化学化工专业英语部分练习参考答案
化学化工专业英语范东生答案

化学化工专业英语范东生答案1.There are several(A)students in our grade.A.hundred.B.hundreds of.C.hundred of.D.hundreds of the.2.Seven minutes for us to finish the jobs(B)quite enough.A.are.B.am.C.is.D.were.3.What()bad news.My parents order me to finish(A)hard work in()bad weather.A.a,such a,such a.B.such,such.C.such,so.D.a,such,such a.4.English is always heard(C)in these days of China.A.taught.B.be taught.C.have taught.D.to be taught.5.Can you tell me how()medicine has(A).A.many,lost.B.much,lost.C.many,been lost.D.much,been lost.6.()New Year is coming,what will you do on(A)New Year‟s Day.A.the.B.;C.The;a.D.The.7.left my pen and now I‟v e no one(C).A.to write.B.to write by.C.to writ for.D.to write with.8.It was too noisy for me(C).A.to go to bed.B.to fall asleep.C.to get to asleepD.to go to asleep.9.What(C)you sing if she plays the piano for you.A.would like.B.did.C.do.D.will.10.When the hard job(C),we‟ll travel to the Great Wall.A.is finished.B.was finished.C.will be finished.D.has been finished.。
化工专业英语试卷参考答案

化学工程与工艺专业英语试题卷参考答案一.Put the following into English or Chinese.1.石油化学制品2. alkali3. sodium carbonate4. 聚合作用5. ammonia6. 药物7. antioxidant8. 聚四氟乙烯9. 环己烷10..carbonmonoxide 11. 乙醇胺12. thermodynamics 13. 光谱学14. refinery 15. 多相的16. isothermal17. 聚氧化亚甲基18. chloride 19. ethanol 20. 聚氯乙烯二.Translation.1. 4 generations under one roof2. to be a winner of the family3. 教务处4.国家助学金5.Principles of chemistry(unit operations)6.生产实习7.graduation thesis8.妇产医院9.transport phenomena10.金窝,银窝,不如自己的狗窝11.normal university 12.综合性大学13.应试教育14.master of business administrator 15.分析化学16.税务局17.party committee 18.专卖店19.chain store(multiple shop) 20.主任医生三、Put the following sentences underlined into ChineseA在20世纪六、七十年代,由于聚乙烯、聚丙烯、尼龙、聚酯环氧树脂等聚合物合成需求量的大量增加,石油化工产品产量呈现爆炸式的增长。
B单一的化工厂产量有从精细化工领域的每年几吨到肥料、石油领域的化工巨头的每年500,000吨。
C一方面,化学生产工业的扩张,另一方面,化学工程与工艺科学的先进,这些使为化工生产奠定了理论基础成为了可能。
化学化工英语试题及答案

化学化工英语试题及答案一、选择题(每题2分,共20分)1. Which of the following is a chemical element?A. WaterB. OxygenC. HydrogenD. Carbon答案:B, C, D2. The chemical formula for table salt is:A. NaOHB. NaClC. HClD. NaHCO3答案:B3. What is the process called when a substance changes from a solid to a liquid?A. SublimationB. VaporizationC. MeltingD. Condensation答案:C4. In the periodic table, which group contains alkali metals?A. Group 1B. Group 2C. Group 17D. Group 18答案:A5. What is the name of the process where a substance decomposes into two or more substances due to heat?A. CombustionB. OxidationC. ReductionD. Decomposition答案:D6. Which of the following is a physical property of a substance?A. ColorB. TasteC. SolubilityD. Reactivity答案:A7. What is the term for a compound that releases hydrogen ions (H+) when dissolved in water?A. BaseB. AcidC. SaltD. Neutral答案:B8. The law of conservation of mass states that in a chemical reaction:A. Mass is lostB. Mass is gainedC. Mass remains constantD. Mass can be converted into energy答案:C9. Which of the following is a type of chemical bond?A. Ionic bondB. Covalent bondC. Hydrogen bondD. All of the above答案:D10. What is the name of the process where a substance absorbs energy and changes from a liquid to a gas?A. MeltingB. VaporizationC. SublimationD. Condensation答案:B二、填空题(每题2分,共20分)1. The symbol for the element iron is ________.答案:Fe2. The pH scale ranges from ________ to ________.答案:0 to 143. A compound that produces a basic solution when dissolvedin water is called a ________.答案:base4. The smallest particle of an element that retains its chemical properties is called a ________.答案:atom5. The process of separating a mixture into its individual components is known as ________.答案:separation6. The study of the composition, structure, and properties of matter is called ________.答案:chemistry7. The process of a substance changing from a gas to a liquid is called ________.答案:condensation8. A(n) ________ reaction is a type of chemical reactionwhere two or more substances combine to form a single product. 答案:synthesis9. The volume of a gas at constant temperature and pressureis directly proportional to the number of ________.答案:moles10. The process of converting a solid directly into a gas without passing through the liquid phase is known as ________. 答案:sublimation三、简答题(每题10分,共30分)1. Explain what is meant by the term "stoichiometry" in chemistry.答案:Stoichiometry is the calculation of the relative quantities of reactants and products in a chemical reaction.It is based on the law of conservation of mass and involvesthe use of balanced chemical equations and the molar massesof substances to determine the amounts of reactants needed to produce a certain amount of product or the amounts ofproducts formed from a given amount of reactant.2. Describe the difference between a physical change and a chemical change.答案:A physical change is a change in the state or form of a substance without altering its chemical composition. Examples include melting, freezing, and boiling. A chemical change, on the other hand, involves a change in the chemical composition of a substance, resulting in the formation of new substances. Examples include combustion and rusting.3. What are the three main types of chemical bonds, and givean example of each.答案:The three main types of chemical bonds are ionic bonds, covalent bonds, and metallic bonds. An ionic bond is formed when electrons are transferred from one atom to another, resulting in the formation of oppositely charged ions. An example is the bond between sodium (Na) and chloride (Cl) in table salt (NaCl). A covalent bond is formed when two atoms share electrons, as seen in water (H2O) where hydrogen atoms share electrons with oxygen. Metallic bonds occur in metals, where a "sea" of delocalized electrons is shared among positively charged metal ions, as in sodium metal。
化工原理习题答案英文

Problems and Solutions Distillation1、 A continuous fractionating column is used to separate 4000kg/h of a mixture of 30percent CS 2and 70 percent CCl 4. Bottom product contain 5 percent CS 2at least, and the rate of recovery of CS 2in the overhead product is 88% by weight,required. Calculate (a) the moles flow of overhead product per hour .(b) the mole fractions of CS 2and CCl 4in the overhead product, respectivelySolution: Form overall material balance1F D W F D W Fx Dx Wx =+'''=+ () (2)Known by the justice of the problem0.88D F Dx Fx ''= (3)Take the place of 3 types and enter 2 types0.880.122880/0.05400028801220/0.880.8840000.30.9431120F F w w FD Fx Fx Wx kg hx D F W kg h Fx x D '''=+'⨯4000⨯0.3=='=-=-='⨯⨯'===F 0.12Fx W=The unit converts :0.943/760.970.943/760.057/154D x ==+(mole fraction )0.97760.0315478.3/112014.3/78.3m M kg kmolD Kmol h =⨯+⨯=∴==2、 A liquid containing 40 mole percent methanol and 60 mole percent wateris to be separated in a continuous fractional column at 1 atm pressure .Calculate the value of q under the three following conditions (a) the feeding is liquid at 40 C (b) the feeding is saturated liquid. The equilibrium data for methanol-water liquid at 1 atm pressure are given in the attached table. If the column is fed with 100koml/h.The molar fractions of methanol in overhead product and bottom product are 0.95 and 0.04,respectively.A reflux ratio at the top of column is 2.5.Calculate (a) the mole flow of overhead product per hour (b) the mole flow of liquid in rectifying column (c) the mole flow of vapor in stripping column .Assume that the constant molar flow applies to this system . Solution: Form overall material balancef D w F D WFx Dx Wx =+=+Solve the eqution we can have :()100(0.40.04)39.6/0.950.0410039.660.4/F w D w F x x D kmol hx x W kmol h--===--=-=And 2.539.699/39.699138.6/L RD kmol hV D L kmol h ==⨯==+=+=These upper values are fixed under the three feed conditions 。
化工专业外语试卷A答案
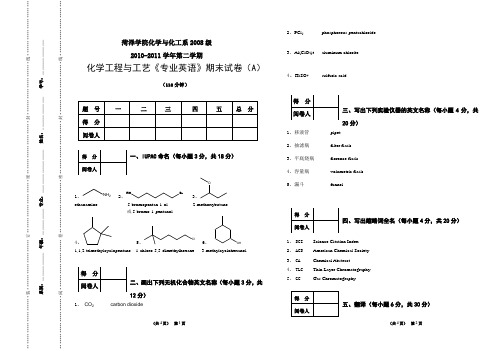
菏泽学院化学与化工系2008级 2010-2011学年第二学期化学工程与工艺《专业英语》期末试卷(A )(110分钟)一、IUPAC 命名(每小题3分,共18分)1、NH 22、HOBr3、Oethanamine 5-bromopentan-1-ol 2-methoxybutane或5-bromo-1-pentanol4、5、Cl6、OH1,1,2-trimethylcyclopentane 1-chloro-5,5-dimethylhexane 3-methylcyclohexanol二、画出下列无机化合物英文名称(每小题3分,共12分)1、 CO 2 carbon dioxide2、PCl 5phosphorous pentachloride3、Al(ClO 2)3 aluminum chlorite4、H 2SO 4sulfuric acid三、写出下列实验仪器的英文名称(每小题4分,共20分)1、移液管pipet2、抽滤瓶 filter flask3、平底烧瓶 florence flask4、容量瓶 volumetric flask5、漏斗funnel四、写出缩略词全名(每小题4分,共20分)1、 SCI Science Citation Index2、 ACS American Chemical Society3、 CA Chemical Abstract4、 TLC Thin Layer Chromatography5、 GCGas Chromatography五、翻译(每小题6分,共30分)························阅·······················卷························密························封························线·························系别:_____________ 年级:____________ 专业:____________________ 姓名:_______________ 学号:························装·······················订························密························封························线·························1、A scientific paper is an organized description of hypotheses, data and conclusions, intended to instruct the reader. Papers are a central part of research. If your research does not generate papers, it might just as well not have been done.科技论文是以向读者论述为目的,对假说、数据和结论所做的系统性描述。
化工专业英语练习题 参考答案

练习一参考答案1将下列句子或段落翻译成英语1)A process is any operation or series of operations that causes a physical or chemical change in asubstance or a mixture of substances .The material that enters a process is referred to as input or feed the process,and that which leaves is called output or product.2)As a chemical engineer,you might be called on to design individual process units (such as reactors,distillation columns,heat exchangers),supervise the operation of a process,or modify a process design to accommodate a change in the feed or in the desired product characteristics.As a rule,to any of these things you must know the amounts,compositions,and conditions of the materials that enter and leave each process unit,and if you are working with an existing units,you must be able to measure enough of these quantities to verify that the process is doing what it was designed to do.3)Founded in 1839from a small production firm for pharmaceutical products,B.Braun has grown steadilyinto a multinational company dealing with medical products,medical technology,pharmaceutical and biotechnology.2将下列句子或段落翻译成汉语1)包括的一系列操作,如混合、蒸发、过滤,无论产物是什么,这些操作都基本同,从而导致了单元操作的概念。
《化工专业英语》答案

《化工专业英语》答案一、词汇翻译1. 盐酸2. 颜料3. 硫酸4. 氢氧化钾5. 氯化钠6. 硝酸铵7. 甲烷8. 硅9. 碳酸钙10. 二氧化碳11.碳酸钙12.乙炔13.氯化钾14.氧化汞15.网格球顶16.晶体学17.对称的18.杂化19.聚氨酯20炸药二、阅读理解1.C2.D3.B4.D5.A;C D C D A四、英译汉1. 也许你主修保健科学,希望从事一个医学或药学方面的工作。
如果这样,你将希望熟悉水溶液的性质,包括血液和其它体液。
分在过去的几十年里,化学家们研制了很多挽救生命的产品,包括在化学疗法中使用的药物和用于对付抗性微生物的新型抗生素2. 在这种土壤中,化合物中的氮是植物生物化学过程中的有限反应物农民通过向土壤中施加氨和其它氮肥以增加这种有限反应物的量,从而大幅提高大豆、小麦和其它作物的产量3. 食品工业中使用盐酸从玉米淀粉中制备玉米糖浆,从骨头中制取明胶。
这些反应包括通过酸的作用将大分子破碎成小分子。
盐酸也存在于胃部中,帮助消化破碎食物,包括将淀粉转化成糖。
4. 在杂货店中出售的清洗液“阿莫尼亚”实际上是氨气的水溶液。
氨水具有一种特征的刺激性气味。
闻氨水气味时要特别小心,让瓶子距离自己的鼻子一段距离,用手轻轻扇动瓶中冒出的氨气,只能闻飘过来的少量氨气。
5. Smalley以擅长网格球顶设计的建筑师巴克敏斯特富勒的名字来命名这个分子为“巴克敏斯特富勒烯”,他提出的结构与足球相同,C60很快又被冠以“巴基球”的昵称。
化学工程与工艺专业英语课后答案(1)

Key to Exercise Unit 1 Chemical Industries1.the Industrial Revolutionanic chemicals3.the contact process4.the Haber process5.synthetic polymers6.intermediates7.artificial fertilizers 8.pesticides9.synthetic fibers10.pharmaceutical11.research and development12.petrochemicalputers14.capital intensiveSome Chemicals Used In Our Daily LifeFood artificial fertilizers, pesticide, veterinary products Health antibiotics, β-blockersClothing synthetic fibers (e.g. polyesters, polyamides),synthetic dyesShelter synthetic polymers (e.g. urea-formaldehyde,polyurethanes),plasticsLeisure plastics and polymers (e.g. nylon)Transport additives (e.g. anti-oxidants, viscosity indeximpovements),polymers, plasticsUnit 2 Research and Development1.R&D2.ideas and knowledge3.process and products4.fundamental5.applied6.product development7.existing product8.pilot plant9. a emerging case10.environmental impact11.energy cost 12.technical support13.process improvement14.effluent treatment15.pharmaceutical16.sufficiently pure17.Reaction18.unreacted material19.by-products20.the product specification21.Product storageUnit 3 Typical Activities of Chemical Engineers1.Mechanical2.electrical3.civil4.scale-upmercial-size6.reactors7.distillation columns8.pumps9.control and instrumentation10.mathematics11.industry12.academia13.steam14.cooling water 15.an economical16.to improve17.P&I Drawings18.Equipment Specification Sheets19.Construction20.capacity and performance21.bottlenecks22.Technical Sales23.new or improved24.engineering methods25.configurationsUnit 4 Sources of Chemicals1.inorganic chemicals2.derive from3.petrochemical processes4.Metallic ores5.extraction process6.non-renewable resource7.renewable sources8.energy source9.fermentation process10.selective 11.raw material12.separation and purification13.food industry14.to be wetted15.Key to success16.Crushing and grinding17.Sieving18.Stirring and bubbling19.Surface active agents20.OverflowingUnit 5 Basic Chemicals1.Ethylene2.acetic acid3.Polymerization4.Polyvinyl acetate5.Emulsion paintHigh-volume sector Low-volume sectorProduction scale tens to hundreds of thousandstons per yeartens to a few thousands tonsper yearProducts / a plant single product multi-products Operation manner continuous batch Price or profit fairly cheap very profitable Usage intermediates end-productsChallengesreduced demand, environment pollutionProducts in the sectorsulphuric acid,phosphorus-containingcompounds,nitrogen-containingcompounds,chlor-alkali,petrochemicals,commodity polymersagrochemicals,dyestuffs,pharmaceuticals,speciality polymersUnit 6 Chlor-Alkali and Related Processes1.Ammonia2.ammonia absorber3.NaCl & NH4OH4.Carbon dioxide5.NH4Cl6.Rotary drier7.Light Na2CO38.WaterProduct Raw materialMajor steps orPrincipal reactionsUsesSoda-ashbrine,limestoneammoniating,carbonating,precipitating,filtering,drying,calciningraw material forglassmaking,sodium silicate;as an alkaliChlorine brine2Na+ + 2Cl - +2H2O →NaOH +Cl2 +H2as water purification, bleaching of wood pulp;production of vinyl chloride, solvents, inorganic chlorine-containing productsCaustic soda brine2Na+ + 2Cl - +2H2O →NaOH +Cl2 +H2for paper-making, manufacture of inorganicchemicals, syntheses of organicchemicals, production of aluminaand soapSulfuric acid elemental sulphurS +O2→ SO2SO2 + O2→ SO3SO3 + H2O → H2SO4feedstock for fertilizers;production of ethanol,hydrofluoric acid,aluminum sulphatesUnit 10 What Is Chemical EngineeringMicroscale (≤10-3m)●Atomic and molecular studies of catalysts●Chemical processing in the manufacture of integrated circuits●Studies of the dynamics of suspensions and microstructured fluidsMesoscale (10-3-102m)●Improving the rate and capacity of separations equipment●Design of injection molding equipment to produce car bumpers madefrom polymers●Designing feedback control systems for bioreactorsMacroscale (>10m)●Operability analysis and control system synthesis for an entire chemicalplant●Mathematical modeling of transport and chemical reactions ofcombustion-generated air pollutants●Manipulating a petroleum reservoir during enhanced oil recoverythrough remote sensing of process data, development and use of dynamicmodels of underground interactions, and selective injection of chemicalsto improve efficiency of recoveryCourse Course contentScience and Math.Chemistry, Physics, Biology, Material Science, Mathematics,Computer InstructionChemical EngineeringThermodynamics, Kinetics, Catalysis,Rector Design and Analysis, Unit Operations, Process Control, Chemical Engineering Laboratories, Design / EconomicsOther Engineering Electrical Engineering, Mechanics, Engineering DrawingHumanities and SocialScience Understand the origins of one’s own culture as well as that ofothersUnit 21 Chemical Industry and Environment1.Atmospheric chemistry2.stratospheric ozone depletion3.acid rain4.environmentally friendly products5.biodegradable6.harmful by-product7.efficiently8.power plant emissions9.different plastics10.recycled or disposed11.acidic waste solutions anic components13.membrane technology14.biotechnology15.microorganismsFrontier Research activities or problems facedIn-site processingField tests; Uncertainties of the process, Adverse environment impactsProcess solidsImprove solids fracture processes,Research on the mechanics of pneumatic and slurry transport, Understand the chemical reaction processes,Equipment design and scale-upSeparation processResearch on:membrane separations, chemical selective separation agents, shape-selective porous solids,traditional separation methodsMaterialsFind construction materials, Develop new process-related materials, Develop less energy intensive materialsDesign and scale-up Complexity, Lack of basic data,。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
化学化工专业英语部分练习参考答案P8.练习答案:(4)I-steel 工字钢;I-shaped I型材;n-region n区p-region p区;T-beam 丁字梁,T型梁P-N-junction P-N结;T-connecting 丁字接头,T形接法A.C.:alternating current 交流电D.C.:direct current 直流电P13.练习答案:一、注意名词单复数的译法1、这台设备已经用了许多年了。
2、空气是各种气体的混合物。
3、许多植物能利用二氧化碳中的氧。
4、带负电荷的机体所含电子数多于质子数。
5、其它蒸发材料(物质)和蒸发过程将简要地加以讨论。
6、这样的一些操作要求物质由气流传递到液体中去。
7、如果不饱和性归因于三键的存在,那么这些化合物都会被称为炔烃。
8、尚未试图在蒸发过程中将蒸汽分馏成各种馏分。
9、许多盐、酸和碱等化合物将被广泛应用于家庭、工业和医药实践中。
10、苯的同系物是那些苯环上含有单烷基(取代一个氢)或多烷基(取代多个氢)的物质。
11、当然,一旦酸失去质子,碱必然接受质子。
因此,酸和碱的反应就是一种质子转移的反应。
这种反应就是我们通常所知的中和反应。
12、这篇文章着重讨论一些原料和成品生产技术上最近的发展。
二、注意词义引申的译法1、叔醇非常难以氧化。
2、含1个到4个碳原子的正烷烃是气体。
3、通常,所有的金属都是良导体,其中银的传导性最好,其次是铜。
4.从两种或任意多种溶液中分离溶质都需要蒸馏的分馏技术。
三、注意词的增译及省译1、橡胶能阻止电流通过。
2、过去每到他轮班时就会给车床上油。
3、从这个意义上讲,结构分析普遍应用于大多数的有机研究。
4、过去在电子尚未被发现以前,人们就假定了不可见电流是从正极流向负极。
5、有人看见这些工人在修理发电机。
6、这些植物提取物的功效已经为早期人类所发现。
7、树木之所以幸存是因为其进化已经使它们成为了高度分隔的有机体。
8、共用电子对必须向相反方向作旋转。
9、室温下汞能在空气中稳定存在,加热条件下,能与氧化合。
10、如果燃烧,燃烧热加上分解热就会产生很热的火焰。
11、汞蕴藏不丰富,分布也不广泛。
12、物理学是研究热、声音、磁、电、光物质性质及其组成的科学。
13、1930年以前《化学文摘》文献的覆盖面要比《化学中心会报》窄得多,因而1930年以前的文献总是可以在这两种文献中查到。
14、有机化合物通常易挥发而且熔点低。
15、事实上所有的物质受热时会膨胀而冷却时会收缩。
热胀冷缩16、所谓“活性组分”适度地加强和扩大了这种化妆品的效果,其进展有很多领域值得用另一篇文章去研究。
P18.练习答案:注意词类转换的译法1、操作机器需要懂得机器的一些性能。
2、电子从锌负极板向铜正极板流动。
3、工作中,他们很注意理论联系实际。
4、使用石油的便利是显而易见的。
5、这些操作仅用于回收或除去溶质。
6、当电子穿过金属导线时,就可得到电能。
7、众所周知木材的强度不如钢。
8、我们也熟悉能量的其它转换。
9、事实上,玻璃比石英易溶得多。
10、日常经验告诉我们重的物体比轻的物体稳定得多。
11、乙醇的快速氧化会导致二氧化碳和水的生成。
12、如果材料是有机的,其化学性质得基于碳。
13、我们发现这台发动机在高速运转着。
14、从盐中制得的氯气,可用于漂白纸张和纺织品。
15、可以把气体溶于液体的过程当作一个物理过程。
16、力、质量、加速度之间的关系可表述如下。
17、操作机器前应在机器的各部分上好润滑油。
18、丙酮用在各种不同的有工业重要性产品的制造中。
19、机器上不规则的各部分可以用超声波清洗得非常干净。
20、碳原子的一个突出性质就是它能够和其它碳原子共用它的电子。
21、蒸发海水生成的食盐,蒸发和结晶出来的晶体形状是远远不同的。
22、气体与固体之不同在于前者的可压缩性比后者大的多。
23、当更多的锌原子溶解时,锌电极所含电子数相应地增多,负电性增强。
24、与无机化合物相反,有机化合物分子间引力弱,因而有机化合物通常不稳定而且熔点低。
25、我们都熟知这样一个事实,即:自然界中没有一件东西会自行开始或停止运动。
26、增溶剂能将不相溶的组分溶在一起形成均匀的溶液。
P22.练习答案:注意句子成分转换的译法1、碳的这种重要性质是有用的。
2、铝合金的热处理有两种类型可用。
3、原油中各种不同烃的沸点各不相同。
4、河流下游的伸展有相当多的变化。
5、这些机器的重量各不相同。
6、这些金属中没有一种金属的传导性比铜强。
7、钠的化学性质非常活泼。
8、在一个吸收系统里,氨是常用的制冷剂。
9、二极管的正向阻抗很小。
10、应注意分离油容器的填充物。
11、有机化合物溶液往往不导电。
12、我们能看见的任何物体所含的分子数都多得难以想象。
13、全世界开始使用相同的数学符号和记号。
14、因此,可以将吸收过程简单地分成两种:物理过程和化学过程。
15、一个共价键中的两个电子自旋相反。
16、也许有人对有机化学所涉及的物质有所了解,但他可能并不知道有机化学触及我们日常生活已达到什么样的程度。
第3课翻译从这些电势可明显看出,从FeO42-到Fe3+反应放出大量的能量,因此FeO42-是一种强氧化剂。
类似地,Fe3+是较弱的氧化剂,Fe3+可被还原成Fe2+,但Fe3+和Fe2+都没有被还原成Fe的趋势。
标准电极电势是以如下表度为准来测量的:~。
H+ + e = H E0 = 0.0 voltsE0值越负的反应其还原能力比氢越强,即是说它们具有强的还原性。
物质半反应的E0值高于+0.8v的,通常认为它们是还原剂,半反应如Fe3+→Fe2+,它们的E0值在+0.8v左右是稳定的(氧化性与还原性相等),而那些E0值低于+0.8v的半反应则有逐渐增强的还原性。
第14课翻译这些分析方法(技术)的使用有三种方式。
【1】实时分析。
此方法是在反应进行中分析物系的组成(如取少量样品或测定这个反应体系)。
【2】骤熄法。
此方法是反应进行一定时间后立即停止反应,然后再分析其组成。
从整个反应体系或从中取出小部分样品,通过骤冷或向混合物中添加大量溶剂,使反应骤熄。
此法只适用于极慢的反应,即在冷却混合物的时间内反应几乎不进行。
【3】流动法。
此方法是将反应物混合于展开槽中。
当随着整个反应混合物流经出口管,反应不断进行。
在沿管道的不同位臵测定其组成(如光学地),即等效于测定混合后不同时间反应混合物的组成。
该方法可以研究在数毫秒内完成的反应,但这需要大量体积的溶液。
第16课翻译【1】传导。
在固体中,由传导方式引起的热流动,是固体的一个分子向另一个分子传递振动能的结果。
而在流体中,还由于动能传递所致。
传导的热传递方式也可从自由电子的运动中产生。
该过程对金属而言相当重要,它解释了它们具有高的热导率。
【2】对流。
由对流方式引起的热流动归因于流体的宏观运动,因而仅限于液体和其他。
其中的自然对流是由体系中的温度梯度造成其密度差别所引起的。
而强制对流是湍流流体中的涡流造成。
【3】辐射。
所有物质将以电磁波的方式向外辐射热能。
当这些辐射能作用于另外一个物体时,将分别发生反射,透过或吸收。
仅是被吸收的这部分(辐射能)才以热的形式在物体中表现出来。
第20课翻译在诸如水的溶剂中,表面活性剂分子以此种方式分布,即它们在界面上的浓度比在溶液内部高,这种状态可归因于它们两性分子的结构(亲水基,憎水基)。
在相边界,表面活性剂分子存在定向排列。
这导致了系统性质的改变,如水和邻相间的界面张力降低,吸湿性改变,又在界面形成双电层。
在溶液内部,当某种表面活性剂的浓度过大时,就会形成表面活性剂的集合体(胶束)。
第23课翻译P139第六段:烷基硫酸钠自其于1930年以来的商业应用至今,一直是最重要的在使用的脂肪基表面活性剂之一。
它们广泛应用于家用产品中,经过复配后用于洗衣、洗碗、地毯清洗等。
剃须膏基化妆品配方中常含有烷基硫酸盐。
十二烷基乙二醇胺硫酸盐和十二烷基三乙醇胺硫酸盐较其他反位的氨类或钠类盐对皮肤的刺激性要小得多。
烷基硫酸钠可用作发泡剂或蛋白中的乳化剂,用于制造药属葵蜜饯及富马酸酸化的果饮。
作为一种食品添加剂,它必须满足包括联邦法规在内的约束。
P140:条状肥皂的商业制造是通过脂肪的皂化作用或用碱中和脂肪酸获得。
传统制造肥皂的方法是采用间歇工艺用碱皂化脂肪制备。
此过程中用碱液加热脂肪;之后便形成了肥皂和甘油基醇。
通过添加盐(产生盐析作用)并之后洗涤的方法使肥皂从甘油基醇中分离出来。
经干燥并铸成条状后得到最终的肥皂。
(目前)皂化方法至今仍在使用,但已(改成)连续工艺,并在工艺的不同阶段使用离心机来进行分离。
这样含有35%水分的肥皂就制造出来了,该产物再进行喷雾干燥。
通过加工提高肥皂的物理性能后,肥皂经挤压最终形成条状。
