教程第五卷:统计物理学》Course of Theoretical Physics Volume five: Statistical Physics六、《朗道理论物理学教程第六卷:流体动力学》Course of Theoretical Physics Volume six: Fluid Mechanics七、《朗道理论物理学教程第七卷:弹性理论》Course of Theoretical Physics Volume seven: Theory of Elasticity八、《朗道理论物理学教程第八卷:连续介质电动力学》Course of Theoretical Physics Volume eight: Electrodynamics of Continuous Media九、《朗道理论物理学教程第九卷:统计物理学》Course of Theoretical Physics Volume nine: Statistical Physics十、《朗道理论物理学教程第十卷:物理动理学》Course of Theoretical Physics Volume ten: Physical Kinetics。
Chapter 2-3 态函数和Shrodinger方程(下)

的单色平面波的叠加:
⎡i ⎤ ϕ (Ρ) exp ⎢ (Ρ ⋅ r − Εt ) ⎥ d Ρ ∫ ⎣ ⎦
Quantum Mechanics ( I )
式中
Ρ2 Ε= 2m
。不难证明
i ∂Ψ 1 i = ϕ (Ρ)Ε exp[ (Ρ ⋅ r − Εt )]d Ρ 3/ 2 ∫ ∂t (2π ) i 1 2 2 2 − ∇ Ψ= ϕ (Ρ)Ρ exp[ (Ρ ⋅ r − Εt )]d Ρ 3/ 2 ∫ (2π )
ˆ ˆ H = H (t )
2.3 Quantum Mechanics ( I ) ※
Peking University
在后一种场合,我们必须用关系式
1 ⎡ e ⎤ H= ⎢ Ρ − c Α(r , t ) ⎥ + eΦ (r , t ) 2m ⎣ ⎦
2.3
2
由关系式(24),便得到如下波动方程:
但方程(22)不满足必须是时间的一阶微分 方程的要求,所以,如不对此重新作出物理 解释,就无法将其作为单粒子的波动方程接 受下来。事实上,一列波能够代表一个且仅 代表一个粒子的动力学状态这件事,只有在 非相对论极限下,亦即在满足粒子数守恒定 律的前提下,才是充分证实了的。一旦跨入 相对论领域,许多概念需要加以审慎地考察 与修正。
Peking University
Quantum Mechanics ( I )
(3). 因为波函数的变数是 r 和 t ,因此波动方程是 关于 r 和 t 的偏微分方程。我们可以要求该方程 不高于二阶,以便一旦初始条件和边界条件给定 后,方程能唯一地确定以后任何时刻的波函数。 因为根据数学物理方程中的斯图姆—刘维定理, 二阶正规的偏微分方程的解,存在唯一性定理成 立。 (4). 波动方程必须是对时间的一阶微分方程。只 有这样,一旦指明了初始时刻的Ψ ,则在以后时 间的变化才能被唯一确定。但是一阶(时间)微 分方程描述不可逆过程(如热传导、扩散方程) 无波动形式解,除非方程系数含虚数i,故要求为 复数Ψ 。
量子力学英文格里菲斯Chapter2PPT课件
Once we have found the separable solutions, then, we can immediately construct a much more general solution, of the form
It so happens that every solution to the (time dependent) Schrödinger equation can be written in this form — it is simply a matter of finding the right constants (c1, c2, c3, c4, …)so as to fit the initial conditions for the problem at hand.
4
Now the left side is a function of t alone, and the right side is a function of x alone.
5
The only way this can be possibly be true is if both sides are in fact constant, we shall call the separation constant E. Then
But before we get to that we would like to consider further the question:
7
What’s so great about separable solution ?
可分离的解(即 (x,t)=(x) f(t) )为何如此重要?
After all, most solutions to the (time-dependent)
Feynman 费曼物理学讲义英文版 2至5章

2Basic Physics2–1IntroductionIn this chapter, we shall examine the most fundamental ideas that we have about physics—the nature of things as we see them at the present time. We shall not discuss the history of how we know that all these ideas are true; you will learn these details in due time.The things with which we concern ourselves in science appear in myriad forms, and with a multitude of attributes. For example, if we stand on the shore and look at the sea, we see the water, the waves breaking, the foam, the sloshing motion of the water, the sound, the air, the winds and the clouds, the sun and the blue sky, and light; there is sand and there are rocks of various hardness and permanence, color and texture. There are animals and seaweed, hunger and disease, and the observer on the beach; there may be even happiness and thought. Any other spot in nature has a similar variety of things and influences. It is always as complicated as that, no matter where it is. Curiosity demands that we ask questions, that we try to put things together and try to understand this multitude of aspects as perhaps resulting from the action of a relatively small number of elemental things and forces acting in an infinite variety of combinations.For example: Is the sand other than the rocks? That is, is the sand perhaps nothing but a great number of very tiny stones? Is the moon a great rock? If we understood rocks, would we also understand the sand and the moon? Is the wind a sloshing of the air analogous to the sloshing motion of the water in the sea? What common features do different movements have? What is common to different kinds of sound? How many different colors are there? And so on. In this way we try gradually to analyze all things, to put together things which at first sight look different, with the hope that we may be able to reduce the number of different things and thereby understand them better.A few hundred years ago, a method was devised to find partial answers to such questions. Observation, reason, and experiment make up what we call the scientific method. We shall have to limit ourselves to a bare description of our basic view of what is sometimes called fundamental physics, or fundamental ideas which have arisen from the application of the scientific method.What do we mean by ―understanding‖ something? We can imagine that this complicated array of moving things which constitutes ―the world‖ is something like a great chess game being played by the gods, and we are observers of the game. We do not know what the rules of the game are; all we are allowed to do is to watch the playing. Of course, if we watch long enough, we may eventually catch on to a few of the rules. The rules of the game are what we mean by fundamental physics. Even if we knew every rule, however, we might not be able to understand why a particular move is made in the game, merely because it is too complicated and our minds are limited. If you play chess you must know that it is easy to learn all the rules, and yet it is often very hard to select the best move or to understand why a player moves as he does. Soit is in nature, only much more so; but we may be able at least to find all the rules. Actually, we do not have all the rules now. (Every once in a while something like castling is going on that we still do not understand.) Aside from not knowing all of the rules, what we really can explain in terms of those rules is very limited, because almost all situations are so enormously complicated that we cannot follow the plays of the game using the rules, much less tell what is going to happen next. We must, therefore, limit ourselves to the more basic question of the rules of the game. If we know the rules, we consider that we ―understand‖ the world.How can we tell whether the rules which we ―guess‖ at are really right if we cannot analyze the game very well? There are, roughly speaking, three ways. First, there may be situations where nature has arranged, or we arrange nature, to be simple and to have so few parts that we can predict exactly what will happen, and thus we can check how our rules work. (In one corner of the board there may be only a few chess pieces at work, and that we can figure out exactly.)A second good way to check rules is in terms of less specific rules derived from them. For example, the rule on the move of a bishop on a chessboard is that it moves only on the diagonal. One can deduce, no matter how many moves may be made, that a certain bishop will always be on a red square. So, without being able to follow the details, we c an always check our idea about the bishop’s motion by finding out whether it is always on a red square. Of course it will be, for a long time, until all of a sudden we find that it is on a black square (what happened of course, is that in the meantime it was captured, another pawn crossed for queening, and it turned into a bishop on a black square). That is the way it is in physics. For a long time we will have a rule that works excellently in an over-all way, even when we cannot follow the details, and then some time we may discover a new rule. From the point of view of basic physics, the most interesting phenomena are of course in the new places, the places where the rules do not work—not the places where they do work! That is the way in which we discover new rules.The third way to tell whether our ideas are right is relatively crude but probably the most powerful of them all. That is, by rough approximation. While we may not be able to tell why Alekhine moves this particular piece, perhaps we can roughly understand that he is gathering his pieces around the king to protect it, more or less, since that is the sensible thing to do in the circumstances. In the same way, we can often understand nature, more or less, without being able to see what every little piece is doing, in terms of our understanding of the game.At first the phenomena of nature were roughly divided into classes, like heat, electricity, mechanics, magnetism, properties of substances, chemical phenomena, light or optics, x-rays, nuclear physics, gravitation, meson phenomena, etc. However, the aim is to see complete nature as different aspects of one set of phenomena. That is the problem in basic theoretical physics, today—to find the laws behind experiment; to amalgamate these classes. Historically, we have always been able to amalgamate them, but as time goes on new things are found. We were amalgamating very well, when all of a sudden x-rays were found. Then we amalgamated some more, and mesons were found. Therefore, at any stage of the game, it always looks rathermessy. A great deal is amalgamated, but there are always many wires or threads hanging out in all directions. That is the situation today, which we shall try to describe.Some historic examples of amalgamation are the following. First, take heat and mechanics. When atoms are in motion, the more motion, the more heat the system contains, and so heat and all temperature effects can be represented by the laws of mechanics. Another tremendous amalgamation was the discovery of the relation between electricity, magnetism, and light, which were found to be different aspects of the same thing, which we call today the electromagnetic field. Another amalgamation is the unification of chemical phenomena, the various properties of various substances, and the behavior of atomic particles, which is in the quantum mechanics of chemistry.The question is, of course, is it going to be possible to amalgamate everything, and merely discover that this world represents different aspects of one thing? Nobody knows. All we know is that as we go along, we find that we can amalgamate pieces, and then we find some pieces that do not fit, and we keep trying to put the jigsaw puzzle together. Whether there are a finite number of pieces, and whether there is even a border to the puzzle, is of course unknown. It will never be known until we finish the picture, if ever. What we wish to do here is to see to what extent this amalgamation process has gone on, and what the situation is at present, in understanding basic phenomena in terms of the smallest set of principles. To express it in a simple manner, what are things made of and how few elements are there?2–2Physics before 1920It is a little difficult to begin at once with the present view, so we shall first see how things looked in about 1920 and then take a few things out of that picture. Before 1920, our world picture was something like this: The ―stage‖ on which the universe goes is the three-dimensional space of geometry, as described by Euclid, and things change in a medium called time. The elements on the stage are particles, for example the atoms, which have some properties. First, the property of inertia: if a particle is moving it keeps on going in the same direction unless forces act upon it. The second element, then, is forces, which were then thought to be of two varieties: First, an enormously complicated, detailed kind of interaction force which held the various atoms in different combinations in a complicated way, which determined whether salt would dissolve faster or slower when we raise the temperature. The other force that was known was a long-range interaction—a smooth and quiet attraction—which varied inversely as the square of the distance, and was called gravitation. This law was known and was very simple. Why things remain in motion when they are moving, or why there is a law of gravitation was, of course, not known.A description of nature is what we are concerned with here. From this point of view, then, a gas, and indeed all matter, is a myriad of moving particles. Thus many of the things we saw while standing at the seashore can immediately be connected. First the pressure: this comes from the collisions of the atoms with the walls or whatever; the drift of the atoms, if they are all moving in one direction on the average, is wind; the random internal motions are the heat. There are waves of excess density,where too many particles have collected, and so as they rush off they push up piles of particles farther out, and so on. This wave of excess density is sound. It is a tremendous achievement to be able to understand so much. Some of these things were described in the previous chapter.What kinds of particles are there? There were considered to be 92 at thattime: 92 different kinds of atoms were ultimately discovered. They had different names associated with their chemical properties.The next part of the problem was, what are the short-range forces? Why does carbon attract one oxygen or perhaps two oxygens, but not three oxygens? What is the machinery of interaction between atoms? Is it gravitation? The answer is no. Gravity is entirely too weak. But imagine a force analogous to gravity, varying inversely with the square of the distance, but enormously more powerful and having one difference. In gravity everything attracts everything else, but now imagine that there are two kinds of ―things,‖ and that this new force (which is the electrical force, of course) has the property that likes repel but unlikes attract. The ―thing‖ that carries this stron g interaction is called charge.Then what do we have? Suppose that we have two unlikes that attract each other, a plus and a minus, and that they stick very close together. Suppose we have another charge some distance away. Would it feel any attraction? It would feel practically none, because if the first two are equal in size, the attraction for the one and the repulsion for the other balance out. Therefore there is very little force at any appreciable distance. On the other hand, if we get very close with the extra charge, attraction arises, because the repulsion of likes and attraction of unlikes will tend to bring unlikes closer together and push likes farther apart. Then the repulsion will be less than the attraction. This is the reason why the atoms, which are constituted out of plus and minus electric charges, feel very little force when they are separated by appreciable distance (aside from gravity). When they come close together, they can ―see inside‖ each other and rearrange their charges, with the result that they have a very strong interaction. The ultimate basis of an interaction between the atoms is electrical. Since this force is so enormous, all the plusses and all minuses will normally come together in as intimate a combination as they can. All things, even ourselves, are made of fine-grained, enormously strongly interacting plus and minus parts, all neatly balanced out. Once in a while, by accident, we may rub off a few minuses or a few plusses (usually it is easier to rub off minuses), and in those circumstances we find the force of electricity unbalanced, and we can then see the effects of these electrical attractions.To give an idea of how much stronger electricity is than gravitation, consider two grains of sand, a millimeter across, thirty meters apart. If the force between them were not balanced, if everything attracted everything else instead of likes repelling, so that there were no cancellation, how much force would there be? There would be a force of three million tons between the two! You see, there is very, very little excess or deficit of the number of negative or positive charges necessary to produce appreciableelectrical effects. This is, of course, the reason why you cannot see the difference between an electrically charged or uncharged thing—so few particles are involved that they hardly make a difference in the weight or size of an object.With this picture the atoms were easier to understand. They were thought to have a ―nucleus‖ at the center, which is positively electrically charged and very massive, and the nucleus is surrounded by a certain number of ―electrons‖ which are very light and negatively charged. Now we go a little ahead in our story to remark that in the nucleus itself there were found two kinds of particles, protons and neutrons, almost of the same weight and very heavy. The protons are electrically charged and the neutrons are neutral. If we have an atom with six protons inside its nucleus, and this is surrounded by six electrons (the negative particles in the ordinary world of matter are all electrons, and these are very light compared with the protons and neutrons which make nuclei), this would be atom number six in the chemical table, and it is called carbon. Atom number eight is called oxygen, etc., because the chemical properties depend upon the electrons on the outside, and in fact only upon how many electrons there are. So the chemical properties of a substance depend only on a number, the number of electrons. (The whole list of elements of the chemists really could have been called 1, 2, 3, 4, 5, etc. Instead of saying ―carbon,‖ we could say ―element six,‖ meaning six electrons, but of course, when the elements were first discovered, it was not known that they could be numbered that way, and secondly, it would make everything look rather complicated. It is better to have names and symbols for these things, rather than to call everything by number.)More was discovered about the electrical force. The natural interpretation of electrical interaction is that two objects simply attract each other: plus against minus. However, this was discovered to be an inadequate idea to represent it. A more adequate representation of the situation is to say that the existence of the positive charge, in some sense, distorts, or creates a ―condition‖ in space, so that when we put the negative charge in, it feels a force. This potentiality for producing a force is called an electric field. When we put an electron in an electric field, we say it is ―pulled.‖ We then have two rules: (a) charges make a field, and (b) charges in fields have forces on them and move. The reason for this will become clear when we discuss the following phenomena: If we were to charge a body, say a comb, electrically, and then place a charged piece of paper at a distance and move the comb back and forth, the paper will respond by always pointing to the comb. If we shake it faster, it will be discovered that the paper is a little behind, there is a delay in the action. (At the first stage, when we move the comb rather slowly, we find a complication which is magnetism. Magnetic influences have to do with charges in relative motion, so magnetic forces and electric forces can really be attributed to one field, as two different aspects of exactly the same thing. A changing electric field cannot exist without magnetism.) If we move the charged paper farther out, the delay is greater. Then an interesting thing is observed. Although the forces between two charged objects should go inversely as the square of the distance, it is found, when we shake acharge, that the influence extends very much farther out than we would guess at first sight. That is, the effect falls off more slowly than the inverse square.Here is an analogy: If we are in a pool of water and there is a floating cork very close by, we can move it ―directly‖ by pushing the water with another cork. If you looked only at the two corks, all you would see would be that one moved immediately in response to the motion of the other—there is some kind of ―interaction‖ between them. Of course, what we really do is to disturb the water; the water then disturbs the other cork. We could make up a ―law‖ that if you pushed the water a little bit, an object close by in the water would move. If it were farther away, of course, the second cork would scarcely move, for we move the water locally. On the other hand, if we jiggle the cork a new phenomenon is involved, in which the motion of the water moves the water there, etc., and waves travel away, so that by jiggling, there is an influence very much farther out, an oscillatory influence, that cannot be understood from the direct interaction. Therefore the idea of direct interaction must be replaced with the existence of the water, or in the electrical case, with what we call the electromagnetic field.The electromagnetic field can carry waves; some of these waves are light, others are used in radio broadcasts, but the general name is electromagnetic waves. These oscillatory waves can have various frequencies. The only thing that is really different from one wave to another is the frequency of oscillation. If we shake a charge back and forth more and more rapidly, and look at the effects, we get a whole series of different kinds of effects, which are all unified by specifying but one number, the number of oscillations per second. The usual ―pickup‖ that we get from electric currents in the circuits in the walls of a building have a frequency of about onehundred cycles per second. If we increase the frequency to 500 or 1000 kilocycles (1 kilocycle=1000cycles) per second, we are ―on the air,‖ for this is the frequency range which is used for radio broadcasts. (Of course it has nothing to do with the air! We can have radio broadcasts without any air.) If we again increase the frequency, we come into the range that is used for FM and TV. Going still further, we use certain short waves, for example for radar. Still higher, and we do not need an instrument to ―see‖ the stuff, we can see it with the human eye. In the range offrequency from 5×1014 to 1015 cycles per second our eyes would see the oscillation of the charged comb, if we could shake it that fast, as red, blue, or violet light, depending on the frequency. Frequencies below this range are called infrared, and above it, ultraviolet. The fact that we can see in a particular frequency range makes that part of the electromagnetic spectrum no more impressive than the other parts from a physicist’s standpoint, but from a human standpoint, of course, it is more interesting. If we go up even higher in frequency, we get x-rays. X-rays are nothing but very high-frequency light. If we go still higher, we get gamma rays. These two terms, x-rays and gamma rays, are used almost synonymously. Usually electromagnetic rays coming from nuclei are called gamma rays, while those of high102 Electrical disturbanceField 5×105 – 106 Radio broadcastWaves 108 FM —TV 1010 Radar5×1014 – 1015 Light1018X-rays Particle 1021γ-rays, nuclear 1024γ-rays, ―artificial‖ 1027 γ-rays, in cosmic rays2–3Quantum physicsHaving described the idea of the electromagnetic field, and that this field cancarry waves, we soon learn that these waves actually behave in a strange way whichseems very unwavelike. At higher frequencies they behave much morelike particles! It is quantum mechanics , discovered just after 1920, which explainsthis strange behavior. In the years before 1920, the picture of space as athree-dimensional space, and of time as a separate thing, was changed by Einstein,first into a combination which we call space-time, and then still further intoa curved space-time to represent gravitation. So the ―stage‖ is changed intospace-time, and gravitation is presumably a modification of space-time. Then it wasalso found that the rules for the motions of particles were incorrect. The mechanicalrules of ―inertia‖ and ―forces‖ are wrong —Newton’s laws are wrong —in the world ofatoms. Instead, it was discovered that things on a small scale behave nothinglike things on a large scale. That is what makes physics difficult —and very interesting.It is hard because the way things behave on a small scale is so ―unnatural‖; we haveno direct experience with it. Here things behave like nothing we know of, so that it isimpossible to describe this behavior in any other than analytic ways. It is difficult, andtakes a lot of imagination.Quantum mechanics has many aspects. In the first place, the idea that a particlehas a definite location and a definite speed is no longer allowed; that is wrong. Togive an example of how wrong classical physics is, there is a rule in quantummechanics that says that one cannot know both where something is and how fast it ismoving. The uncertainty of the momentum and the uncertainty of the position are complementary, and the product of the two is bounded by a small constant. We canwrite the law like this: ΔxΔp≥ℏ/2, but we shall explain it in more detail later. This rule is the explanation of a very mysterious paradox: if the atoms are made out of plus and minus charges, why don’t the minus charges simply sit on top of the plus charges (they attract each other) and get so close as to completely cancel them out? Why are atoms so big? Why is the nucleus at the center with the electrons around it? It was first thought that this was because the nucleus was so big; but no, the nucleus is verysmall. An atom has a diameter of about 10−8 cm. The nucleus has a diameter ofabout 10−13 cm. If we had an atom and wished to see the nucleus, we would have to magnify it until the whole atom was the size of a large room, and then the nucleus would be a bare speck which you could just about make out with the eye, but very nearly all the weight of the atom is in that infinitesimal nucleus. What keeps the electrons from simply falling in? This principle: If they were in the nucleus, we would know their position precisely, and the uncertainty principle would then require that they have a very large (but uncertain) momentum, i.e., a very large kinetic energy. With this energy they would break away from the nucleus. They make a compromise: they leave themselves a little room for this uncertainty and then jiggle with a certain amount of minimum motion in accordance with this rule. (Remember that when a crystal is cooled to absolute zero, we said that the atoms do not stop moving, they still jiggle. Why? If they stopped moving, we would know where they were and that they had zero motion, and that is against the uncertainty principle. We cannot know where they are and how fast they are moving, so they must be continually wiggling in there!) Another most interesting change in the ideas and philosophy of science brought about by quantum mechanics is this: it is not possible to predict exactly what will happen in any circumstance. For example, it is possible to arrange an atom which is ready to emit light, and we can measure when it has emitted light by picking up a photon particle, which we shall describe shortly. We cannot, however, predict when it is going to emit the light or, with several atoms, which one is going to. You may say that this is because there are some internal ―wheels‖ which we have not looked at closely enough. No, there are no internal wheels; nature, as we understand it today, behaves in such a way that it is fundamentally impossible to make a precise prediction of exactly what will happen in a given experiment. This is a horrible thing; in fact, philosophers have said before that one of the fundamental requisites of science is that whenever you set up the same conditions, the same thing must happen. This is simply not true, it is not a fundamental condition of science. The fact is that the same thing does not happen, that we can find only an average, statistically, as to what happens. Nevertheless, science has not completely collapsed. Philosophers, incidentally, say a great deal about what is absolutely necessary for science, and it is always, so far as one can see, rather naive, and probably wrong. For example, some philosopher or other said it is fundamental to the scientific effort that if an experimentis performed in, say, Stockholm, and then the same experiment is done in, say, Quito, the same results must occur. That is quite false. It is not necessary that science do that; it may be a fact of experience, but it is not necessary. For example, if one of the experiments is to look out at the sky and see the aurora borealis in Stockholm, you do not see it in Quito; that is a different phenomenon. ―But,‖ you say, ―that is something that has to do with the outside; can you close yourself up in a box in Stockholm and pull down the shade and get any difference?‖ Surely. If we take a pendulum on a universal joint, and pull it out and let go, then the pendulum will swing almost in a plane, but not quite. Slowly the plane keeps changing in Stockholm, but not in Quito. The blinds are down, too. The fact that this happened does not bring on the destruction of science. What is the fundamental hypothesis of science, the fundamental philosophy? We stated it in the first chapter: the sole test of the validity of any idea is experiment. If it turns out that most experiments work out the same in Quito as they do in Stockholm, then those ―most experiments‖ will be used to formulate some general law, and those experiments which do not come out the same we will say were a result of the environment near Stockholm. We will invent some way to summarize the results of the experiment, and we do not have to be told ahead of time what this way will look like. If we are told that the same experiment will always produce the same result, that is all very well, but if when we try it, it does not, then it does not. We just have to take what we see, and then formulate all the rest of our ideas in terms of our actual experience.Returning again to quantum mechanics and fundamental physics, we cannot go into details of the quantum-mechanical principles at this time, of course, because these are rather difficult to understand. We shall assume that they are there, and go on to describe what some of the consequences are. One of the consequences is that things which we used to consider as waves also behave like particles, and particles behave like waves; in fact everything behaves the same way. There is no distinction between a wave and a particle. So quantum mechanics unifies the idea of the field and its waves, and the particles, all into one. Now it is true that when the frequency is low, the field aspect of the phenomenon is more evident, or more useful as an approximate description in terms of everyday experiences. But as the frequency increases, the particle aspects of the phenomenon become more evident with the equipment with which we usually make the measurements. In fact, although we mentioned many frequencies, no phenomenon directly involving a frequency has yet been detected above approximately 1012 cycles per second. We only deduce the higher frequencies from the energy of the particles, by a rule which assumes that the particle-wave idea of quantum mechanics is valid.Thus we have a new view of electromagnetic interaction. We have a new kind of particle to add to the electron, the proton, and the neutron. That new particle is called a photon. The new view of the interaction of electrons and photons that is electromagnetic theory, but with everything quantum-mechanically correct, is called quantum electrodynamics. This fundamental theory of the interaction of light and matter, or electric field and charges, is our greatest success so far in physics. In。
北京大学物理系英语教材

北京大学物理系英语教材Beijing University Physics Department English TextbookIntroduction:The Beijing University Physics Department English textbook is designed to be a comprehensive resource for students studying physics in English at Beijing University. This textbook aims to provide students with a solid foundation in the principles and concepts of physics while simultaneously improving their English language skills. The content of this textbook has been carefully selected to ensure an effective and efficient learning experience for students.Chapter 1: Introduction to PhysicsIn this chapter, students will be introduced to the fundamental concepts of physics. They will learn about the scientific method, units and measurements, and the basic principles that govern the behavior of matter and energy. Through interactive exercises and real-life examples, students will develop a strong understanding of the key principles that form the building blocks of physics.Chapter 2: MechanicsThe study of mechanics is essential in understanding the motion of objects. This chapter delves into the laws of motion, including Newton's laws, kinematics, and dynamics. Students will explore various types of motion, such as linear, circular, and rotational motion. Through practicalexamples and problem-solving exercises, students will gain a deeper understanding of how forces and motion interact.Chapter 3: ThermodynamicsThermodynamics is the study of heat and energy transfer. In this chapter, students will learn about the laws of thermodynamics and their applications. Concepts such as temperature, heat, entropy, and energy conservation will be explored in detail. Students will also be introduced to various thermodynamic processes and cycles, enabling them to analyze and solve problems related to energy transfer.Chapter 4: ElectromagnetismThis chapter focuses on the principles of electromagnetism. Students will learn about electric fields, magnetic fields, and the relationship between them. They will explore topics such as electromagnetic induction, electric circuits, and the behavior of charged particles in magnetic fields. The chapter also covers important concepts like Gauss's law, Ampere's law, and Faraday's law.Chapter 5: OpticsOptics is the branch of physics that deals with the behavior of light. In this chapter, students will study the properties of light, including reflection, refraction, and diffraction. They will explore the principles of geometric optics and learn about the formation of images by mirrors and lenses. The chapter also covers topics such as interference, polarization, and the wave nature of light.Chapter 6: Modern PhysicsThe final chapter of the textbook introduces students to the fascinating world of modern physics. They will explore topics such as quantum mechanics, the theory of relativity, and the structure of atoms. Students will gain insights into the behavior of particles at the atomic and subatomic levels, and understand the fundamental principles that govern the physical world.Conclusion:The Beijing University Physics Department English textbook provides students with a comprehensive and accessible guide to physics in English. By combining the principles of physics with English language learning, this textbook aims to enhance students' understanding of physics while improving their language skills. With its clear explanations, engaging examples, and challenging exercises, this textbook is a valuable resource for students at Beijing University and beyond.。
数学专业英语第二版的课文翻译

2-A Why study geometry Many leading institutions of higher learning have recognized that positive benefits can be gained by all who study this branch of mathematics. This is evident from the fact that they require study of geometry as a prerequisite to matriculation in those schools. 许多居于领导地位的学术机构承认,所有学习这个数学分支的人都将得到确实的受益,许多学校把几何的学习作为入学考试的先决条件,从这一点上可以证明。
Geometry had its origin long ago in the measurement by the Babylonians and Egyptians of their lands inundated by the floods of the Nile River. The greek word geometry is derived from geo, meaning “earth” and metron, meaning “measure” . As early as 2000 . we find the land surveyors of these people re-establishing vanishing landmarks and boundaries by utilizing the truths of geometry . 几何学起源于很久以前巴比伦人和埃及人测量他们被尼罗河洪水淹没的土地,希腊语几何来源于geo ,意思是”土地“,和metron 意思是”测量“。
费曼物理学讲义 英文版
费曼物理学讲义英文版Richard Feynman's Lectures on Physics - English VersionIntroduction:Richard Feynman, a renowned physicist, was known for his exceptional ability to explain complex scientific concepts in a simple and engaging manner. His lectures on physics have been compiled into a series of books known as "Feynman Lectures on Physics." This article will provide an overview of the English version of his lectures, highlighting their significance and impact on the field of physics.1. Historical Background:The English version of "Feynman Lectures on Physics" was first published in 1964, and it quickly gained popularity among both students and professionals in the field. Feynman's unique teaching style, coupled with his deep understanding of physics, made these lectures a valuable resource for anyone interested in learning the subject.2. Content and Structure:The lectures are divided into three volumes, covering various topics in physics. Volume 1 focuses on mechanics, radiation, and heat. Volume 2 delves into electromagnetism and matter, while Volume 3 explores quantum mechanics. Each volume is further divided into chapters, addressing specific concepts and theories.3. Clarity and Accessibility:One of the key strengths of the English version of Feynman's lectures is its clarity and accessibility. Feynman believed in simplifying complex ideas without sacrificing their essence. He used analogies, real-life examples, and vivid illustrations to make the concepts easily understandable for readers. This approach makes the lectures accessible to a wide range of audiences, from students with little background in physics to seasoned professionals.4. Practical Applications:Feynman's lectures not only provide a solid foundation in theoretical physics but also emphasize the practical applications of the concepts. He often includes real-life examples and experiments to illustrate how physics is relevant in everyday life. This approach helps readers grasp the significance of the theories and encourages them to apply their knowledge to solve practical problems.5. Pedagogical Approach:Feynman's pedagogical approach in the English version of his lectures is highly effective. He encourages active learning by incorporating exercises and problems at the end of each chapter. These exercises allow readers to test their understanding and reinforce their knowledge. Additionally, Feynman includes historical background and anecdotes, making the lectures engaging and enjoyable to read.6. Influence and Legacy:The English version of "Feynman Lectures on Physics" has had a profound impact on the field of physics. It has inspired countless students and researchers, shaping their understanding of the subject. Feynman's teaching style, which emphasizes conceptual understanding and intuition, has become a model for science education. Many universities and educational institutions still use these lectures as a reference for teaching physics.7. Criticisms and Limitations:While the English version of Feynman's lectures is highly regarded, some critics argue that it may not cover all the topics in sufficient depth. As Feynman himself admitted, the lectures were initially designed for undergraduate students and may not fully satisfy the needs of advanced researchers. However, the lectures serve as an excellent starting point for further exploration and provide a solid conceptual foundation.Conclusion:The English version of "Feynman Lectures on Physics" is a valuable resource for anyone interested in understanding the fundamental principles of physics. Richard Feynman's ability to simplify complex concepts, coupled with his engaging teaching style, makes these lectures accessible and enjoyable. Whether you are a student, a professional, or simply curious about the wonders of the universe, these lectures are a must-read.。
量子力学答案
Quantum Mechanics Chapter22.1 a ) the energy eigenvalues ofelectron is 22222n n E maπ= The energy of the electron in the ground state is 2212382E eV maπ== Thenthenergy of the electron in the excited state is222241522E eV maπ== b) Because the electron is confined in the ground state in a one-dimensionalbox of width 1010m -the ground state of electron is 1x aπψ=1010a m -= so when 0x = or 1010x -=, 10ψ= , 210ψ=there is not an electron at 0x = or 1010x -=, so no force on the walls of thebox, the average force on walls is zero .2.2 a)the dimensional particle ′s energy equation should be :()()()p p r r E r ψψ∧H =()()()()222222ipx ipxp p p p p x x x x m m ψψψ∧∧H ====2()()2p p p E r r mψψ= ()()()p p x x E x ψψ∧∴H = So it has proved the question . b) the specific form of (),p x t ψis ()2222,i p ipx ip tipx t mmp x t e ee ψ--=∙=then we can verify ()(),0ipxp p x e x ψψ= is tight.2.3 a)()21aa x dx ψ-=⎰ then , 22sin 1aax A dx a π-⎛⎫= ⎪⎝⎭⎰so A =b) ()x x a πψ⎛⎫=⎪⎝⎭ then , ()'0x x a πψ⎛⎫== ⎪⎝⎭so, we can kown2xk aπππ=+ k z ∈,a x a -≤≤ 2a x ∴=±c) ()2222011sin 4aa x x dx dx a a πψ⎛⎫== ⎪⎝⎭⎰⎰ d)()()21sin 0aaaax x x x x dx x dx a a πψψ-*--⎛⎫=== ⎪⎝⎭⎰⎰0x x a πψ--⎛⎫⎛⎫⎪∴== ⎪ ⎪⎝⎭⎝⎭∴the probability density of finding the particle at this particular value of x -is 20x ψ-⎛⎫= ⎪⎝⎭.2.4 a) ()22y y Aeψ-= and 12m y x ω⎛⎫= ⎪⎝⎭()22m x x Aeωψ-∴= Accoding to ()()x x ψψ∧H =EThen , ()()()22222122d x m x x x m dx ψωψψ-+=E ()()()()222222122m m x x m x x x m ωωψψωψψ⎛⎫∴--++=E ⎪⎝⎭12ω∴E =b) ()21x dx ψ+∞-∞=⎰221m x A edx ω+∞--∞∴=⎰then ,1A= so, 14m A ωπ⎛⎫= ⎪⎝⎭we get the ()x ψ is ()2142m x m x eωωψπ-⎛⎫= ⎪⎝⎭c) ()()1222222m x m x x x x dx x edx m ωωψψπω+∞+∞---*-∞-∞⎛⎫===⎪⎝⎭⎰⎰so the average potential energy :()()221124V x m x x dx ψωψω+∞-*-∂∞==⎰d) (i) we can take the hydrogen atom as the linear harmonic oscillator . the energy eigenvalues of the harmonic oscillator is 12n n ω⎛⎫E =+ ⎪⎝⎭0,1,2,3n =If 0n =,then the associated zero-point energyIs 210115.271022v J ω-E ===⨯ (ii) according to (C), 2223.151022x m m mvω--===⨯2.5 Before the well expands to 2a suddenly, the ground state with energy 22122ma πE =and the wave function is ()()1,0xx x aπψψ==When the well expands to 2a , the energy eigenvalue and eigenstate are22228n n ma πε= and ()2n n xx aπϕ= Because the well expands so fast that the wave function could notchange, then , 1n ε=E , we get the value of n , 2n = Expand the wave function (),0x ψ with ()n x ϕ : ()(),0nnnx c x ψϕ=∑ and2n =.So ()()22202aax c x x dx dx a πϕψ*===⎰ The probability to obtain the value of 1E is 2212c =.。
《结构化学》教学大纲(英文版)
‘Structural Chemistry ’Course SyllabusCourse Code:09040001Course Category:Major BasicMajors:ChemistrySemester:SpringTotal Hours:54 Hours Credit:3Lecture Hours:54 HoursTextbooks:《Structural Chemistry》孙墨珑编著,东北林业大学出版社。
I.Introduction to Structural ChemistryThe major targets this course includes the followings: (1) to introduce the material structure of the basic concepts, basic theory, and basic methods for learning “Structural Chemistry”; (2) to explore the relationship between the microstructures and properties of atoms, molecules, and crystals; (3) to systematically clarify the essence of the periodic law of elements; (4) to deeply and qualitatively clarify the essence of the chemical bonds. This course introduces the basic principles of quantum mechanics and their applications in simple systems, structure of atoms, molecules, and crystals, symmetry of molecular orbitals, molecular orbital theory, and ligand field theory, etc. After learning this course, the students should be able to analyze and solve the basic chemistry problems from the point of view of quantum mechanics.II.Table of contentsSection I (Chapter 1) Basic knowledge of quantum mechanics1.1 Failures of classical mechanics1)Black-body radiation & Planck’s solution;2)Ph otoelectric effect & Einstein’s theory;3)Hydrogen spectrum & Bohr’s model.1.2Characteristics of the motion of microscopic particles1)Wave-particle duality;2)Uncertainty principle.1.3The basic postulates of quantum mechanics1)Postulate 1: wavefunction;2)Postulate 2: Hermitian operators;3)Postulate 3: Schrödinger equation;4)Postulate 4: linearity and superposition;5)Postulate 5: Pauli exclusion principle.1.4Applications of quantum mechanics in simple cases1)Free particle in one-dimensional (1D) box;2)Applications of the 1D-box model in simple chemical systems;3)Free particle in two-dimensional (2D) & three-dimensional (3D) box;4)Tunneling & scanning tunneling microscopy (STM).Section II (Chapter 2) Structures and properties of atoms2.1 One-electron atom: H atom1)The Schrödinger equation of H atoms;2)Solution of the Schrödinger equation of H atom.2.2Quantum numbers1)Principle quantum number, n;2)Angular momentum quantum number, l;3)Magnetic quantum number, m;4)Zeeman effect.2.3Wavefunction and electron cloud1)Radial distribution;2)Angular distribution;3)Spatial distribution.2.4 Structure of multi-electron atoms1)The Schrödinger equation of multi-electron atoms•Self-consistent field method;•Central field approximation.2)The building-up principles and electron configuration of multi-electron atoms•Pauli exclusion principle;•Principle of minimum energy;Hund’s rule.2.5Electron spin and Pauli exclusion principle2.6Atomic spectroscopy1)Orbital-spin coupling;2)Spectroscopic terms & term symbol;3)Derivation of atomic term.4)Hund’s rule on the spectroscopic terms;2.7Atomic properties1)Energy of ionization;2)Electron affinity;3)Electronegativity.Section III (Chapters 3-6) Structures and properties of molecules Chapter 3 Geometric structure of molecules─Molecular symmetry & symmetry point group3.1Symmetry elements and symmetry operations1)Symmetry elements and symmetry operations;2)Combination rules of symmetry elements;3.2Point groups & symmetry classification of molecules3.3Point groups & groups multiplication3.4Applications of molecular symmetry1)Chirality & optical activity;2)Polarity & dipole moment.Chapter 4 S tructure of biatomic molecules (X2 & XY)4.1 Linear variation method and structure of H2+ ion1) Shrödinger equation of H2+ ion;2) Linear variation method;3) Treatment of H2+ ion using linear variation method;4) Solutions of H2+ ion.4.2 Molecular orbital theory and diatomic molecules1) Molecular orbital theory;2) Structure of homonuclear diatomic molecules (X2);3) Structure of heteronuclear diatomic molecules (XY).4.3 Valence bond (VB) theory and H2 moleculeChapter 5 Structure of polyatomic molecules (A)5.1 Structure of Methane (CH4)1) Delocalized molecular orbitals of methane (CH4);2) Localized molecular orbitals of methane (CH4).5.2 Molecular orbital hybridization1) Theory of molecular orbital hybridization;2) Construction of hybrid orbitals;3) Structure of AB n molecules;4) Molecular stereochemistry: valence shell electron-pair repulsion (VSEPR)model.5.3 Delocalized molecular orbital theory─Hückel molecular orbital (HMO) theory1) HMO method & conjugated systems;2) HMO treatment for butadiene;3) HMO treatment for cyclic conjugated polyene (C n H n);4) Molecular diagrams;5) Delocalized π bonds.5.4 Structure of electron deficient molecules5.5 Symmetry of molecular orbitals and symmetry rules for molecular reactions5.6 Molecular spectroscopy1)Infrared absorption spectroscopy: molecular vibrations;2)Raman scattering spectroscopy: molecular vibrations;3)Fluorescence spectroscopy: electronic transitions;4)NMR spectroscopy: nuclear magnetic resonances.Chapter 6 Structure of polyatomic molecules (B), coordination compounds 6.1 Crystal field theory6.2 CO and N2 coordination complexes6.3 Organic metal complexes1) Zeise’s salts;2) Sandwich complexes.6.4 Clusters1) Transition-metal cluster compounds2) Carbon clusters and nanotubesSection IV (Chapters 7-9) Structure of crystalsChapter 7 Basics of crystallography7.1 Periodicity and lattices of crystal structure1) Characteristics of crystal structure;2) Lattices and unit cells;3) Bravais lattices and unit cells of crystals;4) Real crystals & crystal defects.7.2 Symmetry in crystal structure1) Symmetry elements and symmetry operations;2) Point groups (32) and space groups (230).7.3 X-Ray diffraction of crystals1) X-ray diffraction of crystals•Laue equation;•Bragg’s law;•Reciprocal lattice.2) Instrumentation of X-ray diffraction;3) Applications of X-Ray diffraction•Single crystal diffraction: crystal structure determination;•Powder diffraction: qualitative & quantitative analysis of crystalline materialsChapter 8 Crystalline solids, I: metals and alloys8.1 Close Packing of Spheres1) Close packing of identical spheres;2) Packing density;3) Interstices.8.2 Structures and Properties of Pure Metals8.3 Structures and Properties of AlloyChapter 9 Crystalline solids, II: ionic crystals9.1 Packing of Ions;9.2 Crystal Structure of Some Typical Ionic Compounds9.3 Trend of Variation of Ionic Radii9.4 Pauling Rule of Ionic Crystal Structure9.5 Crystals of Functional Materials1) Nonlinear optical materials;2) Magnetic materials;3) Conductive polymers;4) Semiconductors: band gap and photocatalysisIII.Table of ScheduleReferences[1] 王荣顺主编,东北师范大学等,《结构化学》,高等教育出版社,2003年。
Sakurai. Modern Quantumn Mechanics 习题答案(chapter 1 )
3
∞
< x 2 >=
−∞
2 ∫ dx' < α | x' > x' < x'| α >
y = x '−< x > ∞
=
−∞
∫ dy < α | y + < x >> ( y + < x >)
2
< y + < x >| α >
= d 2 + < x >2 < (∆x) 2 >= d 2 Also : h2 < (∆p) >= 4d 2
^
^
h ⎛ cos γ ⎜ 2⎜ ⎝ sin γ
sin γ ⎞ ⎟ − cos γ ⎟ ⎠
⎛ c1 ⎞
h ⎛ cos γ ⎜ 2⎜ ⎝ sirγ
(1) 、求: S x = 解答: S x =
γ⎞ ⎛ ⎜ cos ⎟ sirγ ⎞⎛ c1 ⎞ h ⎛ c1 ⎞ 2⎟ ⎟⎜ ⎜c ⎟ ⎟ ⇒ψ = ⎜ ⎜c ⎟ ⎟ = 2⎜ γ − cpsγ ⎟ ⎜ ⎠⎝ 2 ⎠ ⎝ 2⎠ ⎜ sin ⎟ ⎟ 2⎠ ⎝
⎛ ⎜ ⎜ ⎜ 当 A‘=-a 时,对应 B’=b,要求α=0,γ=iβ,取归一化得 − a, b = ⎜ ⎜ ⎜i ⎜ ⎝ ⎞ ⎟ 0 ⎟ 1 ⎟ ⎟ 2 ⎟ 1 ⎟ ⎟ 2⎠
5
⎛ ⎞ ⎜ ⎟ ⎜ 0 ⎟ ⎜ 1 ⎟ 当 A‘=-a 时,对应 B‘=-b,要求α=0,γ=—iβ,取归一化得 − a,−b = ⎜ ⎟ ⎜ 2 ⎟ ⎜− i 1 ⎟ ⎜ ⎟ 2⎠ ⎝
4
⎛α ⎞ ⎜ ⎟ (3) 、解答:因为 A,B 对易,所以有共同本征态,设其共同本征态为 ⎜ β ⎟ ,本征值为 A`, ⎜γ ⎟ ⎝ ⎠
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
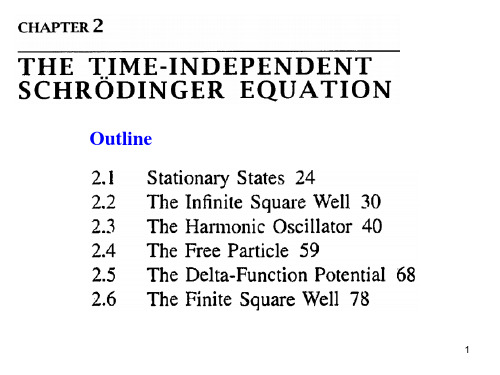
量子力学与统计物理(Quantum mechanics and Statistical Physics)黄建明电子工程学院北京邮电大学,北京100876北京邮电大学北京1008761J. –M. Huang目录第章量子力学的诞生第一章第二章波函数和Schrodinger 方程第三章一维定态问题第四章量子力学中的力学量第五章态和力学量表象第六章近似方法第七章量子跃迁第八章自旋与全同粒子2J. –M. Huang第二章波函数第章波数Sc od ge和Schrodinger 方程§1z 1 波函数的统计解释z§2 态叠加原理3z§3 力学量的平均值和算符的引进z§4 Schrodinger 方程z§5 粒子流密度和粒子数守恒定律z§6 定态Schrodinger方程3J. –M. Huang§1 波函数的统计解释(一)波函数(二)波函数的解释(三)波函数的性质4J. –M. Huang(2) ψ如何体现波粒二象性的?(3) ψ描写的是什么样的波呢?5J. –M. HuangP P电子源感OO光屏(1)两种错误的看法1. 波由粒子组成QQ如水波,声波,由分子密度疏密变化而形成的一种分布。
这种看法是与实验矛盾的,它不能解释长时间单个电子衍射实验。
电子一个一个的通过小孔,但只要时间足够长,底片上增加呈现出衍射花纹。
这说明电子的波动性并不是许多电子在空间聚集在一起时才有的现象一起时才有的现象,单个电子就具有波动性。
事实上,正是由于单个电子具有波动性,才能理解氢原子(只含一个电子!)中电子运动的稳定性以及能量量子化这样一波由粒子组成的看法夸大了粒子性的一面,而抹杀了粒子的波动性的一面,具有片面性。
些量子现象。
6J. –M. Huang,2. 粒子由波组成z电子是波包。
把电子波看成是电子的某种实际结构,是三维空间中。
涉。
连续分布的某种物质波包。
因此呈现出干涉和衍射等波动现象。
波包的大小即电子的大小,波包的群速度即电子的运动速度。
z什么是波包?波包是各种波数(长)平面波的迭加。
平面波描写自由粒子,其特点是充满整个空间,这是因为平面波振幅与位置无关。
由粒子其特点是充满整个空间这是因为平面波振幅与位置无关如果粒子由波组成,那么自由粒子将充满整个空间,这是没有意义的,与实验事实相矛盾。
zz实验上观测到的电子,总是处于一个小区域内。
例如在一个原子内,其广延不会超过原子大小≈1Å。
z电子究竟是什么东西呢?是粒子?还是波?“电子既不是粒子也不是波”,既不是经典的粒子也不是经典的波,但是我们也可以说,电子既是粒子也是波,它是粒子和波动二重性矛盾的可以说“电子既是粒子也是波它是粒子和波动二重性矛盾的统一。
”这个波不再是经典概念的波,粒子也不是经典概念中的粒子。
7J. –M. Huang经典概念中 1.有一定质量、电荷等“颗粒性”的属性; 粒子意味着2.有确定的运动轨道,每一时刻有一定位置和速度。
1实在的物理量的空间分布作周期性的变化;经典概念中1.实在的物理量的空间分布作周期性的变化; 波意味着2.干涉、衍射现象,即相干叠加性。
1.入射电子流强度小,开始显示电子的微粒性,长时间亦显示衍射电子的衍射实验:图样;2.入射电子流强度大,很快显示衍射图样.P P电子源感光QQ O8J. –M. Huang 屏结论:z衍射实验所揭示的电子的波动性是:许多电子在同一个实验中的统计结果,或者是一个电子在许多次相同实验中的统计结果。
z波函数正是为了描述粒子的这种行为而引进的,在此基础上,Born提出了波函数意义的统计解释。
在电子衍射实验中,照相底片上r点附近衍射花样的强度∼正比于该点附近感光点的数目该点附近感光点的数目,∼正比于该点附近出现的电子数目,∼正比于电子出现在r点附近的几率。
点附近的几率9J. –M. Huang玻恩“概率波”说(1954年诺贝尔奖)10J. –M. HuangΨ(r)描述,与光学相似,衍射花纹的强假设衍射波波幅用描述与光学相似衍射花纹的强度则用|Ψ(r)|2描述,但意义与经典波不同。
|Ψ(r)|2的意义是代表电子出现在r点附近几率的大小,确切的说,|Ψ(r)|2ΔxΔyΔz表示在r点处,体积元ΔxΔyΔz中找到粒子的几率。
波函数在空间某点的强度(振幅绝对值的平方)和在这点找到粒子的几率成比例。
据此,描写粒子的波可以认为是几率波,反映微观客体运动的一种统计规律性,波函数Ψ(r)有时也称为几率幅。
这动的种统计规律性波函数()有时也称为几率幅就是首先由Born提出的波函数的几率解释,它是量子力学的基本原理。
11J. –M. Huang(三)波函数的性质(1)几率和几率密度根据波函数的几率解释波函数有如下重要性质根据波函数的几率解释,波函数有如下重要性质:•在t 时刻r点,d τ = dx dy dz体积内,找到由波函数Ψ (r, t) 描写的粒子的几率是:z d W(r, t) = C|Ψ (r, t)|2dτ,其中C是比例系数。
在t时刻r点,单位体积内找到粒子的几率(几率密度):ω(r,t)={dW(r,t)/dτ}=C|Ψ(r,t)|2,在体积V 内,t 时刻找到粒子的几率为:W(t) = ∫V dW= ∫Vω( r, t ) dτ= C∫V|Ψ (r,t)|2dτ12J. –M. Huang•不满足这一要求。
关于自由粒子波函数如何归一化问不满足这要求关于自由粒子波函数如何归化问题,以后再予以讨论。
13J. –M. Huang•这与经典波不同。
经典波波幅增大一倍(原来的2倍),则相应的波动能量将为原来的4倍,因而代表完全不同的波动状态。
经典波无归一化问题。
14J. –M. Huang归一化常数z若Ψ (r , t ) 没有归一化,∫∞|Ψ (r , t )|2dτ= A (A 是大于零的常数),则有∫∞|(A)-1/2Ψ (r , t )|2dτ= 1z注意:对归一化波函数仍有一个模为一的因子不定性。
若Ψ(r,t)是归一化波函数,那末,exp{iα}Ψ(r,t)也是归一化波函数(其中α是实数),与前者描述同一也是归一化波函数(其中α是实数)与前者描述同一几率波。
也就是说,(A)-1/2Ψ(r,t)是归一化的波函数,与Ψ(r,t)描写同一几率波,(A)-1/2称为归一化因子。
15J. –M. Huangi′)()()()(000x x x f x x x f −=−δδ)(||)(x a ax δδ=dx e p p xp p x x x x )(21)(−∞∞−∫=′−==πδ16J. –M. Huangedx t x t x ′−=ΨΨ′δ)(p p ′−=δ)(),(),(x x p p p p x x ∞−∫)(x x p p ′−δ平面波可归一化为函数x x )()()()(000x x x f x x x f −=−δδ17J. –M. Huang注意:这样归一化后的平面波其模的平方仍不表示几率密度,依然只是表示平面波所描写的状态在空间各点找到粒子的几率相同。
18J. –M. Huang§2 态叠加原理(一)态叠加原理(二)动量空间(表象)的波函数()()19J. –M. Huang()态叠加原理(一)态叠加原理z微观粒子具有波动性,会产生衍射图样。
而干涉和衍射的本质在于波的叠加性,即可相加性,两个相加波的干涉的结果产生衍射。
因此,同光学中波的叠加原理一样,量子力学中也存在波叠加中波的叠加原理一样原理。
因为量子力学中的波,即波函数决定体系,,的状态,称波函数为状态波函数,所以量子力学的波叠加原理称为态叠加原理。
20J. –M. Huang考虑电子双缝衍射PΨ1ΨS 1一个电子有Ψ1 和Ψ2两种可能的状态Ψ是Ψ2S 2电子源感光两种可能的状态,Ψ 是这两种状态的叠加。
z Ψ= C 1Ψ1+ C 2Ψ2也是电子的可能状态。
屏z 空间找到电子的几率则是:z |Ψ|2= |C 1Ψ1+ C 2Ψ2|2= (C 1*Ψ1*+ C 2*Ψ2*) (C 1Ψ1+ C 2Ψ2) =|C 2+|C 2+[C **+C **z|C 1Ψ1|+ |C 2Ψ2|+ [C 1C 2Ψ1Ψ2+ C 1C 2Ψ1Ψ2]电子穿过狭缝电子穿过狭缝相干项1出现在P点的几率密度2出现在P点的几率密度正是由于相干项的出现,才产生了衍射花纹。
21J. –M. Huang一般情况下如果是体系的可能状态那末它们一般情况下,如果Ψ1和Ψ2是体系的可能状态,那末它们的线性叠加Ψ=C 1Ψ1+C 2Ψ2也是该体系的一个可能状态.•其中C 1和C 2是复常数,这就是量子力学的态叠加原理。
态叠加原理一般表述:若ΨΨ2,..., Ψ,...是体系的一系列可能的状态1, 2 ,,n ,,则这些态的线性叠加Ψ= C 1Ψ1+ C 2Ψ2+ ...+ C n Ψn + ...(其中C 1, C 2 ,...,C n ,...为复常数)也是体系的一个可能状态。
处于Ψ态的体系,部分的处于Ψ1态,部分的处于Ψ2态...,部分的处于Ψn ,...22J. –M. Huang而衍射图样正是这些平面波叠加干涉的结果。
23J. –M. HuangC()z C(p,t)是以动量p为自变量的波函数,动量空间波函数,动量表象波函数;z二者描写同一量子状态。
24若Ψ (r,t)已归一化,则C(p, t)也是归一化的d t c t c d t c G G GG G 2∗∝=证明:rd t r r t p c p G G G G G ),()(),(ΨΦ=∗∞∞−∫pp p p p ),(),(|),(|∝−∫∫pd r d r t r r d r t r p pG G G G G G G G G ]')'(),'(][)(),([∗∗ΦΨΦΨ=∫∫∫∫∫∫∗∗ΦΦΨΨ=p d r r r d r d t r t r p p G G G G G G G G G )'()('),'(),(∗G G G G G G ∫∫−ΨΨ=)'('),'(),(r r r d r d t r t r δ∫=ΨΨ=∗1),(),(r d t r t r G G G 关系式其中使用了−=ΦΦ∫∗δ)'()'()(r r p d r r p pGG G G G G G 函数的目的。
平面波归一化为由此我们也可以看出把−δ25J. –M. Huang具有类似的物理含义与),(),(t r t r c G G Ψ点附近时刻粒子出现在r t r d t r t r dW GG G G Ψ=2|),(|),(体积元内的几率;r d G→pd t r c t p dW GG G G =2|),(|),(体积元内的几率。
